Characterization of a Choline-Gated Chloride Channel (LGC-40) from Haemonchus contortus Highlights a Novel Cholinergic Binding Site
Abstract
1. Introduction
2. Methods
2.1. Cloning of Hco-Lgc-40 and Sequence Analysis
2.2. Expression of Hco-LGC-40 and Electrophysiological Analysis
2.3. Modelling of the Hco-LGC-40 Binding Pocket
3. Results and Discussion
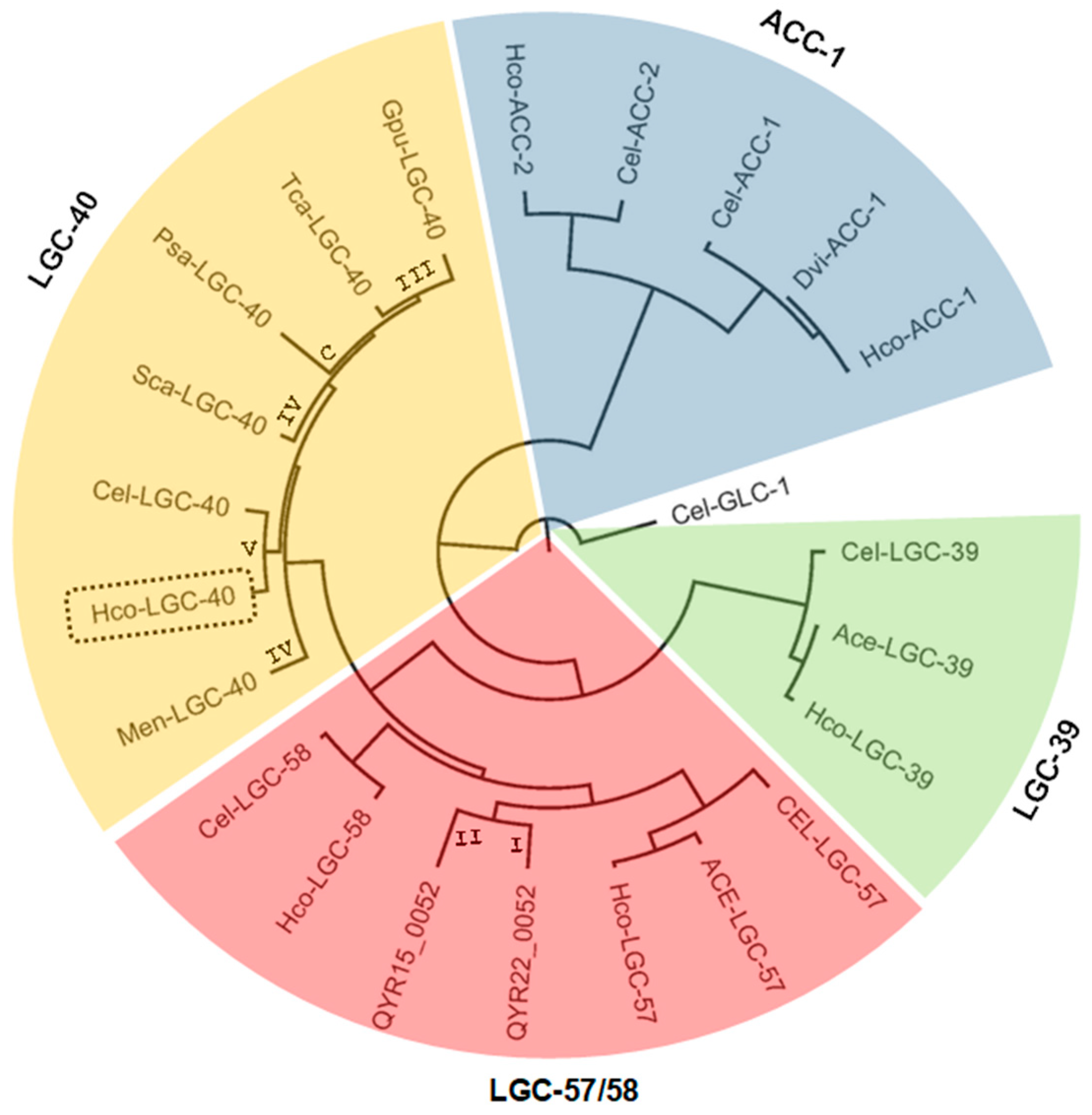
3.1. Cloning of Hco-Lgc-40 and Sequence Analysis
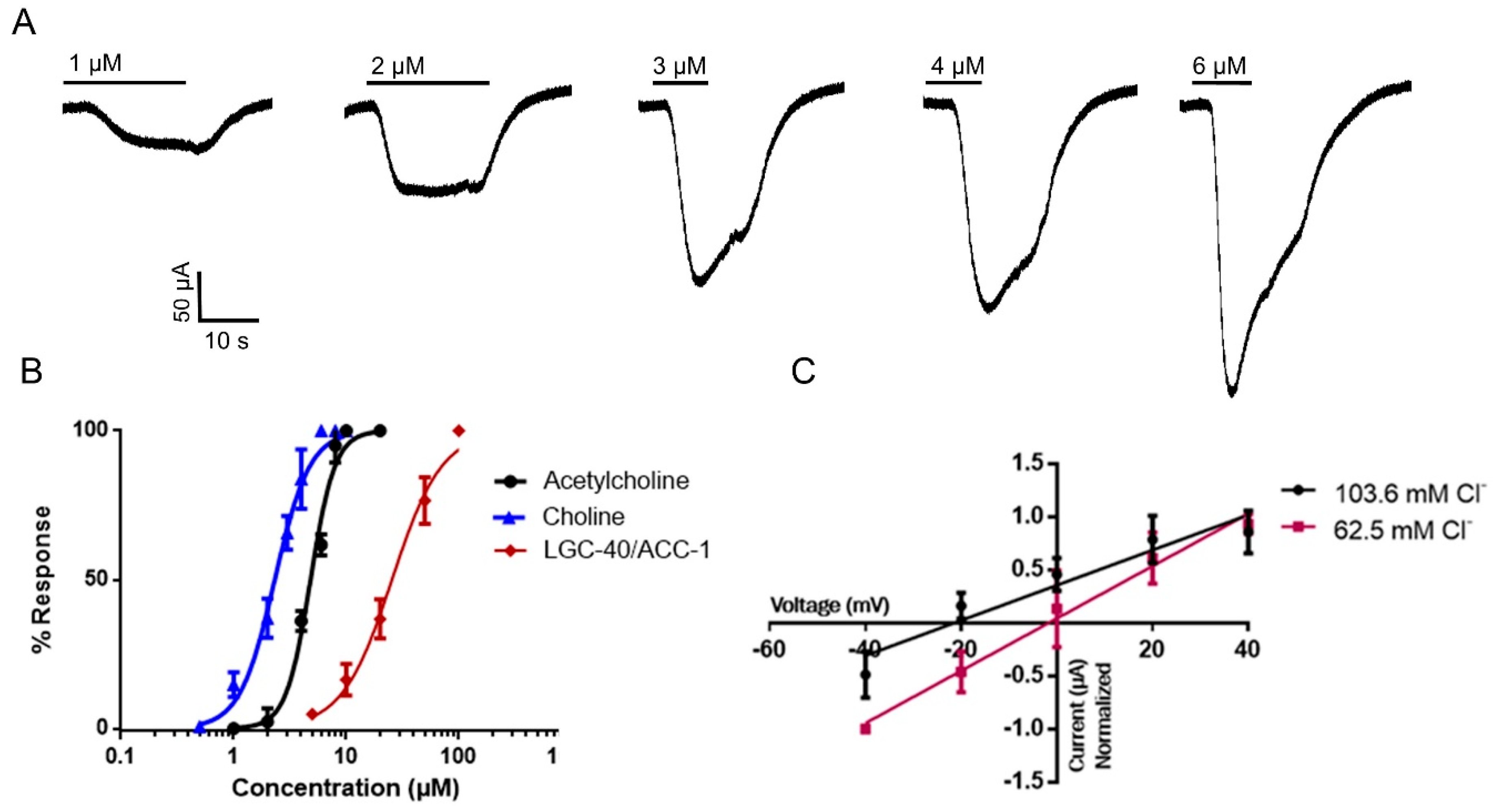
3.2. Expression of Hco-LGC-40 and Electrophysiological Analysis
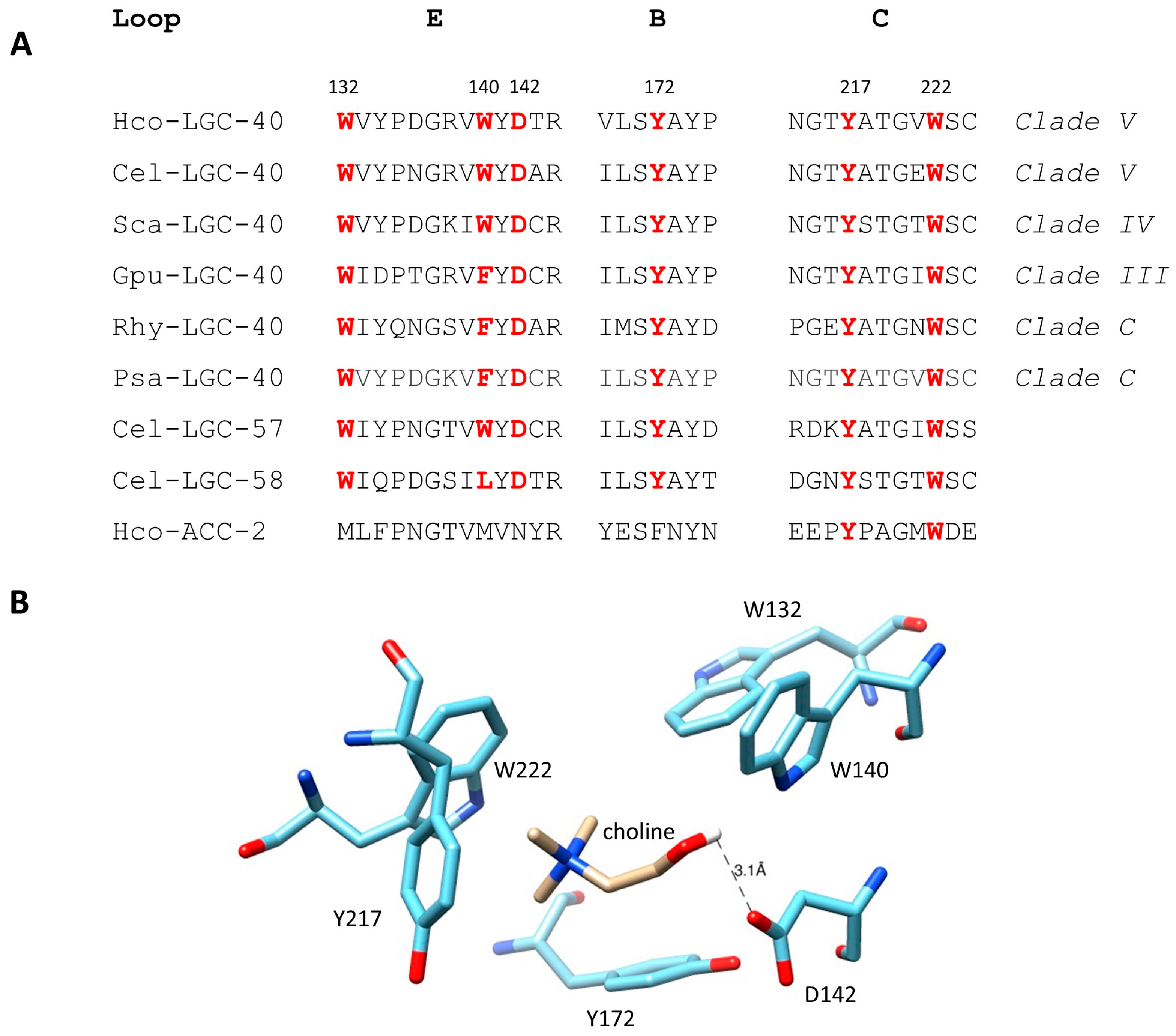
3.3. Modelling of the Hco-LGC-40 Binding Pocket
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choudhary, S.; Kashyap, S.S.; Martin, R.J.; Robertson, A.P. Advances in our understanding of nematode ion channels as potential anthelmintic targets. Int. J. Parasitol. Drugs Drug Resist. 2022, 18, 52–86. [Google Scholar] [CrossRef] [PubMed]
- Bamber, B.A.; Beg, A.A.; Twyman, R.E.; Jorgensen, E.M. The Caenorhabditis elegans unc-49 locus encodes multiple subunits of a heteromultimeric GABA receptor. J. Neurosci. 1999, 19, 5348–5359. [Google Scholar] [CrossRef] [PubMed]
- Putrenko, I.; Zakikhani, M.; Dent, J.A. A family of acetylcholine-gated chloride channel subunits in Caenorhabditis elegans. J. Biol. Chem. 2005, 280, 6392–6398. [Google Scholar] [CrossRef] [PubMed]
- Ringstad, N.; Abe, N.; Horvitz, H.R. Ligand-Gated chloride channels are receptors for biogenic amines in C. elegans. Science 2009, 325, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Habibi, S.; Nazareth, K.; Nichols, J.; Varley, S.; Forrester, S.G. The Haemonchus contortus LGC-39 subunit is a novel subtype of an acetylcholine-gated chloride channel. Int. J. Parasitol. Drugs Drug Resist. 2023, 22, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Hardege, I.; Morud, J.; Courtney, A.; Schafer, W.R. A Novel and Functionally Diverse Class of Acetylcholine-Gated Ion Channels. J. Neurosci. 2023, 43, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Weston, D.; Patel, B.; Van Voorhis, W.C. Virulence in Trypanosoma cruzi infection correlates with the expression of a distinct family of sialidase superfamily genes. Mol. Biochem. Parasitol. 1999, 98, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Abdelmassih, S.A.; Cochrane, E.; Forrester, S.G. Evaluating the longevity of surgically extracted Xenopus laevis oocytes for the study of nematode ligand-gated ion channels. Invertebr. Neurosci. 2018, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Šali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Kusano, K.; Miledi, R.; Stinnakre, J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J. Physiol. 1982, 328, 143–170. [Google Scholar] [CrossRef] [PubMed]
- Callanan, M.K.; Habibi, S.A.; Law, W.J.; Nazareth, K.; Komuniecki, R.L.; Forrester, S.G. Investigating the function and possible biological role of an acetylcholine-gated chloride channel subunit (ACC-1) from the parasitic nematode Haemonchus contortus. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Habibi, S.A.; Blazie, S.M.; Jin, Y.; Forrester, S.G. Isolation and characterization of a novel member of the ACC ligand-gated chloride channel family, Hco-LCG-46, from the parasitic nematode Haemonchus contortus. Mol. Biochem. Parasitol. 2020, 237, 111276. [Google Scholar] [CrossRef] [PubMed]
- Habibi, S.A.; Callanan, M.; Forrester, S.G. Molecular and pharmacological characterization of an acetylcholine-gated chloride channel (ACC-2) from the parasitic nematode Haemonchus contortus. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 518–525. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazareth, K.; Habibi, S.; Forrester, S.G. Characterization of a Choline-Gated Chloride Channel (LGC-40) from Haemonchus contortus Highlights a Novel Cholinergic Binding Site. Parasitologia 2025, 5, 12. https://doi.org/10.3390/parasitologia5010012
Nazareth K, Habibi S, Forrester SG. Characterization of a Choline-Gated Chloride Channel (LGC-40) from Haemonchus contortus Highlights a Novel Cholinergic Binding Site. Parasitologia. 2025; 5(1):12. https://doi.org/10.3390/parasitologia5010012
Chicago/Turabian StyleNazareth, Kristen, Sarah Habibi, and Sean G. Forrester. 2025. "Characterization of a Choline-Gated Chloride Channel (LGC-40) from Haemonchus contortus Highlights a Novel Cholinergic Binding Site" Parasitologia 5, no. 1: 12. https://doi.org/10.3390/parasitologia5010012
APA StyleNazareth, K., Habibi, S., & Forrester, S. G. (2025). Characterization of a Choline-Gated Chloride Channel (LGC-40) from Haemonchus contortus Highlights a Novel Cholinergic Binding Site. Parasitologia, 5(1), 12. https://doi.org/10.3390/parasitologia5010012




