An Investigation into Crithmum maritimum L. Leaves as a Source of Antioxidant Polyphenols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Plant Extraction
2.4. Optimization through Response Surface Methodology (RSM) and Experimental Design
2.5. Total Polyphenol Content (TPC)
2.6. Reducing Power (PR, FRAP Assay)
2.7. Antiradical Activity (AAR, DPPH Assay)
2.8. Pigments (Total Carotenoids and Total Chlorophylls) and Color Analysis
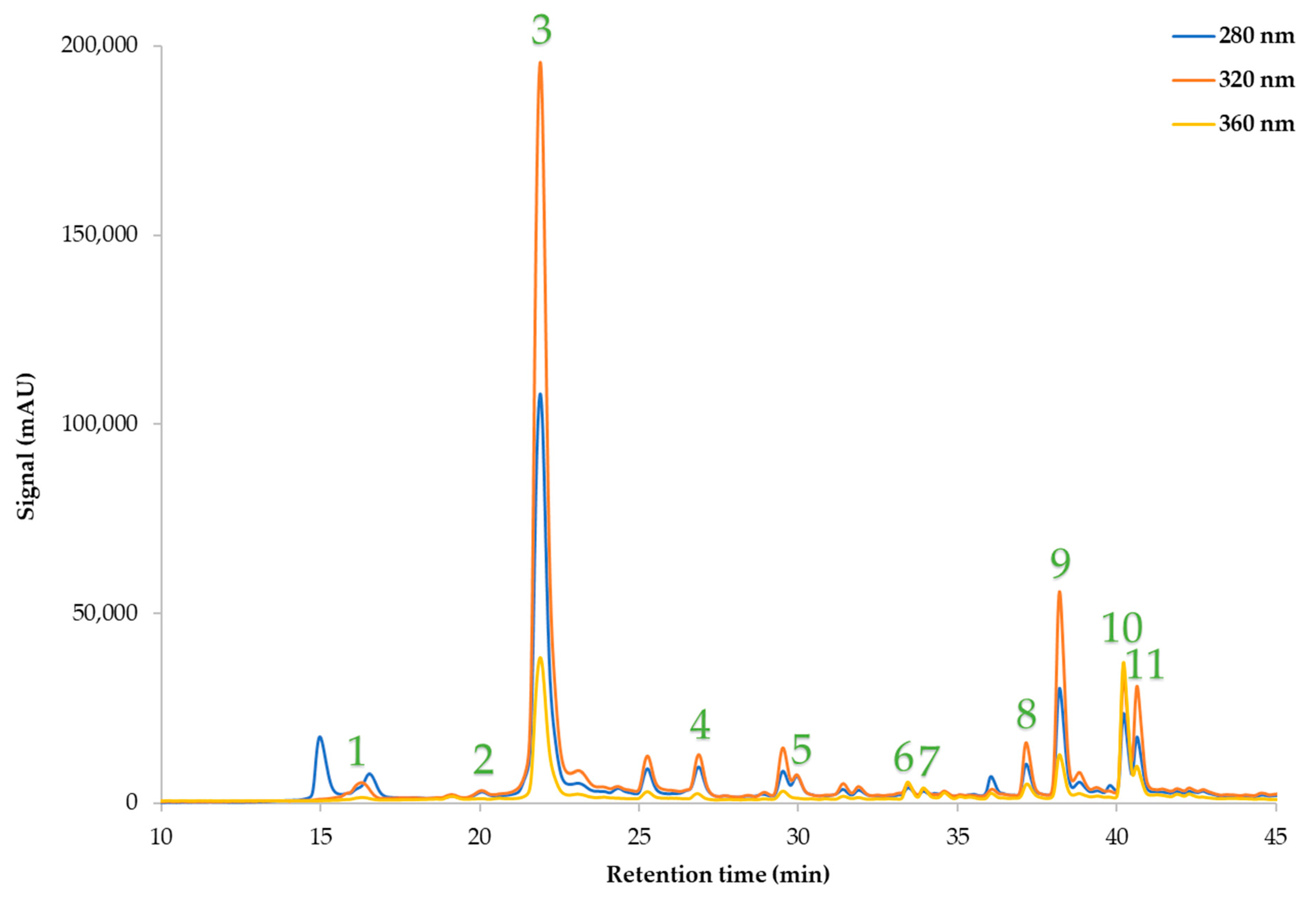
2.9. HPLC Determination of Polyphenolic Compounds
2.10. Statistical Analysis
3. Results and Discussion
3.1. Extraction Optimization
3.2. Total Polyphenol Content, Antioxidant Activity, and Pigment Analysis of the Extracts
3.3. Optimal Extraction Conditions
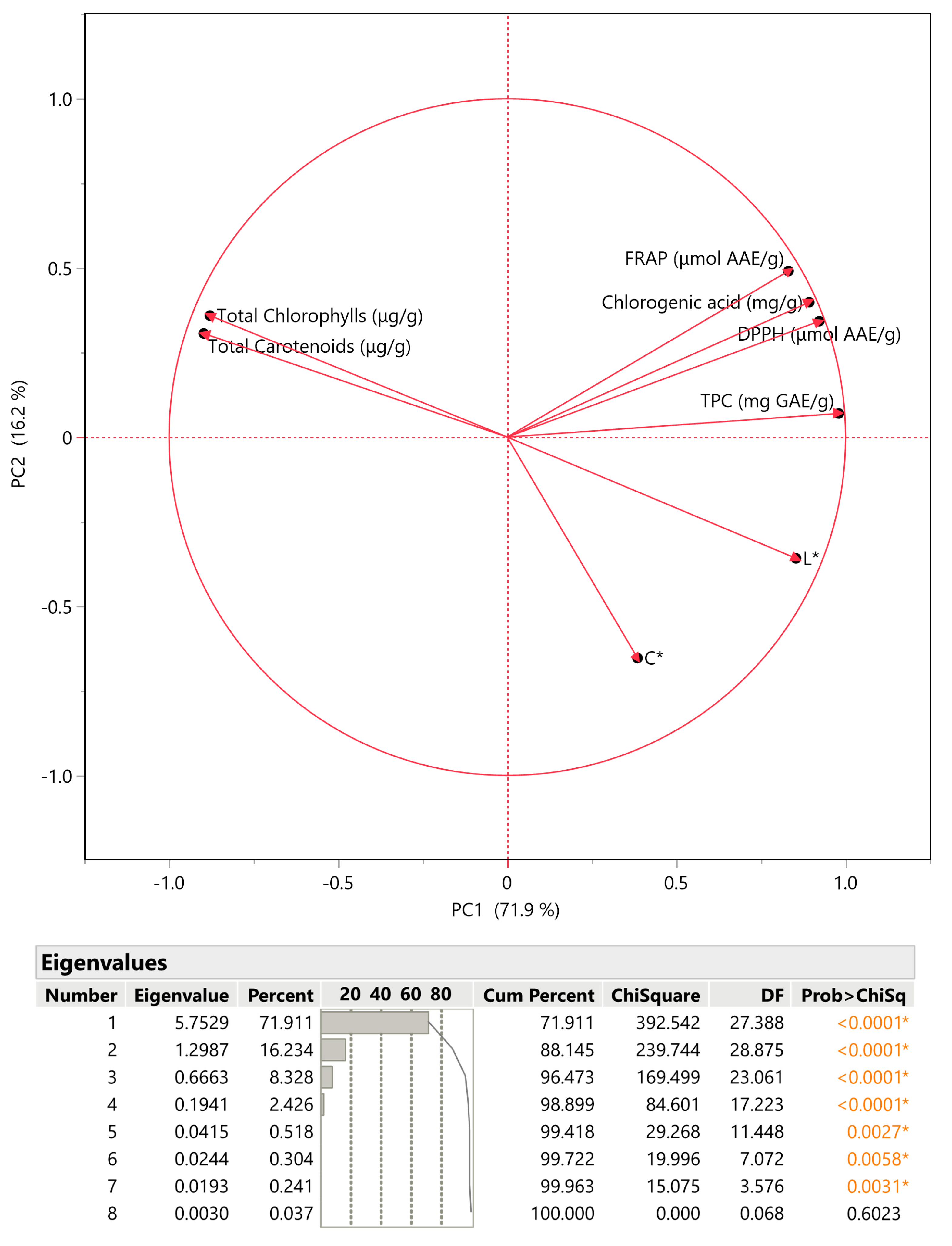
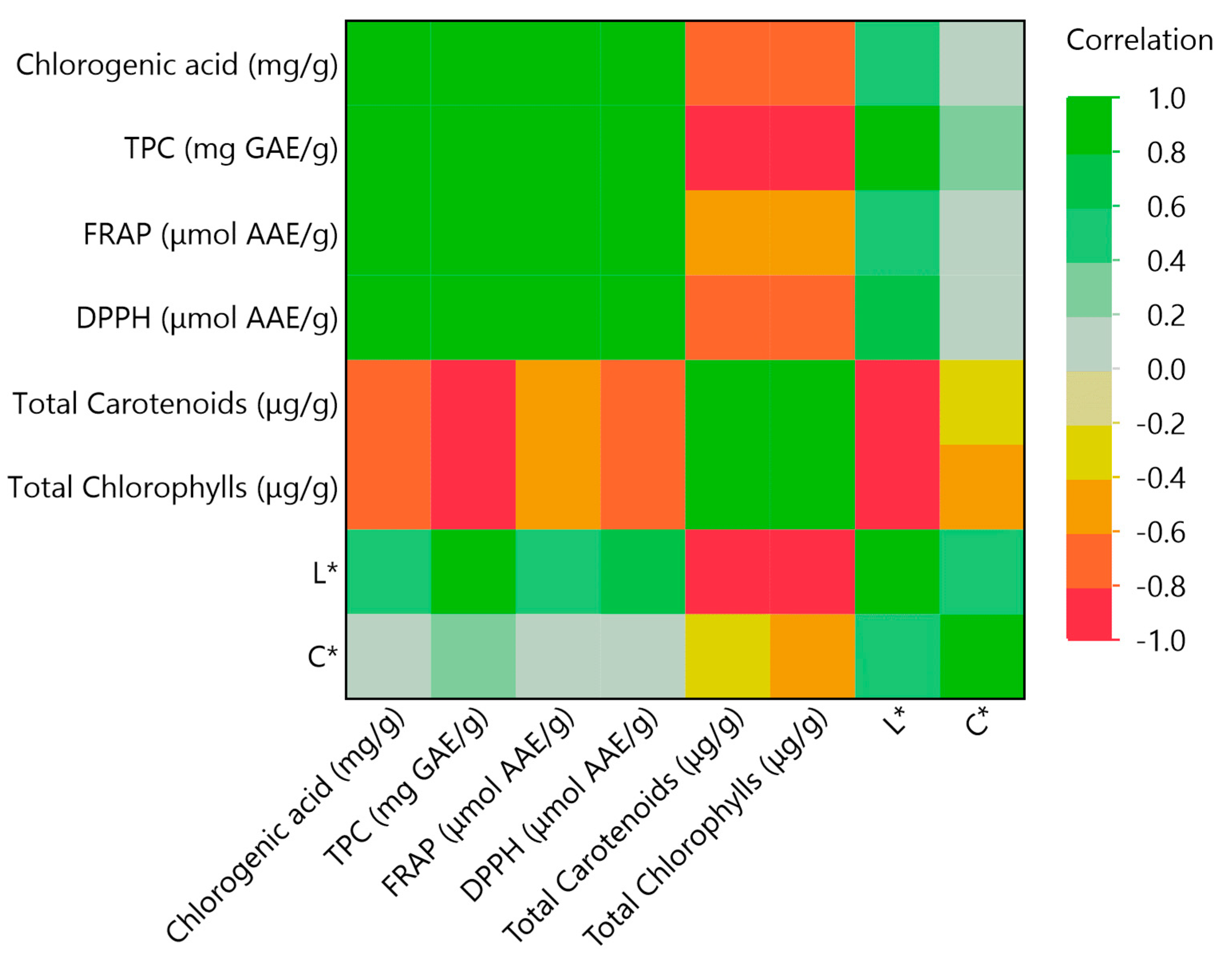
3.4. Principal Component Analysis (PCA) and Multivariate Correlation Analysis (MCA)
3.5. Partial Least Squares (PLS) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lokhande, V.H.; Suprasanna, P. Prospects of Halophytes in Understanding and Managing Abiotic Stress Tolerance. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 29–56. ISBN 978-1-4614-0815-4. [Google Scholar]
- Agudelo, A.; Carvajal, M.; del Carmen Martinez-Ballesta, M. Halophytes of the Mediterranean Basin—Underutilized Species with the Potential to Be Nutritious Crops in the Scenario of the Climate Change. Foods 2021, 10, 119. [Google Scholar] [CrossRef]
- Shaer, H.; Attia-Ismail, S. Halophytic and Salt Tolerant Feedstuffs in the Mediterranean Basin and Arab Region: An Overview; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2015; pp. 21–36. ISBN 978-1-4987-0921-7. [Google Scholar]
- Martins-Noguerol, R.; Matías, L.; Pérez-Ramos, I.M.; Moreira, X.; Francisco, M.; Pedroche, J.; DeAndrés-Gil, C.; Gutiérrez, E.; Salas, J.J.; Moreno-Pérez, A.J.; et al. Soil Physicochemical Properties Associated with the Yield and Phytochemical Composition of the Edible Halophyte Crithmum maritimum. Sci. Total Environ. 2023, 869, 161806. [Google Scholar] [CrossRef]
- Marongiu, B.; Maxia, A.; Piras, A.; Porcedda, S.; Tuveri, E.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L. Isolation of Crithmum maritimum L. Volatile Oil by Supercritical Carbon Dioxide Extraction and Biological Assays. Nat. Prod. Res. 2007, 21, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Accogli, R.; Tomaselli, V.; Direnzo, P.; Perrino, E.V.; Albanese, G.; Urbano, M.; Laghetti, G. Edible Halophytes and Halo-Tolerant Species in Apulia Region (Southeastern Italy): Biogeography, Traditional Food Use and Potential Sustainable Crops. Plants 2023, 12, 549. [Google Scholar] [CrossRef] [PubMed]
- Vekiari, S.; Ouzounidou, G. An Overview of the Wild Plants Consumed in the Island of Crete, Greece. In Global Perspectives on Underutilized Crops; Ozturk, M., Hakeem, K.R., Ashraf, M., Ahmad, M.S.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 259–285. ISBN 978-3-319-77776-4. [Google Scholar]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible Halophytes of the Mediterranean Basin: Potential Candidates for Novel Food Products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Lupidi, G.; Cianfaglione, K.; Dauvergne, X.; Bruno, M.; Benelli, G. Efficacy of Sea Fennel (Crithmum maritimum L., Apiaceae) Essential Oils against Culex quinquefasciatus Say and Spodoptera littoralis (Boisd.). Ind. Crops Prod. 2017, 109, 603–610. [Google Scholar] [CrossRef]
- Martins-Noguerol, R.; Pérez-Ramos, I.M.; Matías, L.; Moreira, X.; Francisco, M.; García-González, A.; Troncoso-Ponce, A.M.; Thomasset, B.; Martínez-Force, E.; Moreno-Pérez, A.J. Crithmum maritimum Seeds, a Potential Source for High-Quality Oil and Phenolic Compounds in Soils with No Agronomical Relevance. J. Food Compos. Anal. 2022, 108, 104413. [Google Scholar] [CrossRef]
- Sousa, G.; Alves, M.I.; Neves, M.; Tecelão, C.; Ferreira-Dias, S. Enrichment of Sunflower Oil with Ultrasound-Assisted Extracted Bioactive Compounds from Crithmum maritimum L. Foods 2022, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Nabet, N.; Boudries, H.; Chougui, N.; Loupassaki, S.; Souagui, S.; Burló, F.; Hernández, F.; Carbonell-Barrachina, Á.A.; Madani, K.; Larbat, R. Biological Activities and Secondary Compound Composition from Crithmum maritimum Aerial Parts. Int. J. Food Prop. 2017, 20, 1843–1855. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Mousavi Khaneghah, A.; Gavahian, M.; Marszałek, K.; Eş, I.; Munekata, P.E.S.; Ferreira, I.C.F.R.; Barba, F.J. Understanding the Potential Benefits of Thyme and Its Derived Products for Food Industry and Consumer Health: From Extraction of Value-Added Compounds to the Evaluation of Bioaccessibility, Bioavailability, Anti-Inflammatory, and Antimicrobial Activities. Crit. Rev. Food Sci. Nutr. 2019, 59, 2879–2895. [Google Scholar] [CrossRef]
- Sharma, P.; Vishvakarma, R.; Gautam, K.; Vimal, A.; Kumar Gaur, V.; Farooqui, A.; Varjani, S.; Younis, K. Valorization of Citrus Peel Waste for the Sustainable Production of Value-Added Products. Bioresour. Technol. 2022, 351, 127064. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Delgado Adámez, J.; Gamero Samino, E.; Valdés Sánchez, E.; González-Gómez, D. In Vitro Estimation of the Antibacterial Activity and Antioxidant Capacity of Aqueous Extracts from Grape-Seeds (Vitis vinifera L.). Food Control 2012, 24, 136–141. [Google Scholar] [CrossRef]
- Souid, A.; Della Croce, C.M.; Frassinetti, S.; Gabriele, M.; Pozzo, L.; Ciardi, M.; Abdelly, C.; Hamed, K.B.; Magné, C.; Longo, V. Nutraceutical Potential of Leaf Hydro-Ethanolic Extract of the Edible Halophyte Crithmum maritimum L. Molecules 2021, 26, 5380. [Google Scholar] [CrossRef] [PubMed]
- Meot-Duros, L.; Magné, C. Antioxidant Activity and Phenol Content of Crithmum maritimum L. Leaves. Plant Physiol. Biochem. 2009, 47, 37–41. [Google Scholar] [CrossRef]
- Veršić Bratinčević, M.; Kovačić, R.; Popović, M.; Radman, S.; Generalić Mekinić, I. Comparison of Conventional and Green Extraction Techniques for the Isolation of Phenolic Antioxidants from Sea Fennel. Processes 2023, 11, 2172. [Google Scholar] [CrossRef]
- Jallali, I.; Megdiche, W.; M’Hamdi, B.; Oueslati, S.; Smaoui, A.; Abdelly, C.; Ksouri, R. Changes in Phenolic Composition and Antioxidant Activities of the Edible Halophyte Crithmum maritimum L. with Physiological Stage and Extraction Method. Acta Physiol. Plant. 2012, 34, 1451–1459. [Google Scholar] [CrossRef]
- Makrygiannis, I.; Athanasiadis, V.; Bozinou, E.; Chatzimitakos, T.; Makris, D.P.; Lalas, S.I. Combined Effects of Deep Eutectic Solvents and Pulsed Electric Field Improve Polyphenol-Rich Extracts from Apricot Kernel Biomass. Biomass 2023, 3, 66–77. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimization of the Extraction Parameters for the Isolation of Bioactive Compounds from Orange Peel Waste. Sustainability 2022, 14, 13926. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Bozinou, E.; Lalas, S.I. Response Surface Optimization for the Enhancement of the Extraction of Bioactive Compounds from Citrus limon Peel. Antioxidants 2023, 12, 1605. [Google Scholar] [CrossRef] [PubMed]
- Ayour, J.; Alahyane, A.; Harrak, H.; Neffa, M.; Taourirte, M.; Benichou, M. Assessment of Nutritional, Technological, and Commercial Apricot Quality Criteria of the Moroccan Cultivar “Maoui” Compared to Introduced Spanish Cultivars “Canino” and “Delpatriarca” towards Suitable Valorization. J. Food Qual. 2021, 2021, e6679128. [Google Scholar] [CrossRef]
- Gregor, J.; Maršálek, B. Freshwater Phytoplankton Quantification by Chlorophyll a: A Comparative Study of In Vitro, In Vivo and In Situ Methods. Water Res. 2004, 38, 517–522. [Google Scholar] [CrossRef]
- Cesa, S.; Carradori, S.; Bellagamba, G.; Locatelli, M.; Casadei, M.A.; Masci, A.; Paolicelli, P. Evaluation of Processing Effects on Anthocyanin Content and Colour Modifications of Blueberry (Vaccinium spp.) Extracts: Comparison between HPLC-DAD and CIELAB Analyses. Food Chem. 2017, 232, 114–123. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimized Isolation Procedure for the Extraction of Bioactive Compounds from Spent Coffee Grounds. Appl. Sci. 2023, 13, 2819. [Google Scholar] [CrossRef]
- Sultana, H.; Chetia, A.; Saikia, A.; Khan, N. An Updated Review on Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Sch. Acad. J. Pharm. 2023, 12, 2320–4206. [Google Scholar] [CrossRef]
- Fadjare Frempong, T.; Owusu Boadi, N.; Badu, M. Optimization of Extraction Conditions for Polyphenols from the Stem Bark of Funtumia elastica (Funtum) Utilizing Response Surface Methodology. AAS Open Res. 2021, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhumakanova, B.S.; Korona-Głowniak, I.; Skalicka-Woźniak, K.; Ludwiczuk, A.; Baj, T.; Wojtanowski, K.K.; Józefczyk, A.; Zhaparkulova, K.A.; Sakipova, Z.B.; Malm, A. Phytochemical Fingerprinting and In Vitro Antimicrobial and Antioxidant Activity of the Aerial Parts of Thymus marschallianus Willd. and Thymus seravschanicus Klokov Growing Widely in Southern Kazakhstan. Molecules 2021, 26, 3193. [Google Scholar] [CrossRef]
- Bonifácio-Lopes, T.; Vilas-Boas, A.; Machado, M.; Costa, E.M.; Silva, S.; Pereira, R.N.; Campos, D.; Teixeira, J.A.; Pintado, M. Exploring the Bioactive Potential of Brewers Spent Grain Ohmic Extracts. Innov. Food Sci. Emerg. Technol. 2022, 76, 102943. [Google Scholar] [CrossRef]
- Cicci, A.; Bravi, M. Chapter 14—Leveraging Novel Green Solvents to Drive Conceptual and Practical Biorefinery Innovation. In Studies in Surface Science and Catalysis; Basile, A., Centi, G., Falco, M.D., Iaquaniello, G., Eds.; Catalysis, Green Chemistry and Sustainable Energy; Elsevier: Amsterdam, The Netherlands, 2020; Volume 179, pp. 243–259. [Google Scholar]
- Jallali, I.; Zaouali, Y.; Missaoui, I.; Smeoui, A.; Abdelly, C.; Ksouri, R. Variability of Antioxidant and Antibacterial Effects of Essential Oils and Acetonic Extracts of Two Edible Halophytes: Crithmum maritimum L. and Inula crithmoїdes L. Food Chem. 2014, 145, 1031–1038. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Šimat, V.; Ljubenkov, I.; Burčul, F.; Grga, M.; Mihajlovski, M.; Lončar, R.; Katalinić, V.; Skroza, D. Influence of the Vegetation Period on Sea Fennel, Crithmum maritimum L. (Apiaceae), Phenolic Composition, Antioxidant and Anticholinesterase Activities. Ind. Crops Prod. 2018, 124, 947–953. [Google Scholar] [CrossRef]
- Shin, L.E.R.; Zzaman, W.; Kuang, Y.T.; Bhat, R. Influence of Dehydration Techniques on Physicochemical, Antioxidant and Microbial Qualities of Ipomoea aquatica Forsk.: An Underutilized Green Leafy Vegetable. J. Food Process. Preserv. 2015, 39, 1118–1124. [Google Scholar] [CrossRef]
- Sarrou, E.; Siomos, A.S.; Riccadona, S.; Aktsoglou, D.-C.; Tsouvaltzis, P.; Angeli, A.; Franceschi, P.; Chatzopoulou, P.; Vrhovsek, U.; Martens, S. Improvement of Sea Fennel (Crithmum maritimum L.) Nutritional Value through Iodine Biofortification in a Hydroponic Floating System. Food Chem. 2019, 296, 150–159. [Google Scholar] [CrossRef]
- Renna, M.; Gonnella, M.; Caretto, S.; Mita, G.; Serio, F. Sea Fennel (Crithmum maritimum L.): From Underutilized Crop to New Dried Product for Food Use. Genet. Resour. Crop Evol. 2017, 64, 205–216. [Google Scholar] [CrossRef]
- Labiad, M.H.; Giménez, A.; Varol, H.; Tüzel, Y.; Egea-Gilabert, C.; Fernández, J.A.; Martínez-Ballesta, M. del C. Effect of Exogenously Applied Methyl Jasmonate on Yield and Quality of Salt-Stressed Hydroponically Grown Sea Fennel (Crithmum maritimum L.). Agronomy 2021, 11, 1083. [Google Scholar] [CrossRef]
- Martins-Noguerol, R.; Matías, L.; Pérez-Ramos, I.M.; Moreira, X.; Muñoz-Vallés, S.; Mancilla-Leytón, J.M.; Francisco, M.; García-González, A.; DeAndrés-Gil, C.; Martínez-Force, E.; et al. Differences in Nutrient Composition of Sea Fennel (Crithmum maritimum) Grown in Different Habitats and Optimally Controlled Growing Conditions. J. Food Compos. Anal. 2022, 106, 104266. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of Extraction Time, Temperature and Solvent on Concentration and Antioxidant Activity of Grape Marc Phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.-V.N.; Prabhakar, S. Techniques and Modeling of Polyphenol Extraction from Food: A Review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Oxygen Radical Absorbance Capacities of Grape/Wine Industry Byproducts and Effect of Solvent Type on Extraction of Grape Seed Polyphenols. J. Food Compos. Anal. 2006, 19, 41–48. [Google Scholar] [CrossRef]
- Lapornik, B.; Prošek, M.; Golc Wondra, A. Comparison of Extracts Prepared from Plant By-Products Using Different Solvents and Extraction Time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Sánchez-Moreno, C.; Saura-Calixto, F. Effect of Temperature on the Free Radical Scavenging Capacity of Extracts from Red and White Grape Pomace Peels. J. Agric. Food Chem. 1998, 46, 2694–2697. [Google Scholar] [CrossRef]
- Ross, C.F.; Hoye, C., Jr.; Fernandez-Plotka, V.C. Influence of Heating on the Polyphenolic Content and Antioxidant Activity of Grape Seed Flour. J. Food Sci. 2011, 76, C884–C890. [Google Scholar] [CrossRef]
- Ayour, J.; Sagar, M.; Alfeddy, M.N.; Taourirte, M.; Benichou, M. Evolution of Pigments and Their Relationship with Skin Color Based on Ripening in Fruits of Different Moroccan Genotypes of Apricots (Prunus armeniaca L.). Sci. Hortic. 2016, 207, 168–175. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Tang Nguyen, V.; Van Vuong, Q.; Bowyer, M.C.; Scarlett, C.J. Bioactive Compound Yield and Antioxidant Capacity of Helicteres hirsuta Lour. Stem as Affected by Various Solvents and Drying Methods. J. Food Process. Preserv. 2017, 41, e12879. [Google Scholar] [CrossRef]
- Costa, C.; Padalino, L.; Spinelli, S.; Serio, F.; Del Nobile Matteo, A.; Conte, A. Study of the Efficacy of Two Extraction Techniques from Crithmum maritimum and Salicornia europaea. J. Food Nutr. Res. 2018, 6, 456–463. [Google Scholar] [CrossRef]
- Giungato, P.; Renna, M.; Rana, R.; Licen, S.; Barbieri, P. Characterization of Dried and Freeze-Dried Sea Fennel (Crithmum maritimum L.) Samples with Headspace Gas-Chromatography/Mass Spectrometry and Evaluation of an Electronic Nose Discrimination Potential. Food Res. Int. 2019, 115, 65–72. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus Flavonoids: Molecular Structure, Biological Activity and Nutritional Properties: A Review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]



| Independent Variables | Coded Units | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Liquid-to-solid ratio (mL/g) | X1 | 10 | 25 | 40 |
| C (%, v/v) | X2 | 0 | 50 | 100 |
| t (min) | X3 | 30 | 90 | 150 |
| T (°C) | X4 | 20 | 50 | 80 |
| Design Point | Independent Variables | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 (Liquid-to-Solid Ratio, mL/g) | X2 (C,% v/v) | X3 (t, min) | X4 (T, °C) | Chlorogenic Acid (mg/g) | TPC (mg GAE/g) | FRAP (μmol AAE/g) | DPPH (μmol AAE/g) | |
| 1 | −1 (10) | 0 (50) | 0 (90) | −1 (20) | 6.15 | 8.34 | 45.24 | 11.08 |
| 2 | 0 (25) | 1 (100) | 1 (150) | 0 (50) | 0.59 | 4.33 | 20.80 | 2.73 |
| 3 | 1 (40) | −1 (0) | 0 (90) | 0 (50) | 1.30 | 9.22 | 19.89 | 9.03 |
| 4 | 0 (25) | 0 (50) | 1 (150) | −1 (20) | 4.77 | 8.19 | 39.52 | 10.54 |
| 5 * | 0 (25) | −1 (0) | 0 (90) | −1 (20) | −1.25 | 5.72 | −6.27 | −1.43 |
| 6 | −1 (10) | 0 (50) | 1 (150) | 0 (50) | 7.73 | 10.93 | 67.52 | 14.64 |
| 7 | 0 (25) | 1 (100) | −1 (30) | 0 (50) | 1.02 | 4.01 | 24.18 | 3.46 |
| 8 | 0 (25) | 0 (50) | 1 (150) | 1 (80) | 9.54 | 14.38 | 82.07 | 20.56 |
| 9 | 0 (25) | −1 (0) | 0 (90) | 1 (80) | 4.27 | 11.72 | 28.20 | 12.40 |
| 10 | 1 (40) | 1 (100) | 0 (90) | 0 (50) | 0.95 | 5.24 | 24.30 | 3.76 |
| 11 | 0 (25) | 1 (100) | 0 (90) | 1 (80) | 1.06 | 5.39 | 33.67 | 3.98 |
| 12 | 0 (25) | 0 (50) | −1 (30) | 1 (80) | 8.25 | 12.87 | 82.15 | 19.40 |
| 13 | 0 (25) | 0 (50) | 0 (90) | 0 (50) | 8.18 | 11.96 | 76.92 | 17.65 |
| 14 | 0 (25) | 0 (50) | 0 (90) | 0 (50) | 8.18 | 11.96 | 76.92 | 17.65 |
| 15 | 0 (25) | 0 (50) | −1 (30) | −1 (20) | 6.44 | 9.97 | 50.12 | 11.38 |
| 16 | 0 (25) | −1 (0) | −1 (30) | 0 (50) | 1.76 | 9.35 | 23.50 | 6.17 |
| 17 | −1 (10) | 0 (50) | −1 (30) | 0 (50) | 7.74 | 10.63 | 74.86 | 14.52 |
| 18 | −1 (10) | 0 (50) | 0 (90) | 1 (80) | 8.77 | 12.56 | 79.38 | 15.66 |
| 19 | −1 (10) | 1 (100) | 0 (90) | 0 (50) | 0.69 | 2.82 | 20.45 | 1.83 |
| 20 * | 0 (25) | 1 (100) | 0 (90) | −1 (20) | −0.01 | 2.30 | −6.46 | −0.22 |
| 21 | −1 (10) | −1 (0) | 0 (90) | 0 (50) | 2.31 | 8.60 | 19.57 | 3.76 |
| 22 | 0 (25) | −1 (0) | 1 (150) | 0 (50) | 1.82 | 8.75 | 16.20 | 7.23 |
| 23 | 1 (40) | 0 (50) | 0 (90) | −1 (20) | 5.09 | 9.54 | 44.17 | 10.24 |
| 24 | 1 (40) | 0 (50) | −1 (30) | 0 (50) | 7.54 | 12.59 | 74.95 | 18.08 |
| 25 | 1 (40) | 0 (50) | 1 (150) | 0 (50) | 7.17 | 12.01 | 71.61 | 18.28 |
| 26 | 0 (25) | 0 (50) | 0 (90) | 0 (50) | 8.18 | 11.96 | 76.92 | 17.65 |
| 27 | 1 (40) | 0 (50) | 0 (90) | 1 (80) | 9.06 | 14.40 | 84.62 | 23.69 |
| Design Point | Independent Variables | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 (Liquid-to-Solid Ratio, mL/g) | X2 (C, % v/v) | X3 (t, min) | X4 (T, °C) | Total Carotenoids (μg/g) | Total Chlorophylls (μg/g) | L* | C* | |
| 1 | −1 (10) | 0 (50) | 0 (90) | −1 (20) | 0.75 | 0.39 | 58.6 | 25.5 |
| 2 | 0 (25) | 1 (100) | 1 (150) | 0 (50) | 9.03 | 20.10 | 30.1 | 25.3 |
| 3 | 1 (40) | −1 (0) | 0 (90) | 0 (50) | 0.54 | 0.31 | 58.5 | 28.4 |
| 4 | 0 (25) | 0 (50) | 1 (150) | −1 (20) | 1.26 | 1.73 | 37.9 | 21.8 |
| 5 | 0 (25) | −1 (0) | 0 (90) | −1 (20) | 0.97 | 0.59 | 55.8 | 31.0 |
| 6 | −1 (10) | 0 (50) | 1 (150) | 0 (50) | 0.57 | 0.47 | 50.3 | 32.0 |
| 7 | 0 (25) | 1 (100) | −1 (30) | 0 (50) | 8.83 | 19.66 | 32.8 | 31.8 |
| 8 | 0 (25) | 0 (50) | 1 (150) | 1 (80) | 0.36 | 0.26 | 60.9 | 35.0 |
| 9 | 0 (25) | −1 (0) | 0 (90) | 1 (80) | 0.54 | 0.47 | 63.9 | 28.1 |
| 10 | 1 (40) | 1 (100) | 0 (90) | 0 (50) | 5.39 | 12.10 | 40.3 | 37.8 |
| 11 | 0 (25) | 1 (100) | 0 (90) | 1 (80) | 8.91 | 18.65 | 32.3 | 24.5 |
| 12 | 0 (25) | 0 (50) | −1 (30) | 1 (80) | 0.41 | 0.44 | 52.2 | 26.5 |
| 13 | 0 (25) | 0 (50) | 0 (90) | 0 (50) | 0.45 | 0.47 | 55.2 | 25.1 |
| 14 | 0 (25) | 0 (50) | 0 (90) | 0 (50) | 0.46 | 0.42 | 55.6 | 30.5 |
| 15 | 0 (25) | 0 (50) | −1 (30) | −1 (20) | 0.95 | 1.11 | 45.1 | 27.2 |
| 16 | 0 (25) | −1 (0) | −1 (30) | 0 (50) | 1.07 | 0.80 | 54.9 | 30.2 |
| 17 | −1 (10) | 0 (50) | −1 (30) | 0 (50) | 0.48 | 0.34 | 47.6 | 23.5 |
| 18 | −1 (10) | 0 (50) | 0 (90) | 1 (80) | 1.03 | 0.80 | 53.3 | 35.4 |
| 19 | −1 (10) | 1 (100) | 0 (90) | 0 (50) | 11.10 | 28.45 | 25.0 | 6.2 |
| 20 | 0 (25) | 1 (100) | 0 (90) | −1 (20) | 8.32 | 18.91 | 32.8 | 27.1 |
| 21 | −1 (10) | −1 (0) | 0 (90) | 0 (50) | 2.06 | 1.34 | 45.4 | 29.5 |
| 22 | 0 (25) | −1 (0) | 1 (150) | 0 (50) | 1.31 | 3.12 | 57.4 | 27.9 |
| 23 | 1 (40) | 0 (50) | 0 (90) | −1 (20) | 1.30 | 2.33 | 45.8 | 22.1 |
| 24 | 1 (40) | 0 (50) | −1 (30) | 0 (50) | 0.41 | 0.55 | 55.8 | 25.9 |
| 25 | 1 (40) | 0 (50) | 1 (150) | 0 (50) | 0.59 | 0.85 | 57.9 | 26.4 |
| 26 | 0 (25) | 0 (50) | 0 (90) | 0 (50) | 0.45 | 0.49 | 55.9 | 25.8 |
| 27 | 1 (40) | 0 (50) | 0 (90) | 1 (80) | 0.36 | 0.38 | 64.0 | 28.1 |
| Design Point | Independent Variables | Responses | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 (Liquid-to-Solid Ratio, mL/g) | X2 (C,% v/v) | X3 (t, min) | X4 (T, °C) | NCA | CCGA | CFA | CA | QGA | RT | FA | NARN | KG | HES | |
| 1 | −1 (10) | 0 (50) | 0 (90) | −1 (20) | 0.21 | 0.06 | 0.15 | 0.06 | 0.09 | 0.10 | 0.13 | 0.91 | 0.15 | 0.06 |
| 2 | 0 (25) | 1 (100) | 1 (150) | 0 (50) | nd * | 0.02 | 0.05 | 0.03 | 0.03 | 0.05 | 0.06 | 0.41 | 0.44 | 0.02 |
| 3 | 1 (40) | −1 (0) | 0 (90) | 0 (50) | 0.01 | 0.08 | 0.12 | 0.10 | nd | 0.04 | 0.04 | nd | nd | 0.01 |
| 4 | 0 (25) | 0 (50) | 1 (150) | −1 (20) | 0.09 | 0.06 | 0.18 | 0.08 | 0.07 | 0.09 | 0.10 | 0.43 | 0.38 | 0.03 |
| 5 | 0 (25) | −1 (0) | 0 (90) | −1 (20) | nd | 0.06 | 0.07 | 0.11 | nd | 0.01 | 0.03 | nd | 0.02 | nd |
| 6 | −1 (10) | 0 (50) | 1 (150) | 0 (50) | 0.22 | 0.05 | 0.14 | 0.07 | 0.10 | 0.09 | 0.19 | 1.58 | 0.12 | 0.16 |
| 7 | 0 (25) | 1 (100) | −1 (30) | 0 (50) | nd | 0.02 | 0.04 | 0.02 | 0.02 | 0.04 | 0.04 | 0.30 | 1.61 | 0.75 |
| 8 | 0 (25) | 0 (50) | 1 (150) | 1 (80) | 0.29 | 0.06 | 0.16 | 0.08 | 0.09 | 0.09 | 0.23 | 1.06 | 0.87 | 0.74 |
| 9 | 0 (25) | −1 (0) | 0 (90) | 1 (80) | 0.49 | 0.08 | 0.16 | 0.08 | 0.02 | 0.05 | 0.13 | 0.24 | 0.07 | 0.11 |
| 10 | 1 (40) | 1 (100) | 0 (90) | 0 (50) | nd | 0.02 | 0.06 | 0.03 | 0.01 | 0.05 | 0.06 | 0.34 | 1.84 | 0.86 |
| 11 | 0 (25) | 1 (100) | 0 (90) | 1 (80) | nd | 0.02 | 0.05 | 0.02 | 0.04 | 0.06 | 0.07 | 0.57 | 1.74 | 0.84 |
| 12 | 0 (25) | 0 (50) | −1 (30) | 1 (80) | 0.23 | 0.06 | 0.17 | 0.09 | 0.10 | 0.10 | 0.21 | 1.58 | 1.30 | 0.24 |
| 13 | 0 (25) | 0 (50) | 0 (90) | 0 (50) | 0.19 | 0.07 | 0.17 | 0.08 | 0.09 | 0.10 | 0.19 | 1.45 | 0.83 | 0.15 |
| 14 | 0 (25) | 0 (50) | 0 (90) | 0 (50) | 0.19 | 0.06 | 0.17 | 0.09 | 0.09 | 0.10 | 0.20 | 1.54 | 0.75 | 0.16 |
| 15 | 0 (25) | 0 (50) | −1 (30) | −1 (20) | 0.16 | 0.07 | 0.17 | 0.08 | 0.09 | 0.10 | 0.16 | 1.05 | 1.07 | 0.08 |
| 16 | 0 (25) | −1 (0) | −1 (30) | 0 (50) | 0.01 | 0.07 | 0.11 | 0.08 | nd | 0.01 | 0.03 | nd | 0.01 | nd |
| 17 | −1 (10) | 0 (50) | −1 (30) | 0 (50) | 0.20 | 0.06 | 0.14 | 0.07 | 0.10 | 0.10 | 0.18 | 1.56 | 0.19 | 0.14 |
| 18 | −1 (10) | 0 (50) | 0 (90) | 1 (80) | 0.27 | 0.05 | 0.15 | 0.07 | 0.10 | 0.09 | 0.21 | 1.31 | 0.21 | 0.55 |
| 19 | −1 (10) | 1 (100) | 0 (90) | 0 (50) | nd | 0.14 | 0.02 | 0.01 | 0.03 | 0.04 | 0.04 | 0.38 | 0.01 | 0.02 |
| 20 | 0 (25) | 1 (100) | 0 (90) | −1 (20) | nd | 0.02 | 0.04 | 0.02 | nd | 0.03 | 0.04 | 0.16 | 1.20 | 0.56 |
| 21 | −1 (10) | −1 (0) | 0 (90) | 0 (50) | 0.11 | 0.07 | 0.11 | 0.03 | 0.02 | 0.03 | 0.02 | 0.04 | nd | nd |
| 22 | 0 (25) | −1 (0) | 1 (150) | 0 (50) | 0.04 | 0.07 | 0.13 | 0.04 | nd | 0.03 | 0.03 | 0.01 | nd | nd |
| 23 | 1 (40) | 0 (50) | 0 (90) | −1 (20) | 0.09 | 0.07 | 0.18 | 0.09 | 0.06 | 0.09 | 0.12 | 0.55 | 0.87 | 0.04 |
| 24 | 1 (40) | 0 (50) | −1 (30) | 0 (50) | 0.18 | 0.07 | 0.18 | 0.09 | 0.08 | 0.10 | 0.21 | 1.50 | 2.26 | 0.15 |
| 25 | 1 (40) | 0 (50) | 1 (150) | 0 (50) | 0.18 | 0.07 | 0.18 | 0.09 | 0.08 | 0.10 | 0.20 | 1.41 | 0.69 | 0.17 |
| 26 | 0 (25) | 0 (50) | 0 (90) | 0 (50) | 0.20 | 0.07 | 0.16 | 0.08 | 0.09 | 0.10 | 0.20 | 1.47 | 0.65 | 0.16 |
| 27 | 1 (40) | 0 (50) | 0 (90) | 1 (80) | 0.21 | 0.06 | 0.18 | 0.09 | 0.08 | 0.10 | 0.23 | 1.17 | 1.61 | 0.55 |
| Responses | Second-Order Polynomial Equations (Models) | R2 | p | Eq. |
|---|---|---|---|---|
| Chlorogenic acid | Y = −2.37 + 0.006X1 + 0.28X2 − 0.002X3 + 0.1X4 − 0.001X12 − 0.003X22 − 0.0001X32 − 0.0007X42 + 0.0004X1X2 − 0.0001X1X3 + 0.0008X1X4 − 0.0001X2X3 − 0.0007X2X4 + 0.0004X3X4 | 0.9723 | <0.0001 | (6) |
| TPC | Y = 3.68 + 0.09X1 + 0.16X2 − 0.02X3 + 0.1X4 − 0.001X12 − 0.002X22 − 0.0001X32 − 0.0005X42 + 0.0006X1X2 − 0.0002X1X3 + 0.0004X1X4 + 0.0001X2X3 − 0.0005X2X4 + 0.0005X3X4 | 0.9587 | <0.0001 | (7) |
| FRAP | Y = −30.75 + 0.27X1 + 2.06X2 − 0.05X3 + 1.6X4 − 0.01X12 − 0.02X22 − 0.0006X32 − 0.01X42 + 0.001X1X2 + 0.001X1X3 + 0.004X1X4 + 0.0003X2X3 + 0.001X2X4 + 0.002X3X4 | 0.967 | <0.0001 | (8) |
| DPPH | Y = −10.05 + 0.1X1 + 0.58X2 + 0.02X3 + 0.27X4 − 0.004X12 − 0.005X22 − 0.0001X32 − 0.002X42 − 0.001X1X2 + 0.0001X1X3 + 0.005X1X4 − 0.0002X2X3 − 0.002X2X4 + 0.0003X3X4 | 0.9496 | < 0.0001 | (9) |
| Responses | Optimal Conditions | ||||
|---|---|---|---|---|---|
| Maximum Predicted Response | Liquid-to-Solid Ratio (mL/g) (X1) | C (%, v/v) (X2) | t (min) (X3) | T (°C) (X4) | |
| Chlorogenic acid (mg/g) | 9.62 ± 1.36 | 25 | 40 | 145 | 80 |
| TPC (mg GAE/g) | 14.94 ± 1.76 | 35 | 35 | 130 | 80 |
| FRAP (μmol AAE/g) | 85.21 ± 9.28 | 35 | 50 | 90 | 75 |
| DPPH (μmol AAE/g) | 24.31 ± 4.1 | 40 | 40 | 100 | 80 |
| Variables | PLS Model Values | Experimental Values |
|---|---|---|
| Chlorogenic acid (mg/g) | 9.34 | 9.35 ± 0.63 |
| TPC (mg GAE/g) | 14.87 | 15.11 ± 0.14 |
| FRAP (μmol AAE/g) | 83.45 | 85.52 ± 3.2 |
| DPPH (μmol AAE/g) | 24.28 | 25.57 ± 1.25 |
| Parameters | Optimal Extract |
|---|---|
| Total Carotenoids (μg/g) | 0.32 ± 0.01 |
| Total Chlorophylls (μg/g) | 0.62 ± 0.04 |
| L* | 69.7 ± 0.8 |
| C* | 29.7 ± 0.8 |
| Polyphenolic compounds (mg/g) | |
| Neochlorogenic acid | 0.24 ± 0.02 |
| Cryptochlorogenic acid | 0.09 ± 0.01 |
| Caffeic acid | 0.21 ± 0.01 |
| Coumaric acid | 0.24 ± 0.01 |
| Quercetin 3-O-galactoside | 0.11 ± 0.01 |
| Rutin | 0.15 ± 0.01 |
| Ferulic acid | 0.26 ± 0.01 |
| Naringin | 1.24 ± 0.05 |
| Kaempferol 3-glycoside | 1.81 ± 0.08 |
| Hesperidin | 0.79 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzimitakos, T.; Athanasiadis, V.; Makrygiannis, I.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. An Investigation into Crithmum maritimum L. Leaves as a Source of Antioxidant Polyphenols. Compounds 2023, 3, 532-551. https://doi.org/10.3390/compounds3040038
Chatzimitakos T, Athanasiadis V, Makrygiannis I, Kalompatsios D, Bozinou E, Lalas SI. An Investigation into Crithmum maritimum L. Leaves as a Source of Antioxidant Polyphenols. Compounds. 2023; 3(4):532-551. https://doi.org/10.3390/compounds3040038
Chicago/Turabian StyleChatzimitakos, Theodoros, Vassilis Athanasiadis, Ioannis Makrygiannis, Dimitrios Kalompatsios, Eleni Bozinou, and Stavros I. Lalas. 2023. "An Investigation into Crithmum maritimum L. Leaves as a Source of Antioxidant Polyphenols" Compounds 3, no. 4: 532-551. https://doi.org/10.3390/compounds3040038
APA StyleChatzimitakos, T., Athanasiadis, V., Makrygiannis, I., Kalompatsios, D., Bozinou, E., & Lalas, S. I. (2023). An Investigation into Crithmum maritimum L. Leaves as a Source of Antioxidant Polyphenols. Compounds, 3(4), 532-551. https://doi.org/10.3390/compounds3040038











