Abstract
Background: Anaemia in adolescents can be influenced by parasitic infections, systemic inflammation, and nutritional status. Objective: To determine whether C-reactive protein (CRP), nutritional status, and infection with Ascaris lumbricoides, Giardia lamblia, or Trichuris trichiura are associated with anaemia in adolescent athletes from Tacna compared to non-athletes. Methods: A cross-sectional study was conducted involving 250 male football players aged 13–18 years and 150 age-matched non-athletes. Haemoglobin, haematocrit, ferritin, serum iron, CRP, and parasitic status were measured; mean comparisons and logistic regression were applied. Results: Anaemia was more prevalent among athletes than non-athletes (30% vs. 18%; p < 0.001). Infected athletes showed lower haemoglobin (11.9 ± 1.1 g/dL) and higher CRP (5.0 ± 1.9 mg/L) levels compared to non-infected athletes (13.8 ± 1.0 g/dL and 2.2 ± 1.1 mg/L; p < 0.001). Logistic regression identified CRP as an independent predictor of anaemia (adjusted OR = 1.20; 95% CI: 1.08–1.38; p < 0.001), while parasitic infections showed no direct association after adjustment. Underweight status was associated with a higher prevalence of anaemia (36%). Conclusions: Systemic inflammation emerged as the main factor associated with anaemia in this population, with parasitic infections contributing indirectly by increasing inflammation. Periodic deworming, iron supplementation, and CRP monitoring are recommended to reduce the burden of anaemia in adolescent athletes from endemic regions.
1. Introduction
Sports practice provides evident benefits—improved cardiovascular function, greater musculoskeletal strength, and reduced risk of chronic diseases; however, high-performance athletes face demands that can alter their haematological parameters [1]. Monitoring these variations is essential to safeguard health and optimise performance [2].
Blood indicators—erythrocyte count, haemoglobin, and leukocytes—reflect general health status [3]. Anaemia is common among adolescent athletes, as intense oxygen demand coincides with rapid growth and high nutritional requirements [4,5].
In rural areas of Latin America, anaemia prevalence exceeds 30%, and Tacna is no exception [6,7]. There, Ascaris lumbricoides, Giardia lamblia, and Trichuris trichiura affect up to 40% of schoolchildren, compromising iron absorption and triggering systemic inflammation [8,9].
Inflammation-induced interleukin-6 stimulates hepcidin synthesis, a hormone that blocks iron absorption and mobilisation, resulting in microcytic anaemia despite apparently normal iron stores [10,11] This parasite → inflammation → hepcidin → anaemia axis constitutes the central hypothesis of this study.
Recent studies confirm that parasitic infections remain key determinants of anaemia in adolescents. Hamdy et al. [12] found that Blastocystis sp. infection significantly reduced haemoglobin (mean 11.2 ± 1.1 g/dL vs. 12.8 ± 1.0 g/dL in controls) and ferritin levels in Egyptian schoolchildren, along with decreased serum zinc and copper levels [12]. In Ethiopia, a meta-analysis of 24 studies indicated that hookworm infections doubled the risk of anaemia in school-aged children (OR = 2.01; 95% CI: 1.52–2.65) [13]. Ipa et al. (2024) reported that in Indonesia, 32.8% of children infected with soil-transmitted helminths presented with microcytic hypochromic anaemia compared to 10.3% of uninfected children [14]. Similarly, El-Sharkawy and Mohamed (2024) observed lower mean haemoglobin (11.7 ± 0.8 g/dL) and significantly reduced serum ferritin levels (p < 0.001) in Egyptian adolescents infected with intestinal parasites [15]. These recent findings underscore the importance of evaluating the impact of parasitic infections on anaemia in vulnerable populations such as adolescent athletes, a group with high metabolic demands and frequent exposure to risk conditions.
This study evaluates the relationship between C-reactive protein (CRP), nutritional status, and parasitic infections by Ascaris lumbricoides, Giardia lamblia, and Trichuris trichiura and their association with anaemia in adolescent football players in Tacna, using a non-athlete group as a reference. The findings aim to guide interventions involving deworming, iron supplementation, and inflammation control in at-risk populations.
Although Necator americanus and Ancylostoma duodenale are strongly associated with anaemia due to chronic intestinal blood loss, these parasites were excluded from this study due to their absence in the Tacna region, as evidenced by local parasitological surveillance studies in reservoir hosts [16]. Conversely, Ascaris lumbricoides causes anaemia by consuming nutrients and causing malabsorption, particularly of vitamin A and iron, thus reducing erythropoiesis [8]. Giardia lamblia induces villous atrophy and interferes with iron and folate absorption in the duodenum and proximal jejunum, leading to microcytic anaemia [17]. Trichuris trichiura causes chronic blood loss due to its traumatic attachment to the colonic mucosa, resulting in iron-deficiency anaemia proportional to parasite load [9].
Main objective: To determine whether systemic inflammation, nutritional status, and infections by A. lumbricoides, G. lamblia, and T. trichiura are associated with anaemia prevalence in adolescent athletes from Tacna.
Working hypotheses:
H1.
Elevated CRP levels predict anaemia independently of age and BMI.
H2.
The presence of any of the studied parasites is associated with lower haemoglobin concentrations through an increase in systemic inflammation.
To test these hypotheses, haemoglobin, haematocrit, ferritin, serum iron, CRP, and parasitic status were measured, and their interrelationships were analysed using multivariable models.
2. Materials and Methods
2.1. Study Design and Population
A descriptive, cross-sectional study was conducted with single-point collection of blood and stool samples. The study included 250 males aged 13–18 years from football academies in Tacna, Peru, and 150 non-athlete adolescents matched by age, sex, and socioeconomic status. The reference group was not exposed to competitive training.
Both groups were assessed for anaemia prevalence, infections with Ascaris lumbricoides, Giardia lamblia, and Trichuris trichiura, and biomarkers including haemoglobin, C-reactive protein (CRP), ferritin, and serum iron. The comparison aimed to identify significant differences in anaemia, parasitic infections, and inflammation.
The study was conducted in two phases:
- Field phase: Informative sessions were held at the academies to obtain informed consent from participants and their legal guardians.
- Laboratory phase: Samples were processed and analysed in the Biology Laboratory of the Faculty of Sciences.
The final cohort comprised 400 adolescents selected based on predefined inclusion and exclusion criteria. All participants underwent a general medical examination and laboratory tests after a fasting period. The protocol was designed to ensure the validity and reliability of the results.
2.1.1. Inclusion Criteria
The participants included were healthy males aged 13–18 years who had been continuously participating in football training for at least three months prior to sample collection. Additionally, informed consent was required from both the participant and their legal guardian, and participants had to have no diagnosed chronic diseases or antiparasitic treatments in the three months preceding the study.
2.1.2. Exclusion Criteria
Exclusion criteria included the presence of chronic diseases that could alter haematological parameters, inactivity in sports training for more than three months prior to sample collection, and the use of medications that could modify haematological or inflammatory markers, such as glucocorticoids, statins, or fibrates. Participants were also excluded if they presented elevated inflammatory markers due to non-parasitic causes, such as a high erythrocyte sedimentation rate, as well as those who failed to provide signed informed consent or did not comply with fasting requirements for evaluations.
Of the 300 athletes evaluated across four academies in Tacna, 250 met the inclusion criteria. Of them, 10 were excluded due to chronic diseases, 20 due to recent antiparasitic treatment, and 20 for incomplete laboratory records or questionnaires.
2.1.3. Measurements Performed
Several parameters were evaluated in participants, including haematological biomarkers such as haemoglobin, haematocrit, and erythrocyte count, as well as C-reactive protein (CRP) as an inflammatory marker. Coproparasitological examinations were performed to detect infections with Ascaris lumbricoides, Giardia lamblia, and Trichuris trichiura. Finally, nutritional status was determined by measuring serum iron and ferritin levels.
2.2. Sample Collection, Transport, and Processing
Venous blood and stool samples were collected under sterile conditions following the guidelines of the Peruvian Ministry of Health [7]. and the Clinical and Laboratory Standards Institute [18]. Blood was collected using sterile 21 G needles and Greiner Bio-One Vacuette® vacuum tubes (Greiner Bio-One GmbH, Kremsmünster, Austria), maintained at 4 °C, and processed within a maximum of 4 h to minimise haemolysis. Stool samples (~10 g per participant) were collected in sterile containers and transported to the laboratory at 4–8 °C. Blood samples were centrifuged to separate serum, while stool samples were examined using the centrifugal flotation technique. All samples were stored at ≤5 °C until analysis.
2.3. Anthropometric Data and Nutritional Status
Height was measured using a Seca 213 stadiometer (Seca GmbH & Co. KG., Hamburg, Germany) and weight was measured with a calibrated Tanita BC-601 digital scale (Tanita Corporation, Tokyo, Japan). Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2), according to World Health Organization (WHO) guidelines (2013) [6]. BMI values between 18.5 and 24.9 kg/m2 were considered normal; athletes outside this range were classified as underweight or overweight.
Additionally, heart rate and blood pressure were recorded using a heart rate monitor and manual sphygmomanometer, respectively, following the American Heart Association protocol to ensure measurement consistency.
Physical activity levels were obtained using a structured questionnaire that quantified weekly exercise hours. Nutritional status was assessed using anthropometric measurements and biochemical markers. Athletes with normal BMI but low serum iron or ferritin levels were functionally classified as underweight, as these biochemical markers reflect their nutritional and haematological status.
References for employed techniques:
Anthropometric measurements and BMI calculation: WHO (2013) [6].
Nutritional classification and BMI cut-off points: WHO (2013) [6].
2.4. Detection of Parasitic Infection
Parasitic detection was carried out using standard flotation and Lugol’s iodine staining methods, following protocols described by Bowman [19]. These procedures allowed for the identification of Ascaris lumbricoides, Giardia lamblia, and Trichuris trichiura.
2.4.1. Sample Collection
Each participant provided approximately 5 g of fresh stool in a sterile container. To avoid contamination and degradation, samples were maintained at 4 °C and processed within four hours after collection; during transportation, they were kept between 4 and 8 °C [20].
2.4.2. Sample Preparation
In the laboratory, stool samples were concentrated via flotation using either saline or sucrose solution. Ten millilitres of solution was mixed with the sample and centrifuged at 1500 rpm for 10 min. After discarding the supernatant, the sediment was resuspended in 1 mL of saline for microscopic examination.
2.4.3. Microscopic Examination
A drop of the sediment was placed on a glass slide, covered with a coverslip, and observed under a light optical microscope: first under the 10× objective for general localisation and then under the 40× objective for detailed identification.
- Giardia lamblia: Oval cysts of 8–12 µm with a distinct double wall.
- Ascaris lumbricoides: Oval eggs measuring 55–75 µm with a thick yellowish shell.
- Trichuris trichiura: Barrel-shaped eggs of 50–54 µm with tapered ends.
2.4.4. Data Recording and Analysis
Observations were recorded in a structured datasheet, indicating presence/absence and estimated parasite load. Subsequently, data were analysed to correlate parasitic infections with haemoglobin levels and other haematological parameters [21].
2.5. Biomarkers for the Evaluation of Anaemia and Parasitic Infections
Blood samples were collected in EDTA tubes (Becton Dickinson, Franklin Lakes, NJ, USA) following standard protocols and were kept uncentrifuged to preserve the accuracy of haematological results [12]. Complete blood counts were performed using an automated analyser, generating values for haemoglobin (Hb), haematocrit (Hct), and other relevant parameters.
The study focused on six key biomarkers: Hb, Hct, corpuscular indices (MCV, MCH, MCHC), C-reactive protein (CRP), and ferritin. These markers allow for the detection of haematological and inflammatory alterations associated with anaemia and parasitic infections, as well as the evaluation of the combined impact of intense exercise and parasitosis in adolescent athletes.
2.5.1. Haemoglobin (Hb)
Hb was measured in two stages: initial screening using a Peak Instruments UV-Vis spectrophotometer with a Sigma-Aldrich kit, and confirmation with a Sysmex automated analyser (absorbance at 540 nm; results expressed in g/dL) [22].
2.5.2. Haematocrit and Erythrocyte Indices
Hct was determined through microcentrifugation. MCV, MCH, and MCHC indices were automatically calculated using the Genrui KT-62 analyser and were expressed in fL, pg, and g/dL, respectively, allowing for the assessment of erythrocyte morphology and function [17].
2.5.3. C-Reactive Protein (CRP)
CRP levels were quantified using an immunoturbidimetric assay (Roche Diagnostics GmbH, Mannheim, Germany, CRP kit). Absorbance was recorded at 540 nm, and the results are expressed in mg/L. Measurements were performed on a BS-200 chemistry analyser (Mindray, Shenzhen, China) [23].
2.5.4. Ferritin
Serum ferritin was determined using ELISA (Abcam Ferritin kit). Serum samples were incubated on plates pre-coated with anti-ferritin antibodies; after adding the enzyme conjugate, absorbance was measured at 450 nm, and results are expressed in ng/mL according to standard reference ranges [24].
2.5.5. Procedures and Technical References
- Sample collection and handling: CLSI guidelines for blood [18]; coproparasitological protocol by García (2016) for stool samples [20].
- Parasitic detection: centrifugal flotation and Lugol staining to identify cysts and eggs of Ascaris lumbricoides, Giardia lamblia, and Trichuris trichiura [19].
- Haemoglobin: quantified using an automated haematology analyser (Sysmex Corporation, 2018) [22].
- Ferritin: measured using ELISA following FDA guidelines (2020) [24].
- C-reactive protein (CRP): determined using immunoturbidimetric assay (Beckman Coulter, 2017) [23].
- Erythrocyte indices (MCV, MCH, and MCHC): automatically calculated according to Schmidt et al., 2014 [17].
Anaemia was defined according to WHO (2011) criteria as haemoglobin < 12 g/dL in adolescents [6]. Data were processed using SPSS v26 [25], and homogeneity and normality were verified following the recommendations of Tabachnick and Fidell [26].
2.5.6. Formulas Applied by the GENRUI KT-6610 Analyser
MCV (fL) = (haematocrit [%] × 10)/erythrocyte count (million/µL)
MCH (pg) = (haemoglobin [g/dL] × 10)/erythrocyte count (million/µL)
MCHC (g/dL) = (haemoglobin [g/dL] × 100)/haematocrit (%)
2.6. Statistical Analysis
Data were analysed using SPSS v26 (IBM, Armonk, NY, USA) [25]. Normality was assessed with the Shapiro–Wilk test, and homogeneity of variances was assessed with Levene’s test. Continuous variables are presented as the mean ± SD, while categorical variables are presented as percentages. Group differences were evaluated using appropriate mean comparison tests, adjusting for unequal variances when required. The association between anaemia (Hb < 12 g/dL) and predictor variables (CRP, BMI, age) was examined through binary logistic regression; odds ratios (OR) with 95% confidence intervals (CI) were calculated. Statistical significance was set at p < 0.05. Recommendations for statistical 237 treatment were followed as per Tabachnick and Fidell [26].
2.7. Ethical Statement
This project was approved by the Research Ethics Committee of Hipólito Unanue Regional Hospital in Tacna, Peru (protocol 010-CIEI-2023, 4 May 2023). The study was conducted in accordance with the principles of the Declaration of Helsinki, ensuring the protection of participants’ rights, privacy, and well-being. Informed consent was obtained from all adolescents—or from their legal guardians when applicable—prior to any data collection or sample handling.
3. Results
Table 1 summarises the baseline profile. The 250 male athletes (15.5 ± 1.4 years) showed a 30% prevalence of anaemia (Hb < 12 g/dL) and 12% were underweight. Mean haemoglobin was 11.5 ± 1.2 g/dL, notably lower than that of non-athletes (13.0 ± 1.1 g/dL). CRP levels reached 10.2 ± 2.8 mg/L compared to 5.4 ± 2.1 mg/L in controls, indicating higher systemic inflammation. Parasite load was also greater in athletes (35% vs. 18%), dominated by Ascaris lumbricoides (25%). These data confirm that athletes had a less favourable nutritional and infectious baseline status.

Table 1.
Demographic, nutritional, and parasitic profile of the study cohort.
Table 2 shows that parasitic infection worsens the haematological profile of athletes. Infected individuals had haemoglobin of 11.9 ± 1.1 g/dL and haematocrit of 35.5 ± 2.8%, in contrast to 13.8 ± 1.0 g/dL and 40.2 ± 2.5% in non-infected athletes (p < 0.001). MCV decreased to 80.0 ± 5.2 fL and MCHC to 31.6 ± 1.3 g/dL, while CRP increased to 5.0 ± 1.9 mg/L. Consequently, anaemia prevalence rose from 5% to 28%.

Table 2.
Haematological and inflammatory profile of athletes stratified by parasitic infection status (Panel A) and C-reactive protein level, CRP (Panel B).
Panel B reveals a clear inflammatory gradient. With CRP < 3 mg/L, the mean haemoglobin was 13.4 ± 0.9 g/dL and anaemia prevalence was 8%. At CRP levels between 3 and 10 mg/L, haemoglobin decreased to 12.6 ± 1.1 g/dL and anaemia prevalence reached 18%. With CRP > 10 mg/L, haemoglobin dropped to 11.8 ± 1.2 g/dL and anaemia reached 28% (p < 0.001). These findings confirm that systemic inflammation induced by parasitic infection is the main factor associated with reduced haemoglobin in this cohort of adolescent athletes.
Table 3 shows a gradient between haemoglobin (Hb) and iron markers. When Hb was <12 g/dL, mean ferritin level was 18.5 ng/mL and that of serum iron was 46.2 µg/dL; MCV, MCH, and MCHC were 74.8 fL, 24.5 pg, and 31.0 g/dL, respectively. With intermediate Hb levels (12–14 g/dL), ferritin levels increased to 51.5 ng/mL and serum iron levels increased to 73.5 µg/dL; concurrently, MCV reached 84.2 fL, MCH reached 27.5 pg, and MCHC reached 33.0 g/dL. In the >14 g/dL range, values rose consistently: ferritin rose to 80.2 ng/mL, serum iron rose to 94.5 µg/dL, MCV rose to 90.8 fL, MCH rose to 29.0 pg, and MCHC rose to 34.5 g/dL. All trends were significant (p < 0.001), indicating that iron availability and erythrocyte size improved proportionally with increasing haemoglobin levels.

Table 3.
Iron markers and erythrocyte indices by haemoglobin range in adolescent athletes.
Table 4 shows how parasite type and nutritional status affect haemoglobin levels and anaemia prevalence.

Table 4.
Influence of parasite type (Panel A) and nutritional status (Panel B) on haemoglobin and anaemia prevalence in adolescent athletes.
Panel A. Athletes without infection maintained Hb levels of 13.7 ± 1.1 g/dL with only 5% anaemia prevalence. Hb decreased to 12.0 ± 1.1 g/dL with Ascaris lumbricoides and to 11.5 ± 1.0 g/dL with Giardia lamblia; anaemia prevalence increased to 28% and 35%, respectively. Trichuris trichiura produced intermediate values (Hb 11.8 ± 1.1 g/dL; anaemia 30%). Mixed infections resulted in the worst profile (Hb 11.3 ± 1.2 g/dL; anaemia 45%). This trend was significant (p < 0.001).
Panel B. Underweight status (BMI < 18.5 kg/m2) was associated with Hb of 11.8 ± 1.1 g/dL and 30% anaemia prevalence. With normal BMI (18.5–24.9 kg/m2), Hb increased to 13.1 ± 1.0 g/dL and anaemia prevalence decreased to 12%. Overweight athletes (≥25 kg/m2) showed intermediate values (Hb 12.6 ± 1.0 g/dL; anaemia 18%). This trend was also significant (p < 0.001). These results confirm that parasitic burden and malnutrition exacerbate haematological deficits in adolescent athletes.
Table 5 presents the logistic regression model for predicting anaemia. After simultaneous adjustment for age and BMI, C-reactive protein (CRP) remained the only independent predictor: each 1 mg/L increase raised the probability of anaemia by 20% (adjusted OR = 1.20; 95% CI: 1.08–1.38; p < 0.001). Neither BMI (OR = 0.98; 95% CI: 0.91–1.06; p = 0.250) nor age (OR = 1.02; 95% CI: 0.95–1.07; p = 0.330) showed significant associations. These results confirm that systemic inflammation, rather than body weight status or age, is the main statistical determinant of anaemia in the adolescent athletes studied.

Table 5.
Predictors of anaemia in adolescent athletes: logistic regression (n = 250).
Figure 1 illustrates the correlations between haematological, biochemical, and parasitic markers. Haemoglobin showed a strong negative correlation with anaemia (r = −0.80) and parasitic infection (r = −0.60). CRP was positively associated with both anaemia (r = 0.65) and infection (r = 0.58), supporting the role of inflammation as a link between parasitosis and decreased haemoglobin. Ferritin had a moderate positive correlation with haemoglobin (r = 0.50) and a negative correlation with anaemia (r = −0.55), confirming the influence of iron stores. Finally, haematocrit and haemoglobin were closely correlated (r = 0.85), while infection showed a positive association with anaemia (r = 0.70). Overall, the heatmap reinforces the hypothesis that systemic inflammation induced by parasites reduces iron availability and precipitates anaemia in adolescent athletes.
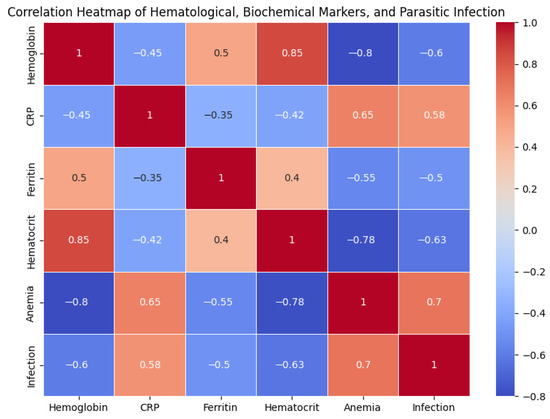
Figure 1.
Correlation heatmap of haematological, biochemical, and parasitic infection markers in adolescent athletes.
4. Discussion
This study confirms that the combination of systemic inflammation and intestinal parasitosis is the main determinant of anaemia in adolescent athletes from Tacna, aligning with evidence from various Middle Eastern and North African settings. For example, in Egyptian schoolchildren, helminth infections reduced haemoglobin levels by 1.5 g/dL and doubled anaemia prevalence independently of BMI [8]. Similarly, in our cohort, haemoglobin levels decreased by 1.9 g/dL and anaemia prevalence increased from 5% to 28% when comparing non-infected to infected athletes, reinforcing the external validity of these findings. Furthermore, in Iranian children infected with Giardia lamblia, parasite eradication increased ferritin levels by 22 ng/mL and normalised mean corpuscular volume [17]; comparably, our haemoglobin gradient showed ferritin levels increasing from 18.5 ng/mL to 80.2 ng/mL as haemoglobin improved.
Although the prevalence of anaemia among Tacna athletes (30%) exceeds the 15–20% reported in Saudi adolescents, the study by Alkhaldy et al. (2021) identified a 36% prevalence of subclinical iron deficiency in this population [15]. This “hidden” deficiency mirrors the pattern observed in athletes infected with Ascaris lumbricoides in our study, whose mean ferritin (35 ng/mL) approximates the threshold below which response to supplementation decreases if helminthiasis is not simultaneously treated [9]. In the CoDe-STH trial, Clarke et al. (2019) [27] demonstrated that community deworming programmes achieved greater anaemia reduction than school-only interventions; by analogy, community-wide coverage in Tacna could benefit athletes and their families, preventing reinfections and mitigating the chronic inflammation responsible for hepcidin induction [15]. The central role of hepcidin is supported by the strong association between C-reactive protein (CRP) and anaemia detected in the multivariate model: each additional 1 mg/L of CRP increased anaemia probability by 20%, while body weight status lost significance. This finding aligns with the pathophysiological framework described by Nemeth and Ganz (2010), who noted that IL-6 stimulates hepcidin transcription, blocking iron export into circulation [10]. Furthermore, transient hepcidin peaks after intense exercise, documented in endurance athletes may become chronic in the presence of parasitosis, as suggested by the doubled baseline CRP found in our football players [15].
The type of parasite also proved decisive. Giardia and mixed infections reduced haemoglobin levels below 11.5 g/dL, while Ascaris caused a more moderate decrease; this pattern reflects the heterogeneity observed in rural Yemen, where parasite load correlated directly with anaemia severity [15]. Although Trichuris trichiura showed an intermediate effect in our sample, the literature suggests that high infection intensity can cause subclinical blood loss and exacerbate microcytosis [9]. Moreover, the absence of blood-feeding helminths such as Ancylostoma duodenale explains why haemoglobin decline was not more drastic, but confirms that iron supplementation alone is unlikely to reverse the condition without adequate inflammation control.
Our findings confirm that CRP is a stronger predictor of anaemia than BMI or age, while parasitic infections showed an indirect association through their link to inflammation. This relationship concurs with recent studies from other regions: Hamdy et al. (2024) [12] found that Egyptian children infected with Blastocystis sp. presented with microcytic anaemia, reduced ferritin, and lower serum zinc [12]; in Ethiopia, a meta-analysis showed that hookworm infections doubled childhood anaemia risk [13]. Ipa et al. (2024) reported that in Indonesia, children with soil-transmitted helminths had a mean haemoglobin of 10.5 ± 1.2 g/dL and anaemia prevalence three times higher than uninfected children [14], while El-Sharkawy and Mohamed (2024) found that parasitised Egyptian adolescents had 35% lower ferritin levels and higher iron-deficiency anaemia prevalence [15]. These findings from African and Asian populations reinforce the external validity of our results and highlight the need for integrated interventions. In line with WHO recommendations (2023) [21], we propose biannual deworming programmes with albendazole, nutritional education focused on improving iron bioavailability, and periodic CRP monitoring to identify athletes requiring specific anti-inflammatory interventions. Implementing these strategies could substantially reduce anaemia burden among physically active adolescents living in endemic regions of Latin America and the Middle East.
5. Conclusions
This study among adolescent athletes in Tacna demonstrates that parasitic infections induce systemic inflammation, leading to anaemia. Athletes infected with Ascaris lumbricoides or Giardia lamblia showed lower haemoglobin levels, higher anaemia prevalence, and elevated C-reactive protein (CRP) levels compared to uninfected peers. These findings confirm that parasite-induced inflammation reduces iron bioavailability and compromises erythropoiesis.
To mitigate this problem, three actions are recommended: periodic deworming, iron supplementation, and routine monitoring of CRP levels. Implementing these measures could decrease anaemia rates and protect the health and performance of adolescents living in regions in which parasitic infections and malnutrition are endemic.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/parasitologia5030039/s1, Table S1: Full statistics for iron markers and erythrocyte indices by haemoglobin range in adolescent athletes; Table S2: Ferritin and serum iron concentrations by parasite type and BMI category; Table S3: Distribution of CRP categories among infected and non-infected athletes.
Author Contributions
Conceptualisation and original idea, K.G.Y.S. and A.B.R.P.; experimentation, V.F.C.Q. and C.W.R.A.; methodology, L.L.L.; analytical analysis of the samples, A.B.R.P.; review and editing, K.G.Y.S. and V.F.C.Q.; formal analysis, C.W.R.A.; investigation, L.L.L. and A.B.R.P.; resources, A.B.R.P.; data curation, K.G.Y.S.; writing—original draft preparation, L.L.L. and C.W.R.A.; writing—review and editing, A.B.R.P. and V.F.C.Q.; supervision, L.L.L. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the Universidad Nacional Jorge Basadre Grohmann through the “Programme of economic subsidies for expenses associated with the publication of scientific articles in indexed journals”. N°010-2024-MINEDU.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Hospital Regional Hipolito Unanue, Tacna-Peru (protocol code 010-CIEI-2023 dated 4 May 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Acknowledgments
We thank the departmental football league of Tacna for the use of their facilities for the execution of data collection.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Suedekum, N.A.; Dimeff, R.J. Iron and the Athlete. Curr. Sports Med. Rep. 2005, 4, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Sim, M.; Badenhorst, C.E.; Dawson, B.; Govus, A.D.; Abbiss, C.R.; Swinkels, D.W.; Trinder, D. Iron Status and the Acute Post-Exercise Hepcidin Response in Athletes. PLoS ONE 2014, 9, e93002. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.A.; Fisher, S.A.; Doree, C.; Di Angelantonio, E.; Roberts, D.J. Oral or Parenteral Iron Supplementation to Reduce Deferral, Iron Deficiency and/or Anaemia in Blood Donors. Cochrane Database Syst. Rev. 2014, 2014, CD009532. [Google Scholar] [CrossRef] [PubMed]
- Hersey, J.C.; Wohlgenant, K.C.; Arsenault, J.E.; Kosa, K.M.; Muth, M.K. Effects of front-of-package and shelf nutrition labeling systems on consumers. Nutr. Rev. 2013, 71, 1–14. [Google Scholar] [CrossRef] [PubMed]
- DellaValle, D.M. Iron Supplementation for Female Athletes: Effects on Iron Status and Performance Outcomes. Curr. Sports Med. Rep. 2013, 12, 234–239. [Google Scholar] [CrossRef] [PubMed]
- WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2011; Available online: https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1 (accessed on 10 May 2025).
- MINSA. Boletín Epidemiológico Nacional 2020; Ministerio de Salud del Perú: Lima, Peru, 2020. Available online: https://www.minsa.gob.pe. (accessed on 24 May 2025).
- Stephenson, L.S.; Latham, M.C.; Ottesen, E.A. Malnutrition and Parasitic Helminth Infections. Parasitology 2000, 121 (Suppl. 1), S23–S38. [Google Scholar] [CrossRef] [PubMed]
- Crompton, D.W.T.; Nesheim, M.C. Nutritional Impact of Intestinal Helminthiasis During the Human Life Cycle. Annu. Rev. Nutr. 2002, 22, 35–59. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin and Iron Regulation, 10 Years Later. Blood 2011, 117, 4425–4433. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron Deficiency Anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, D.A.; Fahmey, S.S.; El Wahab, W.M.A.; Mohamed, S.S.; Mohamed, Y.A. Effect of Blastocystis sp. Infection on Hematological Parameters and Trace Element Status in Children with Iron-Deficiency Anaemia. J. Parasit. Dis. 2024, 48, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Setegn, A.; Wondmagegn, Y.M.; Damtie, W.A.; Abebe, W.; Geremew, G.W.; Alemayehu, T.T.; Wassie, Y.A.; Kibralew, G.; Fentahun, S.; Mengistie, B.A.; et al. Hookworm infection and its determinants among schoolchildren in Ethiopia: A systematic review and meta-analysis. BMC Infect. Dis. 2024, 24, 1420. [Google Scholar] [CrossRef] [PubMed]
- Ipa, M.; Isnani, T.; Girsang, V.I.; Amila; Harianja, E.S.; Purba, Y.; Wandra, T.; Budke, C.M.; Purba, I.E. Soil-Transmitted Helminth Infections and Anaemia in Children Attending Government-Run Schools on Samosir Island, Indonesia. Parasite Epidemiol. Control 2024, 25, e00344. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, H.A.; Mohamed, S.E. Prevalence of Intestinal Parasites and Their Impact on Iron-Status Indicators among Egyptian Adolescents. Parasitol. United J. 2024, 17, 123–131. Available online: https://puj.journals.ekb.eg/article_409223.html (accessed on 20 May 2024).
- Cahuana, L.Y.C. Prevalencia de Ectoparásitos y Enteroparásitos en Caninos (Canis familiaris) del Distrito de Calana—Tacna, 2016. Bachelor’s Thesis, Universidad Nacional Jorge Basadre Grohmann, Tacna, Peru, 2016. Available online: https://repositorio.unjbg.edu.pe/server/api/core/bitstreams/bb35ae64-0161-444b-aa60-73ff994e7de8/content (accessed on 12 April 2024).
- Schmidt, M.; Solgaard, P.; Gammelgaard, B.; Hansen, S.H. Fundamentals of Clinical Chemistry. In Introduction to Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 99–116. [Google Scholar]
- Clinical and Laboratory Standards Institute. CLSI Document H21-A5; Collection, Transport, and Processing of Blood Specimens for Testing Plasma-Based Coagulation Assays and Molecular Hemostasis Assays; Approved Guideline—Fifth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Bowman, D.D. Georgis’ Parasitology for Veterinarians, 10th ed.; Elsevier: St. Louis, MO, USA, 2014. [Google Scholar]
- García, L.S. Diagnostic Medical Parasitology, 6th ed.; ASM Press: Washington, DC, USA, 2016. [Google Scholar]
- WHO. Guideline: Daily Iron Supplementation in Adult Women and Adolescent Girls; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/i/item/9789241510196 (accessed on 26 April 2024).
- Sysmex Corporation. Automated Hematology Analyzer XN-Series; Sysmex Corporation: Kobe, Japan, 2018. [Google Scholar]
- Coulter, B. CRP Latex Reagent Kit Package Insert; Beckman Coulter Inc.: Brea, CA, USA, 2017. [Google Scholar]
- FDA. Bioanalytical Method Validation Guidance for Industry; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2020. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 24 May 2025).
- IBM Corp. IBM SPSS Statistics for Windows, Version 26.0; IBM Corp.: Armonk, NY, USA, 2019. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 6th ed.; Pearson: Boston, MA, USA, 2014. [Google Scholar]
- Clarke, N.E.; Ng-Nguyen, D.; Traub, R.J.; Clements, A.C.; Halton, K.; Anderson, R.M.; Gray, D.J.; Coffeng, L.E.; Kaldor, J.M.; Vaz Nery, S. A cluster-randomised controlled trial comparing school and community-based deworming for soil-transmitted helminth control in school-age children: The CoDe-STH trial protocol. BMC Infect Dis. 2019, 19, 822. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).