Abstract
This study is part of a project devoted to determining the scent of all the orchid species present in Basilicata. All the analyses were performed by using the solid-phase microextraction technique coupled with gas chromatography-mass spectrometry. The scent of eight species belonging to the Orchis genus was investigated. In the case of O. anthropophora, caryophyllene, tetradecanal and hexadecanal were the main components of the aroma; in O. purpurea, 3,5-dimethoxytoluene and elemicin were found; in O. italica, caryophyllene and 4-(3-hydroxy-2-methoxyphenyl)butan-2-one were found; in O. pauciflora, linalool and 1,4-dimethoxybenzene were found; in O. mascula, linalool was found; in O. quadripunctata, penta- and heptadecane were found; in O. provincialis, β-farnesene and farnesal were found; and in O. pallens, curcumene was the main product.
1. Introduction
Within the families of flowering plants, the Orchidaceae family is one of largest, with more than 28,000 species. The scent of some orchids has a relevant importance in perfume industries. However, the emission of volatile organic compounds from an orchid can have a relevant role in the life of the plant considering the possible effect of these compounds in attracting pollinators, or in defense against pathogens. One third of all the orchid species are food-deceptive species because the flowers do not contain nectar and the volatile organic compounds emitted mimic the floral signal of rewarding plants to attract pollinators. Furthermore, many compounds show antimicrobial and antifungal activities [1]. Volatile organic compounds are mainly terpenes, phenylpropanoid derivatives and fatty acid derivatives.
Some years ago, we started a project devoted to determining the floral scent of all the orchid species found in Basilicata (Southern Italy). The main feature of this study is the use of the same chemical method in order to determine the scent. Several methods can be used to determine VOCs. For example, FT-IR has been used to determine the composition of volatile mixtures [2,3], and, probably, GC-MS and FT-IR can be considered complementary methods in the analysis of complex mixtures [4]. We decided to use the solid-phase microextraction (SPME) procedure [5]. SPME analysis needs the exposure of a fiber, contained in the needle of a syringe, to a scent. The adsorbed components are then thermally desorbed into the injection sector of the gas chromatographic apparatus. The use of a single procedure allows obtaining a homogeneous dataset, also considering that SPME can suffer from the different absorption rates of the single components in the fiber [5]. This way, significant results were obtained in the characterization of the scent of Platanthera bifolia subsp. osca [6,7,8], Platanthera chlorantha [7,8], Cephalanthera orchids [9], Serapias orchids [8,10], Gymnadenia orchids [11], Barlia robertiana [8] and Neotinea orchids [12].
In this article, we want to continue with the realization of our project, showing the chemical composition of Orchis species found in Basilicata. This orchid genus is a very common one, where it is diffused in all of Europe, and in Basilicata, there are nine species, Orchis anthropophora, O. italica, O. mascula, O. pallens, O. pauciflora, O. provincialis, O. purpurea, O. quadripunctata and O. simia. In this study, we will report the results obtained in the determination of the scent in O. anthropophora, O. purpurea, O. italica, O. pauciflora, O. mascula, O. quadripunctata, O. provincialis and O. pallens.
In some of these species, some previous results have been reported in the literature. Nilsson reported a data headspace analysis of O. mascula, showing the presence of tricyclene (23.6%), α-pinene (15.6%) and E-ocimene (30.5%) as the main components, and linalool in a low quantity (2.4%) [13,14]. The same species, in a headspace analysis of the scent, showed the presence of limonene (8.37%), 1,8-cineole (11.74%), E-ocimene (23.25%) and linalool (11.89%) [15]. In a work where hexane extracts were considered, pentacosane (12.07%), heptacosane (46.00%) and nonacosane (27.97%) were determined by the same research group [16]. The same species has been analyzed using SPME (with PDMS-DVB fiber), showing the presence of limonene (11.67%), E-ocimene (26.68%) and linalool (13.15%) [17,18]. In an analysis of O. mascula through SPME (with PDMS-DVB fiber) where white and purple flowers were analyzed, the authors found in purple flowers (Z)-3-hexenyl acetate (6.46%), limonene (10.67%), E-ocimene (22.68%) and linalool (13.15%), while in the white flowers, the same compounds were found in a different ratio (12.20%, 12.88%, 16.30%, 3.46%) [19]. In O. italica, only one article reported the composition of the scent, obtained through hexane extraction, where tricosane (37.05%), pentacosane (16.88%), heptacosane (20.65%), nonacosane (8.14%) and 5-pentacosene (5.44%) were observed [16]. The same article also examined the scent of O. provincialis, showing the presence of pentacosane (12.07%), heptacosane (46.00%) and nonacosane (27.97%) [16]. Schiestl and Cozzolino also examined the scent of O. quadripunctata and found tricosane (14.86%), pentacosane (29.62%), heptacosane (32.51%) and nonacosane (9.21%) [16]. Headspace analysis of O. pauciflora showed in its scent nonanal (4.88%), 2-methyl-6-methylene-3,7-octadien-2-ol (30.49%), myrcene (25.87%) and E-ocimene (8.26%) [15]. Two articles were related to the analysis of the aroma components of O. simia: In the first one, where dynamic headspace analysis was performed, ethyl acetophenone (6.79%), α-pinene (32.68%), β-pinene (6.10%), sabinene (5.23%), myrcene (5.45%), eucalyptol (7.89%) and linalool (7.41%) were found [20]. In the second article, where SPME was used, nonanal (5.47%), (Z)-3-hexenyl acetate (3.21%), decanal (2.15%), α-pinene (11.49%), myrcene (7.12%), limonene (4.26%) and β-phellandrene (9.03%) were found [19]. The scent of O. pallens has been determined in a work where SPME (with Carbowax-PDMS fiber) was used; in this case, phenethyl alcohol, β-farnesene, α-farnesene and farnesol were determined [21]. Finally, in a headspace analysis of O. anthropophora, nonanal (9.46%), undecane (8.72%), benzeneacetaldehyde (4.87%), α-pinene (5.77%), limonene (5.22%), 1,8-cineole (7.49%), β-caryophyllene (22.33%) and caryophyllodienol (9.91%) were found as components of its aroma [22,23], while in a study where hexane extracts were examined, tricosane (10.82%), pentacosane (24.47%), heptacosane (26.92%), nonacosane (8.00%), 9-pentacosene (8.50%) and 9-heptacosene (7.68%) were found [16].
The above-reported data show that very different results can be obtained by using different GC-MS analytical methods able to characterize the aroma components, showing that the use of a homogenous method can provide valuable information on the scent of these species. In this work, the same HS-SPME-GC-MS method was used in order to characterize the scent of eight species of the Orchis genus.
2. Experimental Section
2.1. Plant Material
The sample of Orchis anthropophora was collected at Tolve (PZ) (359 m a.s.l.) on 18 April 2018. The sample of Orchis italica was collected at Tolve (PZ) (343 m a.s.l.) on 23 April 2018. The sample of Orchis mascula was collected at Sasso di Castalda (PZ) (1090 m a.s.l.) on 22 May 2018. The sample of Orchis pallens was collected at Serra di Crispo at Terranova del Pollino (PZ) (1882 m a.s.l.) on 18 June 2018. The sample of Orchis pauciflora was collected at Madonna di Sasso at Sasso di Castalda (PZ) (1882 m a.s.l.) (1330 m a.s.l.) on 9 May 2018. The sample of Orchis provincialis was collected at Sasso di Castalda (PZ) (1069 m a.s.l.) on 30 April 2018. The sample of Orchis purpurea was collected at the campus of the University of Basilicata at Macchia Roma in Potenza (714 m a.s.l.) on 5 May 2018. The sample of Orchis quadripunctata was collected at Bosco Ralle at Satriano di Lucania (PZ) (1034 m a.s.l.) on 11 June 2018. The plants were collected by Vito Antonio Romano.
The plants were successively used for further studies on the impollination, fertility and germination of the plants. After these studies, the plants were not in condition to be collected in a herbarium. However, these species can be recognized without ambiguities on the basis of their properties, well documented in Figure 1 and Figure 2.

Figure 1.
(a) Orchis anthropophora; (b) Orchis purpurea; (c) Orchis italica; (d) Orchis pauciflora. Photos of V. A. Romano.
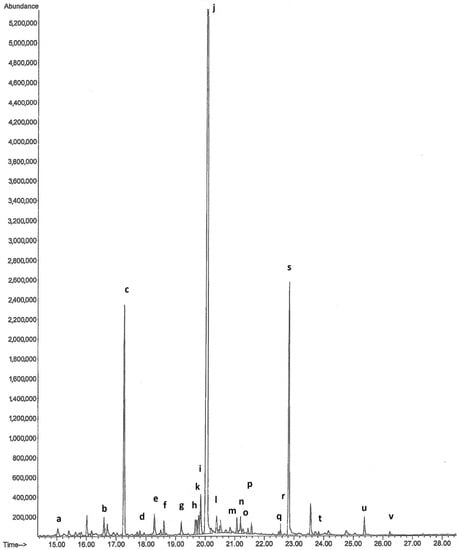
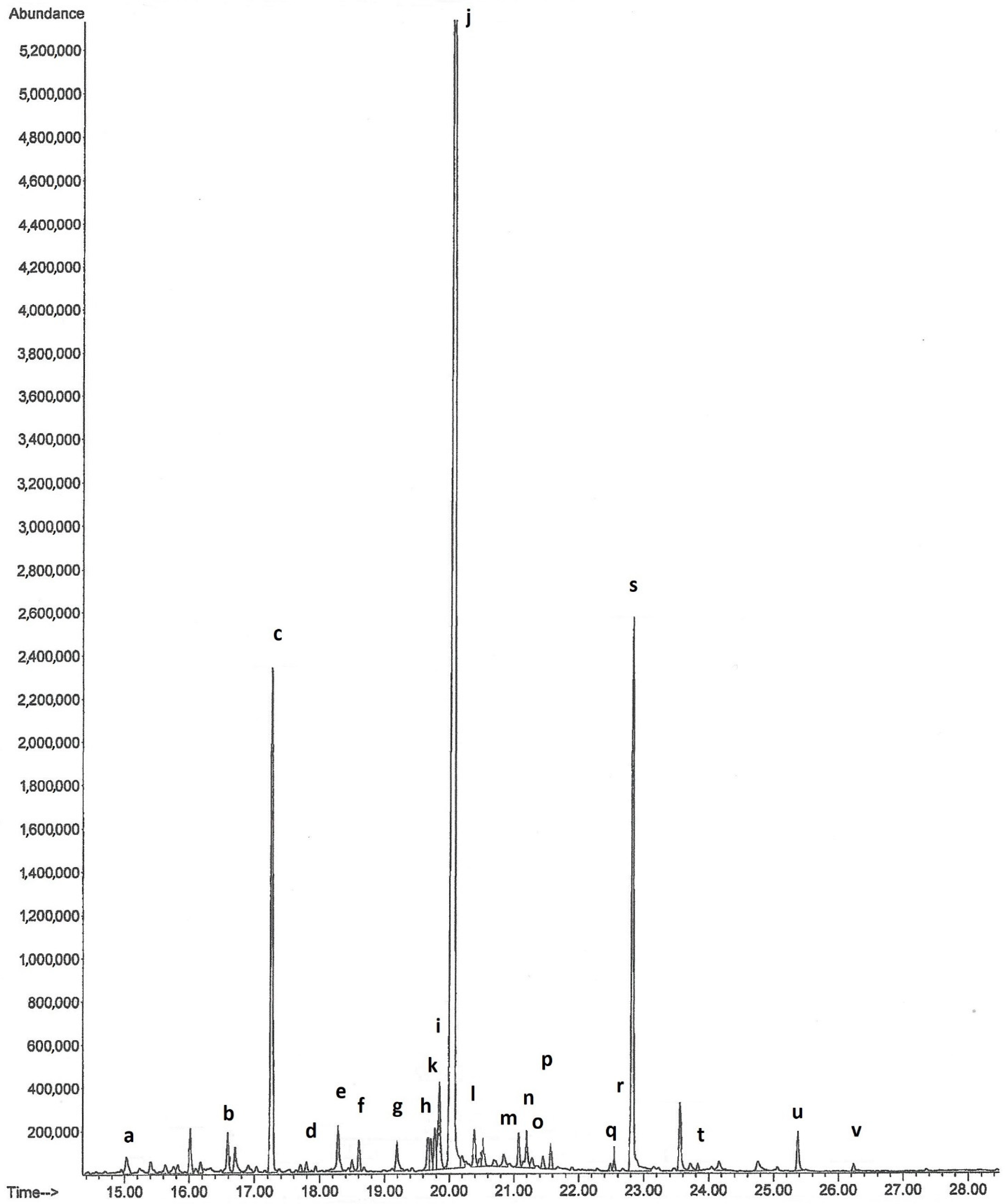
Figure 2.
Chromatogram of volatile organic compounds from Orchis anthropophora: (a) tridecane; (b) tetradecane; (c) caryophyllene; (d) humulene; (e) pentadecane; (f) tridecanal; (g) 1-(1-methylethyl)-5-methyl-1,2,3,4-tetrahydronaphthalene; (h) 2-allyl-4-methylphenol; (k) ethyl dodecanoate; (i) hexadecane; (j) tetradecanal; (l) megastigmatrienone; (m) 4-(1-methylethyl)-1,6-dimethyl-1,2,3,4-tetrahydronaphthalene; (n) heptadecane; (o) pristane; (p) pentadecanal; (q) ethyl tetradecanoate; (r) octadecane; (s) hexadecanal; (t) nonadecane; (u) isopropyl palmitate; (v) heneicosane.
To prevent plant damage to the whole plant from a population in Basilicata, a large portion of soil all around the plant was removed from its habitat and placed in a greenhouse for a few days of acclimatization.
Following this period, for three days, the plant was placed under a bell jar. In view of the fact that the investigated taxa are rare wild plants, in order to preserve the species, we chose to use a single plant for our analysis.
2.2. Analysis of Volatile Organic Compounds
SPME [4] analysis of eight different samples of Orchis was performed. This way, the identified plants were collected and inserted in a glass jar for 24 h where a fiber (DVB/CAR/PDMS) and SPME syringe were also present. After this time, the fiber was desorbed in a gas chromatographic apparatus equipped with a quadrupole mass spectrometer detector. A 50/30 μm DVB/CAR/PDMS module with a 1 cm fiber (57328-U, Supelco, Milan, Italy) was employed to determine VOCs. The SPME fiber was maintained in the bell jar for 24 h. The analytes were desorbed in the splitless injector at 250 °C for 2 min. Analyses were accomplished with an HP 6890 Plus gas chromatograph equipped with a Phenomenex Zebron ZB-5 MS capillary column (30 m × 0.25 mm i.d. × 0.25 μm FT) (Agilent, Milan, Italy). An HP 5973 mass selective detector (Agilent) was utilized with helium at 0.8 mL/min as the carrier gas. The analyses were performed by using a splitless injector. The splitless injector was maintained at 250 °C, and the detector at 230 °C. The oven was held at 40 °C for 2 min, then gradually warmed, 8 °C/min, up to 250 °C and held for 10 min. Tentative identification of aroma components was based on mass spectra and Wiley 11 and NIST 14 library comparison. A single VOC peak was considered as identified when its experimental spectrum matched with a score over 90% present in the library. All the analyses were performed in triplicate.
To avoid contamination on the sample due, for example, to volatile organic compounds emitted from the soil, analysis of Orchis anthropophora was conducted on a single flower without the presence of soil, showing that this type of contamination does not exist. Otherwise, all the analyses were carried out by inserting the flowering plant in a glass bell jar and isolating the plant from the soil.
3. Results
Orchis anthropophora (Figure 1a) returned the results reported in Table 1 and Figure 2. The main volatile organic compounds detected were caryophyllene (11.32%), tetradecanal (57.17%) and hexadecanal (12.10%). Caryophyllene has a scent described as sweet, woody and terpenic, while the aroma of tetradecanal is described as fatty, waxy, amber, incense, citrus peel and musk.

Table 1.
SPME-GC-MS analysis of Orchis species.
The SPME analysis of Orchis purpurea (Figure 1b) showed that the main components of the aroma were aromatic compounds such as 3,5-dimethoxytoluene (35.29%) and elemicin (4.76%) (Table 1). The scent of elemicin is described as spicy and floral. When Orchis italica (Figure 1c) was examined, eucalyptol (3.93%), caryophyllene (47.29%) and 4-(3-hydroxy-2-methoxyphenyl)butan-2-one (4.96%) were the most abundant compounds found in the scent (Table 1). The scent of eucalyptol is recognized as eucalyptus, herbal, camphoreous and medicinal. Orchis pauciflora (Figure 1d) showed the presence of linalool (26.12%), 1,4-dimethoxybenzene (15.01%), germacrene D (9.34%) and 6,10,14-trimethyl-4,8,12-tetradecadrienal (3.60%). The scent of linalool is described as citrus, floral, sweet and woody, while that of 1,4-dimethoxybenzene is sweet, green and hay.
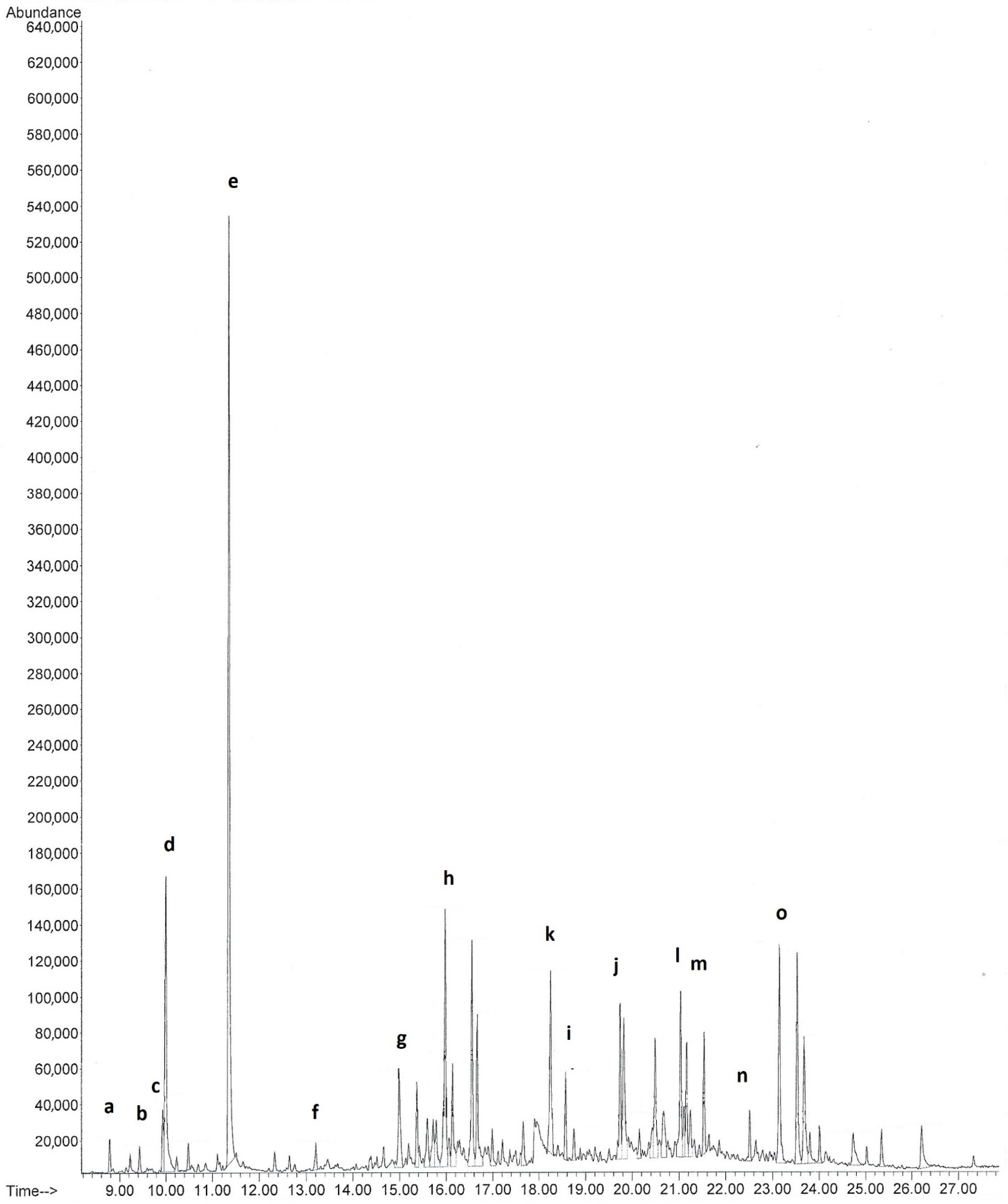
The SPME analysis of the scent of Orchis mascula (Figure 3a) showed the presence of eucalyptol (7.80%), linalool (21.28%), tetradecane (3.70%), pentadecane (4.41%) and 6,10,14-trimethyl-2-pentadecanone (Table 1 and Figure 4). Orchis quadripunctata (Figure 3b) had a scent composition where only hydrocarbons were found. Thus, tridecane (9.70%), tetradecane (9.38%), pentadecane (26.85%) and heptadecane were the main components of the scent (Table 1). In the case of Orchis provincialis (Figure 3c), the volatile organic compounds found in the SPME analysis were β-farnesene (44.16%), 3,7,11-trimethyl-2,6,10-dodecatrienal (29.25%) and 6,10,14-trimethyl-2-pentadecanone (6.32%) (Table 1). β-Farnesene’s scent is described as woody, citrus, herbal and sweet. Finally, the aroma components of Orchis pallens (Figure 3d) were α-zingiberene (14.67%), di-epi-α-cedrene (10.64%), β-curcumene (33.29%) and diethyltoluamide (13.95%) (Table 1). α-Zingiberene has a scent described as spicy, fresh and sharp, while that of di-epi-α-cedrene is described as woody, cedar, sweet and fresh.

Figure 3.
(a) Orchis mascula; (b) Orchis quadripunctata; (c) Orchis provincialis; (d) Orchis pallens. Photos of V. A. Romano.
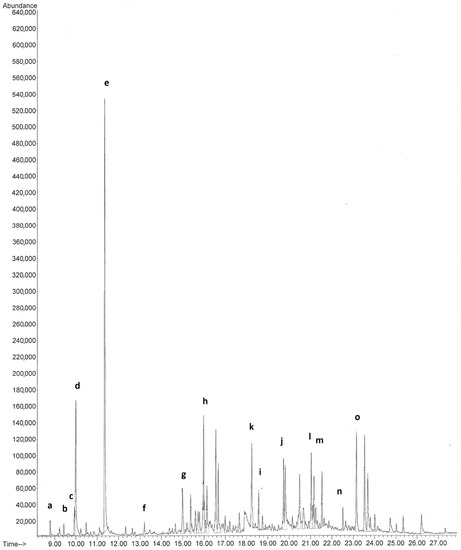
Figure 4.
Chromatogram of volatile organic compounds from Orchis mascula; (a) β-terpinene; (b) 2,2,4,6,6-pentamethyl-3-heptene; (c) limonene; (d) eucalyptol; (e) linalool; (f) dodecane; (g) tridecane; (h) tetradecane; (k) germacrene D; (i) β-sesquiphellandrene; (j) hexadecane; (l) heptadecane; (m) pristane; (n) octadecane; (o) 6,10,14-trimethyl-2-pentadecanone.
4. Discussion
It is interesting to note the large differences between our reported results and those reported in the Introduction section. For O. anthropophora, two different analyses are available [16,20]. While in the work of Cozzolino [16], only hydrocarbons with an extremely high molecular weight were found, the other article [20] reported that β-caryophyllene was the main component. In our study, β-caryophyllene was present, but the main component was tetradecanal. In the case of O. purpurea, no other results on the composition of the scent are available. For O. italica, only an article published by Cozzolino is available [16], and, also in this case, only high-molecular weight hydrocarbons were found. In our experiment, on the contrary, β-caryophyllene was the main component of the scent.
The scent of O. pauciflora has been determined through headspace analysis, showing the presence of 2-methyl-6-methylene-3,7-octadiene-2-ol as the main component [15]. However, in our analysis, linalool and 1,4-dimethoxybenzene were found as the main components. O. mascula was the object of an intense study where several different analytical methods were used. This way, headspace analysis returned E-ocimene as the main component of the scent [13,14]. This result was confirmed by SPME analysis [15,17]. In our analysis, as it is evident considering Figure 4, linalool was the main component of the aroma. For O. quadripunctata, the work of Schiestl and Cozzolino found only hydrocarbons [16]. Only hydrocarbons were found in this work, but with a significant difference in the molecular weight of the detected compounds. In the case of O. provincialis, the work of Schiestl and Cozzolino determined only the presence of hydrocarbons [17], while the presence of relevant amounts of β-farnesene was determined in this study. Finally, while an SPME analysis of the scent of O. pallens found phenethyl alcohol, farnesene and farnesol [21], our analysis of the same species found β-curcumene as the main component.
5. Conclusions
This work shows the analysis of Orchis samples from Basilicata. The analyses were performed by using the same procedure and the same fiber in SPME-GC-MS, which allowed achieving a homogenous dataset. The analyses showed different scent compositions from those determined on samples deriving from different sites. These observed differences, when SPME of other headspace techniques is used, can depend both on the different absorption rates of the analytes on the fiber and on the variation in the scent due to natural adaptation of the plant to different environmental conditions, due, for example, to different pollination insects. A completely different consideration can be found in the work of Schiestl and Cozzolino, where a completely different analytical method was used (hexane extraction of labellum, and GC-MS analysis of the extracts). In their case, only hydrocarbons were determined. Probably, their analytical procedure was not the correct method for the determination of the orchid scent.
Author Contributions
Conceptualization, M.D. and V.A.R.; methodology, R.R.; investigation, R.R., L.V. and M.M.; data curation, R.L.; writing—original draft preparation, M.D. and V.A.R.; writing—review and editing, M.D., V.A.R. and R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramya, M.; Jang, S.; An, H.-R.; Lee, S.-Y.; Park, P.-M.; Park, P.H. Volatile Organic Compounds from Orchids: From Synthesis and Function to Gene Regulation. Int. J. Mol. Sci. 2020, 21, 1160. [Google Scholar] [CrossRef] [Green Version]
- Apolonski, A.; Maiti, K.S. Towards a standard operating procedure for revealing hidden volatile organic compounds in breath: The Fourier-transform IR spectroscopy case. Appl. Opt. 2021, 60, 4217–4224. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.S.; Lewton, M.; Fill, E.; Apolonskiy, A. Sensitive spectroscopic breath analysis by water condensation. J. Breath Res. 2018, 12, 046003. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.S.; Lewton, M.; Fill, E.; Apolonski, A. Human beings as islands of stability: Monitoring body states using breath pro-files. Sci. Rep. 2019, 9, 16167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawliszyn, J. Solid-Phase Microextraction: Theory and Practice; VCH: New York, NY, USA, 1997. [Google Scholar]
- D’Auria, M.; Racioppi, R. Characterization of the volatile fraction of mastic oil and mastic gum. Nat. Prod. Res. 2020, 1–4. [Google Scholar] [CrossRef]
- D’Auria, M.; Lorenz, R.; Racioppi, R.; Romano, V.A. Fragrance components of Platanthera bifolia subsp. osca. Nat. Prod. Res. 2017, 31, 1612–1619. [Google Scholar] [CrossRef]
- D’Auria, M.; Lorenz, R.; Mecca, M.; Racioppi, R.; Romano, V.A.; Viggiani, L. Fragrance components of Platanthera bifolia subsp. osca and Platanthera chlorantha collected in several sites in Italy. Nat. Prod. Res. 2020, 34, 2857–3861. [Google Scholar] [CrossRef]
- D’Auria, M.; Fascetti, S.; Racioppi, R.; Romano, V.A.; Rosati, L. Orchids from Basilicata: The Scent. In Reference Series in Phytochemistry; Springer Science and Business Media LLC: Berlin, Germany, 2020; pp. 1–22. [Google Scholar]
- D’Auria, M.; Lorenz, R.; Mecca, M.; Racioppi, R.; Romano, V.A. Aroma components of Cephalanthera orchids. Nat. Prod. Res. 2021, 35, 174–177. [Google Scholar] [CrossRef]
- D’Auria, M.; Lorenz, R.; Mecca, M.; Racioppi, R.; Romano, V.A. The composition of the aroma of Serapias orchids in Basilicata (Southern Italy). Nat. Prod. Res. 2020, 1–5. [Google Scholar] [CrossRef]
- D’Auria, M.; Lorenz, R.; Mecca, M.; Racioppi, R.; Romano, V.A.; Viggiani, L. Fragrance components of Gymnadenia conopsea and Gymnadenia odoratissima collected at several sites in Italy and Germany. Nat. Prod. Res. 2020, 1–5. [Google Scholar] [CrossRef]
- D’Auria, M.; Lorenz, R.; Mecca, M.; Racioppi, R.; Romano, V.A.; Viggiani, L. The scent of Neotinea orchids from Basilicata (Southern Italy). Nat. Prod. Res. 2021, 1–3. [Google Scholar] [CrossRef]
- Nilsson, L.A. Anthecology of Orchis mascula (Orchidaceae). Nord. J. Bot. 1983, 3, 157–179. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R.; Honnay, O.; Hutchings, M.J. Biological Flora of the British Isles:Orchis mascula(L.) L. J. Ecol. 2009, 97, 360–377. [Google Scholar] [CrossRef]
- Salzmann, C.C.; Cozzolino, S.; Schiestl, F.P. Floral scent in food-deceptive orchids: Species specificity and sources of variabil-ity. Plant Biol. 2007, 9, 720–729. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Cozzolino, S. Evolution of sexual mimicry in the orchid subtribe orchidinae: The role of preadaptations in the attraction of male bees as pollinators. BMC Evol. Biol. 2008, 8, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dormont, L.; Delle-Vedove, R.; Bessière, J.-M.; Hossaert-Mc Key, M.; Schatz, B. Rare white-flowered morphs increase the re-productive success of common purple morphs in a food-deceptive orchid. New Phytol. 2010, 185, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Dormont, L.; Fort, T.; Bessière, J.-M.; Proffit, M.; Hidalgo, E.G.; Buatois, B.; Schatz, B. Sources of floral scent variation in the food-deceptive orchid Orchis mascula. Acta Oecol. 2020, 107, 103600. [Google Scholar] [CrossRef]
- Dormont, L.; Delle-Vedove, R.; Bessière, J.-M.; Schatz, B. Floral scent emitted by white and coloured morphs in orchids. Phytochemistry 2014, 100, 51–59. [Google Scholar] [CrossRef]
- Schatz, B.; Geoffroy, A.; Dainat, B.; Bessière, J.-M.; Buatois, B.; Hossaert-Mckey, M.; Selosse, M.-A. A case study of modified interactions with symbionts in a hybrid mediterranean orchid. Am. J. Bot. 2010, 97, 1278–1288. [Google Scholar] [CrossRef]
- Barták, P.; Bednář, P.; Čáp, L.; Ondráková, L.; Stránský, Z. SPME-A valuable tool for investigation of flower scent. J. Sep. Sci. 2003, 26, 715–721. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R.; Hutchings, M.J. Biological flora of the British Isles: Orchis anthropophora (L.) All. (Aceras anthropophorum (L.) W.T. Aiton). J. Ecol. 2011, 99, 1551–1565. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).