Anti-Inflammatory Nanocarriers Based on SWCNTs and Bioactive Molecules of Oregano: An In Silico Study
Abstract
1. Introduction
2. Computational Methods
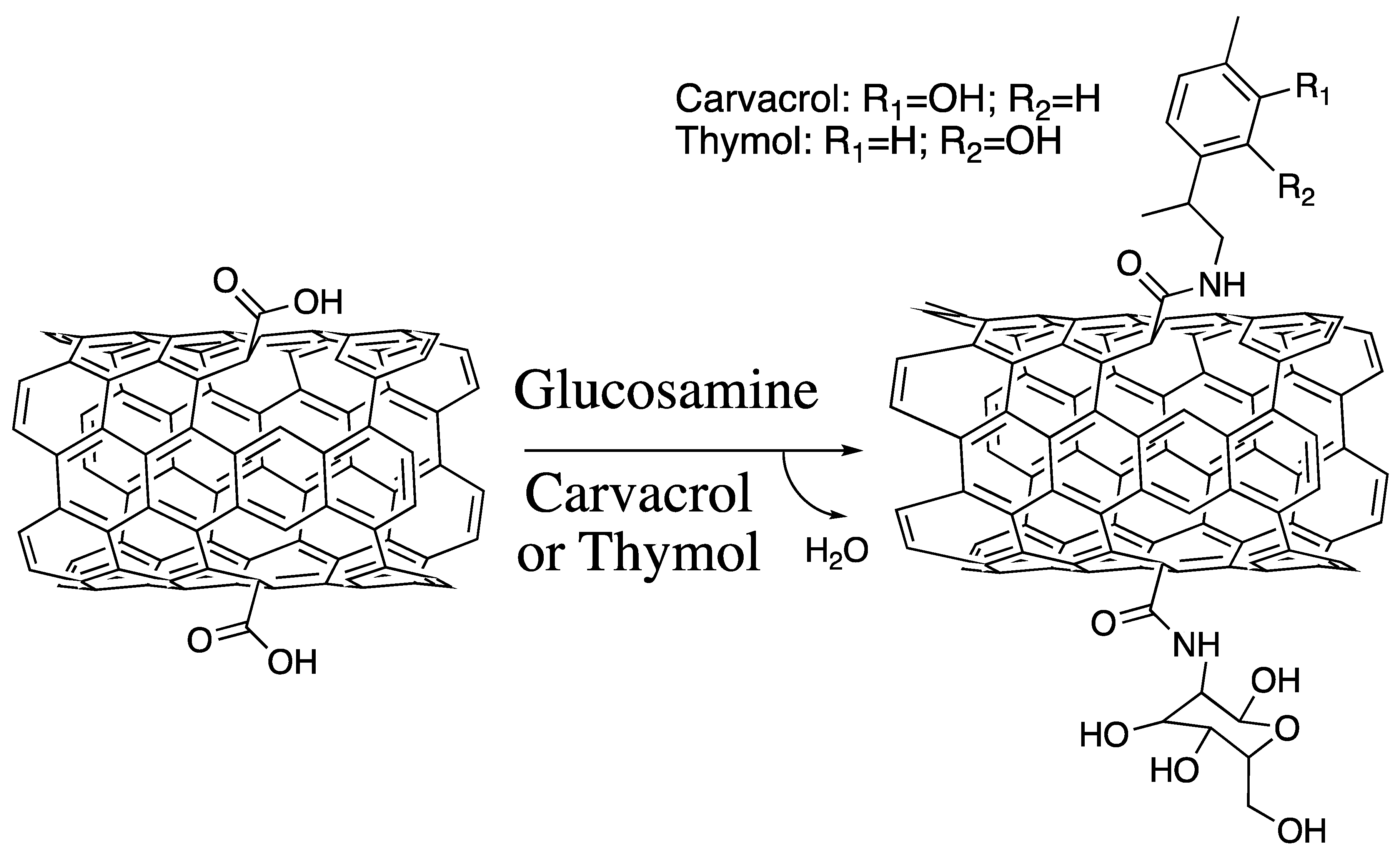
2.1. Functionalization, Binding Energies, and Chemical Descriptors
2.2. Solubility in Water
2.3. Molecular Docking
3. Results and Discussion
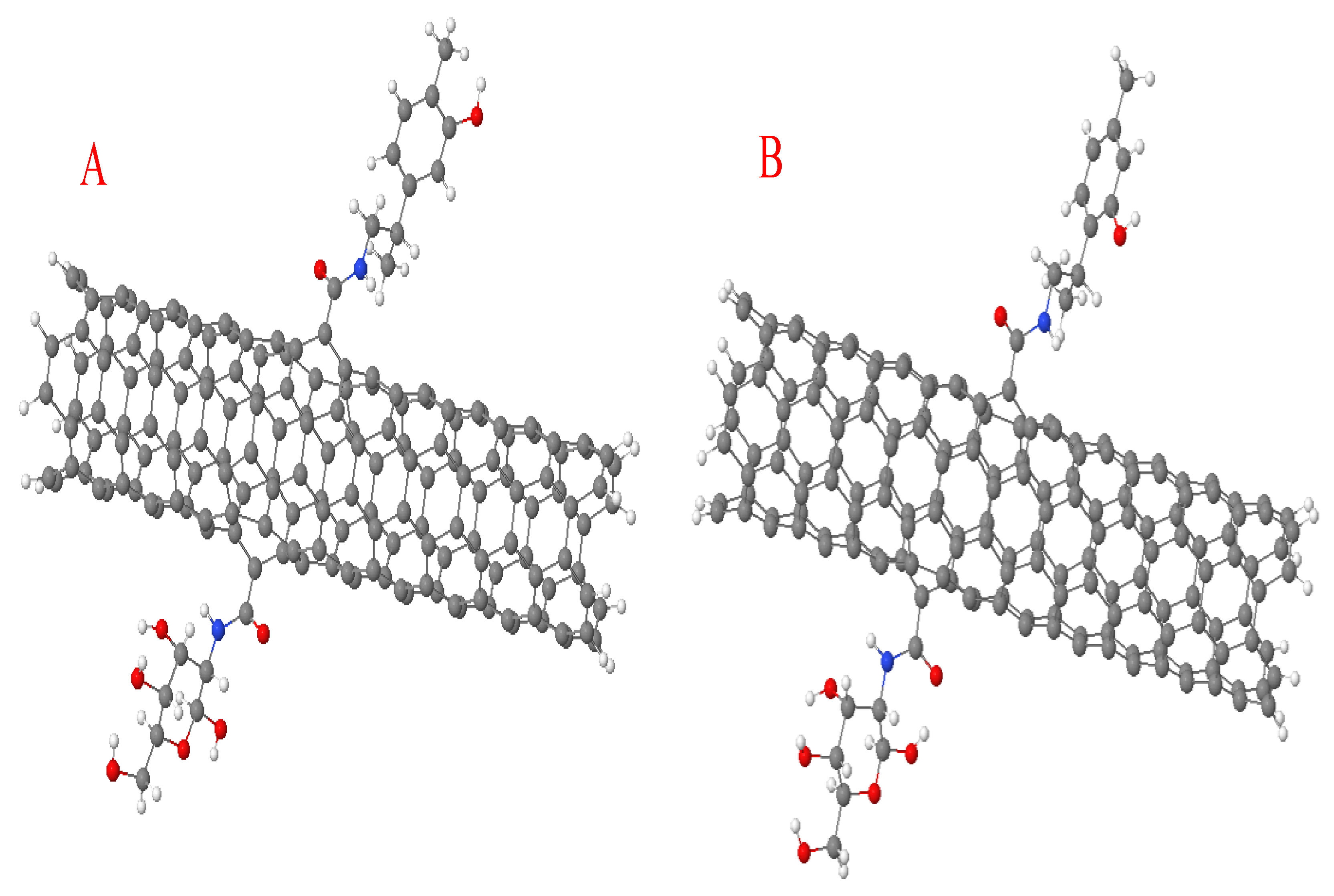
3.1. Functionalization and Binding Energies
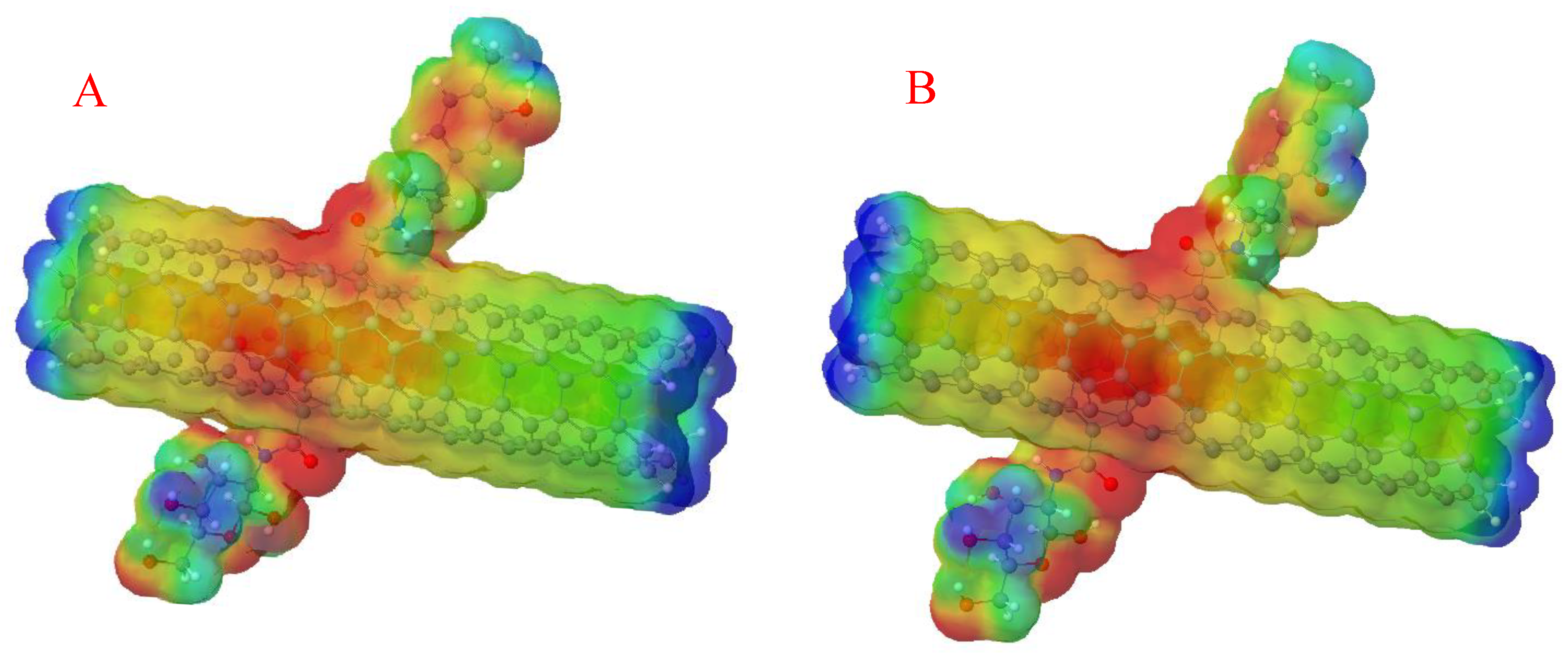
3.2. Global Chemical Descriptors
3.3. Solubility in Water
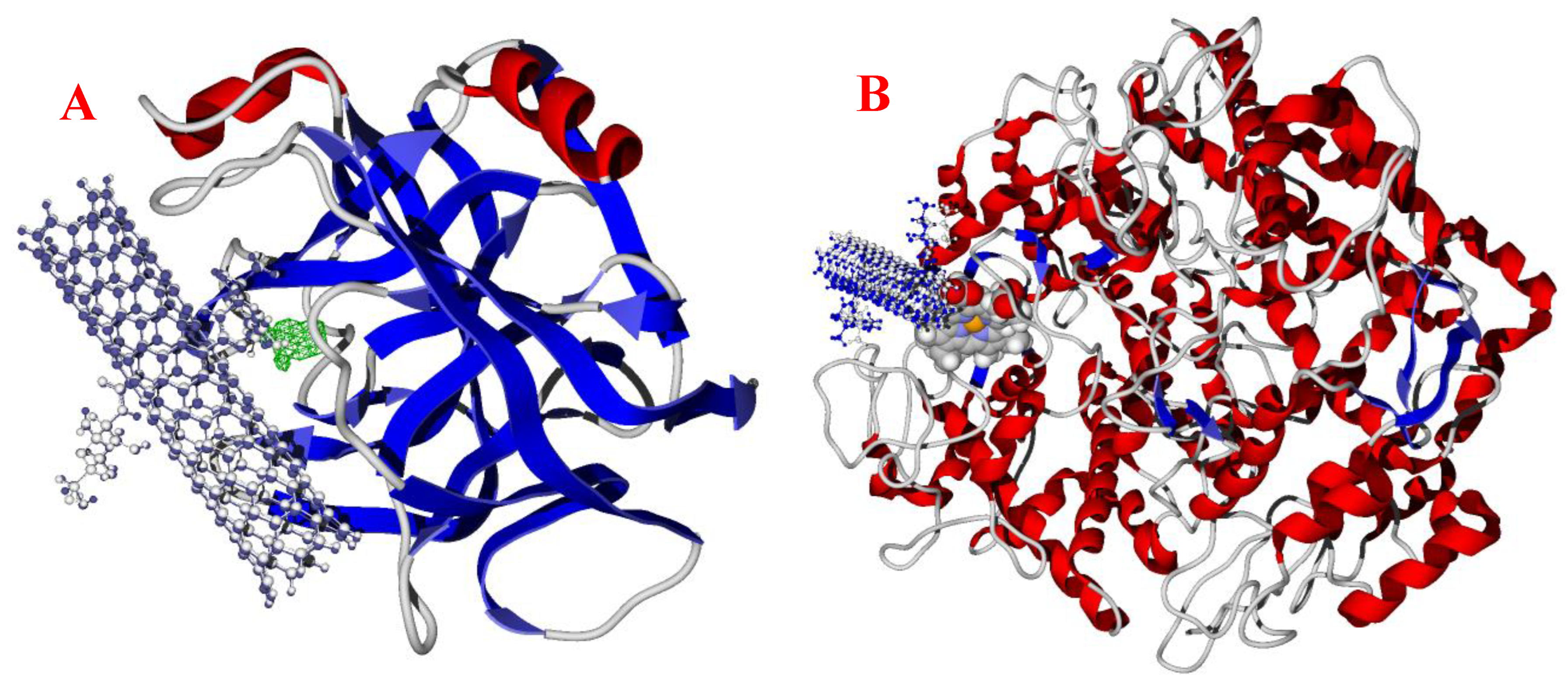
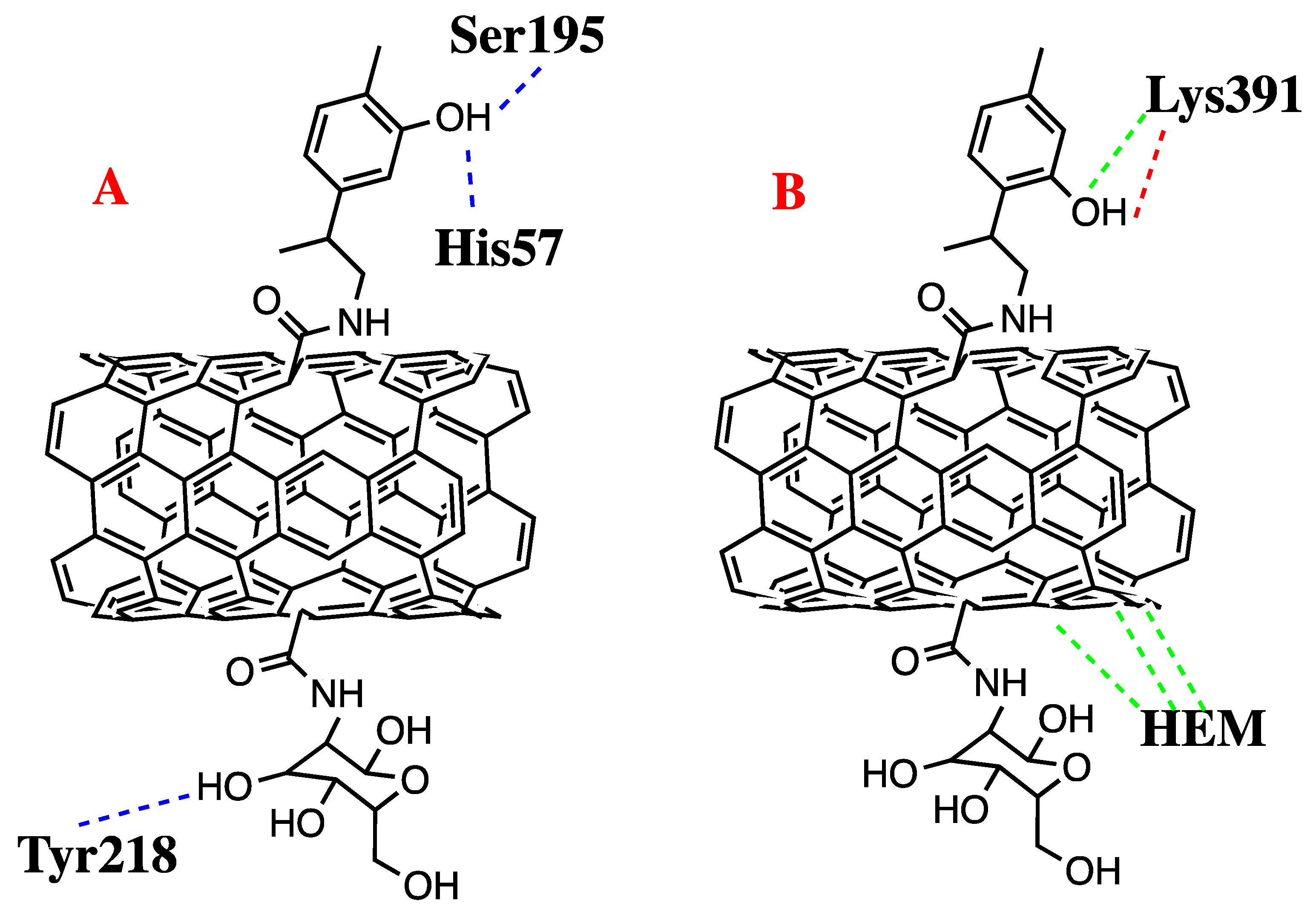
3.4. Molecular Docking
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gautam, N.; Mantha, A.K.; Mittal, S. Essential oils and their constituents as anticancer agents: A mechanistic view. BioMed Res. Int. 2014, 1, 154106. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Sun, C.; Huang, S.; Zhou, Q. Carvacrol suppresses proliferation and invasion in human oral squamous cell carcinoma. OncoTargets Ther. 2016, 9, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Ruiz, M.C.; Sánchez, R.A.S.; Ramos, S.V.; Muñoz, J.V.T.; Nevárez-Moorillón, G.V. Antifungal effect of Mexican oregano (Lippia berlandieri Schauer) essential oil on a wheat flour-based Medium. J. Food Sci. 2012, 77, M441–M445. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I. An overview of the biological effects of some mediterranean essential oils on human health. BioMed Res. Int. 2017, 1, 9268468. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef]
- Sarac, N.; Ugur, A. Antimicrobial activities of the essential oils of Origanum onites L., Origanum vulgare L. subspecies hirtum (Link) Ietswaart, Satureja thymbra L., and Thymus cilicicus Boiss. & Bal. growing wild in Turkey. J. Med. Food 2008, 11, 568–573. [Google Scholar]
- Prieto, J.M.; Lacopini, P.; Cioni, P.; Chericoni, S. In vitro activity of the essential oils of Origanum vulgare, Satureja montana and their main constituents in peroxynitrite-induced oxidative processes. Food Chem. 2007, 104, 889–895. [Google Scholar] [CrossRef]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevao-Silva, C.F.; Carvalho, M.D.D.B.; Cunha, J.M.; Grespan, R.; Cuman, R.K.N. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evid.-Based Compl. Alt. 2012, 1, 657026. [Google Scholar]
- Sobral, M.V.; Xavier, A.L.; Lima, T.C.; De Sousa, D.P. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014, 1, 953451. [Google Scholar] [CrossRef]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Baygar, T.; Ozgul Onal, M.; Ozturk, F. Comparison of ultrastructural changes and the anticarcinogenic effects of thymol and carvacrol on ovarian cancer cells: Which is more effective? Ultrastruct. Pathol. 2020, 44, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Ortega-Nieblas, M.M.; Robles-Burgueño, M.R.; Acedo-Félix, E.; González-León, A.; Morales-Trejo, A.; Vázquez-Moreno, L. Chemical composition and antimicrobial activity of oregano (Lippia palmeri S. Wats) essential oil. Rev. Fitotec. Mex. 2011, 34, 11–17. [Google Scholar] [CrossRef]
- Nostro, A.; Papalia, T. Antimicrobial activity of carvacrol: Current progress and future prospectives. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Nagoor-Meeran, M.F.; Javed, H.; Al-Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
- Can-Baser, K.H. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar] [CrossRef] [PubMed]
- Ben-Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.; Alberda, M.; Hoekstra, F.A.; Smid, E.J. Adaptation of the food-borne pathogen Bacillus cereus to carvacrol. Arch. Microbiol. 2000, 174, 233–238. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef] [PubMed]
- Arunasree, K.M. Anti-proliferative effects of carvacrol on a human metastatic breast cancer cell line, MDA-MB 231. Phytomedicine 2010, 17, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Li, X.; Cao, Y.; Qi, H.; Li, L.; Zhang, Q.; Sun, H. Carvacrol inhibits proliferation and induces apoptosis in human colon cancer cells. Anticancer. Drugs 2015, 26, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Bahuguna, A.; Kumar, P.; Bajpai, V.K.; Kang, S.C. In vitro and in vivo antitumor potential of carvacrol nanoemulsion against human lung adenocarcinoma A549 cells via mitochondrial mediated apoptosis. Sci. Rep. 2018, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Pérez, K.M.; Medina, D.I.; Narayanan, J.; Parra-Saldívar, R.; Iqbal, H.M.N. Synthesis and Nano-Sized Characterization of Bioactive Oregano Essential Oil Molecule-Loaded Small Unilamellar Nanoliposomes with Antifungal Potentialities. Molecules 2021, 26, 2880. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kim, Y.S.; Kim, E.K.; Hwang, J.W.; Jeong, J.H.; Dong, X.; Park, P.J. Anticancer effect of thymol on AGS human gastric carcinoma cells. J. Microbiol. Biotechnol. 2016, 26, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, D.A.A.K.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Current medicinal chemistry. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Liolios, C.C.; Gortzi, O.; Lalas, S.; Tsaknis, J.; Chinou, I. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 2009, 112, 77–83. [Google Scholar] [CrossRef]
- Coimbra, M.; Isacchi, B.; van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Bilia, R. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef]
- Díaz-Cervantes, E.; García-Revilla, M.A.; Robles, J.; Aguilera-Granja, F. Solubility of functionalized single-wall carbon nanotubes in water: A theoretical study. Theor. Chem. Acc. 2017, 136, 127. [Google Scholar] [CrossRef]
- Díaz-Cervantes, E.; Robles, J.; Aguilera-Granja, F. Understanding the structure, electronic properties, solubility in water, and protein interactions of three novel nano-devices against ovarian cancer: A computational study. J. Nanopart. Res. 2018, 20, 266. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules, 1st ed.; Oxford Science Publications: Cary, NC, USA, 1989. [Google Scholar]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Avogadro: An Open-Source Molecular Builder and Visualization Tool, Version 1.2.0.
- Svensson, M.; Humbel, S.; Froese, R.D.J.; Matsubara, T.; Sieber, S.; Morokuma, K. ONIOM: A Multilayered Integrated MO + MM Method for Geometry Optimizations and Single Point Energy Predictions. A Test for Diels−Alder Reactions and Pt(P(t-Bu)3)2 + H2 Oxidative Addition. J. Phys. Chem 1996, 100, 19357–19363. [Google Scholar] [CrossRef]
- Humbel, S.; Sieber, S.; Morokuma, K. The IMOMO method: Integration of different levels of molecular orbital approximations for geometry optimization of large systems: Test for n-butane conformation and SN2 reaction: RCl + Cl−. J. Chem. Phys. 1996, 105, 1959–1967. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Mod. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed]
- Binkley, J.S.; Pople, J.A.; Hehre, W.J. Self-Consistent Molecular Orbital Methods. 21. Small Split-Valence Basis Sets for First-Row Elements. J. Am. Chem. Soc. 1980, 102, 939–947. [Google Scholar] [CrossRef]
- Schwabe, T.; Grimme, S. Theoretical thermodynamics for large molecules: Walking the thin line between accuracy and computational cost. Acc. Chem. Res. 2008, 41, 569–579. [Google Scholar] [CrossRef]
- Onsager, L. Electric Moments of Molecules in Liquids. J. Am. Chem. Soc. 1936, 58, 1486–1493. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Yousef, G.M.; Obiezu, C.V.; Jung, K.; Stephan, C.; Scorilas, A.; Diamandis, E.P. Differential expression of kallikrein gene 5 in cancerous and normal testicular tissues. Urology 2002, 60, 714–718. [Google Scholar] [CrossRef]
- Wang, J.L.; Limburg, D.; Matthew, J.; Graneto, J.; Springer, J.; Hamper, J.R.B.; Liao, S.; Pawlitz, J.L.; Kurumbail, R.G.; Maziasz, T.; et al. The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: The second clinical candidate having a shorter and favorable human half-life. Bioorg. Med. Chem. Lett. 2010, 20, 7159–7163. [Google Scholar] [CrossRef]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, C. GEMDOCK: A generic evolutionary method for molecular docking. Proteins 2004, 55, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor-Granados, T.; Díaz-Cervantes, E.; Soto-Arredondo, K.J.; Martínez-Alfaro, M.; Robles, J.; García-Revilla, M.A. Binding of Pb-Melatonin and Pb-(Melatonin-metabolites) complexes with DMT1 and ZIP8: Implications for lead detoxification. DARU 2019, 27, 137–148. [Google Scholar] [CrossRef] [PubMed]
| Molecule | ΔΔGrx (eV) | |
|---|---|---|
| No Dispersion | Dispersion | |
| Carva-SWCNT-Gluc | −1.75 | −2.55 |
| Thymol-SWCNT-Gluc | −1.81 | −3.06 |
| Molecule | * I | A | χ | η | s | ω | EGap |
|---|---|---|---|---|---|---|---|
| Carvacrol | 7.72 | 1.67 | 4.69 | 3.03 | 0.33 | 3.64 | 4.31 |
| Thymol | 7.72 | 1.60 | 4.66 | 3.06 | 0.33 | 3.54 | 4.24 |
| Carva-SWCNT-Gluc | 5.04 | 3.18 | 4.11 | 0.93 | 1.08 | 9.11 | 0.08 |
| Thymol-SWCNT-Gluc | 5.20 | 3.16 | 4.18 | 1.02 | 0.98 | 8.53 | 0.26 |
| COOH-SWCNT44-COOH [31] | 4.25 | 4.12 | 4.19 | 0.06 | 15.42 | 35.22 | 0.01 |
| Molecule | ∆∆Gsolv (eV) |
|---|---|
| Carva-SWCNT-Gluc | −1.28 |
| Thymol-SWCNT-Gluc | −1.25 |
| Carvacrol | −0.17 |
| Thymol | −0.17 |
| Ligand | * Target | Energy | LE | Hbond | Electro | VdW |
|---|---|---|---|---|---|---|
| Carva-SWCNT-Gluc | KLK5 | −14.92 | −0.07 | −0.05 | 0.01 | 23.06 |
| Cox2 | −22.63 | −0.11 | −0.26 | −0.05 | −0.59 | |
| Thymol-SWCNT-Gluc | KLK5 | −16.70 | −0.08 | −0.05 | −0.05 | 8.39 |
| Cox2 | −22.08 | −0.11 | −0.11 | −0.08 | −1.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Cervantes, E.; Monjaraz-Rodríguez, A.; Aguilera-Granja, F. Anti-Inflammatory Nanocarriers Based on SWCNTs and Bioactive Molecules of Oregano: An In Silico Study. Nanomanufacturing 2022, 2, 176-185. https://doi.org/10.3390/nanomanufacturing2040012
Díaz-Cervantes E, Monjaraz-Rodríguez A, Aguilera-Granja F. Anti-Inflammatory Nanocarriers Based on SWCNTs and Bioactive Molecules of Oregano: An In Silico Study. Nanomanufacturing. 2022; 2(4):176-185. https://doi.org/10.3390/nanomanufacturing2040012
Chicago/Turabian StyleDíaz-Cervantes, Erik, Alejandra Monjaraz-Rodríguez, and Faustino Aguilera-Granja. 2022. "Anti-Inflammatory Nanocarriers Based on SWCNTs and Bioactive Molecules of Oregano: An In Silico Study" Nanomanufacturing 2, no. 4: 176-185. https://doi.org/10.3390/nanomanufacturing2040012
APA StyleDíaz-Cervantes, E., Monjaraz-Rodríguez, A., & Aguilera-Granja, F. (2022). Anti-Inflammatory Nanocarriers Based on SWCNTs and Bioactive Molecules of Oregano: An In Silico Study. Nanomanufacturing, 2(4), 176-185. https://doi.org/10.3390/nanomanufacturing2040012