Abstract
Non-symmetrical chiral 4,4′-bipyridines have recently found interest in organocatalysis and medicinal chemistry. In this regard, the development of efficient methods for their synthesis is highly desirable. Herein, a series of non-symmetrical atropisomeric polyhalogenated 4,4′-bipyridines were prepared and further functionalized by using cross-coupling reactions. The desymmetrization step is based on the N-oxidation of one of the two pyridine rings of the 4,4′-bipyridine skeleton. The main advantage of this methodology is the possible post-functionalization of the pyridine N-oxide, allowing selective introduction of chlorine, bromine or cyano groups in 2- and 2′-postions of the chiral atropisomeric 4,4′-bipyridines. The crystal packing in the solid state of some newly prepared derivatives was analyzed and revealed the importance of halogen bonds in intermolecular interactions.
1. Introduction
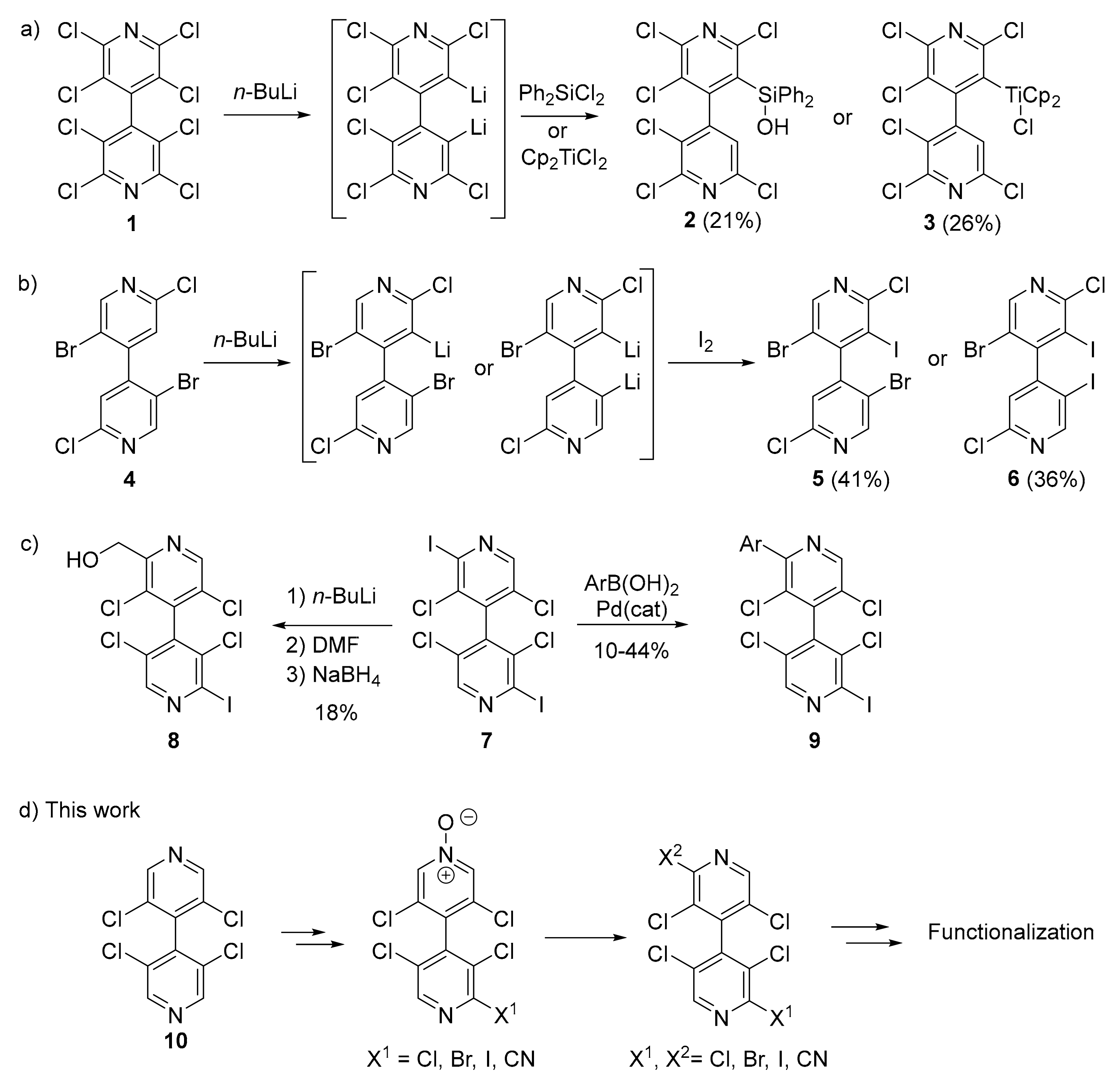
4,4′-Bipyridines are useful ligands for the design of coordination polymers and metal-organic frameworks (MOFs) [1], and are key components in the preparation of viologens [2,3]. In contrast to 2,2′-bipyridines for which a large number of chiral derivatives were developed [4,5,6], chiral 4,4′-bipyridines were much less explored. In the latter derivatives, the chirality can be brought by specific functions on the pyridine rings [7,8] or by atropisomery [9,10]. Indeed, atropisomeric 4,4′-bipyridines are the particular case where rotation around the pyridyl-pyridyl bond is blocked by the presence of three or four substituents. They were first used for the preparation of metallo-supramolecular squares [9] and some years later for building chiral MOFs [11]. These last years, our groups were involved in the development of halogenated chiral 4,4′-bipyridines and in the study of their performances as halogen- [12,13] and chalcogen [14,15] bond donors in different applications such as organocatalysis [16] and medicinal chemistry [17].
Particularly, we have shown that homocoupling reactions represent straightforward ways for the synthesis of symmetrical chiral 4,4′-bipyridines [10,18]. However, these methods are not relevant for the synthesis of the non-symmetrical derivatives; therefore, desymmetrization processes have to be employed. Very limited methods for the desymmetrization of the 4,4′-bipyridine framework to non-symmetrical chiral 4,4′-bipyridines were reported. During their work on the 3,3′-dilithiation of octachloro-4,4′-bipyridine 1, Foulger and Wakefield observed that quenching with dichlorodiphenylsilane and di-π-cyclopentadienyltitanium dichloride generated 4,4′-bipyridines 2 and 3, respectively [19] (Figure 1a). Later, we showed that 3-mono- and 3,5′-dilithiation of 2,2′-dibromo-5,5′-dichloro-4,4′-bipyridine 4 and subsequent electrophilic trapping furnished 4,4′-bipyridines 5 and 6, respectively [20] (Figure 1b). Finally, very recently, we used lithiation and cross-coupling reactions in 2-position of 2,2′-diiodo-3,3′,5,5′-tetrachloro-4,4′-bipyridine 7 as non-selective routes to 4,4′-bipyridines 8 and 9 [17] (Figure 1c). It is worth mentioning that in all these examples the yields were low to moderate.
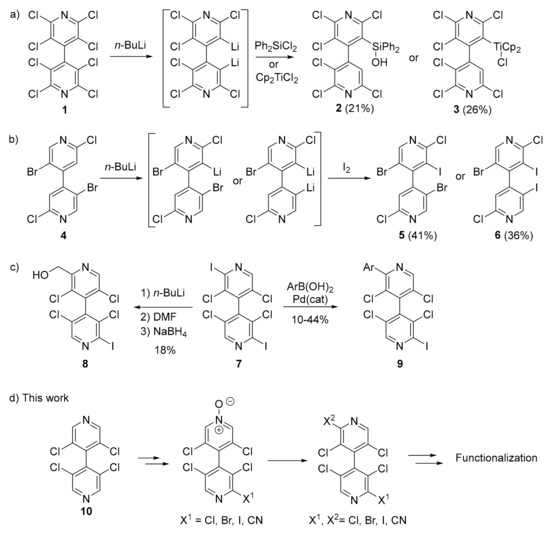
Figure 1.
Non-symmetrical chiral 4,4′-bipyridines obtained by desymmetrization of parent 4,4′-bipyridines in the literature (a–c) and in the present work (d).
Herein, the N-oxidation as a straightforward and operatively easy method was used to desymmetrize 3,3′,5,5′-tetrachloro-4,4′-bipyridine 10. The advantage of the reported methodology relies on the chemical transformations allowed by the pyridine N-O function, such as halogenation and cyanation [21]. The halogenated 4,4′-bipyridines were further functionalized through metal-catalyzed coupling reactions (Figure 1d). Moreover, X-ray diffraction analysis of selected compounds revealed very interesting solid-state packing features.
2. Materials and Methods
2.1. General Information
Proton (1H NMR) and carbon (13C NMR) nuclear magnetic resonance spectra were recorded on a Bruker Avance III instrument operating at 300, 400, or 500 MHz (Bruker Corporation, Billerica, MA, USA). The chemical shifts are given in parts per million (ppm) on the delta scale. The solvent peak was used as reference values for 1H NMR (CDCl3 = 7.26 ppm) and for 13C NMR (CDCl3 = 77.16 ppm). Data are presented as follows: Chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, quint = quintet, m = multiplet, b = broad), integration, and coupling constants (J/Hz). High-resolution mass spectra (HRMS) data were recorded on a micrOTOF spectrometer (Bruker Corporation, Billerica, MA, USA) equipped with an orthogonal electrospray interface (ESI). Analytical thin layer chromatography (TLC plates from Merck KGaA, Darmstadt, Germany) was carried out on silica gel 60 F254 plates with visualization by ultraviolet light. Reagents and solvents were purified using standard means. Tetrahydrofuran (THF) was distilled from sodium metal/benzophenone and freshly used. Dry dichloromethane was obtained by passing through activated alumina under a positive pressure of argon using GlassTechnology GTS100 devices. Dry dioxane (over molecular sieve) was purchased from Aldrich, triethylamine and diisopropylamine were distilled over CaH2 and stored over KOH under an argon atmosphere. Anhydrous reactions were carried out in flame-dried glassware and under an argon atmosphere. All other chemicals were used as received.
2.2. Syntheses
2.2.1. 3,3′,5,5′-Tetrachloro-4,4′-Bipyridine 10
To a solution of diisopropylamine (27.5 mmol, 3.85 mL) in THF (200 mL) at −40 °C was added a solution of n-BuLi (1.6 M in hexanes, 27.5 mmol, 17.2 mL) and the mixture was stirred for 20 min. A solution of 3,5-dichloropyridine 11 (50 mmol, 7.4 g) in THF (100 mL) was added during 1 h while maintaining the temperature close to −40 °C. The temperature of the cloudy solution was slowly raised to −15 °C (during 1h) to give a homogeneous dark red solution. The temperature was lowered to −78 °C then a solution of I2 (30 mmol, 7.62 g) in THF (50 mL) was slowly added. After 10 min at −78 °C, the temperature was raised to room temperature and the reaction was quenched by the addition of aqueous saturated solution of Na2S2O3 (100 mL). Water was added (200 mL) and the mixture was extracted three times with ethyl acetate (3 × 200 mL). The organic phases were combined, washed with brine (100 mL) and dried over MgSO4. After concentration, the crude compound was purified by chromatography on silica gel (cyclohexane/ethyl acetate 95/5) to give 4,4′-bipyridine 10 (3.64 g) and compound 12 (2.7 g). Compound 12 was diluted with ethyl acetate (200 mL) and I2 (18 mmol, 4.57g) was added and the mixture was stirred overnight at room temperature in an open flask. The reaction was quenched by the addition of aqueous saturated solution of Na2S2O3 (25 mL). Water was added (50 mL) and the organic phases washed with brine (100 mL) and dried over anhydrous MgSO4. After concentration, the crude compound was purified by chromatography on silica gel (cyclohexane/ethyl acetate 95/5) to give 4,4′-bipyridine 10 (2.4 g). A total mass of 6.04 g of 10 was obtained which corresponds to an overall yield of 82%. The NMR data for 10 are in complete agreement with the literature [22].
3,3′,5,5′-Tetrachloro-1,4-dihydro-4,4′-bipyridine (12). Yellow solid, mp 155–157 °C. 1H NMR (500 MHz, CD3COCD3) δ 8.57 (s, 1H), 8.56 (s, 1H), 7.55 (s, 1H, NH), 6.56 (s, 1H), 6.55 (s, 1H), 5.87 (s, 1H). 13C NMR (126 MHz, CD3COCD3) δ 150.7, 148.3, 142.5, 134.3, 134.0, 127.7, 102.8, 50.6. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C10H6Cl5N2 294.9285, found: 294.9327 (Figure S1).
2.2.2. 3,3′,5,5′-Tetrachloro-[4,4′-Bipyridine] 1-Oxide 13
4,4′-Bipyridine 10 (12.38 mmol, 3.64 g) was dissolved in CH2Cl2 (62 mL) at room temperature. m-CPBA (m-chloro-perbenzoic acid) (77% purity, 12.38 mmol, 2.8 g) was added and the mixture was stirred for 24 h. After dilution with CH2Cl2 (140 mL), the mixture was washed with NaOH 1M (2 × 100 mL) and with brine. The organic phase was dried over MgSO4, concentrated and purified by chromatography on silica gel (CH2Cl2/methanol 99/1) to give respectively the starting compound 10 (850 mg, 23%), compound 13 (2.0 g, 54% Yield) and the bis N-oxide 14 (925 mg, 23%).
3,3′,5,5′-Tetrachloro-[4,4′-bipyridine] 1-oxide (13). White solid, mp 218–220 °C. 1H NMR (500 MHz, CDCl3) δ 8.68 (s, 2H), 8.31 (s, 2H). 13C NMR (126 MHz, CDCl3) δ 148.0, 139.0, 138.3, 132.9, 132., 130.2. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C10H5Cl4N2O 308.9150, found: 308.9153 (Figure S2).
3,3′,5,5′-Tetrachloro-[4,4′-bipyridine] 1,1′-dioxide (14). White solid, mp 270–272 °C. 1H NMR (500 MHz, CDCl3) δ 8.31 (s, 4H). 13C NMR (126 MHz, CDCl3) δ 138.3, 133.7, 128.9. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C10H5Cl4N2O2 324.9100, found: 324.9095 (Figure S3).
2.2.3. 2-Bromo-3,3′,5,5′-Tetrachloro-4,4′-Bipyridine 15
3,3′,5,5′-Tetrachloro-[4,4′-bipyridine] 1-oxide 13 (6.37 mmol, 1.91 g) was dissolved in dibromoethane (15 mL) and triethylamine (12.73 mmol, 1.7 mL) was added. The mixture was cooled to −40 °C and oxalyl bromide (12.73 mmol, 1.8 mL) was slowly added while maintaining the temperature below −35 °C. After stirring 1h at −40 °C, methanol (1 mL) was added and temperature was raised to room temperature. A saturated solution of ammonium chloride (20 mL) was added and the mixture was extracted with CH2Cl2 (3 × 50 mL). The organic phase was dried over MgSO4, filtered and concentrated under vacuum. The crude compound was purified by chromatography on silica gel (pentane/CH2Cl2 7/3 to 2/1) to give pure 4,4′-bipyridine 15 (2.18 g, 94% yield). White solid, mp 100–102 °C. 1H NMR (500 MHz, CDCl3) δ 8.68 (s, 2H), 8.48 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 148.1, 147.2, 142.1, 140.7, 140.4, 132.9, 130.9, 130.6. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C10H4BrCl4N2 370.8306, found: 370.8302 (Figure S4).
2.2.4. 3,3′,5,5′-Tetrachloro-[4,4′-Bipyridine]-2-Carbonitrile 16
3,3′,5,5′-Tetrachloro-[4,4′-bipyridine] 1-oxide 13 (1 mmol, 310 mg) was dissolved in acetonitrile (2 mL) and triethylamine (0.5 mL). Trimethylsilylcyanide (3 mmol, 375 μL) was slowly added and the mixture was refluxed at 100 °C for 24 h. The temperature lowered to 0 °C, a solution of NaOH (5 M, 20 mL) was slowly added and the mixture was extracted with CH2Cl2 (3 × 30 mL). The organic phase was dried over MgSO4, filtered and concentrated under vacuum. The crude compound was purified by chromatography on silica gel (pentane/ethyl acetate 7/3) to give 4,4′-bipyridine 16 (267 mg, 83% yield). White solid, mp 99–101 °C. 1H NMR (500 MHz, CDCl3) δ 8.76 (s, 1H), 8.71 (s, 2H). 13C NMR (126 MHz, CDCl3) δ 149.1, 148.2, 141.9, 138.7, 135.40, 135.35, 132.3, 130.9. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C11H3Cl4N3 317.9154, found: 317.9151 (Figure S5).
2.2.5. 2-Bromo-3,3′,5,5′-Tetrachloro-[4,4′-Bipyridine] 1-Oxide 17
2-Bromo-3,3′,5,5′-tetrachloro-4,4′-bipyridine 15 (5.36 mmol, 2.0 g) was dissolved in CH2Cl2 (28 mL) at room temperature. m-CPBA (77% purity, 11 mmol, 2.47 g) was added and the mixture was stirred for 60 h. After dilution with CH2Cl2 (100 mL), the mixture was washed with NaOH 1M (2 × 80 mL) and with brine. The organic phase was dried over MgSO4, concentrated and purified by chromatography on silica gel (CH2Cl2/methanol 99/1) to give compound 17 (1.72 g, 83%). White solid, mp 212–214 °C. 1H NMR (500 MHz, CDCl3) δ 8.48 (s, 1H), 8.31 (s, 2H). 13C NMR (126 MHz, CDCl3) δ 147.2, 141.1, 140.8, 138.4, 133.6, 132.6, 131.3, 130.4. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C10H3BrCl4N2O 386.8256, found: 386.8265 (Figure S6).
2.2.6. 3,3′,5,5′-Tetrachloro-2′-Cyano-[4,4′-Bipyridine] 1-Oxide 18
Procedure 2.2.5. was used starting from 3,3′,5,5′-tetrachloro-[4,4′-bipyridine]-2-carbonitrile 16 (0.5 mmol, 160 mg) and m-CPBA (77% purity, 1 mmol, 224 mg) in CH2Cl2 (2.5 mL). After purification by chromatography on silica gel (CH2Cl2/methanol 98/2), compound 18 was obtained (158 mg, 94%). White solid, mp 193–195 °C. 1H NMR (500 MHz, CDCl3) δ 8.78 (s, 1H), 8.32 (s, 2H). 13C NMR (126 MHz, CDCl3) δ 149.2, 141.0, 138.5, 136.2, 136.1, 132.7, 132.3, 128.4, 113.9. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C11H3Cl4N3O 333.9103, found: 333.9091 (Figure S7).
2.2.7. 2-Bromo-2′,3,3′,5,5′-Pentachloro-4,4′-Bipyridine 19
Procedure 2.2.3. was used starting from 2-bromo-3,3′,5,5′-tetrachloro-[4,4′-bipyridine] 1-oxide 17 (0.179 mmol, 70 mg), oxalyl chloride (0.358 mmol, 31 μL), triethylamine (0.358 mmol, 50 μL) in CH2Cl2 (0.5 mL). After purification by chromatography on silica gel (pentane/ethyl acetate 97/3), compound 19 was obtained (51 mg, 70%). White solid, mp 119–121 °C. 1H NMR (500 MHz, CDCl3) δ 8.50 (s, 1H), 8.49 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 148.7, 147.3, 146.9, 142.9, 142.2, 140.8, 132.6, 130.3, 129.9, 129.8. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C10H2BrCl5N2 404.7917, found: 404.7921 (Figure S8).
2.2.8. 2′,3,3′,5,5′-Pentachloro-[4,4′-Bipyridine]-2-Carbonitrile 20
Procedure 2.2.3. was used starting from 3,3′,5,5′-tetrachloro-[4,4′-bipyridine]-2-carbonitrile 16 (0.179 mmol, 60 mg), oxalyl chloride (0.358 mmol, 31 μL), triethylamine (0.358 mmol, 50 μL) in CH2Cl2 (0.5 mL). After purification by chromatography on silica gel (pentane/ethyl acetate 95/5), compound 20 was obtained (45 mg, 71%). Colorless syrup. 1H NMR (500 MHz, CDCl3) δ 8.78 (s, 1H), 8.53 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 149.2, 149.0, 147.1, 142.1, 141.2, 135.1, 132.4, 129.9, 129.7, 113.9. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C11H2Cl5N3 351.8764, found: 351.8774 (Figure S9).
2.2.9. 3,3′,5,5′-Tetrachloro-2′-Iodo-[4,4′-Bipyridine] 1-Oxide 21
In a dry tube were placed 2-bromo-3,3′,5,5′-tetrachloro-[4,4′-bipyridine] 1-oxide 17 (4.11 mmol, 1.6 g), NaI (8.22 mmol, 1.23 g), CuI (0.411 mmol, 78 mg) and (rac)-trans-cyclohexane-1,2-diamine (0.822 mmol, 117 mg). The tube was evacuated and filled with argon before addition of degassed dioxane (15 mL). The tube was sealed with a screw cap and heated at 120 °C for 60 h. After cooling to room temperature, NH4OH (20 mL) and H2O (40 mL) were added then the product was extracted with dichloromethane (3 × 50 mL). After drying over MgSO4, filtration and concentration, the crude compound was purified by chromatography on silica gel (CH2Cl2/methanol 99/1) to give compound 21 (1.29 g, 72%). White solid, mp 244–246 °C. 1H NMR (500 MHz, CDCl3) δ 8.47 (s, 1H), 8.30 (s, 2H). 13C NMR (126 MHz, CDCl3) δ 147.8, 139.2, 138.3, 138.0, 132.6, 131.8, 130.7, 119.4. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C10H3Cl4IN2O 434.8117, found: 434.8109 (Figure S10).
2.2.10. 2,3,3′,5,5′-Pentachloro-2′-Iodo-4,4′-Bipyridine 22
Procedure 2.2.3. was used starting from 3,3′,5,5′-tetrachloro-2′-iodo-[4,4′-bipyridine] 1-oxide 21 (0.179 mmol, 78 mg), oxalyl chloride (0.358 mmol, 31 μL), triethylamine (0.358 mmol, 50 μL) in CH2Cl2 (0.5 mL). After purification by chromatography on silica gel (pentane/ethyl acetate 97/3), compound 22 was obtained (65 mg, 80%). White solid, mp 140–142 °C. 1H NMR (500 MHz, CDCl3) δ 8.48 (s, 1H), 8.49 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 148.7, 147.9, 146.9, 143.1, 140.4, 137.0, 130.8, 129.8, 129.7, 119.5. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C10H2Cl5IN2 452,7778, found: 452.7749 (Figure S11).
2.2.11. 2′-Bromo-3,3′,5,5′-Tetrachloro-[4,4′-Bipyridine]-2-Carbonitrile 23
Procedure 2.2.3. was used starting from 3,3′,5,5′-tetrachloro-2′-cyano-[4,4′-bipyridine] 1-oxide 18 (0.297 mmol, 100 mg), oxalyl bromide (0.595 mmol, 85 μL), triethylamine (0.595 mmol, 80 μL) in CH2Br2 (1 mL). After purification by chromatography on silica gel (pentane/ethyl acetate 95/5), compound 23 was obtained (107 mg, 90%). White solid, mp 131–133 °C. 1H NMR (500 MHz, CDCl3) δ 8.77 (s, 1H), 8.52 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 149.2, 147.4, 142.2, 141.0, 140.7, 135.06, 135.05, 132.5, 132.4, 130.2, 113.9. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C11H2BrCl4N3 395.8259, found: 395.8277 (Figure S12).
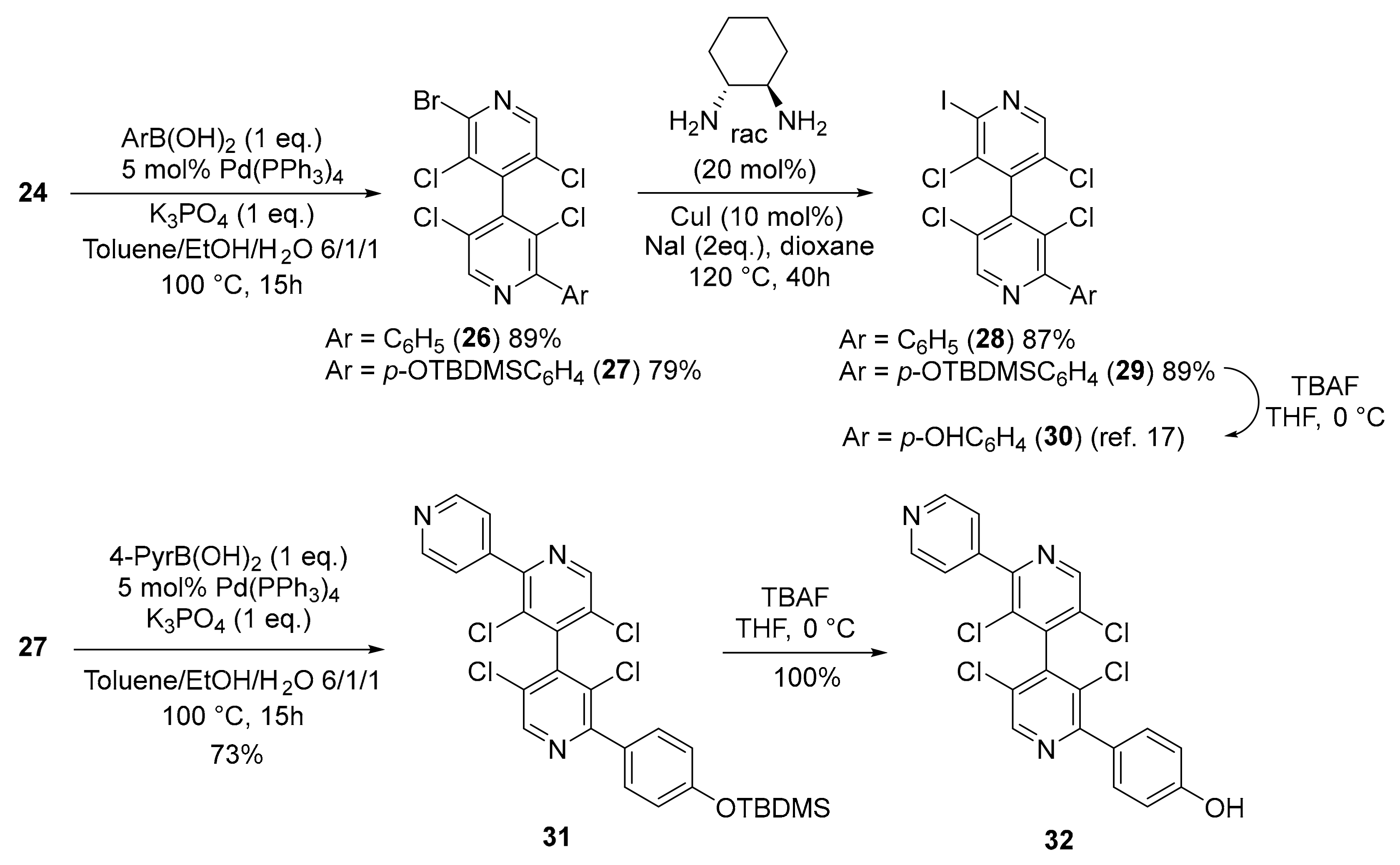
2.2.12. 2-Bromo-3,3′,5,5′-Tetrachloro-2′-Iodo-4,4′-Bipyridine 24
Procedure 2.2.3. was used starting from 3,3′,5,5′-tetrachloro-2′-iodo-[4,4′-bipyridine] 1-oxide 21 (1.74 mmol, 760 mg), oxalyl bromide (3.49 mmol, 496 μL), triethylamine (3.49 mmol, 463 μL) in CH2Br2 (4.2 mL). After purification by chromatography on silica gel (pentane/CH2Cl2 7/3), compound 24 was obtained (710 mg, 81%). White solid, mp 165–167 °C. 1H NMR (500 MHz, CDCl3) δ 8.414 (s, 1H), 8.410 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 147.9, 147.3, 142.6, 140.8, 140.5, 137.0, 132.5, 130.8, 130.2, 119.5. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C10H2BrCl4IN2 496.7273, found: 496.7242 (Figure S13).
2.2.13. 3,3′,5,5′-Tetrachloro-2′-Iodo-[4,4′-Bipyridine]-2-Carbonitrile 25
Procedure 2.2.4. was used starting from 3,3′,5,5′-tetrachloro-2′-iodo-[4,4′-bipyridine] 1-oxide 21 (0.395 mmol, 172 mg), acetonitrile (1.5 mL), triethylamine (0.4 mL) and trimethylsilylcyanide (1.18 mmol, 150 μL). After purification by chromatography on silica gel (pentane/ethyl acetate 95/5), compound 25 was obtained (110 mg, 62%). White solid, mp 144–146 °C. 1H NMR (500 MHz, CDCl3) δ 8.77 (s, 1H), 8.51 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 149.2, 148.0, 142.4, 138.9, 137.0, 135.1, 135.0, 132.4, 130.7, 119.7, 114.0. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C11H2Cl4IN3 443.8120, found: 443.8121 (Figure S14).
2.2.14. 2-Bromo-3,3′,5,5′-Tetrachloro-2′-Phenyl-4,4′-Bipyridine 26
In a dry tube were placed 2-bromo-3,3′,5,5′-tetrachloro-2′-iodo-4,4′-bipyridine 24 (0.3 mmol, 151.5 mg), Pd(PPh3)4 (0.015 mmol, 17.3 mg), phenylboronic acid (0.3 mmol, 36.6 mg) and K3PO4 (0.3 mmol, 64 mg). The tube was evacuated and filled with argon before addition of degassed mixture of toluene/water/ethanol (6:1:1, 4 mL). The tube was sealed with a screw cap and heated at 100 °C for 15 h. The cooled reaction mixture was diluted with water (10 mL) and extracted with CH2Cl2 (3 × 15 mL). After drying over MgSO4, filtration and concentration, the crude compound was purified by chromatography on silica gel (pentane/ethyl acetate 98/2) to give compound 26 (122 mg, 89%). Colorless syrup. 1H NMR (500 MHz, CDCl3) δ 8.76 (s, 1H), 8.49 (s, 1H), 7.78–7.25 (m, 2H), 7.52–7.46 (m, 3H). 13C NMR (126 MHz, CDCl3) δ 156.2, 147.5, 147.2, 143.1, 141.7, 140.7, 137.0, 132.8, 130.6, 129.6, 129.5, 129.1, 128.7, 128.4. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C16H7BrCl4N2 446.8619, found: 446.8625 (Figure S15).
2.2.15. 2-Bromo-2′-(4-((Tert-Butyldimethylsilyl)oxy)phenyl)-3,3′,5,5′-Tetrachloro-4,4′-Bipyridine 27
Procedure 2.2.14. was used starting from 2-bromo-3,3′,5,5′-tetrachloro-2′-iodo-4,4′-bipyridine 24 (0.3 mmol, 151.5 mg), Pd(PPh3)4 (0.015 mmol, 17.3 mg), 4-((tert-butyldimethylsilyl)oxy)phenyl boronic acid (0.3 mmol, 75.7 mg) and K3PO4 (0.3 mmol, 64 mg). After purification by chromatography on silica gel (pentane/ethyl acetate 98/2), compound 27 was obtained (137 mg, 79%). Colorless syrup. 1H NMR (500 MHz, CDCl3) δ 8.72 (s, 1H), 8.49 (s, 1H), 7.69 (d, J = 8.5 Hz, 2H), 6.94 (d, J = 8.5 Hz, 2H), 1.00 (s, 9H), 0.24 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 157.1, 155.8, 147.3, 147.2, 143.3, 141.6, 140.7, 132.9, 131.1, 130.7, 130.0, 128.5, 128.4, 120.0, 25.8, 18.4, −4.2. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C22H22BrCl4N2OSi 576.9433, found: 576.9428 (Figure S16).
2.2.16. 2″-(4-((Tert-Butyldimethylsilyl)oxy)phenyl)-3′,3″,5′,5″-Tetrachloro-4,2′:4′,4″-Terpyridine 31
Procedure 2.2.14. was used starting from 2-bromo-3,3′,5,5′-tetrachloro-2′-iodo-4,4′-bipyridine 24 (0.143 mmol, 82.5 mg), Pd(PPh3)4 (0.007 mmol, 8.3 mg), 4-pyridine boronic acid (0.215 mmol, 31 mg) and K3PO4 (0.215 mmol, 46 mg). After purification by chromatography on silica gel (pentane/ethyl acetate 7/3), compound 31 was obtained (60 mg, 73%). Colorless syrup. 1H NMR (500 MHz, CDCl3) δ 8.80 (s, 1H), 8.78 (d, J = 5.5 Hz, 2H), 8.74 (s, 1H), 8.76–8.66 (m, 4H), 6.95 (d, J = 8.5 Hz, 2H), 1.00 (s, 9H), 0.24 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 157.1, 155.9, 153.2, 150.1, 147.9, 147.3, 144.7, 142.9, 141.8, 131.09, 131.05, 130.1, 129.5, 128.7, 128.6, 124.0, 120.0, 25.8, 18.4, −4.2. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C27H26Cl4N3OSi 576.0594, found: 576.0589 (Figure S17).
2.2.17. 4-(3′,3″,5′,5″-Tetrachloro-[4,2′:4′,4″-Terpyridin]-2″-yl)phenol 32
2″-(4-((Tert-butyldimethylsilyl)oxy)phenyl)-3′,3″,5′,5″-tetrachloro-4,2′:4′,4″-terpyridine 31 (0.078 mmol, 45 mg) was dissolved in THF (2 mL) and the solution was cooled to 0 °C. A tetra-butylammonium fluoride (TBAF) solution (1M in THF, 0.117 mmol, 117 μL) was slowly added and the mixture was stirred at 0 °C for 30 min. After addition of a saturated solution of NH4Cl (1 mL), the temperature was raised to ambient. Water was added (5 mL) and the mixture was extracted with CH2Cl2 (3 × 10 mL). The organic phase was washed with brine, dried over MgSO4, filtered and concentrated. After filtration on silica gel (ethyl acetate), compound 32 was obtained (36 mg, 100%). White solid, mp 254–256 °C. 1H NMR (500 MHz, DMSO-d6) δ 9.998 (broad s, 1H), 9.11 (s, 1H), 8.99 (s, 1H), 8.77 (d, J = 6.0 Hz, 2H), 7.73 (d, J = 6.0 Hz, 2H), 7.62 (d, J = 9.0 Hz, 2H), 6.90 (d, J = 9.0 Hz, 2H). 13C NMR (126 MHz, DMSO-d6) δ 158.8, 155.4, 153.1, 150.0, 148.2, 147.5, 143.9, 142.0, 141.1, 131.1, 130.2, 128.4, 127.6, 127.4, 127.2, 115.1. HRMS (ESI-TOF) [M + H]+ m/z: Calcd. for C21H12Cl4N3O 461.9729, found: 461.9701 (Figure S18).
2.3. Single Crystal X-ray Diffraction
2.3.1. Crystallizations and Analysis
Suitable crystals of compounds 13, 18 and 24 were obtained by slow evaporation of a dichloromethane/hexane (1:1) solution. The solid-state structures were determined by single crystal X-ray diffraction at low temperature (T = 100 K).
2.3.2. Crystal Data
CCDC 2077648, 2077649 and 2077650 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 14 April 2021) (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
Crystal Data for 24 C10H2BrCl4IN2 (M = 498.75 g/mol): Monoclinic, space group P21/c (no. 14), a = 7.9297(2) Å, b = 15.6388(4) Å, c = 11.4938(3) Å, β =105.632(2)°, V = 1372.64(6) Å3, Z = 4, T = 100(2) K, μ(MoKα) = 6.002 mm−1, Dcalc = 2.413 g/cm3, 51767 reflections measured (2.254° ≤ Θ ≤ 36.150°), 6552 unique (Rint = 0.0298), which were used in all calculations. The final R1 was 0.0239 (I > 2σ(I)) and wR2 was 0.0548 (all data). CCDC 2077649.
Crystal Data for 18 C11H3Cl4N3 (M = 334.96 g/mol): Monoclinic, space group P21/c (no. 14), a = 7.2662(2) Å, b = 12.0325(4) Å, c = 14.4319(5) Å, β = 93.491(3)°, V = 1259.45(7) Å3, Z = 4, T = 100(2) K, μ(MoKα) = 0.931 mm−1, Dcalc = 1.767 g/cm3, 48102 reflections measured (2.205° ≤ Θ ≤ 33.667°), 5000 unique (Rint = 0.0424), which were used in all calculations. The final R1 was 0.0323 (I > 2σ(I)) and wR2 was 0.0732 (all data). CCDC 2077650.
Crystal Data for 13 C10H4Cl4N2 (M = 309.95 g/mol): Monoclinic, space group P21/c (no. 14), a = 7.2697(2) Å, b = 11.5462(3) Å, c = 14.0808(4) Å, β = 97.804(3)°, V = 1170.96(6) Å3, Z = 4, T = 100(2) K, μ(MoKα) = 0.991 mm−1, Dcalc = 1.758 g/cm3, 48887 reflections measured (2.290° ≤ Θ ≤ 37.162°), 6021 unique (Rint = 0.0444), which were used in all calculations. The final R1 was 0.0384 (I > 2σ(I)) and wR2 was 0.0840 (all data). CCDC 2077648.
2.4. Isolated Molecule Calculations
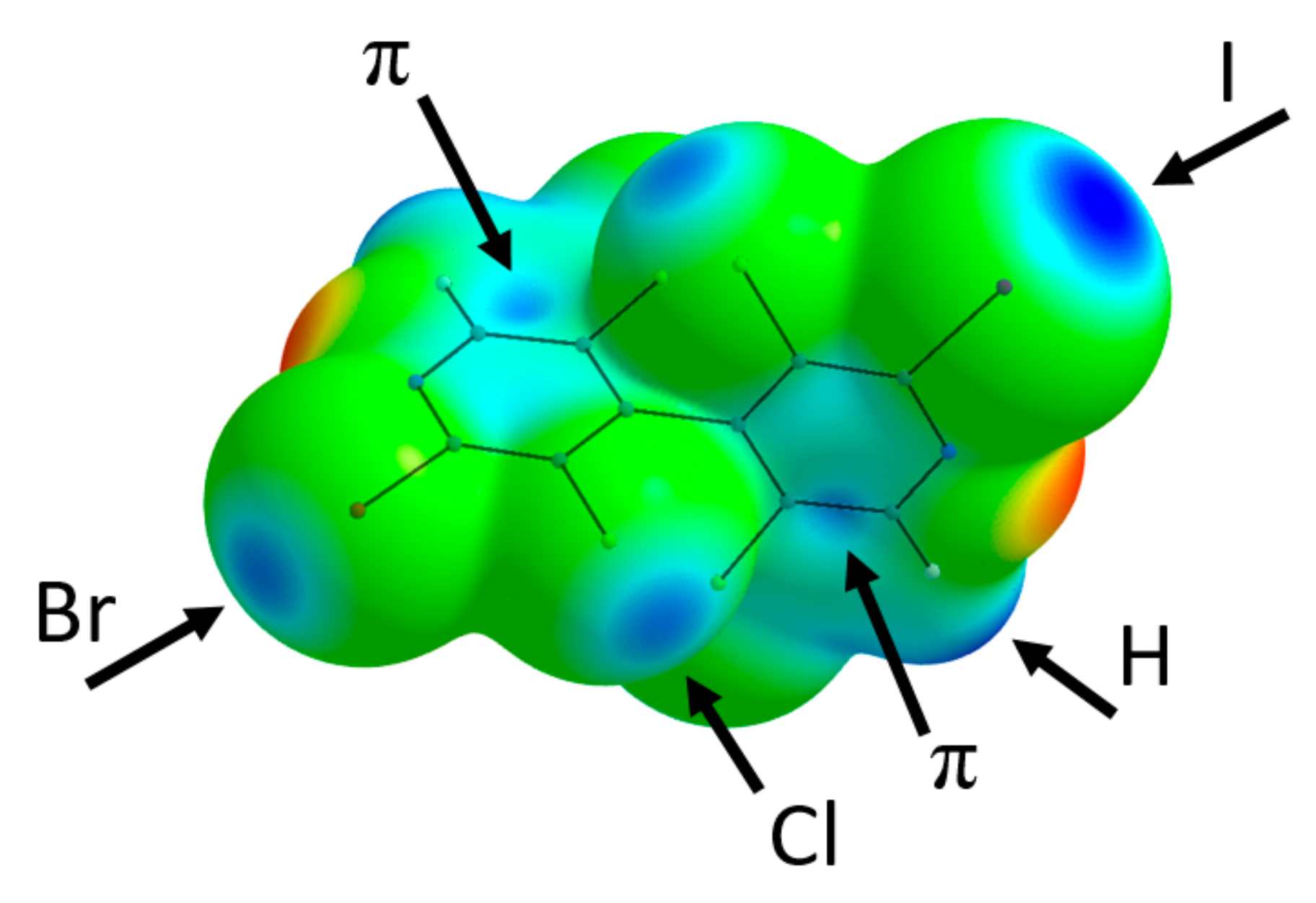
Molecular structures of 24, 18 and 13 were optimized with Gaussian09 software at DFT level of theory using the B3LYP functional completed with D3 dispersion correction and the Def2TZVPP basis set. Frequency calculations were performed in order to check that true energy minimum were obtained. Electrostatic maps (Figure 2, Figures S31 and S32) were drawn using the AIMAll software, and locations of ESP extrema VS,max were searched using MultiWFN program (Table S4). Integrated atomic charges (AIMAll) are depicted on Figures S33–S35.
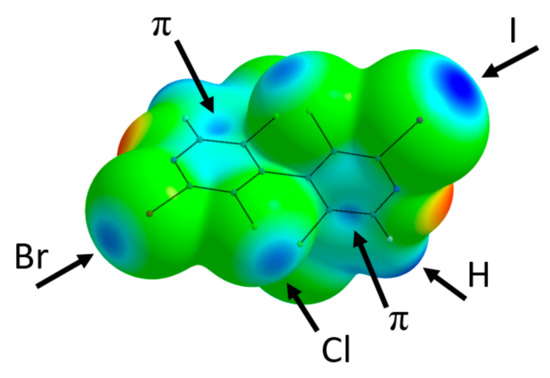
Figure 2.
Electrostatic potential mapped on the ρ = 0.002 a.u. electron density isosurface of 24. Coloring from red = −0.05 a.u. to blue = +0.05 a.u. Black arrows point to some representative electropositive σ- and π-holes.
3. Results
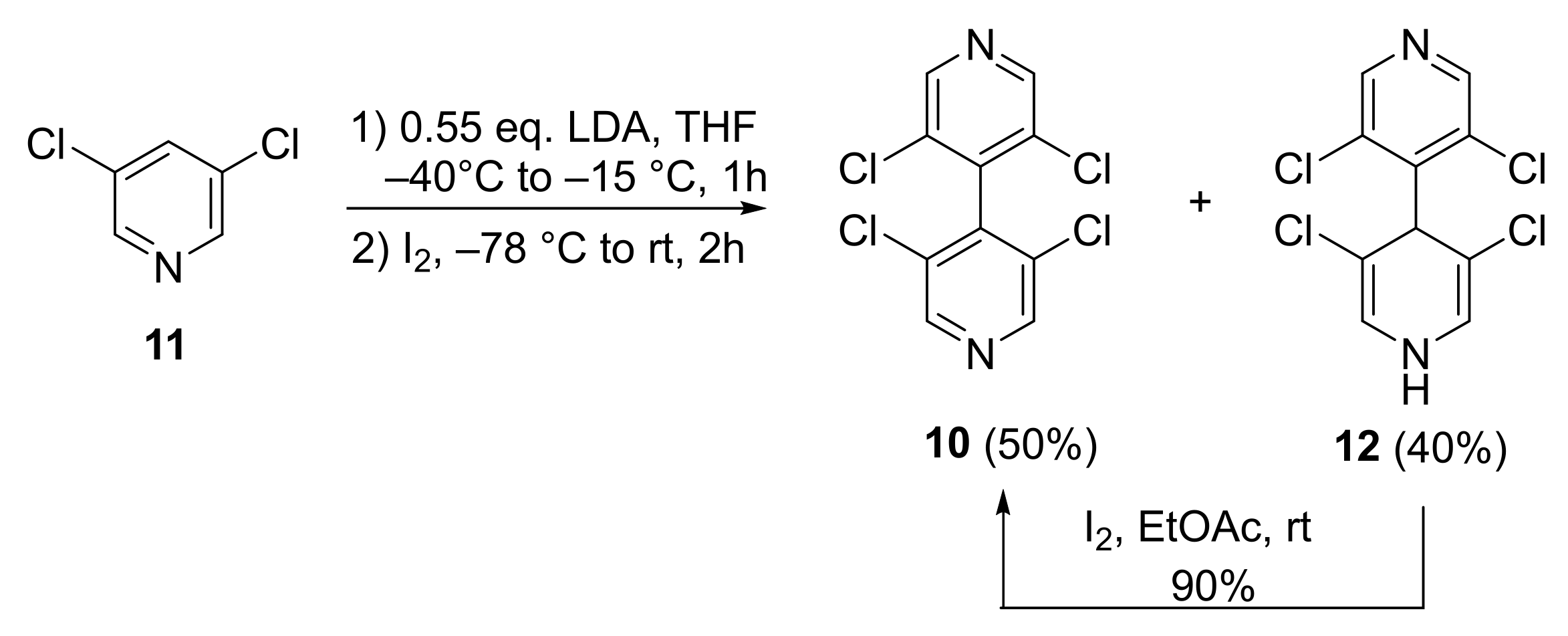
3.1. Improved Synthesis of 3,3′,5,5′-Tetrachloro-4,4′-Bipyridine 10
The starting 4,4′-bipyridine 10 was already prepared by us with 44% yield by dimerization of 3,5-dichlopyridine 11 [22]. When the synthesis was reproduced on large scale (50 mmol), the yield was improved to 50%. Moreover, during the chromatographic purification, the more polar non-rearomatized compound 12 was also isolated with 40% yield. Compound 12 could be oxidized with I2 in ethyl acetate at room temperature for 12 h, furnishing 10 with 90% yield. Overall, the yield of 10 in the dimerization process could be increased to 82% yield (Scheme 1).
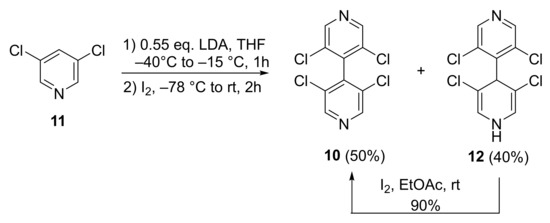
Scheme 1.
Improved synthesis of 3,3′,5,5′-tetrachloro-4,4′-bipyridine 10.
3.2. Desymmetrization of 3,3′,5,5′-Tetrachloro-4,4′-Bipyridine 10
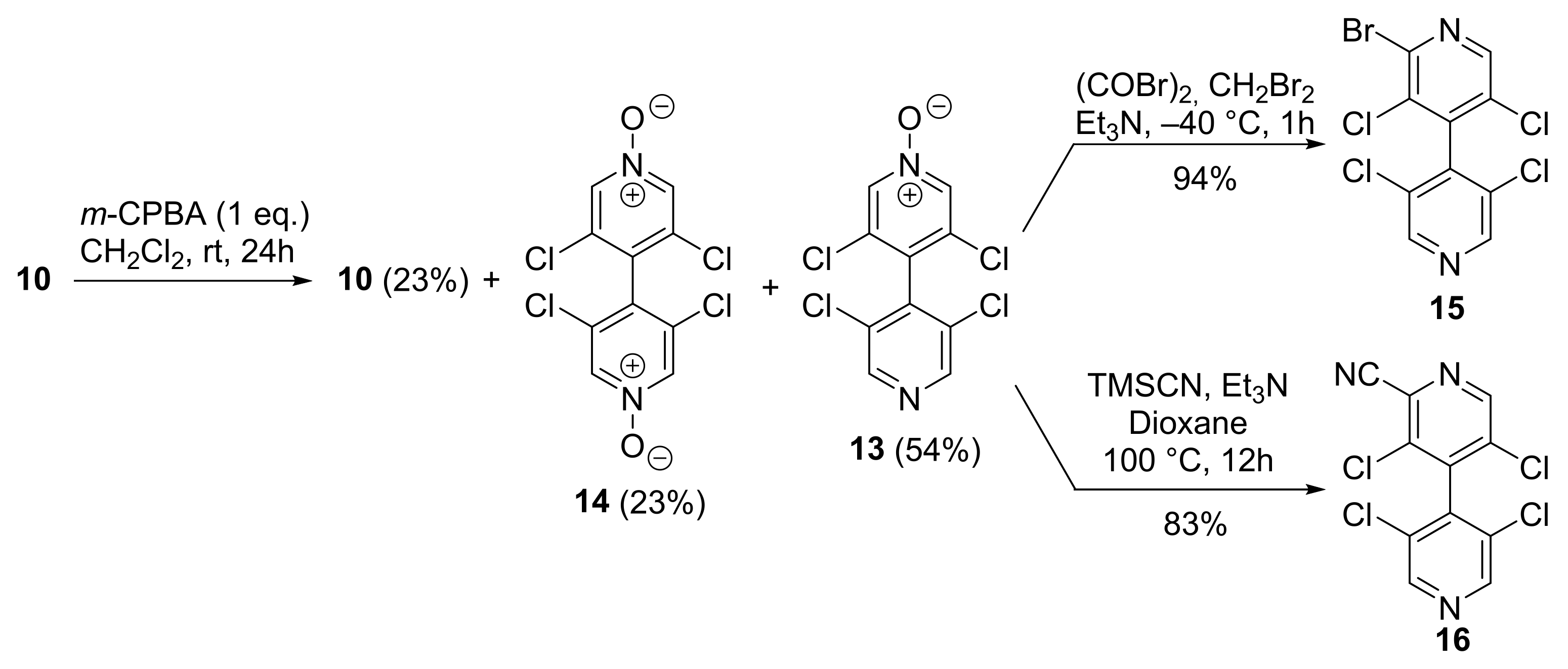
The mono N-oxidation of 4,4′-bipyridine 10 was performed using 1 equivalent of m-CPBA in CH2Cl2 (0.2 M) at room temperature. After treatment using NaOH 1M to remove the benzoic acid, the crude mixture was purified by chromatography on silica gel to give the expected mono-oxidized 4,4′-bipyridine 13 with 54% yield. Unreacted 4,4′-bipyridine 10 (23%) as well as doubly oxidized 4,4′-bipyridine 14 (23%) were also obtained after the purification. Based on the recovery of the starting 4,4′-bipyridine 10, the yield of 14 was of 75%. The N-oxide 13 could be further transformed to the pentahalogenated 4,4′-bipyridine 15 in very good yields by using oxalyl bromide with Et3N in CH2Br2 at low temperature [23], and to the cyano derivative 16 by using TMSCN/Et3N in refluxing dioxane [24] (Scheme 2).
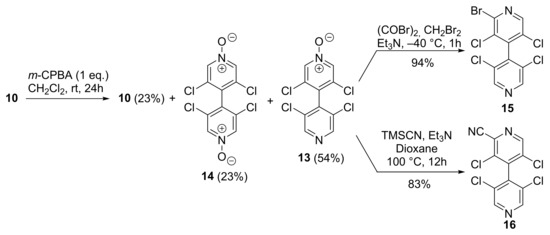
Scheme 2.
Mono-oxidation of 4,4′-bipyridine 10 and its functionalization.
3.3. New Chiral Non-Symmetrical 4,4′-Bipyridines Based on the 3,3′,5,5′-Tetrachloro-4,4′-Bipyridine Core
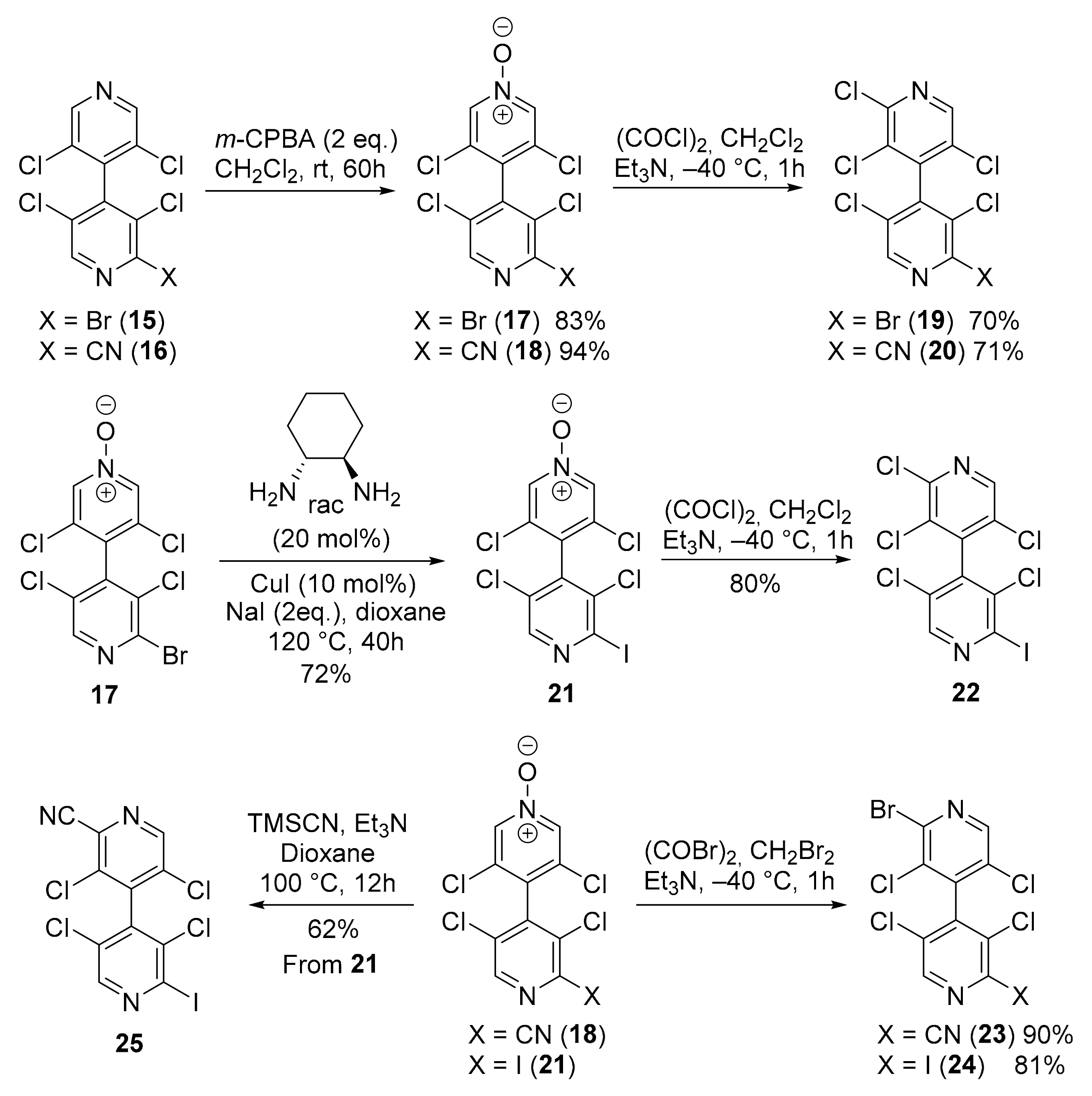
4,4′-Bipyridines 15 and 16 were submitted to a new sequence of N-oxidation/halogenation in order to access to novel chiral non-symmetrical 4,4′-bipyridines. Thus, regioselective N-oxidation of the less substituted ring of 15 and 16 furnished, respectively, 4,4′-bipyridine N-oxides 17 and 18 in high yields. Chlorination of N-oxides 17 and 18 using oxalyl chloride with Et3N in CH2Cl2 at low temperature [23] afforded 4,4′-bipyridines 19 and 20 in good yields. Alternatively, N-oxide 17 was transformed by a copper-catalyzed Finkelstein reaction [25] to the iodinated 4,4′-bipyridine N-oxide 21 which was further submitted to the chlorination to provide 4,4′-bipyridine 22. N-oxides 18 and 21 were also brominated as described in Scheme 2 to give two other chiral non-symmetrical 4,4′-bipyridines 23 and 24 in good yields (Scheme 3). Finally, 4,4′-bipyridine 21 was transformed in good yield to the iodinated 4,4′-bipyridine 25 by using TMSCN/Et3N in refluxing dioxane.
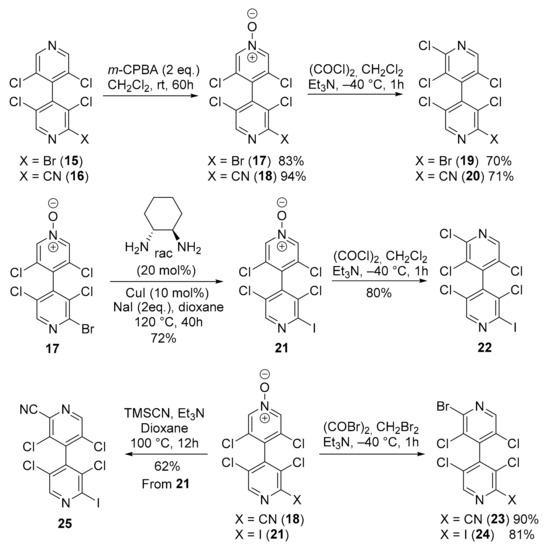
Scheme 3.
Synthesis of six new chiral non-symmetrical 4,4′-bipyridines.
3.4. Cross-Coupling Reactions with 4,4′-Bipyridine 24
4,4′-Bipyridine 24 bearing two different halogens (Br and I) in 2 and 2′-positions was chosen to perform selective cross-coupling reactions. In particular, 4,4′-bipyridines 28 and 30, which were recently described as good transthyretin fibrillogenesis inhibitors [17], could be obtained in excellent yields and with very high purity by successive Suzuki and Finkelstein reactions, followed by silyl group deprotection in the case of 30. Moreover, their enantiomers could be separated by HPLC on chiral stationary phase [26,27]. High selectivity for mono-coupling products 26 and 27 was obtained by using the Suzuki coupling [28] with one equivalent of arylboronic acid, letting the C–Br bond untouched for a further coupling reaction such as the copper-catalyzed Finkelstein Br/I exchange [18]. Moreover, a new Suzuki cross-coupling of 4,4′-bipyridine 27 with 4-pyridylboronic acid delivered compound 31, which, after silyl group deprotection, furnished the chiral 4,4′-bipyridine 32, bearing two different functional groups (Scheme 4).
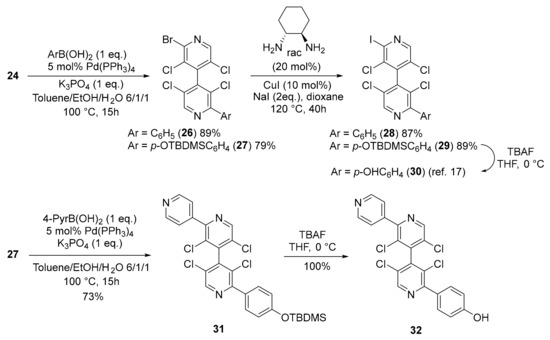
Scheme 4.
Cross-coupling reactions with 4,4′-bipyridine 24.
3.5. X-ray Diffraction Analysis
One non-symmetrical 4,4′-bipyridine (24) and two 4,4′-bipyridine N-oxydes (13 and 18) solid state structures were determined by single X-ray diffraction.
2-Bromo-3,3′,5,5′-tetrachloro-2′-iodo-4,4′-bipyridine 24 crystallizes in P21/c space group with one molecule in the asymmetric unit. A positional disorder (corresponding to two different orientations of the molecule) exchanges the Br and I halogen atom positions, the major component having a population of 0.7441(13). According to the energy packing analysis performed with CrystalExplorer software [29], the solid-state structure of 24 is primarily determined by the formation of intermolecular interactions with a predominance of stabilizing dispersion over electrostatic contributions (Table S1, entries 1–6). These contributions involve first the formation of strongly bound dimers centered on an inversion center where π⋯π stacking occurs (N7-pyridine interplane distance of 3.272 Å) (Table S1, entry 1 and Figure S19), with a complementary electrostatic interaction between the electropositive C12-H12 group (integrated atomic charges of Q(C12) = +0.59 and Q(H12) = +0.06) and the electronegative crown of Br1 (Q = −0.02), as evidenced from the electrostatic potential mapped on the isodensity molecular surface (Figure 2). In this dimer, the electronegative region about N7 is also near the electropositive area of Cl1, although the σ-hole of the halogen is not optimally oriented (C3-Cl1⋯N7 = 127.59°). The geometry of the next two intermolecular interactions (x, −y + 3/2, z − 1/2 and 1 + x, −y + 3/2, z + 1/2, Table S1, entries 2–3) can also be understood from electrostatic point of view, since electronegative regions (respectively Cl3 with Q(Cl3) = −0.17 and N1 with Q(N1) = −1.12) are close to electropositive area (respectively C6 with Q(C6) = +0.59 and C12 with Q(C12) = +0.59) (Figures S20 and S21).
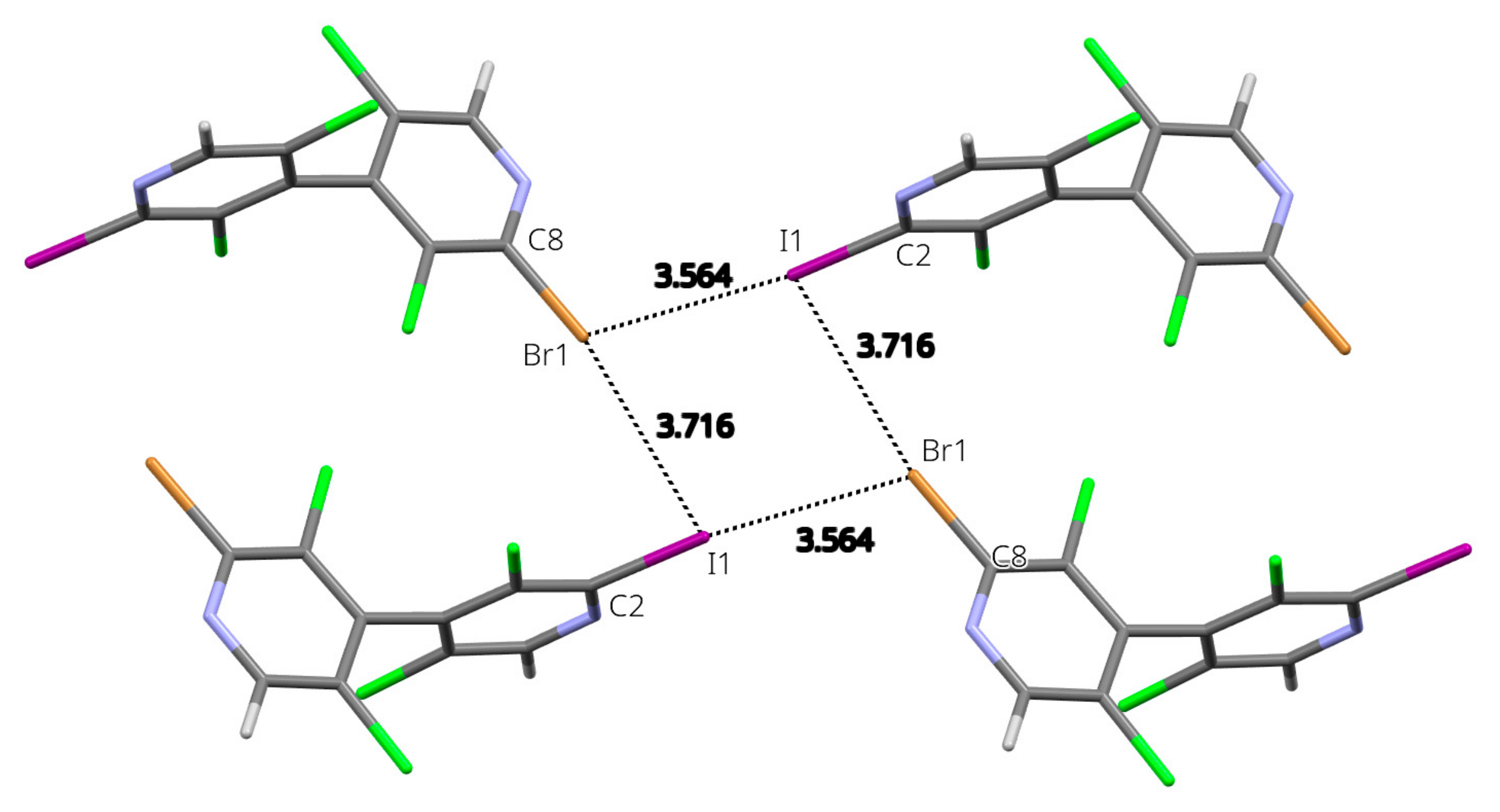
The most remarkable feature of the molecular packing is the formation of a four-membered ring involving four I/Br atoms engaged in type-II halogen⋯halogen bonds (Figure 3). Although the I/Br positional disorder can induce bias in the modeled interatomic distances, these latter show a clear interpenetration of the van der Waals spheres and an interaction geometry typical of type-II hal⋯hal bonds [30,31] (I1⋯Br1 = 3.564(2) Å RR = 0.93; C2-I1⋯Br1 = 170.22(7)°; I1⋯Br1-C8 = 114.6(1)°; Br1⋯I1 = 3.716(3) Å RR = 0.97; C8- Br1⋯I1 = 170.4(1)°; Br1⋯I1-C2 = 81.45(6)°)). Noticeably, the shortest type-II hal⋯hal bond is obtained when the σ-hole of the I atom points toward the crown of the Br atom; indeed, the σ-hole is more electropositive for iodine than for bromine (Figure 2 and Table S4), whereas the electronegative crown is more pronounced for the latter. From the intermolecular energy decomposition (Table S1), these interactions appear with predominant electrostatic contribution (entry 7: −1 + x, y, −1 + z), but also with major dispersion contribution along with significant electrostatic contribution (entry 4: 1 − x, 1 − y, 1 − z). This is due to the fact that in the former dimer the molecules are well separated (interacting only through the halogen atoms), whereas in the latter a close proximity of polarizable halogen atoms and aromatic rings is observed.
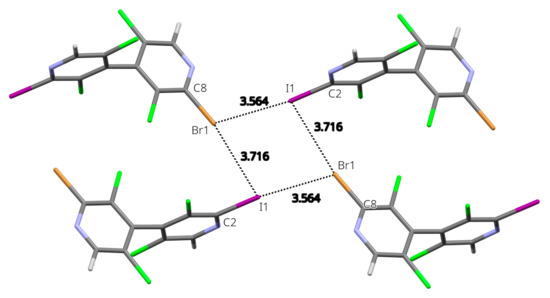
Figure 3.
Four-membered ring involving four I/Br atoms (x, y, z; −1 + x, y, −1 + z; −x, 1 − y, −z; 1 − x, 1 − y, 1 − z) engaged in type-II halogen⋯halogen bonds in the crystal structure of 24. Representative distances are given in Å. Only the major component disorder is shown.
In order to rationalize the observed positional disorder that involves the iodine and bromine atoms, DFT calculations were undertaken on the isolated tetramer centered about the type-II hal⋯hal bond’s four-membered ring (Figure S36). From the results (Table S5), it appears that the most stable configuration is obtained for the observed largest component disorder (labelled IBrIBr), where the halogen atoms alternate in the sequence Hal1=I, Hal2=Br, Hal3=I, Hal4=Br, as depicted on Figure 3. However, the other configurations are not excessively less stabilizing, with the most unfavorable case (IIII) being only 8.92 kJ/mol higher in energy. Boltzmann populations calculated, taking into account all the possible configurations, finally lead to an equivalent average disorder population of 0.675, in remarkable qualitative agreement with the refined population parameter (0.7441(13)). This may indicate that the observed dissymmetry in the disorder population (i.e., different from 50/50) results from the preferential interaction of the σ-hole of iodine atom toward the crown of bromine at the short distance, at the expense of the reverse situation (interaction of bromine σ-hole toward the crown of iodine).
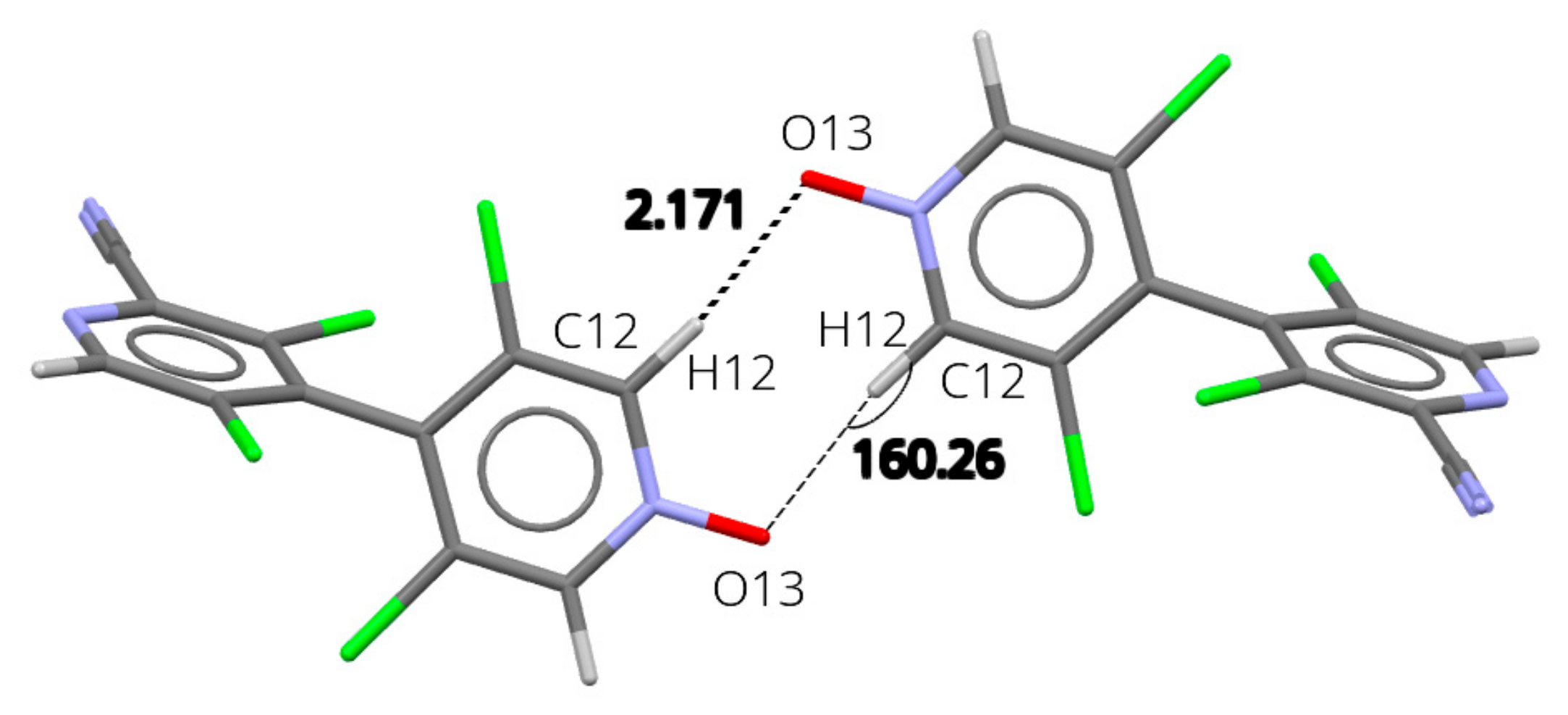
N-oxides 13 and 18 are isostructural, crystallizing in P21/c space group with similar unit cell parameters. Indeed, they present similar crystal packing, with notable differences only about the substituent in 2 position (-H in 13, -CN in 18) (Figures S22 and S23). The analysis of intermolecular interaction energies (Tables S2 and S3) shows that in both structures the main interaction (entry 1) corresponds to the formation of a strong cyclic R2,2(8) hydrogen bond motif about an inversion center, involving the oxygen atom of the N-O as the acceptor (Figure 4; 18: C12-H12⋯O13 = 2.17 Å; 160°; 13: 2.07 Å; 162°).
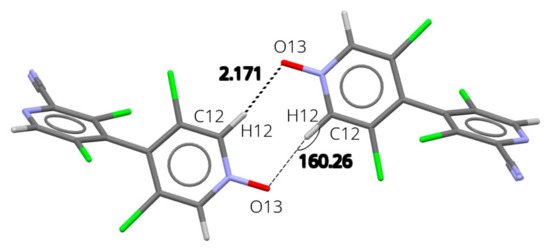
Figure 4.
Packing in the solid-state structure of 18. Interaction between x, y, z, and −1 + x, 1−y, −z molecules. Representative distance is given in Å and angle in °.
The main differences between the two structures concern the next two most intense interactions involving 2 − x, 1 − y,1 − z and 1 − x, 1 − y,1 − z molecules. The first dimer is strongly stabilizing in 18, with the cyano groups of the two neighboring molecules arranged in a head-to-tail manner (Figure S24), associated with a large electrostatic component (Table S2, entry 2); on the contrary, in 13 the corresponding molecules does not present any contact below van der Waals limit and dispersion is the main stabilizing contribution to the interaction (Table S3, entry 2 and Figure S25). The second dimer (with 1 − x, 1 − y,1 − z, Tables S2 and S3, entry 3) is twice more stabilizing in 18 than in 13 and involves a contact at van der Waals limit between the Cl1 and C14 atoms of the cyano group, the crown of the halogen atom pointing in the direction of the positively charged carbon atom (C3-Cl1⋯C14 = 3.442 Å, 110.04°; Q(C14) = 0.94) (Figure S26); in such a way, both electrostatic and dispersion components are more stabilizing in 18, while in 13 a C3-Cl1⋯Cl1 contact at van der Waals limit (3.490 Å, 78.83°) implies the crown of both halogen atoms in a unfavorable disposition (Figure S27).
The next interaction (Tables S2 and S3, entry 5; x, −y + 3/2, z − 1/2) evidences also some differences between the structures, although to a lesser extent. In both structures this dimer displays a halogen bond between the σ-hole of Cl3 and the O13 oxygen atom of the N-oxide group (18: C9-Cl3⋯O13 = 3.152 Å RR = 0.96, 156.54°; 13: 3.021 Å, RR = 0.92, 157.94°; Figures S28 and S29). However, in 18 a second halogen bond participates in the stabilization, the σ-hole of Cl2 being properly oriented toward the nitrogen atom of the cyano group (C5-Cl2⋯N15 = 3.128 Å, RR = 0.95, 156.31°).
The last significant interaction implies 1 − x, y + 1/2, −z + 1/2 molecule (Tables S2 and S3, entry 6) and is slightly more stabilizing for 13 than for 18 due to a more negative electrostatic contribution. In this latter Cl2 atom presents three contacts at distances smaller than van der Waals limits with this neighbor, namely with N7 (3.179 Å, RR = 0.96), C8 (3.380 Å, RR = 0.98) and C12 (3.426 Å, RR = 0.99), but with an orientation that does not involves the halogen σ-hole (C5-Cl2⋯N7 = 109.10°) (Figure S30). The electrostatic stabilization may then result from the head-to-tail relative orientation of the two molecules which bear a dipole moment parallel to their long N-N axis in the case of 13 (1.87 D), whereas in 18 the molecular dipole moment (3.42 D) is almost parallel to the –CN group. In this situation, the neighboring molecules have almost orthogonal dipole moments and thus a reduced overall electrostatic stabilization.
4. Discussion
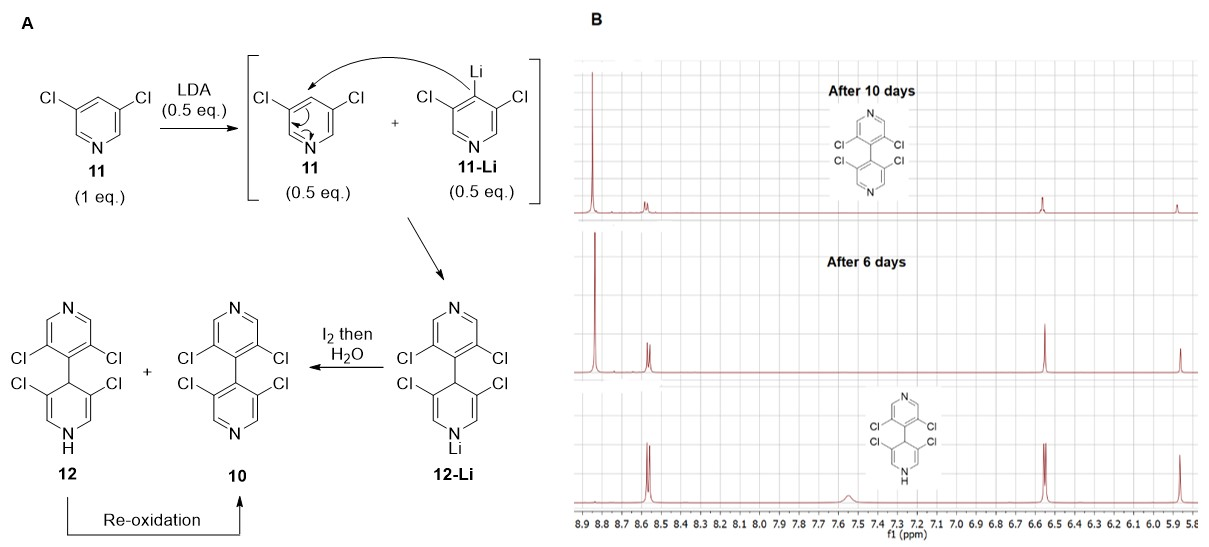
The synthesis of 3,3′,5,5′-tetrachloro-4,4′-bipyridine 10 [22] was greatly improved by adjusting the amount of LDA to 0.55 equivalent with regard to 3,5-dichloropyridine 11. Moreover, a large quantity (44%) of dihydropyridine 12 was also isolated during the reaction, confirming the proposed mechanism for the dimerization process [22]. Indeed, after deprotonation of half equivalent of 11, the nucleophilic lithiated 11-Li added in 4-position of the remaining half equivalent of 11 to give 12-Li, which after oxidation with iodine and hydrolysis delivered 10 and non-oxidized 12 (Figure 5A). After purification, dihydropyridine 12 was very stable in the solid state with no noticeable oxidation after three months on the bench at ambient temperature. In acetone solution, a slow oxidation occurred with almost complete formation of 4,4′-bipyridine 10 after 10 days, as shown by 1H NMR (Figure 5B).
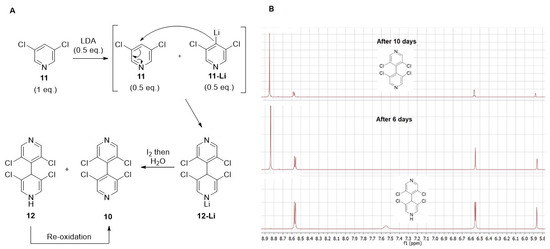
Figure 5.
(A) Proposed mechanism for the formation of 4,4′-bipyrine 10; (B) 1H NMR spectra showing oxidation of dihydropyridine 12 in acetone over time.
The desymmetrization route of 4,4′-bipyridines used in this study is based on the N-oxidation of one of the two pyridine nitrogens in the presence of one equivalent of m-CPBA. N-oxidation of electron-deficient pyridines generally requires harsher conditions that necessitate prior N-activation with trifluoroacetic acid anhydride [32,33]. As expected, this first N-oxidation is not selective; however, the chemical nature of the three compounds of the reaction mixture (starting material with free nitrogens, mono N-oxide and bis N-oxide) allowed for simple purification with easy recovery of unreacted starting material. The N-oxidation of the electron-deficient pentasubstituted 4,4′-bipyridines 15 and 16 was highly selective with no formation of the bis N-oxide. The high selectivity of the reaction is due to both electronic and steric factors. Indeed, the trisubstituted pyridine ring is electronically impoverished by the three electron-withdrawing groups (Cl, Cl and Br or CN) and the N-pyridine is sterically hindered by the substituent in 2-position (Br or CN).
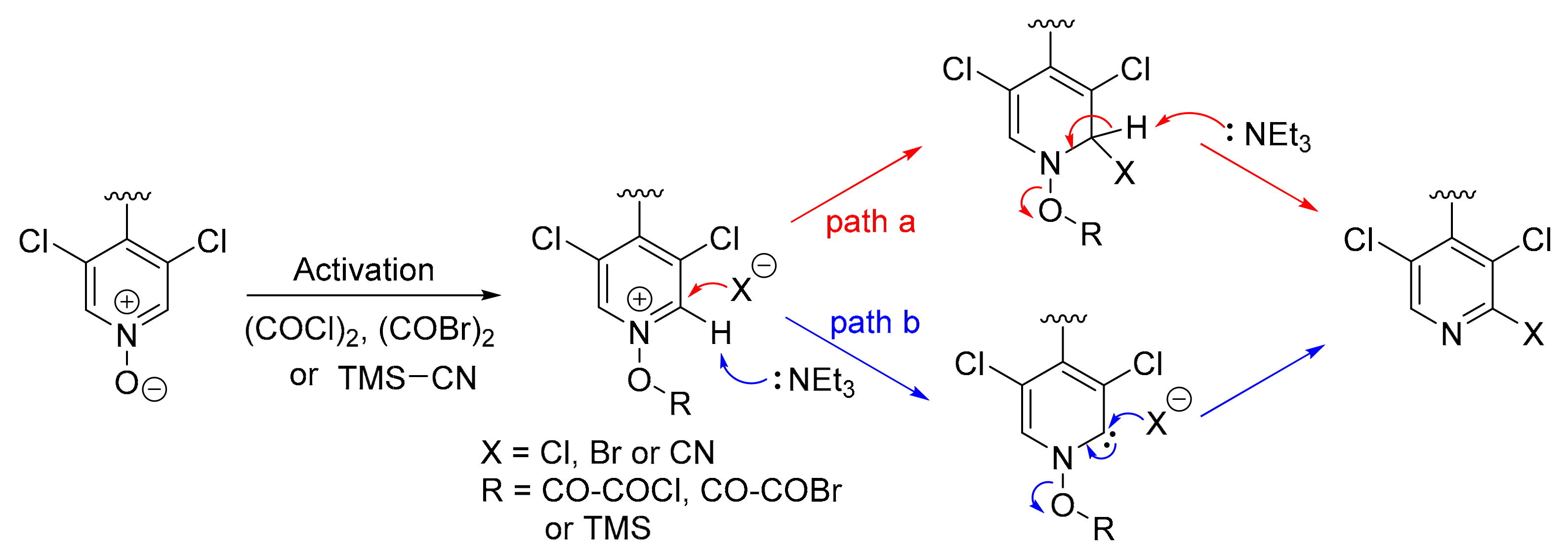
The choice of the pyridine N-oxides in our synthesis was also guided by the numerous known methodologies for their functionalization [21]. Halogenation and cyanation of 4,4′-bipyridine N-oxides used in this work are examples of such reactions which efficiently provided 4,4′-bipyridines functionalized in 2-position with Cl, Br and CN. A general mechanism can be proposed for these transformations (Figure 6). After activation of the N-oxide by (COCl)2, (COBr)2 or TMSCN, the intermediate may evolve following two different paths. In path a, the generated nucleophile during the first step (X− = Cl−, Br− or CN−) attacks the carbon in 2-position followed by base-assisted elimination of RO− to give the functionalized pyridine. In path b, the deprotonation of the acidic hydrogen in 2-position first occurs to generate a carbene intermediate which is trapped by the nucleophile with concomitant elimination of RO−.
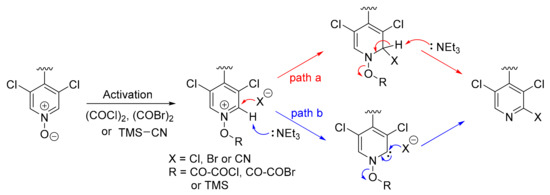
Figure 6.
Proposed mechanism for the functionalization of pyridine N-oxides.
The installed functions in 2,2′-positions represent a potential entry to new functional groups by nucleophilic substitution of the chlorine, hydrolysis of the cyano group and cross-coupling reaction with the C-Br bond. In the latter case, we have used the copper-catalyzed Finkelstein reaction to exchange bromine by iodine, and the Suzuki reaction to introduce aryl groups in 2-positions. In this regard, 4,4′-bipyridine 24 possessing both bromine and iodine in 2,2′-positions is a compound of choice in order to selectively introduce two different aryl groups on the 3,3′,5,5′-tetrachloro-4,4′-bipyridine scaffold. This was highlighted by the synthesis of 4,4′-bipyridine 32 whose supramolecular arrangement in solution and in the solid state is currently under investigation in our laboratory.
5. Conclusions
A new methodology for the synthesis of chiral non-symmetrical 4,4′-bipyridines was developed. It is based on the successive functionalization of 2- and 2′-positions of 4,4′-bipyridines by using the sequence N-oxidation then halogenation or cyanation. Starting from 3,3′,5,5′-tetrachloro-4,4′-bipyridine, all the six possible non-symmetrical isomers bearing Cl, Br, I or CN in 2- and 2′-positions were efficiently prepared. The isomer bearing bromine and iodine was used as the key component for the improved synthesis of biologically active 2-iodinated 4,4′-bipyridines and for the preparation of 4,4′-bipyridines with two different aryl groups in 2- and 2′-positions. This work opens new directions toward the synthesis of non-symmetrical ligands for the design of supramolecular materials, such as coordination polymers and metal-organic frameworks (MOFs).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/compounds1020006/s1, Figure S1: 3,3′,5,5′-Tetrachloro-1,4-dihydro-4,4′-bipyridine (12), Figure S2: 3,3′,5,5′-Tetrachloro-[4,4′-bipyridine] 1-oxide (13), Figure S3: 3,3′,5,5′-Tetrachloro-[4,4′-bipyridine] 1,1′-dioxide (14), Figure S4: 2-Bromo-3,3′,5,5′-tetrachloro-4,4′-bipyridine (15), Figure S5: 3,3′,5,5′-Tetrachloro-[4,4′-bipyridine]-2-carbonitrile (16), Figure S6: 2-Bromo-3,3′,5,5′-tetrachloro-[4,4′-bipyridine] 1-oxide (17), Figure S7: 3,3′,5,5′-Tetrachloro-2′-cyano-[4,4′-bipyridine] 1-oxide (18), Figure S8: 2-Bromo-2′,3,3′,5,5′-pentachloro-4,4′-bipyridine (19), Figure S9: 2′,3,3′,5,5′-Pentachloro-[4,4′-bipyridine]-2-carbonitrile (20), Figure S10: 3,3′,5,5′-Tetrachloro-2′-iodo-[4,4′-bipyridine] 1-oxide (21), Figure S11: 2,3,3′,5,5′-Pentachloro-2′-iodo-4,4′-bipyridine (22), Figure S12: 2′-Bromo-3,3′,5,5′-tetrachloro-[4,4′-bipyridine]-2-carbonitrile (23), Figure S13: 2-Bromo-3,3′,5,5′-tetrachloro-2′-iodo-4,4′-bipyridine (24), Figure S14: 3,3′,5,5′-Tetrachloro-2′-iodo-[4,4′-bipyridine]-2-carbonitrile (25), Figure S15: 2-Bromo-3,3′,5,5′-tetrachloro-2′-phenyl-4,4′-bipyridine (26), Figure S16: 2-Bromo-2′-(4-((tert-butyldimethylsilyl)oxy)phenyl)-3,3′,5,5′-tetrachloro-4,4′-bipyridine (27), Figure S17: 2″-(4-((Tert-butyldimethylsilyl)oxy)phenyl)-3′,3″,5′,5″-tetrachloro-4,2′:4′,4″-terpyridine (31), Figure S18: 4-(3′,3″,5′,5″-Tetrachloro-[4,2′:4′,4″-terpyridin]-2″-yl)phenol (32), Figure S19: Packing in the solid-state structure of 24. Interaction between x, y, z, and 2 − x, 1 − y, 1 − z molecules. Representative distance is given in Å. Only the major component disorder is shown, Figure S20: Packing in the solid-state structure of 24. Interaction between x, y, z, and x, −y + 3/2, z − 1/2 molecules. Representative distance is given in Å. Only the major component disorder is shown, Figure S21: Packing in the solid-state structure of 24. Interaction between x, y, z, and 1 + x, −y + 3/2, z + 1/2 molecules. Representative distance is given in Å. Only the major component disorder is shown, Figure S22: Superimposition of the molecular environment about a central molecule (displayed as ball and sticks) showing the isostructural relationship between 18 (colored as atom type) and 13 (light gray), Figure S23: Focus on the region centered on –CN group in 18, showing the largest differences between 18 (colored as atom type) and 13 (light gray) crystal structures, Figure S24: Packing in the solid-state structure of 18. Interaction between x, y, z, and 2 − x, 1 − y, 1 − z molecules. Representative distances are given in Å, Figure S25: Packing in the solid-state structure of 13. Interaction between x, y, z, and 2 − x, 1 − y, 1 − z molecules. Representative distance is given in Å, Figure S26: Packing in the solid-state structure of 18. Interaction between x, y, z, and 1 − x, 1 − y, 1 − z molecules. Representative distances are given in Å, Figure S27: Packing in the solid-state structure of 13. Interaction between x, y, z, and 1 + x, −y + 3/2, z + 1/2 molecules. Representative distances are given in Å, Figure S28: Packing in the solid-state structure of 18. Interaction between x, y, z, and x, −y + 3/2, z − 1/2 molecules. Representative distances are given in Å, Figure S29: Packing in the solid-state structure of 13. Interaction between x, y, z, and x, −y + 3/2, z − 1/2 molecules. Representative distances are given in Å, Figure S30: Packing in the solid-state structure of 13. Interaction between x, y, z, and 1 − x, y + 1/2, −z + 1/2 molecules. Representative distances are given in Å, Figure S31: Electrostatic potential mapped on the ρ = 0.002 a.u. electron density isosurface of 13. Coloring from red = −0.05 a.u. to blue = +0.05 a.u., Figure S32: Electrostatic potential mapped on the ρ = 0.002 a.u. electron density isosurface of 18. Coloring from red = −0.05 a.u. to blue = +0.05 a.u., Figure S33: Integrated Bader atomic charges for isolated 24 molecule, Figure S34: Integrated Bader atomic charges for isolated 13 molecule, Figure S35: Integrated Bader atomic charges for isolated 18 molecule, Figure S36: Tertamer extracted from the experimental structure of 24 and used for the modeling of I/Br substation disorder. HalX/HalX′ are I/Br or Br/I, Table S1: Intermolecular interaction energies (kJ/mol) in the packing of solid state structure of 24. R is the distance between molecular centroids (mean atomic position) in Å, Table S2: Intermolecular interaction energies (kJ/mol) in the packing of solid state structure of 18. Largest differences between 18 and 13 are highlighted in bold. R is the distance between molecular centroids (mean atomic position) in Å, Table S3. Intermolecular interaction energies (kJ/mol) in the packing of solid state structure of 13 Largest differences between 18 and 13 are highlighted in bold. R is the distance between molecular centroids (mean atomic position) in Å, Table S4. Electrostatic potential maxima (VS,max; kcal/mol) on the molecular surface ρ = 0.002 a.u. of 24, Table S5: Type-II hal⋯hal bonds four membered ring tetramer relative energies. Hal1-2-3-4 numbering corresponds to Figure S18.
Author Contributions
Conceptualization, V.M. and P.P.; methodology, V.M.; X-ray analysis, E.A. and E.W.; data curation, V.M.; writing—original draft preparation, V.M.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All the data are given in the article and in the associated supporting information.
Acknowledgments
We thank University of Strasbourg and CNRS for financial support. “The Plateforme de Mesures de Diffraction et Diffusion des rayons X of Université de Lorraine” is thanked for providing access to crystallographic facilities. The EXPLOR mesocentre is thanked for computing facilities (Project 2019CPMXX0984).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Biradha, K.; Sarkar, M.; Rajput, L. Crystal engineering of coordination polymers using 4,4′-bipyridine as a bond between transition metal atoms. Chem. Commun. 2006, 40, 4169–4179. [Google Scholar] [CrossRef] [PubMed]
- Striepe, L.; Baumgartner, T. Viologens and their application as functional materials. Chem. Eur. J. 2017, 23, 16924–16940. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, R. Mono-and di-quaternized 4,4′-bipyridine derivatives as key building blocks for medium- and environment-responsive compounds and materials. Molecules 2019, 25, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chelucci, G.; Thummel, R.P. Chiral 2,2′-bipyridines, 1,10-phenanthrolines, and 2,2′:6′,2″-terpyridines: Syntheses and applications in asymmetric homogeneous catalysis. Chem. Rev. 2002, 102, 3129–3170. [Google Scholar] [CrossRef]
- Fletcher, N.C. Chiral 2,2′-bipyridines: Ligands for asymmetric induction. J. Chem. Soc. Perkin Trans. 1 2002, 16, 1831–1842. [Google Scholar] [CrossRef]
- Bednářová, E.; Malatinec, S.; Kotora, M. Applications of Bolm’s ligand in enantioselective synthesis. Molecules 2020, 25, 958. [Google Scholar] [CrossRef] [Green Version]
- Sbircea, L.; Sharma, N.D.; Clegg, W.; Harrington, R.W.; Horton, P.N.; Hursthouse, M.B.; Apperley, D.C.; Boyd, D.R.; James, S.L. Chemoenzymatic synthesis of chiral 4,4′-bipyridyls and their metal–organic frameworks. Chem. Commun. 2008, 43, 5538–5540. [Google Scholar] [CrossRef]
- Jouaiti, A.; Hosseini, M.W.; Kyritsakas, N. Non-centrosymmetric packing of 1-d coordination networks based on chirality. Chem. Commun. 2002, 17, 1898–1899. [Google Scholar] [CrossRef]
- Mamane, V.; Aubert, E.; Peluso, P.; Cossu, S. Synthesis, resolution, and absolute configuration of chiral 4,4′-bipyridines. J. Org. Chem. 2012, 77, 2579–2583. [Google Scholar] [CrossRef]
- Rang, A.; Engeser, M.; Maier, N.M.; Nieger, M.; Lindner, W.; Schalley, C.A. Synthesis of axially chiral 4,4′-bipyridines and their remarkably selective self-assembly into chiral metallo-supramolecular squares. Chem. Eur. J. 2008, 14, 3855–3859. [Google Scholar] [CrossRef]
- Aubert, E.; Abboud, M.; Doudouh, A.; Durand, P.; Peluso, P.; Ligresti, A.; Vigolo, B.; Cossu, S.; Pale, P.; Mamane, V. Silver(I) coordination polymers with 3,3′,5,5′-tetrasubstituted 4,4′-bipyridine ligands: Towards new porous chiral materials. RSC Adv. 2017, 7, 7358–7367. [Google Scholar] [CrossRef] [Green Version]
- Peluso, P.; Mamane, V.; Aubert, E.; Dessì, A.; Dallocchio, R.; Dore, A.; Pale, P.; Cossu, S. Insights into halogen bond-driven enantioseparations. J. Chromatogr. A 2016, 1467, 228–238. [Google Scholar] [CrossRef]
- Peluso, P.; Mamane, V.; Dallocchio, R.; Dessì, A.; Villano, R.; Sanna, D.; Aubert, E.; Pale, P.; Cossu, S. Polysaccharide-based chiral stationary phases as halogen bond acceptors: A novel strategy for detection of stereoselective σ-hole bonds in solution. J. Sep. Sci. 2018, 41, 1247–1256. [Google Scholar] [CrossRef]
- Peluso, P.; Gatti, C.; Dessì, A.; Dallocchio, R.; Weiss, R.; Aubert, E.; Pale, P.; Cossu, S.; Mamane, V. Enantioseparation of fluorinated 3-arylthio-4,4′-bipyridines: Insights into chalcogen and π-hole bonds in high-performance liquid chromatography. J. Chromatogr. A 2018, 1567, 119–129. [Google Scholar] [CrossRef]
- Peluso, P.; Dessì, A.; Dallocchio, R.; Sechi, B.; Gatti, C.; Chankvetadze, B.; Mamane, V.; Weiss, R.; Pale, P.; Aubert, E.; et al. Enantioseparation of 5,5′-dibromo-2,2′-dichloro-3-selanyl-4,4′-bipyridines on polysaccharide-based chiral stationary phases: Exploring chalcogen bonds in liquid-phase chromatography. Molecules 2021, 26, 221. [Google Scholar] [CrossRef]
- Weiss, R.; Aubert, E.; Peluso, P.; Cossu, S.; Pale, P.; Mamane, V. Chiral chalcogen bond donors based on the 4,4′-bipyridine scaffold. Molecules 2019, 24, 4484. [Google Scholar] [CrossRef] [Green Version]
- Dessì, A.; Peluso, P.; Dallocchio, R.; Weiss, R.; Andreotti, G.; Allocca, M.; Aubert, E.; Pale, P.; Mamane, V.; Cossu, S. Rational design, synthesis, characterization and evaluation of iodinated 4,4′-bipyridines as new transthyretin fibrillogenesis inhibitors. Molecules 2020, 25, 2213. [Google Scholar] [CrossRef]
- Mamane, V.; Peluso, P.; Aubert, E.; Cossu, S.; Pale, P. Chiral hexahalogenated 4,4′-bipyridines. J. Org. Chem. 2016, 81, 4576–4587. [Google Scholar] [CrossRef]
- Foulger, N.J.; Wakefield, B.J. Polyhalogenoaromatic compounds. XXIX. Hexachloro-5,5′-dilithio-4,4′-bipyridine as an intermediate for organometallic and organic syntheses. J. Organomet. Chem. 1974, 69, 161–167. [Google Scholar] [CrossRef]
- Mamane, V.; Aubert, E.; Peluso, P.; Cossu, S. Lithiation of prochiral 2,2′-dichloro-5,5′-dibromo-4,4′-bipyridine as a tool for the synthesis of chiral polyhalogenated 4,4′-bipyridines. J. Org. Chem. 2013, 78, 7683–7689. [Google Scholar] [CrossRef]
- Kutasevich, A.V.; Perevalov, V.P.; Mityanov, V.S. Recent progress in non-catalytic C–Hunctionalization of heterocyclic N-oxides. Eur. J. Org. Chem. 2021, 2021, 357–373. [Google Scholar] [CrossRef]
- Abboud, M.; Mamane, V.; Aubert, E.; Lecomte, C.; Fort, Y. Synthesis of polyhalogenated 4,4′-bipyridines via a simple dimerization procedure. J. Org. Chem. 2010, 75, 3224–3231. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, J.; Hwang, T.L.; Chen, M.J.; Tedrow, J.S.; Farrell, R.P.; Bio, M.M.; Cui, S. Highly regioselective halogenation of pyridine N-oxide: Practical access to 2-halo-substituted pyridines. Org. Lett. 2015, 17, 2948–2951. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.W.; Giese, M.W.; Coghlan, M.J. An intra/intermolecular Suzuki sequence to benzopyridyloxepines containing geometrically pure exocyclic tetrasubstituted alkenes. Org. Lett. 2008, 10, 2701–2704. [Google Scholar] [CrossRef] [PubMed]
- Klapars, A.; Buchwald, S.L. Copper-catalyzed halogen exchange in aryl halides: An aromatic Finkelstein reaction. J. Am. Chem. Soc. 2002, 124, 14844–14845. [Google Scholar] [CrossRef] [PubMed]
- Peluso, P.; Sechi, B.; Lai, G.; Dessì, A.; Dallocchio, R.; Cossu, S.; Aubert, E.; Weiss, R.; Pale, P.; Mamane, V.; et al. Comparative enantioseparation of chiral 4,4′-bipyridine derivatives on coated and immobilized amylose-based chiral stationary phases. J. Chromatogr. A 2020, 1625, 461303. [Google Scholar] [CrossRef] [PubMed]
- Dallocchio, R.; Sechi, B.; Dessì, A.; Chankvetadze, B.; Cossu, S.; Mamane, V.; Weiss, R.; Pale, P.; Peluso, P. Enantioseparations of polyhalogenated 4,4′-bipyridines on polysaccharide-based chiral stationary phases and molecular dynamics simulations of selector–selectand interactions. Electrophoresis 2021. [Google Scholar] [CrossRef] [PubMed]
- Perdomo Rivera, R.; Ehlers, P.; Ohlendorf, L.; Torres Rodríguez, E.; Villinger, A.; Langer, P. Chemoselective synthesis of arylpyridines through Suzuki–Miyaura cross-coupling reactions. Eur. J. Org. Chem. 2018, 8, 990–1003. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17 (2017). University of Western Australia. Available online: https://hirshfeldsurface.net (accessed on 26 February 2021).
- Desiraju, G.R.; Parthasarathy, R. The nature of halogen⋯halogen interactions: Are short halogen contacts due to specific attractive forces or due to close packing of nonspherical atoms? J. Am. Chem. Soc. 1989, 111, 8725–8726. [Google Scholar] [CrossRef]
- Metrangolo, P.; Resnati, G. Type II halogen⋯halogen contacts are halogen bonds. IUCrJ 2014, 1, 5–7. [Google Scholar] [CrossRef]
- Zhu, X.; Kreutter, K.D.; Hu, H.; Player, M.R.; Gaul, M.D. A novel reagent combination for the oxidation of highly electron deficient pyridines to N-oxides: Trifluoromethanesulfonic anhydride/sodium percarbonate. Tetrahedron Lett. 2008, 49, 832–834. [Google Scholar] [CrossRef]
- Caron, S.; Do, N.D.; Sieser, J.E. A practical, efficient, and rapid method for the oxidation of electron deficient pyridines using trifluoroacetic anhydride and hydrogen peroxide–urea complex. Tetrahedron Lett. 2000, 41, 2299–2302. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).