Abstract
Metal–organic frameworks (MOFs) represent the largest class of materials among crystalline porous materials ever developed, and have attracted attention as core materials for separation technology. Their extremely uniform pore aperture and nearly unlimited structural and chemical characteristics have attracted great interest and promise for applying MOFs to adsorptive and membrane-based separations. This paper reviews the recent research into and development of MOF membranes for gas separation. Strategies for polycrystalline membranes and mixed-matrix membranes are discussed, with a focus on separation systems involving hydrocarbon separation, CO2 capture, and H2 purification. Challenges to and opportunities for the industrial deployment of MOF membranes are also discussed, providing guidance for the design and fabrication of future high-performance membranes. The contributions of the underlying mechanism to separation performance and adopted strategies and membrane-processing technologies for breaking the selectivity/permeability trade-off are discussed.
1. Introduction
Research, development, and demonstration tests for the practical application of metal–organic frameworks (MOFs) are underway, involving companies and universities in various fields [1,2,3,4,5]. MOFs are porous materials consisting of coordination bonds between metal ions and multifunctional organic ligands. Unparalleled properties and functions (e.g., storage, adsorption, separation, catalytic, electromagnetic, and optical properties) can be exhibited by tuning their framework composition and pore structure. As companies begin to produce and market MOFs, products are being created that exploit their properties. Queen’s University Belfast start-ups, MOF Technologies and DECCO, have applied MOFs to a product that keeps fruit and vegetables fresh [6]. The role of MOFs is to store and release 1-methylcyclopropene, which inhibits the action of ethylene that ripens fruit and vegetables, as required. NuMat Technologies, a start-up company from Northwestern University, has commercialized a MOF as a gas cylinder that can store and safely transport toxic gases for the semiconductor industry [7]. Atomis, a start-up company from Kyoto University, is in the process of gaining approval for the commercial use of a MOF-based high-pressure gas container, CubiTan®. SyncMOF, a start-up company from Nagoya University, is in the process of commercializing a MOF-based gas separation system, MOFclean. Transaera, a start-up company from Massachusetts Institute of Technology, is in the process of commercializing dehumidifying air conditioning systems using MOFs. Svante and Crimeworks are also piloting the application of MOFs in direct air capture (DAC), which captures CO2 directly from the atmosphere. Thus, large-scale applications of MOFs are expected to expand.
To date, more than 14,000 unique MOFs, comprising more than 350 topologies, have been synthesized [8]. In addition, hundreds of thousands more have been computationally predicted [9]. The number of possible MOF structures may range from millions to billions [10]. When a new structure is proposed, it is important to properly characterize it in order to support its application and to understand its performance in the desired process. Different physical and chemical information can be obtained by different techniques, the choice of which depends on the type of material being studied and the equipment available. The available analytical techniques for MOFs include X-ray diffraction, X-ray photoelectron spectroscopy, X-ray energy-dispersive spectroscopy, thermogravimetry, differential thermal analysis, differential scanning calorimetry, Fourier transform infrared spectroscopy, Raman spectroscopy, scanning electron microscopy, transmission electron microscopy, nuclear magnetic resonance, and gas adsorption–desorption isotherm measurements. Of these, gas adsorption–desorption isotherm measurements are particularly essential for separation applications. Iacomi et al. used a high-throughput method to process 32,000 adsorption isotherms from the NIST Database of Novel and Emerging Adsorbent Materials [11] to predict potential separation applications [12] (Figure 1). Note that N2 and Ar adsorption at cryogenic temperatures is the measurement for the characterization of a porous structure. Figure 1 clearly indicates that the application of MOFs for separation is directed towards H2 purification, CO2 capture, and hydrocarbon separation.
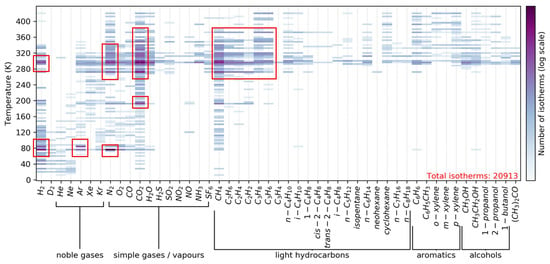
Figure 1.
High-throughput screening data on adsorption isotherms of MOFs. The number of measured isotherms is shown for each adsorbate used and measurement temperature. Color indicates the number of isotherms on a logarithmic scale. Reprinted with permission from Ref. [12]. Copyright 2020 American Chemical Society.
The present review provides insights into membrane separation technologies for gas separation using MOF-based membranes. The application studies of MOF-based membranes are growing for a wide range of target gas mixtures. Although there are several excellent reviews on the application of MOFs in separation membranes [13,14,15,16], this review focuses on (1) both adsorption properties and membrane performances, and (2) MOF-based membranes both as polycrystalline forms and as fillers in mixed-matrix membranes. The review starts with a brief discussion about the adsorption performance of MOFs, followed by the challenges faced by MOFs in terms of membrane fabrication and performance. With regard to the polycrystalline membranes of MOFs, this review provides insight into strategies to develop the molecular sieving capabilities of MOFs by intergrowing crystals. With regard to the polycrystalline membranes of MOFs, this review provides in-depth knowledge on strategies to exploit the molecular sieving capabilities of MOFs by intergrowing crystals. In addition, it also gives in-depth knowledge on strategies to improve the performance of mixed-matrix membranes by modifying the properties of fillers and polymers. Extensive gas transport data from retrospective and the latest literature on H2 purification, CO2 capture, and hydrocarbon separation using MOF-based membranes are compiled, plotted, and analyzed. Finally, future directions in the field of gas separation are discussed to support the development of MOF-based membranes with improved performance.
2. Characteristics of MOFs
2.1. Structural Flexibility
Some MOFs have flexible pore structures. It is known that the pore structure changes when gas is adsorbed. Some of these MOFs exhibit unique adsorption behavior in that they behave as nonporous materials under low-gas-pressure conditions and show no adsorption performance. On the other hand, when the gas pressure reaches a certain threshold pressure (so-called gate-opening pressure), they change to a porous structure, resulting in a rapid increase in adsorption. The gate-opening-type adsorption behavior, which is not observed in conventional porous materials, depends on the combination of metal ions and ligands constituting the framework. Various types of structural flexibility have been reported [17], for example, (1) changes in pore shape from a rhombic structure to a square structure and vice versa, (2) changes in the relative position of interpenetrating structures, (3) the stretching and shrinking of lattice layers, and (4) the rotation of ligands at the pore aperture. Furthermore, adsorption behavior has been reported to vary with crystal size and shape. For example, [Cu2(bdc)2(bpy)]n (bdc = benzene-1,4-dicarboxylic acid, bpy = 4,4′-dipyridyl) [18] and ZIF-8 [19] have been reported to exhibit higher gate-opening pressure with smaller crystals.
2.2. Structural Stability
The thermal and chemical stability of materials is one of the most important properties not only for membrane separation, but also for many industrial applications. Due to the instability of the metal–ligand coordination bond, the structure of many MOFs is degraded by moisture in the air. In order to prevent the collapse of the network structure due to the hydrolysis reactions of the metal–ligand coordination bonds or ligand substitution reactions, it is effective to have either a strong coordination bond that is thermodynamically stable or a kinetically stable structure using large steric hindrance. Basically, when the coordination environment with the ligand is the same, metal ions with higher valence and charge density form a more stable framework. This tendency is explained according to the HSAB theory and supported by many findings in MOF studies [20]. According to the HSAB theory, carboxylic acid ligands can be regarded as hard bases that form stable complexes with hard acid metal ions such as Al3+, Cr3+, Fe3+, Ti4+, and Zr4+. MIL series and UiO-66 are well-known MOFs with high structural stability synthesized by such a combination (Figure 2 and Table 1). Imidazolate and azolate ligands of soft bases form relatively stable structures together with divalent metal ions of soft acids such as Zn2+, Co2+, and Cu2+. The most representative example is the ZIF series, which is composed of Zn2+ and imidazolate [21].
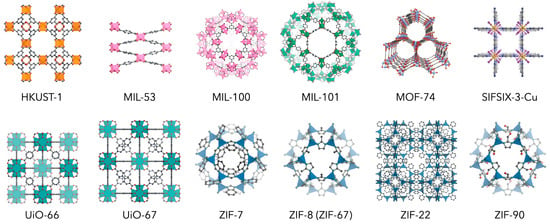
Figure 2.
Representative MOFs with relatively high structural stability.

Table 1.
Brief overview of structural parameters of commercially available MOFs.
3. Hydrocarbon Adsorption on MOFs
3.1. Olefins and Paraffins
The first MOF investigated for potential application in olefin/paraffin separation was HKUST-1, which consists of a paddle-wheel Cu(II) dimer and 1,3,5-benzenetricarboxylate as building blocks. Wang et al. measured the adsorption isotherms of C2H4 and C2H6 on HKUST-1 at 295 K and showed that C2H4 is preferentially adsorbed [22]. Water molecules are coordinated to the metal site of HKUST-1 and dehydration forms coordinatively unsaturated open metal sites [23]. Lamia et al. found that C2H4 is adsorbed due to the interaction between the π-electrons of C2H4 and the partially positively charged open metal site, whereas C2H6, which has no C=C double bond, has a low binding affinity to the open metal site, resulting in a selective separation function [24].
MOFs with open metal sites include frameworks of the MIL series, such as MIL-53, MIL-96, and MIL-100, and MOF-74. The MIL series, consisting of trivalent transition metals such as Fe(III), Cr(III), Al(III), and V(III), has been widely studied as a MOF for gas separation. Compared to divalent metals, trivalent transition metals have stronger bonds to ligands and can form more chemically stable structures [25]. However, the strong bonding between the metal and the ligand makes it difficult to synthesize MOFs with high crystallinity, and synthetic methods that satisfy the conditions for spontaneous self-assembly by reversible “weak bonding” are required. For example, MOFs have been synthesized under strongly acidic conditions using HF or HCl [26,27,28,29], or by a solvothermal method at high temperatures (100~ °C) [30,31,32].
The MIL series has trivalent metal sites with high electrophilicity and is excellent for the adsorption of electron-rich olefins. Yoon et al. reported that MIL-100(Fe) can be applied to C3H6/C3H8 separation [33]. Lee et al. reported that MIL-101(Cr), from which terephthalate anions were removed by treatment with NH4F solution, showed C2H4/C2H6 selectivity ~4 [34]. In addition, attempts to improve the selectivity by using the interaction between Cu(I) or Ag(I) sites and C=C bonds of C2H4 have been reported by depositing Cu nanoparticles on the pore surface of MIL-101(Cr) [35], or by introducing a functional group (-SO3Ag) as a building block ligand [36]. Similarly, Kim et al. obtained a C3H6/C3H8 selectivity of ~13 by modifying MIL-100(Fe) with Cu(I) [37].
MOF-74 is a honeycomb structure composed of Mg(II), Mn(II), Ni(II), Co(II), Zn(II), Cu(II) or Fe(II), and 2,5-dihydroxyterephthalate as building blocks. Bao et al. first investigated Mg-MOF-74 for the separation of C2H4/C2H6 and C3H6/C3H8 (Figure 3) [38]. Bae et al. compared the influence of metal sites on the adsorption selectivity of C3H6/C3H8 using Mg-, Mn-, and Co-MOF-74. The effect of metal sites on the adsorption selectivity of C3H6/C3H8 was compared, and it was reported that the selectivity was higher for Mg (selectivity 4.5) < Mn (24) < Co (46) [39]. The influence of the type of phthalate ligand of MOF-74 on the olefin/paraffin separation was also studied, and the replacement of 2,5-dihydroxyterephthalate with 4,6-dihydroxyisophthalate resulted in a higher C2H4/C2H6 (>259) and C3H6/C3H8 selectivity (>55) of Fe-MOF-74 [40].
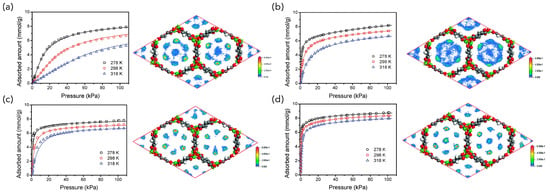
Figure 3.
Experimentally measured adsorption isotherms and simulated equilibrium snapshots (at 100 kPa) of (a) C2H6, (b) C2H4, (c) C3H8, and (d) C3H6 in Mg-MOF-74. All adsorbates were preferentially adsorbed by the open metal sites and each metal could adsorb one molecule. Reprinted with permission from ref. [38]. Copyright 2011 American Chemical Society.
Olefin-selective adsorption using open metal sites of MOFs is enhanced by increasing the charge density of coordinatively unsaturated open metal sites. However, these MOFs exhibit very high enthalpies of adsorption (>tens of kJ/mol) and suffer a significant energy penalty in adsorbent regeneration. Furthermore, such MOFs may decrease their adsorption capacity in the presence of water.
Most MOFs without open metal sites do not show the selective adsorption of olefins/paraffins, with the notable exception of NOTT-300, which is composed of [AlO4(OH)2] and biphenyl-3,3′,5,5′-tetracarboxylate as building blocks. NOTT-300 exhibits a very high C2H4/C2H6 selectivity of 48.7, while its low enthalpy of adsorption, approximately 16 kJ/mol. The energy penalty for regeneration is also reduced [41].
The use of adsorbents that selectively adsorb paraffins saves energy by eliminating the adsorption–desorption cycle required for olefin recovery. However, C2H6 has a smaller quadrupole moment and larger dynamic molecular size than C2H4, making selective adsorption generally more difficult. On the other hand, the selective adsorption of C2H6 has been reported in several MOFs. ZIF-7, composed of Zn(II) and benzimidazolate, has been reported to adsorb C2H6 (and C3H8 compared to C3H6) at lower pressures than C2H4, although there is no large difference in saturation adsorption capacity for olefins and paraffins [42,43].
MAF-49, which is composed of Zn(II) and the triazole ligand bis(5-amino-1H-1,2,4-triazol-3-yl)methane and has one-dimensional zigzag channels, is also known to preferentially adsorb C2H6 [44]. The enthalpy of C2H6 adsorption by MAF-49 (60 kJ/mol) is higher than that of C2H4 (48 kJ/mol), and it preferentially adsorbs C2H6 in the low-pressure region, where C-H⋯N hydrogen bonds and electrostatic interactions occur between electronegative nitrogen atoms and C2H6 (Figure 4). On the other hand, for C2H4, it was concluded that steric hindrance and electrostatic repulsion occur between the C-H of C2H4 and the methylene group of the ligand. Therefore, the placement of multiple polar functional groups at appropriate positions in the framework may be effective in achieving the desired selective separation.
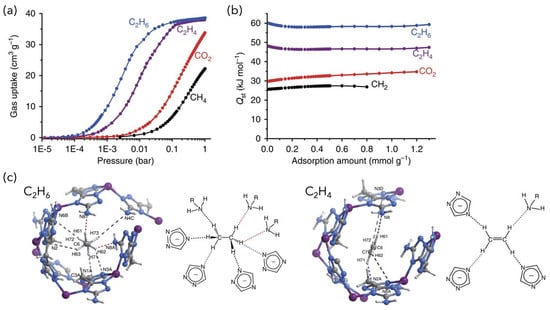
Figure 4.
(a) Experimentally measured adsorption isotherms and (b) adsorption enthalpy of C2H6, C2H4, CO2, and CH4 in MAF-49. (c) Preferential adsorption sites and host–guest interactions for C2H6 and C2H4 in MAF-49. Reproduced from ref. [44] with permission. Copyright 2015 Springer Nature.
3.2. Other Hydrocarbons
The separation of 1,3-butadiene from C4 hydrocarbon mixtures is essential to produce synthetic rubber. However, C4 isomers have close boiling points, and some components form azeotropic mixtures. Kishida et al. discussed the possibility of separating 1,3-butadiene from C4 hydrocarbons by a MOF [45]. The synthesized MOF is called SD-65 and has an interpenetrating structure in which Zn(II) is coordinated to two components: 5-nitroisophthalate and 1,2-di(4-pyridyl)ethylene. SD-65 adsorbed almost no n-C4H10, i-C4H10, 1-butene, isobutene, trans-2-butene, or cis-2-butene (adsorption capacity ~2.5 cm3/g at approximately 1 bar), while it adsorbed 40 cm3/g of 1,3-butadiene. The pore structure remains closed until the pressure of 1,3-butadiene is about 0.6 bar, at which point the pore structure rapidly transitions to an open pore structure and butadiene is adsorbed. Other MOFs have been investigated for 1,3-butadiene separation, all of which have potential, but there are still many issues to be solved to meet the separation selectivity requirements [45,46,47,48,49].
The separation of linear/branched hydrocarbons using MOFs has also been studied. Pan et al. reported that a MOF composed of paddlewheel Cu(II) dimer and 4,4′-(hexafluoroisopropylidene)bis-(benzoic acid) adsorbs C3H8, C3H6, and n-C4H10, while i-C4H10, n-pentane, i-pentane, n-Hexane, and 3-methylpentane are not adsorbed [50]. Peralta et al. reported the separation of linear/branched hydrocarbons by ZIF-8 [51]. ZIF-8 adsorbs n-hexane and 3-methylpentane, but not 2,2-dimethylbutane.
The MIL series, including MIL-47 and MIL-53, has also been studied for xylene isomer separation [52,53,54,55,56]. MIL-47 and MIL-53 have the same crystal topology consisting of [MO4(OH)2] and phthalic acid. MIL-47, which is composed of V(III), has a rigid structure, whereas MIL-53, which is composed of Al(III), Cr(III), and Fe(III), shows a unique flexibility called the breathing effect. The p-xylene/m-xylene separation by MIL-47 showed a selectivity of 2.9. On the other hand, MIL-53(Al) could not separate p-xylene and m-xylene.
UiO-66, composed of zirconium and terephthalic acid, is well known for its excellent chemical and thermal stability. UiO-66 preferentially adsorbs branched hydrocarbons (2,2-dimethylbutane and 2,3-dimethylbutane) over linear hydrocarbons (n-hexane) [57]. This unique adsorption behavior is attributed to the 6–7 Å triangular lattice of the channel pores of UiO-66, which is believed to be responsible for the preferential adsorption of o-xylene over p-xylene.
High-purity C2H2 is an important raw material for the production of a variety of valuable chemicals. C2H2 production inevitably involves trace or large amounts of CO2 (1–50%), making C2H2/CO2 separation extremely important in the petrochemical industry. Since the realization of the first example of MOFs for C2H2 adsorption by Matsuda et al. [58], MOFs showing a highly selective separation of C2H2/CO2 through powerful strategies of pore tuning and pore functionalization have been actively investigated. Pei et al. reported that a Hoffmann-type MOF with two oppositely adjacent open metal sites, denoted as ZJU-74a, showed a much higher uptake of C2H2 than CO2 at 296 K, with a high C2H2/CO2 selectivity of 36.5 [59]. GCMC simulations revealed that two oppositely adjacent open Ni(II) sites can tightly sandwich the C2H2 molecule, providing a strong interaction. Zhang et al. reported that Cu(I)-chelated UiO-66-(COOH)2 exhibits a much steeper C2H2 adsorption with 0.9 mmol g−1 at 298 K and 0.01 bar [60]. GCMC simulations indicated that the steep C2H2 adsorption is mainly attributed to a strong interaction between the chelated Cu(I) sites and C2H2 molecules. Recently, Ye et al. reported a control strategy of hydrogen-bonding nanotraps on the pore surface of MIL-160 to overcome the trade-off in C2H2/CO2 separation [61]. GCMC simulations revealed that C2H2 molecules are trapped on the MIL-160 pore surface via C−H≡C−Hδ+⋯Oδ− hydrogen bonds, while CO2 molecules weakly interact with the pore surface via electrostatic interaction. In contrast to C2H2-selective adsorption, processes that selectively eliminate CO2 in C2H2/CO2 separations are more desirable, as high-purity C2H2 can be obtained directly in a single adsorption step with much lower energy consumption. Ma et al. reported a hydro-stable Tm(III)-based MOF with a high CO2 uptake of 5.83 mmol g–1 and a CO2/C2H2 selectivity of 17.5 at 298 K and 1 bar [62].
Current regulations regarding the low concentration of aromatic VOCs in indoor air and industrial effluents have accelerated the development of MOF-based adsorbents capable of capturing trace levels of aromatic VOCs [63,64]. The hydrophobic properties of the BUT series with a double-walled metal–dipyrazolate framework make it a promising adsorbent for capturing aromatic VOCs from indoor air. BUT-66 showed twice the benzene adsorption capacity compared to carboxene at moderate temperatures. More recently, research on VOC treatment has extended to innovations in the catalytic capacity of MOFs. Dong et al. demonstrated that isomorphous bimetallic MOFs denoted as PCN-250(Fe2M; M = Co2+, Ni2+, Mn2+) have great potential as high-performance porous catalysts for both O3 degradation and VOC removal [65]. Among these materials, PCN-250(Fe2Co) exhibited the highest O3 degradation rate (100%) and could serve as a unique and promising multifunctional material for air purification. The introduction of high porosity into O3 decomposition catalysts can impart multifunctional properties such as adsorption, separation, and sensing, which is beneficial for practical air purification.
4. CO2 Capture and H2 Purification
Since global CO2 emissions from energy conversion, such as power generation, account for more than 40% of total global CO2 emissions, the decarbonization of energy conversion is crucial to reducing emissions [66]. CO2 separation and capture processes in the power-generation sector can be classified into pre-combustion, post-combustion, and oxy-fuel combustion (Figure 5) [67]. The most mature technology for capturing CO2 after combustion is chemical absorption using monoethanolamine (MEA). However, the energy cost of CO2 separation and capture is high, even for power plants that use the captured CO2 for enhanced oil recovery (EOR) [68]. Carbon pricing through “carbon taxes” and “emissions trading” has been introduced as a measure to reduce CO2 emissions. The cap-and-trade European Union Emissions Trading Scheme (EU-ETS) has become the most recognized carbon market in the world, with the EU-ETS price exceeding EUR 50/t-CO2 in May 2021. Many international organizations, including the International Energy Agency (IEA) and the International Renewable Energy Agency (IRENA), have stated that carbon pricing will spur innovation in low-carbon technologies and increase the potential for new technologies to replace existing technologies [69]. Membrane separation is considered a promising next-generation separation technology because it can operate continuously (no need to regenerate separators), consumes less energy than other separation methods, and can be easily integrated into existing technologies due to its compact equipment [70].
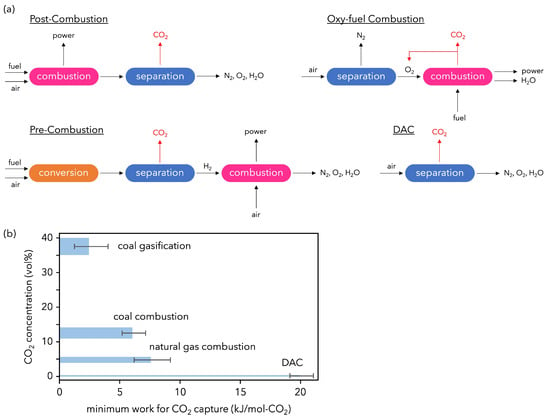
Figure 5.
(a) Conceptual diagram of carbon capture configurations. (b) Minimum work as a function of CO2 concentration. These were calculated from a combination of the first and second laws of thermodynamics required for a given capture rate (50–90%) and CO2 purity (80–99%) from a gas mixture with a known CO2 concentration [71,72].
Membrane gas separation was commercialized in the late 1970s for hydrogen separation and has since been applied to carbon dioxide separation from natural gas, biogas, and landfill gas, air separation (nitrogen-enriched gas and oxygen-enriched gas production), and air dehumidification. However, membrane separation as a CO2 separation and recovery technology for CO2 Capture, Utilization and Storage (CCUS) has only been studied up to bench scale with a few exceptions. In addition, the minimum work required increases sharply as CO2 becomes more dilute in the gas mixture (Figure 5). DAC has recently received attention as a potential negative-emissions technology. Proponents emphasize the need for DAC to remove CO2 if global warming overshoots target limits. If a carbon tax is to be introduced, promoting DAC may be a better solution than retrofitting current methods of coal- and natural gas-fired power generation. Realistically, however, with the performance of current separation technologies, it may be more cost-effective and less energy intensive to capture CO2 pre- and post-combustion.
The global energy crisis caused by increasing energy consumption calls for sustainable alternative energy sources. Although H2 is the most promising energy source with high energy density and CO2-free emissions, more than 90% of H2 is produced by the steam reforming process of natural gas using fossil fuel hydrocarbons, which emit CO2 [73]. The produced H2 is expected to be used as energy in fuel cell vehicles (FCVs), FC buses, and power generation; however, to date it has been used in large-scale processes in various industries, including petrochemicals, electronics, metallurgy, steelmaking, pharmaceuticals, and the production of raw material chemicals. For example, H2 is used in large quantities in petroleum complexes to remove sulfur from crude oil, in the petrochemical sector as an additive in the production of plastics and other resins, and in steel mills as an additive for bright annealing, which gives stainless steel and other surfaces a luster. High-purity H2 is essential as a reducing-atmosphere and carrier gas in the manufacture of semiconductor wafers, liquid crystal and plasma displays, and fiber optics. However, large-scale H2 production inevitably generates by-products. Thus, conventional H2 production requires separation and purification processes (Table 2), increasing the overall cost of H2 production. In particular, the purification process increases. This additional cost is one of the drawbacks of using H2 as a fuel. Therefore, energy-efficient and cost-effective methods for separating H2 from less desirable products must be developed. H2 storage in microporous materials via adsorption methods has been actively investigated. Activated carbons, zeolites, and MOFs are promising candidates for H2 storage. MOFs with larger surface areas, pore volume, and suitable pore size distribution have greater H2 storage capacity (Table 3) [21,74,75,76,77,78,79,80,81,82,83,84]. In addition, the surface properties of MOFs can be controlled by organic linker substitution and interface-grafting methods, resulting in improved H2 storage performance. However, it is only at low temperatures (≈77 K) that these nanomaterials including activated carbons, zeolites, and MOFs show their highest storage capacity. This is because the physical interaction of H2 with solid surfaces is negligible at practical temperatures and pressures (≈STP).

Table 2.
Various sources and gas mixtures for H2 production.

Table 3.
H2 storage capacity of typical MOFs at 77 K and 1 bar.
Membrane technology is the most promising alternative as it is lower cost, consumes less energy, and is easy to operate continuously compared to other conventional methods such as fractional/cryogenic distillation and pressure/temperature swing adsorption. To date, intensive research has been conducted for H2 separation and recovery using conventional materials, ranging from organic polymers to inorganic materials such as palladium-based metals, silica, zeolites, and carbon. Polymers such as silicone rubber, cellulose acetate, polysulfone, and polyimide have mainly been used as membrane materials. Recently, porous membranes with sub-nanometer-sized pores have been extensively studied, with silica and zeolite membranes receiving much attention. Mixed-matrix membranes (MMMs), in which MOFs are mixed with polymer matrix as filler, have also been actively studied. Pre-combustion is primarily intended for use in integrated gasification combined cycles (IGCCs), a process in which coal and natural gas are partially oxidized to produce natural gas vapor. Fuel gas is purified by separating and recovering CO2 from synthesis gas (consisting primarily of H2 and CO) produced by the partial oxidation of coal and natural gas, or by the steam reforming of natural gas to produce H2 and CO2 by reacting CO with aqueous gas shift. Since high-pressure gas is the separation target (mainly CO2/H2) in pre-combustion, equipment such as vacuum pumps is not required, saving energy and cost. However, the separation membrane must be durable under high temperature and high pressure. In addition, since H2 has a smaller molecular size than CO2, H2-selective permeation membranes have mainly been studied. On the other hand, post-conversion targets the separation of combustion exhaust gas generated from boilers in power plants at relatively low pressure, which requires the installation of vacuum pumps and compressors, making it difficult to achieve significant energy conservation and cost reduction compared to existing technologies. For energy conservation and cost reduction, high permeability is required for separation membranes from the viewpoint of reducing the required membrane area.
5. MOF-Based Membranes
5.1. Types of Membranes
Separation membranes based on MOF can be broadly classified into two categories. One is a polycrystalline membrane composed of MOF alone, and the other is a mixed-matrix membrane (MMM) in which MOF is mixed with a polymer membrane as a filler. Similar to porous inorganic membranes such as silica and zeolite membranes, MOF polycrystalline membranes are often formed on porous ceramic supports to ensure the mechanical strength of the membrane. MOFs are often compared and discussed with zeolites because of their similarities with zeolites in terms of crystalline porous structure. The MMM, on the other hand, is a strategy to improve membrane performance by synergistically combining the excellent processability of polymers with the porous properties of MOF fillers (Figure 6).
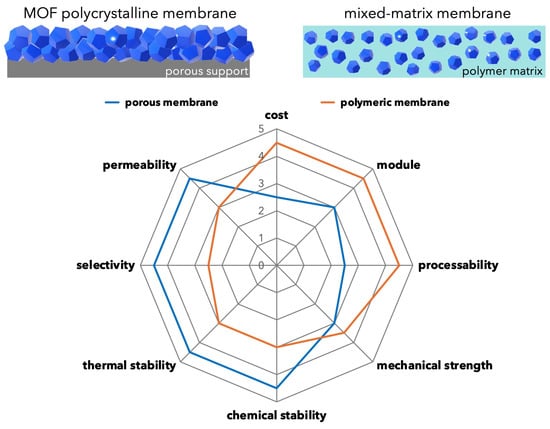
Figure 6.
Schematic illustration of polycrystalline membrane and MMM. Radar chart showing the advantages and disadvantages of porous and polymeric membranes. The ideal vision for MMM is to improve membrane performance by combining the advantages of porous and polymeric materials.
MOF polycrystalline membranes exhibit high separation performance by selecting the optimum structure for the separation target because the only membrane permeation pathway for gas molecules is through the pores of the MOF. However, nonselective permeation often occurs due to the formation of grain boundaries between crystals, pinholes, and intracrystalline defects. In order to fabricate membranes with dense grain boundaries, polycrystalline membranes are generally prepared by using seed crystals via the secondary growth method [85,86,87,88]. Although pioneering studies of MOF membrane formation reported in the late 2000s did not lead to the reporting of gas permeation results, these studies stimulated research on polycrystalline MOF membranes, and various membrane-preparation methods have been reported.
MMM is a membrane in which MOF fillers are dispersed in a polymer matrix. The dispersion state of the polymer and filler greatly affects the performance of the membrane [89]. MMMs may be prepared on supports, but they differ from MOF polycrystalline membranes in that the processability of polymers can be used to fabricate freestanding membranes. Since MOFs contain organic ligands, they are expected to interact well with the polymer matrix and inhibit microvoid formation between the filler and the polymer. The use of highly porous MOFs as fillers is expected to improve membrane permeability. However, to improve permeability, it is necessary to increase the MOF filler content. However, as filler content increases, the mechanical properties and processability of polymers decrease. In general, the smaller the fillers, the more likely they are to aggregate. If interface defects are formed in the MMM due to non-uniform dispersion caused by the aggregation of fillers and/or poor interaction between the filler and polymer, gas molecules will preferentially diffuse through the defects and separation performance will be degraded. In order to suppress filler agglomeration and poor dispersion in the polymer matrix, a technique to control the filler/polymer heterointerface at the molecular level is required.
5.2. MOF Membrane-Preparation Method and Points to Consider
If MOFs can be thinned so that there are no voids between crystals, they can be applied as separation membranes. However, fabricating polycrystalline membranes is not so easy. It must be noted that cracks, pinholes, and intra-crystal defects between crystals cause non-selective permeation, and that large areas must be achieved with thin membranes. Various methods have been proposed for preparing MOF membranes (Figure 7).
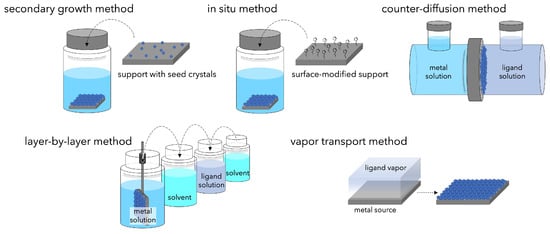
Figure 7.
Schematic of the methods developed for synthesis of continuous MOF membranes.
To fabricate continuous polycrystalline membranes on a support, a dense heterogeneous nucleation field must exist on the support surface. The secondary growth method is often used, in which pre-prepared seed crystals are loaded on the support surface and grown to form continuous films. Seeding techniques such as dip coating [90], slip coating [91], and rubbing [92] are used, followed by solvothermal or hydrothermal synthesis. In general, it is important to uniformly load seed crystals on the support surface and to make thin membranes (<1 μm); seed crystals of about 100 nm are required to allow sufficient crystal intergrowth [93]. The secondary growth method is an effective way to promote the formation of dense heterogeneous nuclei, which is important for thin membrane growth, but it still poses a challenge in terms of adhesion between membrane and support.
To address the issue of adhesion between membrane and support, modification of the support surface with compounds that bind the MOF crystals and the support has been used [94,95,96,97]. These compounds have one end that can coordinate with the nodes constituting the MOF and the other end that can covalently bond with the support. The functional groups immobilized on the support cause the heterogeneous nucleation of MOFs and promote crystal growth, resulting in continuous MOF membranes with a high degree of crystallinity and relatively thin membrane thickness. The chemical modification method is also effective when using polymers as supports in addition to ceramic supports [98,99]. Besides surface modification by covalent bonding, the strategy of coating carbon nanotubes (CNTs), which have the important feature of the uniform distribution of π-π bonds, has attracted attention for membrane assembly. Wei et al. reported that pre-immobilized 2-methylimidazole readily reacts with Zn2+ to form an oriented ZIF-8 membrane on CNT-coated support [100].
Another method has been proposed to solve the problem of adhesion between the membrane and the support by growing and immobilizing MOFs in the pores of the porous support. The counter-diffusion method is used to deposit MOFs in the pores of the support [101,102,103]. In the counter-diffusion method, the solutions of metal ions and organic ligands are supplied from opposite sides of the support, and the MOF layer is formed at the interface where the diffusing raw materials come into contact by chemical potential gradient.
Grain boundary defects are one of the most important problems in continuous polycrystalline membranes. The difference in the coefficient of thermal expansion between MOF crystals and the support is a source of stress and causes defects in the membrane. Membrane defects can also occur during the activation process of MOF. The ZIF-78 membrane, synthesized using N,N-dimethylformamide as the reaction solvent, easily forms membrane defects when activated at 100 °C under vacuum [104]. Therefore, it is effective to bring the coefficients of thermal expansion of the two materials closer, but such a combination is not always possible. On the other hand, it has been demonstrated that membrane defects can be reduced by optimizing the cooling rate after membrane formation at high temperature [105]. To solve this problem, it is effective to replace the remaining reaction solvent with a solvent with a low boiling point and surface tension, such as methanol or ethanol, before heating the MOF under vacuum. In addition, various post-synthetic modifications have been investigated to suppress the generation of membrane defects and to repair defects that have occurred.
5.3. Olefin/Paraffin Separation
MOFs have potential for a wide range of separation targets due to their excellent pore structure and composition, as well as the diversity of their synthesis and membrane production methods. Although MOFs appear promising for olefin/paraffin separation, only a few MOF membranes are currently available. While they have been demonstrated to be effective for the separation of C3H6/C3H8, few have been reported to be able to efficiently separate C2H4/C2H6 (Table 4) [100,106,107,108,109,110].

Table 4.
C2H4/C2H6 separation performance of MOF polycrystalline membranes.
ZIF-8, which is composed of Zn(II) and 2-methylimidazolate as building blocks and has an SOD structure, has been the most studied MOF for C3H6/C3H8 separation. The effective pore size of ZIF-8 is 4.0–4.2 Å, but even 1,2,4-trimethylbenzene of approximately 7.6 Å enters the pores [111], suggesting a lack of sharp molecular sieving. Indeed, the selectivity of CO2/CH4 separation by the ZIF-8 membrane is only about 5 [112]. On the other hand, the structural flexibility of ZIF-8 works effectively in C3H6/C3H8 separation, showing a sharp cut-off between C3H6 and C3H8 molecular sizes. The diffusion selectivity of C3H6/C3H8 in ZIF-8 is theoretically estimated to be approximately 125 [113], and various studies on ZIF-8 membranes have been conducted with this value as a benchmark. Pan et al. first reported the separation of C2/C3 hydrocarbons (C2H6/C3H8, C2H4/C3H6, and C2H4/C3H8) using a ZIF-8 membrane prepared on a porous alumina disc [114]. Meanwhile, at the same time, Bux et al. reported a selectivity of only 2.8 for C2H4/C2H6 separation [106]. Subsequently, intensive research on ZIF-8 membranes was undertaken after Zhang et al. showed that the pore size of ZIF-8 was effective for C3H6/C3H8 separation by estimating the diffusion coefficient [115]. Furthermore, doping 2-aminobenzimidazole (ambz) [116] and ionic liquid (IL)/Ag+ [109] into ZIF-8 could improve the membrane performance. ZIF-8 is mostly employed as a leading polycrystalline membrane candidate for C3H6/C3H8 separation, and few membranes other than ZIF-8 have been reported so far (Table 5) [93,97,100,102,103,109,117,118,119,120,121,122,123,124,125], while MOFs other than ZIF-8 have been used as fillers for MMMs (Table 6) [126,127,128,129,130,131,132,133,134,135]. It is important to explore the possibilities of other MOFs and optimize the interaction between the MOF filler and the polymer matrix with the help of computational simulations. Various improvements have been made to meet the separation performance requirements, such as optimizing secondary growth and activation conditions, and devising unique membrane-preparation methods.

Table 5.
C3H6/C3H8 separation performance of MOF polycrystalline membranes.

Table 6.
C2H4/C2H6 and C3H6/C3H8 separation performances of typical MOF-based MMMs.
Brown et al. devised an interfacial microfluidic membrane-processing (IMMP) method to fabricate ZIF-8 membranes on polyamide-imide hollow fibers (Torlon®) [117]. In the IMMP method, an aqueous solution of 2-methylimidazole is fed to one side of the hollow fiber and a 1-octanol solution of zinc nitrate is continuously fed to the opposite side for counter-diffusion to form a ZIF-8 membrane at an incompatible interface. This method is promising for the scale-up and mass production of membranes, as it is low cost and can process several hollow fibers with high specific surface area simultaneously. In their initial report, the C3H6/C3H8 selectivity was only 12 due to the presence of membrane defects. Thereafter, the C3H6/C3H8 selectivity reached 180 (at a feed gas pressure of 1 bar) [118] by controlling the membrane formation and optimizing the membrane growth process and the microstructure of the hollow fibers. It was also confirmed that the selectivity of 90 was maintained even when the feed gas pressure was 9.5 bar.
Besides controlling the microstructure between neighboring crystals, it is also important to control the structural flexibility of MOFs to improve the separation selectivity of polycrystalline membranes. For ZIFs, the framework flexibility caused by the rotation of the ligands allows larger molecules to permeate, resulting in a reduced molecular sieving effect. ZIF-8 membranes have been demonstrated to be extremely effective in separating C3H6/C3H8 (Table 5). This separation performance is due to the “swing effects” of 2-methylimidazole of a six-membered window, which enlarges the aperture. In contrast, four-membered windows along the <100> direction have a very small aperture. Theoretically, only He and H2 can pass through the four-membered windows. However, simulation studies suggest that the four-membered window aperture could also be enlarged by the swing effects. The {100}-orientated ZIF-8 membrane prepared on a CNT-coated support has four-membered windows, demonstrating improved performance in C2H4/C2H6 separation based on the “swing effects” (Table 4). On the other hand, the C3H6/C3H8 separation performance of the {100}-orientated ZIF-8 membrane was lower than that of the randomly oriented membrane. Tanaka et al. showed that the structural flexibility of ZIF-8 varies depending on the crystal size [19] and proposed that the membrane performance can be tuned by the size of the primary particles constituting the polycrystalline membrane [97]. On the other hand, the kinetic properties of the ZIF ligand are altered by substituting the metal nodes. ZIF-67 has the same crystal topology as ZIF-8, with Co(II) as a node instead of Zn. It is known that the bonding of Co(II)-2-methylimidazolate is stronger than that of Zn(II)-2-methylimidazolate, which makes ZIF-67 more rigid than ZIF-8 and limits the rotation of the ligand. Kwon et al. grew ZIF-67 heteroepitaxially on a ZIF-8 seed layer and then ZIF-8 on a ZIF-67 layer to prepare a membrane with a trilayer ZIF-8/ZIF-67/ZIF-8 structure (Figure 8) and demonstrated that extremely high C3H6/C3H8 selectivity is achieved [119].
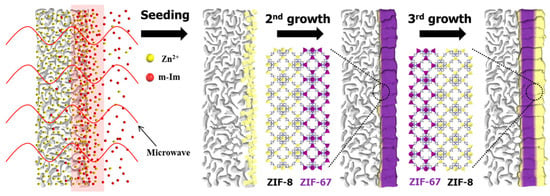
Figure 8.
Schematic of ZIF-8/ZIF-67/ZIF-8 membrane synthesis via heteroepitaxial growth. Reprinted with permission from ref. [119]. Copyright 2015 American Chemical Society.
Zhou et al. devised a fast current-driven synthesis (FCDS) method and fabricated ZIF-8 membranes on anodic alumina oxide (AAO) [120]. In the FCDS method, it was found that the formation of ZIF-8 was promoted by the DC current, resulting in the formation of lattice-distorted ZIF-8_Cm as a crystalline polymorph (Figure 9). ZIF-8_Cm, which accounts for 60–70% of the membranes formed, shows higher rigidity and better C3H6/C3H8 separation than the common cubic ZIF-8_I3m, resulting in the highest C3H6/C3H8 selectivity among ZIF-8 membranes reported so far. The methods have been reported to provide sharp molecular sieving ability by suppressing the structural flexibility characteristic of MOFs. Hou et al. reported the preparation of crystal-oriented Co-Zn bimetallic ZIF membranes (Zn(100−x)Cox-ZIF) by the FCDS method and the effect of Co2+ on the framework flexibility [121]. Co2+ acts as a rigidity factor affecting the framework flexibility, and the resulting bimetallic Zn82Co18-ZIF membrane exhibited high C3H6/C3H8 selectivity due to the balance between the grain boundary structure and framework rigidity. Wang et al. applied an inhibited Ostwald ripening (IOR) technique to the FCDS method to produce ultra-thin ZIF-8 membranes [110]. The IOR strategy enables the efficient control of grain size and uniformity, resulting in ultra-thin membranes, simply by adding polymer-based inhibitors to the precursor. The IOR strategy is expected to be a platform technology for the membrane fabrication of MOFs because of its distinct straightforward and generic features.
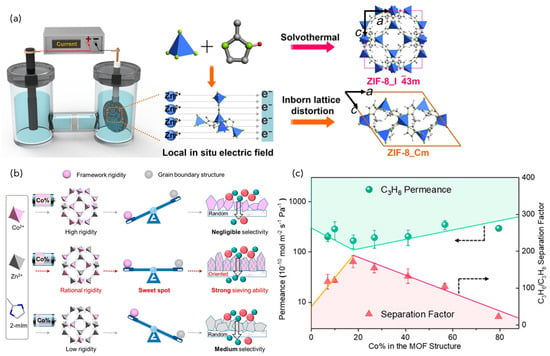
Figure 9.
(a) Schematic of the electrochemical cell for ZIF-8 membrane growth by FCDS. The solvothermal route assembles normal I3m phase. The inborn lattice distortion occurs and the stiff polymorph ZIF-8_Cm is formed via FCDS. Reproduced from ref. [120] with permission. Copyright 2018 American Association for the Advancement of Science. (b) Schematic of the design and (c) C3H6/C3H8 separation performance of bimetallic Zn(100−x)Cox-ZIF membranes. Reproduced from ref. [121] with permission. Copyright 2020 American Chemical Society.
Eum et al. proposed a unique “all-nanoporous hybrid membrane (ANHM)” concept to achieve high permeability and selectivity that cannot be reached with single-phase nanoporous materials alone [125]. ANHM is a membrane concept inspired by a conventional polymer-based MMM, but is new in that it combines two or more nanoporous materials of different morphologies into a single membrane (Figure 10). The use of nanosheets of MFI zeolite as a counterpart to the MOF and modifying the layering achieved different performance-enhancement routes (e.g., improved permeability, selectivity, or both).
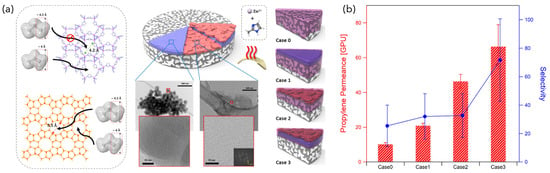
Figure 10.
(a) Schematic of the ANHM fabrication and membrane architectures. Case 0: ZIF-8 only; Case 1: ZIF-8 with MFI nanoparticles; Case 2: ZIF-8 with MFI nanosheets; and Case 3: ZIF-8 with MFI nanoparticles and MFI nanosheets. (b) Equimolar binary C3H6/C3H8 separation performances of Cases 0–3 membranes. Reprinted with permission from ref. [125]. Copyright 2020 American Chemical Society.
Although the selectivity of ZIF membranes for C3H6/C3H8 separation has improved significantly, from tens up to about three hundred, the permeability is still in the order of 10−8 mol m−2 Pa−1 s−1 (Table 5). The main reason for this is the membrane thickness, which for most ZIF-8 membranes is several to tens of micrometers. In contrast, Li et al. developed a gel–vapor deposition (GVD) method that combines the sol–gel and chemical vapor deposition methods to fabricate extremely thin ZIF-8 membranes (17~ nm) in PVDF hollow fiber (Figure 11) [123]. The ZIF-8 membranes prepared by the GVD method showed relatively high C3H6/C3H8 selectivity and one to three orders of magnitude higher permeability than conventional membranes. Ma et al. developed an all-gas phase process for ZIF-8 membrane production [124]. In this method, an ultrathin ZnO layer is deposited on a support by atomic layer deposition (ALD), and then the ZnO layer is converted to ZIF-8 by 2-methylimidazole vapor treatment. The membrane thickness and microstructure are controlled by the number of ALD cycles.
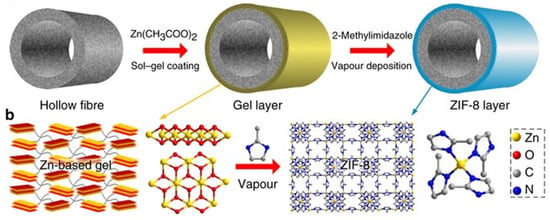
Figure 11.
Schematic of GVD fabrication of ultrathin ZIF-8 membrane. Reprinted with permission from ref. [123]. Copyright 2017 Springer Nature.
5.4. Other Hydrocarbon Separations
Eum et al. applied the IMMP method, which produced ZIF-8 membranes on polyamide-imide hollow fibers, to carbon hollow fibers to produce ZIF-90 membranes [136]. In general, polymer supports have poor chemical resistance and swell when exposed to organic compounds. In contrast, ZIF-90 membranes fabricated on chemically inert carbon hollow fibers exhibited high chemical resistance. ZIF-90, which is composed of Zn(II) and 2-imidazolecarboxaldehyde, has the same crystal topology as ZIF-8, and its crystallographic pore size (3.5 Å) is not much different from that of ZIF-8. On the other hand, its effective pore size (5.0 Å) is larger than ZIF-8 due to its structural flexibility. The ZIF-90 membrane showed n-C4H10/i-C4H10 selectivity of 12 and n-C4H10 permeability of 6.0 × 10−8 mol m−2 Pa−1 s−1, indicating its potential for the separation of butane isomers.
Huang et al. prepared MIL-160 membranes on porous alumina discs modified with polydopamine and applied them to the separation of xylene isomers [137]. The MIL-160 membranes showed p-xylene/o-xylene selectivity of 38.5 and p-xylene permeation flux of 467 g m−2 h−1. MIL-160, which is composed of [AlO4(OH)2] and 2,5-furan-dicarboxylate as building blocks, has an effective pore size of 5 to 6 Å and shows higher adsorption enthalpy and diffusivity for p-xylene than for o-xylene. Therefore, MIL-160 membranes are effective for xylene isomer separation and are promising candidates for thermal and chemical stability. In addition, the high thermal and chemical stability of MIL-160 membranes is effective for the separation of xylene isomers.
5.5. CO2 Separation
Commercially available PolyactiveTM [138] and Pebax® [139] membranes are PEO-based block copolymers with high CO2 permselectivity. PEO-based membranes exhibit a high CO2/N2 permselectivity of over 40 due to the high affinity of ether oxygen for CO2. However, the CO2 permeability is not high enough to be of practical use. Due to the relatively low CO2 permeability, when PEO membranes are used for CO2 capture from flue gases emitted from coal-fired power plants, the membrane thickness should be reduced to less than 200 nm to achieve a CO2 permeance of more than 1000 GPU, which is required for practical use (Figure 12) [140]. MOF-based membrane research targeting CO2 separation has been actively investigated [141]. HKUST-1, MIL-53, MIL-100, and MIL-101 are candidates for combustion flue gas, natural gas purification, and hydrogen purification due to their higher CO2 adsorption capacity than typical zeolites (Table 7) [32,142,143,144,145,146,147,148,149,150,151,152,153].
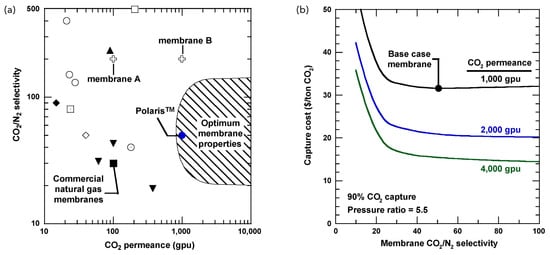
Figure 12.
(a) Trade-off relationship between selectivity and permeability in membrane separation, using CO2/N2 separation as an example. (b) Effect of CO2/N2 selectivity on the cost of CO2 capture, estimated assuming a case of 90% capture of CO2 in the flue gas at a pressure ratio of 5.5. Reprinted with permission from ref. [140]. Copyright 2009 Elsevier B.V.

Table 7.
CO2 adsorption capacity of typical zeolites and MOFs.
Since H2/CO2 is the main separation target pre-combustion and the molecular size of H2 is smaller than that of CO2, research has focused on H2-selective permeation membranes. Table 8 shows the top data for H2/CO2 separation using MOF polycrystalline membranes [98,99,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170]. MOFs with suitable pore size and high CO2 affinity can be candidates for CO2/N2 separation. CAU-1 with amino groups is one of them (Table 9) [162,171,172,173,174,175,176,177,178]. The structure of CAU-1 consists of distorted octahedral and tetrahedral cages, which are connected by a triangular window with an opening diameter of 3–4 Å. The amino groups in the CAU-1 framework interact with CO2 through acid–base interactions, resulting in improved CO2/N2 separation performance. Efficient CO2/CH4 separation is very important in natural gas and biogas refining. Corrosion control is important in pipeline transportation, and CO2 is corrosive in the presence of water vapor; thus, it must be kept at low concentrations. Currently, membrane separation accounts for only 10% of the natural-gas-refining market. If membranes with high permeability and selectivity can be developed, membrane separation may be superior to chemical absorption in natural gas and biogas purification; polycrystalline membranes such as ZIF-8, IRMOF-1, MIL-53-NH2, and UiO-66 have been reported for CO2/CH4 separation applications (Table 10) [171,172,173,175,177,179,180,181,182,183,184,185,186]. However, it has been noted that many MOF polycrystalline membranes have low CO2/CH4 ideal separation factors. ZIF-8 and MIL-96 have been considered suitable for CO2/CH4 separation because their pore entrance diameters are between the molecular sizes of CO2 and CH4. However, it should be noted that some MOFs have flexible structures and exhibit dynamic pore characteristics. For example, the effective pore size of ZIF-8 is 4.0–4.2 Å, which is larger than the molecular sizes of CO2, N2, and CH4, and thus does not allow for sharp molecular sieving for CO2/N2 and CO2/CH4. Ligands with polarizable functional groups and metal nodes with high valence, such as Zr4+, Al3+, Cr3+, and Fe3+, show high adsorption to gas molecules with large quadrupole moments, such as CO2. On the other hand, strong adsorption may result in low diffusion coefficients. So far, the separation performance of MOF polycrystalline membranes often falls within the trade-off range of higher permeability but lower selectivity, compared to zeolite membranes. On the other hand, Fan et al. reported that, besides gas affinity, shape, and size, the flexible behavior of MOFs also influences the gas adsorption process, enabling the effective separation of light hydrocarbons [186]. They demonstrated that the MIL-160/CAU-10-F membrane with a more polar fluorine-functional group improved CO2/CH4 separation selectivity by 10.7% compared to MIL-160.

Table 8.
H2/CO2 separation performance of MOF polycrystalline membranes (* single-gas permeation test).

Table 9.
CO2/N2 separation performance of MOF polycrystalline membranes (* single-gas permeation test).

Table 10.
CO2/CH4 separation performance of MOF polycrystalline membranes (* single-gas permeation test).
On the other hand, separation membranes that exceed the upper limit of polymer membrane performance have been reported by using MOFs as fillers in mixed-matrix membranes. The combination of polymer matrix and filler is very important. Note that the introduction of fillers can alter the arrangement and free volume of the polymer chains and cause interfacial defects between filler/filler and filler/matrix (Figure 13). Since the affinity between the filler and the polymer matrix plays an important role in the processability and performance of the membrane, the compatibility of both components must also be considered. It has been reported that dispersing MOF fillers in the polymer matrix without interfacial defects improves the separation performance of MMMs due to the molecular sieving effect derived from the uniform pores of the filler (Table 11) [187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204]. Recently, it has also been reported that the synergistic effects of different fillers can be obtained by adding MOF fillers, together with graphene oxide (GO) [194] and ionic liquids (ILs) [195], to polymer matrices.
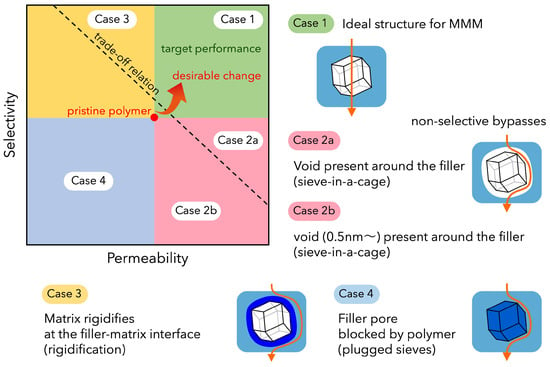
Figure 13.
Relationship between filler/matrix interface structure and MMM separation performance.

Table 11.
CO2 separation performance of typical MOF-based MMMs.
6. Outlook and Challenges
The experimental findings reported in this field indicate that although MOF-based membranes are a useful option for gas separation, there are still challenges that must be overcome. In general, the separation performances of MOF-based membranes are studied using single-gas permeation tests. In industrial processes, gases are of course present as mixtures, so membrane performance should be evaluated using practical gas mixture conditions. Investigating the membrane durability under practical operating conditions is essential to determine the possibility for the social implementation of MOF-based membranes in gas separation. For example, the temperature of syngas produced in steam reforming processes can reach 200 °C, and its pressure can be maintained at at least 5–10 bar, and even higher. In addition, membranes must also be chemically resistant to corrosive and acidic gases (e.g., H2S in natural gas purification, SOx and NOx in CO2 capture from flue gas). It is undeniable that many MOFs may degrade under humid conditions and their gas separation performance may be reduced. Therefore, a stable membrane that can operate under high temperature and high pressure for a long-term practical operation is required. One strategy to improve the stability is to functionalize MOF-based membranes to change their resistance for potentially damaging contaminants. Although significant studies have been reported that subjected ZIF-8/polybenzimidazole MMMs to long-term gas separation testing for more than 50 days under more realistic conditions (e.g., in the absence of sweep gas) [205], there have been limited studies investigating the stability of MOF-based membranes.
Stable and reliable membranes are expected to improve the separation process, increase efficiency, and reduce costs, thus facilitating membrane technology transfer to industry. From this perspective, Robeson’s upper bounds are not necessarily indicative of the economic feasibility of membranes. The membrane-manufacturing process needs to be continuously optimized and fine-tuned to improve the productivity and scalability of high-performance membranes. By integrating automation and robotics into the membrane-manufacturing process, standardized protocols and procedures can help minimize variation among different production batches and improve the reproducibility of membranes. On an industrial scale, to increase membrane area, polycrystalline membranes would need to be housed in monolithic modules and follow fiber membranes in tubular modules and flat-sheet membranes in spiral-wound or pleated modules. In addition, tests to compare the performance of flat-sheet and hollow fiber membranes are needed, and new membrane materials and membrane modules need to be considered. In addition, research and development should be expedited to produce low-cost membranes, with a focus on commercial scalability and life-cycle analysis.
Many computational studies on MOF-based membranes serve as a starting point for listing promising membranes, though they rely on several assumptions [206,207,208,209,210,211,212,213]. Many studies use rigid frameworks to predict gas separation performance. The use of generic force fields and the omission of framework flexibility is a very common approach. It should be noted that while generic force fields are sufficient for large-scale membrane-screening studies, more accurate calculations require specialized force fields. The inclusion of flexibility effects may improve the accuracy of the design of MOFs and membrane performance prediction. However, although considering framework flexibility can be computationally costly, it would be reasonable to perform such simulations for a limited number of screened membranes with high performance. The construction of computational studies on MOFs, coupled with the accumulation of experimental data on membranes, would enable the design of promising membranes and the prediction of separation performance, accelerating the social implementation of MOF-based membranes.
7. Conclusions
The development of membrane separation using MOFs has been active due to the rapid increase in the number of studies on MOFs, from synthesis and structural design to application. Relatively long-term durability tests have also been conducted at the laboratory level. Although various MOF-based membranes have been fabricated, a common issue is how to achieve thin membrane formation without generating defects such as pinholes, cracks, and grain boundaries. To this end, it is important to understand the formation mechanism of MOFs based on complexation reactions between metal ions and ligands, and to develop the elementary processes of membrane formation, which can control nucleation and crystal growth. Such fundamental understanding will be the driving force for the next step toward the practical application of separation membranes based on MOFs.
Funding
This study was supported by the Kansai University Fund for Supporting Outlay Research Centers, 2021.
Acknowledgments
M.S. acknowledges Kansai University’s “Scholars from Overseas” program. S.T. acknowledges the support of JKA and its promotion funds from KEIRIN RACE (Grant No. 2023M-412) and the FY2023 research grant program of the Carbon Recycling Fund Institute, Japan.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Tissot, A.; Serre, C. Metal-organic frameworks: From ambient green synthesis to applications. Bull. Chem. Soc. Jpn. 2021, 94, 2623–2636. [Google Scholar] [CrossRef]
- Zulkifli, Z.I.; Lim, K.L.; Teh, L.P. Metal-organic frameworks (MOFs) and their applications in CO2 adsorption and conversion. ChemistrySelect 2022, 7, e202200572. [Google Scholar] [CrossRef]
- Petit, C. Present and future of MOF research in the field of adsorption and molecular separation. Curr. Opin. Chem. Eng. 2018, 20, 132–142. [Google Scholar] [CrossRef]
- Editorial article. Frameworks for commercial success. Nat. Chem. 2016, 8, 987. [CrossRef]
- Chung, Y.G.; Haldoupis, E.; Bucior, B.J.; Haranczyk, M.; Lee, S.; Zhang, H.D.; Vogiatzis, K.D.; Milisavljevic, M.; Ling, S.L.; Camp, J.S.; et al. Advances, updates, and analytics for the computation-ready, experimental metal-organic framework database: CoRE MOF 2019. J. Chem. Eng. Data 2019, 64, 5985–5998. [Google Scholar] [CrossRef]
- Anderson, R.; Gómez-Gualdrón, D.A. Increasing topological diversity during computational “synthesis” of porous crystals: How and why. CrystEngComm 2019, 21, 1653–1665. [Google Scholar] [CrossRef]
- Bobbitt, N.S.; Shi, K.H.; Bucior, B.J.; Chen, H.Y.; Tracy-Amoroso, N.; Li, Z.; Sun, Y.Z.S.; Merlin, J.H.; Siepmann, J.I.; Siderius, D.W.; et al. MOFX-DB: An online database of computational adsorption data for nanoporous materials. J. Chem. Eng. Data 2023, 68, 483–498. [Google Scholar] [CrossRef]
- NIST/ARPA-E Database of Novel and Emerging Adsorbent Materials. Available online: https://adsorption.nist.gov/isodb/index.php#home (accessed on 25 January 2024).
- Iacomi, P.; Llewellyn, P.L. Data mining for binary separation materials in published adsorption isotherms. Chem. Mater. 2020, 32, 982–991. [Google Scholar] [CrossRef]
- Demir, H.; Aksu, G.O.; Gulbalkan, H.C.; Keskin, S. MOF membranes for CO2 capture: Past, present and future. Carbon Capture Sci. Technol. 2022, 2, 100026. [Google Scholar] [CrossRef]
- Ban, Y.J.; Cao, N.; Yang, W.S. Metal-organic framework membranes and membrane reactors: Versatile separations and intensified processes. Research 2020, 2020, 1583451. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.H.; Asinger, P.A.; Lee, M.J.; Han, G.; Rodriguez, K.M.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-based membranes for gas separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef]
- Xu, X.; Hartanto, Y.; Zheng, J.; Luis, P. Recent advances in continuous MOF membranes for gas separation and pervaporation. Membranes 2022, 12, 1205. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef]
- Sakata, Y.; Furukawa, S.; Kondo, M.; Hirai, K.; Horike, N.; Takashima, Y.; Uehara, H.; Louvain, N.; Meilikhov, M.; Tsuruoka, T.; et al. Shape-memory nanopores induced in coordination frameworks by crystal downsizing. Science 2013, 339, 193–196. [Google Scholar] [CrossRef]
- Tanaka, S.; Fujita, K.; Miyake, Y.; Miyamoto, M.; Hasegawa, Y.; Makino, T.; Van der Perre, S.; Remi, J.C.S.; Van Assche, T.; Baron, G.V.; et al. Adsorption and diffusion phenomena in crystal size engineered ZIF-8 MOF. J. Phys. Chem. C 2015, 119, 28430–28439. [Google Scholar] [CrossRef]
- Devic, T.; Serre, C. High valence 3p and transition metal based MOFs. Chem. Soc. Rev. 2014, 43, 6097–6115. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Wang, Q.M.; Shen, D.M.; Bulow, M.; Lau, M.L.; Deng, S.G.; Fitch, F.R.; Lemcoff, N.O.; Semanscin, J. Metallo-organic molecular sieve for gas separation and purification. Microporous Mesoporous Mater. 2002, 55, 217–230. [Google Scholar] [CrossRef]
- Lin, K.S.; Adhikari, A.K.; Ku, C.N.; Chiang, C.L.; Kuo, H. Synthesis and characterization of porous HKUST-1 metal organic frameworks for hydrogen storage. Int. J. Hydrogen Energy 2012, 37, 13865–13871. [Google Scholar] [CrossRef]
- Lamia, N.; Jorge, M.; Granato, M.A.; Almeida, P.F.A.; Chevreau, H.; Rodrigues, A.E. Adsorption of propane, propylene and isobutane on a metal–organic framework: Molecular simulation and experiment. Chem. Eng. Sci. 2009, 64, 3246–3259. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Gohari, D.P.; Depauw, H.; Coudert, F.X.; Vrielinck, H.; Van Der Voort, P.; Leus, K. Mixed-metal metal–organic frameworks. Chem. Soc. Rev. 2019, 48, 2535–2565. [Google Scholar] [CrossRef]
- Hong, D.Y.; Hwang, Y.K.; Serre, C.; Férey, G.; Chang, J.S. Porous chromium terephthalate MIL-101 with coordinatively unsaturated sites: Surface functionalization, encapsulation, sorption and catalysis. Adv. Funct. Mater. 2009, 19, 1537–1552. [Google Scholar] [CrossRef]
- Jiang, D.; Keenan, L.L.; Burrows, A.D.; Edler, K.J. Synthesis and post-synthetic modification of MIL-101(Cr)-NH2 via a tandem diazotisation process. Chem. Commun. 2012, 48, 12053–12055. [Google Scholar] [CrossRef]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Segakweng, T.; North, B.C.; Mathe, M.; Kang, X. Modulated synthesis of chromium-based metal-organic framework (MIL-101) with enhanced hydrogen uptake. Int. J. Hydrogen Energy 2014, 39, 12018–12023. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P. Stable metal–organic frameworks: Design, synthesis, and applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- Latroche, M.; Surblé, S.; Serre, C.; Mellot-Draznieks, C.; Llewellyn, P.L.; Lee, J.H.; Chang, J.S.; Jhung, S.H.; Férey, G. Hydrogen storage in the giant-pore metal–organic frameworks MIL-100 and MIL-101. Angew. Chem. Int. Ed. 2006, 45, 8227–8231. [Google Scholar] [CrossRef]
- Horcajada, P.; Surblé, S.; Serre, C.; Hong, D.Y.; Seo, Y.K.; Chang, J.S.; Grenèche, J.M.; Margiolaki, I.; Férey, G. Synthesis and catalytic properties of MIL-100(Fe), an iron(iii) carboxylate with large pores. Chem. Commun. 2007, 2820–2822. [Google Scholar] [CrossRef]
- Llewellyn, P.L.; Bourrelly, S.; Serre, C.; Vimont, A.; Daturi, M.; Hamon, L.; De Weireld, G.; Chang, J.S.; Hong, D.Y.; Hwang, Y.K. High uptakes of CO2 and CH4 in mesoporous metal—Organic frameworks MIL-100 and MIL-101. Langmuir 2008, 24, 7245–7250. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Seo, Y.K.; Hwang, Y.K.; Chang, J.S.; Leclerc, H.; Wuttke, S.; Bazin, P.; Vimont, A.; Daturi, M.; Bloch, E. Controlled reducibility of a metal–organic framework with coordinatively unsaturated sites for preferential gas sorption. Angew. Chem. Int. Ed. 2010, 49, 5949–5952. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yoon, J.W.; Seo, Y.K.; Kim, M.B.; Lee, S.K.; Lee, U.H.; Hwang, Y.K.; Bae, Y.S.; Chang, J.S. Effect of purification conditions on gas storage and separations in a chromium-based metal-organic framework MIL-101. Microporous Mesoporous Mater. 2014, 193, 160–165. [Google Scholar] [CrossRef]
- Chang, G.; Bao, Z.; Ren, Q.; Deng, S.; Zhang, Z.; Su, B.; Xing, H.; Yang, Y. Fabrication of cuprous nanoparticles in MIL-101: An efficient adsorbent for the separation of olefin–paraffin mixtures. RSC Adv. 2014, 4, 20230–20233. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Krishna, R.; Wu, Z.; Ma, D.; Shi, Z.; Pham, T.; Forrest, K.; Space, B.; Ma, S. Highly selective adsorption of ethylene over ethane in a MOF featuring the combination of open metal site and π-complexation. Chem. Commun. 2015, 51, 2714–2717. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Yoon, T.U.; Kim, E.J.; Yoon, J.W.; Kim, S.Y.; Yoon, J.W.; Hwang, Y.K.; Chang, J.S.; Bae, Y.S. Facile loading of Cu (I) in MIL-100 (Fe) through redox-active Fe (II) sites and remarkable propylene/propane separation performance. Chem. Eng. J. 2018, 331, 777–784. [Google Scholar] [CrossRef]
- Bao, Z.; Alnemrat, S.; Yu, L.; Vasiliev, I.; Ren, Q.; Lu, X.; Deng, S. Adsorption of ethane, ethylene, propane, and propylene on a magnesium-based metal–organic framework. Langmuir 2011, 27, 13554–13562. [Google Scholar] [CrossRef]
- Bae, Y.S.; Lee, C.Y.; Kim, K.C.; Farha, O.K.; Nickias, P.; Hupp, J.T.; Nguyen, S.T.; Snurr, R.Q. High propene/propane selectivity in isostructural metal–organic frameworks with high densities of open metal sites. Angew. Chem. Int. Ed. 2012, 51, 1857–1860. [Google Scholar] [CrossRef]
- Bachman, J.E.; Kapelewski, M.T.; Reed, D.A.; Gonzalez, M.I.; Long, J.R. M2(m-dobdc) (M = Mn, Fe, Co, Ni) metal–organic frameworks as highly selective, high-capacity adsorbents for olefin/paraffin separations. J. Am. Chem. Soc. 2017, 139, 15363–15370. [Google Scholar] [CrossRef]
- Yang, S.; Ramirez-Cuesta, A.J.; Newby, R.; Garcia-Sakai, V.; Manuel, P.; Callear, S.K.; Campbell, S.I.; Tang, C.C.; Schroder, M. Supramolecular binding and separation of hydrocarbons within a functionalized porous metal–organic framework. Nat. Chem. 2014, 7, 121–129. [Google Scholar] [CrossRef]
- Gücüyener, C.; van den Bergh, J.; Gascon, J.; Kapteijn, F. Ethane/ethene separation turned on its head: Selective ethane adsorption on the metal−organic framework ZIF-7 through a gate-opening mechanism. J. Am. Chem. Soc. 2010, 132, 17704–17706. [Google Scholar] [CrossRef]
- Van den Bergh, J.; Gucuyener, C.; Pidko, E.A.; Hensen, E.J.M.; Gascon, J.; Kapteijn, F. Understanding the anomalous alkane selectivity of ZIF-7 in the separation of light alkane/alkene mixtures. Chem. Eur. J. 2011, 17, 8832–8840. [Google Scholar] [CrossRef]
- Liao, P.Q.; Zhang, W.X.; Zhang, J.P.; Chen, X.M. Efficient purification of ethene by an ethane-trapping metal-organic framework. Nat. Commun. 2015, 6, 8697. [Google Scholar] [CrossRef]
- Kishida, K.; Okumura, Y.; Watanabe, Y.; Mukoyoshi, M.; Bracco, S.; Comotti, A.; Sozzani, P.; Horike, S.; Kitagawa, S. Recognition of 1,3-butadiene by a porous coordination polymer. Angew. Chem. Int. Ed. 2016, 55, 13784–13788. [Google Scholar] [CrossRef]
- Zhu, W.; Kapteijn, F.; Moulijn, J.A.; Jansen, J.C. Selective adsorption of unsaturated linear C4 molecules on the all-silica DD3R. Phys. Chem. Chem. Phys. 2000, 2, 1773–1779. [Google Scholar] [CrossRef]
- Hartmann, M.; Kunz, S.; Himsl, D.; Tangermann, O.; Ernst, S.; Wagener, A. Adsorptive separation of isobutene and isobutane on Cu3(BTC)2. Langmuir 2008, 24, 8634–8642. [Google Scholar] [CrossRef]
- Lange, M.; Kobalz, M.; Bergmann, J.; Lässig, D.; Lincke, J.; Möllmer, J.; Möller, A.; Hofmann, J.; Krautscheid, H.; Staudt, R.; et al. Structural flexibility of a copper-based metal–organic framework: Sorption of C4-hydrocarbons and in situ XRD. J. Mater. Chem. A 2014, 2, 8075–8085. [Google Scholar] [CrossRef]
- Ye, Z.M.; He, C.T.; Xu, Y.T.; Krishna, R.; Xie, Y.; Zhou, D.D.; Zhou, H.L.; Zhang, J.P.; Chen, X.M. A new isomeric porous coordination framework showing single-crystal to single-crystal structural transformation and preferential adsorption of 1,3-butadiene from C4 hydrocarbons. Cryst. Growth Des. 2017, 17, 2166–2171. [Google Scholar] [CrossRef]
- Pan, L.; Olson, D.H.; Ciemnolonski, L.R.; Heddy, R.; Li, J. Separation of hydrocarbons with a microporous metal–organic framework. Angew. Chem. Int. Ed. 2006, 45, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Peralta, D.; Chaplais, G.; Simon-Masseron, A.; Barthelet, K.; Pirngruber, G.D. Separation of C6 paraffins using zeolitic imidazolate frameworks: Comparison with zeolite 5A. Ind. Eng. Chem. Res. 2012, 51, 4692–4702. [Google Scholar] [CrossRef]
- Alaerts, L.; Kirschhock, C.E.; Maes, M.; van der Veen, M.A.; Finsy, V.; Depla, A.; Martens, J.A.; Baron, G.V.; Jacobs, P.A.; Denayer, J.F.M.; et al. Selective Adsorption and separation of xylene isomers and ethylbenzene with the microporous vanadium(IV) terephthalate MIL-47. Angew. Chem. Int. Ed. 2007, 46, 4293–4297. [Google Scholar] [CrossRef]
- Alaerts, L.; Maes, M.; Jacobs, P.A.; Denayer, J.F.M.; De Vos, D.E. Activation of the metal-organic framework MIL-47 for selective adsorption of xylenes and other difunctionalized aromatics. Phys. Chem. Chem. Phys. 2008, 10, 2979–2985. [Google Scholar] [CrossRef] [PubMed]
- Alaerts, L.; Maes, M.; Giebeler, L.; Jacobs, P.A.; Martens, J.A.; Denayer, J.F.M.; Kirschhock, C.E.A.; De Vos, D.E. Selective adsorption and separation of ortho-substituted alkylaromatics with the microporous aluminum terephthalate MIL-53. J. Am. Chem. Soc. 2008, 130, 14170–14178. [Google Scholar] [CrossRef] [PubMed]
- Finsy, V.; Verelst, H.; Alaerts, L.; De Vos, D.; Jacobs, P.A.; Baron, G.V.; Denayer, J.F.M. Pore-filling-dependent selectivity effects in the vapor-phase separation of xylene isomers on the metal−organic framework MIL-47. J. Am. Chem. Soc. 2008, 130, 7110–7118. [Google Scholar] [CrossRef] [PubMed]
- Finsy, V.; Christine, E.A.; Kirschhock, C.E.; Vedts, G.; Maes, M.; Alaerts, L.; De Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Framework breathing in the vapour-phase adsorption and separation of xylene isomers with the metal–organic framework MIL-53. Chem. Eur. J. 2009, 15, 7724–7731. [Google Scholar] [CrossRef] [PubMed]
- Bárcia, P.S.; Guimarães, D.; Mendes, P.A.P.; Silva, J.A.C.; Guillerm, V.; Chevreau, H.; Serre, C.; Rodrigues, A.E. Reverse shape selectivity in the adsorption of hexane and xylene isomers in MOF UiO-66. Microporous Mesoporous Mater. 2011, 139, 67–73. [Google Scholar] [CrossRef]
- Matsuda, R.; Kitaura, R.; Kitagawa, S.; Kubota, Y.; Belosludov, R.V.; Kobayashi, T.C.; Sakamoto, H.; Chiba, T.; Takata, M.; Kawazoe, Y.; et al. Highly controlled acetylene accommodation in a metal-organic microporous material. Nature 2005, 435, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Shao, K.; Wang, J.X.; Wen, H.M.; Yang, Y.; Cui, Y.; Krishna, R.; Li, B.; Qian, G. A chemically stable Hofmann-type metal−organic framework with sandwich-like binding sites for benchmark acetylene capture. Adv. Mater. 2020, 32, 1908275. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, K.; Yang, L.; Li, L.; Hu, E.; Yang, L.; Shao, K.; Xing, H.; Cui, Y.; Yang, Y.; et al. Benchmark C2H2/CO2 separation in an ultra-microporous metal–organic framework via copper(I)-alkynyl chemistry. Angew. Chem. Int. Ed. 2021, 60, 15995. [Google Scholar] [CrossRef]
- Ye, Y.X.; Xian, S.K.; Cui, H.; Tan, K.; Gong, L.S.; Liang, B.; Pham, T.; Pandey, H.; Krishna, R.; Lan, P.C.; et al. Metal-organic framework based hydrogen-bonding nanotrap for efficient acetylene storage and separation. J. Am. Chem. Soc. 2022, 144, 1681–1689. [Google Scholar] [CrossRef]
- Ma, D.Y.; Li, Z.; Zhu, J.X.; Zhou, Y.P.; Chen, L.L.; Mai, X.F.; Liufu, M.L.; Wu, Y.B.; Li, Y.W. Inverse and highly selective separation of CO2/C2H2 on a thulium-organic framework. J. Mater. Chem. A 2020, 8, 11933–11937. [Google Scholar] [CrossRef]
- Xie, L.H.; Liu, X.M.; He, T.; Li, J.R. Metal-organic frameworks for the capture of trace aromatic volatile organic compounds. Chem 2018, 4, 1911–1927. [Google Scholar] [CrossRef]
- He, T.; Kong, X.J.; Bian, Z.X.; Zhang, Y.Z.; Si, G.R.; Xie, L.H.; Wu, X.Q.; Huang, H.L.; Chang, Z.; Bu, X.H.; et al. Trace removal of benzene vapour using double-walled metal-dipyrazolate frameworks. Nat. Mater. 2022, 21, 689–695. [Google Scholar] [CrossRef]
- Dong, C.; Yang, J.J.; Xie, L.H.; Cui, G.L.; Fang, W.H.; Li, J.R. Catalytic ozone decomposition and adsorptive VOCs removal in bimetallic metal-organic frameworks. Nat. Commun. 2022, 13, 4991. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change. World Meteorological Organization Technical Document: Geneva, Switzerland, 2018. Available online: http://www.ipcc.ch/report/sr15/ (accessed on 25 January 2024).
- Siegelman, R.L.; Kim, E.J.; Long, J.R. Porous materials for carbon dioxide separations. Nat. Mater. 2021, 20, 1060–1072. [Google Scholar] [CrossRef]
- Schlissel, D.W.D.; Wamsted, D. Holy Grail of Carbon Capture Continues to Elude Coal Industry. Inst. Energy Econ. Financ. Anal. 2018. Available online: https://ieefa.org/resources/holy-grail-carbon-capture-continues-elude-coal-industry (accessed on 25 January 2024).
- Refinitiv Carbon Market Survey 2021. Available online: https://www.refinitiv.com/ (accessed on 24 June 2021).
- Kamio, E.; Yoshioka, T. Membrane separation technology for CO2 separation and recovery in Japan. Membrane 2017, 42, 2–10. [Google Scholar] [CrossRef]
- Wilcox, J. Carbon Capture; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Wilcox, J.; Haghpanah, R.; Rupp, E.C.; He, J.J.; Lee, K. Advancing adsorption and membrane separation processes for the gigaton carbon capture challenge. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 479–505. [Google Scholar] [CrossRef]
- Panda, P.K.; Sahoo, B.; Ramakrishna, S. Hydrogen production, purification, storage, transportation, and their applications: A review. Energy Technol. 2023, 11, 2201434. [Google Scholar] [CrossRef]
- Yan, Y.; Lin, X.; Yang, S.H.; Blake, A.J.; Dailly, A.; Champness, N.R.; Hubberstey, P.; Schröder, M. Exceptionally high H2 storage by a metal–organic polyhedral framework. Chem. Commun. 2009, 1025–1027. [Google Scholar] [CrossRef]
- Feldblyum, J.I.; Wong-Foy, A.G.; Matzger, A.J. Non-interpenetrated IRMOF-8: Synthesis, activation, and gas sorption. Chem. Commun. 2012, 48, 9828–9830. [Google Scholar] [CrossRef]
- Sumida, K.; Brown, C.M.; Herm, Z.R.; Chavan, S.; Bordiga, S.; Long, J.R. Hydrogen storage properties and neutron scattering studies of Mg2(dobdc)-a metal-organic framework with open Mg2+ adsorption sites. Chem. Commun. 2011, 47, 1157–1159. [Google Scholar] [CrossRef]
- Lin, X.; Jia, J.H.; Zhao, X.B.; Thomas, K.M.; Blake, A.J.; Walker, G.S.; Champness, N.R.; Hubberstey, P.; Schröder, M. High H2 adsorption by coordination-framework materials. Angew. Chem. Int. Ed. 2006, 45, 7358–7364. [Google Scholar] [CrossRef]
- Chen, B.L.; Ockwig, N.W.; Millward, A.R.; Contreras, D.S.; Yaghi, O.M. High H2 adsorption in a microporous metal-organic framework with open metal sites. Angew. Chem. Int. Ed. 2005, 44, 4745–4749. [Google Scholar] [CrossRef]
- Wang, X.S.; Ma, S.Q.; Rauch, K.; Simmons, J.M.; Yuan, D.Q.; Wang, X.P.; Yildirim, T.; Cole, W.C.; Lopez, J.J.; de Meijere, A.; et al. Metal-organic frameworks based on double-bond-coupled di-isophthalate linkers with high hydrogen and methane uptakes. Chem. Mater. 2008, 20, 3145–3152. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal−organic frameworks. J. Am. Chem. Soc. 2006, 128, 1304–1315. [Google Scholar] [CrossRef]
- Park, H.J.; Lim, D.W.; Yang, W.S.; Oh, T.R.; Suh, M.P. A highly porous metal–organic framework: Structural transformations of a guest-free MOF depending on activation method and temperature. Chem. Eur. J. 2011, 17, 7251–7260. [Google Scholar] [CrossRef]
- Liu, J.Q.; Liu, G.L.; Gu, C.Y.; Liu, W.C.; Xu, J.W.; Li, B.H.; Wang, W.J. Rational synthesis of a novel 3,3,5-c polyhedral metal-organic framework with high thermal stability and hydrogen storage capability. J. Mater. Chem. A 2016, 4, 11630–11634. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Millward, A.R.; Park, K.S.; Yaghi, O.M. Hydrogen sorption in functionalized metal−organic frameworks. J. Am. Chem. Soc. 2004, 126, 5666–5667. [Google Scholar] [CrossRef]
- Yuan, D.Q.; Zhao, D.; Sun, D.F.; Zhou, H.C. An isoreticular series of metal-organic frameworks with dendritic hexacarboxylate ligands and exceptionally high gas-uptake capacity. Angew. Chem. Int. Ed. 2010, 49, 5357–5361. [Google Scholar] [CrossRef]
- Hermes, S.; Schroder, F.; Chelmowski, R.; Wöll, C.; Fischer, R.A. Selective nucleation and growth of metal−organic open framework thin films on patterned COOH/CF3-terminated self-assembled monolayers on Au(111). J. Am. Chem. Soc. 2005, 127, 13744–13745. [Google Scholar] [CrossRef]
- Arnold, M.; Kortunov, P.; Jones, D.J.; Nedellec, Y.; Kärger, J.; Caro, J. Oriented crystallisation on supports and anisotropic mass transport of the metal-organic framework manganese formate. Eur. J. Inorg. Chem. 2007, 2007, 60–64. [Google Scholar] [CrossRef]
- Scherb, C.; Schodel, A.; Bein, T. Directing the structure of metal–organic frameworks by oriented surface growth on an organic monolayer. Angew. Chem. Int. Ed. 2008, 47, 5777–5779. [Google Scholar] [CrossRef]
- Gascon, J.; Aguado, S.; Kapteijn, F. Manufacture of dense coatings of Cu3(BTC)2 (HKUST-1) on α-alumina. Microporous Mesoporous Mater. 2008, 113, 132–138. [Google Scholar] [CrossRef]
- Lin, R.J.; Hernandez, B.V.; Ge, L.; Zhu, Z.H. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, G.; Pan, Y.; Lai, Z. Synthesis of highly c-oriented ZIF-69 membranes by secondary growth and their gas permeation properties. J. Membr. Sci. 2011, 379, 46–51. [Google Scholar] [CrossRef]
- Zheng, B.; Pan, Y.; Lai, Z.; Huang, K.W. Molecular dynamics simulations on gate opening in ZIF-8: Identification of factors for ethane and propane separation. Langmuir 2013, 29, 8865–8872. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Highly permeable zeolite imidazolate framework-8 membranes for CO2/CH4 separation. J. Am. Chem. Soc. 2010, 132, 76–78. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.; Shin, H.; Yoo, S.J.; Kwon, H.T.; Kim, J. Zeolitic imidazolate framework ZIF-8 films by ZnO to ZIF-8 conversion and their usage as seed layers for propylene-selective ZIF-8 membranes. J. Ind. Eng. Chem. 2019, 72, 374–379. [Google Scholar] [CrossRef]
- Huang, A.; Bux, H.; Steinbach, F.; Caro, J. Molecular-sieve membrane with hydrogen permselectivity: ZIF-22 in LTA topology prepared with 3-aminopropyltriethoxysilane as covalent linker. Angew. Chem. Int. Ed. 2010, 49, 4958–4961. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Guerrero, V.V.; Barnett, G.V.; Jeong, H.K. Synthesis of zeolitic imidazolate framework films and membranes with controlled microstructures. Langmuir 2010, 26, 14636–14641. [Google Scholar] [CrossRef]
- Tanaka, S.; Shimada, T.; Fujita, K.; Miyake, Y.; Kida, K.; Yogo, K.; Denayer, J.F.M.; Sugita, M.; Takewaki, T. Seeding-free aqueous synthesis of zeolitic imidazolate framework-8 membranes: How to trigger preferential heterogeneous nucleation and membrane growth in aqueous rapid reaction solution. J. Membr. Sci. 2014, 472, 29–38. [Google Scholar] [CrossRef]
- Tanaka, S.; Okubo, K.; Kida, K.; Sugita, M.; Takewaki, T. Grain size control of ZIF-8 membranes by seeding-free aqueous synthesis and their performances in propylene/propane separation. J. Membr. Sci. 2017, 544, 306–311. [Google Scholar] [CrossRef]
- Li, W.; Yang, Z.; Zhang, G.; Fan, Z.; Meng, Q.; Shen, C.; Gao, C. Stiff metal–organic framework–polyacrylonitrile hollow fiber composite membranes with high gas permeability. J. Mater. Chem. A 2014, 2, 2110–2118. [Google Scholar] [CrossRef]
- Li, W.; Meng, Q.; Zhang, C.; Zhang, G. Metal–organic framework/PVDF composite membranes with high H2 permselectivity synthesized by ammoniation. Chem. Eur. J. 2015, 21, 7224–7230. [Google Scholar] [CrossRef]
- Wei, R.C.; Liu, X.W.; Zhou, Z.Y.; Chen, C.L.; Yuan, Y.Y.; Li, Z.; Li, X.; Dong, X.L.; Lu, D.W.; Han, Y.; et al. Carbon nanotube supported oriented metal organic framework membrane for effective ethylene/ethane separation. Sci. Adv. 2022, 8, eabm6741. [Google Scholar] [CrossRef]
- Yao, J.; Dong, D.; Li, D.; He, L.; Xu, G.; Wang, H. Contra-diffusion synthesis of ZIF-8 films on a polymer substrate. Chem. Commun. 2011, 47, 2559–2561. [Google Scholar] [CrossRef]
- Know, H.T.; Jeong, H.K. In situ synthesis of thin zeolitic–imidazolate framework ZIF-8 membranes exhibiting exceptionally high propylene/propane separation. J. Am. Chem. Soc. 2013, 135, 10763–10768. [Google Scholar]
- Hara, N.; Yoshimune, M.; Negishi, H.; Haraya, K.; Hara, S.; Yamaguchi, T. Diffusive separation of propylene/propane with ZIF-8 membranes. J. Membr. Sci. 2014, 450, 215–223. [Google Scholar] [CrossRef]
- Dong, X.; Huang, K.; Liu, S.; Ren, R.; Jin, W.; Lin, Y.S. Synthesis of zeolitic imidazolate framework-78 molecular-sieve membrane: Defect formation and elimination. J. Mater. Chem. 2012, 22, 19222–19227. [Google Scholar] [CrossRef]
- Guerrero, V.V.; Yoo, Y.; McCarthy, M.C.; Jeong, H.K. HKUST-1membranes on porous supports using secondary growth. J. Mater. Chem. 2010, 20, 3938–3943. [Google Scholar] [CrossRef]
- Bux, H.; Chmelik, C.; Krishna, R.; Caro, J. Ethene/ethane separation by the MOF membrane ZIF-8: Molecular correlation of permeation, adsorption, diffusion. J. Membr. Sci. 2011, 369, 284–289. [Google Scholar] [CrossRef]
- Sánchez, E.P.V.; Gliemann, H.; Haas-Santo, K.; Ding, W.J.; Hansjosten, E.; Wohlgemuth, J.; Woll, C.; Dittmeyer, R. α-Al2O3-supported ZIF-8 SURMOF membranes: Diffusion mechanism of ethene/ethane mixtures and gas separation performance. J. Membr. Sci. 2019, 594, 117421. [Google Scholar] [CrossRef]
- Sun, Y.W.; Ji, T.T.; Gao, Y.L.; Yan, J.H.; He, Y.F.; Xu, G.L.; Yan, F.Y.; Bian, Q.; Liu, Y. Freezing contra-diffusion: A new protocol for synthesizing Co-gallate MOF membranes toward superior ethylene/ethane separation performance. ACS Mater. Lett. 2023, 5, 558–564. [Google Scholar] [CrossRef]
- Yang, K.; Ban, Y.J.; Yang, W.S. Layered MOF membranes modified with ionic liquid/AgBF4 composite for olefin/paraffin separation. J. Membr. Sci. 2021, 639, 119771. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, Y.; Liu, Y.T.; Wu, H.; Zhao, M.G.; Ren, Y.X.; Pu, Y.C.A.; Li, W.P.; Wang, S.Y.; Song, S.Q.; et al. Ultrathin ZIF-8 membrane through inhibited Ostwald ripening for high-flux C3H6/C3H8 separation. Adv. Funct. Mater. 2022, 32, 2208064. [Google Scholar] [CrossRef]
- Zhang, K.; Lively, R.P.; Zhang, C.; Chance, R.R.; Koros, W.J.; Sholl, D.S.; Nair, S. Exploring the framework hydrophobicity and flexibility of ZIF-8: From biofuel recovery to hydrocarbon separations. J. Phys. Chem. Lett. 2013, 4, 3618–3622. [Google Scholar] [CrossRef]
- Bux, H.; Chmelik, C.; van Baten, J.M.; Krishna, R.; Caro, J. Novel MOF-membrane for molecular sieving predicted by IR-diffusion studies and molecular modeling. Adv. Mater. 2010, 22, 4741–4743. [Google Scholar] [CrossRef]
- Li, K.; Olson, D.H.; Seidel, J.; Emge, T.J.; Gong, H.; Zeng, H.; Li, J. Zeolitic imidazolate frameworks for kinetic separation of propane and propene. J. Am. Chem. Soc. 2009, 131, 10368–10369. [Google Scholar] [CrossRef]
- Pan, Y.; Lai, Z. Sharp separation of C2/C3 hydrocarbon mixtures by zeolitic imidazolate framework-8 (ZIF-8) membranes synthesized in aqueous solutions. Chem. Commun. 2011, 47, 10275–10277. [Google Scholar] [CrossRef]
- Zhang, C.; Lively, R.P.; Zhang, K.; Johnson, J.R.; Karvan, O.; Koros, W.J. Unexpected molecular sieving properties of zeolitic imidazolate framework-8. J. Phys. Chem. Lett. 2012, 3, 2130–2134. [Google Scholar] [CrossRef]
- Song, E.Y.; Wei, K.F.; Lian, H.Q.; Hua, J.X.; Tao, H.X.; Wu, T.T.; Pan, Y.C.; Xing, W.H. Improved propylene/propane separation performance under high temperature and pressures on in-situ ligand-doped ZIF-8 membranes. J. Membr. Sci. 2021, 617, 118655. [Google Scholar] [CrossRef]
- Brown, A.J.; Brunelli, N.A.; Eum, K.; Rashidi, F.; Johnson, J.R.; Koros, W.J.; Jones, C.W.; Nair, S. Interfacial microfluidic processing of metal-organic framework hollow fiber membranes. Science 2014, 345, 72–75. [Google Scholar] [CrossRef]
- Eum, K.; Ma, C.; Rownaghi, A.; Jones, C.W.; Nair, S. ZIF-8 membranes via interfacial microfluidic processing in polymeric hollow fibers: Efficient propylene separation at elevated pressures. ACS Appl. Mater. Interfaces 2016, 8, 25337–25342. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.K.; Lee, A.S.; An, H.S.; Lee, J.S. Heteroepitaxially grown zeolitic imidazolate framework membranes with unprecedented propylene/propane separation performances. J. Am. Chem. Soc. 2015, 137, 12304–12311. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Li, L.; Duan, Y.; Hou, Q.; Zhang, L.; Ding, L.X.; Xue, J.; Wang, H.; Caro, J. Paralyzed membrane: Current-driven synthesis of a metal-organic framework with sharpened propene/propane separation. Sci. Adv. 2018, 4, eaau1393. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.Q.; Zhou, S.; Wei, Y.Y.; Caro, J.; Wang, H.H. Balancing the grain boundary structure and the framework flexibility through bimetallic metal-organic framework (MOF) membranes for gas separation. J. Am. Chem. Soc. 2020, 142, 9582–9586. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Wei, Y.Y.; Lyu, L.X.; Hou, Q.Q.; Caro, J.; Wang, H.H. Flexible polypropylene-supported ZIF-8 membranes for highly efficient propene/propane separation. J. Am. Chem. Soc. 2020, 142, 20915–20919. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Su, P.; Li, Z.; Xu, Z.; Wang, F.; Ou, H.; Zhang, J.; Zhang, G.; Zeng, E. Ultrathin metal–organic framework membrane production by gel–vapour deposition. Nat. Commun. 2017, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kumar, P.; Mittal, N.; Khlyustova, A.; Daoutidis, P.; Mkhoyan, K.A.; Tsapatsis, M. Zeolitic imidazolate framework membranes made by ligand-induced permselectivation. Science 2018, 361, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Eum, K.; Yang, S.W.; Min, B.; Ma, C.; Drese, J.H.; Tamhankar, Y.; Nair, S. All-nanoporous hybrid membranes: Incorporating zeolite nanoparticles and nanosheets with zeolitic imidazolate framework matrices. ACS Appl. Mater. Interfaces 2020, 12, 27368–27377. [Google Scholar] [CrossRef]
- Liu, D.H.; Xiang, L.; Chang, H.; Chen, K.; Wang, C.Q.; Pan, Y.C.; Li, Y.S.; Jiang, Z.Y. Rational matching between MOFs and polymers in mixed matrix membranes for propylene/propane separation. Chem. Eng. Sci. 2019, 204, 151–160. [Google Scholar] [CrossRef]
- Sun, Y.X.; Tian, L.; Qiao, Z.H.; Geng, C.X.; Guo, X.Y.; Zhong, C.L. Surface modification of bilayer structure on metal-organic frameworks towards mixed matrix membranes for efficient propylene/propane separation. J. Membr. Sci. 2022, 648, 120350. [Google Scholar] [CrossRef]
- Lee, T.H.; Jung, J.G.; Kim, Y.J.; Roh, J.S.; Yoon, H.W.; Ghanem, B.S.; Kim, H.W.; Cho, Y.H.; Pinnau, I.; Park, H.B. Defect engineering in metal-organic frameworks towards advanced mixed matrix membranes for efficient propylene/propane separation. Angew. Chem. Int. Ed. 2021, 60, 13081–13088. [Google Scholar] [CrossRef]
- Song, S.Q.; Jiang, H.F.; Wu, H.; Zhao, M.G.; Guo, Z.Y.; Li, B.Y.; Ren, Y.X.; Wang, Y.H.; Ye, C.M.; Guiver, M.D.; et al. Weakly pressure-dependent molecular sieving of propylene/propane mixtures through mixed matrix membrane with ZIF-8 direct-through channels. J. Membr. Sci. 2022, 648, 120366. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.J.; Liu, G.P.; Belmabkhout, Y.; Adil, K.; Eddaoudi, M.; Koros, W. Conformation-controlled molecular sieving effects for membrane-based propylene/propane separation. Adv. Mater. 2019, 31, 1807513. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.N.; Liu, G.Z.; Liu, G.P.; Jin, W.Q. UTSA-280 metal-organic framework incorporated 6FDA-polyimide mixed-matrix membranes for ethylene/ethane separation. AIChE J. 2022, 68, e17688. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Samarasinghe, S.A.S.C.; Li, W.; Goh, K.; Bae, T.H. Leveraging nanocrystal HKUST-1 in mixed-matrix membranes for ethylene/ethane separation. Membranes 2020, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- He, R.R.; Cong, S.Z.; Peng, D.L.; Zhang, Y.T.; Shan, M.X.; Zhu, J.Y.; Wang, J.; Gascon, J. Enhanced compatibility and selectivity in mixed matrix membranes for propylene/propane separation. AIChE J. 2023, 69, e17948. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, L.T.; Zhang, K.; Fan, W.D.; Fan, L.L.; Kang, Z.X.; Sun, D.F. Fabrication of mixed matrix membranes with regulated MOF fillers via incorporating guest molecules for optimizing light hydrocarbon separation performance. CrystEngComm 2022, 24, 7658–7668. [Google Scholar] [CrossRef]
- Shen, Q.; Cong, S.Z.; He, R.R.; Wang, Z.; Jin, Y.H.; Li, H.; Cao, X.Z.; Wang, J.; Van der Bruggen, B.; Zhang, Y.T. SIFSIX-3-Zn/PIM-1 mixed matrix membranes with enhanced permeability for propylene/propane separation. J. Membr. Sci. 2019, 588, 117201. [Google Scholar] [CrossRef]
- Eum, K.; Ma, C.; Koh, D.Y.; Rashidi, F.; Li, Z.; Jones, C.W.; Lively, R.P.; Nair, S. Zeolitic imidazolate framework membranes supported on macroporous carbon hollow fibers by fluidic processing techniques. Adv. Mater. Interfaces 2017, 4, 1700080. [Google Scholar] [CrossRef]
- Wu, X.; Wei, W.; Jiang, J.; Caro, J.; Huang, A. High-flux high-selectivity metal–organic framework MIL-160 membrane for xylene isomer separation by pervaporation. Angew. Chem. Int. Ed. 2018, 57, 15354–15358. [Google Scholar] [CrossRef]
- Rahman, M.M.; Abetz, C.; Shishatskiy, S.; Martin, J.; Müller, A.J.; Abetz, V. CO2 selective PolyActive membrane: Thermal transitions and gas permeance as a function of thickness. ACS Appl. Mater. Interfaces 2018, 10, 26733–26744. [Google Scholar] [CrossRef]
- Embaye, A.S.; Martínez-Izquierdo, L.; Malankowska, M.; Téllez, C.; Coronas, J. Poly(ether-block-amide) copolymer membranes in CO2 separation applications. Energy Fuels 2021, 35, 17085–17102. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.Q.; Wei, X.T.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Metal organic framework membranes for carbon dioxide separation. Chem. Eng. Sci. 2015, 124, 3–19. [Google Scholar] [CrossRef]
- Dunne, J.; Myers, A.L. Adsorption of gas mixtures in micropores: Effect of difference in size of adsorbate molecules. Chem. Eng. Sci. 1994, 49, 2941–2951. [Google Scholar] [CrossRef]
- Pakseresht, S.; Kazemeini, M.; Akbarnejad, M.M. Equilibrium isotherms for CO, CO2, CH4 and C2H4 on the 5A molecular sieve by a simple volumetric apparatus. Sep. Purif. Technol. 2002, 28, 53–60. [Google Scholar] [CrossRef]
- Maghsoudi, H.; Soltanieh, M.; Bozorgzadeh, H.; Mohamadalizadeh, A. Adsorption isotherms and ideal selectivities of hydrogen sulfide and carbon dioxide over methane for the Si-CHA zeolite: Comparison of carbon dioxide and methane adsorption with the all-silica DD3R zeolite. Adsorption 2013, 19, 1045–1053. [Google Scholar] [CrossRef]
- Calleja, G.; Pau, J.; Calles, J.A. Pure and multicomponent adsorption equilibrium of carbon dioxide, ethylene, and propane on ZSM-5 zeolites with different Si/Al ratios. J. Chem. Eng. Data 1998, 43, 994–1003. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Synthesis of SAPO-34 crystals in the presence of crystal growth inhibitors. J. Phys. Chem. B 2008, 112, 16261–16265. [Google Scholar] [CrossRef]
- Lin, J.B.; Nguyen, T.T.T.; Vaidhyanathan, R.; Burner, J.; Taylor, J.M.; Durekova, H.; Akhtar, F.; Mah, R.K.; Ghaffari-Nik, O.; Marx, S.; et al. A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture. Science 2021, 374, 1464–1469. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal−organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Bourrelly, S.; Llewellyn, P.L.; Serre, C.; Millange, F.; Loiseau, T.; Férey, G. Different adsorption behaviors of methane and carbon dioxide in the isotypic nanoporous metal terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc. 2005, 127, 13519–13521. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Benin, A.I.; Jakubczak, P.; Willis, R.R.; LeVan, M.D. CO2/H2O adsorption equilibrium and rates on metal−organic frameworks: HKUST-1 and Ni/DOBDC. Langmuir 2010, 26, 14301–14307. [Google Scholar] [CrossRef]
- Mason, J.A.; Sumida, K.; Herm, Z.R.; Krishna, R.; Long, J.R. Evaluating metal–organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 2011, 4, 3030–3040. [Google Scholar] [CrossRef]
- Shekhah, O.; Belmabkhout, Y.; Chen, Z.; Guillerm, V.; Cairns, A.; Adil, K.; Eddaoudi, M. Made-to-order metal-organic frameworks for trace carbon dioxide removal and air capture. Nat. Commun. 2014, 5, 4228. [Google Scholar] [CrossRef]
- Venna, S.R.; Zhu, M.Q.; Li, S.G.; Carreon, M.A. Knudsen diffusion through ZIF-8 membranes synthesized by secondary seeded growth. J. Porous Mater. 2014, 21, 235–240. [Google Scholar] [CrossRef]
- Zhou, S.; Zou, X.; Sun, F.; Ren, H.; Liu, J.; Zhang, F.; Zhao, N.; Zhu, G. Development of hydrogen-selective CAU-1 MOF membranes for hydrogen purification by ‘dual-metal-source’ approach. Int. J. Hydrogen Energy 2013, 38, 5338–5347. [Google Scholar] [CrossRef]
- Nian, P.; Liu, H.; Zhang, X. Bottom-up fabrication of two-dimensional Co-based zeolitic imidazolate framework tubular membranes consisting of nanosheets by vapor phase transformation of Co-based gel for H2/CO2 separation. J. Membr. Sci. 2019, 573, 200–209. [Google Scholar] [CrossRef]
- Ben, T.; Lu, C.; Pei, C.; Xu, S.; Qiu, S. Polymer-supported and free-standing metal–organic framework membrane. Chem. Eur. J. 2012, 18, 10250–10253. [Google Scholar] [CrossRef]
- Kang, Z.; Xue, M.; Fan, L.; Huang, L.; Guo, L.; Wei, G.; Chen, B.; Qiu, S. Highly selective sieving of small gas molecules by using an ultra-microporous metal–organic framework membrane. Energy Environ. Sci. 2014, 7, 4053–4060. [Google Scholar] [CrossRef]
- Wang, X.; Chi, C.; Zhang, K.; Qian, Y.; Gupta, K.M.; Kang, Z.; Jiang, J.; Zhao, D. Reversed thermo-switchable molecular sieving membranes composed of two-dimensional metal-organic nanosheets for gas separation. Nat. Commun. 2017, 8, 14460. [Google Scholar] [CrossRef]
- Li, W.; Su, P.; Zhang, G.; Shen, C.; Meng, Q. Preparation of continuous NH2–MIL-53 membrane on ammoniated polyvinylidene fluoride hollow fiber for efficient H2 purification. J. Membr. Sci. 2015, 495, 384–391. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, X.; Gao, X.; Fan, S.; Sun, F.; Ren, H.; Zhu, G. Hydrogen selective NH2-MIL-53(Al) MOF membranes with high permeability. Adv. Funct. Mater. 2012, 22, 3583–3590. [Google Scholar] [CrossRef]
- Wang, N.; Mundstock, A.; Liu, Y.; Huang, A.; Caro, J. Amine-modified Mg-MOF-74/CPO-27-Mg membrane with enhanced H2/CO2 separation. Chem. Eng. Sci. 2015, 124, 27–36. [Google Scholar] [CrossRef]
- Fan, S.; Sun, F.; Xie, J.; Guo, J.; Zhang, L.; Wang, C.; Pan, Q.; Zhu, G. Facile synthesis of a continuous thin Cu(bipy)2(SiF6) membrane with selectivity towards hydrogen. J. Mater. Chem. A 2013, 1, 11438–11442. [Google Scholar] [CrossRef]
- Knebel, A.; Sundermann, L.; Mohmeyer, A.; Strauß, I.; Friebe, S.; Behrens, P.; Caro, J. Azobenzene guest molecules as light-switchable CO2 valves in an ultrathin UiO-67 membrane. Chem. Mater. 2017, 29, 3111–3117. [Google Scholar] [CrossRef]
- Li, W.; Meng, Q.; Li, X.; Zhang, C.; Fan, Z.; Zhang, G. Non-activation ZnO array as a buffering layer to fabricate strongly adhesive metal–organic framework/PVDF hollow fiber membranes. Chem. Commun. 2014, 50, 9711–9713. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, J.; Wang, J.; Bai, J.; Yin, H.; Yuan, B.; Lu, J.; Zhang, Y.; Zhou, L.; Duan, C. Deposition of chemically modified α-Al2O3 particles for high performance ZIF-8 membrane on a macroporous tube. Chem. Commun. 2012, 48, 5977–5979. [Google Scholar] [CrossRef]
- Huang, A.; Liu, Q.; Wang, N.; Caro, J. Highly hydrogen permselective ZIF-8 membranes supported on polydopamine functionalized macroporous stainless-steel-nets. J. Mater. Chem. A 2014, 2, 8246–8251. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, D.; Liu, Z.; Zhong, C. Synthesis of zeolitic imidazolate framework membrane using temperature-switching synthesis strategy for gas separation. Ind. Eng. Chem. Res. 2016, 55, 7164–7170. [Google Scholar] [CrossRef]
- Huang, A.; Wang, N.; Kong, C.; Caro, J. Organosilica-functionalized zeolitic imidazolate framework ZIF-90 membrane with high gas-separation performance. Angew. Chem. Int. Ed. 2012, 51, 10551–10555. [Google Scholar] [CrossRef]
- Huang, A.; Chen, Y.; Wang, N.; Hu, Z.; Jiang, J.; Caro, J. A highly permeable and selective zeolitic imidazolate framework ZIF-95 membrane for H2/CO2 separation. Chem. Commun. 2012, 48, 10981–10983. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y.; Ban, Y.; Yang, W. Two-dimensional metal–organic framework nanosheets for membrane-based gas separation. Angew. Chem. Int. Ed. 2017, 56, 9757–9761. [Google Scholar] [CrossRef]
- Yin, H.; Wang, J.; Xie, Z.; Yang, J.; Bai, J.; Lu, J.; Zhang, Y.; Yin, D.; Lin, J.Y.S. A highly permeable and selective amino-functionalized MOF CAU-1 membrane for CO2–N2 separation. Chem. Commun. 2014, 50, 3699–3701. [Google Scholar] [CrossRef]
- Hara, N.; Yoshimune, M.; Negishi, H.; Haraya, K.; Hara, S.; Yamaguchi, T. Metal–organic framework membranes with layered structure prepared within the porous support. RSC Adv. 2013, 3, 14233–14236. [Google Scholar] [CrossRef]
- Rui, Z.; James, J.B.; Kasik, A.; Lin, Y.S. Metal-organic framework membrane process for high purity CO2 production. AIChE J. 2016, 62, 3836–3841. [Google Scholar] [CrossRef]
- Dou, Z.; Cai, J.; Cui, Y.; Yu, J.; Xia, T.; Yang, Y.; Qian, G. Preparation and gas separation properties of metal-organic framework membranes. Z. Anorg. Allg. Chem. 2015, 641, 792–796. [Google Scholar] [CrossRef]
- Wu, W.; Li, Z.; Chen, Y.; Li, W. Polydopamine-modified metal–organic framework membrane with enhanced selectivity for carbon capture. Environ. Sci. Technol. 2019, 53, 3764–3772. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Liu, J.; Hou, J.; Zhang, Y. Enzyme-embedded metal–organic framework membranes on polymeric substrates for efficient CO2 capture. J. Mater. Chem. A 2017, 5, 19954–19962. [Google Scholar] [CrossRef]
- Jomekian, A.; Behbahani, R.M.; Mohammadi, T.; Kargari, A. Innovative layer by layer and continuous growth methods for synthesis of ZIF-8 membrane on porous polymeric support using poly(ether-block-amide) as structure directing agent for gas separation. Microporous Mesoporous Mater. 2016, 234, 43–54. [Google Scholar] [CrossRef]
- Liu, M.; Xie, K.; Nothling, M.D.; Gurr, P.A.; Tan, S.S.L.; Fu, Q.; Webley, P.A.; Qiao, G.G. Ultrathin Metal–Organic Framework Nanosheets as a Gutter Layer for Flexible Composite Gas Separation Membranes. ACS Nano 2018, 12, 11591–11599. [Google Scholar] [CrossRef]
- Kong, C.; Du, H.; Chen, L.; Chen, B. Nanoscale MOF/organosilica membranes on tubular ceramic substrates for highly selective gas separation. Energy Environ. Sci. 2017, 10, 1812–1819. [Google Scholar] [CrossRef]
- Nan, J.; Dong, X.; Wang, W.; Jin, W. Formation mechanism of metal–organic framework membranes derived from reactive seeding approach. Microporous Mesoporous Mater. 2012, 155, 90–98. [Google Scholar] [CrossRef]
- Yeo, Z.Y.; Chai, S.P.; Zhu, P.W.; Mohamed, A.R. Development of a hybrid membrane through coupling of high selectivity zeolite T on ZIF-8 intermediate layer and its performance in carbon dioxide and methane gas separation. Microporous Mesoporous Mater. 2014, 196, 79–88. [Google Scholar] [CrossRef]
- Li, J.; Cao, W.; Mao, Y.; Ying, Y.; Sun, L.; Peng, X. Zinc hydroxide nanostrands: Unique precursors for synthesis of ZIF-8 thin membranes exhibiting high size-sieving ability for gas separation. CrystEngComm 2014, 16, 9788–9791. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, Y.; Wang, N.; Li, Y.; Pan, J.H.; Yang, W.; Caro, J. Significantly enhanced separation using ZIF-8 membranes by partial conversion of calcined layered double hydroxide precursors. ChemSusChem 2015, 8, 3582–3586. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, H.; Ma, Q.; Mo, K.; Mao, H.; Feldhoff, A.; Cao, X.; Li, Y.; Pan, F.; Jiang, Z. A MOF glass membrane for gas separation. Angew. Chem. 2020, 132, 4395–4399. [Google Scholar] [CrossRef]
- Cacho-Bailo, F.; Etxeberría-Benavides, M.; Karvan, O.; Téllez, C.; Coronas, J. Sequential amine functionalization inducing structural transition in an aldehyde-containing zeolitic imidazolate framework: Application to gas separation membranes. CrystEngComm 2017, 19, 1545–1554. [Google Scholar] [CrossRef]
- Fan, W.D.; Ying, Y.P.; Peh, S.B.; Yuan, H.Y.; Yang, Z.Q.; Yuan, Y.D.; Shi, D.C.; Yu, X.; Kang, C.J.; Zhao, D. Multivariate polycrystalline metal–organic framework membranes for CO2/CH4 separation. J. Am. Chem. Soc. 2021, 143, 17716–17723. [Google Scholar] [CrossRef]
- Mubashir, M.; Fong, Y.Y.; Leng, C.T.; Keong, L.K. Optimization of spinning parameters on the fabrication of NH2-MIL-53(Al)/cellulose acetate (CA) hollow fiber mixed matrix membrane for CO2 separation. Sep. Purif. Technol. 2019, 215, 32–43. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Mixed matrix membranes based on amine and non-amine MIL-53(Al) in Pebax® MH-1657 for CO2 separation. Sep. Purif. Technol. 2018, 200, 177–190. [Google Scholar] [CrossRef]
- Sabetghadam, A.; Liu, X.L.; Orsi, A.F.; Lozinska, M.M.; Johnson, T.; Jansen, K.M.B.; Wright, P.A.; Carta, M.; McKeown, N.B.; Kapteijn, F.; et al. Towards high performance metal–organic framework–microporous polymer mixed matrix membranes: Addressing compatibility and limiting aging by polymer doping. Chem. Eur. J. 2018, 24, 12796–12800. [Google Scholar] [CrossRef]
- Fan, Y.F.; Yu, H.Y.; Xu, S.; Shen, Q.C.; Ye, H.M.; Li, N.W. Zn(II)-modified imidazole containing polyimide/ZIF-8 mixed matrix membranes for gas separations. J. Membr. Sci. 2020, 597, 117775. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Comparison between ZIF-67 and ZIF-8 in Pebax® MH-1657 mixed matrix membranes for CO2 separation. Sep. Purif. Technol. 2020, 235, 116150. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Zhang, S.; Hu, L.; Jin, J. Interfacial design of mixed matrix membranes for improved gas separation performance. Adv. Mater. 2016, 28, 3399–3405. [Google Scholar] [CrossRef]
- Nafisi, V.; Hagg, M.B. Development of dual layer of ZIF-8/PEBAX-2533 mixed matrix membrane for CO2 capture. J. Membr. Sci. 2014, 459, 244–255. [Google Scholar] [CrossRef]
- Dong, L.; Chen, M.; Li, J.; Shi, D.; Dong, W.; Li, X.; Bai, Y. Metal-organic framework-graphene oxide composites: A facile method to highly improve the CO2 separation performance of mixed matrix membranes. J. Membr. Sci. 2016, 520, 801–811. [Google Scholar] [CrossRef]
- Li, H.; Tuo, L.; Yang, K.; Jeong, H.K.; Dai, Y.; He, G.; Zhao, W. Simultaneous enhancement of mechanical properties and CO2 selectivity of ZIF-8 mixed matrix membranes: Interfacial toughening effect of ionic liquid. J. Membr. Sci. 2016, 511, 130–142. [Google Scholar] [CrossRef]
- Jeazet, H.B.T.; Sorribas, S.; Román-Marín, J.M.; Zornoza, B.; Téllez, C.; Coronas, J. Increased selectivity in CO2/CH4 separation with mixed-matrix membranes of polysulfone and mixed-MOFs MIL-101(Cr) and ZIF-8. Eur. J. Inorg. Chem. 2016, 2016, 4363–4367. [Google Scholar] [CrossRef]
- Xin, Q.P.; Ouyang, J.Y.; Liu, T.Y.; Li, Z.; Li, Z.; Liu, Y.C.; Wang, S.F.; Wu, H.; Jiang, Z.Y.; Gao, X.Z. Enhanced interfacial interaction and CO2 separation performance of mixed matrix membrane by incorporating polyethylenimine-decorated metal–organic frameworks. ACS Appl. Mater. Interfaces 2015, 7, 1065–1077. [Google Scholar] [CrossRef]
- Japip, S.; Xiao, Y.; Chung, T.S. Particle-size effects on gas transport properties of 6FDA-Durene/ZIF-71 mixed matrix membranes. Ind. Eng. Chem. Res. 2016, 55, 9507–9517. [Google Scholar] [CrossRef]
- Hao, L.; Liao, K.S.; Chung, T.S. Photo-oxidative PIM-1 based mixed matrix membranes with superior gas separation performance. J. Mater. Chem. A 2015, 3, 17273–17281. [Google Scholar] [CrossRef]
- Ghalei, B.; Sakurai, K.; Kinoshita, Y.; Wakimoto, K.; Isfahani, A.P.; Song, Q.; Doitomi, K.; Furukawa, S.; Hirao, H.; Kusuda, H.; et al. Enhanced selectivity in mixed matrix membranes for CO2 capture through efficient dispersion of amine-functionalized MOF nanoparticles. Nat. Energy 2017, 2, 17086. [Google Scholar] [CrossRef]
- Yu, G.L.; Zou, X.Q.; Sun, L.; Liu, B.S.; Wang, Z.Y.; Zhang, P.P.; Zhu, G.S. Constructing connected paths between UiO-66 and PIM-1 to improve membrane CO2 separation with crystal-like gas selectivity. Adv. Mater. 2019, 31, 1806853. [Google Scholar] [CrossRef]
- Venna, S.R.; Lartey, M.; Li, T.; Spore, A.; Kumar, S.; Nulwala, H.B.; Luebke, D.R.; Rosi, N.L.; Albenze, E. Fabrication of MMMs with improved gas separation properties using externally-functionalized MOF particles. J. Mater. Chem. A 2015, 3, 5014–5022. [Google Scholar] [CrossRef]
- Jiang, X.; Li, S.; He, S.; Bai, Y.; Shao, L. Interface manipulation of CO2–philic composite membranes containing designed UiO-66 derivatives towards highly efficient CO2 capture. J. Mater. Chem. A 2018, 6, 15064–15073. [Google Scholar] [CrossRef]
- Tien-Binh, N.; Vinh-Thang, H.; Chen, X.Y.; Rodrigue, D.; Kaliaguine, S. Crosslinked MOF-polymer to enhance gas separation of mixed matrix membranes. J. Membr. Sci. 2016, 520, 941–950. [Google Scholar] [CrossRef]
- Perea-Cachero, A.; Etxeberría-Benavides, M.; David, O.; Deacon, A.; Johnson, T.; Malankowska, M.; Téllez, C.; Coronas, J. Pre-combustion gas separation by ZIF-8-polybenzimidazole mixed matrix membranes in the form of hollow fibres-long-term experimental study. R. Soc. Open Sci. 2021, 8, 210660. [Google Scholar] [CrossRef]
- Haldoupis, E.; Nair, S.; Sholl, D.S. Efficient calculation of diffusion limitations in metal organic framework materials: A tool for identifying materials for kinetic separations. J. Am. Chem. Soc. 2010, 132, 7528–7539. [Google Scholar] [CrossRef]
- Daglar, H.; Keskin, S. Computational screening of metal-organic frameworks for membrane-based CO2/N2/H2O separations: Best materials for flue gas separation. J. Phys. Chem. C 2018, 122, 17347–17357. [Google Scholar] [CrossRef]
- Krishna, R. Methodologies for screening and selection of crystalline microporous materials in mixture separations. Sep. Purif. Technol. 2018, 194, 281–300. [Google Scholar] [CrossRef]
- Keskin, S.; Altinkaya, S.A. A review on computational modeling tools for MOF-based mixed matrix membranes. Computation 2019, 7, 36. [Google Scholar] [CrossRef]
- Daglar, H.; Keskin, S. Recent advances, opportunities, and challenges in high-throughput computational screening of MOFs for gas separations. Coord. Chem. Rev. 2020, 422, 213470. [Google Scholar] [CrossRef]
- Li, W.; Liang, T.G.; Lin, Y.C.; Wu, W.X.; Li, S. Machine learning accelerated high-throughput computational screening of metal-organic frameworks. Prog. Chem. 2022, 34, 2619–2637. [Google Scholar]
- Gulbalkan, H.C.; Aksu, G.O.; Ercakir, G.; Keskin, S. Accelerated discovery of metal-organic frameworks for CO2 capture by artificial intelligence. Ind. Eng. Chem. Res. 2023, 63, 37–48. [Google Scholar] [CrossRef]
- Demir, H.; Keskin, S. A new era of modeling MOF-based membranes: Cooperation of theory and data science. Macromolec. Mater. Eng. 2024, 309, 2300225. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).