Abstract
Leishmaniasis is a tropical and subtropical disease caused by protozoans of the genus Leishmania, primarily transmitted to humans through the bite of sandflies. This neglected disease poses a serious global health challenge due to its spectrum of clinical manifestations, which can lead to potentially fatal outcomes. In Honduras, four clinical forms of leishmaniasis are present: ulcerative cutaneous leishmaniasis (UCL), mucosal leishmaniasis (ML), non-ulcerated cutaneous leishmaniasis (NUCL), and visceral leishmaniasis (VL). This study aims to identify spatial patterns of these four clinical forms of the disease in Honduras, utilizing epidemiological data from 2009 to 2016. Geographic Information System (GIS) analysis was employed for spatial assessment. Moran’s I was used to evaluate the data and reveal patterns, while Hot Spot Analysis identified statistically significant spatial clusters of high and low values. For UCL and NUCL, all Global Moran’s I p-values were below 0.001 throughout the study period. For VL, p-values were under 0.001 in 2010, 2013, and 2016. For ML, p-values were below 0.05 in 2009, 2011, 2014, and 2015. In conclusion, our findings demonstrate geographical segregation among the different clinical forms of leishmaniasis.
1. Introduction
Leishmaniasis is a tropical and subtropical disease caused by protozoans of the genus Leishmania (Kinetoplastida: Trypanosomatidae), primarily transmitted to humans through sandfly bites. In 2023, 272,098 new cases of cutaneous leishmaniasis and 11,922 of visceral leishmaniasis were reported in 200 countries worldwide [1]. This neglected disease represents a serious world health problem, particularly in Latin America, due to its broad spectrum of clinical manifestations that can result in fatal outcomes [2,3]. In Honduras, four clinical forms of leishmaniasis are recognized: ulcerative cutaneous leishmaniasis (UCL), non-ulcerative cutaneous leishmaniasis (NUCL), mucosal leishmaniasis (ML), and visceral leishmaniasis (VL), each determined by clinical characteristics, their geographic distribution, parasite species, and vectors involved [4,5,6,7,8,9].
The first case of VL reported in Honduras occurred in 1977 in the southern region of the country [10]. Fourteen years later, in 1988, a variant of cutaneous leishmaniasis caused by Leishmania (L.) infantum chagasi [6] was identified in the same area. In contrast, the UCL and ML forms are primarily found along the Caribbean coast [11]. In Honduras, three species of Leishmania have been reported: Leishmania (L.) infantum chagasi [8,12,13], which is associated with both VL and NUCL, and is predominantly distributed in the southern part of the country; Leishmania (V.) braziliensis [12], which is associated with UCL and ML, and is found in the northeastern region; and Leishmania (V.) panamensis [12], which is associated only with UCL and circulates in the northwestern part of the country.
Despite the prevalence of the disease in this region, the spatial and temporal distribution of leishmaniasis throughout Honduras has not yet been studied. Therefore, this study aims to identify spatial patterns of the different clinical forms of leishmaniasis reported in Honduras, based on epidemiological data from 2009 to 2016. The data were provided by the National Health Secretary’s Chagas and Leishmania National Program and analyzed at the sub-regional government level (local municipalities). We also evaluated the characteristics of the patterns (clustered, dispersed, or random) and identified spatial clusters within the country.
2. Materials and Methods
2.1. Study Area and Data
Honduras is located in northern Central America (15°11′60″ N 86°14′30.9″ W), and has a population of 9,880,507 across 111,890 km2 [14]. The climate is tropical, with an estimated average annual rainfall of 1500 mm. The yearly average maximum and minimum temperatures are 35 °C and 19 °C, respectively. Honduras is divided into 18 regional governments known as departments and 298 municipalities (Figure 1).
Figure 1.
Map of Honduras showing the geographical position in Central America.
Leishmaniasis is a reportable disease under the surveillance of the Honduran Ministry of Public Health. All leishmaniasis cases registered at the municipality level for the period 2009–2016 were provided by the National Health Secretary’s Chagas and Leishmania National Program (http://www.salud.gob.hn/site/index.php, accessed on 20 October 2023). The database does not include names or personal information of patients.
The incidence data were categorized as cutaneous leishmaniasis (UCL and NUCL), ML, and VL. Cases of UCL and NUCL are confirmed through microscopic analysis of the biopsy or cutaneous scraping, which usually reveals the parasite in its amastigote stage. VL is confirmed based on clinical symptomatology or via an rK39-positive quick test that utilizes bone marrow material for microscopic analysis of the parasite in its amastigote stage, according to the “Resolution DGM-M04:2016, Integral Approach Manual of Leishmaniasis in Honduras” [15]. The database includes the confirmed cases of the four clinical forms of leishmaniasis in Honduras during 2009 and 2016, along with age group classifications.
2.2. Leishmaniasis Morbidity Analysis
The population data by year for calculating the incidence rate of leishmaniasis (2009–2016) were obtained from the Honduran National Institute of Statistics (https://ine.gob.hn/bases-de-datos/, accessed on 20 October 2023). The geographic layers for the country outline and administrative subdivisions were obtained from the Center for Spatial Sciences at the University of California. These layers contained a single-class vector format (polygons). The disease incidence rate was calculated from the total number of cases per 10,000 inhabitants in each municipality, divided by the total number of people at risk within the municipality.
2.3. Spatial Analysis
The information on the four clinical forms of leishmaniasis reported in Honduras was mapped and analyzed using a geographic information system (ArcGIS Pro 2.5.1) [16]. Incidence maps were created to highlight the distribution of leishmaniasis at the municipality level, and Global Moran’s I was used to evaluate whether the geographic pattern of incidence was clustered, dispersed, or random, based on the calculation of spatial autocorrelation of the data. Finally, a Hot Spot Analysis based on the Getis–Ord Gi* statistic was employed to identify statistically significant spatial clusters of high and low incidence values. The Getis–Ord Gi* statistic uses the null hypothesis of randomization as the basis for the test of statistical significance.
3. Results
3.1. Distribution of Cases and Incidence
Between 2009 and 2016, 15,345 cases of leishmaniasis were confirmed in 112 municipalities across 14 (77.7%) of the 18 Honduran departments. The department with the highest number of cases was Olancho, with 4064 cases (26.5%), with an incidence rate of 75.2, whilst Copan was the department with the lowest number of cases (7 cases, 0.04%, incidence rate 0.7, as was Lempira (Table 1). UCL was the most prevalent form of the disease in the country, with 9167 cases (59.73%), followed by NUCL with 6055 cases (39.45%), ML with 92 cases (0.59%), and VL with 31 cases (0.20%) (Figure 2). The distribution of incidence remained stable from 2011 to 2015, followed by an increase in 2016, with 2652 cases (an incidence rate of 3.86 per 10,000 inhabitants) (Figure 3).

Table 1.
Epidemiological overview of leishmaniasis in Honduras, during the period 2009–2016.
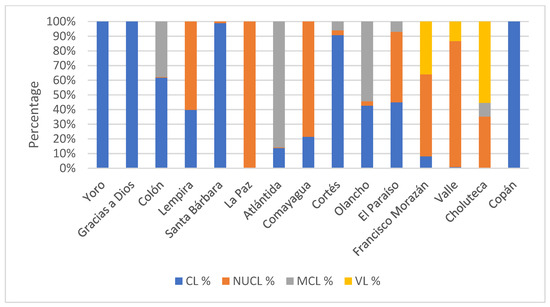
Figure 2.
Distribution of leishmaniasis cases by clinical form and health region registered in Honduras during the period 2009–2016. CL (cutaneous leishmaniasis), NUCL (non-ulcerated cutaneous leishmaniasis), ML (mucocutaneous leishmaniasis), and VL (visceral leishmaniasis).
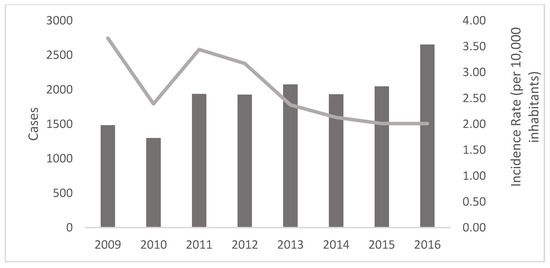
Figure 3.
Distribution of cases (bars) and incidence rate (line) of leishmaniasis registered in Honduras during the period 2009–2016.
3.2. Age and Gender
The gender incidence could not be analyzed for the entire period, as the only available information began in 2013. A significant statistical difference was found between female and male cases (p < 0.0001), with 3288 cases (37.78%) in females and 5415 cases (52.48%) in males. The incidence rates between both groups also showed a significant statistical difference (p = 0.006) (Table 2).

Table 2.
Cases and incidence rate of leishmaniasis according to gender during the period 2013–2016 in Honduras.
The age group with the highest number of cases was 20–49 years, with 3336 cases (38.33%), followed by 10–19 years with 2884 cases (33.13%). The lowest number of cases was in the age group of <5 years, with 724 cases (8.31%). A significant statistical difference was found among the age groups (p = 0.0001) (Table 3).

Table 3.
Cases and incidence rate of leishmaniasis by age group during the period 2013–2016 in Honduras.
3.3. Hot Spot and Cluster Analysis
3.3.1. Incidence Distribution
The incidence rates of UCL, ML, NUCL, and VL varied between 0 and 75, 0 and 35, 0 and 135, and 0 and 2 cases per 10,000 inhabitants, respectively.
In the three years analyzed (Figure 4), UCL presented an incidence of 35 or greater in the departments of Olancho, Colón, El Paraíso, and Francisco Morazán. ML was only recorded in 2012, showing a rate of ≥35 in one of its municipalities, Choluteca. The NUCL had a rate of ≥65 in the Choluteca department, affecting up to three municipalities (2009 and 2012), and in one municipality of Valle in 2016, located in the southern part of the country. VL exhibited a similarly low incidence rate across all three years, also being concentrated in the southern region.
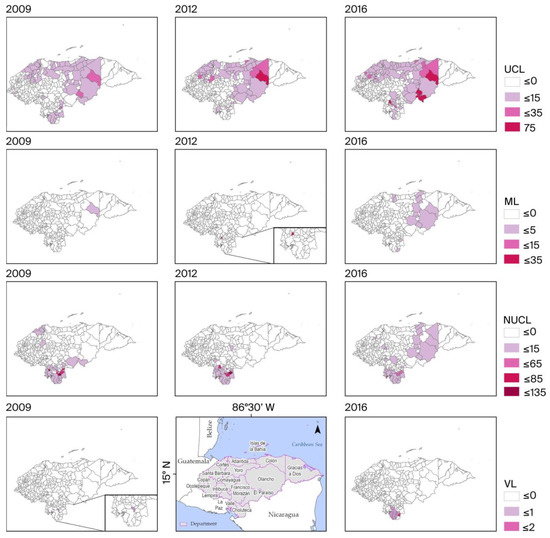
Figure 4.
Incidence rate distribution. Note: No available data for VL incidence in 2012.
3.3.2. Global Spatial Autocorrelation (Global Moran’s I)
Table 4 displays the results of the global spatial autocorrelation test for Global Moran’s I of all clinical forms of leishmaniasis. For UCL and NUCL, spatial autocorrelation was significant (p-value <0.001 throughout the whole period), as was the case for VL in 2010, 2013, and 2016. There is less than a 1% likelihood that this cluster pattern could result from random chance. For ML, significant autocorrelation occurred in 2009, 2011, 2014, and 2015 (p-value <0.05).

Table 4.
Global Spatial Autocorrelation (Global Moran’s I).
3.3.3. Hot Spot Analysis (Getis–Ord Gi*)
The identification of hot spots for each clinical form of leishmaniasis was based on the Getis–Ord Gi* statistic using a fixed distance band in ArcGIS software. The Gi*z-score ranged from 1.67 to 17.23, while the GiPValues ranged from 0 to 0.09 for the entire period, indicating a clustered pattern with 95% confidence.
According to Table 5, the results show that the clinical forms NUCL_09 to NUCL_16 exhibit Z-scores between 5.8 and 11.9 with p < 0.01, indicating a high spatial concentration of elevated values, interpreted as highly significant hot spots. This behavior suggests the presence of geographic areas where the cases or values associated with these forms occur more frequently than would be expected by chance. In contrast, the forms UCL_09 to UCL_16 mostly display Z-scores near zero and non-significant p-values (p > 0.05), indicating a random spatial distribution, except for UCL_10, which shows moderate clustering (Z = 2.269; p < 0.05). Similarly, the ML and VL forms exhibit mixed results: some could not be evaluated due to a lack of spatial variation (Error 000906), while others, such as VL_10, VL_13, and VL_16, show highly significant spatial clustering (Z > 4; p < 0.01).

Table 5.
Global Hot-Cold Pattern Analysis Using Getis-Ord General G.
The Getis–Ord Gi* maps for UCL and ML reflect critical points mainly located in the eastern part of the country, specifically in the departments of Colón and Olancho for most of the entire period (Gi*-score > 1.6, p-values < 0.05), except for the San José municipality in the Choluteca department in 2012, located in the southern region. Since 2016, both clinical forms of leishmaniasis have shown a hot spot trend in some municipalities in the southern departments of Honduras, such as Choluteca (ML) and Francisco Morazán (UCL). Conversely, NUCL and VL exhibited a statistically significant hot-spot spatial pattern only in the southern part of the country across all observations during the period. In contrast, there was a noticeable decrease in the probability of clustering of hot spots or cold spots for all four clinical forms toward the western part of the country (Figure 5).
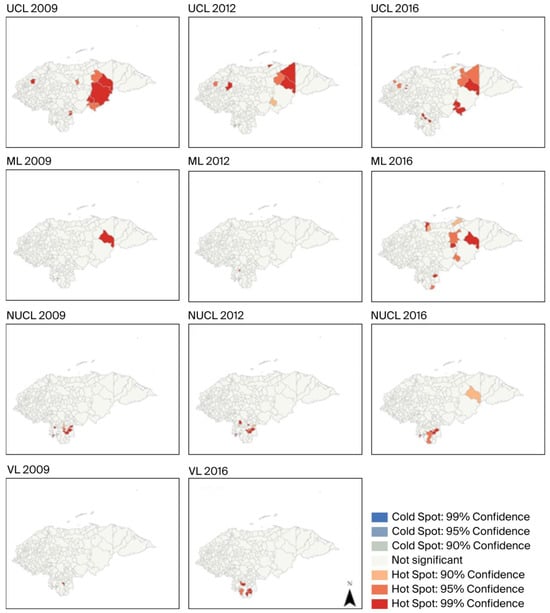
Figure 5.
Local hot spots of four clinical forms of leishmaniasis identified using the Getis-Ord Gi* statistic. Note: No available data for VL incidence in 2012.
4. Discussion
This study provides the first description of the spatial distribution of leishmaniasis across the departments and municipalities of Honduras. An analysis of the morbidity of the four clinical forms present in the country was conducted using data from two official sources [15]. In Honduras, it is mandatory to report leishmaniasis weekly, monthly, and individually during case investigations [15]. Even so, the actual number of patients remains unknown, and the Leishmania species is not reported in most departments. Consequently, the absolute magnitude, potential distribution and the risks associated with the disease are still unclear. Between 2009 and 2016, 15,345 cases of leishmaniasis were reported, primarily concentrated in Olancho, Choluteca, Colón, and Valle. The rural population was the most vulnerable, as noted in other studies [2,4,17]. A significant statistical difference was discovered ( = 246.86, p < 0.0001) between males and females from 2013 to 2016, with the 20–49 years age group having the highest number of cases (38.33%). This may be attributed to the agricultural activities of men, which increase the likelihood of occurrence of the disease in this demographic, similar to findings from other studies conducted in Latin America [18,19,20].
During the period (2009–2016) under analysis, UCL was the predominant clinical form of the disease (60.37%), similar to the incidence in Costa Rica, Panamá, and Nicaragua [21], which are also located in Central America. The NUCL, or atypical variant of cutaneous leishmaniasis caused by Leishmania (L.) infantum, has been observed in Honduras [6,8,22], Nicaragua [23,24], Costa Rica [24,25], and Paraguay [26]. It was the second most prevalent clinical form of the disease in Honduras.
Our data also showed that morbidity from the disease during the study period increased by 60% compared to that during the period from 2001 to 2008 [21]. This increase was primarily due to improved health service coverage by the Honduran Health Secretary, as well as ongoing active case surveillance in endemic areas. The data presented here offer a new perspective on the distribution of various clinical forms of leishmaniasis as well as the species associated with their transmission in Honduras.
Currently, there is no reference regarding the distribution of Leishmania species in Honduras. Specific studies have primarily focused on the NUCL form, addressing histopathological and immunological findings [8,27,28,29,30], vector behavior [5,7,31,32], and the parasite species involved in transmission [9,22,28]. Geographically, the spatial analysis revealed segregation of the clinical forms of the disease, with higher probabilities of VL occurring in semiarid areas in the southern parts of the country, similar to reports from Nicaragua [23] and Costa Rica [25]. ML and UCL are prevalent in humid areas in the northeast, with climate zones similar to those found in Costa Rica [33,34] and Panamá [33,34,35]. Based on this evidence, it is essential to conduct research that correlates the incidence of clinical forms of leishmaniasis with biophysical variables.
The thematic maps in Figure 4 illustrate the spatial distribution of the incidence rate of the four clinical forms of leishmaniasis in the years 2009, 2012, and 2016, calculated per 10,000 inhabitants. Studies utilizing spatial analysis tools are relatively new, and this is the first time they have been applied in Honduras; however, reports of these tools being used in South America exist [36,37,38]. UCL has been present throughout Honduras in all three years, with the highest values recorded in 2016 in Patuca and Dulce Nombre de Culmí, both municipalities in the Olancho department, as well as in Trojes in the El Paraíso department. In 2012, Dulce Nombre de Culmí registered the highest rate, along with Santa Fé and Santa Cruz de Yojoa, which belong to the Colón and Cortés departments, respectively. In 2009, San Antonio in the El Paraíso department had the highest incidence, followed by Dulce Nombre de Culmí and Patuca. The analysis reveals the distribution of CL incidence in regions with similar temperature and rainfall fluctuation. Studies have been conducted across endemic countries in the New World [39,40,41]. The occurrence of ML was mostly localized; the municipality of Dulce Nombre de Culmí represents the primary transmission focus for this clinical form of leishmaniasis. Optimal associations have been reported between areas with the presence of CL/ML [42]. These associations may be related to optimal environments for the sandfly populations reported in the region, with less variation in humidity levels [43,44].
In 2009, the departments of Choluteca and El Paraíso exhibited remarkably high incidence rates of NUCL. The highest values were recorded in Choluteca’s municipalities of Apacilagua, Orocuina, and Morolica, along with San Francisco de Coray in Valle. The peak in 2012 shifted to Morolica, Amapala, and Reitoca from the Francisco Morazán department. By 2016, the incidence rate had significantly decreased compared to previous years, while Morolica and Orocuina also showed a decline; Amapala maintained a rate similar to its 2012 values.
Regarding the incidence of VL disease, no cases were reported for 2012. Overall, the disease did not show representative values for other years. The few records available correspond exclusively to the Choluteca department. In 2009, there were only cases in Orocuina, while in 2016, one case was reported in each of the following municipalities: Concepción de María, Santa Ana de Yusguare, Pespire, El Corpus, Namasigue, Marcovia, and Choluteca. This confirms that the incidence was highly localized to this single department and exhibited a very low rate. Similar reports have been described primarily in South American countries [37,45].
The Getis-Ord Gi* maps from 2009 to 2016 for UCL and ML indicate hot spots mainly in the northeast of Honduras. This same index groups several focal municipalities in the south, specifically for NUCL and VL during the same period (Figure 5). No cold spots were detected, given a high percentage without statistical significance for all Leishmania cases in the years with recorded observations. In 2016, the sample increased in hot spots for all four clinical forms of leishmaniasis in the southern region of Honduras, along with a slight increase in municipalities with VL cases compared to those in previous years. Meanwhile, in 2012, there was a dramatic decrease in hot spot areas in the south for ML and VL. In all cases, the results are highly influenced by the weighted average of neighboring municipalities. This allows for testing the occurrence of temporal probability in the Cartesian plane and the high-high associations (hot spots) in Figure 5.
The results of the technical spatial statistics based on GIS illustrate the spatial behavior of the different forms of leishmaniasis from 2009 to 2016 in Honduras. This constitutes a baseline for future investigations regarding the biophysical factors of the terrain that can directly affect this distribution pattern, such as soil types, topography, vegetation, and regional climate, for a comprehensive analysis of the disease.
Although the methodology is based on the general principles of spatial statistics, the spatial diffusion patterns and the detection of hot spots provide valuable insights for health planning and predicting future disease behavior based on the distribution and hot spots observed over several years in Honduran municipalities. On the other hand, although our analysis describes the incidence of the disease in Honduras, we found that one of the limitations of our study lies in the uncertainty of the true magnitude of leishmaniasis, which is due to the underreporting of cases, the absence of reports on Leishmania species in the country, and the lack of biophysical variables for a complete understanding of the proposed spatial analysis.
5. Conclusions
In conclusion, findings from this study demonstrate geographical segregation among the different clinical forms of leishmaniasis, where precipitation and temperature serve as predictive factors for the distribution of the disease. Further investigations, which include Leishmania species typing, vector identification, and their association with different clinical forms, will enhance our understanding of the geographical distribution of leishmaniasis in Honduras.
Author Contributions
Conceptualization, W.S.-O., S.A.-V. and M.D.L.; methodology, W.S.-O., S.A.-V., G.M.-L., C.M.S.-P. and G.V.A.-F.; validation, G.M.-L. and C.A.R.-M.; formal analysis, M.D.L., W.S.-O., S.A.-V. and G.M.-L.; investigation, C.Z., O.N.Z. and E.A.H.; resources, W.S.-O. and S.A.-V.; data curation, G.M.-L. and C.Z.; writing—original draft preparation, M.D.L., W.S.-O., S.A.-V., G.M.-L. and E.A.H.; writing—review and editing, W.S.-O., S.A.-V., G.M.-L., C.A.R.-M., C.Z., O.N.Z., E.A.H., C.M.S.-P., G.V.A.-F. and M.D.L.; visualization, W.S.-O.; supervision, S.A.-V. and M.D.L.; project administration, W.S.-O.; funding acquisition, W.S.-O. and S.A.-V. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this study was provided by the Microbiology Research Institute, Facultad de Ciencias, Universidad Nacional Autónoma de Honduras (Project Number: PI-350-DICITH).
Institutional Review Board Statement
The project was approved by the Ethics Committee of the Master’s Program in Infectious and Zoonotic Diseases of the School of Microbiology of the National Autonomous University of Honduras (protocol number 04-2023, approval date: 27 June 2023).
Informed Consent Statement
Consent to participate was not necessary due to (a) the absence of personal information; (b) the study’s contribution to public health; and (c) the absence of any harm to the participants. The data were obtained from the Honduran Ministry of Health and do not include personal information about patients.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We thank the National Chagas and Leishmaniasis Program, Ministry of Health of Honduras for providing one of the databases that allowed us to carry out this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| UCL | Ulcerative Cutaneous Leishmaniasis |
| ML | Mucosal Leishmaniasis |
| NUCL | Non-Ulcerated Cutaneous Leishmaniasis |
| VL | Visceral Leishmaniasis |
References
- Jain, S.; Madjou, S.; Junerlyn, F. Global leishmaniasis surveillance updates 2023: 3 years of the NTD road map. Wkly. Epidemiol. Rec. 2024, 45, 653–669. [Google Scholar]
- Alvar, J.; Velez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Herrera, G.; Teheran, A.; Pradilla, I.; Vera, M.; Ramirez, J.D. Geospatial-temporal distribution of Tegumentary Leishmaniasis in Colombia (2007–2016). PLoS Negl. Trop. Dis. 2018, 12, e0006419. [Google Scholar] [CrossRef]
- Hotez, P.J.; Woc-Colburn, L.; Bottazzi, M.E. Neglected tropical diseases in Central America and Panama: Review of their prevalence, populations at risk and impact on regional development. Int. J. Parasitol. 2014, 44, 597–603. [Google Scholar] [CrossRef]
- Mejia, A.; Matamoros, G.; Fontecha, G.; Sosa-Ochoa, W. Bionomic aspects of Lutzomyia evansi and Lutzomyia longipalpis, proven vectors of Leishmania infantum in an endemic area of non-ulcerative cutaneous leishmaniasis in Honduras. Parasit. Vectors 2018, 11, 15. [Google Scholar] [CrossRef]
- Ponce, C.; Ponce, E.; Morrison, A.; Cruz, A.; Kreutzer, R.; McMahon-Pratt, D.; Neva, F. Leishmania donovani chagasi: New clinical variant of cutaneous leishmaniasis in Honduras. Lancet 1991, 337, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Ochoa, W.; Varela Amador, J.; Lozano-Sardaneta, Y.; Rodriguez Segura, G.; Zuniga Valeriano, C.; Araujo, G.V.; Sandoval Pacheco, C.M.; Laurenti, M.D.; Galvis-Ovallos, F. Detection of Leishmania infantum DNA in Pintomyia evansi and Lutzomyia longipalpis in Honduras. Parasit. Vectors 2020, 13, 593. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Ochoa, W.; Zuniga, C.; Chaves, L.F.; Araujo Flores, G.V.; Sandoval Pacheco, C.M.; Ribeiro da Matta, V.L.; Pereira Corbett, C.E.; Tobias Silveira, F.; Dalastra Laurenti, M. Clinical and Immunological Features of Human Leishmania (L.) infantum-Infection, Novel Insights Honduras, Central America. Pathogens 2020, 9, 554. [Google Scholar] [CrossRef] [PubMed]
- Silveira, F.T.; Flores, G.V.A.; Pacheco, C.M.S.; Sosa-Ochoa, W.; Vasconcelos Dos Santos, T.; Sousa, E.C.; Valeriano, C.Z.; da Matta, V.L.; Gomes, C.M.C.; Ramos, P.K.; et al. A comprehensive phenotypic and genotypic taxonomic review of Leishmania (Leishmania) poncei n. sp. (Kinetoplastea: Trypanosomatidae): A novel agent of cutaneous (non-ulcerated) and visceral leishmaniasis in Honduras, Central America. Trop. Dis. Travel. Med. Vaccines 2025, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Nuernberger, S.; Ramos, C. Leishmaniasis visceral. Informe del primer caso en Honduras. Rev. Médica Hondureña 1974, 42, 234–241. [Google Scholar]
- Risco Oliva, G.E.; Fuentes González, O.; Núñez, F. Leishmaniosis cutánea en la Región Sanitaria No. 3, República de Honduras, enero 1998-Septiembre 2002. Rev. Cuba. Hig. Y Epidemiol. 2009, 47, 27. [Google Scholar]
- Montalvo, A.M.; Fraga, J.; Tirado, D.; Blandon, G.; Alba, A.; Van der Auwera, G.; Velez, I.D.; Muskus, C. Detection and identification of Leishmania spp.: Application of two hsp70-based PCR-RFLP protocols to clinical samples from the New World. Parasitol. Res. 2017, 116, 1843–1848. [Google Scholar] [CrossRef] [PubMed]
- Neva, F.A.; Ponce, C.; Ponce, E.; Kreutzer, R.; Modabber, F.; Olliaro, P. Non-ulcerative cutaneous leishmaniasis in Honduras fails to respond to topical paromomycin. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Estadística, I.N.d.; Presidencial, H.S.d.E.e.e.D. XVI Censo de Población y V de Vivienda: Características Económicas de la Población Interrelacionadas; Instituto Nacional de Estadística: Madrid, Spain, 2002; Volume 5. [Google Scholar]
- Secretaria de Salud. Manual Para el Abordaje Integral de Las Leishmaniasis en Honduras, 12016th ed.; Montalvan, W., Medina Ramos, F., Mercedes Tercero, D., Fajardo, G.E., Eds.; Línea Creatica: Tegucigalpa, Honduras, 2016; Volume 89. [Google Scholar]
- ESRI. How Hot Spot Analysis (Getis-Ord Gi*) Works. Available online: https://pro.arcgis.com/en/pro-app/latest/tool-reference/spatial-statistics/h-how-hot-spot-analysis-getis-ord-gi-spatial-stati.htm (accessed on 8 March 2023).
- Hotez, P.J.; Remme, J.H.; Buss, P.; Alleyne, G.; Morel, C.; Breman, J.G. Combating tropical infectious diseases: Report of the Disease Control Priorities in Developing Countries Project. Clin. Infect. Dis. 2004, 38, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Cincura, C.; de Lima, C.M.F.; Machado, P.R.L.; Oliveira-Filho, J.; Glesby, M.J.; Lessa, M.M.; Carvalho, E.M. Mucosal leishmaniasis: A Retrospective Study of 327 Cases from an Endemic Area of Leishmania (Viannia) braziliensis. Am. J. Trop. Med. Hyg. 2017, 97, 761–766. [Google Scholar] [CrossRef]
- Augusto de Oliveira Guerra, J.; Vale Barbosa Guerra, M.D.G.; Vasconcelos, Z.S.; da Silva Freitas, N.; Rodrigues Fonseca, F.; Celso Andrade da Silva Júnior, R.; Soares da Silva, A.; Sampaio, V.; Gonçalves Maciel, M.; de Sousa Melo Cavalcante, M.; et al. Socioenvironmental aspects of the Purus Region—Brazilian Amazon: Why relate them to the occurrence of American Tegumentary Leishmaniasis? PLoS ONE 2019, 14, e0211785. [Google Scholar] [CrossRef] [PubMed]
- Medina-Morales, D.A.; Machado-Duque, M.E.; Machado-Alba, J.E. Epidemiology of Cutaneous Leishmaniasis in a Colombian Municipality. Am. J. Trop. Med. Hyg. 2017, 97, 1503–1507. [Google Scholar] [CrossRef]
- Maia-Elkhoury, A.N.S.; EYadón, Z.; Idali Saboyá Díaz, M.; de Fátima de Araújo Lucena, F.; Gerardo Castellanos, L.; JSanchez-Vazquez, M. Exploring Spatial and Temporal Distribution of Cutaneous Leishmaniasis in the Americas, 2001–2011. PLoS Negl. Trop. Dis. 2016, 10, e0005086. [Google Scholar] [CrossRef]
- Noyes, H.; Chance, M.; Ponce, C.; Ponce, E.; Maingon, R. Leishmania chagasi: Genotypically similar parasites from Honduras cause both visceral and cutaneous leishmaniasis in humans. Exp. Parasitol. 1997, 85, 264–273. [Google Scholar] [CrossRef]
- Belli, A.; Garcia, D.; Palacios, X.; Rodriguez, B.; Valle, S.; Videa, E.; Tinoco, E.; Marin, F.; Harris, E. Widespread atypical cutaneous Leishmaniasis caused by Leishmania (L.) chagasi in Nicaragua. Am. J. Trop. Med. Hyg. 1999, 61, 380–385. [Google Scholar] [CrossRef][Green Version]
- Convit, J.; Ulrich, M.; Pérez, M.; Hung, J.; Castillo, J.; Rojas, H.; Viquez, A.; Araya, L.N.; Lima, H.D. Atypical cutaneous leishmaniasis in Central America: Possible interaction between infectious and environmental elements. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Zeledon, R.; Hidalgo, H.; Viquez, A.; Urbina, A. Atypical cutaneous leishmaniasis in a semiarid region of north-west Costa Rica. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 786. [Google Scholar] [CrossRef] [PubMed]
- Salvioni, O.D.; Pereira, J.; Sander, M.; Gómez, C. Molecular detection of Leishmania infantum in atypical cutaneous lesions from Paraguayan patients. J. Dermatol. Clin. Res. 2017, 3, 1104. [Google Scholar]
- Sandoval Pacheco, C.M.; Araujo Flores, G.V.; Favero Ferreira, A.; Sosa Ochoa, W.; Ribeiro da Matta, V.L.; Zuniga Valeriano, C.; Pereira Corbett, C.E.; Dalastra Laurenti, M. Histopathological features of skin lesions in patients affected by non-ulcerated or atypical cutaneous leishmaniasis in Honduras, Central America. Int. J. Exp. Pathol. 2018, 99, 249–257. [Google Scholar] [CrossRef]
- Sosa-Ochoa, W.; Zuniga, C.; Flores, G.V.A.; Pacheco, C.M.S.; Corbett, C.E.P.; Silveira, F.T.; Laurenti, M.D. Cohort study of human infection by Leishmania (L.) infantum chagasi in southern Honduras, Central America. Trans. R. Soc. Trop. Med. Hyg. 2025. [Google Scholar] [CrossRef]
- Araujo Flores, G.V.; Sandoval Pacheco, C.M.; Tomokane, T.Y.; Sosa Ochoa, W.; Zuniga Valeriano, C.; Castro Gomes, C.M.; Corbett, C.E.P.; Laurenti, M.D. Evaluation of Regulatory Immune Response in Skin Lesions of Patients Affected by Nonulcerated or Atypical Cutaneous Leishmaniasis in Honduras, Central America. Mediat. Inflamm. 2018, 2018, 3487591. [Google Scholar] [CrossRef]
- Cardoso, C.A.; Araujo, G.V.; Sandoval, C.M.; Nogueira, P.M.; Zuniga, C.; Sosa-Ochoa, W.H.; Laurenti, M.D.; Soares, R.P. Lipophosphoglycans from dermotropic Leishmania infantum are more pro-inflammatory than those from viscerotropic strains. Mem. Inst. Oswaldo Cruz. 2020, 115, e200140. [Google Scholar] [CrossRef]
- Carrasco, J.; Morrison, A.; Ponce, C. Behaviour of Lutzomyia longipalpis in an area of southern Honduras endemic for visceral/atypical cutaneous leishmaniasis. Ann. Trop. Med. Parasitol. 1998, 92, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.G.; Ward, R.D.; Dougherty, M.J.; Maignon, R.; Ponce, C.; Ponce, E.; Noyes, H.; Zeledon, R. Comparison of the sex-pheromone components of Lutzomyia longipalpis (Diptera: Psychodidae) from areas of visceral and atypical cutaneous leishmaniasis in Honduras and Cost Rica. Ann. Trop. Med. Parasitol. 1996, 90, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Pascual, M. Climate cycles and forecasts of cutaneous leishmaniasis, a nonstationary vector-borne disease. PLoS Med. 2006, 3, e295. [Google Scholar] [CrossRef] [PubMed]
- González, K.; Calzada, J.E.; Saldaña, A.; Rigg, C.A.; Alvarado, G.; Rodríguez-Herrera, B.; Kitron, U.D.; Adler, G.H.; Gottdenker, N.L.; Chaves, L.F.; et al. Survey of wild mammal hosts of cutaneous leishmaniasis parasites in panamá and costa rica. Trop. Med. Health 2015, 43, 75–78. [Google Scholar] [CrossRef]
- Yamada, K.; Valderrama, A.; Gottdenker, N.; Cerezo, L.; Minakawa, N.; Saldaña, A.; Calzada, J.E.; Chaves, L.F. Macroecological patterns of American Cutaneous Leishmaniasis transmission across the health areas of Panamá (1980–2012). Parasite Epidemiol. Control 2016, 1, 42–55. [Google Scholar] [CrossRef]
- Marchi, M.N.A.; Caldart, E.T.; Martins, F.D.C.; Freire, R.L. Spatial analysis of leishmaniasis in Brazil: A systematized review. Rev. Inst. Med. Trop. 2019, 61, e68. [Google Scholar] [CrossRef]
- de Araujo, V.E.; Pinheiro, L.C.; Almeida, M.C.; de Menezes, F.C.; Morais, M.H.; Reis, I.A.; Assuncao, R.M.; Carneiro, M. Relative risk of visceral leishmaniasis in Brazil: A spatial analysis in urban area. PLoS Negl. Trop. Dis. 2013, 7, e2540. [Google Scholar] [CrossRef]
- Santos, M.F.d.; Lorenz, C.; Chiaravalotti-Neto, F.; Lima-Camara, T.N. Spatial analysis of American cutaneous leishmaniasis in the state of Amazonas. Rev. Saúde Pública 2024, 58, 11. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Jirmanus, L.; Junior, N.N.; Machado, P.R.; Glesby, M.J.; Ko, A.I.; Carvalho, E.M.; Schriefer, A.; Weinberger, D.M. Forecasting temporal dynamics of cutaneous leishmaniasis in Northeast Brazil. PLoS Negl. Trop. Dis. 2014, 8, e3283. [Google Scholar] [CrossRef]
- Franke, C.R.; Staubach, C.; Ziller, M.; Schluter, H. Trends in the temporal and spatial distribution of visceral and cutaneous leishmaniasis in the state of Bahia, Brazil, from 1985 to 1999. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Munoz Morales, D.; Suarez Daza, F.; Franco Betancur, O.; Martinez Guevara, D.; Liscano, Y. The Impact of Climatological Factors on the Incidence of Cutaneous Leishmaniasis (CL) in Colombian Municipalities from 2017 to 2019. Pathogens 2024, 13, 462. [Google Scholar] [CrossRef] [PubMed]
- Tarnas, M.C.; Abbara, A.; Desai, A.N.; Parker, D.M. Ecological study measuring the association between conflict, environmental factors, and annual global cutaneous and mucocutaneous leishmaniasis incidence (2005–2022). PLoS Negl. Trop. Dis. 2024, 18, e0012549. [Google Scholar] [CrossRef] [PubMed]
- Carrasco López, B.E. Diversidad de especies Lutzomyia spp. como potenciales vectores de leishmanniasis en las comunidades de San José de la montaña y Nueva Esperanza del municipio de Dulce Nombre de Culmi. Bachelor’s Thesis, Universidad Nacional de Agricultura de Honduras, Catacamas, Honduras, 2011. [Google Scholar]
- Lawyer, P.; Killick-Kendrick, M.; Rowland, T.; Rowton, E.; Volf, P. Laboratory colonization and mass rearing of phlebotomine sand flies (Diptera, Psychodidae). Parasite 2017, 24, 42. [Google Scholar] [CrossRef]
- Castillo-Castaneda, A.; Herrera, G.; Ayala, M.S.; Fuya, P.; Ramirez, J.D. Spatial and Temporal Variability of Visceral Leishmaniasis in Colombia, 2007 to 2018. Am. J. Trop. Med. Hyg. 2021, 105, 144–155. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).