Abstract
Chitin (usually derived from aq. arthropods like shrimp Pandalus borealis) acts as a potent metal sorbent in both environmental monitoring and retention applications such as wastewater purification or nuclear fuel reprocessing. Given this established (starting in the 1970s) use of chitin and the fact that adsorption of metal ions/complexes to chitin does increase the currents observed in metal-centered redox couples by a factor of about 10, it is straightforward to conceive self-organized (by adsorption modified by adding certain ligands bridging M and chitin) surface films which exert electrical information processing by means of inner-sphere redox processes. Preliminary work is shown concerning the influence of ligands—including some possibly acting as inner-sphere-transfer agents, like caffeic acid—on metal ion retention by chitin. Another ligand is reported to enhance current flow into electrodes (i.e., electron injection from some reducing cation). These inner-sphere redox processes, in turn, can be controlled by creating or removing a chain of conjugated double bonds, e.g., by Diels–Alder reactions. Devices admitting corresponding reagents in a controlled manner and appropriate array then act as NAND gates, thus being components capable of performing each kind of classical computation. Applications in environmental analysis and “green” computing for simple purposes like electronic keys are suggested. The empirical basis for these conclusions includes studies on the influences of ligand additions on M adsorption (Mn, Ni, several REEs…) on chitin; some of these bridging ligands, like caffeinate and ferulate, can reversibly react with appropriate dienes. At the employed concentrations, distances among adsorbed metal ions are 1–3 nm, meaning that the charge-flow control takes spacer ligands like carotenoids. Practical setups are pointed to, using evidence from ligand-augmented metal ion–chitin interactions, which might combine oxidizing (Ce) and optically address reducing (Eu) metal ions into a framework for coligand-controlled charge flow.
1. Introduction
Structures which bring about information processing and corresponding reactions are outright typical for animals, but among living beings, plants, phytoplankton, and certain bacteria are also capable of responding to their environment after receiving all optical, chemical, and mechanical “signals”. Hence, biopolymers and organs from different organisms were investigated as possible backbones of or precursors for information-processing devices, including muscles, cellulose, and, recently, wood or nerve membranes. Many of these setups, starting with 17th- and 18th-century experiments by, e.g., Boyle, Galvani, and Volta obviously raise ethical issues. Work using “scrap” biopolymers obtained in food processing or during wood or paper production or recycling is less critical. Quite recently [1], (balsa) wood was processed by adding conductive polymers to obtain a structure that acts as a transistor (Figure 1).
Figure 1.
Note this assembly exactly matches that of classical transistors while we want to describe a full-scale logical gate produced by modifying chitin, without altering its internal composition (i.e., no removal of lignin, phenols, quinones, or peptides, respectively). From [1] via internet public announcement by PNAS, Lund University (Sweden).
Obviously, this approach could be extended to other porous or “open-structure” biopolymers like spongy structures or bone, respectively. Another such biopolymer is chitin, usually taken from crab and shrimp peeling. During the last 10 years, our workgroup cumulated a lot of data and technical experience on modifying chitin, which can be applied to the topic of information processing, also. The chitin used in this work is scrap according to the above definition: no animal is harmed or killed just to obtain chitin. Applications of chitin modified by metal ions for purposes of information processing are the key topic of this paper.
Previous work on the electrical properties associated with chitin dealt with the piezoelectric [2], thermoelectric, and even photovoltaic [3] properties of native arthropod chitin while people learned how to deposit and activate semiconductor materials on various supportive surfaces, e.g., by pyrolysis or protolysis (employing PH3, AsH3, H2S, or H2Se as acids) of volatile organometal or metal trichloride or metal alcoholate compounds; for example, GaAs is made in this manner almost exclusively [4]. Concerning probable semiconductor properties, information processing, or energy conversion, chitin samples from certain arthropods are distinguished by quite diverse features. These phenomena include:
- Extreme thermal and electrochemical stability of chitin [5,6,7];
- Efficient adsorption of even minute traces of metal ions or their complexes [8];
- Activation/enhancement of electron transfer to/from metal ions or their complexes in an adsorbed state [7];
- Ligand exchange can be realized with the metal ion sticking to chitin.
There is precedent for uncommon modes of behavior of chitin modified by biology; the most remarkable effects have been hitherto observed in shrimp Palaeomon(etes) spp. ([9], Figure 2) and hornet Vespa orientalis ([3] Figure 3).

Figure 2.
A shrimp of genus Palaemon (formerly Palaeomonetes). Chitin taken from closely related P. varians was demonstrated to exhibit piezoelectric effects in living shrimps equipped with an electrode at the inner side of chitin cladding [2]. P. varians lives in all seawater, brackish water, and tidal pools, with changes in metal-ion concentrations producing both electrochemical signals and sizable pH excursions at the chitin surface. Photo by Enrique Dans (Spain, obtained from Internet, Wikimedia).

Figure 3.
An Asian hornet Vespa orientalis eating a piece of bacon. The photoactive part of the chitin shroud is the dark-brown area located between the wings. It is distinguished by both a peculiar microstructure and a very high level of xanthopterin producing the distinctive color.
These properties were thought to be linked to unspecified semiconductor properties of chitin at least for shrimp [9] and wasps ([3] and Figure 3) with the precise effects probably due to components other than the very polysaccharide, namely quinones or pterins. They are not attributed to semiconductor crystals, some of which are readily produced by biota (Fe3O4, both modifications of ZnS) nor to metalliferous complexes, peptides, or coordination polymers. It should be noted that in purified shrimp chitin from Pandalus borealis, there are sizable amounts of redox active metal ions (typical contents: 15 µg/g Fe, 10 µg/g Ti, and 4 µg/g Co, and some Cu, 1.5 µg/g (own measurements; [10]) yet the solution is redox silent until some of these or other metal ions were deliberately added to the solution, either as simple salts (chlorides, perchlorates, or triflates readily dissolve in DMF) or as complexes [6,7]. Apparently, the “indigenous” metals are present in a form that precludes substantial electrochemical activity, while those adsorbed afterward display enhanced electrochemical behavior. The speciation form of the former likely is carbonate or oxide particles.
With semiconductor properties shown for chitin in both shrimp ([2,9]) and hornets [3], and evidence for thermoelectric effects in the latter system, also [3], treating chitin with hot water caused about a doubled release of preabsorbed metal ions. Chitin adsorption of redox-active species like methylene blue or indicators like Nile Blue can be much increased by abrading the upper surface [2]. This work showed that (certain samples of) chitin will respond to thermal or photonic excitation by the production of a potential (some 50 mV with a positive bias on the inner side) while anions remain outside; apparently, both heating and exposition to light cause a similar dissociation of complexes’ or adsorbed acids’ protons and metal ions, which then pass through the chitin membrane to the inner side. Either dissociation would increase with both T and quantum flux while both effects are enhanced by structures about 5 µm in diameter, which resemble some parabolic mirrors [11]. Figure 4 was redrawn by this author following the description in [11]; the hornet is thought to produce the “solar cell” and “thermoelectric” performances in the chitin layer located in between the wings (Figure 3). It was described ([3,11]) that the presence of proteobacteria colonies under this part of the carapace was crucial for producing the effect (Figure 4):
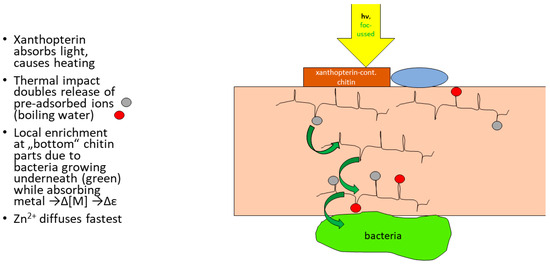
Figure 4.
The kinked lines in the chitin layer symbolize single biopolymer strands and their acetamido groups, respectively. The latter acetamido groups make the difference with respect to cellulose both in terms of nitrogen content and metal adsorption capabilities. Grey and red circles denote metal ions. The blue ellipsoid denotes a water drop from which—like from dust—meta ions can be taken up into chitin. Like in “common” ecosystems, chitin will respond to an outer (below) sink represented by microorganisms with an enrichment of the metal ion undergoing uptake with a local enrichment, increasing the ratio of metal ions bound to chitin at the bottom to that captured in or next to the water. This difference in “load” gives rise to an electrochemical dilution potential. The observed value of some 30–40 mV corresponds to a factor of some 10–20 in divalent ions. Digby [2,9] and Ishay et al. [3] commonly used Cu metal electrodes introduced into the carapace.
The strands of chitin are fairly loosely packed in their common structure, enabling both hopping conduction of metal ions among the parallel strands of chitin in bulk [12], producing pronounced capacitor activity [2], and causing the chitin surface to respond to changes of pressure [9]. Upon photo- or thermodissociation, binding voids are produced for both the cat- and the anions which are closed, partly by enhanced adsorption to chitin, and partly by solvation. The isoelectric point of chitin is pH = 4.6 [13], meaning surface is negatively charged and accomodating cations and cation complexes in normal conditions. Since this effect is increased by pressure, a seemingly piezoelectric effect also is implied, which was observed by Digby in assisting in keeping Palaemon varians at a given water depth. In addition, chitin (or chitosan) was shown to be a useful dielectric material in capacitors operating at up to 15 V [14].
In the experiments described by Digby and the Israeli authors, electrodes were placed on their outer chitin claddings. These indicate piezoelectric, photovoltaic, and even Seebeck effect (i.e., thermoelectric) phenomena [9,11], producing voltages of up to some 50 mV in either case. While the effects observed in Vespa orientalis are not understood in terms of a biological function or any associated selection advantage beyond some contribution to catabolic metabolism by making use of sunlight, piezoelectricity in Palaeomonetes was associated with maintaining a certain depth underwater (some 17 m; [2]). Conversely, these voltages are large enough to betray locations of aquatic arthropods to predators that employ electric sensors for hunting, such as platypuses, weakly electric fishes, or (in the ocean and in estuaries) sharks or rays. Since these signals are created right at the borderline to water and, thus, probably are much stronger than those getting out from nerve- or muscle-action potentials, they might create a problem for arthropods when trying to evade such predators. Yet, it probably takes additional measures for technical information processing on chitin; that is, setups that produce larger voltages and currents.
In fact, the remarkable thermal and oxidant stability of chitin, and it being a powerful sorbent to bind many different metal ions [8], their complexes, and environmental speciation forms [7,10,15], can be exploited to prepare semiconductors and sensors [16] right on chitin interfaces. At some 300 °C, many different heterocyclic volatiles are formed, including acetamidofurane, its ring rearrangement product 3-acetylpyrrole, and pyridine, benzene, toluene, etc., [5] but electropolymerization of the latter furane or pyrrole [17] is hardly conceivable in these circumstances. Accordingly, other techniques must be employed, starting with the spontaneous and very effective adsorption of diverse [8,15] metal ions from either water or organic solvents to chitin. Neutral organometal species and metal carbonyls also bind, enhanced by adding iodine or some other oxidant, (i.e., likely by exchange of iodoligand) enabling thermal construction of conducting lines (control electrodes). Yet, one should think about whether it is feasible to move one step ahead by producing a modified surface, as it is in planar microelectronics for performing logical operations beyond mere switching or amplification of currents.
[M(CO)n] + I2 → [MI2(CO)n−1] + CO↑ → better adsorption (M = Fe, Mo, or W, n = 5 (Fe) or 6)
Whether or not the observations reported above are due to chitin containing typical (peptides, phenols [2], and ZnCO3 [3]) or non-common (xanthopterin in V. orientalis) admixtures making it act as a semiconductor, results from our workgroup on ready-to-do modifications of some chitin surfaces strongly suggest that modified chitin can be used in electronics very much in the same manner in which semiconductors have been used for some 70 years now. Such modifications can be achieved by simple adsorption of metal ions to chitin and then fitting the appropriate ligands to them that are involved in inner-sphere charge transfer to other metal centers or electrodes.
2. Material and Methods
2.1. Detailed Explanations of Methods
Chitin is exposed to the solution in circular arrangements of flakes (1 cm diameter) obtained from shrimp peeling (Sigma-Aldrich, St. Louis, MO, USA; donor organism = Pandalus borealis [Iceland coastal waters]) in a 50/50 v. aq. DMF buffer solution that contains aniline and anilinium trifluoroacetate PhNH3CF3CO2 in equimolar amounts. Exposure time is 10 min previously determined [15] to be sufficient for equilibration. The cations (M2+ Mn and Ni, M3+ La, Sm, Eu, Gd, Dy, and Yb; Ce with an unspecified oxidation state) are adsorbed to chitin without and with different levels (0.5, one and 2 mmol/L protonated ligand/L of solvent mixture) of ligands glycine, citric, malic, malonic, caffeic, ferulic, humic, and acetohydroxamic acids, and of NCS− (NaSCN) [14]. The adsorption is measured by dissolving the surface layer of chitin after exposition and aq. washing using 1.5 M/L Li salts in N,N-dimethyl formamide. The solution is absorbed by a bag containing an acid form of the cation exchanger Amberlite H-120 which works in DMF quite as well as in protic solvents, even though the acidity of arenesulfonic acids or even H2SO4 in DMF is not very pronounced. There is a linear correlation among σY in hydroxoacids H-OY, like acetic, nitric acids, or phenol, and pKa values in water and DMF [7], namely,
and
for neutral acids (cations and anions like HSO4− behave differently in DMF). Yet, glycine (solubility limit in DMF = 41 mmol/L) acts as a buffer in DMF like it does in water but can increase the adsorption of certain ions like La3+ or Eu3+ [15]. The other acids were not studied in DMF in much detail. In pure water, the zero-zeta potential of chitin is pH = 4.6 [12].
pKa(H2O) = 11.03–15.0 σ
pKa(DMF) = 22.91–22.68 σ
Adsorption (changes) caused by different levels of nine different ligands were studied by measuring the total adsorption of metals when there are 0.5, one or two mmol/L of dissolved protonated ligand (except for thiocyanate where NaSCN was used, rather than the corresponding acid which here is unstable). At H0 = 5.8 in the solvent mixture, the chitin surface can be assumed to be negatively charged with certainty. Hence, e.g., protonated glycine or the zwitterion will attach to the interface by the ammonio group, whereas the terminal (remote from the surface) carboxylate augments and enhances Mx+ binding; see Figure 5.
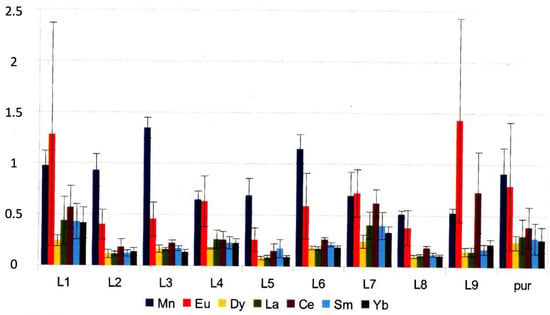
Figure 5.
From [15]. Meanings of L1 through L9 are given in text. Only few ligand-metal combinations containing potentially conducting ligands cause an enhanced binding to the solvent mixture-chitin interface. These are selected for constructing logical building-stones.
In addition, diene components such as 2-cyano-furane-5-carboxylate, which still must be shown to cause similar increases with respect to adsorption, can be added in a controlled manner. Once both are present in the adlayer, Diels–Alder reactions will stop charge transfer (see below). Figure 5 shows previously obtained [14] results on changes in the adsorption of metal ions upon ligand addition to chitin:
The metals Mn2+, Ce (unspecified oxidation state), REE3+ La, Sm, Eu, Dy, and Yb. [Mn+] are added into a solvent = 1 mmol/L; [LH] = 0.5 mmol/L. Light was excluded in measurements to avoid photoreduction of Eu by ligands like glycine, humic, malonic, and caffeic acids, which previously were shown ([7,16]) to undergo Eu-mediated photooxidation. L1–L9 = glycine, citric, malic, humic acid, SCN−, malonic, caffeic, ferulic, and acetohydroxamic acids, respectively; “pur” (pure) means there is no added ligand other than the anion of the metal salt (i.e., either perchlorate or trifluoromethanesulfonate, not chloride or nitrate, which both form REE complexes). Note that the “simple” behavior (i.e., all ligands keep metal ions from adsorption simply by complexation) is observed with Sm(III) only, except perhaps for L1 = glycine and L7 = caffeinate (see below). Eu (bright red) is distinguished by photochemistry. One can obtain a first idea of the effects caused by adding (small amounts of) some ligand or its protonated form by comparing the size of the rightmost septet of bars (no ligand) with the other ones (more to the left). With Sm (light blue), there is an increase only for L1 (glycine) and L7 (caffeic acid), whereas, in other cases, the multitude of ligands enhance chitin retention.
L7 and L8 (ferulic acid) are examples of bridging, inner-sphere-active unsaturated aromatic ligands (derivatives of cinnamate), which—unlike NCS− (L5)—will react with 1,3-cyclohexadiene or other cis-cis dienes like furanes or pyrrols.
Concentrations given on the left side of the diagram refer to that in the solution obtained after double ion exchange. The total dilute HNO3 volume is 6 mL; that is, 1 µg/L means 6 ng of metal ions were recovered from chitin. The recovery rate is about 65%, and the dilution factor caused by workup is some 25 (0.35 mL DMF, 6 mL HNO3 dil., cp. [12]). The mutual distance of adsorbed ions is between about 1 and 3 nm, i.e., larger than the saccharide ring diameter.
2.2. Detailed Explanation of Methods
The cations (M2+ Mn and Ni, M3+ La, Sm, Eu, Gd, Dy, and Yb; Ce with an unspecified oxidation state) are adsorbed to chitin without and with different levels (0.5, 1, and 2 mmol/L protonated ligand/L of solvent mixture) of ligands glycine, citric, malic, malonic, caffeic, ferulic, humic, and acetohydroxamic acids, and of NCS− (NaSCN) in a 50/50 v. aq. DMF buffer solution that contains aniline and anilinium trifluoroacetate PhNH3CF3CO2 in equimolar amounts. The adsorption is measured by dissolving the surface layer of chitin after exposition and aq. washing using 1.5 M/L Li salts in N,N-dimethyl formamide. The solution is absorbed by a bag containing the acid form of cation exchanger Amberlite H-120, which works in DMF much like in aq. media.
3. Results
Materials and techniques applied in the studies that corroborate the described design were outlined in previous papers [7,10]. The main techniques included adsorbate analysis by ICP/MS following the surface dissolution of chitin and double ion exchange, headspace GC/MS analysis of volatiles at the chitin surface, and cyclic voltammetry. Most of this work was completed in the solvent N,N-dimethyl formamide, with perchlorates or hexafluorophosphates of different cations as conducting salts.
Seven metals (Mn, REEs) were studied with respect to (nine different) ligands and a control (50% v/v aq. DMF) causing increased or decreased adsorption. Some metal ions undergo redox reactions readily when adsorbed to chitin; hence, ligands known to promote inner-sphere transfer might combine with adsorption effects. This should hold for NCS−, malonate, caffeinate, and possibly humic acids among the above ligand set, whereas acac− was demonstrated to improve electron injection from photochemically generated Eu2+ to indium or tantalum electrodes up to a saturation concentration of [Hacac] = 0.25 mol/L (the solubility limit of Hacac in water is 1.2 M/L). acac- can be replaced by other 3-keto enolates like (anion of) 1,3-cyclohexanedione.
This does define a range of suitable interactions at a chitin surface. The emphasis lies on the four ligands, among which some additionally enhance the adsorption of certain redox-active anions. The key ambient acceptors within native chitin would be Fe(III), Cu(II), and Ti(IV), although dissolved chitin as such does not produce an electrochemical signal at all. The general setting of effects is summarized in Figure 5 (above) and Table 1:

Table 1.
Enhanced (or decreased, marked by -) binding of redox-active Mijky+ to chitin interface induced by the addition of inner-sphere-active ligands L4, L7, L8 (L1 [glyc−] often produces significant binding effects, but there is no evidence for enhanced electron transfer between whatever metal ion/complex couples); 0 means no significant effect on extent of adsorption. [Mn+] in solvent = 1 mmol/L. [LH] = 0.5 mmol/L.
It turns out that L7 (caffeic acid) is actually well-suited to pass electrons from REE2+ Sm, Eu, or Yb to Ce(IV), while the latter can be made by either oxidant (MnO2 + acid, electrode, OCl−) and chitin, withstanding all these reagents while Eu2+ can be prepared by photochemical reduction, thereby converting optical (plus chemical; are there one or two different bridging ligands capable of reacting with one another?) into electric signals.
L4 and L7 undergo photooxidation by Eu(III); hence, there is an additional (in effect, optoelectronic) pathway into electron injection in the system. Details on the mutual distance among binding sites for the respective elements and the corresponding redox potentials are given in Table 2 below.

Table 2.
Association tendency towards chitin as affected by the addition of ligands that are potentially involved in electron shuttles to a supportive electrode.
L7 and L8 (ferulic acid) are examples of bridging, inner-sphere-active unsaturated aromatic ligands (derivatives of cinnamate) which—unlike NCS− (L5)—will react with 1.3-cyclohexadiene, or furans and pyrrols.
The concentrations given on the left side of the diagram refer to that in the solution obtained after double ion exchange. The total dilute HNO3 volume is 6 mL; that is, 1 µg/L means 6 ng of metal ions were recovered from chitin. The recovery rate is about 65%, and the dilution factor caused by the workup is 25 (0.35 mL DMF, 6 mL HNO3 dil., 65% average recovery [12,15]). The mutual distance of adsorbed ions is between about 1 and 3 nm, i.e., larger than the saccharide ring diameter (cp. Table 2 below).
Assuming diffusion inside the chitin layers to be a random process, which still gives rise to pronounced diffusion fronts with Al or Co salts [18], and this places the diffusion front at some 10 µm below the surface after several weeks (i.e., about one megasec), one can assume that within 1 s only sites can be reached by intruding cations which are located some 10 nm or less below the surface (random walk distance by diffusion increases by t1/2). The mutual distance among metal ions by saturation in chitin is about 3 nm. Cations that can be reduced inside chitin with these 10 nm would interact by direct contact with intruding ions unless perturbation of the surface by illumination will stop, causing parts of the ions obtained from the adlayer (H+ or Mx+) to return rather than proceed to the opposite interface and be used by bacteria.
Among the metals which most readily undergo adsorption to chitin, Cr, V, Ce, Eu, Cu, Ni, Fe, and, in non-aq. conditions (DMF solution dissolving chitin by Li salt addition, then “metalated” by adding some REE salt), also Yb, Sm, and Tm produce redox transitions [16] providing something other than the metals described by Digby. Bi or Mn probably just provide (colloidal) metal when decreasing the applied potential accordingly, but many of these former processes are reversible in terms of electrochemistry when run on chitin [15]. Divalent V, Cr, Eu, or Cu were described to be involved in inner-sphere redox processes occurring via “unsaturated” ligands before [18,19], acting as either donors or acceptors (Cu) of electrons.
The previous work in my group [15] revealed the following superposition of ligand effects concerning adsorption on chitin with their potential use in inner-sphere charge transfers (Table 2):
The study was run in water–DMF 50/50 by volume buffered to a H0 value of 5.8.
Electronics or current-dependent information processing is about the controlled (switched) transfer of charge carriers (usually electrons or defect electrons [“holes”] the effective mass and mobility of which may vary within a wide range, up to the speed of sound in conductive solids [some 5 km/s] and up to some 20 electron masses) between different entities to fulfill logical operations as defined by Boole’s algebra. Among different basic networks of switches, the NAND gate provides a universal backbone for the construction of logical operators and entire computers, just lacking storage functions beyond the “positions” of the “switches”. We now show that such elements can be made by modifications of chitin which are straightforward to realize. Then, the conduction properties of the bulk material are insignificant (conductivity of chitin does suffice [2,9] while that of the adlayer and the chance to manipulate them become most important. It was shown previously [7,13,15] that chitin
- (1)
- can be modified in this manner due to its excellent adsorption properties for metal ions, complexes, and (less so) for metal carbonyls, biomethylation products;
- (2)
- displays semiconductor properties itself [2,3,9]
Thus, it is anticipated that corresponding structures will be readily built and controlled, later on using additional features. These structures are built in a two-step procedure: adsorption of metal ions to the interfaces and then connecting them by (one of the) “conductive” ligands; if both are present, they will react with one another and thus block the current flow. The addition of either ligand can be taken as an input at one branch of the NAND gate. Here, established replicator chemistry [20] gives ideas about which kinds of reactions might turn “conductive” bridging ligands into something no longer capable of inner-sphere processes with either redox-active partner ions, complexes, or electrodes. This does include Diels–Alder or 1,3-dipole reactions [20]. Replication implies that crosswise addition of reagents is reversible in mild conditions. DA reactions mean that formerly conjugated organic or heterorganic backbones—thus suited for efficient inner-sphere transfer—are turned into such containing isolated double bonds in a cyclohexene motif, which means conduction comes to an end once both reagents are present at the chitin surface (→not an AND function). However, the effects caused by furane-2,5-dicarboxylate (the symmetrical ligand is to be preferred vs. others because “wrong” binding to either Mx+ or chitin cannot occur) are not yet investigated.
The devices to be described here are considered to represent some interbreeding between the classical Taube hetero-metal complex bridges fulfilling inner-sphere electron transfer at remarkably high rates [12] and simple sorbate structures which form on chitin spontaneously by adsorption of metal ions by chitin and subsequent ligand exchange in a bound state. The range of potentials that can be addressed, at least in a DMF solvent, is very large (>4.5 V).
The enhancement of current flow by adsorption to chitin, which is a general feature observed with several newly introduced ligands, including hydroxamate at Fe, glycinate at Ni (log β ≈ 5.8 at common conditions [21], and different complexes of Eu, was mentioned before.
In a photoactivated system, Eu2+ finally donates an electron to trivalent V, Cr, or Fe, or to I3−, or to an electrode [ongoing own work to be reported later in more detail], or to nitrobenzenes [16]. Sometimes, novel transitions turn up at very negative potentials (≪−1 V vs. SCE) [7,20]; however, they do not represent metal deposition and, accordingly, usually are reversible. The upper end of the potential range is given not by oxidative attack towards chitin (which does not even react with classical saccharide-decomposing agents like Pb(IV) acetate or periodate [22], nor is it stained by either OsO4 [after hydrolysis, chitosan does react, though] or Ag+) but by decomposition of ligands and solvent (you can both measure the CeIII/IV redox couple in DMF [+1.63 V vs. SCE] and dissolve Ce(CF3SO3)4 in DMF to obtain a bright yellow solution, but this color will fade within minutes) at ≤some 1.7 V vs. SCE.
Metal-ion adsorption to chitin dissolved in DMF/Li+ as such does produce only one “parasitic” electrochemical signal at −1.50 V [7,10,14], which does not depend on the kind of added metal ion (including redox silent ones) and is attributed to increased acidity of some saccharide OH group (or possibly the NH group of the acetamido moiety upon co-ordination. Traces of water that exist in both 99.8% DMF and on chitin are likely intercepted by the Li salts dissolved in DMF at the 1.5 mol/L level, enabling electrochemical stability and causing appropriate reactions with strongly reducing species other than Sm2+, which the latter is remarkably tolerant towards present water, e.g., using aq. amine solutions for certain reduction processes rather than liquid ammonia or the like. The solvated proton is more stable in DMF by some 0.19 eV than in water [22]. Thus, the standard redox couple
would have ε ≈ −0.43 V vs. SCE in a solution having H0 = 0 in DMF (which “DMF-NHE” is hard, if at all to make even using very strong acids because, e.g., H2SO4 is just a moderately strong acid in DMF [23]).
2 (DMF)nH)+ + 2 e− ↔ H2 + 2 n DMF
We correlated the acidities of hydroxyacids HOZ in water and DMF (glycine acts as a buffer at H0 = 11.0 [15]) with one another and with the σ Hammett constants of the attached groups (CH3CO, NO2, and the like, Equations (2) and (3) above). The above value of a probably H-related reduction potential does imply pKa ≈ 18 in DMF; typical pKa values of sugars and oligosaccharides in water are about 12; however [21], without Mx+ addition, such solutions become more acidic, too.
There is evidence for the formation of oligonuclear complexes on chitin by the mere number of electrochemical transitions observed in spite of the fairly large average distance (3.0 nm) among metal ions in “fully metalated” chitin; i.e., there is substantial scatter around this average value even though the maximum uptake of metals ≠ Pb, Al, Zn [24], Ce, and Eu [25]) is remarkably constant (45 µmol/g).
Apparently, some clusters of metal ions form on chitin, which might be linked by ligands such as donor-substituted peptides (e.g., polyaspartic acid and phytochelatin) or polyamines. The observed “extra” electrochemical transitions occurring on chitin may be related to some particular ions within such an assembly or represent conduction over small areas of the chitin surface. Anyway, likely one ligand attached to one metal ion carries most of the charge transfer, and this feature will persist when some “conductive” ligand is added. Which are such ligands? The list does include dienes. In DMF, Tm adsorbed to chitin was shown by us to undergo reversible electrochemical reduction at −2.3 V vs. SCE. We have not tried adding nitriles so far. On the other hand, an “uncommon” redox potential value (compared to other solvents, including CH3OH and water, and to the more common Sm or Yb redox couples) was reported for Tm in acetonitrile [17], while it is uncertain what they actually measured then, likely a reversible formation of 2,4-diiminopentyl-3-amine or one of its deprotonated forms.
Note added in proof: although 4-oxopent-2-enolate-2 (acac−) is not a classical bridging ligand that readily fulfills electron transfer but rather undergoes exchange between the redox partners [CoIII(en)(acac)n]q+ and Cr2+ as such [26], its use in an electrochemical cell where Eu2+ from the photochemical reduction of Eu(III) by organics such as ethanol does donate electrons to some metal electrode (Ta or In, dark or illuminated) does increase maximum currents and simultaneously decrease the activation barrier for electron injection from Eu2+ to metal (aq. electrolyte, NaClO4 conducting salt). The size of the latter activation barrier is estimated from changes in the current caused by heating or cooling the device. The addition of acetylacetone causes a sizable increase in current anyway, giving proof that it acts as a conductive ligand. Experiments with derivatives of caffeic acid and specific ligands e.g., for enhanced charge transfer to nickel metal or Ni2+ are underway.
An amplifying device can be constructed (Figure 6) due to the fact that currents of, e.g., Fe hydroxamatocomplexes [7] or Ni chloro- and glycinatocomplexes [27] will increase massively (by a factor of 5–12) when some chitin is dissolved by adding Li+ to the N,N-dimethyl formamide (DMF) solution, which the biopolymer then “captures” the metal ions or complexes until saturation is reached.
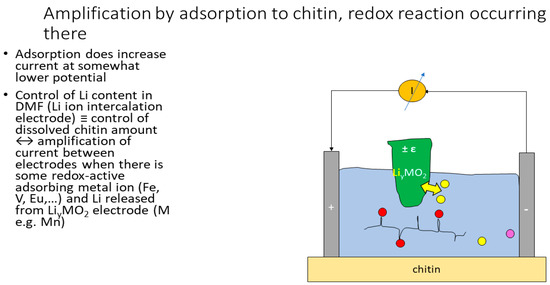
Figure 6.
The electrode material LiyMO2 (green) does absorb Li cations from or release them into the DMF solution depending on change of an electric potential. Thus, the applied voltage does control both the solubility of chitin in DMF and by binding of the metal ions and the amplification of the current. In effect, this is a transistor operating on a solution of a biopolymer rather than on semiconductors. Redox-active ions (red, violet) can exist either bound to chitin or in solution; the former sustain much higher currents at given concentrations and sometimes even additional redox transitions ([7,27]. The yellow circle with a crossing arrow denotes an amperemeter.
Electron flow causing uptake or discharge of Li ions from the control (“gate”) electrode material thus translates into dissolution of chitin and, thus, amplification of the current flowing “horizontally” mediated by changing oxidation state of some ion or complex adsorbing to (also dissolved) chitin.
4. Discussion
As was shown by Taube already in 1959 [19], unsaturated ligands (maleinate, terephthalate, and oxalate), the former of which also undergoes Diels–Alder or 1,3-dipolar reactions, are capable of transferring electrons much faster than happens along isomeric (i.e., fumarate and hydrogenfumarate) or saturated (f.e., succinate) bridges (from Cr2+ to Co(III)-pentammine-heteroligand species in water [26]). Since we could show how products of the adsorption of metal ions can be modified by both electrochemistry and ligand exchange, two differently “metalated” chitin surfaces can make controlled electron transfer if the bridging ligands represent dienes fitted with donor ligand functions and dienophiles like maleinate, maleic imides, etc. The (reversible) Diels–Alder reaction between both maleinate or maleimide (or imines when using reduced Tm complexes) and some 1,3-cyclohexadiene then will block the current flow between chitin fitted with an appropriate metal ion by adsorption (e.g., Cu2+) and a positively biased backward electrode and some metal electrode distanced by a molecular spacer.
4.1. Logical Structures Which Can Be Made on Chitin
In native chitin, there are substantial amounts of possible charge carriers, both forming semiconductors and capable of accepting another electron to cause “hopping conduction” (which also is observed in certain mixed-valence semiconductors like magnetite), like quinones and sometimes pterins. The observed semiconductor properties, including photovoltaic and Seebeck effects, might be due to this. Added metal ions follow a certain pathway (i.e., chitin claddings of arthropods are not symmetrical in terms of metal ion transport) which gives rise to pH gradients after changing the outside metal-ion activity or composition [9]. Such an asymmetry is required for information processing. The following suggestion, which is based on the literature and our experience with modified chitin, shows how such things might work. It is probably pointless to try to convert the surface adlayer of metal ions into some semiconductor by unsaturated bridging ligands to obtain a diode or transistor from two or more layers of differently modified chitin because the distances among metal ions are too large.
4.2. Extension from Amplifiers (Transistors) to NAND Gates
The amplification of electron flows by interaction with dissolved or interfacial chitin can be used to build an amplifier. Taking advantage of this effect, the change of adsorption amounts associated with redox-active ions binding to chitin (Figure 5, Table 2) can be combined with the inner-sphere conducting ability and the Diels–Alder reactivity of certain of these ligands (caffeinate and ferulate) to obtain a full-scale NAND gate which responds to additions of cis-dienes, such as furane- or pyrrole-based ligands or vice versa. This NAND gate, in turn, would provide a fundament for universal computation, not just for an electrochemical sensor as might be inferred from the previous remarks. Rather, one would obtain a logical gate in a straightforward, experimentally less demanding manner.
More complex logical operations and mathematical procedures which may directly integrate the processing of environmental data obtained by the very adsorption of metal ions on chitin imply that a larger (≥4) number of NAND gates be integrated. Hence, the question arises how the NAND information processing can be repeated. A positive current should cause the release of some more Eu(III) at the “end” of a NAND gate, enabling the same chemistry to be realized again.
Acetyl acetonate acts as an efficient additive that facilitates and, thus, enhances current flow from Eu2+ into an indium electrode in water, but, (electron shuttle activity) likely, acac is more difficult to control by additions, at least not in a reversible manner. In mixed ligand systems, usually L´ other than acac− (e.g., azobenzothiazoles) affect inner-sphere electron transfer. This does match the expectation concerning molecular structure. The volatility of neutral acac complexes, which enabled both REE separation by GC/MS and the use of [REE(acac)3] as antiknock agents in petrol, does suggest there are few sites for charge transfer. Electron transfer from Eu2+ to In is enhanced by adding Hacac, reducing the activation barrier for e injection to ≤0.1 eV, but it does undergo by-reactions with aldehydes formed by alcohol photooxidation to afford isopentyl acetate (typical pear juice fragrance) with the REE Eu promoting a photoinduced Tishchenko coupling while acac− does undergo hydrogenation.
In addition, unsaturated ligands can become involved in inner-sphere electron transfer (distances here are smaller than with making bridges among metal ions on chitin) while possibly reacting with other representatives of this group of molecules or anions. Reported examples of such bridging ligands include maleinate and terephthalate [17], while the isomers are about as poor conductors in the [CoIIIL(NH3)5]q+/Cr2+ or -CrCl+ couple as saturated ligands like succinate are [19]. Since conjugation within the ligand backbones is crucial for fast inner-sphere electron transfer, any addition- or ring-forming reaction that attacks this conjugated π–bond system can block electron transfer. The next Figure 7 shows the general setup:
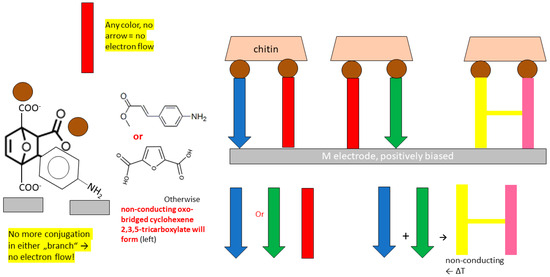
Figure 7.
Principle and chemical realization of a NAND gate that responds to Diels–Alder reaction which takes place once the “other” inner-sphere-conductive ligand is added. Furan-2,5-dicarboxylate is available from wood processing. There is current only when one (not no, not both) ligands bridge chitin-binding metal ions (brown ball) and a metal (micro)electrode. DA reactions are thermally reversible (reactivation, bottom right).
NAND gates (Figure 8) are universal pieces for assembling logical gates which, thus, can fulfill arbitrary functions. However, the measured potential changes are significant but too small for direct secondary processing by semiconductors or relay switches; in fact, Digby´s corresponding experiments used voltages sometimes 1000–2000 times larger than those produced on and by (also dead [3,11]) hornets. Thus, while, generally speaking, chitin can be used for such processes, the actual setup cannot be reduced any further when trying to exploit this nevertheless remarkable effect. Some reported examples of blocked charge flow due to DA reactions are taken from replicator chemistry [20] which implies that additions be reversible; that is, conduction can be reactivated—most simply by thermal treatment once the logical function is fulfilled once. Unlike polyacrylonitrile, chitin is unlikely to provide any conductive polymer secondary backbone structures upon thermal or oxidative treatment itself and, hence, takes surface modification. However, once metal ions are attached to chitin (which occurs spontaneously even from very dilute solutions in water or organic solvents [7,10,28,29] and, hence, can be realized by either photolithography or inkjet printing), they can be subjected to ligand exchange and also to redox reactions within a very broad range of redox potentials.
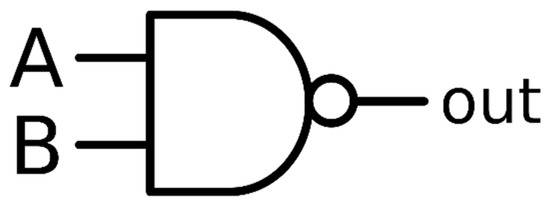
Figure 8.
Description of a simple NAND gate (there are also versions where only one out of >2 inputs will cause a positive reading). Here A and B are conductive entities that link chitin and adsorbed (clusters of) metal ions to an electrode (right; “out”) but are independently introduced by ligation on the metal-modified chitin interface such as to avoid mutual chemical reactions.
This potential window also includes the formation of some more reducing REE2+ ions (Tm, Dy, and Nd) which then can promote interesting coupling reactions, especially since Eu and Yb will never be reduced to the metal (Eu and Yb are the only REEs to spontaneously dissolve in liquid NH3 due to the exceptionally high stability and correspondingly low formation potential of the M2+ ions [30]), and photochemically (photooxidation of appropriate organics [31] by blue–violet light) produced Eu2+ either detaches from chitin or injects electrons into a metal electrode (meeting some activation barrier) or can reduce other metal centers like trivalent Cr, V, or Fe. Now, we arrive in the field of chitin-surface-dependent optoelectronics.
Some very interesting ene-capturing complexes capable of both inner-sphere electron transfer and donating one electron would be η2π2-C6R5Y, where Y, e.g., CN, NH2, OCH3, COOR´, or COO− while MII = Ru(NH3)52+ or Os(NH3)52+, prefer binding to a formal double bond within the aromatic ring to interacting with the “common” Y donor group [32]. Both Ru and Os can undergo reversible Diels–Alder reactions in this framework while capable of donating an electron. When fully using the range of redox potentials accessible on chitin in DMF (down to −2.7 V vs. SCE at least), some typical “conductive” functional groups of a ligand, namely, a nitrile, would undergo (partly reversible) reductive coupling mediated by Dy- or Tm salts affording either N-rich heterocycles, including triazines or other products (2,4′-diminopentyl-3-amine with R = CH3 and M = Tm), the reaction then becoming reversible as is that of imines/enamines with 1,3-cyclohexadiene [33] (for secondary charge flow control purposes) which adducts are unlikely to conduct charges between chitin and a metal counter electrode as good as nitriles would do. For the foreseeable time, one should focus on the studied caffeinate and similar bridges plus competing furan- or pyrrole-based ligands, which also permit storage of some conductive state.
In fact, Dy [14,29,34] and Tm [8] adsorb to chitin as well as the other REEs, save Ce and Eu (which bind to larger extents [26]), and the electrochemical reduction of Tm on chitin was demonstrated by us in preliminary experiments where, however, nitriles were not yet added. Another feature that might be used in the current control is also the activation of one OH group in the chitin polysaccharide rings by complexation of whichever metal ions. This gives rise to an irreversible, reductive electrochemical signal at −1.5 V vs. SCE in DMF. Except for Mn0/Mn2+ or Yb2+/3+, this does not overlap with any electrochemical signals produced on chitin by metal (complex) trapping so far. Otherwise, unmodified chitin is electrochemically completely silent over a potential range of >4 V (from some −2.6 V in DMF up to solvent breakdown).
There can also be planar, bis-chitin constructions that represent a diode or a transistor, whereas entire logical gates can be made from chitin modified with certain complexes and a counter-electrode from metals or semiconductors that are bridged with some pair of ligands involved in inner-sphere electron transfer like maleinate or cis-2-dimethylamino-3-tert-butyl-2,4-pentadiene-carboxylate-1 (made from tert-butyl-vinyl ketone and N,N-dimethylglycinate by Knoevenagel condensation), with a diene reacting with this dienophile used to block electron flow. As a result, there will be a current only if one or the other ligand is present, with the Diels–Alder adduct undergoing cycloreversion when heated. Thus, such an artificial synapse can be reactivated while a simple diene (e.g., 1,3-cyclohexadiene or terpenoid phellandrene) can be used to stop electron flow, also acting as a switch.
It shall be shown that, beyond preparing chitin-based varieties of diodes or transistors, it is likely feasible to construct entire fundamental (i.e., NAND-) gates using
- chitin;
- a redox-active metal ion (best in several oxidation states, such as vanadium);
- two kinds of bridging ligands which, in addition, can react with each other (but not simply dimerize);
- a metal- or semiconductor-based counter-electrode.
The term “nanomanufactoring” does suggest reducing the sizes of corresponding entities to almost molecular dimensions, and there are ideas on how this might be completed concerning chitin from the mode of operation of a scanning–tunneling microscope. For now, preparing corresponding interfaces by inkjet-printing which normally means unit sizes of 85 µm/dot (while the distance between both electrodes actually is a few nm) (≡300 dots/inch) will do, also with respect to applications as a sensor in environmental studies. On chitin “saturated” with most metal ions (there are somewhat higher levels for Pb, Al, Ce, or Eu), the average mutual distance between two adsorbed metal ions (or those that already migrated into bulk chitin) is about 3 nm. Hence, if one wants to link metal ions on/in chitin for conducting structures in the manner of some 1D- or 2D-(semi-)conductor, spacers are needed. Here two chitin surfaces modified in a different way are brought into close contact. However, it should also be feasible to have an asymmetrical chitin/metal adsorbate-(other) metal sandwich control current flows by enabling or avoiding chemistry among the bridging ligands.
Recent experiments on spontaneous backoxidation of photoproduced Eu2+ on an indium electrode in my workgroup showed that the photocurrent greatly increased after a brief reduction caused by fluorescent deviation of energy caused by [Eu(acac)]2+ formation. There is precedent for the transfer of acac ligands during intermetal electron transfer [26]. Apparently, acetylacetonate also is a ligand that causes effective charge transfer to an electrode.
Instead, known properties of surface-modified chitin must be combined with Boolean logic to accomplish an operating system through the switching times of the original system that compare well with that of the wood-based transistor; that is, on the order of 1 s. Voltages of order 1 V or more instead are to be produced by electrochemical processes at either chitin or a metal- or carbon-fleece electrode.
4.3. Sensor Applications [15]
Some slight modifications of the setup can be used for sensors. The unique mode of photooxidation mediated by Eu(III), that is, CH- or NH-bond abstraction affording Eu2+ [7,10], which in turn does reduce trivalent V, Cr, or Fe complexes [35], would enable implementation of optoelectronics producing a full-scale NAND device once again, with activation by light and dienophilic bridging groups where carboxylate does bind to Eu preferentially [36] and catechols bind to metals like In (our favorite electrode material due to acceptably efficient charge injection by Eu2+) or Fe.
Additional products leaked or removed from the few nm gap identified by GC/MS give a record of molecular information processing previously completed. Information can also be stored by oxidizing the DA adduct, such as to cleave the C=C bond left in the formed (2,5-bridged) cyclohexene.
Chitin/metal ion; µ-ligand–metal electrode assemblies may be stacked to construct more complicated arrangements of NAND gates in terms of molecular computing. Likewise, the ligand bridge reaction/current can be terminated by the additions of some dienophile (including oleic acid, for example) or, conversely, a diene like the above cis-2-dimethylamino-3-tert-butyl-2,4-pentadiene-carboxylate-1 ligand or some terpene, to enable sensing of such functional groups.
Stacking of such devices provides more complex logical functions. The reactivation of a logical device after being used takes electrochemical processes. Digby used high negative potentials while the addition of pH-indicator dyes (Nile Blue [2,9]) showed both strong adsorption and some buffer activity of the chitin interface. Digby postulated a role for both Fe and quinones in the chitin matrix for charge transport and showed that cation exchange is also involved in creating some pH gradient (outer side alkalinic, inner side acidic). The alkalinity of the outer side was thought to combine with carboanhydrase activity (which, however, is rather small) and access of Ca2+ to yield CaCO3 (lime) in outside alkalinic conditions finally reinforcing the chitin. The entire process of calcification was thought to be electrochemical in origin which would take sizable current flows per unit of area. However, if this interpretation is correct, chitin would qualify as the optimum biogenic backbone for electronics, including the issue of attaching bias electrodes. This kind of metal counter-electrode demands low activation barriers for electron injection by the use of suitable bridging ligands [37].
While the spontaneous potential measured between identical electrodes on either side can be attributed to pH gradients influencing the same kind of electrode, and electrokinetic water transport was also described by Digby (1967) [2], and some ions placed at the chitin surface will give rise to diffusion fronts in the bulk weeks or months later, like Al or Co [12,37], eventually to turn up on the backside of a thin chitin structure, long-lasting logical structures should mainly rely on elements at least parts of which tend to stick to the chitin surface, such as Pb, Ni, Cu. Conceivably, large ligand structures, e.g., with naphthyl- or triphenylsilyl side chains, or the likely conductive isocyanoterpenoids isolated from marine sponges, will also avoid the active metal center “diving” into the interior chitin structure but keep it where it is needed. In addition, such properly chosen side chains would avoid “horizontal” (parallel to the chitin surface) charge flow and act as the spacers while not sterically inhibiting addition reactions controlling the charge flow unless bound directly to or near the double bonds.
Similar studies concerning derivatives of cinnamate ion concerned the “leakage” of conduction band electrons from quantum dots made from CdSe (4 molecular layers) [38]; here, electron flow rates correlate well with the rate of tunneling expected for a square-well potential and a band gap of some 3 eV (1.67 eV in bulk CdSe), suggesting that actual inner-sphere transfer is rather negligible, or the barrier is higher. The contribution of inner-sphere conduction is thus assumed to be minor. The estimated oxidation potential of the cinnamate ion is some 1.5 V vs. SCE, and that of alkyl cinnamates or cinnamic aldehyde is about 1.9–2.1 V from the empirical eq.
and the σp values tabulated by Hansch et al. (1971) [39].
ε = 1.88 + 1.40 σ [V vs. SCE]
Photoexcited Eu(III) [or UO22+] would thus enable the abstraction of one electron from the HOMO (addressed by the electrochemical procedure) and, in turn, cause conduction, with the electrons eventually passed over to Ce(IV) or oxidized Mn via the two (like) bridges and the chitin space in between.
The logical functions to be implemented in chitin are universal insofar as NAND gates can actually be prepared by anyone by the outlined procedures and combinations. An obvious disadvantage is that reactivation of a once-used logical gate for exerting the next logical operation probably must be completed by heating the device. However, in both of the systems based on Tm and nitriles and certain inner-sphere electron flows between two metal-modified, ligand-(pair) bridged chitin layers can be “erased” by changing the potential of one electrode or the chitin layer accordingly after exerting the “calculation”, much like a nerve axon must be “set back” into possible excitation by back-diffusion of Na or K ions. Using such electrical modes of “reversible computing”, such devices will exert logical operations much faster than NA-based calculations [40] copying (polymerase chain reaction, reproduction in bacteria each taking a couple of minutes), or even biopolymer-based switches like those described by Tran et al. (“balsawood transistor”), because just three atom- or molecule layers are involved; hence, not much charge is required to alter the state of the intercalated material. The above density of metal ions on chitin (mutual distance of about 3 nm) translates into a metal-ion area density of some 1013/cm2; accordingly, some 1013 electrons or about 1–2 µC/cm2 are required to “switch” the system (or to restore the active state ready for another logical operation). In our electrochemical studies with chitin solutions, typical currents were several µA/cm2. Thus, one would expect the transformations to happen within less than 1 s.
A practical application that exploits the properties of electronic keys: keys and smart cards contain hardware encoding some numbers to be unlocked by a corresponding PIN. For security reasons, some kinds of application (such as tickets giving access to a single event or given train/airplane travel route) structures may be preferred which do not leave behind “electronic waste” while storing some information on the (legitimate) holder of the ticket. Chitin can be burnt or subjected to enzyme degradation while the metal/ligand modification is in µg- to sometimes ng ranges only, and hence will not cause any pollution issues.
Author Contributions
Conceptualization: S.F.; investigation S.F. and F.B., writing, editing: S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
First: we are grateful for the invitation to write this paper. Second, of course, my (SF) most thanks and respect for their stubborn readiness to overcome both technical difficulties and sometimes conceptional vagueness with often simple but powerful means goes to my team during the last ten years or so, and of course to the lab-staff at Zittau. In addition, the comments of three anonymous referees helped much to render the text more clear and comprehensible.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| β | complex formation constant here applied to the first step of complexation; that is, Mx+ + L ↔ [ML]x+. Usually log β is tabulated, e.g., in the equation: Log β = a*Σσ + b. Ligand designations: see the list in the explanation of Figure 5. |
| NHE | normal hydrogen electrode: the potential of an electrode formed from hydrogen (H2) gas circulating around some metal at ambient pressure and pH = 0. Because it is cumbersome to work with, it is hardly used outside of fuel cells (there, however, solutions are either more acidic, i.e., viscous phosphoric acid) or even alkalinic (conc. KOH) and almost never employed in pH/H0 determination. |
| Pzzp | point of zero zeta potential. At this pH or (in non-aq. media) H0 value, the surface of some solid—not necessarily an electrode—is uncharged. For chitin = pH 4.6 [12], for Au = pH 3, for all other materials (metals) in water it is approximately the pzzp of the corresponding oxide or hydroxide which can cover a large range: W pH = 0.5, Mg, and REEs about 10. Below pzzp, the interface is protonated and thus positively charged while at pH > pzzp it is deprotonated (acts as an acid) and thus becomes negatively charged. Of course, this does control the adsorption of cat- vs. anions and thus the sign of the electrokinetic (=zeta) potential. |
| REE | rare earth elements: metals with Z = 57…71 (La–Lu). They are distinguished by (fairly) similar chemical properties, not excluding that differences can be used for the detection of environmental parameters. |
| REY | REEs (see above) and yttrium |
| SCE | saturated calomel electrode (Hg2Cl2 + 2e−/2 Hg + 2Cl−), ε = +0.241 V vs. NHE. Chloride ion activity in SCE setup is that of a saturated aq. KCl solution; the potential of 1 M KCl/calomel/Hg is SCE +39 mV |
| σ | the substituent constant introduced by L.P. Hammett to understand chemical equilibria (acidity constants) and kinetics of substitution in/at/of aromatic compounds by either cations, other electrophils, or free radicals. |
References
- Tran, V.C.; Mastantuoni, G.G.; Zabihipour, M.; Li, L.; Berglund, L.; Berggren, M.; Engquist, I. Electrical current modulation in wood electrochemical transistor. Proc. Natl. Acad. Sci. USA 2023, 120, e2218380120. [Google Scholar] [CrossRef] [PubMed]
- Digby, P.B.S. Semi-conduction and electrode processes in biological material. I. Crustacea and certain soft-bodied forms. Transact. Linn. Soc. Lond. 1965, 176, 504–525. [Google Scholar]
- Ishay, J.S.; Rosenzweig, E.; Litinetsky, L.; Kirshboim, S. The solar cell in hornet cuticle: Nanometer to micrometer scale. J. Electron. Microsc. 2000, 49, 559–568. [Google Scholar] [CrossRef]
- Lum, R.M.; Klingert, J.K. Thermochemistry of alkylarsine compounds used as arsenic precursors in metalorganic vapor phase epitaxy. J. Appl. Phys. 1989, 66, 3820–3823. [Google Scholar] [CrossRef]
- Stankiewicz, B.A.; Van Bergen, P.F.; Duncan, I.J.; Carter, J.F.; Briggs, D.E.; Evershed, R.P. Recognition of chitin and proteins in invertebrate cuticles using analytical pyrolysis/gas chromatography and pyrolysis/gas chromatography/mass spectrometry. Rapid Comm. Mass Spectrom. 1996, 10, 1747–1757. [Google Scholar] [CrossRef]
- Stankiewicz, B.A.; Mastalerz, M.; Hof, C.H.J.; Bierstedt, A.; Flannery, M.B.; Briggs, D.E.G.; Evershed, R.P. Biodegradation of the chitin-protein complex in crustacean cuticle. J. Org. Geochem. 1998, 28, 67–76. [Google Scholar] [CrossRef]
- Blind, F.; Fränzle, S. Chitin as a sorbent superior to other biopolymers: Features and applications in environmental research, energy conversion, and understanding evolution of animals. Polysaccharides 2021, 2, 773–794. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Rocchetti, R.; Marangio, G. Separation of zirconium, niobium, cerium and ruthenium on chitin and chitosan columns for the determination of cesium in nuclear fuel solutions. J. Radioanal. Chem. 1972, 10, 17–25. [Google Scholar] [CrossRef]
- Digby, P.B.S. Calcification and its mechanism in the shore-crab, Carcinus maenas (L.). Proc. Linn. Soc. Lond. 1967, 178, 129–146. [Google Scholar] [CrossRef]
- Fränzle, S. Estimating and predicting chemical potentials, distributions, speciation modes and mobilities of radiometals in soil, water and biomass. J. Environ. Radioact. 2013, 102, 109–116. [Google Scholar] [CrossRef]
- Kirshboim, S.; Ishay, J.S. Silk produced by hornets: Thermophotovoltaic properties—A review. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 127, 1–20. [Google Scholar] [CrossRef]
- Gebauer, T. Methodische Optimierung des Übertrags von Metallionen aus Umweltprobenmodellen auf Chitinoberflächen und von Diesen zu Zwecken Analytischen Biomonitorings Sowie Untersuchungen zur Diffusion/Ausbreitung von Analyten in Chitinproben. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2016. [Google Scholar]
- Klapiszewski, Ł.; Wysokowski, M.; Majchrzak, I.; Szatkowski, T.; Nowacka, M.; Siwi´nska-Stefa´nska, K.; Szwarc-Rzepka, K.; Bartczak, P.; Ehrlich, H.; Jesionowski, T. Preparation and Characterization of Multifunctional Chitin/Lignin Materials. J. Nanomat. 2013, 2013, 425726. [Google Scholar] [CrossRef]
- Nainggolan, I.; Nasution, T.I.; Putri, S.R.E.; Azdena, D.; Balyan, M.; Agusnar, H. Study on chitosan film properties as a green dielectric. IOP Confer. Ser. Mater. Chem. Eng. 2018, 309, 012801. [Google Scholar] [CrossRef]
- Erler, M. Untersuchung des Bindungsverhaltens Ausgewählter Elemente und Ihrer Bodenrelevanten Komplexe an Chitin. Master’s Thesis, IHI Zittau/Technische Universität Dresden, Dresden, Germany, 2020. [Google Scholar]
- Junold, M. Versuche zur Isolation und Identifikation Boden Bürtiger Organischer Säuren Mithilfe Eines Photochemischen Sensors. Master’s Thesis, IHI Zittau/Technische Universität Dresden, Dresden, Germany, 2021. [Google Scholar]
- Couffin, F. Potentiels d´Oxydo-Reduction des Elements Lanthanides et Actinides dans les Solvants Organiques; CEA-BIB 233; French Scientific Institution CEA: Paris, France, 1980.
- Zhou, M.; Pagels, M.; Geschke, B.; Heinze, J. Electropolymerization of Pyrrole and Electrochemical Study of Polypyrrole. 5. Controlled Electrochemical Synthesis and Solid-State Transition of Well-Defined Polypyrrole Variants. J. Phys. Chem. B 2002, 106, 1006573. [Google Scholar] [CrossRef]
- Taube, H. Recent Progress in the Study of Inner-Sphere Electron-Transfer Reactions; DeGruyter: Berlin, Germany, 1970. [Google Scholar]
- Tobe, M.L. Reaktionsmechanismen der Anorganischen Chemie; Verlag Chemie: Weinheim, Germany, 1976. [Google Scholar]
- Vidonne, A.; Philp, D. Making molecules make themselves—The chemistry of artificial replicators. Eur. J. Org. Chem. 2009, 5, 593–610. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamic functions of transfer of single ions from water to nonaqueous and mixed solvents: Part 1—Gibbs free energies of transfer to nonaqueous solvents. Pure Appl. Chem. 1983, 55, 977–1021. [Google Scholar] [CrossRef]
- Kolthoff, I.M.; Chantooni, M.K.; Smagowski, H. Acid-base strength in N,N-dimethylformamide. Anal. Chem. 1970, 42, 1622–1628. [Google Scholar] [CrossRef]
- Bauer, A. Orientierende Untersuchungen zur Bindung von Metallionen an Chitin und zur davon abhängigen Eignung von Arthropoden zur Bestimmung von Metallionenkonzentrationen in der Umwelt. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2014. [Google Scholar]
- Kuppusamy, V.; Balasubramanian, R. Single and binary biosorption of cerium and europium onto crab shell particles. Chem. Engin J. 2010, 163, 337–343. [Google Scholar]
- Balahura, R.J.; Lewis, N.A. Substituent effects in electron transfer reactions: The preparation and chromium(II) reduction of 3-formylpentane-2,4-dionatobis(ethylenediamine)cobalt(III). Preparation of the linkage isomer acetylbutane-1,3-dionatobis(ethylenediamine)cobalt(II). Can. J. Chem. 1975, 53, 1154–1164. [Google Scholar] [CrossRef]
- Retschke, D. Orientierende Untersuchungen zur Adsorption von Schwermetallen (Nickel) unter dem Einfluss Ausgewählter Komplexliganden Sowie in Arealen Potenzieller und Manifester Methanogenese. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2017. [Google Scholar]
- Budelmann, P. Verbreitung der Flusskrebse (Decapoda) in der südlichen Oberlausitz und die Eignung des invasiven Kamberkrebses (Orconectes limosus) für Chitin-basiertes Monitoring von Schwermetallen in limnischen Ökosystemen. Master‘s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2021. [Google Scholar]
- Nief, F. Non-classical divalent lanthanoid complexes. Dalton Transact. 2010, 39, 6589–6598, (dissolution of Eu and Yb metals in liquid NH3). [Google Scholar] [CrossRef]
- Blind, F. Orientierende Untersuchungen zur Platinmetall freien Aktivierung von CH-Bindungen für Europium basierte Brennstoffzellenanwendungen. Master‘s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2018. [Google Scholar]
- Harman, W.D.; Taube, H. The selective hydrogenation of benzene to cyclohexene on pentaammineosmium(II). J. Am. Chem. Soc. 1988, 110, 7906–7907. [Google Scholar] [CrossRef]
- Heintzelman, G.R.; Meigh, I.R.; Mahajan, Y.R.; Weinreb, S.M. Diels-Alder Reactions of Imino Dienophiles. Org. React. 2005, 65, 141–599. [Google Scholar] [CrossRef]
- Carlyle, D.W. Kinetics and Mechanisms of Some Rapid Substitution and Oxidation-Reduction Reactions of Iron(III), Europium (II). Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1968. [Google Scholar]
- Vold, I.M.N.; Christensen, B.E. Periodate oxidation of chitosans with different chemical compositions. Carbohydrate Res. 2005, 340, 679–684. [Google Scholar] [CrossRef]
- Wood, S.A. The aqueous geochemistry of the rare-earth elements: Critical stability constants for complexes with simple carboxylic acids at 25 °C and 1 bar and their application to nuclear waste management. Engin. Geol. 1993, 34, 229–259. [Google Scholar] [CrossRef]
- Fronaeus, S.; Johansson, C.L. Kinetics of the electrode processes between copper amalgam and Copper(II) glyoxylate and pyruvate solutions. Electroanal. Chem. Interfac. Electrochem. 1975, 60, 29–39. [Google Scholar] [CrossRef]
- Fränzle, S.; Erler, M.; Blind, F.; Ariuntsetseg, L.; Narangarvuu, D. Chitin adsorption in environmental monitoring: Not an alternative to moss monitoring but a method providing (lots of) bonus information. J. Sci. Arts. Univ. Valahia 2019, 19, 659–674. [Google Scholar]
- Wijtmans, M.; Rosenthal, S.J.; Zwanenburg, B. Visible Light Excitation of CdSe Nanocrystals Triggers the Release of Coumarin from Cinnamate Surface Ligands. J. Am. Chem. Soc. 2006, 128, 11720–11726. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 97, 165–195. [Google Scholar] [CrossRef]
- Adleman, L.M. Molecular computation of solutions to combinatorial problems. Science 1994, 266, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).