Solution-Processed Bilayered ZnO Electron Transport Layer for Efficient Inverted Non-Fullerene Organic Solar Cells
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Device Fabrication
2.3. Characterization Techniques
3. Results and Discussion
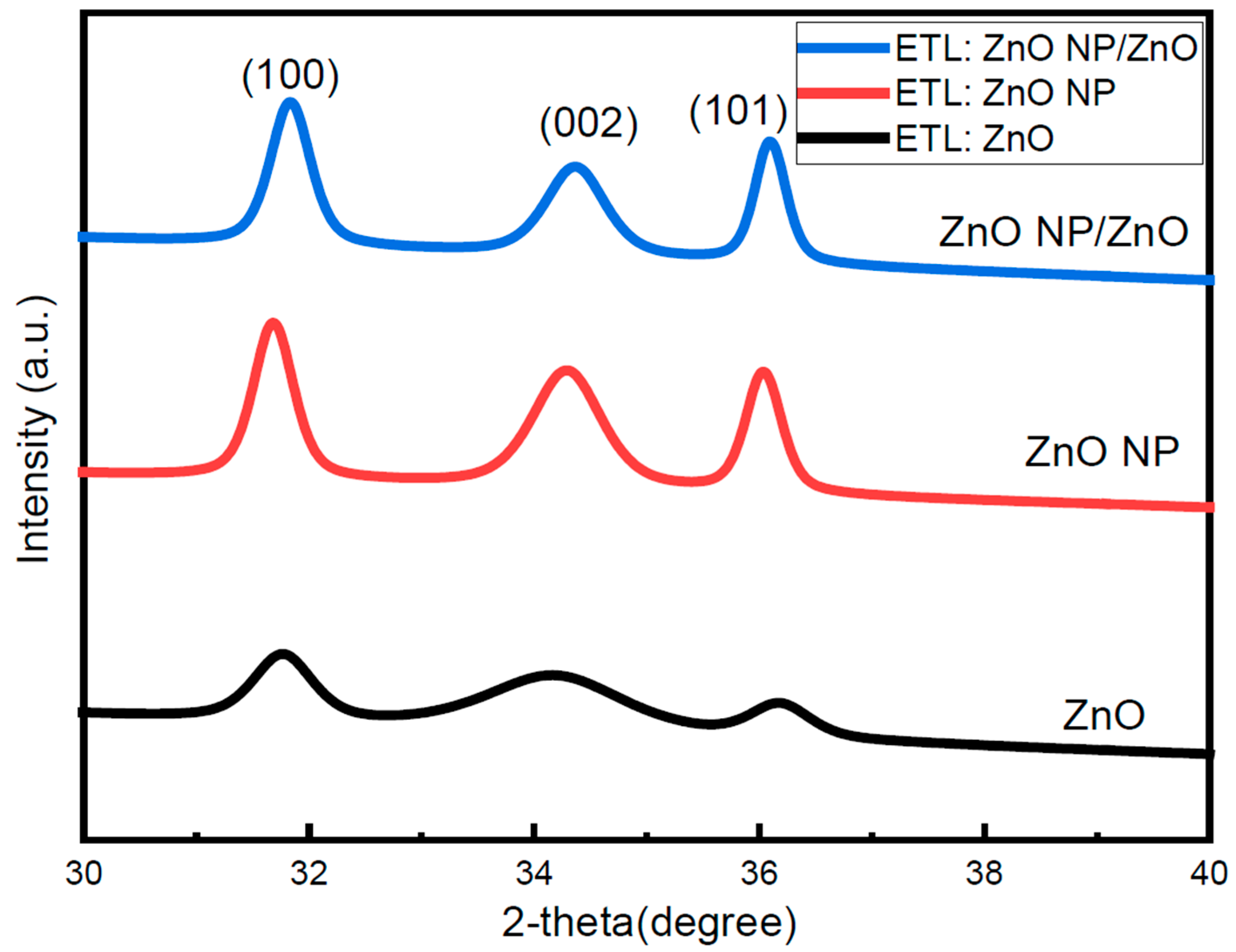
3.1. Structural Properties
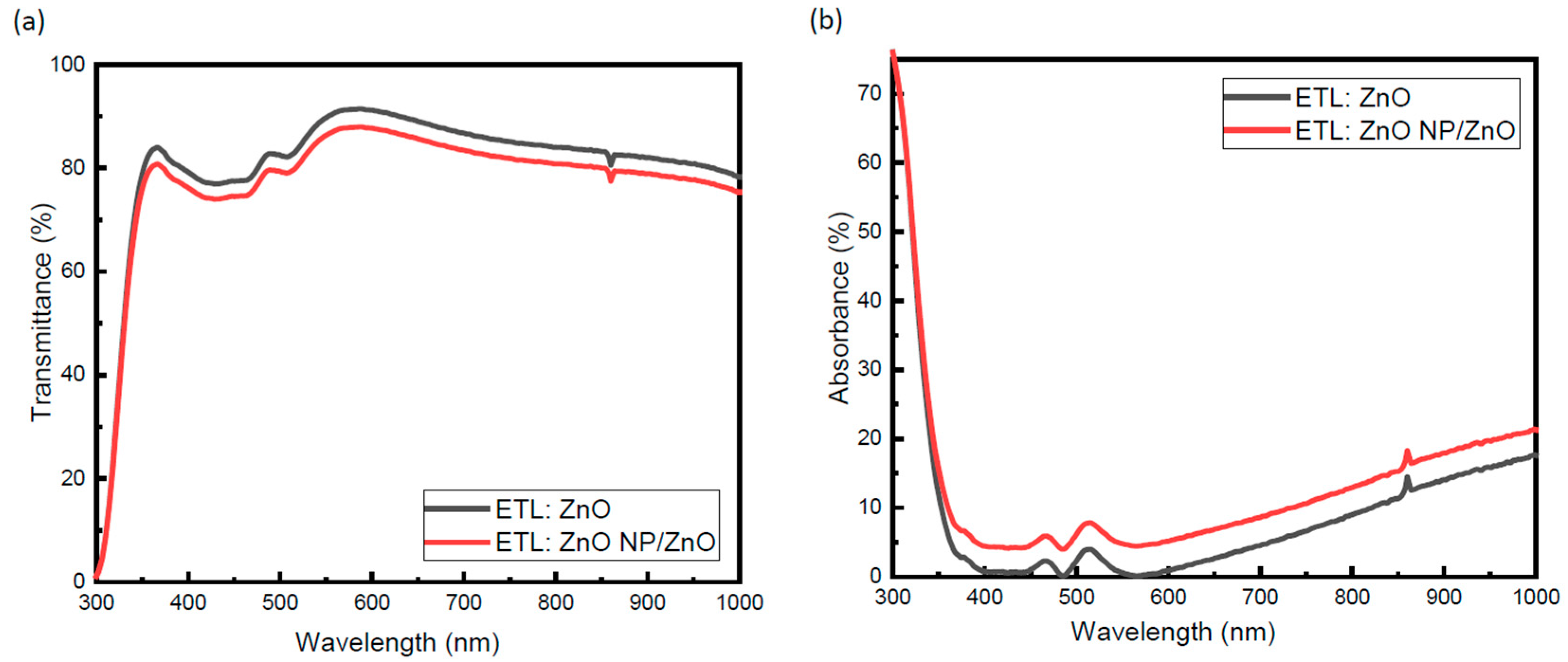
3.2. Optical Properties
3.3. Morphological Properties
3.4. Elemental States
3.5. Photovoltaic Performances
3.6. Stability Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yip, H.-L.; Jen, A.K.-Y. Recent Advances in Solution-Processed Interfacial Materials for Efficient and Stable Polymer Solar Cells. Energy Environ. Sci. 2012, 5, 5994–6011. [Google Scholar] [CrossRef]
- Ongul, F. Solution-Processed Inverted Organic Solar Cell Using V2O5 Hole Transport Layer and Vacuum Free EGaIn Anode. Opt. Mater. 2015, 50, 244–249. [Google Scholar] [CrossRef]
- Fu, H.; Li, Y.; Yu, J.; Wu, Z.; Fan, Q.; Lin, F.; Woo, H.Y.; Gao, F.; Zhu, Z.; Jen, A.K.-Y. High Efficiency (15.8%) All-Polymer Solar Cells Enabled by a Regioregular Narrow Bandgap Polymer Acceptor. J. Am. Chem. Soc. 2021, 143, 2665–2670. [Google Scholar] [CrossRef]
- Sun, C.; Pan, F.; Bin, H.; Zhang, J.; Xue, L.; Qiu, B.; Wei, Z.; Zhang, Z.-G.; Li, Y. A Low Cost and High Performance Polymer Donor Material for Polymer Solar Cells. Nat. Commun. 2018, 9, 743. [Google Scholar] [CrossRef]
- Chen, J.-D.; Cui, C.; Li, Y.-Q.; Zhou, L.; Ou, Q.-D.; Li, C.; Li, Y.; Tang, J.-X. Single-Junction Polymer Solar Cells Exceeding 10% Power Conversion Efficiency. Adv. Mater. 2015, 27, 1035–1041. [Google Scholar] [CrossRef]
- He, Z.; Xiao, B.; Liu, F.; Wu, H.; Yang, Y.; Xiao, S.; Wang, C.; Russell, T.P.; Cao, Y. Single-Junction Polymer Solar Cells with High Efficiency and Photovoltage. Nat. Photon 2015, 9, 174–179. [Google Scholar] [CrossRef]
- Chen, H.; Hu, D.; Yang, Q.; Gao, J.; Fu, J.; Yang, K.; He, H.; Chen, S.; Kan, Z.; Duan, T.; et al. All-Small-Molecule Organic Solar Cells with an Ordered Liquid Crystalline Donor. Joule 2019, 3, 3034–3047. [Google Scholar] [CrossRef]
- Zhou, R.; Jiang, Z.; Yang, C.; Yu, J.; Feng, J.; Adil, M.A.; Deng, D.; Zou, W.; Zhang, J.; Lu, K.; et al. All-Small-Molecule Organic Solar Cells with over 14% Efficiency by Optimizing Hierarchical Morphologies. Nat. Commun. 2019, 10, 5393. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Y.; Meng, L.; Han, C.; Wan, X.; Chen, Y. Recent Progress in All-Small-Molecule Organic Solar Cells. Small 2023, 19, 2205594. [Google Scholar] [CrossRef] [PubMed]
- Eisner, F.; Seitkhan, A.; Han, Y.; Khim, D.; Yengel, E.; Kirmani, A.R.; Xu, J.; de Arquer, F.P.G.; Sargent, E.H.; Amassian, A.; et al. Solution-Processed In2O3/ZnO Heterojunction Electron Transport Layers for Efficient Organic Bulk Heterojunction and Inorganic Colloidal Quantum-Dot Solar Cells. Sol. RRL 2018, 2, 1800076. [Google Scholar] [CrossRef]
- Bashir, R.; Kashif Bilal, M.; Bashir, A.; Zhao, J.; Asif, S.U.; Ahmad, W.; Xie, J.; Hu, W. A Low-Temperature Solution-Processed Indium Incorporated Zinc Oxide Electron Transport Layer for High-Efficiency Lead Sulfide Colloidal Quantum Dot Solar Cells. Nanoscale 2021, 13, 12991–12999. [Google Scholar] [CrossRef]
- Ren, X.; Yang, D.; Yang, Z.; Feng, J.; Zhu, X.; Niu, J.; Liu, Y.; Zhao, W.; Liu, S.F. Solution-Processed Nb:SnO2 Electron Transport Layer for Efficient Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 2421–2429. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei, H.; Li, B.; Wan, J.; et al. Low-Temperature Solution-Processed Tin Oxide as an Alternative Electron Transporting Layer for Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2015, 137, 6730–6733. [Google Scholar] [CrossRef]
- Ma, J.; Lin, Z.; Guo, X.; Zhou, L.; Su, J.; Zhang, C.; Yang, Z.; Chang, J.; Liu, S.; Hao, Y. Low-Temperature Solution-Processed ZnO Electron Transport Layer for Highly Efficient and Stable Planar Perovskite Solar Cells with Efficiency Over 20%. Sol. RRL 2019, 3, 1900096. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Elumalai, N.K.; Upama, M.B.; Wang, D.; Chan, K.H.; Wright, M.; Xu, C.; Haque, F.; Uddin, A. Low Temperature Processed ZnO Thin Film as Electron Transport Layer for Efficient Perovskite Solar Cells. Sol. Energy Mater. Sol. Cells 2017, 159, 251–264. [Google Scholar] [CrossRef]
- Jørgensen, M.; Norrman, K.; Krebs, F.C. Stability/Degradation of Polymer Solar Cells. Sol. Energy Mater. Sol. Cells 2008, 92, 686–714. [Google Scholar] [CrossRef]
- Salsberg, E.; Aziz, H. Degradation of PEDOT:PSS Hole Injection Layers by Electrons in Organic Light Emitting Devices. Org. Electron. 2019, 69, 313–319. [Google Scholar] [CrossRef]
- Vohra, V.; Kawashima, K.; Kakara, T.; Koganezawa, T.; Osaka, I.; Takimiya, K.; Murata, H. Efficient Inverted Polymer Solar Cells Employing Favourable Molecular Orientation. Nat. Photon 2015, 9, 403–408. [Google Scholar] [CrossRef]
- Small, C.E.; Chen, S.; Subbiah, J.; Amb, C.M.; Tsang, S.-W.; Lai, T.-H.; Reynolds, J.R.; So, F. High-Efficiency Inverted Dithienogermole–Thienopyrrolodione-Based Polymer Solar Cells. Nat. Photon 2012, 6, 115–120. [Google Scholar] [CrossRef]
- Sun, Y.; Seo, J.H.; Takacs, C.J.; Seifter, J.; Heeger, A.J. Inverted Polymer Solar Cells Integrated with a Low-Temperature-Annealed Sol-Gel-Derived ZnO Film as an Electron Transport Layer. Adv. Mater. 2011, 23, 1679–1683. [Google Scholar] [CrossRef]
- Kyaw, A.K.K.; Sun, X.W.; Jiang, C.Y.; Lo, G.Q.; Zhao, D.W.; Kwong, D.L. An Inverted Organic Solar Cell Employing a Sol-Gel Derived ZnO Electron Selective Layer and Thermal Evaporated MoO3 Hole Selective Layer. Appl. Phys. Lett. 2008, 93, 221107. [Google Scholar] [CrossRef]
- Cowan, S.R.; Schulz, P.; Giordano, A.J.; Garcia, A.; MacLeod, B.A.; Marder, S.R.; Kahn, A.; Ginley, D.S.; Ratcliff, E.L.; Olson, D.C. Chemically Controlled Reversible and Irreversible Extraction Barriers Via Stable Interface Modification of Zinc Oxide Electron Collection Layer in Polycarbazole-Based Organic Solar Cells. Adv. Funct. Mater. 2014, 24, 4671–4680. [Google Scholar] [CrossRef]
- Huang, J.-H.; Wei, H.-Y.; Huang, K.-C.; Chen, C.-L.; Wang, R.-R.; Chen, F.-C.; Ho, K.-C.; Chu, C.-W. Using a Low Temperature Crystallization Process to Prepare Anatase TiO2 Buffer Layers for Air-Stable Inverted Polymer Solar Cells. Energy Environ. Sci. 2010, 3, 654–658. [Google Scholar] [CrossRef]
- Tran, V.-H.; Eom, S.H.; Yoon, S.C.; Kim, S.-K.; Lee, S.-H. Enhancing Device Performance of Inverted Organic Solar Cells with SnO2/Cs2CO3 as Dual Electron Transport Layers. Org. Electron. 2019, 68, 85–95. [Google Scholar] [CrossRef]
- Yang, H.B.; Dong, Y.Q.; Wang, X.; Khoo, S.Y.; Liu, B. Cesium Carbonate Functionalized Graphene Quantum Dots as Stable Electron-Selective Layer for Improvement of Inverted Polymer Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, C.; Ji, G.; Han, Y.; Ismail, I.; Li, H.; Luo, Q.; Yang, J.; Ma, C.-Q. Roll-to-Roll Printed Stable and Thickness-Independent ZnO:PEI Composite Electron Transport Layer for Inverted Organic Solar Cells. Sol. Energy 2019, 193, 102–110. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, C.; Xie, C.; Li, W. Flexible Organic Solar Cells: Materials, Large-Area Fabrication Techniques and Potential Applications. Nano Energy 2021, 89, 106399. [Google Scholar] [CrossRef]
- Wang, J.-C.; Weng, W.-T.; Tsai, M.-Y.; Lee, M.-K.; Horng, S.-F.; Perng, T.-P.; Kei, C.-C.; Yu, C.-C.; Meng, H.-F. Highly Efficient Flexible Inverted Organic Solar Cells Using Atomic Layer Deposited ZnO as Electron Selective Layer. J. Mater. Chem. 2010, 20, 862–866. [Google Scholar] [CrossRef]
- Ko, S.H.; Lee, D.; Kang, H.W.; Nam, K.H.; Yeo, J.Y.; Hong, S.J.; Grigoropoulos, C.P.; Sung, H.J. Nanoforest of Hydrothermally Grown Hierarchical ZnO Nanowires for a High Efficiency Dye-Sensitized Solar Cell. Nano Lett. 2011, 11, 666–671. [Google Scholar] [CrossRef]
- Sanchez, S.; Berson, S.; Guillerez, S.; Lévy-Clément, C.; Ivanova, V. Toward High-Stability Inverted Polymer Solar Cells with an Electrodeposited ZnO Electron Transporting Layer. Adv. Energy Mater. 2012, 2, 541–545. [Google Scholar] [CrossRef]
- Lin, R.; Miwa, M.; Wright, M.; Uddin, A. Optimisation of the Sol–Gel Derived ZnO Buffer Layer for Inverted Structure Bulk Heterojunction Organic Solar Cells Using a Low Band Gap Polymer. Thin Solid Film. 2014, 566, 99–107. [Google Scholar] [CrossRef]
- Soultati, A.; Fakharuddin, A.; Polydorou, E.; Drivas, C.; Kaltzoglou, A.; Haider, M.I.; Kournoutas, F.; Fakis, M.; Palilis, L.C.; Kennou, S.; et al. Lithium Doping of ZnO for High Efficiency and Stability Fullerene and Non-Fullerene Organic Solar Cells. ACS Appl. Energy Mater. 2019, 2, 1663–1675. [Google Scholar] [CrossRef]
- Xu, X.; Xiao, J.; Zhang, G.; Wei, L.; Jiao, X.; Yip, H.-L.; Cao, Y. Interface-Enhanced Organic Solar Cells with Extrapolated T80 Lifetimes of over 20 years. Sci. Bull. 2020, 65, 208–216. [Google Scholar] [CrossRef]
- Rahaman, H.; Sang, B.; Hossain, A.; Hoex, B.; Mota-Santiago, P.; Mitchell, V.D.; Uddin, A.; Stride, J.A. Impact of the Bilayer Electron Transport Layer in the Donor Acceptor Bulk Heterojunctions for Improved Inverted Organic Photovoltaic Performance. Appl. Surf. Sci. 2023, 612, 155669. [Google Scholar] [CrossRef]
- Gong, X. Toward High Performance Inverted Polymer Solar Cells. Polymer 2012, 53, 5437–5448. [Google Scholar] [CrossRef][Green Version]
- Jin, W.-Y.; Ginting, R.T.; Jin, S.-H.; Kang, J.-W. Highly Stable and Efficient Inverted Organic Solar Cells Based on Low-Temperature Solution-Processed PEIE and ZnO Bilayers. J. Mater. Chem. A 2016, 4, 3784–3791. [Google Scholar] [CrossRef]
- Cheng, H.-W.; Raghunath, P.; Wang, K.; Cheng, P.; Haung, T.; Wu, Q.; Yuan, J.; Lin, Y.-C.; Wang, H.-C.; Zou, Y.; et al. Potassium-Presenting Zinc Oxide Surfaces Induce Vertical Phase Separation in Fullerene-Free Organic Photovoltaics. Nano Lett. 2020, 20, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhang, S.; Wang, J.; Zhang, J.; Zhang, D.; Zhang, Y.; Wei, Z.; Tang, Z.; Hou, J.; Zhou, H. Exquisite Modulation of ZnO Nanoparticle Electron Transporting Layer for High-Performance Fullerene-Free Organic Solar Cell with Inverted Structure. J. Mater. Chem. A 2019, 7, 3570–3576. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ko, S.-J.; Eom, S.H.; Kim, H.; Kim, D.W.; Lee, C.; Yoon, S.C. Composite Interlayer Consisting of Alcohol-Soluble Polyfluorene and Carbon Nanotubes for Efficient Polymer Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 14244–14253. [Google Scholar] [CrossRef]
- Upama, M.B.; Elumalai, N.K.; Mahmud, M.A.; Wright, M.; Wang, D.; Xu, C.; Haque, F.; Chan, K.H.; Uddin, A. Interfacial Engineering of Electron Transport Layer Using Caesium Iodide for Efficient and Stable Organic Solar Cells. Appl. Surf. Sci. 2017, 416, 834–844. [Google Scholar] [CrossRef]
- Huang, S.; Kang, B.; Duan, L.; Zhang, D. Highly Efficient Inverted Polymer Solar Cells by Using Solution Processed MgO/ZnO Composite Interfacial Layers. J. Colloid Interface Sci. 2021, 583, 178–187. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, B.; Fang, J.; Zhai, T.; Wang, Y.; Li, G.; Zhang, J.; Wei, Z.; Duhm, S.; Guo, X.; et al. Surface Modification of ZnO Electron Transport Layers with Glycine for Efficient Inverted Non-Fullerene Polymer Solar Cells. Org. Electron. 2019, 70, 25–31. [Google Scholar] [CrossRef]
- Pan, W.; Han, Y.; Wang, Z.; Gong, C.; Guo, J.; Lin, J.; Luo, Q.; Yang, S.; Ma, C.-Q. An Efficiency of 14.29% and 13.08% for 1 cm2 and 4 cm2 Flexible Organic Solar Cells Enabled by Sol–Gel ZnO and ZnO Nanoparticle Bilayer Electron Transporting Layers. J. Mater. Chem. A 2021, 9, 16889–16897. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Elumalai, N.K.; Upama, M.B.; Wang, D.; Soufiani, A.M.; Wright, M.; Xu, C.; Haque, F.; Uddin, A. Solution-Processed Lithium-Doped ZnO Electron Transport Layer for Efficient Triple Cation (Rb, MA, FA) Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 33841–33854. [Google Scholar] [CrossRef]
- Duan, L.; Guli, M.; Zhang, Y.; Yi, H.; Haque, F.; Uddin, A. The Air Effect in the Burn-In Thermal Degradation of Nonfullerene Organic Solar Cells. Energy Technol. 2020, 8, 1901401. [Google Scholar] [CrossRef]
- Xu, C.; Wright, M.; Elumalai, N.K.; Mahmud, M.A.; Wang, D.; Upama, M.B.; Haque, F.; Uddin, A. Highly Crystalline Bilayer Electron Transport Layer for Efficient Conjugated Polymer Solar Cells. Curr. Appl. Phys. 2018, 18, 505–511. [Google Scholar] [CrossRef]
- Upama, M.B.; Elumalai, N.K.; Mahmud, M.A.; Xu, C.; Wang, D.; Wright, M.; Uddin, A. Enhanced Electron Transport Enables over 12% Efficiency by Interface Engineering of Non-Fullerene Organic Solar Cells. Sol. Energy Mater. Sol. Cells 2018, 187, 273–282. [Google Scholar] [CrossRef]
- Rahaman, M.H.; Holland, J.; Hossain, M.A.; Duan, L.; Hoex, B.; Mota-Santiago, P.; Mitchell, V.D.; Uddin, A.; Stride, J.A. Increased Efficiency of Organic Solar Cells by Seeded Control of the Molecular Morphology in the Active Layer. Sol. RRL 2022, 6, 2200184. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Fakhar-e-Alam, M.; Rahim, S.; Atif, M.; Aziz, M.H.; Malick, M.I.; Zaidi, S.S.Z.; Suleman, R.; Majid, A. ZnO Nanoparticles as Drug Delivery Agent for Photodynamic Therapy. Laser Phys. Lett. 2013, 11, 025601. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Vijila, C.; Jose, R.; Ming, K.Z.; Saha, A.; Ramakrishna, S. Simultaneous Improvements in Power Conversion Efficiency and Operational Stability of Polymer Solar Cells by Interfacial Engineering. Phys. Chem. Chem. Phys. 2013, 15, 19057–19064. [Google Scholar] [CrossRef]
- Jao, M.-H.; Liao, H.-C.; Su, W.-F. Achieving a High Fill Factor for Organic Solar Cells. J. Mater. Chem. A 2016, 4, 5784–5801. [Google Scholar] [CrossRef]
- Kumar, E.N.; Jose, R.; Archana, P.S.; Vijila, C.; Yusoff, M.M.; Ramakrishna, S. High Performance Dye-Sensitized Solar Cells with Record Open Circuit Voltage Using Tin Oxide Nanoflowers Developed by Electrospinning. Energy Environ. Sci. 2012, 5, 5401–5407. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Q.; Jiang, L.; Cao, G. ZnO Cathode Buffer Layers for Inverted Polymer Solar Cells. Energy Environ. Sci. 2015, 8, 3442–3476. [Google Scholar] [CrossRef]
- Kim, H.P.; Yusoff, A.R.B.M.; Lee, H.J.; Lee, S.J.; Kim, H.M.; Seo, G.J.; Youn, J.H.; Jang, J. Effect of ZnO:Cs2CO3 on the Performance of Organic Photovoltaics. Nanoscale Res. Lett. 2014, 9, 323. [Google Scholar] [CrossRef]
- Na, S.-I.; Kim, S.-S.; Jo, J.; Kim, D.-Y. Efficient and Flexible ITO-Free Organic Solar Cells Using Highly Conductive Polymer Anodes. Adv. Mater. 2008, 20, 4061–4067. [Google Scholar] [CrossRef]
- Fan, P.; Zhang, D.; Wu, Y.; Yu, J.; Russell, T.P. Polymer-Modified ZnO Nanoparticles as Electron Transport Layer for Polymer-Based Solar Cells. Adv. Funct. Mater. 2020, 30, 2002932. [Google Scholar] [CrossRef]
- Han, C.; Cheng, Y.; Chen, L.; Qian, L.; Yang, Z.; Xue, W.; Zhang, T.; Yang, Y.; Cao, W. Enhanced Performance of Inverted Polymer Solar Cells by Combining ZnO Nanoparticles and Poly[(9,9-Bis(3′-(N,N-Dimethylamino)Propyl)-2,7-Fluorene)-Alt-2,7-(9,9-Dioctyfluorene)] as Electron Transport Layer. ACS Appl. Mater. Interfaces 2016, 8, 3301–3307. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, H.; Shi, J.; Dong, J.; Luo, Y.; Li, D.; Meng, Q. Highly Efficient Planar Perovskite Solar Cells with a TiO2/ZnO Electron Transport Bilayer. J. Mater. Chem. A 2015, 3, 19288–19293. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Herrera-Gomez, A.; Cabrera-German, D.; Dutoi, A.D.; Vazquez-Lepe, M.; Aguirre-Tostado, S.; Pianetta, P.; Nordlund, D.; Cortazar-Martinez, O.; Torres-Ochoa, A.; Ceballos-Sanchez, O.; et al. Intensity Modulation of the Shirley Background of the Cr 3p Spectra with Photon Energies around the Cr 2p Edge. Surf. Interface Anal. 2018, 50, 246–252. [Google Scholar] [CrossRef]
- Yi, H.; Wang, D.; Mahmud, M.A.; Haque, F.; Upama, M.B.; Xu, C.; Duan, L.; Uddin, A. Bilayer SnO2 as Electron Transport Layer for Highly Efficient Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 6027–6039. [Google Scholar] [CrossRef]
- Awan, S.U.; Hasanain, S.K.; Bertino, M.F.; Jaffari, G.H. Ferromagnetism in Li Doped ZnO Nanoparticles: The Role of Interstitial Li. J. Appl. Phys. 2012, 112, 103924. [Google Scholar] [CrossRef]
- Hsieh, P.-T.; Chen, Y.-C.; Kao, K.-S.; Wang, C.-M. Luminescence Mechanism of ZnO Thin Film Investigated by XPS Measurement. Appl. Phys. A 2008, 90, 317–321. [Google Scholar] [CrossRef]
- Wu, C.-K.; Sivashanmugan, K.; Guo, T.-F.; Wen, T.-C. Enhancement of Inverted Polymer Solar Cells Performances Using Cetyltrimethylammonium-Bromide Modified ZnO. Materials 2018, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Dongaonkar, S.; Servaites, J.D.; Ford, G.M.; Loser, S.; Moore, J.; Gelfand, R.M.; Mohseni, H.; Hillhouse, H.W.; Agrawal, R.; Ratner, M.A.; et al. Universality of Non-Ohmic Shunt Leakage in Thin-Film Solar Cells. J. Appl. Phys. 2010, 108, 124509. [Google Scholar] [CrossRef]
- Huang, D.; Li, Y.; Xu, Z.; Zhao, S.; Zhao, L.; Zhao, J. Enhanced Performance and Morphological Evolution of PTB7:PC71BM Polymer Solar Cells by Using Solvent Mixtures with Different Additives. Phys. Chem. Chem. Phys. 2015, 17, 8053–8060. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zhang, Y.; Yi, H.; Haque, F.; Deng, R.; Guan, H.; Zou, Y.; Uddin, A. Trade-Off between Exciton Dissociation and Carrier Recombination and Dielectric Properties in Y6-Sensitized Nonfullerene Ternary Organic Solar Cells. Energy Technol. 2020, 8, 1900924. [Google Scholar] [CrossRef]
- Huang, J.-S.; Chou, C.-Y.; Lin, C.-F. Efficient and Air-Stable Polymer Photovoltaic Devices with \hboxWO_3–\hboxV_2\hboxO_5 Mixed Oxides as Anodic Modification. IEEE Electron. Device Lett. 2010, 31, 332–334. [Google Scholar] [CrossRef]
- Proctor, C.M.; Nguyen, T.-Q. Effect of Leakage Current and Shunt Resistance on the Light Intensity Dependence of Organic Solar Cells. Appl. Phys. Lett. 2015, 106, 083301. [Google Scholar] [CrossRef]
- Garcia-Belmonte, G.; Guerrero, A.; Bisquert, J. Elucidating Operating Modes of Bulk-Heterojunction Solar Cells from Impedance Spectroscopy Analysis. J. Phys. Chem. Lett. 2013, 4, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, Z.; Yang, Y. Low-Work-Function Surface Formed by Solution-Processed and Thermally Deposited Nanoscale Layers of Cesium Carbonate. Adv. Funct. Mater. 2007, 17, 1966–1973. [Google Scholar] [CrossRef]
- You, J.; Chen, C.-C.; Dou, L.; Murase, S.; Duan, H.-S.; Hawks, S.A.; Xu, T.; Son, H.J.; Yu, L.; Li, G.; et al. Metal Oxide Nanoparticles as an Electron-Transport Layer in High-Performance and Stable Inverted Polymer Solar Cells. Adv. Mater. 2012, 24, 5267–5272. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Guo, X.; Wang, Z.; Li, W.; Guo, B.; Ma, W.; Zhang, M.; Li, Y. 10.8% Efficiency Polymer Solar Cells Based on PTB7-Th and PC71BM via Binary Solvent Additives Treatment. Adv. Funct. Mater. 2016, 26, 6635–6640. [Google Scholar] [CrossRef]
- Garcia-Belmonte, G.; Munar, A.; Barea, E.M.; Bisquert, J.; Ugarte, I.; Pacios, R. Charge Carrier Mobility and Lifetime of Organic Bulk Heterojunctions Analyzed by Impedance Spectroscopy. Org. Electron. 2008, 9, 847–851. [Google Scholar] [CrossRef]
- Arredondo, B.; Martín-López, M.B.; Romero, B.; Vergaz, R.; Romero-Gomez, P.; Martorell, J. Monitoring Degradation Mechanisms in PTB7:PC71BM Photovoltaic Cells by Means of Impedance Spectroscopy. Sol. Energy Mater. Sol. Cells 2016, 144, 422–428. [Google Scholar] [CrossRef]
- Duan, L.; Uddin, A. Progress in Stability of Organic Solar Cells. Adv. Sci. 2020, 7, 1903259. [Google Scholar] [CrossRef] [PubMed]
- Tarique, W.B.; Uddin, A. A Review of Progress and Challenges in the Research Developments on Organic Solar Cells. Mater. Sci. Semicond. Process. 2023, 163, 107541. [Google Scholar] [CrossRef]
- Park, S.; Son, H.J. Intrinsic Photo-Degradation and Mechanism of Polymer Solar Cells: The Crucial Role of Non-Fullerene Acceptors. J. Mater. Chem. A 2019, 7, 25830–25837. [Google Scholar] [CrossRef]
- Yang, S.; Yu, H. The Modification of ZnO Surface with Natural Antioxidants to Fabricate Highly Efficient and Stable Inverted Organic Solar Cells. Chem. Eng. J. 2023, 452, 139658. [Google Scholar] [CrossRef]
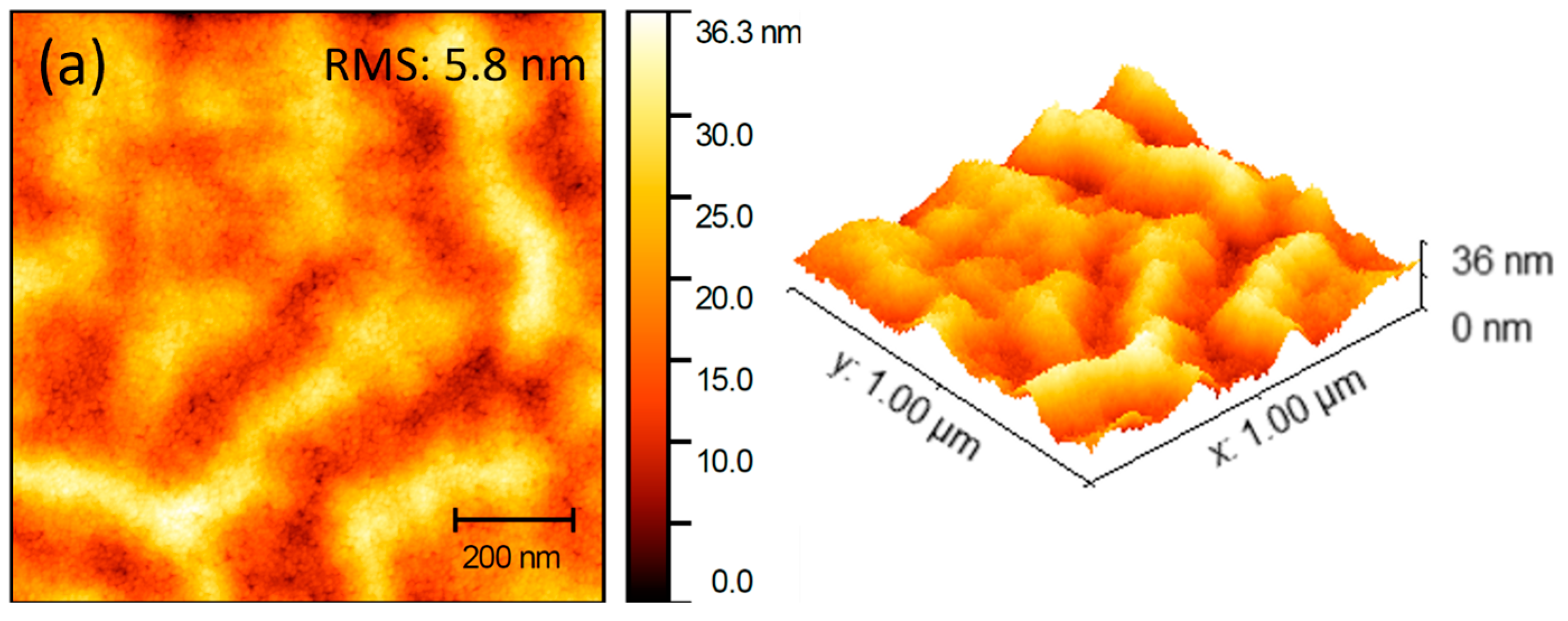
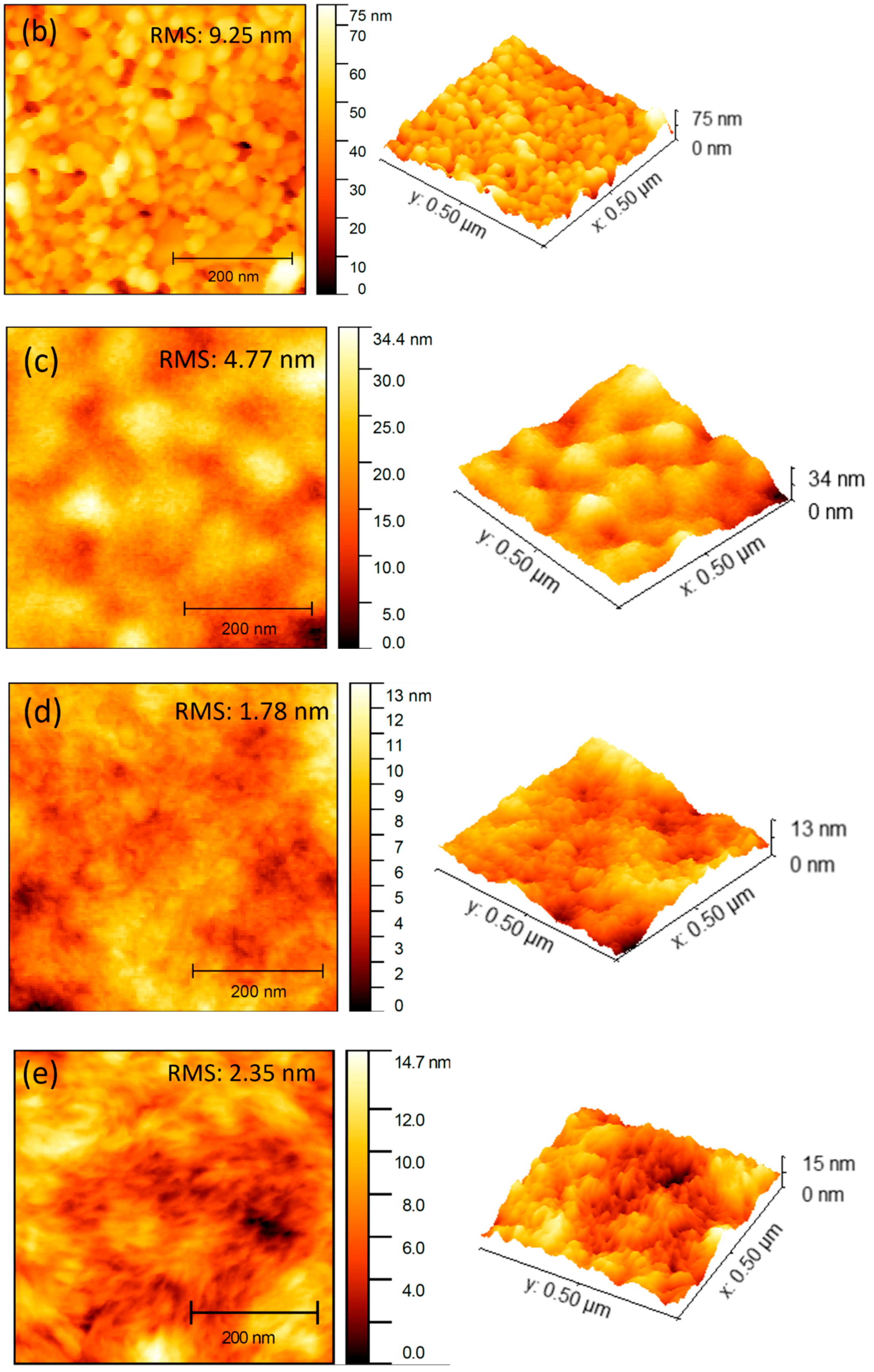
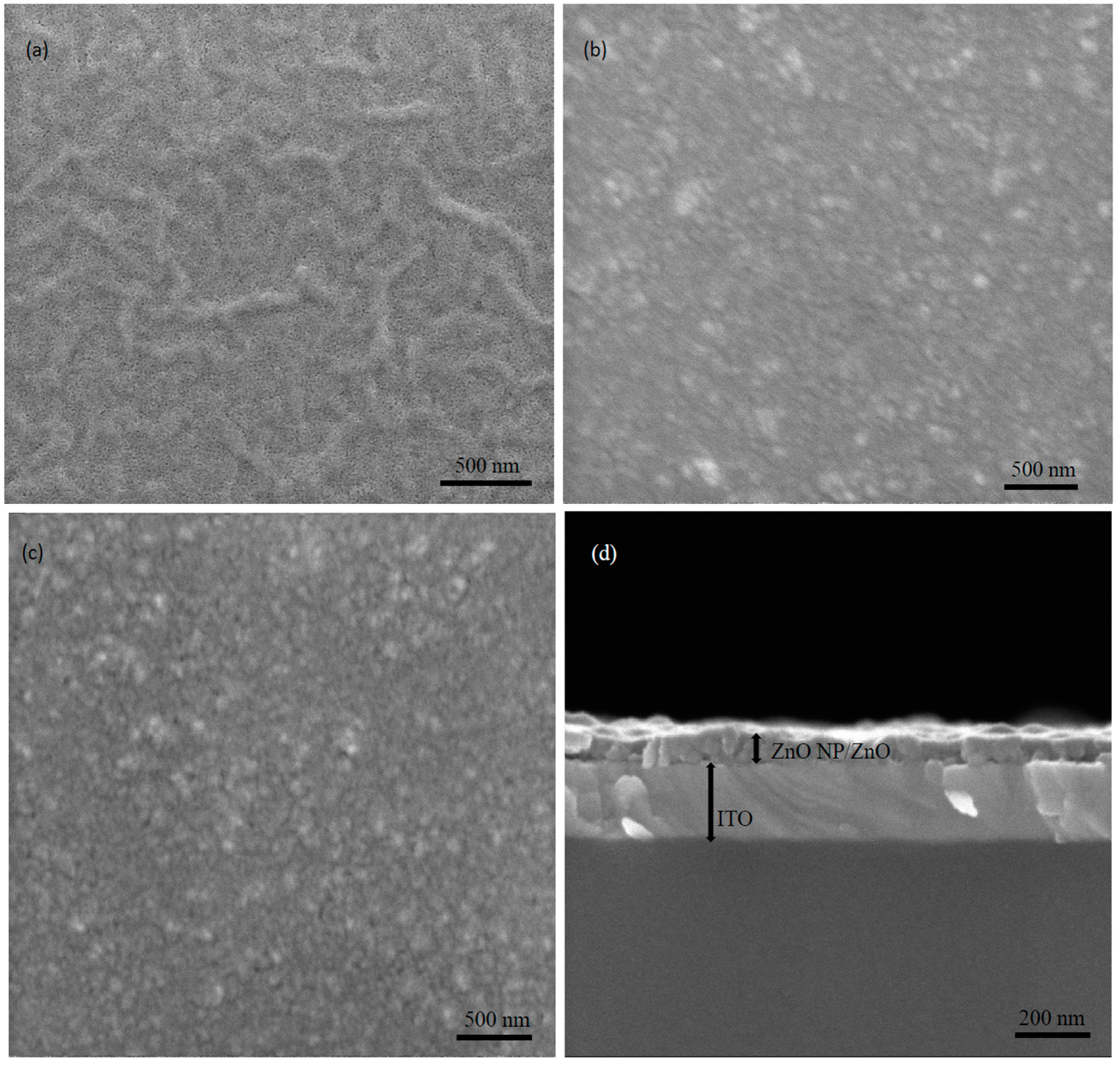
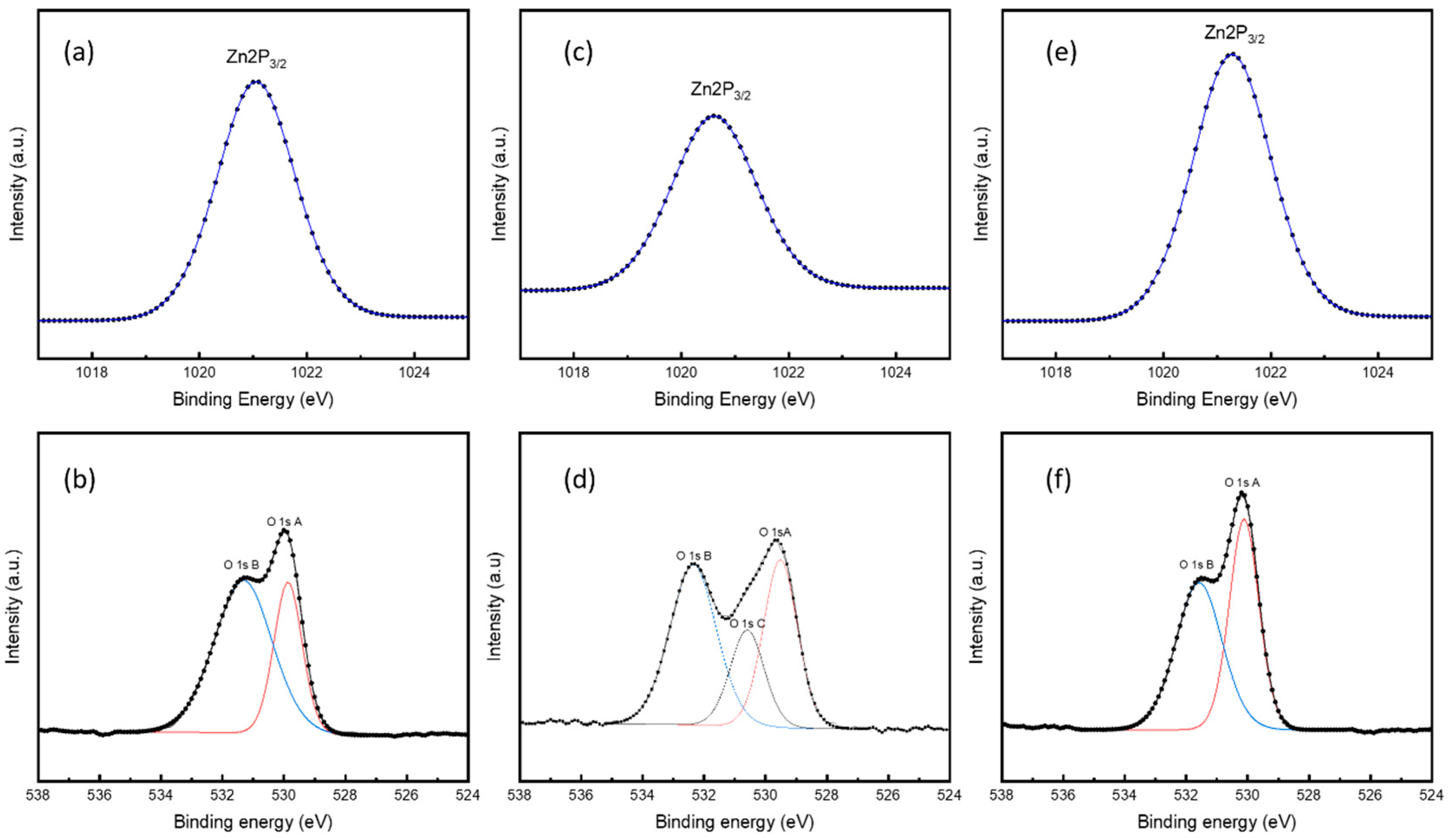
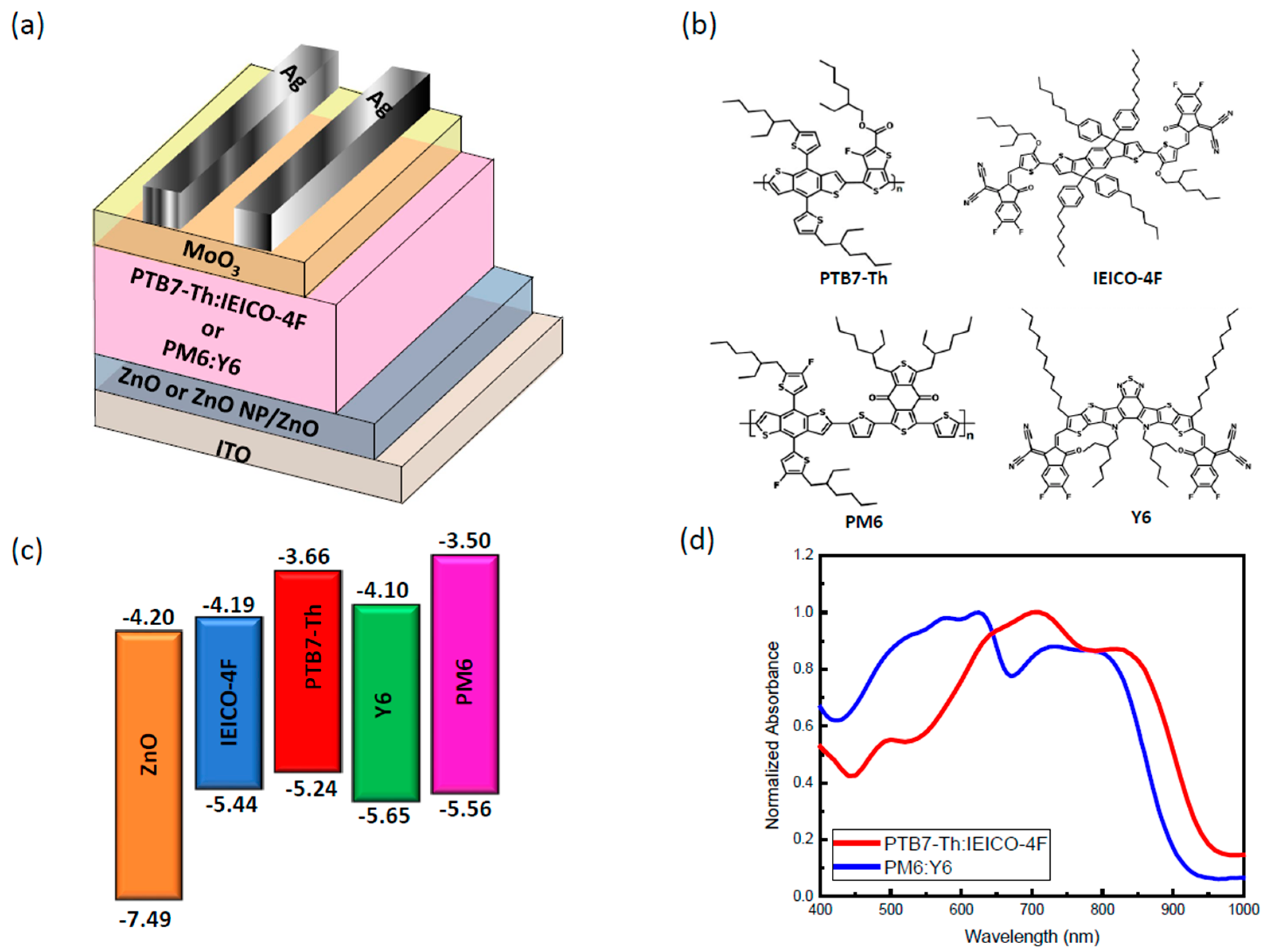

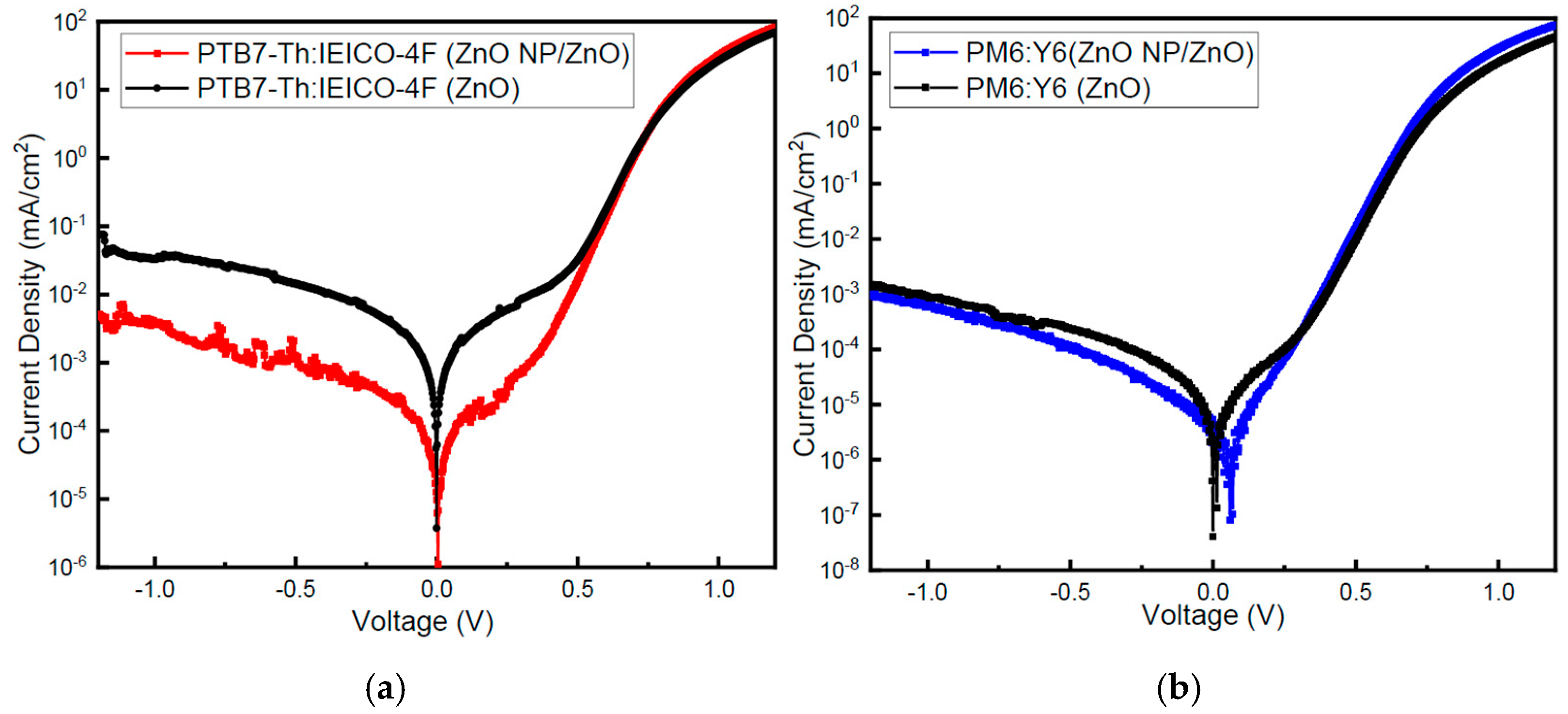



| Electron Transport Layer | Rrms (nm) | Average (nm) |
|---|---|---|
| ITO/ZnO | 5.8 | 18.96 |
| ITO/ZnO NP | 9.25 | 43.51 |
| ITO/ZnO NP/ZnO | 4.77 | 19.49 |
| ITO/ZnO NP/ZnO/PTB7-Th:IEICO-4F | 1.78 | 6.37 |
| ITO/ ZnO NP/ZnO/PM6:Y6 | 2.35 | 7.06 |
| Devices | PCE (%) | Voc (V) | Jsc (mA/cm2) | FF (%) | Rsh (Ωcm−2) | Rs (Ωcm−2) |
|---|---|---|---|---|---|---|
| ZnO/PTB7-Th:IEICO-4F | 9.18 | 0.73 | 22.08 | 56.29 | 356.85 | 6.345 |
| ZnO NP/ZnO/PTB7-Th:IEICO-4F | 10.2 | 0.74 | 23.55 | 58.29 | 380.25 | 5.22 |
| ZnO/PM6:Y6 | 13.95 | 0.80 | 24.92 | 69.33 | 966 | 34.69 |
| ZnO NP/ZnO/PM6:Y6 | 14.6 | 0.80 | 25.97 | 69.63 | 1045.8 | 31.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarique, W.B.; Rahaman, M.H.; Dipta, S.S.; Howlader, A.H.; Uddin, A. Solution-Processed Bilayered ZnO Electron Transport Layer for Efficient Inverted Non-Fullerene Organic Solar Cells. Nanomanufacturing 2024, 4, 81-98. https://doi.org/10.3390/nanomanufacturing4020006
Tarique WB, Rahaman MH, Dipta SS, Howlader AH, Uddin A. Solution-Processed Bilayered ZnO Electron Transport Layer for Efficient Inverted Non-Fullerene Organic Solar Cells. Nanomanufacturing. 2024; 4(2):81-98. https://doi.org/10.3390/nanomanufacturing4020006
Chicago/Turabian StyleTarique, Walia Binte, Md Habibur Rahaman, Shahriyar Safat Dipta, Ashraful Hossain Howlader, and Ashraf Uddin. 2024. "Solution-Processed Bilayered ZnO Electron Transport Layer for Efficient Inverted Non-Fullerene Organic Solar Cells" Nanomanufacturing 4, no. 2: 81-98. https://doi.org/10.3390/nanomanufacturing4020006
APA StyleTarique, W. B., Rahaman, M. H., Dipta, S. S., Howlader, A. H., & Uddin, A. (2024). Solution-Processed Bilayered ZnO Electron Transport Layer for Efficient Inverted Non-Fullerene Organic Solar Cells. Nanomanufacturing, 4(2), 81-98. https://doi.org/10.3390/nanomanufacturing4020006








