Abstract
Anaplasma phagocytophilum causes tick-borne fever (TBF) in ruminants and is considered the most widespread tick-borne pathogen in sheep in Europe. This study aimed to determine the contribution of TBF to lamb mortality in Ireland and to identify factors associated with the risk of co-infection with A. phagocytophilum. Samples from dead lambs submitted to 3 Irish Regional Veterinary Laboratories (RVLs) in 2021 and 2022 were screened for the presence of A. phagocytophilum using real-time PCR. In total, 864 animals were sampled, of which 57 (6.6%) tested positive. The majority of the positive results originated in the northwest of the country; a region generally associated with high rainfall and a high prevalence of ticks and tick-borne infections in sheep. The most common causes of death reported in all lambs (including both TBF-positive and TBF-negative animals) were pneumonia, clostridial infection, and bacteraemia. Pneumonia accounted for 14.5% of deaths including 22.8 and 13.0% of TBF-positive and TBF-negative lambs, respectively. Bacteria from the family Pasteurellaceae were identified in 33.3 and 17.7% of TBF-positive and TBF-negative lambs, respectively. It was concluded that A. phagocytophilum is a possible concomitant infection and contributor to other infectious diseases in sheep, particularly those caused by bacteria in the Pasteurellaceae family. Understanding the prevalence and co-morbid associations of TBF is essential for improving disease surveillance and control strategies in endemic regions.
1. Introduction
Anaplasma phagocytophilum is an obligate intracellular Gram-negative rickettsial organism which is transmitted by ticks [1]. In Ireland, the primary vector of the infectious agent is the hard tick Ixodes ricinus. A. phagocytophilum has a tropism for neutrophils, which it enters actively rather than by phagocytosis [2]. Inside the cytoplasm of the neutrophil and enclosed in a parasitophorous vacuole it evades the host’s intracellular defence mechanisms [3] leading to a marked reduction in circulating neutrophils and reduced activity in infected neutrophils, typically causing profound immunosuppression. The resulting disease, known as tickborne fever (TBF), is characterised by high fever, anorexia and dullness. Clinical signs are usually seen in cattle and sheep, most commonly in young animals, or adults which have not previously been exposed to tick-infested pasture. Less severe infections can lead to reduced daily liveweight gain in fattening lambs. In adult sheep, A. phagocytophilum is associated with abortion and infertility in rams [4]. TBF often occurs in conjunction with other infectious diseases such as Louping Ill [5], polyarthritis or tick pyaemia (due to Staphylococcus aureus), and systemic pasteurellosis [6]. It is assumed that this tendency for co-infection with other pathogens is at least partially due to the immunosuppressive effect of A. phagocytophilum [7].
Ireland has a state-funded network of six regional veterinary laboratories (RVLs) which carry out animal disease surveillance by gathering data from carcases submitted by farmers and veterinary practitioners. A. phagocytophilum is occasionally recorded in sheep and cattle at postmortem examination [8,9]; however, it is likely that many cases go undiagnosed. Sheep carcases are generally referred for postmortem to one of the RVLs when animals have died suddenly, because of larger outbreaks or because an unusual disease syndrome is observed on a farm. Other factors, such as the proximity of the farm to the laboratory, can also have an influence on the submitting vet and farmer’s decision to send carcases for postmortem examination. On the other hand, the clinical presentation of TBF is often vague and due to the lack of pathognomonic signs, is easily confused with other diseases, in particular those of viral origin. Veterinary Research Officers in the veterinary laboratories only submit samples for TBF testing where they suspect A. phagocytophilum may have been involved in contributing to the death, or the severity of the disease involved. In contrast, in the present study every lamb submitted to one of three regional veterinary laboratories in 2021 and 2022 was screened for the presence of A. phagocytophilum. By comparing epidemiological and clinical parameters of TBF-positive and TBF-negative lambs, this study aimed to investigate the most common co-infections and the contribution of TBF to lamb mortality in Ireland.
2. Materials and Methods
2.1. Carcase Submissions and Postmortem Examination
Participating RVLs were located in Sligo (western seaboard), Athlone (central Ireland) and Kilkenny (southeast) (Figure 1). Every lamb carcase less than 12 months of age submitted to one of the 3 RVLs between January 2021 and December 2022 was included in the study. Detailed postmortem findings were recorded by a trained pathologist or veterinary research officer, for each animal. Data recorded at the time of carcase submission included: submission identifier (SDG), RVL, date of submission, herd number (Farm ID), county of origin, sample type, age, sex, animal type, agent, diagnosis, system involved, aetiology, and presence/absence of splenomegaly. In some cases, and at the discretion of the research officer conducting the postmortem examination, additional testing for other potential pathogens consistent with the clinical history and gross pathological findings was carried out using standard diagnostic methods. Diagnosis of the cause of death and most significant agents identified contributing to the disease pathogenesis was recorded following interpretation of all results. In order to avoid potential bias, all lamb carcasses submitted during the study period, even those that had died due to clearly unrelated causes (e.g., trauma or poisoning), were included in the analysis.
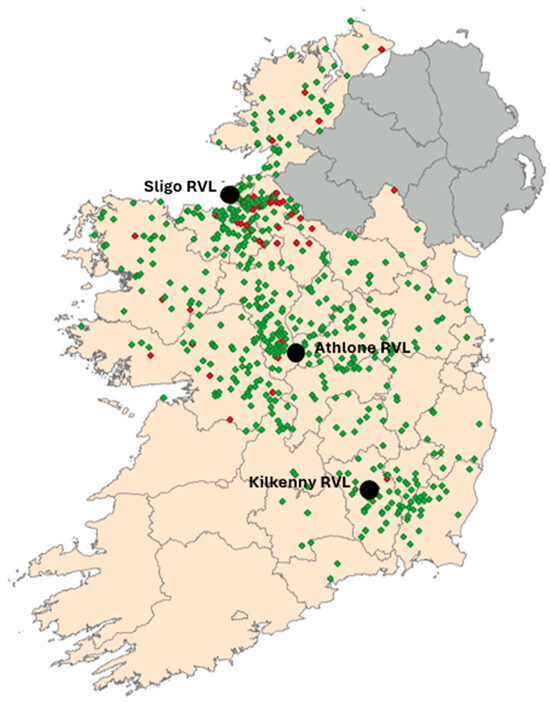
Figure 1.
Map of Ireland showing locations of all submissions analysed during the study with flocks returning positive results in red and negative flocks in green. The locations of the participating Regional Veterinary Laboratories (RVLs) are also shown.
2.2. TaqMan PCR Analysis for the Presence of A. phagocytophilum
For TBF analysis, approximately 200 mg of spleen sample was added to a 2 mL Fastprep™ screw-top container together with 1 mL of saline and Fastprep™ disruption beads. The sample was homogenised in the FastPrep24™ (MP Biomedicals, Irvine, CA 92618, USA) for 30 s, centrifuged (1 min at 5000× g) and purified using the Indimag 48™ (Indical™, Leipzig, Germany) magnetic bead purification system according to the manufacturer’s instructions. PCR testing was carried out using the method previously described [10]. Briefly, TaqMan-based real-time PCR assays were performed using primers 5′-CTCAGAACGAACGCTGG-3′ (forward), 5′-CATTTCTAGTGGCTATCCC-3′ (reverse), and probe HEX-TTGCTATAAAGAATARTTAGTGGCAGACG-BHQ1, targeting the 16S rRNA gene. TaqMan RT-qPCR assays were performed using 10 µL of SensiFAST™ Probe No-ROX One-Step Kit (BioLine™, Meridian Biosciences, Cincinnati, OH 45244, USA) in a total reaction volume of 20 µL. Forward and reverse primers were included at a final concentration of 250 nM each, and the probe was added at a final concentration of 75 nM. The reaction mixture also contained 0.2 µL of reverse transcriptase and 0.4 µL of RNase inhibitor, with nuclease-free water added to a final volume of 15 µL before the addition of the sample. Finally, a 5 µL aliquot of the purified sample was added to each reaction. The thermal cycling profile consisted of an initial reverse transcription step at 50 °C for 20 min, followed by enzyme activation at 95 °C for 5 min. This was followed by 40 cycles of denaturation at 95 °C for 20 s, annealing at 50 °C for 30 s, and extension at 72 °C for 30 s. A reference sample was included as a positive control in each run, while deionized water was used as a negative control. The assay cut-off threshold was set at a Ct value of 40 in keeping with the in-house validation protocol for the assay.
2.3. Data Processing and Statistical Analysis
Lamb age was categorised into four groups: Age Group 1 (0–2 days), Age group 2 (3–28 days), Age group 3 (29–149 days) and Age group 4 (150 days and greater). These age categories were chosen based on the likely occurrence of different disease conditions at different stages of the lamb’s life, i.e., conditions likely related to birth, neonatal conditions, conditions affecting preweaning lambs, and conditions affecting lambs in the post weaning period [11,12]. Stillborn or aborted foetuses were excluded from the study. Linear regression analysis was used to assess the relationship between age of TBF-positive cases and Ct value.
For statistical analysis, the cause of death/diagnosis and the agent which was identified at postmortem examination were reclassified into broader diagnostic and aetiological categories as detailed in Table 1. It is worth pointing out that some diagnostic categories (e.g., GIT disorders) contained both infectious and non-infectious causes as these were frequently reported as multiple contributing factors. In the analysis involving agent category these were separated out. ‘Clostridial diseases’ included all conditions which had a diagnosis directly attributable to clostridial infection or associated toxins.

Table 1.
Summary of the main diagnoses and aetiological agents and how they were grouped together in categories for data analysis. The presence or absence of splenomegaly was also recorded.
Sample variance and associations between biological and diagnostic variables were assessed using chi-square analysis and Factor Analysis of Mixed Data (FAMD), a statistical method used to explore relationships between both qualitative (categorical) and quantitative (numerical) variables. Fisher’s exact test was carried out where more than 20% of cells in the contingency table had expected frequencies of less than 5. Analyses were performed using the “factominer” package [13]. FAMD produces graphical representations of statistical relationships of different variables in a common multidimensional space, each dimension capturing a proportion of the total variance in the dataset. The position of TBF-positive and TBF-negative lambs in the plot reflects their degree of similarity or difference with respect to the included variables. Data analysis and graphical representations were performed using R version 4.0 software [14]. p values < 0.05 were regarded as significant for all analysis.
3. Results
3.1. Geographical Distribution of Submitted Carcases and Flock History of Positive Samples
Over the 24 months period, a total of 864 lamb carcases were submitted for postmortem examination, including 360 lambs in Athlone, 164 in Kilkenny and 340 in Sligo RVL. Of these, 6.6% (n = 57) tested positive for A. phagocytophilum by qPCR (with Ct values ranging between 15 and 39.09). While samples were received from the east, midlands, and northwest of the country, 49% (n = 28) of all positive samples originated in the northwest region (Figure 1).
The fifty-seven positive samples were derived from fifty-three farms with four farms submitting two positives each. The flock size of farms with TBF-positive cases ranged between 10 and 800 with a median flock size of 52.5. Thirty of the positive animals were found dead by the owner, suggesting sudden death, with most of the remainder being recorded as having a short sickness of 1–2 days duration. The exceptions were one owner reporting a sickness of 6 days before death with two others recording durations of illness of 7 days. Most positive submissions came from flocks where multiple deaths (due to various causes) had been recorded recently (2 to 15 within the 30 days preceding the postmortem examination), with only 14 cases arising where this was the only recent death.
3.2. TBF Positivity in Relation to Age and Sex of the Lamb
The percentage TBF positivity in each age category is presented in Table 2. While there was a slight increase in the percentage of lamb carcases that tested positive for A. phagocytophilum in the 3 to 28 days age group, this difference was not significant (p > 0.05). However, there was a weak positive correlation between age and Ct value (Figure 2). The regression model was statistically significant with an R2 value of 0.1751 (F (1, 55) = 11.68, p = 0.0012) explaining 17.51% of the variance in Ct value when plotted against age.

Table 2.
Age categories of TBF-positive submissions compared to all submissions in each category.
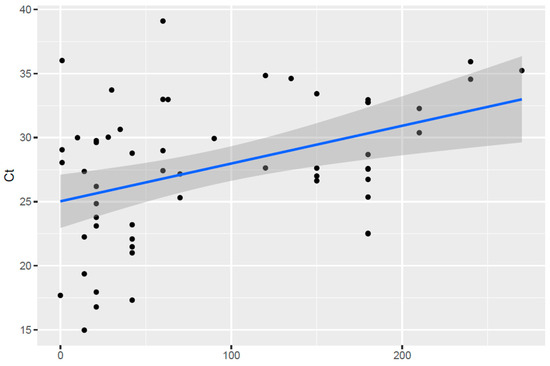
Figure 2.
Correlation between estimated age (in number of days) and qPCR Ct values in TBF-positive lambs. Black dots represent individual data points with the linear regression line represented by the blue line.
Unfortunately, sex was only recorded in 398 lambs. Of these 206 were male and 192 were female. Overall, 19 males (9.2%) and 14 females (7.3%) were TBF-positive. Chi-square analysis indicated there was no significant association between TBF status and sex (X2 (2, n = 864) = 4.04, p = 0.133).
3.3. Multivariate Analysis of Diagnoses Associated with A. phagocytophilum Co-Infection
Factor Analysis of Mixed Data (FAMD) indicated a significant correlation between TBF-positivity and splenomegaly (Figure 3). Splenomegaly was noted in 37 carcases and was significantly more common in TBF-positive cases (8/57 animals; 14%) than it was in TBF-negative cases (29/807 animals; 3.6%) (X2 (1, n = 864) = 14.2, p < 0.001). Other diagnostic findings showed weaker associations with TBF-positivity in the FAMD, including malnutrition/starvation, laryngitis/pharyngitis, and pneumonia. It should be noted that dimensions one and two only explained nine and eight percent of the variances in the data, respectively.
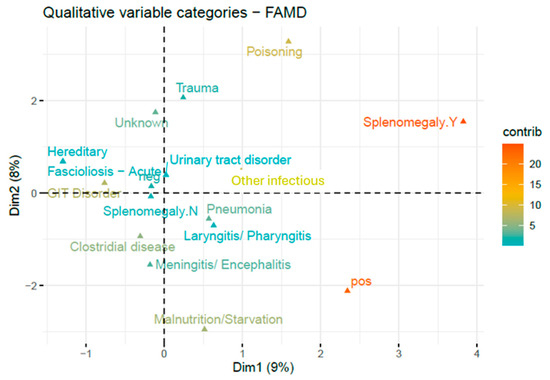
Figure 3.
Two dimensional FAMD of diagnoses and splenomegaly associated with TBF positivity. Relative contributions of categories are indicated by gradient colour. Relative contributions of categories are indicated by gradient colour. Binary variables (e.g., splenomegaly) are represented by their two modalities: presence (Y) and absence (N).
The effect of TBF status on diagnosis of lamb mortality is presented in Table 3. The only postmortem finding that was significantly more frequently reported in TBF-positive cases was malnutrition/starvation (p = 0.048). There were some additional conditions that were also more common in TBF-positive cases, most notably pneumonia (roughly 23% of TBF-positive compared to 14% of TBF-negative lambs, p = 0.064) but these differences were not significant. Conversely, there was a significantly higher incidence of GIT disorders (including both infectious and non-infectious causes) in TBF-negative lambs (p = 0.006). There was no indication that TBF status affected the incidence of fasciolosis, hereditary disorders, meningitis/encephalitis, poisoning, other infectious causes, trauma or ‘unknown’ causes.

Table 3.
Number of records in the different diagnostic categories and percentages of TBF-positive and TBF-negative submissions out of the total number of TBF-positive (n = 57) and TBF-negative submissions (n = 807) in each category.
3.4. Multivariate Analysis of Disease Agents Associated with A. phagocytophilum Co-Infection
FAMD analysis of TBF status and the agent identified at postmortem indicated an association between the presence of Pasteurellaceae and Louping Ill virus (LIV) and TBF positivity (Figure 4). The first was confirmed by chi-square analysis which showed that Pasteurellaceae were significantly more frequently identified as aetiological agents in TBF-positive lambs compared to TBF-negative lambs (X2 (1, n = 864) = 8.52, p = 0.004) (Table 4). While Louping Ill cases were also more common in TBF-positive lambs (1.8% as compared to 0.4%) the overall case numbers were too low to be significant.
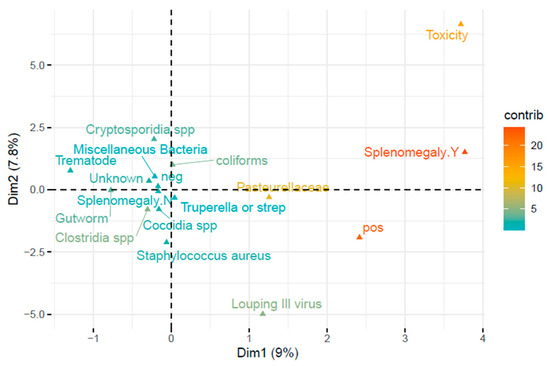
Figure 4.
Two dimensional FAMD of disease agents and splenomegaly associated with TBF positivity. Relative contributions of categories are indicated by gradient colour. Binary variables (e.g., splenomegaly) are represented by their two modalities: presence (Y) and absence (N).

Table 4.
Numbers of records for different aetiological categories and percentages of TBF-positive and TBF-negative submissions out of the total number of TBF-positive (n = 57) and TBF-negative submissions (n = 807) in each category.
For context, LIV was identified as the aetiological agent in four cases, of which three were TBF-negative and one was TBF-positive (X2 (1, n = 864) = 2.21, p = 0.137). All four of these lambs originated in the northwest of the country. The three TBF-negative lambs were a 9-month-old lamb with Flavivirus RNA detected in the CNS, and two 1-month-old lambs with LIV confirmed based on histopathology and detection of RNA in brain tissue. The fourth case was a 4-month-old lamb which tested positive for A. phagocytophilum. In this animal, Louping ill infection was identified based on histopathology of the brain showing characteristic lesions associated with LIV, most notably multifocal lymphocytic meningitis, associated with lymphocytic perivascular cuffing. Interestingly, splenomegaly was not noted in any of the four lambs infected with LIV.
Gutworms were detected more frequently in TBF-negative cases (14.7%) than they were in TBF-positive cases (8.8%), but the difference was not statistically significant (X2 (1, n = 864) = 1.55, p = 0.214). No other statistically significant differences were observed among the remaining aetiological agents.
3.5. Breakdown of Members of the Family Pasteurellaceae Identified as Aetiological Agents in TBF-Positive and TBF-Negative Cases
Of all agents in the Pasteurellaceae family, Biberstenia trehalosi was the most commonly identified (55.6%; 90 of 162 cases), followed by Mannheimia haemolytica (38.9% or 63 cases) (Table 5). Pasteurella spp. including Pasteurella multocida were detected in nine carcases (5.5%). Biberstenia trehalosi was also the most common aetiology associated with a diagnosis of bacteremia/toxaemia/septicemia (47 out of 59 cases) while Mannheimia haemolytica was more often associated with a diagnosis of pneumonia (54 out of a total of 92 pneumonia diagnoses). This was especially the case in TBF-positive lambs, where 81.1% of pneumonia diagnoses were associated with M. haemolytica. However, these differences were not statistically significant (Fisher’s exact test, p = 1.0).

Table 5.
Number of TBF-positive and TBF-negative lambs diagnosed with pneumonia, bacteraemia and ‘other’ conditions and associated aetiological agent.
4. Discussion
While Anaplasma phagocytophilum may be suspected in cattle displaying sudden fever or milk drop, or in sheep as an underlying cause of other diseases such as tick pyaemia or Louping ill, postmortem samples are generally only tested for TBF where A. phagocytophilum is thought to have contributed to the death of the animal and/or gross enlargement of the spleen is noted. In contrast, in this study all lamb carcases of less than 12 months of age were screened in order to clinically characterise cases with underlying A. phagocytophilum infections and to determine whether the contribution of TBF to lamb mortality is, in fact, underestimated.
The overall incidence of A. phagocytophilum in lamb carcases submitted during the study period was 6.6%, exceeding the rates recorded for lambs in national surveillance reports in 2022 (1.9%) and 2023 (2.4%) [8,9]. Most TBF-positive animals originated from the northwest of Ireland, an area that is also known to have a high prevalence of I. ricinus ticks [15]. Ongoing research by the authors also indicates that this region has a high percentage of A. phagocytophilum seropositive flocks (Gilmore et al., in preparation). The majority of TBF-positive samples were associated with multiple concurrent mortalities on the same farm indicating that TBF should be considered as a contributory factor in cases involving multiple sheep deaths from areas of suspected tick activity.
There was a statistically significant but weak positive correlation between the age of the animal and Ct value of the qPCR assay, with older lambs showing higher Ct values than younger animals. This may indicate that many lambs are exposed at a young age (TBF-positivity was highest in the 3 to 28 days age group) and as animals age, circulating bacterial numbers decline as was shown by Granquist and colleagues [16]. Alternatively, it may indicate that infections in older lambs are associated with lower bacteraemia, possibly related to a more developed immune response in older lambs. In any event there was no significant difference in positivity rates between the different age categories. An interesting observation was the fact that the youngest age group (0–2 days olds) had a similar incidence of 6% when compared with the mean of 6.6% in all age groups. In utero infection of these animals, as previously described [17] is the most likely source of infection given their age. This is important both in terms of ewe health and its potential impact on the foetus and any direct impacts on the foetus itself.
Surprisingly splenomegaly was only noted in 14% of TBF-positive animals. While this was significantly higher than splenomegaly rates in TBF-negative animals (3.6%), there was a sizable number of infected carcasses where splenomegaly was not recorded. This is a noteworthy finding as splenomegaly is generally considered a characteristic postmortem finding in TBF cases [18] although it has previously been observed that splenic enlargement can be subtle following experimental infection of naïve sheep [19], suggesting that relying on the presence of splenomegaly alone as a selection criterion for TBF may result in a significant percentage of infected animals going undiagnosed. Consideration should also be given to potential inter-operator variations in the interpretation of splenomegaly and how splenomegaly is defined at postmortem examination.
The significant correlation of A. phagocytophilum with malnutrition/starvation observed in this study probably reflects the detrimental effect TBF has on the ability of young lambs to maintain contact with their mothers [20], compounded by the fact that many tick-infested pastures in Ireland are marginal land.
Our study identified pneumonia as a common cause of mortality among lambs. Pneumonia has previously been reported as a common cause of death in Irish sheep flocks, accounting for around 10.5% [21]. The higher percentage of pneumonia cases observed here (14.5%) is probably attributable to the fact that we only considered young animals which are more susceptible to pneumonia [22]. Regarding the aetiological agent, members of the Family Pasteurellaceae are generally associated with pneumonia in lambs, with one report showing that Mannheimia haemolytica is the most common aetiological agent, being present in 35% of ovine pneumonias, followed by B. trehalosi detected in 24.4% of ovine pneumonias [8]. Mannheimia haemolytica is more commonly associated with pneumonic pasteurellosis whereas B. thehalosi is more commonly associated with septicemic pasteurellosis [22]. Similar results are reported in this study, with M. haemolytica being associated with 59% of pneumonia cases (54 out of a total of 92), while B. trehalosi accounted for 80% of septicaemia cases associated with Pasteurellaceae (47 out of a total 59).
In our study, pneumonia was more common in lamb carcases that were co-infected with A. phagocytophilum (22.8% compared with 13.9%) although this difference failed to be statistically significant (p = 0.064). Considering the immunosuppressive effects of A. phagocytophilum, particularly on neutrophils, we would perhaps have expected a more clear-cut effect. It is possible that this result reflects the opportunistic nature of A. phagocytophilum as just one of several debilitating factors that contribute to lamb mortality. There was a strong association between TBF-positivity and the detection of bacteria in the Family Pasteurellaceae as bacteriological agents. This finding is in keeping with previous reports, where increased susceptibility to pneumonic pasteurellosis caused by M. haemolytica and to septicaemia caused by B. trehalosi have been reported, highlighting the importance of investigating the potential contribution of A. phagocytophilum to outbreaks of pneumonic or septicaemic pasteurellosis in areas of suspected tick activity [6,23].
Louping Ill is a viral disease, the clinical signs of which are thought to be exacerbated when animals are co-infected with TBF [24]. Serological studies have shown LIV to be present in the northwest region of Ireland where 49% of TBF-positive lambs and all four Louping ill cases reported in this study originated from [25]. However, of the four cases only one was TBF-positive. Brodie and colleagues observed that TBF greatly increased susceptibility to LIV, as sheep experimentally co-infected with LIV and A. phagocytophilum died or were euthanised due to the severity of clinical signs, while those only infected with LIV showed mild clinical signs and recovered [6]. To what extent TBF co-infection affects the survival of naturally LIV-infected animals simultaneously exposed to both pathogens will have to be investigated further.
One of the limitations of this study is the potential for selection bias. Even though every lamb submitted to one of the RVLs was tested, we had no control over the decision criteria for submitting lambs to the RVL. This decision could be influenced by factors such as proximity to or familiarity with the local RVL, or increased awareness by some farmers to various diseases including tick-borne diseases. Another reason why the sample set is likely to be biassed is that sheep carcases are mostly referred for postmortem where animals have died under unusual circumstances or as part of disease outbreaks involving multiple deaths. The low number of TBF-positive animals identified in the study was also a limitation as it resulted in statistical results that were often close to, but not quite significant, allowing us to observe trends rather than clear-cut differences.
Nevertheless, we believe that our study provides valuable insights into the effects of TBF on lamb survival in tick-endemic areas.
5. Conclusions
The immunosuppressive effects of A. phagocytophilum due to marked neutropenia and reduced neutrophil function, and increased susceptibility to co-infections are well documented [26]. The results presented here indicate that, in the Irish context, co-infections of TBF with members of the Family Pasteurellaceae may be the most significant contributor to mortality in lambs. This is highlighted by the fact that A. phagocytophilium was significantly associated with cases of pneumonia and septicaemia caused by any members of the Family Pasteurellaceae, and, in particular, M. haemolytica and B. trehalosi. TBF should be considered as a predisposing factor when investigating cases of pneumonic or systemic pasteurellosis in lambs originating from areas with suspected tick activity, particularly if the farm experienced multiple sheep deaths in the recent past.
Splenomegaly, whilst being more commonly recorded in TBF-positive lambs than TBF-negative lambs, was not a consistent finding in all cases of A. phagocytophilum infection and should not be regarded as a pre-requisite for considering co-infection with TBF at postmortem examination.
Author Contributions
Conceptualization, J.M.G., A.Z., J.F.M. and T.W.J.K.; methodology, J.M.G., A.Z., J.F.M., T.W.J.K., S.M.G., S.F., R.F. and M.S.; software, L.D.R.; validation, A.Z. and J.M.G.; formal analysis, J.M.G., A.Z., A.N.-L. and L.D.R.; investigation, S.M.G., R.F., S.F., A.M.F. and M.S.; resources, J.F.M.; data curation, S.M.G., R.F., K.B. and C.M.D.; writing—original draft preparation, J.M.G. and A.Z.; writing—review and editing, A.Z., S.M.G., R.F., T.W.J.K., J.F.M., L.D.R. and A.N.-L.; visualisation, A.N.-L. and L.D.R.; supervision, A.Z.; project administration, J.F.M.; funding acquisition, J.F.M. and T.W.J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This project was part-funded by the Irish Agriculture and Food Development Authority, Teagasc (Teagasc, Moorepark, Fermoy County Cork, P61RX43, Ireland) under the Walsh Fellowship Programme.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We gratefully acknowledge the input of a wide range of staff in the Department of Agriculture Food and The Marine Veterinary Laboratory Service in the areas of study design, sample collection, data recording, postmortem analysis, and ancillary testing without which this project would not have been possible.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Woldehiwet, Z. The natural history of Anaplasma phagocytophilum. Vet. Parasitol. 2010, 167, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Carlyon, J.A.; Latif, D.A.; Pypaert, M.; Lacy, P.; Fikrig, E. Anaplasma phagocytophilum utilizes multiple host evasion mechanisms to thwart NADPH oxidase-mediated killing during neutrophil infection. Infect. Immun. 2004, 72, 4772–4783. [Google Scholar] [CrossRef] [PubMed]
- Rennoll-Bankert, K.E.; Sinclair, S.H.; Lichay, M.A.; Dumler, J.S. Comparison and characterization of granulocyte cell models for Anaplasma phagocytophilum infection. Pathog. Dis. 2014, 71, 55–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum—A widespread multi-host pathogen with highly adaptive strategies. Front. Cell Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R.; Hopkins, B.A.M.; Rocchi, M.S.; Wessels, M.; Floyd, T. High mortality in a sheep flock caused by coinfection of Louping Ill Virus and Anaplasma phagocytophilum. Vet. Rec. Case. Rep. 2020, 8, 2–4. [Google Scholar] [CrossRef]
- Brodie, T.; Holmes, P.; Urquhart, G. Some aspects of tick-borne diseases of British sheep. Vet. Rec. 1986, 118, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Sargison, N.; Edwards, G. Tick infestations in sheep in the UK. In Pract. 2009, 31, 58–65. [Google Scholar] [CrossRef]
- DAFM. 2023 All-Island Animal Disease Surveillance. 2023. Available online: https://www.animalhealthsurveillance.agriculture.gov.ie/currentnews/allislanddiseasesurveillancereport2023.html (accessed on 30 July 2025).
- DAFM. 2022 All-Island Animal Disease Surveillance. 2022. Available online: https://www.animalhealthsurveillance.agriculture.gov.ie/currentnews/allislanddiseasesurveillancereport2022.html (accessed on 30 July 2025).
- Reinbold, J.B.; Coetzee, J.F.; Sirigireddy, K.R.; Ganta, R.R. Detection of Anaplasma marginale and A. phagocytophilum in bovine peripheral blood samples by Duplex Real-Time Reverse Transcriptase PCR Assay. J Clin. Microbiol. 2010, 48, 2424–2432. [Google Scholar] [CrossRef] [PubMed]
- Sargison, N. The lambing percentage. In Sheep Flock Health, 1st ed.; Blackwell Publishing: Oxford, UK, 2008; pp. 1–142. [Google Scholar] [CrossRef]
- Sargison, N. Lamb growth. In Sheep Flock Health, 1st ed.; Blackwell Publishing: Oxford, UK, 2008; pp. 143–227. [Google Scholar] [CrossRef]
- Husson, F.; Josse, J.; Le, S.; Mazet, J. FactoMineR: Multivariate Exploratory Data Analysis and Data Mining. 2017. Available online: https://doi.org/10.32614/CRAN.package.FactoMineR (accessed on 30 July 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 30 July 2025).
- Zintl, A.; Zaid, T.; McKiernan, F.; Naranjo-Lucena, A.; Gray, J.; Brosnan, S.; Browne, J.; O’Connor, J.; Mee, J.; Good, B.; et al. Update on the presence of Ixodes ricinus at the western limit of its range and the prevalence of Borrelia burgdorferi sensu lato. Ticks Tick Borne Dis. 2020, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Granquist, E.G.; Bårdsen, K.; Bergström, K.; Stuen, S. Variant -and individual dependent nature of persistent Anaplasma phagocytophilum infection. Acta Vet. Scand. 2010, 52, 25. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Okstad, W.; Sagen, A.M. Intrauterine transmission of Anaplasma phagocytophilum in persistently infected lambs. Vet. Sci. 2018, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Overås, J.; Lund, A.; Ulvund, M.J.; Waldeland, H. Tick-borne fever as a possible predisposing factor in septicaemic pasteurellosis in lambs. Vet. Rec. 1993, 133, 398. [Google Scholar] [CrossRef] [PubMed]
- Almazán, C.; Fourniol, L.; Rouxel, C.; Alberdi, P.; Gandoin, C.; Lagrée, A.C.; Boulouis, H.J.; De la Fuente, J.; Bonnet, S.I. Experimental Ixodes ricinus-sheep cycle of Anaplasma phagocytophilum NV2Os propagated in tick cell cultures. Front. Vet. Sci. 2020, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Woldehiwet, Z.; Ristic, M. Rickettsial and Chlamydial Diseases of Domestic Animals, 1st ed.; Pergamon Press: Oxford, UK, 1993; pp. 65–88. [Google Scholar]
- Murray, G.M.; Fagan, S.; Murphy, D.; Fagan, J.; Muireagáin, C.O.; Froehlich-Kelly, R.; Barrett, D.J.; Sheehan, M.; Wilson, M.; Brady, C.P.; et al. Descriptive analysis of ovine mortality in sentinel sheep flocks in Ireland. Vet. Rec. 2019, 184, 649. [Google Scholar] [CrossRef] [PubMed]
- Bell, S. Respiratory disease in sheep 1. Differential diagnosis and epidemiology. In Pract. 2008, 30, 200–207. [Google Scholar] [CrossRef]
- Daniel, R.G.; Pugh, K.; Torrens, N.; Carson, A.J.; Wessels, M. Intercurrent tickborne fever infection and Bibersteinia trehalosi septicaemia in a five-week-old lamb. Vet. Rec. 2015, 177, 24. [Google Scholar] [CrossRef] [PubMed]
- Reid, H.W.; Buxton, D.; POW, I.; Brodie, T.A.; Holmes, P.H.; Urquhart, G.M. Response of sheep to experimental concurrent infection with tick-borne fever (Cytoecetes phagocytophila) and Louping-Ill Virus. Res. Vet. Sci. 1986, 41, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.; Collins, D.M.; Mcgrath, G.; Muireagain, C.Ó. Seroprevalence of Louping Ill Virus (LIV) antibodies in sheep submitted for post mortem examination in the North West of Ireland in 2011. Ir. Vet. J. 2012, 65, 20. [Google Scholar] [CrossRef] [PubMed]
- Woldehiwet, Z. Anaplasma phagocytophilum in ruminants in Europe. Ann. N. Y. Acad. Sci. 2006, 1078, 446–460. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).