Abstract
In this study, the extraction of rare earth elements (REEs) from chloride solutions after leaching REE carbonate concentrate with solutions of the mixtures of P507 (2-ethylhexylphosphonic acid mono-2-ethylhexyl ester) and Cyanex 272 (bis(2,4,4-trimethylpentyl)phosphinic acid) (1:1) at various concentrations was experimentally studied. It was shown that the distribution ratios of all REEs decrease with the increasing concentration of these metals in the initial solution, which is associated with the loading of the organic phase. The most significant improvement in the extraction is observed for the heavy group of rare earth elements. The extractability of REEs increases with the increasing atomic number of the element, as is typical for the extraction of these metals with acidic organophosphorus extractants. The data obtained show that the separation factors of adjacent rare earth elements decrease slightly with the increasing concentration of metals in the initial aqueous solution. Increasing the concentration of the extractant mixture does not have a significant effect on the values of the adjacent REE separation factors. The data obtained on the distribution ratios and separation factors made it possible to propose a flow sheet for the separation of rare earth elements with the production of Y, Ho, Tb and Dy.
1. Introduction
Rare earth elements (REEs), including lanthanides, scandium and yttrium, are widely used both in traditional industries and in modern production, since they have a variety of useful and unique properties (for example, electronic, luminescent, magnetic, catalytic, etc.). New applications in electronics and other industries have led to an increase in demand for REEs as well as high purity REE materials. Owing to the similarity in chemical and physical properties, separating REEs is a complicated and urgent problem. Recently, the number of studies on the recovery and separation of REEs using various methods, especially the use of solvent extraction, has been growing. This is due to the fact that solvent extraction is presently one of the major techniques used at the industrial scale for recovery, separation and concentration of metals from aqueous solutions. Particularly, the extraction of rare earth elements with acidic organophosphorus extractants of various kinds is widely used. Among these extractants, bis(2,4,4-trimethylpentyl)phosphinic acid (Cyanex 272), di(2-ethylhexyl)phosphoric acid and 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester (P507) are commonly applied for the recovery and separation of REEs [1,2,3,4,5,6,7]. Sufficiently high separation factors between adjacent elements are observed in P507 extraction systems [5,6,7,8,9], but the separation factors for some lanthanide pairs (such as Nd/Pr, Gd/Eu, Lu/Yb) are very low. Cyanex 272 also exhibits high separation efficiency between adjacent rare earth elements; however, the extraction power of Cyanex 272 towards REEs is low owing to its high pKa value [10]. Moreover, the use of Cyanex 272 is restricted due to the poor physical characteristics of this extractant. To overcome the low extractability of rare earth elements in systems with Cyanex 272, the use of synergistic mixtures with other extractants has been suggested [11,12,13,14,15,16,17,18]. In [19], a binary mixture of P507 and Cyanex 272 was proposed as a synergistic system for the extraction of REEs. It has been found that the separation of heavy lanthanides(III) in the systems involving a binary mixture of P507 and Cyanex 272 was higher than those using P507. Additionally, the stripping processes in the systems involving mixtures of P507 and Cyanex 272 proceeded more easily than with P507 alone. Later, Cytec Industries developed a new extractant called Cyanex 572, which is a mixture of phosphinic (Cyanex 272) and phosphonic (P507) acids (Cytec Industries, 2015) [20]. This reagent was specifically developed for the extraction and purification of rare earth elements. Cyanex 572 provides efficient extraction of heavy REEs simultaneously, with the stripping of these metals from the loaded organic phases using mineral acid solutions with a lower concentration than in the P507 system. It was found that Cyanex 572 achieves the same separation performance as P507 while reducing the acid requirements by more than 30% [21,22,23,24]. In [23], it was reported that Cyanex 572 is a more effective extractant than Cyanex 272 for the separation of Nd(III) from a mixture containing Nd, Tb and Dy. The ability of Cyanex 572 to form complexes with metal ions is between that of phosphonic and phosphinic organic acids [22]. Thus, Cyanex 572 has potential for commercial applications due to the significant reduction in chemical consumption compared to the P507 system.
The aim of this work is to study the use of the mixtures of P507 and Cyanex 272 (1:1) for the extraction and separation of rare earth elements (Ce, Pr, Nd, Sm, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu and Y) from chloride solutions after the leaching of REE carbonate concentrate. In this study, we used a mixture of P507 and Cyanex 272 extractants in a 1:1 ratio, which in its extraction characteristics is identical to Cyanex 572—which is an equimolar mixture of the same extractants.
2. Materials and Methods
The chemicals and reagents used in this study were of analytical grade, and they were used without any purification.
All the extraction studies were carried out in triplicate at 298 K unless otherwise mentioned, and the results indicate the average of three independent measurements.
The initial solution of REE chlorides was obtained by dissolving a concentrate of REE carbonates (Apatit Cherepovets, Cherepovets, Russia, Vologda region) in a 10 M HCl solution. The carbonates were added to 10 M HCl until the aqueous solution was neutralized. The resulting orange precipitate was filtered off, and HCl solution was added 1.5 pH. The resulting solution of REE chlorides had the following composition (%): Ce—1.2, Pr—0.2, Nd—3.4, Sm—19.7, Eu—4.5, Gd—14.5, Tb—1.5, Dy—7.5, Ho—1.1, Er—2.6, Tm—0.2, Yb—1.1, Lu—0.1, Y—41.4.
Solutions of REE chlorides with concentrations from 0.05 to 0.90 mol/L were prepared from the initial solution.
A mixture of P507 (Luoyang, China, Luoyang Zhongda Chemical Co.) and Cyanex 272 (Toronto, ON, Canada, Cytec Solvay Group) in a molar ratio of 1:1 was used as an extractant. Isopar L (fraction C11–C13) was used as a solvent with the addition of 15 vol.% isooctanol. For the extraction, 0.3, 0.6, 0.9 and 1.2 M solutions of extractant in isopar L were prepared. To improve the extraction ability of the mixture of P507 and Cyanex 272, a saponified extractant was used (the degree of saponification of the extractants did not exceed 40%).
The phase contact time on the orbital shaker was 30 min, which was sufficient to establish constant REE distribution ratios.
After phase separation, the aqueous phase concentrations of REEs were determined via inductively coupled plasma optical emission spectroscopy (ICP-OES) using a Shimadzu ICPE-9000 with an analytical error < 5%. The REE concentrations in the organic phase were determined as follows. An aliquot of the organic phase was burned in a muffle furnace at 650 °C. The resulting residue was dissolved in a 6 M nitric acid solution on heating, then the solution (after neutralization) was transferred into a volumetric flask, and the REEs were analyzed by ICP-OES.
The total concentration of REE chlorides in the initial solutions and aqueous phases after extraction was determined via titration with a standard solution of EDTA at 5.6 pH, using xylenol orange as an indicator [25].
The distribution ratio (D) was determined as the ratio of the concentration of REE in the organic phase to that in the aqueous phase and was calculated according to the following equation:
where Co and Caq are the equilibrium concentrations of REE in the organic and aqueous phases, respectively. The separation factor (β) characterizes the separation degree between the two rare earth elements (E1 and E2) in the extraction system and was calculated according to the following equation:
D = Co/Caq,
β = D(E1)/D(E2)
3. Results and Discussion
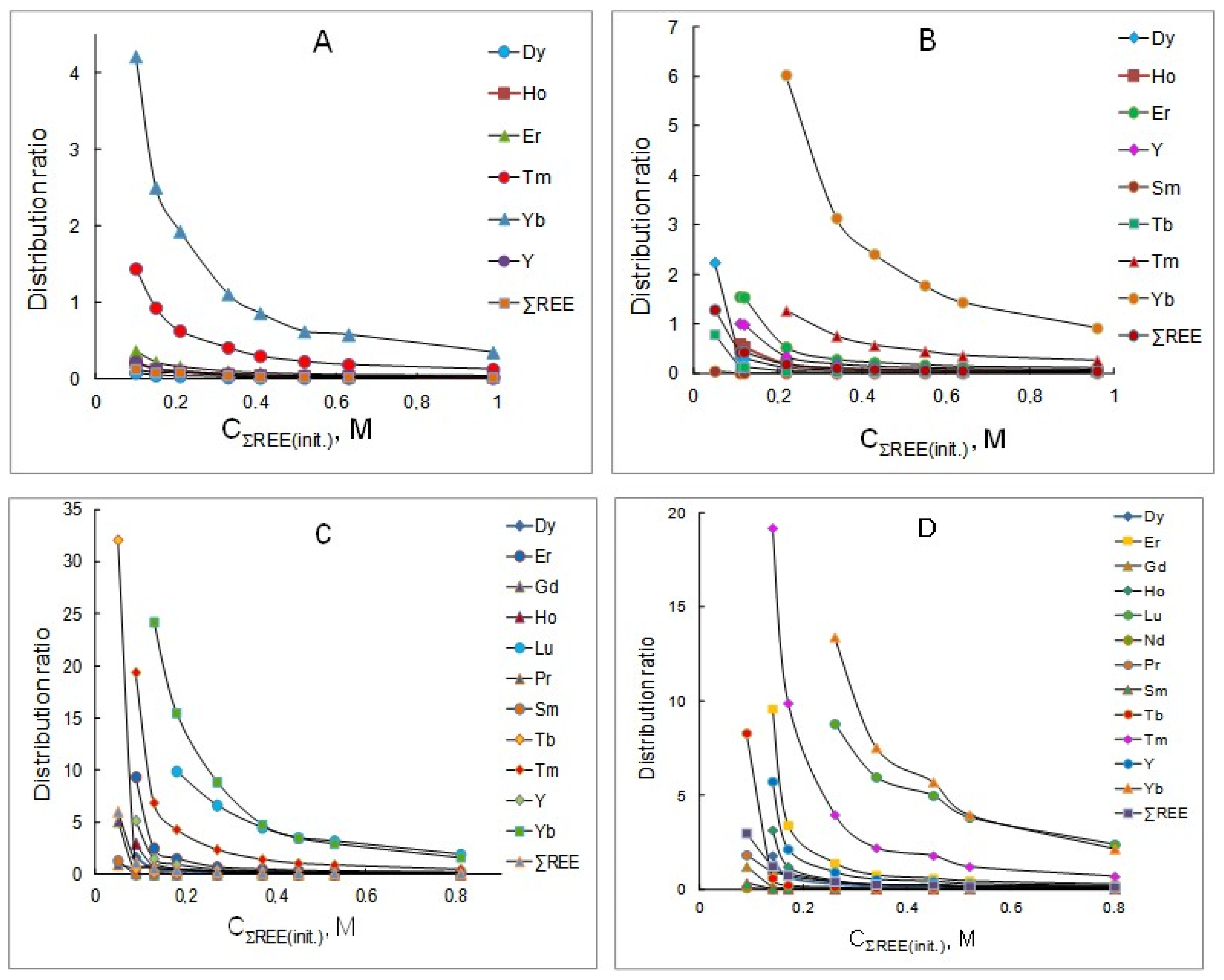
The extraction of rare earth elements from chloride solutions after the leaching of REE carbonate concentrate containing mixtures of these metals, the concentration of which varied in the range of 0.05–0.9 mol/L, with solutions of a mixture of P507 and Cyanex 272 (1:1) at various concentrations was studied. The calculated distribution ratios for each metal are given in Table 1, Table 2, Table 3 and Table 4 and in Figure 1. From the data obtained, it follows that the distribution ratios of all REEs decrease with the increasing concentration of these metals in the initial solution, which is associated with the loading of the organic phase and a decrease in the free concentration of the extractant mixture. An increase in the initial concentration of the extractant mixture leads to an increase in the distribution ratios of REEs. The most significant improvement in the extraction is observed for the heavy group of rare earth elements (Ho, Er, Tm, Yb and Lu). In general, the experimental results obtained are consistent with the literature data [19,20,21,22,23,24,25]. It should be noted that in a wide range of initial concentrations of REE chlorides, the distribution ratio of yttrium exceeds the distribution ratio of dysprosium by 2–4 times, depending on the equilibrium concentration in the aqueous phase. The extractability of rare earth elements increases with the increasing atomic number of the element, as is typical for the extraction of these metals with acidic organophosphorus extractants.

Table 1.
Distribution ratios of REEs depending on the initial concentration of rare earth elements in aqueous solutions in extraction with a 0.3 M solution of a mixture of P507 and Cyanex 272.

Table 2.
Distribution ratios of REEs depending on the initial concentration of rare earth elements in aqueous solutions in extraction with a 0.6 M solution of a mixture of P507 and Cyanex 272.

Table 3.
Distribution ratios of REEs depending on the initial concentration of rare earth elements in aqueous solutions in te extraction with a 0.9 M solution of a mixture of P507 and Cyanex 272.

Table 4.
Distribution ratios of REEs depending on the initial concentration of rare earth elements in aqueous solutions in extraction with a 1.2 M solution of a mixture of P507 and Cyanex 272.
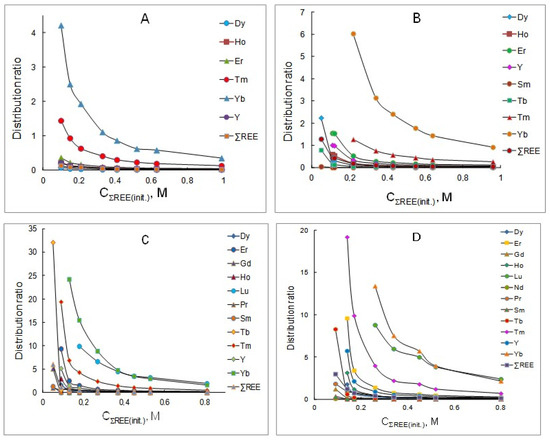
Figure 1.
Dependency of distribution ratios of REEs on the initial concentration of rare earth elements in aqueous solutions at extractant concentrations, mol/L: (A) 0.3; (B) 0.6; (C) 0.9; (D) 1.2.
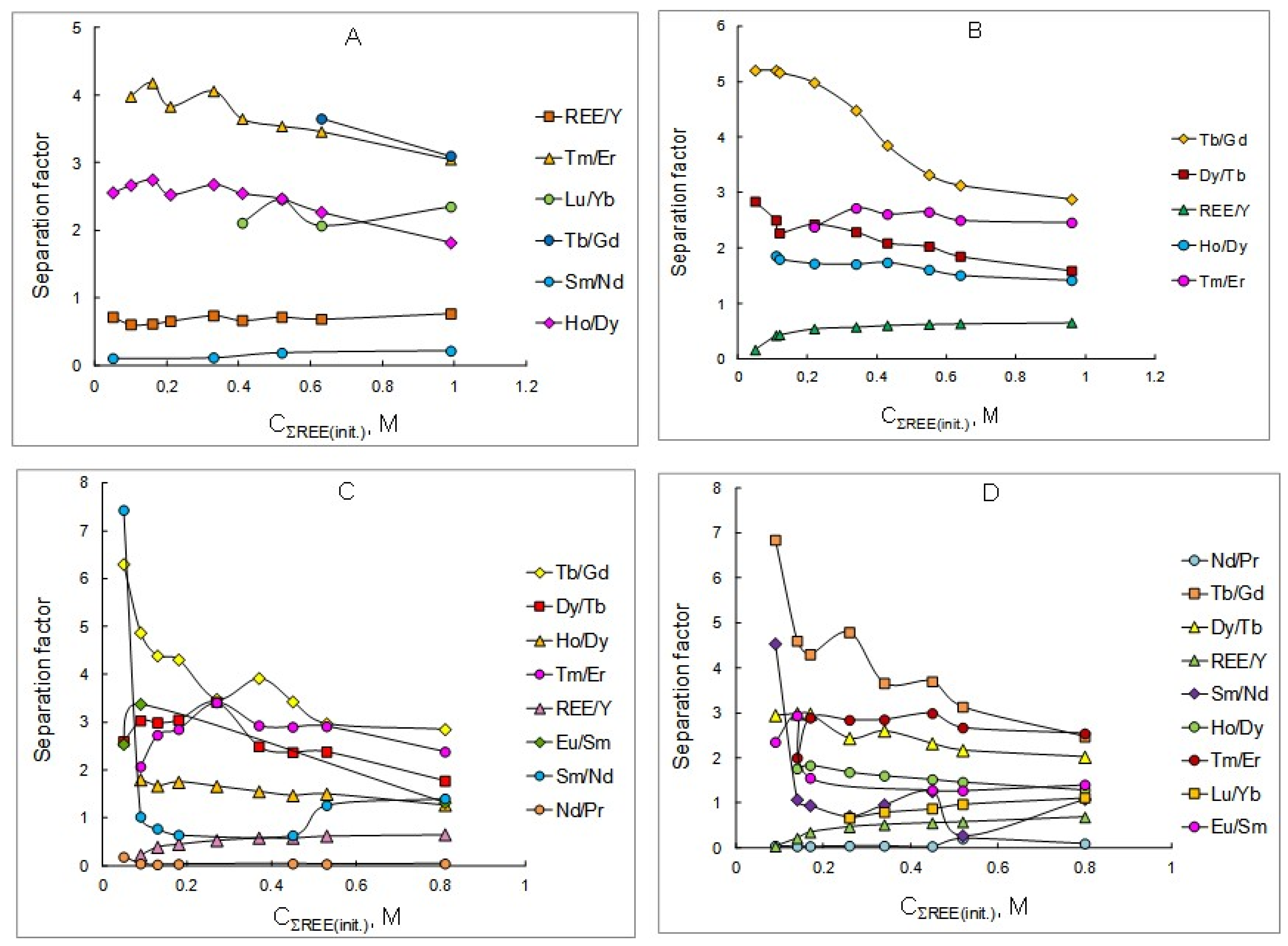
The obtained REE distribution ratios (Table 1, Table 2, Table 3 and Table 4) were used to calculate the separation factors of adjacent REEs depending on the initial concentration of these elements in the initial aqueous solutions in the extraction using solutions of the mixtures of P507 and Cyanex 272 at various concentrations. The results obtained are shown in Figure 2 and in Table 5, Table 6, Table 7 and Table 8.
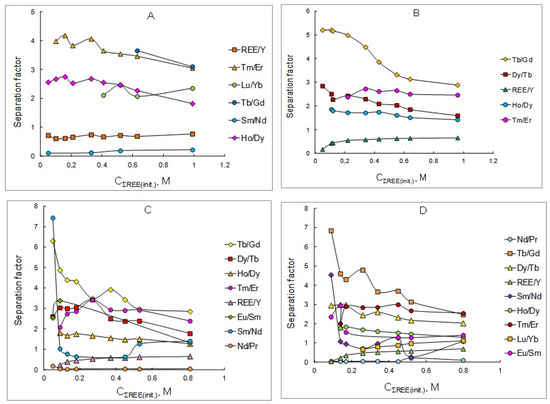
Figure 2.
Dependency of separation factors of REE pairs on the initial concentration of rare earth elements in aqueous solutions at extractant concentrations, mol/L: (A) 0.3; (B) 0.6; (C) 0.9; (D) 1.2.

Table 5.
Separation factors of adjacent REEs depending on the initial concentration of rare earth elements in aqueous solutions in extraction with a 0.3 M solution of a mixture of P507 and Cyanex 272.

Table 6.
Separation factors of adjacent REEs depending on the initial concentration of rare earth elements in aqueous solutions in extraction with a 0.6 M solution of a mixture of P507 and Cyanex 272.

Table 7.
Separation factors of adjacent REEs depending on the initial concentration of rare earth elements in aqueous solutions in extraction with a 0.9 M solution of a mixture of P507 and Cyanex 272.

Table 8.
Separation factors of adjacent REEs depending on the initial concentration of rare earth elements in aqueous solutions in extraction with a 1.2 M solution of a mixture of P507 and Cyanex 272.
As can be seen from the data obtained, the separation factors of adjacent rare earth elements decrease slightly with increasing concentration of metals in the initial aqueous solution. It can be assumed that this is due to the use of isooctanol as an additive, which prevents the formation of associates of extracted species in the organic phase. However, the separation factor of the sum of REEs to yttrium increases with the increasing concentration of metals in the initial aqueous solution. The most significant separation factors are observed for pairs of medium-heavy (Tb/Gd) and heavy group of metals (Tm/Er, Dy/Tb). When using a 0.9 M solution of a mixture of P507 and Cyanex 272, the dysprosium/terbium separation factor reaches a value of 3.42, and the thulium/erbium separation factor reaches 3.41. Increasing the concentration of the extractant mixture does not have a significant effect on the values of the adjacent REEs separation factors as well as the separation factors of the sum of REEs to yttrium. The optimal extractant concentration for the extraction and separation of rare earth elements using a mixture of extractants is 0.6 mol/L. At lower concentrations of the extractant mixture, the REE distribution ratios are not high enough, and at higher concentrations, precipitation may occur, which is unacceptable when using technological processes.
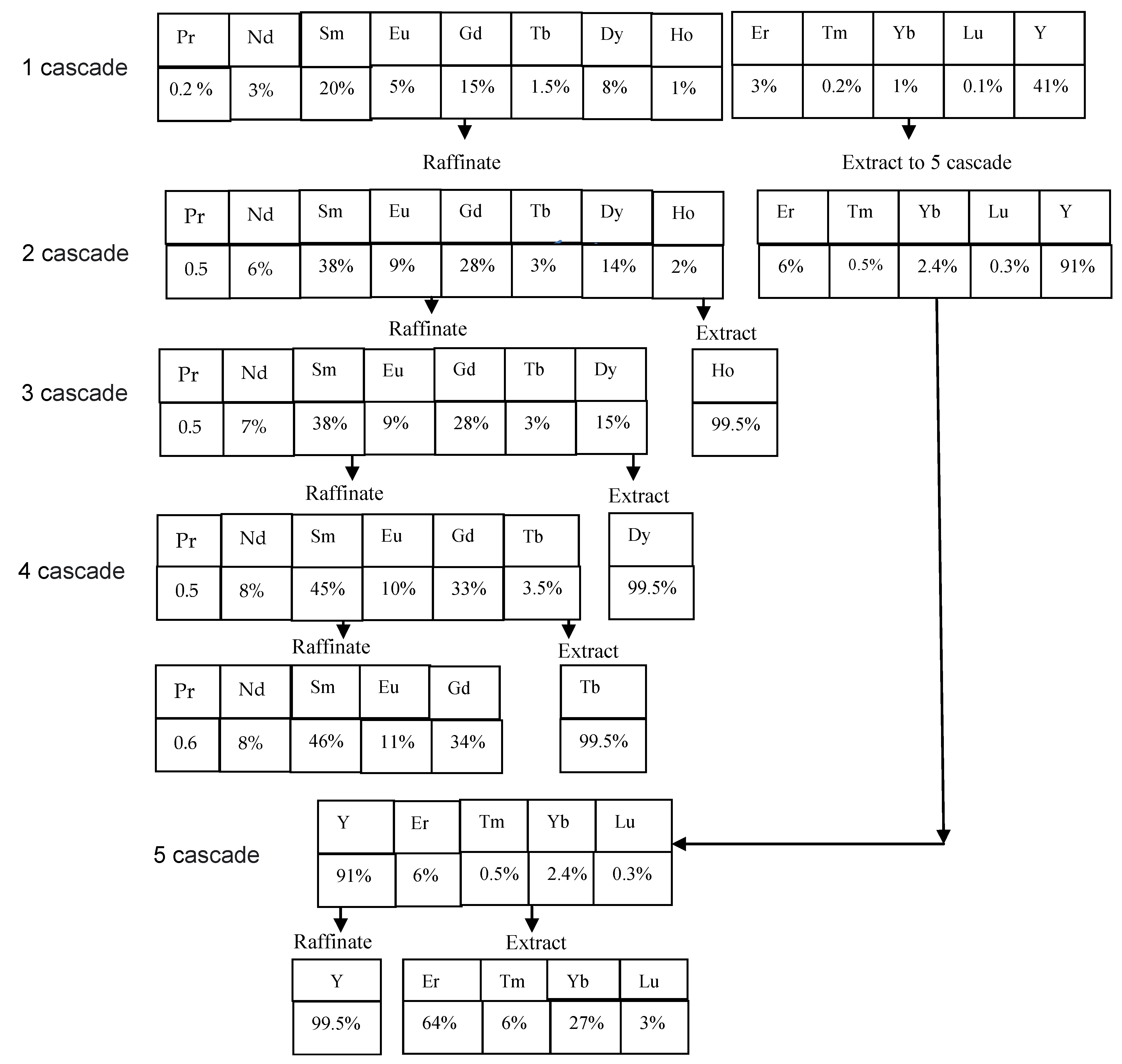
Based on the experimental data obtained (Table 2 and Table 6), a flow sheet for obtaining rare earth elements from chloride solutions after leaching the REE carbonate concentrate has been proposed. For this purpose, the concentrations of REEs in the initial solution, raffinate and extract were calculated based on the material balance using the Excel program. Figure 3 shows a flow sheet of the production of medium-heavy REEs from a concentrate using a 0.6 M equimolar mixture of P507 and Cyanex 272 as an extractant. Under the names of REEs, the percentages of these metals in the stream are given. In the first cascade (25 stages in the extraction zone and 15 stages in the scrubbing zone), REEs are separated along the Er/Ho line. Yttrium and heavy lanthanides (Er, Tm, Yb and Lu) are distributed into the extract, while lanthanides from Pr to Ho remain in the raffinate. Due to the extraction of yttrium, the amount of RREs for further processing is reduced by almost twice. In the second cascade (50 stages in the extraction zone and 30 stages in the scrubbing zone), REEs are separated along the Ho/Dy line to obtain pure holmium in the extract. Holmium is stripped using a solution of hydrochloric acid. Lanthanides from Pr to Dy remain in the raffinate. In the third cascade (35 stages in the extraction zone and 25 stages in the scrubbing zone), REEs are separated along the Dy/Tb line to obtain pure dysprosium in the extract. Dysprosium is stripped using a solution of hydrochloric acid. Lanthanides from Pr to Tb remain in the raffinate. In the fourth cascade (20 stages in the extraction zone and 12 stages in the scrubbing zone), REEs are separated along the Tb/Gd line to obtain pure terbium in the extract. Terbium is stripped using a solution of hydrochloric acid. Lanthanides from Pr to Gd remain in the raffinate. In the fifth cascade (23 stages in the extraction zone and 18 stages in the scrubbing zone), REEs are separated along the Er/Y line to obtain the sum of heavy lanthanides (Er, Tm, Yb and Lu) in the extract. These elements are also stripped using a hydrochloric acid solution. Pure yttrium remains in the raffinate. Stripping was performed in four stages using hydrochloric acid solution with the appropriate concentration in all cascades.
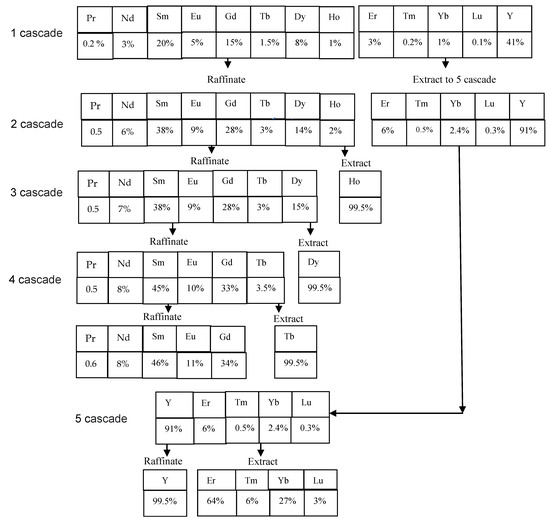
Figure 3.
Proposed flow sheet for the separation of rare earth elements using a 0.6 M solution of a mixture of P507 and Cyanex 272.
4. Conclusions
In this study, the experimental data on the extraction of rare earth elements from chloride solutions after leaching REE carbonate concentrate with solutions of a mixture of P507 and Cyanex 272 (1:1) were obtained. It has been shown that the distribution ratios of all REEs decrease with the increasing concentration of these metals in the initial solution, while increasing the initial concentration of the extractant mixture causes the distribution ratios of REEs to increase. The most significant improvement in the extraction is observed for the heavy group of rare earth elements. The separation factors of adjacent rare earth elements decrease slightly with the increasing concentration of metals in the initial aqueous solution probably due to the use of isooctanol as an additive, which prevents the formation of associates of extracted species in the organic phase. Based on the experimental results obtained, it is concluded that commercial products such as Y, Ho, Tb and Dy can be produced from a concentrate of the medium-heavy group.
The experimental data can be used in the mathematical modeling and optimization of extraction cascades to obtain individual rare earth elements using a mixture of P507 and Cyanex 272.
Author Contributions
Conceptualization, M.A.A.; Methodology, M.A.A. and A.V.N.; Validation, I.A.Y. and V.V.B.; Formal Analysis, M.A.A. and V.V.B.; Writing, Review & Editing, M.A.A. and V.V.B.; Visualization, I.A.Y., A.V.N. and V.V.B.; Project Administration, A.V.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation as part of the State Assignment of St. Petersburg State Institute of Technology (Technical University) and the Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
Author Andrey V. Nechaev was employed by the Join-Stock Company «Group of Companies «Rusredmet». The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kashi, E.; Habibpour, R.; Gorzin, H.; Maleki, A. Solvent extraction and separation of light rare earth elements (La, Pr and Nd) in the presence of lactic acid as a complexing agent by Cyanex 272 in kerosene and the effect of citric acid, acetic acid and Titriplex III as auxiliary agents. J. Rare Earths 2018, 36, 317–323. [Google Scholar] [CrossRef]
- Banda, R.; Jeon, H.S.; Lee, M.S. Solvent extraction separation of La from chloride solution containing Pr and Nd with Cyanex 272. Hydrometallurgy 2012, 121–124, 74–80. [Google Scholar] [CrossRef]
- Yin, S.; Wu, W.; Bian, X.; Zhang, F. Effect of complexing agent lactic acid on the extraction and separation of Pr(III)/Ce(III) with di-(2-ethylhexyl) phosphoric acid. Hydrometallurgy 2013, 131–132, 133–137. [Google Scholar] [CrossRef]
- Michelsen, O.D.; Smutz, M. Separation of yttrium, holmium and herbium with di-2-ethylhexyl-phosphoric acid in chloride and nitrate systems. J. Inorg. Nucl. Chem. 1971, 33, 265–278. [Google Scholar] [CrossRef]
- Kao, H.C.; Yen, P.S.; Juang, R.S. Solvent extraction of La(III) and Nd(III) from nitrate solutions with 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester. Chem. Eng. J. 2006, 119, 167–174. [Google Scholar] [CrossRef]
- Radhika, S.; Kumar, B.N.; Kantam, M.L.; Reddy, B.R. Liquid-liquid extraction and separation possibilities of heavy and light rare-earths from phosphoric acid solutions with acidic organophosphorus reagents. Sep. Purif. Technol. 2010, 75, 295–302. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, W.; Dai, J.; Bian, X. Extraction and separation of Pr(III)/Ce(III) from chloride medium by 2-ethylhexylphosphonic acid mono-(2-ethylhexyl) ester in the presence of two complexing agents. Sep. Purif. Technol. 2016, 51, 778–783. [Google Scholar] [CrossRef]
- Quinn, J.E.; Soldenhoff, K.H.; Stevens, G.W.; Lengkeek, N.A. Solvent extraction of rare earth elements using phosphonic/phosphinic acid mixtures. Hydrometallurgy 2015, 157, 298–305. [Google Scholar] [CrossRef]
- Fontana, D.; Pietrelli, L. Separation of middle rare earths by solvent extraction using 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester as an extractant. J. Rare Earths 2009, 27, 830–833. [Google Scholar] [CrossRef]
- Kolarik, Z. Review: Dissociation, self-association, and partition of monoacidic organophosphorus extractants. Solvent Extr. Ion Exch. 2010, 28, 707–763. [Google Scholar] [CrossRef]
- Wang, Y.L.; Liao, W.P.; Li, D.Q. A solvent extraction process with mixture of CA12 and Cyanex 272 for the preparation of high purity yttrium oxide from rare earth ores. Sep. Purif. Technol. 2011, 82, 197–201. [Google Scholar] [CrossRef]
- Liu, Y.; Jeon, H.S.; Lee, M.S. Solvent extraction of Pr and Nd from chloride solution by the mixtures of Cyanex 272 and amine extractants. Hydrometallurgy 2014, 150, 61–67. [Google Scholar] [CrossRef]
- Liu, Y.; Jeon, H.S.; Lee, M.S. Separation of Pr and Nd from La in chloride solution by extraction with a mixture of Cyanex 272 and Alamine 336. Met. Mater. Int. 2015, 21, 944–949. [Google Scholar] [CrossRef]
- Wang, X.L.; Du, M.; Liu, H. Synergistic extraction study of samarium(III) from chloride medium by mixtures of bis(2,4,4-trimethylpentyl)phosphinic acid and 8-hydroxyquinoline mixtures. Sep. Purif. Technol. 2012, 93, 48–51. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Li, D.; Li, H. Synergistic extraction of rare earths by mixture of bis(2,4,4-trimethylpentyl)phosphinic acid and sec-nonylphenoxy acetic acid. Sep. Purif. Technol. 2006, 50, 30–34. [Google Scholar] [CrossRef]
- Zaheri, P.; Abolghasemi, H.; Maraghe, M.G.; Mohammadi, T. Intensification of europium extraction through a supported liquid membrane using mixture of D2EHPA and Cyanex 272 as carrier. Chem. Eng. Process. 2015, 92, 18–24. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Li, D. Extraction and stripping of rare earths using mixtures of acidic phosphorus based reagents. J. Rare Earths 2011, 29, 413–415. [Google Scholar] [CrossRef]
- Kuang, S.; Zhang, Z.; Li, Y.; Wei, H.; Liao, W. Synergistic extraction and separation of rare earths from chloride medium by the mixture of HEHAPP and D2EHPA. Hydrometallurgy 2017, 174, 78–83. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, X.; Li, D. Synergistic extraction and separation of heavy lanthanide by mixtures of bis(2,4,4-trimethylpentyl)phosphinic acid and 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester. Sep. Sci. Technol. 2005, 40, 2325–2336. [Google Scholar] [CrossRef]
- Wang, Y. The novel extraction process based on Cyanex 572 for separating heavy rare earths from ion-adsorbed deposit. Sep. Purif. Technol. 2015, 151, 303–308. [Google Scholar] [CrossRef]
- Kolar, E.; Catthoor, R.P.R.; Kriel, F.H.; Sedev, R.; Middlemas, S.; Klier, E.; Hatch, G.; Priest, C. Microfluidic solvent extraction of rare earth elements from a mixed oxide concentrate leach solution using Cyanex 572. Chem. Eng. Sci. 2016, 148, 212–218. [Google Scholar] [CrossRef]
- El-Hefny, N.E.; Gasser, M.S.; Emam, S.S.; Mahmoud, W.H.; Aly, H.F. Comparative studies on Y(III) and Dy(III) extraction from hydrochloric and nitric acids by Cyanex 572 as a novel extractant. J. Rare Earths 2018, 36, 1342–1350. [Google Scholar] [CrossRef]
- Pavon, S.; Kutucu, M.; Coll, M.T.; Fortunya, A.; Sastre, A.M. Comparison of Cyanex 272 and Cyanex 572 for the separation of neodymium from a Nd/Tb/Dy mixture by pertraction. J. Chem. Technol. Biotechnol. 2017, 93, 2152–2159. [Google Scholar] [CrossRef]
- Belova, V.V.; Petyaeva, M.M.; Kostanyan, A.E. Extraction of lanthanides from chloride solutions in hexane–isopropanol–water systems using Cyanex 572. Theor. Found. Chem. Eng. 2022, 56, 595–599. [Google Scholar] [CrossRef]
- Lyle, S.J.; Rahman, M.M. Complexometric titration of yttrium and the lanthanons. A comparison of direct methods. Talanta 1963, 10, 1177–1182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).