1. Introduction
In biological detection, achieving rapid, high-quality information without the necessity of labeling is crucial for advancing biotechnology, particularly in addressing complex challenges such as the ongoing novel coronavirus outbreak. Biosensing utilizes biometric technology’s sensitivity to observe and understand the environment, translating observations into measurable physical quantities to assess and preempt hazards [
1]. Biosensors typically comprise three components: a molecular recognition element, a conversion element, and a signal amplification device [
2]. As biosensing continues to advance, new biomedical sensor technologies employing sophisticated sensing techniques and sensitive materials are emerging [
3]. Surface acoustic wave (SAW) biosensors are among the innovations in this field.
In 1885, Lord Rayleigh discovered the transmission mode and properties of a SAW [
4]. SAW technology operates by producing a periodic electric field through the application of alternating current to an interdigital transducer (IDT) [
5]. Then, the surface acoustic wave propagates along the piezoelectric substrate, reaches another IDT output terminal through a delay, and converts the acoustic wave signal into an electrical signal output through the piezoelectric effect [
6]. The orientation of the crystals used, the thickness of the piezoelectric nanomaterials, and the geometry of the metal transducers determine the type of acoustic waves [
6]. SAW biosensors facilitate label-free detection with cost effectiveness, biocompatibility, and energy efficiency. They also feature a straightforward fabrication process and operate across a broad frequency range ideal for biological detection [
3]. Additionally, SAW biosensors offer notable advantages in surface modification. Regarding manufacturing, SAW devices hold significant potential for mass production at a relatively low cost [
7], which makes SAW technology suitable for biological applications.
Recent years have witnessed significant advancements in the manufacture of surface acoustic wave (SAW) biosensors, marking a transformative period in sensor technology [
1]. These sensors capitalize on nanomaterials and microfabrication techniques to enhance their sensitivity and specificity in detecting biological analytes [
8]. The integration of advanced manufacturing processes enables precise control over the sensor architecture, including electrode configurations and material properties, thereby optimizing signal transduction mechanisms [
9]. Moreover, innovations in nanotechnology have facilitated the development of miniaturized SAW devices, which not only reduce manufacturing costs but also enhance portability and integration capabilities into various diagnostic platforms [
8]. These advancements underscore the pivotal role of manufacturing techniques in advancing SAW biosensors towards practical applications in clinical diagnostics, environmental monitoring, and biodefense, promising significant contributions to the healthcare and biotechnology fields [
10]. Researchers developed a surface acoustic wave (SAW) biosensor integrated with Rayleigh wave flow to enhance sensitivity, utilizing SH-SAW to demonstrate high reliability in biosensing applications [
11]. Their study showcases that SAW biosensors significantly improve overall performance and reliability in biosensing applications through surface functionalization with specific biomaterials and controlled unidirectional vortex flow patterns.
Three key steps are essential in fabricating the SAW biosensor: preparing the piezoelectric substrate, creating the sensing area, and preparing the interdigital transducer electrodes [
12]. The manufacturing process of the sensor, depicted in
Figure 1a, involves several key steps [
11]. First, quartz crystals are chosen as the piezoelectric material for their excellent piezoelectric properties capable of generating surface acoustic waves [
11]. In terms of material stack, PDMS (Sylgard 184 organic silicone elastomer) and SU-8 polymer materials are employed, with PDMS used for microfluidic chamber fabrication and SU-8 providing structural support [
11]. The manufacturing strategy includes pouring mixed PDMS into SU-8 molds, curing by heating to obtain elastomeric replicas, and subsequently sealing microfluidic PDMS chambers onto quartz substrates through plasma treatment. Quartz substrates, serving as the base material, not only exhibit excellent piezoelectric properties but also demonstrate outstanding chemical stability, making them highly suitable for SAW sensor applications [
3]. Prior to biofunctionalization, a research team characterized the prepared SAW chips using a vector network analyzer to assess the scattering parameters of their RF signal transmission [
11]. Furthermore, DNA-P was immobilized on gold films through strong Au-S bonds, and the functionalized membrane was characterized using fluorescence microscopy. Simultaneously, gold nanoparticles (AuNPs) were successfully synthesized and conjugated with DNA-T, validated through visible light absorption spectroscopy to confirm DNA-T binding with AuNPs [
11]. In summary, the manufacturing process of the SAW biosensor involves selecting a piezoelectric substrate, fabricating the sensing and structural components, and then functionalizing the sensor.
In this paper, recent advancements in the development of surface acoustic wave (SAW) biosensors are explored, with a particular emphasis placed on the integration of nanomaterials. The enhancement of SAW biosensor performance through the incorporation of these nanomaterials is investigated, focusing on improvements in sensitivity, portability, and compactness. Additionally, recent improvements in SAW biosensor manufacturing techniques are evaluated, highlighting the precise control achieved over sensor dimensions and material properties, as well as scalable and cost-effective production methods. The potential of these advanced SAW biosensors for various clinical and rapid detection applications is demonstrated, with a focus on their capability for the label-free detection of biological entities such as proteins, cells, viruses, and bacteria.
2. Materials and Progress of SAW Biosensors
Choosing suitable materials is crucial in the development of surface acoustic wave (SAW) biosensors due to their direct influence on sensor performance and sensitivity. The materials employed must meet specific criteria such as acoustic impedance matching, biocompatibility, stability, and ease of functionalization for biomolecule immobilization. Commonly used materials include piezoelectric substrates like quartz or lithium niobate, which generate acoustic waves upon electrical excitation. Thin films of materials such as metals (gold and aluminum), polymers (polyimides and SU-8), and ceramics (aluminum nitride) are utilized to modify the sensor surface, enhancing selectivity and sensitivity to targeted biomolecules. Each material’s properties play a pivotal role in optimizing sensor performance, making material selection a critical aspect in the design and fabrication of effective SAW biosensors.
A wide variety of piezoelectric materials exist, both man-made and natural. These crystals exhibit the greatest electromechanical coupling behavior when cut in specific directions. For example, a 36° yx lithium tantalate (LiTaO
3) crystal will produce shear waves, while a 128° yx lithium niobate (LiNbO
3) crystal will produce Rayleigh waves. It is important to understand the nomenclature of these crystal cuts in specific directions. For instance, a 128° yx cut of LiNbO
3 means that the crystal has thickness along the y-direction and then rotates 128° around the x-axis. The same crystal will exhibit different wave speeds when cut along different directions. Normal, uncut LiNbO
3 has a wave velocity of 3488 m/s, whereas a 128° yx cut of LiNbO
3 has a wave velocity of 3979 m/s. Thin films and piezoelectric substrates play a crucial role in fabricating acoustic surface wave (SAW) sensors. Materials such as zinc oxide (ZnO), aluminum nitride (AlN), and piezoelectric ceramics are chosen for depositing thin films on the substrate [
13,
14]. PZT, despite its advantages in good electromechanical coupling coefficients, suffers from high energy loss, low velocity, a poor quality factor, and difficulties in fabrication [
15]. AlN films offer the highest wave speed [
16,
17]. In recent years, the discovery and experimental results for aluminum gallium nitride (AlGaN) have shown that it is well-suited for thin film layers. Commonly used substrate materials in SAW sensors are listed in
Table 1. Typically, materials with lower temperature coefficients (TCs) are preferred over others.
One of the main goals of the biosensor research community is the detection of analytes in extremely small amounts of samples. An ideal microsystem should be able to simultaneously detect several analytes at low concentrations without the need for analytical labels (label-free).
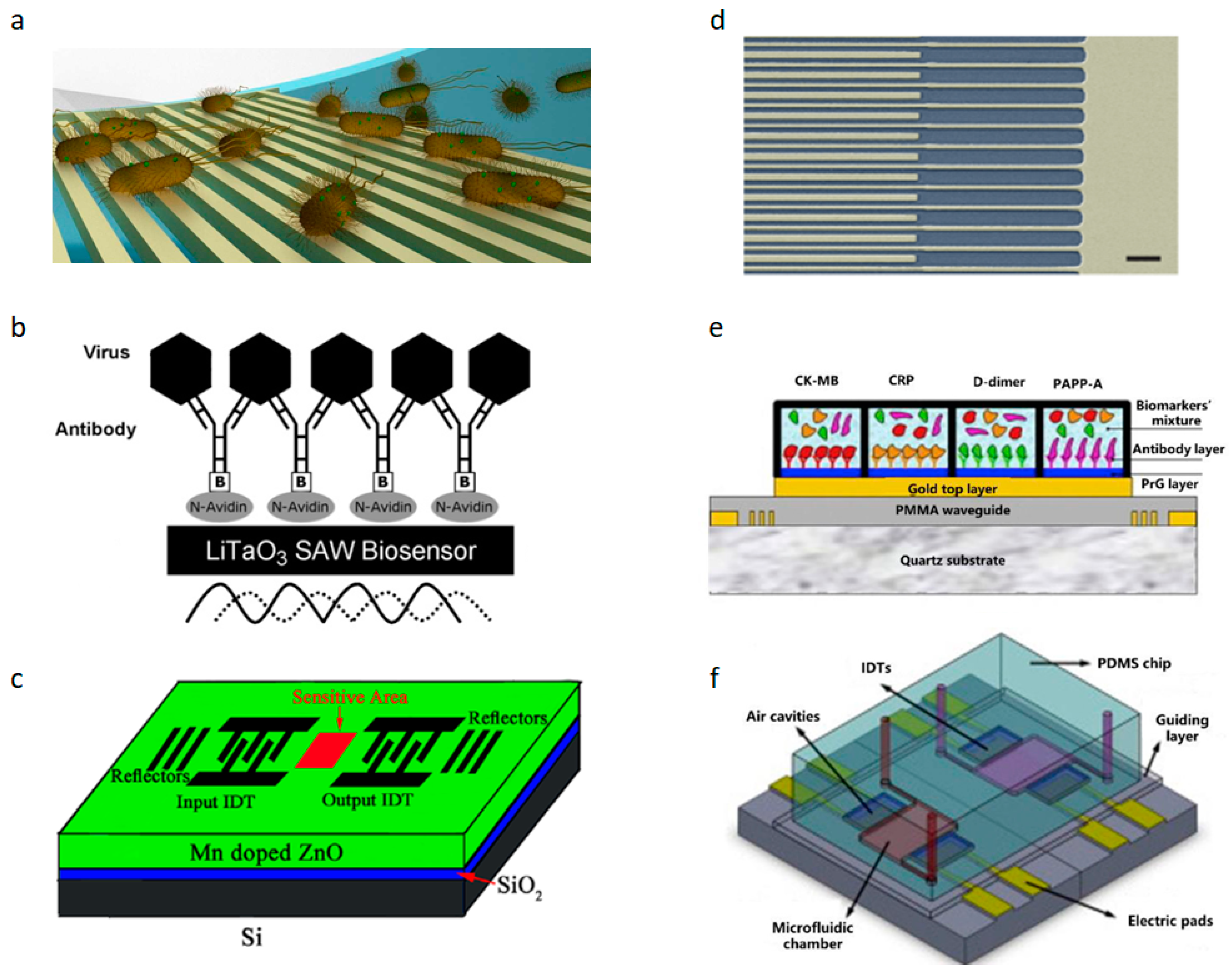
Figure 2a illustrates the fundamental mechanism of using SAW devices for biosensing, which involves functionalizing cells, proteins, and other entities detected within the surface acoustic wave sensing region (delay line) [
1]. UHF-SAW (ultra-high-frequency surface acoustic wave) has been proven to improve the sound intensity, mixing time, signal-to-noise ratio, and more. Surface acoustic wave (SAW) technology has garnered increasing attention in contemporary chip laboratory research in the field of biosensing [
18]. Today, surface acoustic waves (SAWs) serve as a versatile tool for small-scale fluid manipulation in microfluidics and biological applications [
19]. They meet the primary requirements of microfluidics and biosensing due to chip miniaturization and component integration. The exceptional sensitivity and signal-to-noise ratio of SAWs enable their application in lab-on-a-chip (LoC) devices across diverse fields, including amino acids [
20], exosomes [
21], clinical experiments [
22], bacteria and virus detection [
23,
24,
25], environmental monitoring, and protein detection [
7,
26,
27]. Researchers have developed a SH-SAW biosensor for detecting anti-SARS-CoV-2 nucleocapsid antibodies. An evaluation was conducted on rabbit serum collected on different days after injection with the SARS-CoV-2 N protein using both the SH-SAW biosensor and enzyme-linked immunosorbent assay (ELISA) [
28]. Antibodies are utilized as the bio-recognition elements in the biosensor. SAW biosensors have also been applied in the detection of the H1N1 virus, where antibodies specific to viral surface antigens such as hemagglutinin (HA) or neuraminidase (NA) enable specific detection. These biosensors exhibit a low detection limit (LOD) of 1 ng/mL, providing real-time and rapid detection results [
29].
3. Protein Monitoring and Device Fabrication
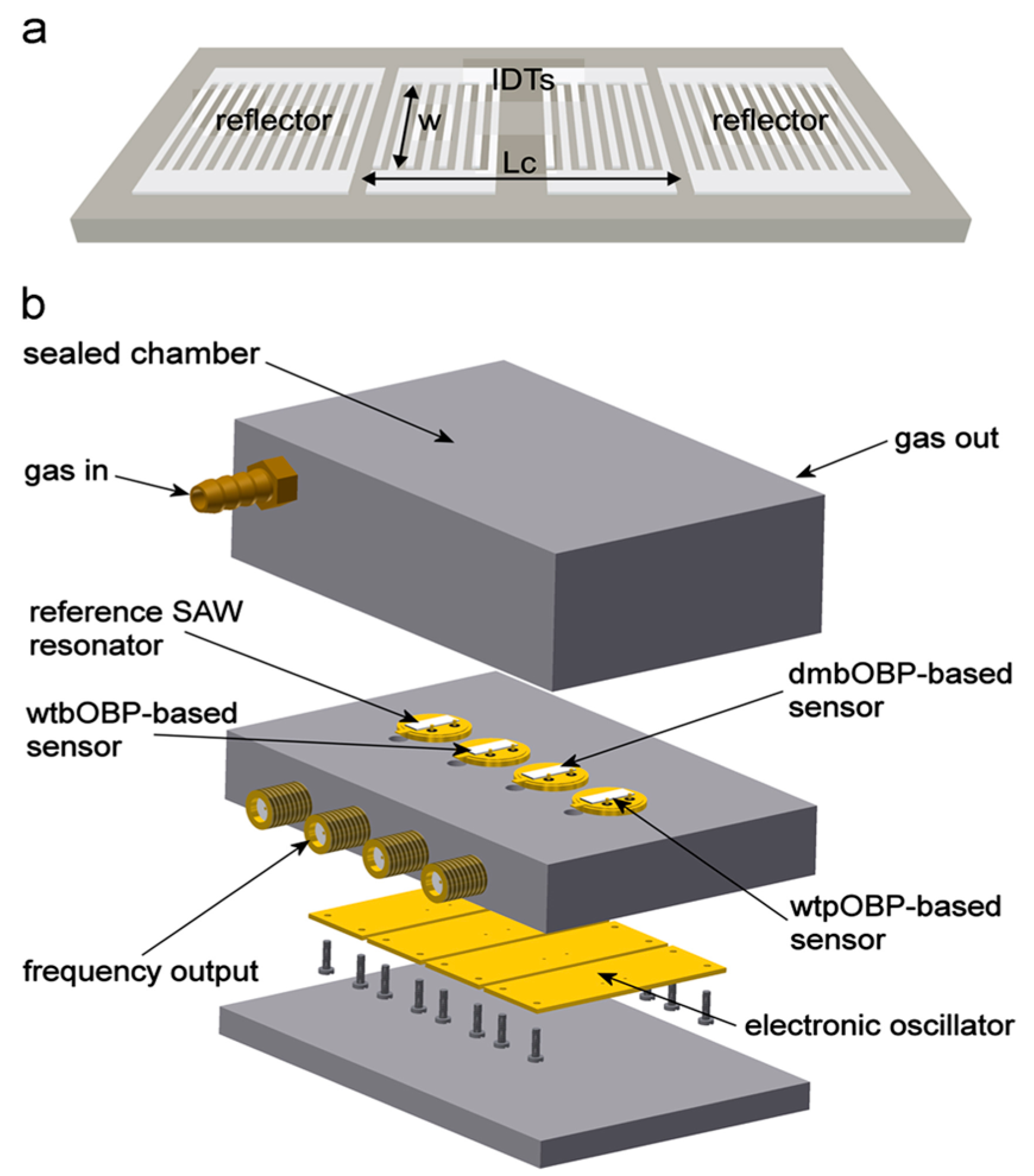
SAW biosensors typically monitor protein concentrations within samples and exhibit high sensitivity and specificity in protein detection, making them invaluable for rapid protein detection. Simultaneously, the demand for label-free protein detection has grown extensively in basic research and clinical practice, where SAW biosensors play a significant role due to their effectiveness in label-free detection. In 2013, F. Di Pietrantonio introduced a novel SAW biosensor array system designed for detecting and identifying various odor molecules with high sensitivity and rapid response times [
35]. The SAW biosensor structure is shown in
Figure 3. The biosensor utilizes radio-frequency magnetron sputtering technology to deposit metal films sourced from high-purity Au and Al targets. Subsequently, a photolithography process defines the sensor’s structure, incorporating an interdigital transducer (IDT) and a reflection grating to both excite and detect Rayleigh waves. The SAW resonator is integrated with a microwave probe to measure the frequency response and monitor changes in SAW velocity, which are crucial for biological and chemical sensing applications. The system is completed with four electronic oscillators housed in a TO39 package and interconnected to the electronic circuit through pin connectors. This biosensor demonstrates high selectivity and real-time monitoring capabilities [
35]. A schematic diagram of the biosensor is shown in
Figure 2b,c.
In 2016, Aleksei Tretjakov and Vitali Syritski first integrated molecularly imprinted polymer (MIP)-based synthetic receptors with a SAW sensing platform for label-free protein detection. Integrating a protein-mip sensing layer with SAW technology cost-effectively fabricates biosensors to analyze biological samples while at high operating frequencies with about an order of magnitude higher mass resolution than the Quartz Crystal Microbalance (QCM) at a lower cost [
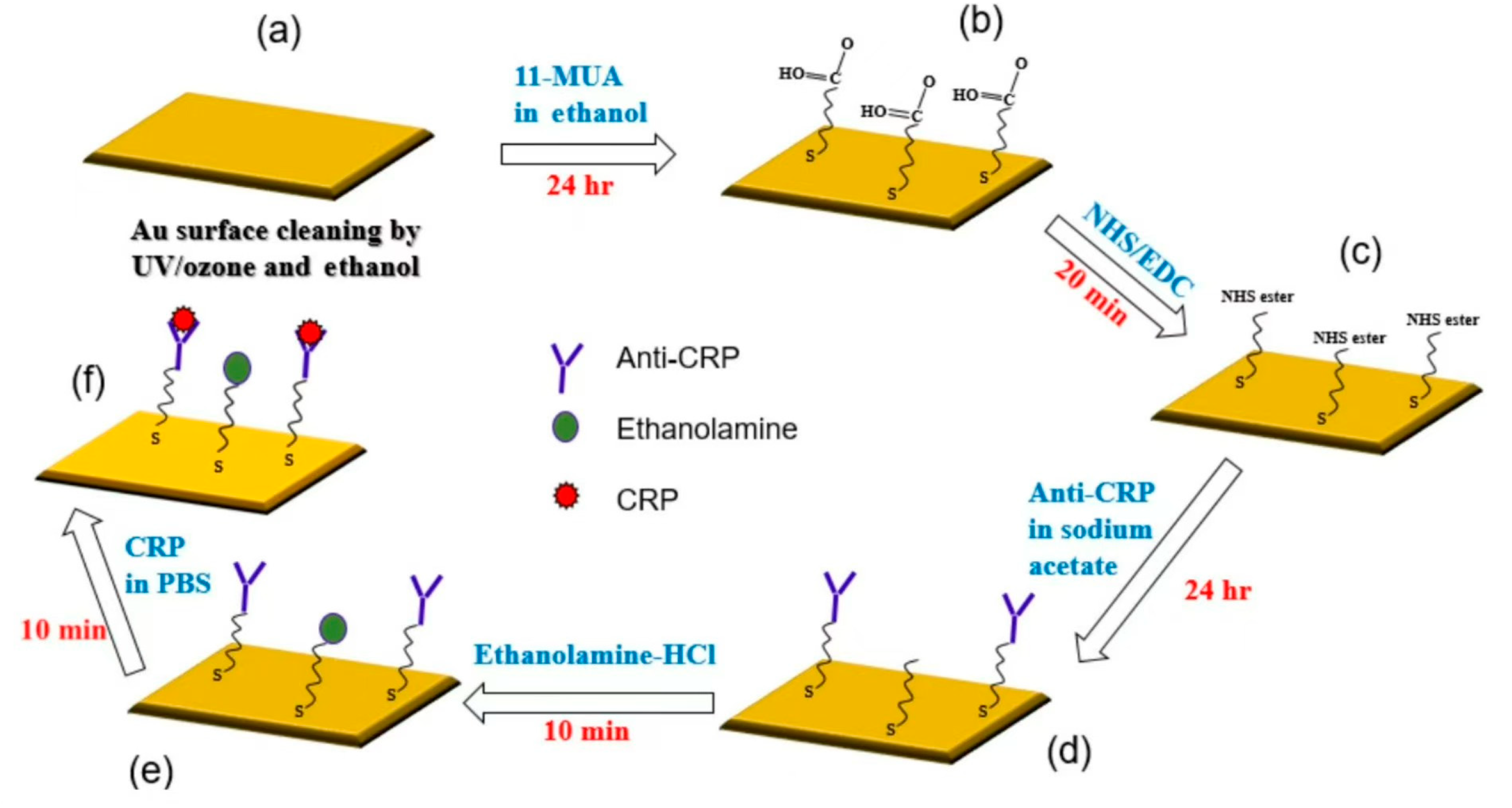
36]. The use of SAW biosensors for monitoring tumor marker proteins for the early detection of cancer is increasingly popular today, with traditional methods such as radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), and fluorescent immunoassay (FIA), as well as chemiluminescence immunoassay (CLIA) that uses SAW biosensors as an alternative detection method due to its complex analytical preparation process, time-consuming and expensive instruments, and high requirements for monitoring personnel. In 2020, researchers developed a SAW biosensor to detect proteins, and the interaction process of sensing is shown in
Figure 4 [
37].
Figure 4 illustrates the process for immobilizing the gold surface and evaluating the CRP/anti-CRP interaction: (a) the gold surface was cleaned with UV/ozone, ethanol, and nitrogen; (b) 11-mercaptoundecanoic acid (11-MUA) was used to form a self-assembled monolayer (SAM) through a 24 h incubation, followed by washing and drying; (c) the SAM was activated with an EDC/NHS mixture and washed with sodium acetate buffer; (d) anti-CRP was immobilized in sodium acetate buffer for 24 h; (e) blocking was performed with ethanolamine-HCl to prevent non-specific binding, followed by washing with PBS buffer; and (f) CRP solutions of varying concentrations were injected for a 10 min interaction, after which the surface was rinsed, dried, and the frequency response was recorded using a vector network analyzer (VNA). The biosensing region of the SAW sensor measures 503 × 2628 μm. The interdigital transducer (IDT) pattern was fabricated on a single 500 μm-thick, 128-degree Y-cut LiNbO
3 wafer, polished on one side, with dimensions of 2 × 2 cm. Aluminum (Al) electrodes with a thickness of 70 nm were deposited using thermal evaporation. The patterning of the electrodes for the SAW IDTs was performed using a conventional lift-off process. A guide layer of silicon dioxide (SiO
2) with a thickness of 100 nm was deposited on top of the device using electron beam evaporation to protect the IDTs and isolate the pattern. Finally, a thin 10 nm layer of gold and a 1 nm chromium (Cr) adhesion layer were deposited on the biosensing region between the two fork-finger transducers by thermal evaporation and lift-off. The printed circuit board (PCB) design measures 4.2 × 1.7 cm, with the chip area being 1.2 × 1.2 cm.
Figure 3 illustrates the steps involved in immobilizing the gold surface and the process of CRP/anti-CRP interaction [
38]. In 2020, P.J. Jandas and Jingting Luo developed a Love mode surface acoustic wave biosensor based on ST-cut quartz for the highly selective, label-free detection of carcinoembryonic antigen (CEA) [
38]. This biosensor demonstrates high selectivity and real-time monitoring capabilities [
35]. Furthermore, in the development of devices, their detection performance can be enhanced by integrating various nanosynthetic materials, opening up new avenues for fabricating more advanced and economical SAW biosensors.
4. Virus and Bacteria Surveillance and Device Fabrication
Detecting bacteria and viruses at concentrations well below health hazard thresholds remains a challenge due to sensitivity, specificity, and selectivity requirements. Established techniques like enzyme-linked immunosorbent assay (ELISA) and surface plasmon resonance (SPR) necessitate analyte pretreatment, with SPR being relatively expensive and unsuitable for portable formats. In contrast, the SAW platform enables the detection of viral biologics without analyte pretreatment, making it viable for portable field applications. SAW biosensors have been employed for the early detection of specific cancer biomarkers and various pathogens causing serious diseases such as AIDS, dengue, and Ebola [
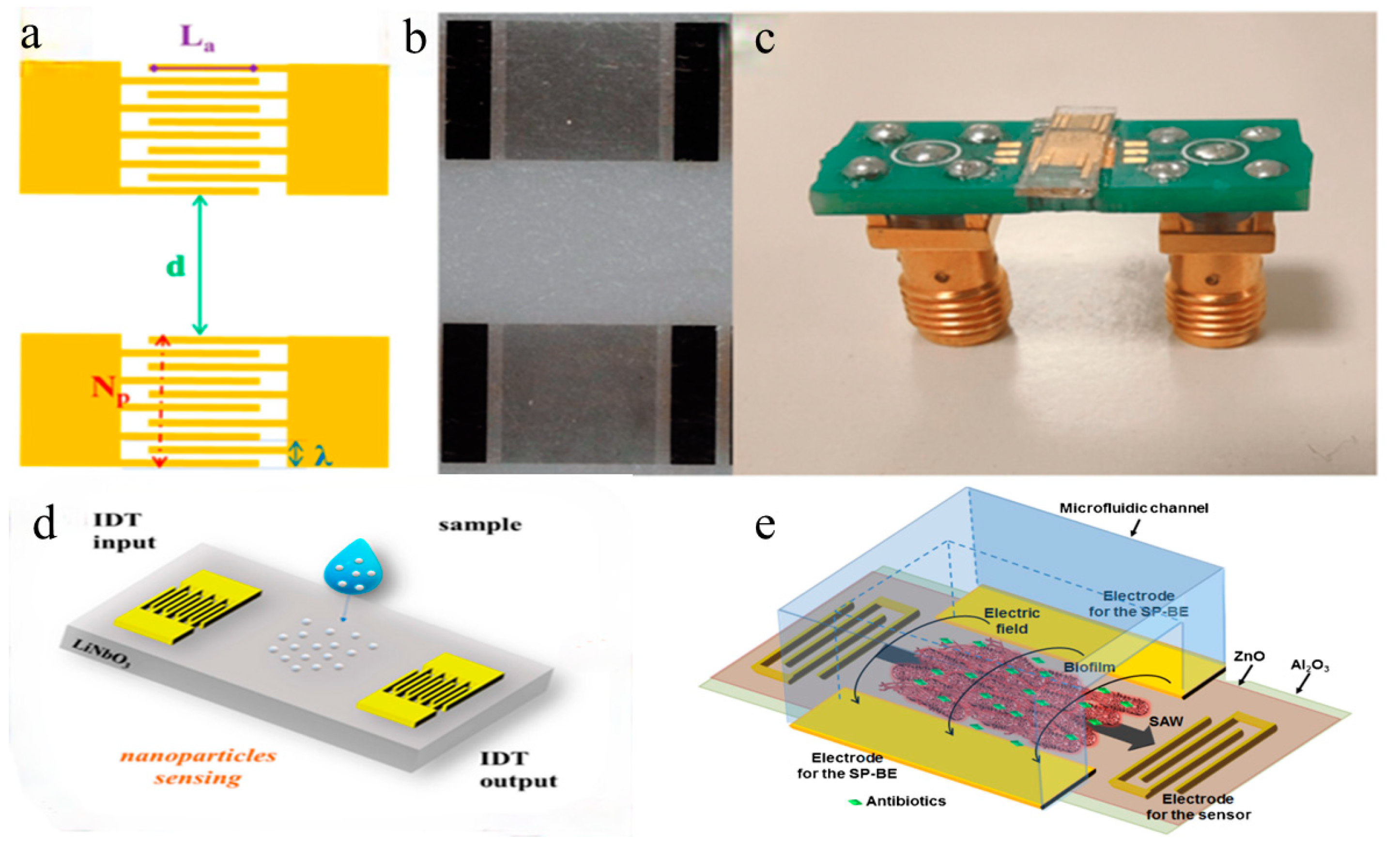
1]. Conventional antibiotic therapy requires 500–5000 times the usual concentration to eliminate infections when bacterial biofilms are involved compared to infections not related to biofilms. Early biofilm detection is critical for effective eradication. In 2016, Young Wook Kim et al. used SAW to establish a microsystem to detect the growth of biofilms [
39]. In 2016, Young Wook Kim et al. utilized surface acoustic wave (SAW) technology to establish a microsystem for detecting the growth of biofilms [
39]. The schematic of the integrated microsystem, which includes the SAW sensor and electrodes for inducing the bioelectric effect, is shown in
Figure 5e. Photolithography was employed to create metal electrodes with a width and spacing of 2 microns to prepare the SAW sensor components. Subsequently, a 500-nanometer-thick zinc oxide (ZnO) piezoelectric film was deposited on the metal electrodes using the atomic layer deposition (ALD) technique. To protect the ZnO film from potential damage caused by bacterial growth media, a layer of aluminum oxide (Al
2O
3) was applied as a protective coating through ALD. The integrated biofilm treatment component involved applying an electric field and antibiotics on a separate set of electrodes on the SAW sensor. This component seamlessly integrated with the SAW sensor to enable real-time monitoring and processing of biofilms. Following this manufacturing process can lead to the development of highly sensitive and reliable SAW sensors for the real-time monitoring and processing of biofilms. The microsystem depicted in
Figure 2d successfully achieves continuous real-time biofilm detection for effective therapy. SAW sensors achieve specific binding with viruses through surface modification, virus capture, and signal detection. Initially, the sensor surface is modified with molecules like antibodies or oligonucleotides capable of binding to target viral components. When the modified sensor contacts a sample containing the virus, specific binding occurs between the virus and immobilized molecules. This binding alters the physical properties of the SAW sensor, such as the velocity, attenuation, and amplitude of the acoustic wave, which are detected via integrated electrodes. These changes serve as a basis for detecting and quantifying the presence of viruses, offering advantages in sensitivity, selectivity, and real-time monitoring for virus detection and diagnostics.
In 2023, researchers utilized SAW sensors to detect simulated nanoparticles/nanoplastics and successfully achieved detection of grapevine leafroll-associated virus 3 (GLRaV-3) [
40].
Figure 5 shows (a) a schematic of a SAW delay line with parametrically optimized design features, (b) optical microscopy images of a SAW delay line, and (c) a SAW device connected to a printed circuit board.
Figure 5d illustrates the SAW device and nanoparticle deposition. SAW sensors operate based on the propagation and reflection of acoustic waves on piezoelectric materials. When an alternating voltage is applied to the interdigital transducers (IDTs) on the piezoelectric material, acoustic waves are generated on the material’s surface. These waves propagate along the surface and are received at the receiver on the other end. Changes to the material’s surface, such as biological recognition events, alter the propagation characteristics of the waves, enabling the detection of these changes. The manufacturing process of SAW sensors includes the following steps: Initially, two identical interdigitated transducers (IDTs) are fabricated on a lithium niobate substrate composed of periodic metal electrodes. Photolithography is then used to coat the lithium niobate substrate with photoresist, followed by exposure and development to pattern the IDTs. After development, a 5-nanometer chromium film and 50-nanometer gold film are thermally evaporated onto the photoresist and subsequently lifted off. Subsequently, the SAW device is bonded to a printed circuit board (PCB) using 25-micrometer aluminum wires. Finally, a network analyzer is used to measure the amplitude and phase of the scattering parameter S12 (transmission signal) to characterize the SAW device. By optimizing the parameters of SAW sensors, their performance can be enhanced for detecting biological and chemical events such as nanoparticles and viruses. These SAW sensors offer the advantages of miniaturization, high sensitivity, and low cost, making them suitable for on-site monitoring and replacing traditional heavy and expensive laboratory instruments.
In 2024, researchers used the piezoelectric material LiNbO
3 as a substrate to fabricate surface acoustic wave (SAW) sensors for rapid detection of the H1N1 virus. This involved preparing input and output interconnected digital transformers (IDTs) on LiNbO
3 crystal substrates. Each IDT consisted of 30 pairs of interconnected electrodes made from aluminum (Al). Fabrication processes such as photolithography and evaporative deposition were employed to deposit these electrodes and additional layers onto the LiNbO
3 substrate. The fabrication process included several sequential layers deposited on the LiNbO
3 substrate. First, an O
2 plasma treatment was applied to generate hydrophilic hydroxyl groups on the substrate’s surface. Following this, an aminopropyltriethoxysilane (APTES) modification layer was introduced, incorporating amino functional groups through condensation reactions with the substrate’s hydroxyl groups. Lastly, a DNA probe was immobilized onto the sensor’s sensitive area by reacting its amino functional groups with those on the APTES modification layer, forming stable covalent bonds [
41]. In 2008, M. Bisoffi and B. Hjelle developed a biosensor using SH-SAW at 325 MHz to monitor class A viral agents [
31]. This biosensor functions as a portable, standalone device for rapid virus detection in field settings. Another example is the 2014 Ebola virus outbreak, during which identification methods included IgM ELISA, RT-PCR, and primarily PCR-based technologies. These methods typically require reagents, refrigeration, and specialized laboratories with trained personnel. In contrast, SAW sensors can promptly detect Ebola virus antigens at the point of care without the need for additional reagents, sample manipulation, or specialized personnel, as illustrated in
Figure 2e. Simple positive/negative results are provided for frontline pandemic workers [
24]. In the context of the current coronavirus pandemic, the SAW biosensor is expected to be a fast, portable, and low-cost tool for detecting it.
5. Cell Monitoring and Device Fabrication
The detection of circulating tumor cells (CTCs) plays a crucial role in the diagnosis and prognostic assessment of tumor metastasis. However, due to the small number of CTCs, common histological methods, radiological assessments, and biomarkers do not provide sufficient information to make a rapid diagnosis. It is of great significance to develop feasible diagnostic methods to accurately, quickly, and easily identify tumor metastases. In 2014, Kai Chang and Yan Pi reported a label-free, high-sensitivity detection method that combines the sensitivity of LSAW (leaky surface acoustic wave) biosensors with the specificity of aptamer recognition for CTC detection and provides a highly versatile platform for CTC detection [
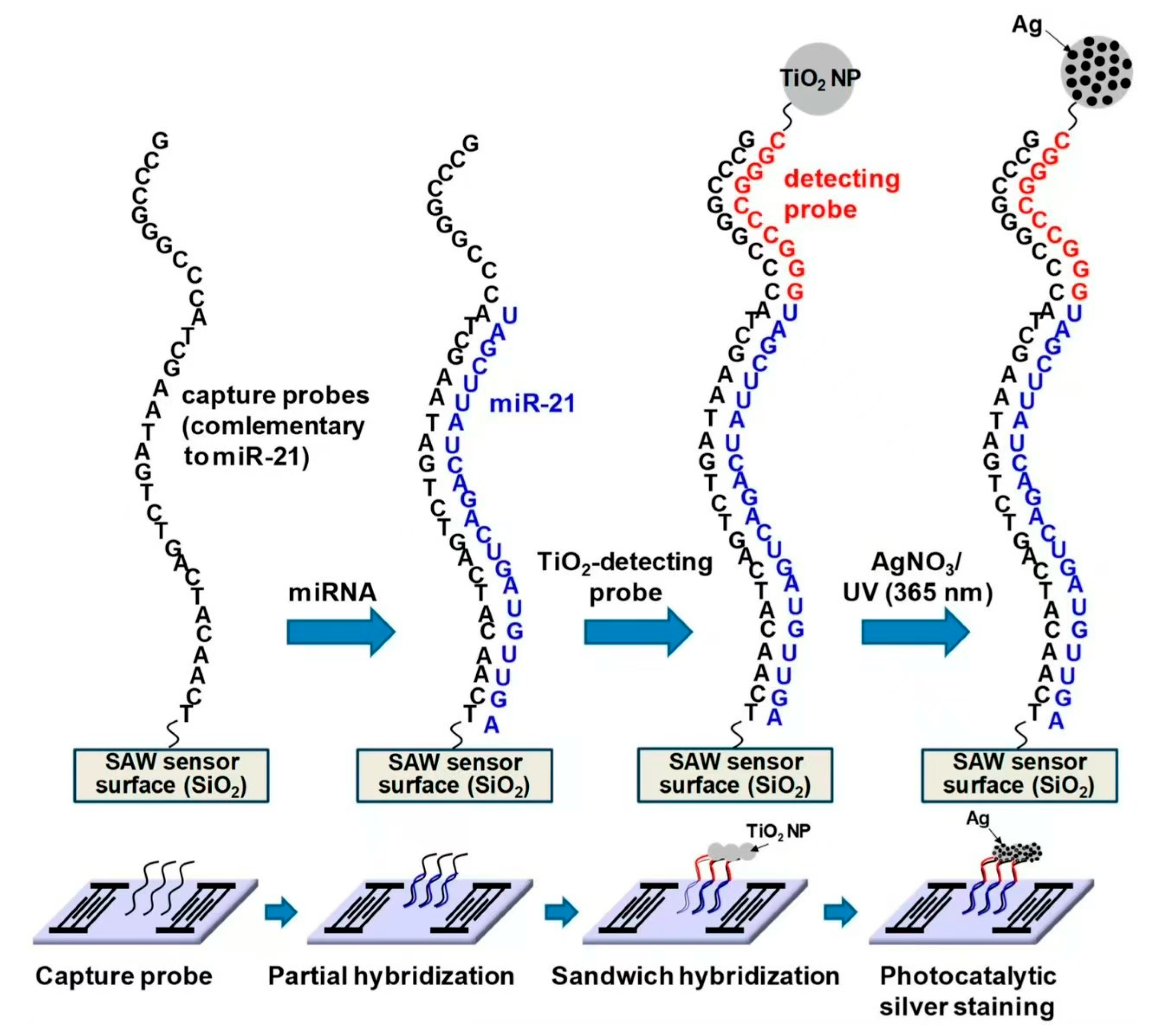
42]. In 2024, researchers developed a device for the simultaneous detection of cancer-origin exosomal microRNAs [
43]. The SAW biosensor detects microRNA molecules within exosomes by measuring changes in acoustic wave signal frequency. The principle involves using a capture probe on the SAW sensor to specifically bind to target microRNA molecules, altering the acoustic wave propagation speed on the sensor surface and thus changing its frequency. By measuring this frequency change, the presence or absence of the target microRNA molecule in the exosome can be determined. The study utilized the method in the schematic depicted in
Figure 6, illustrating the sandwich hybridization format in combination with TiO
2-mediated photocatalytic silver staining on a SAW sensor. The fabrication process of the SAW biosensor includes the following steps: The preparation of the SAW sensor chip involves using a LiTaO
3 wafer as the piezoelectric substrate, with input and output IDT electrodes fabricated on the wafer through photolithography. A SiO
2 bootstrap layer is then deposited on the wafer, followed by a chromium layer for use as an etch mask. Capture probe immobilization proceeds after cleaning and activation of the SiO
2-coated SAW sensor chip, where a 5′-amino-modified DNA capture probe is immobilized on the sensor surface using 3-GPTES (3-aminopropyltriethoxysilane). Temperature and humidity are carefully controlled during this process to prevent evaporation. Detection probe attachment involves dropping a solution containing the detection probe and TiO
2 nanoparticles onto the sensor chip, enabling specific binding to the immobilized capture probe. A fluid control system is constructed using an automatic pump and fluid controller to introduce the sample into the reaction chamber of the sensor chip, with temperature control maintaining stable experimental conditions. Through this fabrication process, highly sensitive and specific SAW biosensors can be prepared for detecting microRNA molecules in exosomes. In 2018, Kaiyue Wang and Wei Zhou developed a microfluidic device featuring a pair of direct interdigital transducers (IDTs) and focused interdigital transducers (FIDTs). The design and fabrication of these transducers employed microelectromechanical systems (MEMS) technology, beginning with the deposition of an aluminum layer onto piezoelectric lithium niobium tantalate (LN, LiNbO
3) substrates to form finger electrodes. For IDTs, the design considerations included the precise wavelength and resonant frequency of surface acoustic waves (SAWs). Similarly, floating interdigital transducers (FIDTs) were fabricated on LN substrates using MEMS technology, with design parameters tailored for transverse surface acoustic waves (TSAWs). Polydimethylsiloxane (PDMS) was employed to create transparent, soft, and chemically resistant microchannels essential for microfluidic device manufacturing. IDTs and FIDTs were securely mounted onto the LN substrate using adhesives or other fixing methods, ensuring alignment with the microchannels. Electrodes were connected to a power supply via wires to facilitate SAW and TSAW generation [
44].
Sound waves are used to isolate different cells that move at a certain frequency due to their different masses and densities. Tumor cells and red blood cells have been separated from the bloodstream by surface acoustic waves (SAWs). As shown in
Figure 2f, tumor cells are concentrated and separated from erythrocytes using SSAW (standing SAW)- and TSAW (traveling pulse SAW)-based multistage sound fields [
44]. The SAW multi-stage acoustic device can sort tumor cells continuously, label-free, and non-invasively compared with traditional methods. By changing the fixed aptamers according to clinical needs, it can detect a wider range of circulating tumor cells (CTCs), offering a promising opportunity for CTC detection. However, before clinical application, more detailed work is required. In 2023, researchers used shear-horizontal surface acoustic wave (SH-SAW) biosensors to analyze red blood cell samples [
45]. This method involves detecting changes in the red blood cell concentration by capturing the cells, as illustrated in the diagram. The process for manufacturing the sensor is as follows: First, a 500-nanometer-thick silicon dioxide (SiO
2) film is coated on a silicate substrate. This layer restricts the propagation of acoustic waves on the chip surface and protects the metal areas from chemical corrosion. Next, the sensor chip undergoes pre-cleaning to remove organic residues and a frequency scan is performed to check its electromechanical functionality. The chip is then subjected to a salinization treatment, which can be achieved via vapor deposition or solution chemistry methods. The purpose of salinization is to make the sensor surface hydrophilic to facilitate red blood cell capture. This process involves immersing the chip in a salinization reagent, followed by drying and curing under appropriate temperature and time conditions. Finally, the salinized sensor is functionalized to specifically capture red blood cells. In 2015, researchers developed a surface acoustic wave (SAW) biosensor for measuring and quantifying cell growth in 2D and 3D cell cultures [
46]. This sensor utilizes changes in the propagation characteristics of acoustic waves in liquid media to measure variations in cell mass. A conceptual diagram is shown in
Figure 7. Specifically, when surface acoustic waves propagate in the liquid medium, their frequency changes, which correlates with mechanical properties of the medium (such as viscosity and density) and electrical properties (such as dielectric constant and conductivity). By measuring these frequency changes, changes in cell density can be quantitatively measured. The manufacturing process of this biosensor includes the following steps: First, interdigital transducers (IDTs) are fabricated on a lithium tantalate (LiTaO
3) substrate using conventional photolithography techniques. Next, a 200-nanometer thick layer of zinc oxide (ZnO) is deposited on the LiTaO
3 substrate using sputtering deposition. Microfluidic wells are then fabricated using traditional PDMS soft lithography techniques. The PDMS wells are bonded to the LiTaO
3 substrate through oxygen plasma treatment. Finally, the fabricated IDTs and PDMS wells are assembled together to form a complete SAW biosensor. Through this manufacturing process, SAW biosensors for measuring cell growth can be produced. These sensors exhibit high sensitivity and reliability, enabling the real-time monitoring and quantification of cell density changes in 2D and 3D cell cultures.
6. Conclusions
This paper explores the versatile applications of SAW biosensors in microbial monitoring, enabled by advancements in synthetic nanomaterials. These biosensors are pivotal for various detection needs, continually benefitting from ongoing improvements and updates in materials. This evolution not only miniaturizes and enhances portability but also improves the overall performance, ensuring accurate microbiological testing. SAW biosensors hold immense promise for enabling convenient home monitoring of diverse microbes. Concurrently, efforts in manufacturing these biosensors involve intricate processes to integrate synthetic nanomaterials onto sensor surfaces. These advancements in fabrication play a crucial role in enhancing sensor reliability and functionality, supporting their wide-ranging applications in microbiological analysis.
Today, sonar devices equipped with high-frequency electronic filter components have been extensively mass-produced. These devices are known for their simplicity of operation and low cost, requiring no expensive optical setups. They are compatible with CMOS and MEMS technologies and are particularly suitable for integration into biosensor chips. Looking ahead, advancements in surface acoustic wave (SAW) biosensors will rely heavily on materials and manufacturing processes. In terms of materials, SAW sensors typically employ highly piezoelectric substrates such as LiNbO3 and LiTaO3, which efficiently generate and receive acoustic wave signals. Moreover, to enhance the sensitivity and selectivity of biosensors, the sensor surfaces are often coated with organic molecules or biomolecules such as proteins or DNA probes, enabling specific binding with target biomolecules. Manufacturing processes for SAW sensors typically involve advanced micro-nanofabrication techniques such as photolithography and physical vapor deposition (PVD) to define and deposit electrodes, filters, and other functional layers. Additionally, surface treatments such as plasma treatment or functional chemical modification are often necessary to enhance biocompatibility and stability, ensuring stable immobilization of biomolecules and long-term sensor performance.
With technological advancements, SAW-based biosensors not only reduce sample requirements but also offer faster response and analysis times, high throughput, automation, and portability, making them indispensable tools in modern bioanalysis and medical diagnostics.