Effect of Particle Size on the Physical Properties of PLA/Potato Peel Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Potato Peel Pre-Treatment
2.2.2. Classification of Dried Potato Peel Powder
2.2.3. Specimen Production
2.3. Characterization
2.3.1. Laser Diffraction Analysis
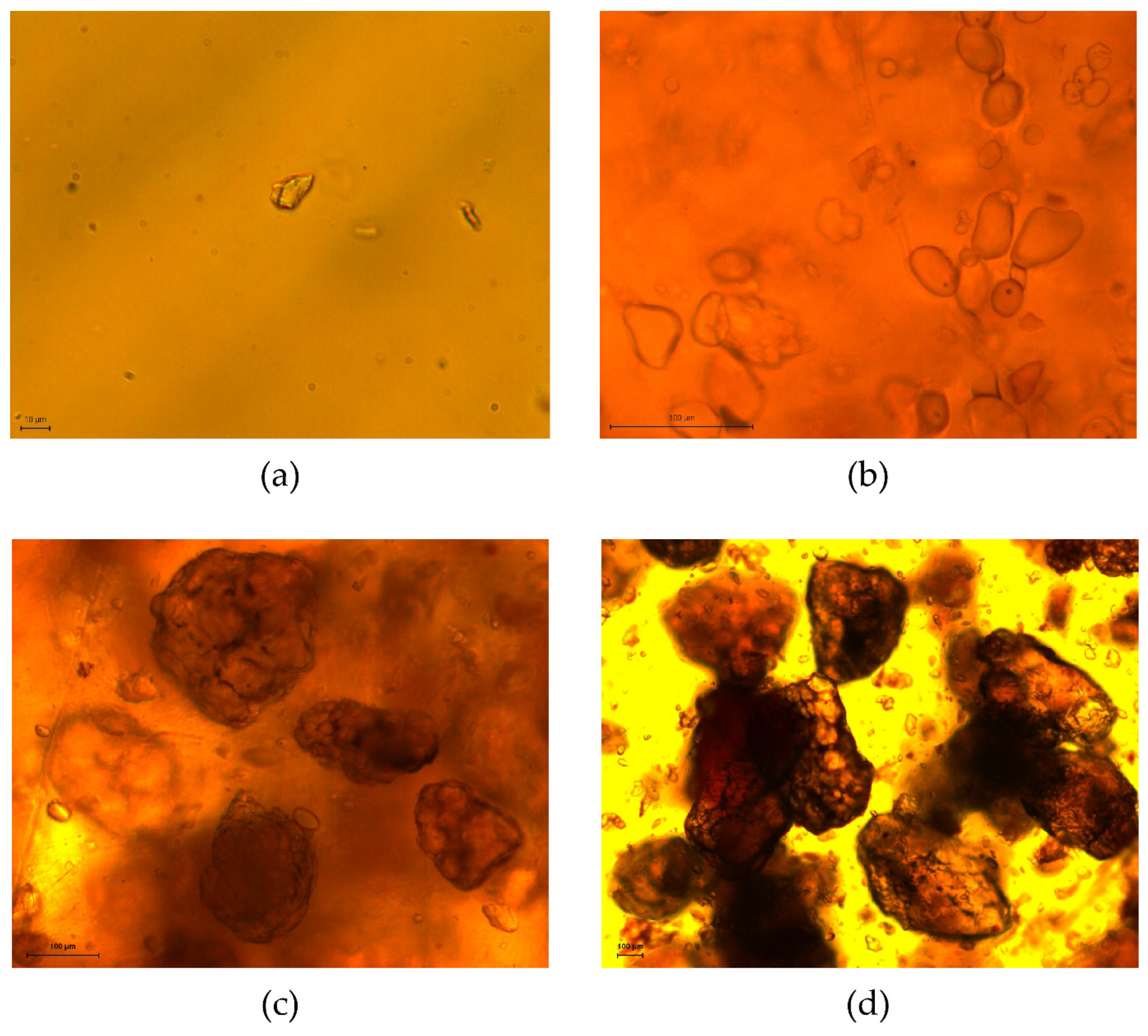
2.3.2. Microscopic Image Analysis
2.3.3. Thickness Measurement
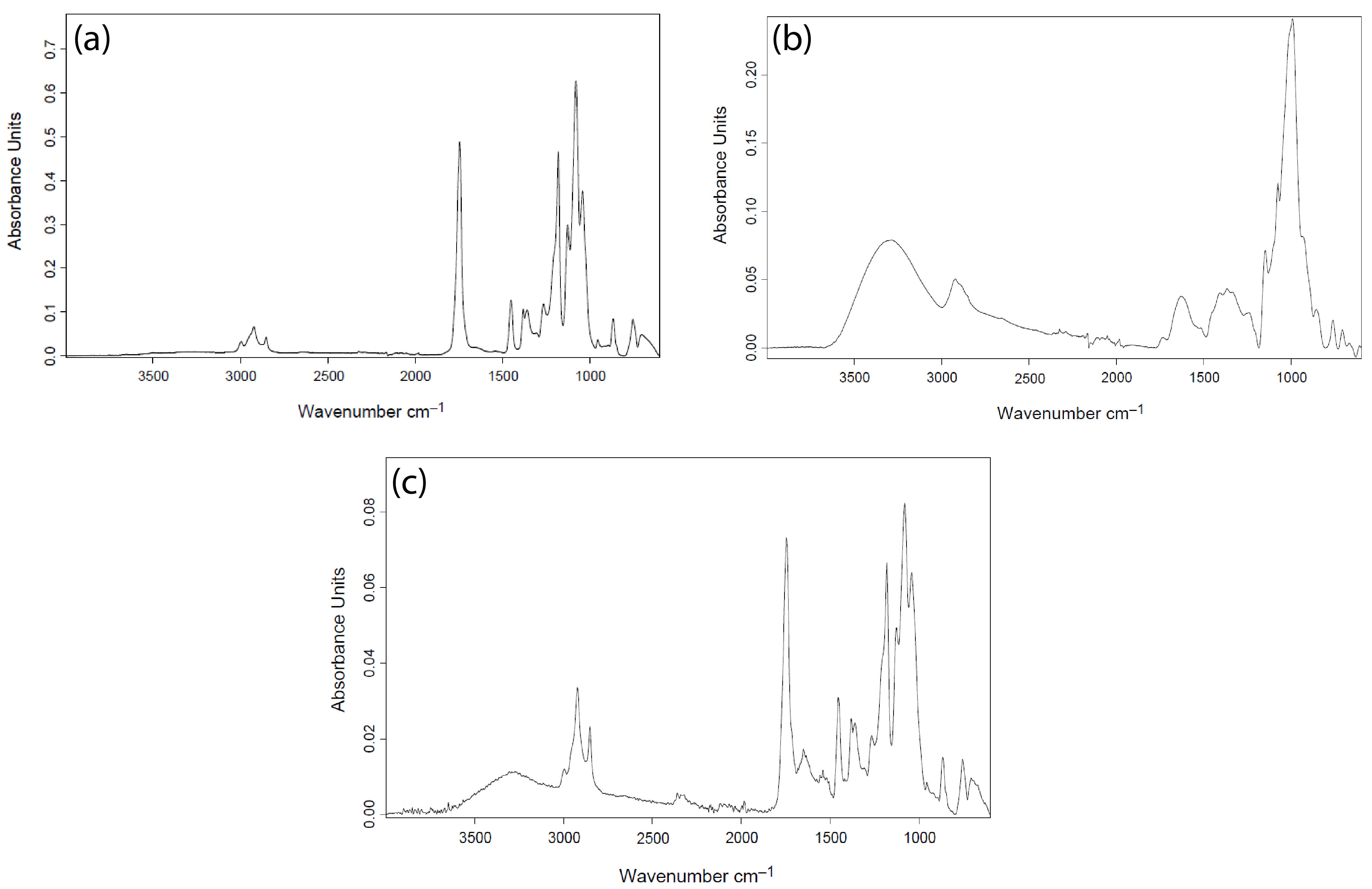
2.3.4. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.3.5. Differential Scanning Calorimetry (DSC)
2.3.6. Tensile Properties
2.3.7. Water Vapor Transmission Rate
2.3.8. Statistical Analysis
3. Results
3.1. Obtained Samples
3.1.1. Classification
3.1.2. Specimen Production
3.2. Characterization
3.2.1. Particle Size-Based Parameters
3.2.2. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
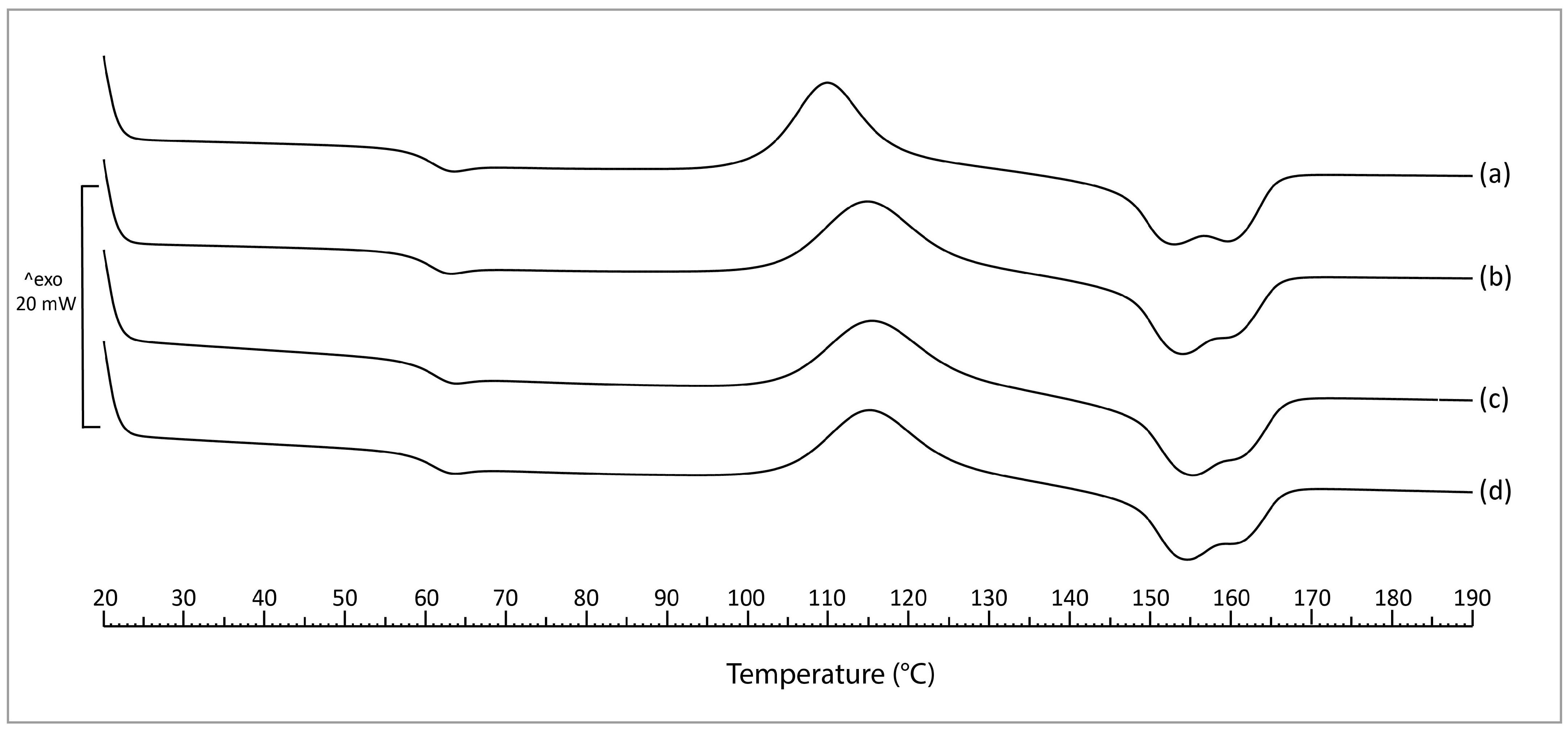
3.2.3. Differential Scanning Calorimetry (DSC)
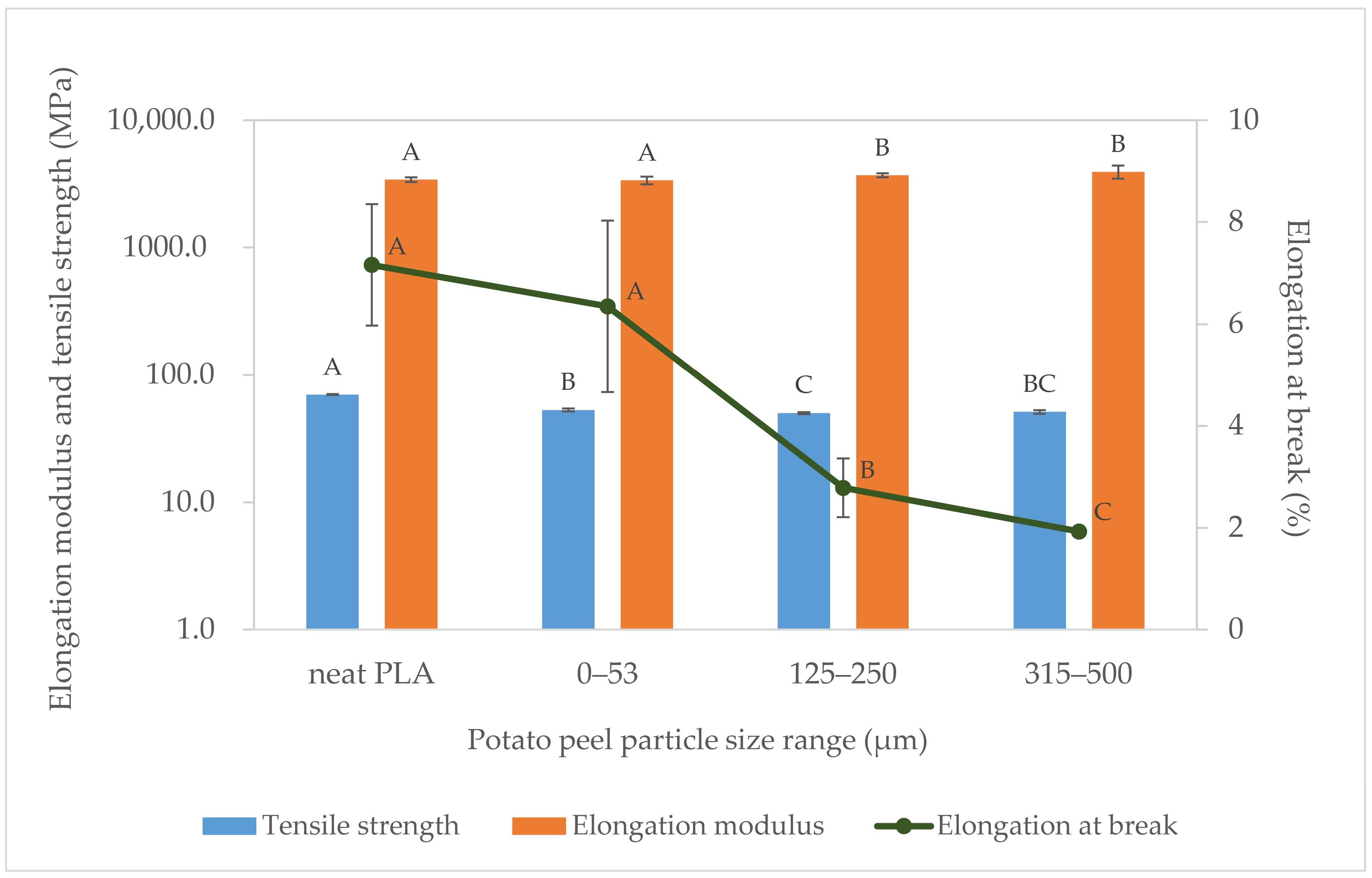
3.2.4. Tensile Properties
3.2.5. Water Vapor Transmission Rate (WVTR)
4. Discussion
4.1. Specimen Production
4.2. Particle Characterization
4.3. ATR-FTIR
4.4. DSC
4.5. Tensile Properties
4.6. Water Vapor Transmission Rate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A versatile biobased polymer for the future with multifunctional properties-From monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Nova-Institute. Global Production Capacities of Bioplastics 2027: (By Material Type). Available online: https://www.european-bioplastics.org/market/#iLightbox[gallery_image_1]/1 (accessed on 19 July 2023).
- Rodríguez-Núñez, J.R.; Madera-Santana, T.J.; Burrola-Núñez, H.; Martínez-Encinas, E.G. Composite materials based on PLA and its applications in food packaging. In Composites Materials for Food Packaging; First published; Cirillo, G., Spizzirri, U.G., Kozlowski, M.A., Eds.; Scrivener Publishing LLC.: Beverly, MA, USA; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2018; pp. 355–399. ISBN 9781119160205. [Google Scholar]
- Li, X.; Lin, Y.; Liu, M.; Meng, L.; Li, C. A review of research and application of polylactic acid composites. J. Appl. Polym. Sci. 2023, 140, e53477. [Google Scholar] [CrossRef]
- Mann, G.S.; Singh, L.P.; Kumar, P.; Singh, S. Green composites: A review of processing technologies and recent applications. J. Thermoplast. Compos. Mater. 2020, 33, 1145–1171. [Google Scholar] [CrossRef]
- Rajeshkumar, G.; Seshadri, S.A.; Devnani, G.L.; Sanjay, M.R.; Siengchin, S.; Maran, J.P.; Al-Dhabi, N.A.; Karuppiah, P.; Mariadhas, V.A.; Sivarajasekar, N.; et al. Environment friendly, renewable and sustainable poly lactic acid (PLA) based natural fiber reinforced composites—A comprehensive review. J. Clean. Prod. 2021, 310, 127483. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Hofifah, S.N.; Girsang, G.C.S.; Putri, S.R.; Budiman, B.A.; Triawan, F.; Al-Obaidi, A.S.M. The effects of rice husk particles size as a reinforcement component on resin-based brake pad Pperformance: From literature review on the use of agricultural waste as a reinforcement material, chemical polymerization reaction of epoxy resin, to experiments. Automot. Exp. 2021, 4, 68–82. [Google Scholar] [CrossRef]
- Battegazzore, D.; Noori, A.; Frache, A. Natural wastes as particle filler for poly(lactic acid)-based composites. J. Compos. Mater. 2019, 53, 783–797. [Google Scholar] [CrossRef]
- Bajwa, D.; Eichers, M.; Shojaeiarani, J.; Kallmeyer, A. Influence of biobased plasticizers on 3D printed polylactic acid composites filled with sustainable biofiller. Ind. Crops Prod. 2021, 173, 114132. [Google Scholar] [CrossRef]
- Palaniyappan, S.; Veeman, D.; Sivakumar, N.K.; Natrayan, L. Development and optimization of lattice structure on the walnut shell reinforced PLA composite for the tensile strength and dimensional error properties. Structures 2022, 45, 163–178. [Google Scholar] [CrossRef]
- Chun, K.S.; Husseinsyah, S.; Osman, H. Mechanical and thermal properties of coconut shell powder filled polylactic acid biocomposites: Effects of the filler content and silane coupling agent. J. Polym. Res. 2012, 19, 9859. [Google Scholar] [CrossRef]
- Battegazzore, D.; Bocchini, S.; Alongi, J.; Frache, A. Plasticizers, antioxidants and reinforcement fillers from hazelnut skin and cocoa by-products: Extraction and use in PLA and PP. Polym. Degrad. Stab. 2014, 108, 297–306. [Google Scholar] [CrossRef]
- Gigante, V.; Cinelli, P.; Righetti, M.C.; Sandroni, M.; Tognotti, L.; Seggiani, M.; Lazzeri, A. Evaluation of mussel shells powder as reinforcement for PLA-based biocomposites. Int. J. Mol. Sci. 2020, 21, 5364. [Google Scholar] [CrossRef]
- Kim, K.-W.; Lee, B.-H.; Kim, H.-J.; Sriroth, K.; Dorgan, J.R. Thermal and mechanical properties of cassava and pineapple flours-filled PLA bio-composites. J. Therm. Anal. Calorim. 2012, 108, 1131–1139. [Google Scholar] [CrossRef]
- Baek, B.-S.; Park, J.-W.; Lee, B.-H.; Kim, H.-J. Development and application of green composites: Using coffee ground and bamboo flour. J. Polym. Environ. 2013, 21, 702–709. [Google Scholar] [CrossRef]
- Battegazzore, D.; Alongi, J.; Frache, A. Poly(lactic acid)-based composites containing natural fillers: Thermal, mechanical and barrier properties. J. Polym. Environ. 2014, 22, 88–98. [Google Scholar] [CrossRef]
- Petinakis, E.; Liu, X.; Yu, L.; Way, C.; Sangwan, P.; Dean, K.; Bateman, S.; Edward, G. Biodegradation and thermal decomposition of poly(lactic acid)-based materials reinforced by hydrophilic fillers. Polym. Degrad. Stab. 2010, 95, 1704–1707. [Google Scholar] [CrossRef]
- Sudamrao Getme, A.; Patel, B. A Review: Bio-fiber’s as reinforcement in composites of polylactic acid (PLA). Mater. Today Proc. 2020, 26, 2116–2122. [Google Scholar] [CrossRef]
- Formela, K.; Zedler, Ł.; Hejna, A.; Tercjak, A. Reactive extrusion of bio-based polymer blends and composites—Current trends and future developments. EXPRESS Polym. Lett. 2018, 12, 24–57. [Google Scholar] [CrossRef]
- Makri, S.P.; Xanthopoulou, E.; Klonos, P.A.; Grigoropoulos, A.; Kyritsis, A.; Tsachouridis, K.; Bikiaris, D.N.; Anastasiou, A.; Deligkiozi, I.; Nikolaidis, N. Effect of micro- and nano-lignin on the thermal, mechanical, and antioxidant properties of biobased PLA–lignin composite films. Polymers 2022, 14, 5274. [Google Scholar] [CrossRef] [PubMed]
- Bundesanstalt für Landwirtschaft und Ernährung. Bericht zur Markt- und Versorgungslage Kartoffeln; Bundesanstalt für Landwirtschaft und Ernährung: Bonn, Germany, 2020. [Google Scholar]
- Miller, K.; Reichert, C.L.; Schmid, M.; Loeffler, M. Physical, chemical and biochemical modification approaches of potato (peel) constituents for bio-based food packaging concepts: A review. Foods 2022, 11, 2927. [Google Scholar] [CrossRef]
- Willersinn, C.; Mack, G.; Mouron, P.; Keiser, A.; Siegrist, M. Quantity and quality of food losses along the Swiss potato supply chain: Stepwise investigation and the influence of quality standards on losses. Waste Manag. 2015, 46, 120–132. [Google Scholar] [CrossRef]
- Dey, T.; Bhattacharjee, T.; Nag, P.; Ritika; Ghati, A.; Kuila, A. Valorization of agro-waste into value added products for sustainable development. Bioresour. Technol. 2021, 16, 100834. [Google Scholar] [CrossRef]
- Khanal, S.; Karimi, K.; Majumdar, S.; Kumar, V.; Verma, R.; Bhatia, S.K.; Kuca, K.; Esteban, J.; Kumar, D. Sustainable utilization and valorization of potato waste: State of the art, challenges, and perspectives. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Borah, P.P.; Das, P.; Badwaik, L.S. Ultrasound treated potato peel and sweet lime pomace based biopolymer film development. Ultrason. Sonochem. 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Kang, H.J.; Min, S.C. Potato peel-based biopolymer film development using high-pressure homogenization, irradiation, and ultrasound. LWT 2010, 43, 903–909. [Google Scholar] [CrossRef]
- Kang, H.J.; Won, M.Y.; Lee, S.J.; Min, S.C. Plasticization and moisture sensitivity of potato peel-based biopolymer films. Food Sci. Biotechnol. 2015, 24, 1703–1710. [Google Scholar] [CrossRef]
- Miller, K.; Silcher, C.; Lindner, M.; Schmid, M. Effects of glycerol and sorbitol on optical, mechanical, and gas barrier properties of potato peel-based films. Packag. Technol. Sci. 2021, 34, 11–23. [Google Scholar] [CrossRef]
- Rommi, K.; Rahikainen, J.; Vartiainen, J.; Holopainen, U.; Lahtinen, P.; Honkapää, K.; Lantto, R. Potato peeling costreams as raw materials for biopolymer film preparation. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Merino, D.; Paul, U.C.; Athanassiou, A. Bio-based plastic films prepared from potato peels using mild acid hydrolysis followed by plasticization with a polyglycerol. Food Packag. Shelf Life 2021, 29, 100707. [Google Scholar] [CrossRef]
- Othman, S.H.; Edwal, S.m.M.; Risyon, N.P.; Basha, R.K.; Talib, R. Water sorption and water permeability properties of edible film made from potato peel waste. Food Sci. Technol. 2017, 37, 63–70. [Google Scholar] [CrossRef]
- Sugumaran, V.; Kapur, G.S.; Narula, A.K. Sustainable potato peel powder–LLDPE biocomposite preparation and effect of maleic anhydride-grafted polyolefins on their properties. Polym. Bull. 2018, 75, 5513–5533. [Google Scholar] [CrossRef]
- Sugumaran, V.; Vimal, K.V.; Kapur, G.S.; Narula, A.K. Preparation and morphological, thermal, and physicomechanical properties of polypropylenepotato peel biocomposites. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Sugumaran, V.; Bhunia, H.; Narula, A.K. Evaluation of biodegradability of potato peel powder based polyolefin biocomposites. J. Polym. Environ. 2018, 26, 2049–2060. [Google Scholar] [CrossRef]
- Patil, A.Y.; Banapurmath, N.R.; Yaradoddi, J.S.; Kotturshettar, B.B.; Shettar, A.S.; Basavaraj, G.D.; Keshavamurthy, R.; Yunus Khan, T.M.; Mathad, S.N. Experimental and simulation studies on waste vegetable peels as bio-composite fillers for light duty applications. Arab. J. Sci. Eng. 2019, 44, 7895–7907. [Google Scholar] [CrossRef]
- Schmid, M.; Herbst, C.; Müller, K.; Stäbler, A.; Schlemmer, D.; Coltelli, M.-B.; Lazzeri, A. Effect of potato pulp filler on the mechanical properties and water vapour transmission rate of thermoplastic WPI/PBS blends. Polym. Plast. Technol. Eng. 2016, 55, 510–517. [Google Scholar] [CrossRef]
- Suaduang, N.; Ross, S.; Ross, G.M.; Pratumshat, S.; Mahasaranon, S. Effect of spent coffee grounds filler on the physical and mechanical properties of poly(lactic acid) bio-composite films. Mater. Today Proc. 2019, 17, 2104–2110. [Google Scholar] [CrossRef]
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Krawczak, A.; Wesoły, K.; Szostak, M. Rotational molding of biocomposites with addition of buckwheat husk filler. Structure-property correlation assessment for materials based on polyethylene (PE) and poly(lactic acid) PLA. Compos. Part B Eng. 2020, 202, 108410. [Google Scholar] [CrossRef]
- Bulota, M.; Budtova, T. PLA/algae composites: Morphology and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2015, 73, 109–115. [Google Scholar] [CrossRef]
- Merkus, H.G. Particle Size Measurements: Fundamentals, Practice, Quality; Springer Science + Business Media B.V.: Dordrecht, The Netherlands, 2009; ISBN 978-1-4020-9015-8. [Google Scholar]
- DIN ISO, 9276-6; Darstellung der Ergebnisse von Partikelgrößenanalysen–Teil 6. Deutsches Institut für Normung e.V., Beuth Verlag GmbH: Berlin, Germany, 2012.
- Barczewski, M.; Hejna, A.; Aniśko, J.; Andrzejewski, J.; Piasecki, A.; Mysiukiewicz, O.; Bąk, M.; Gapiński, B.; Ortega, Z. Rotational molding of polylactide (PLA) composites filled with copper slag as a waste filler from metallurgical industry. Polym. Test. 2022, 106, 107449. [Google Scholar] [CrossRef]
- DIN EN ISO 527-1; Kunststoffe-Bestimmung der Zugeigenschaften: Teil 1: Allgemeine Grundsätze. Deutsches Institut für Normung e.V., Beuth Verlag GmbH: Berlin, Germany, 2012.
- DIN 53122-1; Kunststoffe-Bestimmung der Wasserdampfdurchlässigkeit: Teil 1: Gravimetrisches Verfahren. Deutsches Institut für Normung e.V., Beuth Verlag GmbH: Berlin, Germany, 2001.
- Jasmee, S.; Omar, G.; Othaman, S.S.C.; Masripan, N.A.; Hamid, H.A. Interface thermal resistance and thermal conductivity of polymer composites at different types, shapes, and sizes of fillers: A review. Polym. Compos. 2021, 42, 2629–2652. [Google Scholar] [CrossRef]
- Merkus, H.G.; Cirillo, G.; Kozlowski, M.A.; Spizzirri, U.G. Dispersion of Powders in Air and in Liquids. In Particle Size Measurements; Springer: Dordrecht, The Netherlands; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 117–136. ISBN 9781119160205. [Google Scholar]
- Carter, R.M.; Yan, Y. Measurement of particle shape using digital imaging techniques. J. Phys. Conf. Ser. 2005, 15, 177–182. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.; Ghazali, S.; Heim, H.P.; Feldmann, M. Impact modified PLA-hydroxyapatite composites—Thermo-mechanical properties. Compos. Part A Appl. Sci. Manuf. 2018, 107, 326–333. [Google Scholar] [CrossRef]
- Hassan, M.M.; Koyama, K. Thermomechanical and viscoelastic properties of green composites of PLA using chitin micro-particles as fillers. J. Polym. Res. 2020, 27, 27. [Google Scholar] [CrossRef]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.; Restrepo, I. Study of the structural order of native starch granules using combined FTIR and XRD analysis. J. Polym. Res. 2018, 25, 266. [Google Scholar] [CrossRef]
- Derkacheva, O.; Sukhov, D. Investigation of lignins by FTIR spectroscopy. Macromol. Symp. 2008, 265, 61–68. [Google Scholar] [CrossRef]
- Hussain, M.; Qayum, A.; Zhang, X.; Hao, X.; Liu, L.; Wang, Y.; Hussain, K.; Li, X. Improvement in bioactive, functional, structural and digestibility of potato protein and its fraction patatin via ultra-sonication. LWT 2021, 148, 111747. [Google Scholar] [CrossRef]
- Singh, A.A.; Sharma, S.; Srivastava, M.; Majumdar, A. Modulating the properties of polylactic acid for packaging applications using biobased plasticizers and naturally obtained fillers. Int. J. Biol. Macromol. 2020, 153, 1165–1175. [Google Scholar] [CrossRef]
- Lee, D.S.; Yam, K.L.; Piergiovanni, L. Food Packaging Science and Technology; CRC Press: Hoboken, NJ, USA, 2008; ISBN 9781439894071. [Google Scholar]
- Matissek, R. Lebensmittelsicherheit; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 978-3-662-61898-1. [Google Scholar]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R. Synthesis, Structure and Properties of Poly(Lactic Acid); Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-64229-1. [Google Scholar]
- Le Marec, P.E.; Ferry, L.; Quantin, J.-C.; Bénézet, J.-C.; Bonfils, F.; Guilbert, S.; Bergeret, A. Influence of melt processing conditions on poly(lactic acid) degradation: Molar mass distribution and crystallization. Polym. Degrad. Stab. 2014, 110, 353–363. [Google Scholar] [CrossRef]
- Signori, F.; Coltelli, M.-B.; Bronco, S. Thermal degradation of poly(lactic acid) (PLA) and poly(butylene adipate-co-terephthalate) (PBAT) and their blends upon melt processing. Polym. Degrad. Stab. 2009, 94, 74–82. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R. Industrial Applications of Poly(Lactic Acid); Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-75458-1. [Google Scholar]
- Xu, R. Light scattering: A review of particle characterization applications. Particuology 2015, 18, 11–21. [Google Scholar] [CrossRef]
- Janaka, G.H.A.; Kumara, J.; Hayano, K.; Ogiwara, K. Image analysis techniques on evaluation of particle size distribution of gravel. GEOMATE J. 2012, 3, 290–297. [Google Scholar]
- Samal, S. Effect of shape and size of filler particle on the aggregation and sedimentation behavior of the polymer composite. Powder Technol. 2020, 366, 43–51. [Google Scholar] [CrossRef]
- Aworinde, A.K.; Adeosun, S.O.; Oyawale, F.A.; Akinlabi, E.T.; Akinlabi, S.A. Comparative effects of organic and inorganic bio-fillers on the hydrophobicity of polylactic acid. Results Eng. 2020, 5, 100098. [Google Scholar] [CrossRef]
- Beauson, J.; Schillani, G.; van der Schueren, L.; Goutianos, S. The effect of processing conditions and polymer crystallinity on the mechanical properties of unidirectional self-reinforced PLA composites. Compos. Part A Appl. Sci. Manuf. 2022, 152, 106668. [Google Scholar] [CrossRef]
- Anwer, M.A.; Naguib, H.E.; Celzard, A.; Fierro, V. Comparison of the thermal, dynamic mechanical and morphological properties of PLA-Lignin & PLA-Tannin particulate green composites. Compos. Part B Eng. 2015, 82, 92–99. [Google Scholar]
- Echeverría, C.; Limón, I.; Muñoz-Bonilla, A.; Fernández-García, M.; López, D. Development of highly crystalline polylactic acid with -crystalline phase from the Induced alignment of electrospun fibers. Polymers 2021, 13, 2860. [Google Scholar] [CrossRef] [PubMed]
- Spiridon, I.; Leluk, K.; Resmerita, A.M.; Darie, R.N. Evaluation of PLA–lignin bioplastics properties before and after accelerated weathering. Compos. Part B Eng. 2015, 69, 342–349. [Google Scholar] [CrossRef]
- Gao, Y.; Picot, O.T.; Bilotti, E.; Peijs, T. Influence of filler size on the properties of poly(lactic acid) (PLA)/graphene nanoplatelet (GNP) nanocomposites. Eur. Polym. J. 2017, 86, 117–131. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Zhang, N.; Wang, T.; Hao, M.Y. Analysis of crystallization and melting behavior of composites before and after annealing. IOP Conf. Ser. Mater. Sci. Eng. 2020, 733, 12025. [Google Scholar] [CrossRef]
- Kumar, A.; Tumu, V.R. Physicochemical properties of the electron beam irradiated bamboo powder and its bio-composites with PLA. Compos. Part B Eng. 2019, 175, 107098. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Onuoha, C.; Onyemaobi, O.O.; Anyakwo, C.N.; Onuegbu, G.C. Effect of filler loading and particle size on the mechanical properties of periwinklw shell-filled recycled polypropylene Composites. Am. J. Eng. Res. 2017, 6, 72–79. [Google Scholar]
- Móczó, J.; Pukánszky, B. Particulate fillers in thermoplastics. Encyclopedia of Polymers and Composites; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–35. [Google Scholar]
- Patti, A.; Acierno, D.; Latteri, A.; Tosto, C.; Pergolizzi, E.; Recca, G.; Cristaudo, M.; Cicala, G. Influence of the processing conditions on the mechanical performance of sustainable bio-based PLA compounds. Polymers 2020, 12, 2197. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, A.; Karegarfard, A.; Ashori, A.; Nourbakhsh, A. Effects of particle size and coupling agent concentration on mechanical properties of particulate-filled polymer composites. J. Thermoplast. Compos. Mater. 2010, 23, 169–174. [Google Scholar] [CrossRef]
- Gosh, S.; Viana, J.C.; Reis, R.L.; Mano, J.F. Effect of processing conditions on morphology and mechanical properties of injection-molded poly(l-lactic acid). Polym. Eng. Sci. 2007, 47, 1141–1147. [Google Scholar] [CrossRef]
- Sahayaraj, F.A.; Muthukrishnan, M.; Ramesh, M. Experimental investigation on physical, mechanical, and thermal properties of jute and hemp fibers reinforced hybrid polylactic acid composites. Polym. Compos. 2022, 43, 2854–2863. [Google Scholar] [CrossRef]
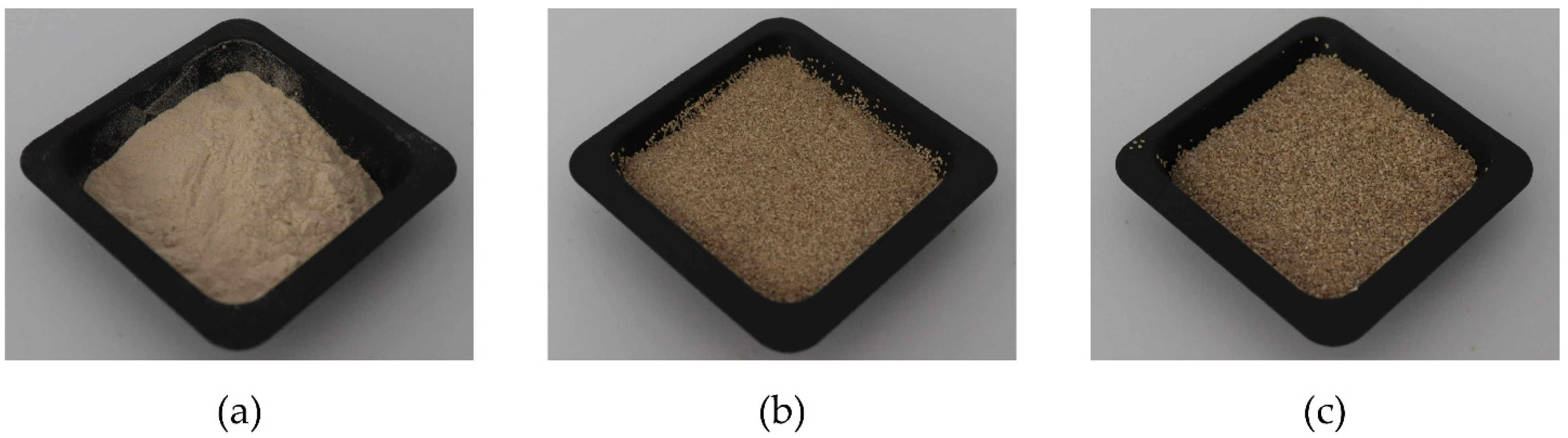
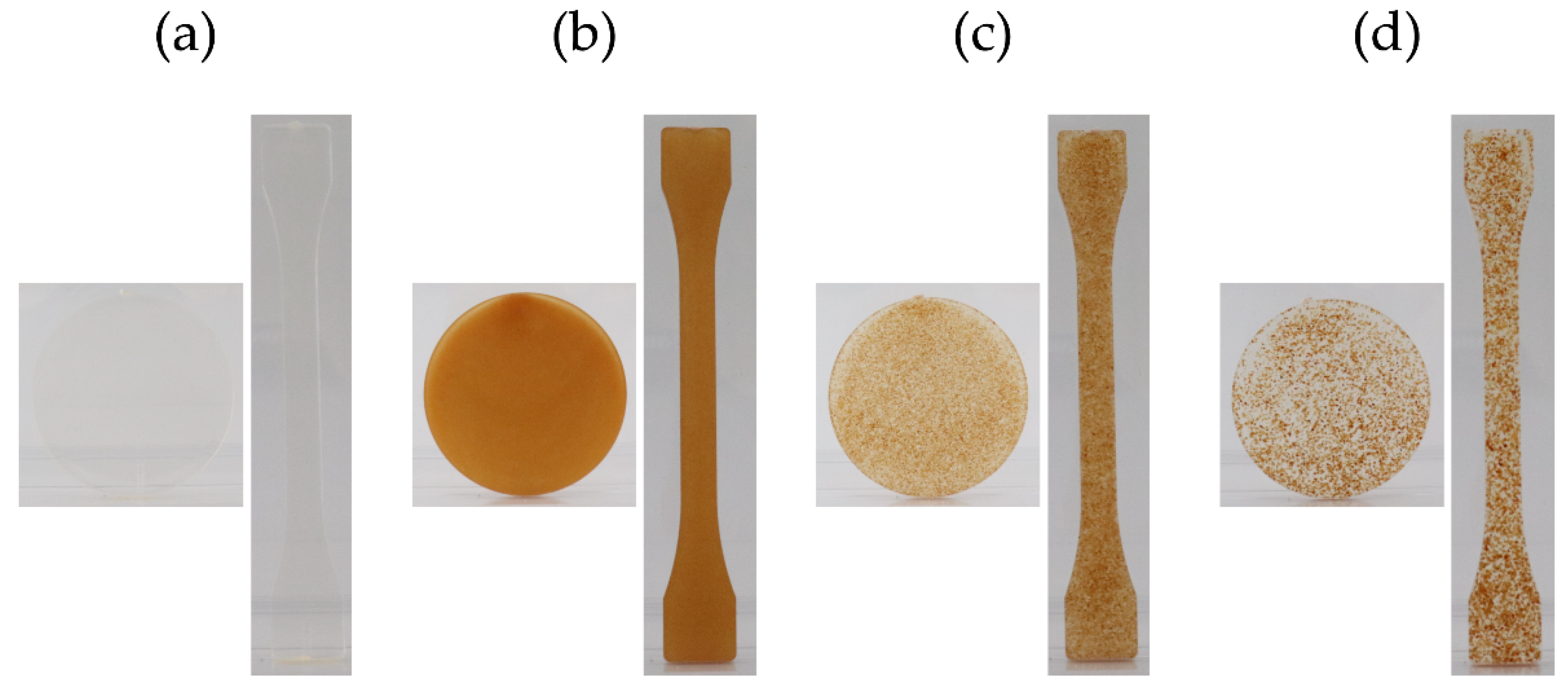

| Pure PLA | PLA:Ppeel 0–53 μm | PLA:PPeel 125–250 μm | PLA:PPeel 315–500 μm | |
|---|---|---|---|---|
| PLA:PPeel ratio | 9:0 | 9:1 | 9:1 | 9:1 |
| Moisture content (%) | 0.3 | 0.9 | 1.1 | 0.7 |
| Extruder screw rotation (rpm) | 50 | 50 | 50 | 50 |
| Extruder temperature (°C) | 210 | 220 | 200 | 200 |
| Injection temperature (°C) | 210 | 210 | 200 | 210 |
| Mold temperature (°C) | 30 | 30 | 50 | 30 |
| Particle Class (μm) | D[3,2] (μm) | Volume (μm³) | Volume Ratio (-) | Surface Area (μm²) | Coarsest Class Equivalent Surface Area (μm²) | Surface Area Ratio (-) | ||
|---|---|---|---|---|---|---|---|---|
| 0–53 | 31 | 15,599 | 3,019 | 9,112,179 | ||||
| 252.7 | 6.3 | |||||||
| 125–250 | 196 | 3,942,456 | 3018.2 | 120,687 | 1,441,212 | 14.5 | ||
| 11.9 | 2.3 | |||||||
| 315–500 | 448 | 47,079,589 | 630,530 | 630,530 | ||||
| Particle Class (μm) | D[3,2] LDA (μm) | D[3,2] DF (μm) | D[3,2] DA (μm) | Aspect Ratio (-) | Roundness (-) |
|---|---|---|---|---|---|
| 0–53 | 31 | 38 | 34 | 0.67 | 0.77 |
| 125–250 | 196 | 193 | 184 | 0.67 | 0.77 |
| 315–500 | 448 | 464 | 446 | 0.68 | 0.77 |
| Sample | Tg | Tcc | Tm | Χc |
|---|---|---|---|---|
| Neat PLA | 59.5 ± 0.3 AB | 109.9 ± 0.1 A | 152.4 ± 0.5 A/159.1 ± 0.3 | 3.2 ± 0.5 AB |
| PLA/PPeel 0–53 μm | 58.9 ± 0.1 A | 114.8 ± 0.3 B | 153.4 ± 0.4 AB | 1.9 ± 0.2 A |
| PLA/PPeel 125–250 μm | 59.5 ± 0.3 B | 115.3 ± 1.5 B | 154.6 ± 0.1 C | 6.3 ± 1.4 C |
| PLA/PPeel 315–500 μm | 59.3 ± 0.2 AB | 115.1 ± 0.8 B | 153.9 ± 0.5 BC | 4.7 ± 0.9 BC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, K.; Reichert, C.L.; Loeffler, M.; Schmid, M. Effect of Particle Size on the Physical Properties of PLA/Potato Peel Composites. Compounds 2024, 4, 119-140. https://doi.org/10.3390/compounds4010006
Miller K, Reichert CL, Loeffler M, Schmid M. Effect of Particle Size on the Physical Properties of PLA/Potato Peel Composites. Compounds. 2024; 4(1):119-140. https://doi.org/10.3390/compounds4010006
Chicago/Turabian StyleMiller, Katharina, Corina L. Reichert, Myriam Loeffler, and Markus Schmid. 2024. "Effect of Particle Size on the Physical Properties of PLA/Potato Peel Composites" Compounds 4, no. 1: 119-140. https://doi.org/10.3390/compounds4010006
APA StyleMiller, K., Reichert, C. L., Loeffler, M., & Schmid, M. (2024). Effect of Particle Size on the Physical Properties of PLA/Potato Peel Composites. Compounds, 4(1), 119-140. https://doi.org/10.3390/compounds4010006








