Amebicides against Acanthamoeba castellanii: The Impact of Organism Models Used in Amebicide Assays
Abstract
1. Introduction
2. Results and Discussion
2.1. Therapies Used for the Treatment of A. castellanii Infections
2.2. Therapies in Research for Pathologies Caused by A. castellanii
2.3. Methods that Are Being Used to Research New Drugs for A. castellanii Infection
2.4. Overview of New Drug Possibilities for the Future
3. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, U.; Anwar, A.; Ong, S.-K.; Anwar, A.; Khan, N.A. Applications of Medicinal Chemistry for Drug Discovery against Acanthamoeba Infections. Med. Res. Rev. 2022, 42, 462–512. [Google Scholar] [CrossRef] [PubMed]
- Winiecka-Krusnell, J.; Dellacasa-Lindberg, I.; Dubey, J.P.; Barragan, A. Toxoplasma Gondii: Uptake and Survival of Oocysts in Free-Living Amoebae. Exp. Parasitol. 2009, 121, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, A.G.; Lira, M. Acanthamoeba Keratitis: A Review of Biology, Pathophysiology and Epidemiology. Ophthalmic Physiol. Opt. 2021, 41, 116–135. [Google Scholar] [CrossRef] [PubMed]
- Varacalli, G.; Di Zazzo, A.; Mori, T.; Dohlman, T.H.; Spelta, S.; Coassin, M.; Bonini, S. Challenges in Acanthamoeba Keratitis: A Review. J. Clin. Med. 2021, 10, 942. [Google Scholar] [CrossRef] [PubMed]
- Ozpinar, N.; Ozpinar, H.; Bakay, B.B.; Tunc, T. Amoebicidal Activity of Benzothiazole on Acanthamoeba castellanii Cysts and Trophozoites and Its Cytotoxic Potentials. Acta Trop. 2020, 203, 105322. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, L.S.; de Freitas, D.; Hofling-Lima, A.L. Ceratite Por Acanthamoeba. Arq. Bras. Oftalmol. 2000, 63, 155–159. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An Update on Acanthamoeba Keratitis: Diagnosis, Pathogenesis and Treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. The Biology of Acanthamoeba Keratitis. Exp. Eye Res. 2021, 202, 108365. [Google Scholar] [CrossRef]
- Baig, A.M. Innovative Methodology in the Discovery of Novel Drug Targets in the Free-Living Amoebae. Curr. Drug Targets 2019, 20, 60–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Wei, Z.; Cao, K.; Zhang, Z.; Liang, Q. The Global Epidemiology and Clinical Diagnosis of Acanthamoeba Keratitis. J. Infect. Public Health 2023, 16, 841–852. [Google Scholar] [CrossRef]
- Passos, R.M.; Cariello, A.J.; Yu, M.C.Z.; Höfling-Lima, A.L. Microbial Keratitis in the Elderly: A 32-Year Review. Arq. Bras. Oftalmol. 2010, 73, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Maschio, V.J.; Chies, F.; Carlesso, A.M.; Carvalho, A.; Rosa, S.P.; Van Der Sand, S.T.; Rott, M.B. Acanthamoeba T4, T5 and T11 Isolated from Mineral Water Bottles in Southern Brazil. Curr. Microbiol. 2015, 70, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, S.; Gallo-Francisco, P.H.; Roque, G.A.S.; Flóro e Silva, M. Granulomas in Parasitic Diseases: The Good and the Bad. Parasitol. Res. 2020, 119, 3165–3180. [Google Scholar] [CrossRef] [PubMed]
- Łanocha-Arendarczyk, N.; Kolasa-Wołosiuk, A.; Wojciechowska-Koszko, I.; Kot, K.; Roszkowska, P.; Krasnodębska-Szponder, B.; Paczkowska, E.; Machaliński, B.; Łuczkowska, K.; Wiszniewska, B.; et al. Changes in the Immune System in Experimental Acanthamoebiasis in Immunocompetent and Immunosuppressed Hosts. Parasit. Vectors 2018, 11, 517. [Google Scholar] [CrossRef]
- Kot, K.; Łanocha-Arendarczyk, N.; Kosik-Bogacka, D. Immunopathogenicity of Acanthamoeba spp. in the Brain and Lungs. Int. J. Mol. Sci. 2021, 22, 1261. [Google Scholar] [CrossRef]
- Kalra, S.K.; Sharma, P.; Shyam, K.; Tejan, N.; Ghoshal, U. Acanthamoeba and Its Pathogenic Role in Granulomatous Amebic Encephalitis. ScienceDirect 2020, 208, 107788. Available online: https://www.sciencedirect.com/science/article/pii/S001448941930311X (accessed on 13 March 2023).
- Alencar e Silva, R.; Almeida Araújo, S.; Freitas e Avelar, I.F.; Homem Pitella, J.E.; de Oliveira, J.T.; Christo, P.P. Granulomatous Amoebic Meningoencephalitis in an Immunocompetent Patient. Arch. Neurol. 2010, 67, 1516–1520. [Google Scholar] [CrossRef]
- Elsheika, H.M.; Siddiqui, R.; Khan, N.A. Drug Discovery against Acanthamoeba Infections: Present Knowledge and Unmet Needs. Pathogens 2020, 9, 405. [Google Scholar] [CrossRef]
- Akhavan, B.J.; Khanna, N.R.; Vijhani, P. Amoxicillin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Purssell, A.; Lau, R.; Boggild, A.K. Azithromycin and Doxycycline Attenuation of Acanthamoeba Virulence in a Human Corneal Tissue Model. J. Infect. Dis. 2017, 215, 1303–1311. [Google Scholar] [CrossRef]
- Makhlouf, Z.; Akbar, N.; Khan, N.A.; Shah, M.R.; Alharbi, A.M.; Alfahemi, H.; Siddiqui, R. Antiamoebic Properties of Ceftriaxone and Zinc-Oxide-Cyclodextrin-Conjugated Ceftriaxone. Antibiotics 2022, 11, 1721. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Liu, X.; Zhang, S.; Pan, B.; Liu, M.-L. Ciprofloxacin Derivatives and Their Antibacterial Activities. Eur. J. Med. Chem. 2018, 146, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Takemori, N.; Ooi, H.-K.; Imai, G.; Hoshino, K.; Saio, M. Possible Mechanisms of Action of Clarithromycin and Its Clinical Application as a Repurposing Drug for Treating Multiple Myeloma. Ecancermedicalscience 2020, 14, 1088. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.W.S.; Dias, C.V.B.; Kogawa, A.C. Status of Physicochemical and Microbiological Analytical Methods of Gatifloxacin: A Review. J. AOAC Int. 2022, 105, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Martín-Navarro, C.M.; López-Arencibia, A.; Arnalich-Montiel, F.; Valladares, B.; Piñero, J.E.; Lorenzo-Morales, J. Evaluation of the in vitro Activity of Commercially Available Moxifloxacin and Voriconazole Eye-Drops against Clinical Strains of Acanthamoeba. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2013, 251, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Miyazaki, M.; Mashima, K.; Takagi, S.; Hara, S.; Kamimura, H.; Jimi, S. The Effects of Silver Sulfadiazine on Methicillin-Resistant Staphylococcus Aureus Biofilms. Microorganisms 2020, 8, 1551. [Google Scholar] [CrossRef]
- Nakaminami, H.; Tanuma, K.; Enomoto, K.; Yoshimura, Y.; Onuki, T.; Nihonyanagi, S.; Hamada, Y.; Noguchi, N. Evaluation of In vitro Antiamoebic Activity of Antimicrobial Agents Against Clinical Acanthamoeba Isolates. J. Ocul. Pharmacol. Ther. 2017, 33, 629–634. [Google Scholar] [CrossRef]
- Johnson-Arbor, K. Ivermectin: A Mini-Review. Clin. Toxicol. 2022, 60, 571–575. [Google Scholar] [CrossRef]
- Chai, J.-Y.; Jung, B.-K.; Hong, S.-J. Albendazole and Mebendazole as Anti-Parasitic and Anti-Cancer Agents: An Update. Korean J. Parasitol. 2021, 59, 189–225. [Google Scholar] [CrossRef]
- Dingsdag, S.A.; Hunter, N. Metronidazole: An Update on Metabolism, Structure–Cytotoxicity and Resistance Mechanisms. J. Antimicrob. Chemother. 2018, 73, 265–279. [Google Scholar] [CrossRef]
- Palić, S.; Beijnen, J.H.; Dorlo, T.P.C. An Update on the Clinical Pharmacology of Miltefosine in the Treatment of Leishmaniasis. Int. J. Antimicrob. Agents 2022, 59, 106459. [Google Scholar] [CrossRef]
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Elekhnawy, E.; Batiha, G.E.-S. Nitazoxanide and COVID-19: A Review. Mol. Biol. Rep. 2022, 49, 11169–11176. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Ghimire, R.; Lamichhane, P. Efficacy and Safety of Paromomycin for Visceral Leishmaniasis: A Systematic Review. J. Trop. Med. 2021, 2021, 8629039. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.A.; Lira, M.G.S.; Licá, I.C.L.; Frazão, G.C.C.G.; dos Santos, V.A.F.; Filho, A.C.C.M.; Rodrigues, J.G.M.; Miranda, G.S.; Carvalho, R.C.; Nascimento, F.R.F. Praziquantel: An Update on the Mechanism of Its Action against Schistosomiasis and New Therapeutic Perspectives. Mol. Biochem. Parasitol. 2022, 252, 111531. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Blanco, M.E.; Aguilera-Martínez, S.L.; Ventura-Juarez, J.; Pérez-Serrano, J.; Casillas-Casillas, E.; Barba-Gallardo, L.F. Effectiveness of Polyclonal Antibody Immunoconjugate Treatment with Propamidine Isethionate for Amoebic Keratitis in Golden Hamsters. J. Parasitol. Res. 2023, 2023, 3713368. [Google Scholar] [CrossRef]
- Muzny, C.A.; Van Gerwen, O.T.; Legendre, D. Secnidazole: A Treatment for Trichomoniasis in Adolescents and Adults. Expert Rev. Anti Infect. Ther. 2022, 20, 1067–1076. [Google Scholar] [CrossRef]
- Pereira Sousa, J.C.; Kogawa, A.C. Overview of Analytical Methods for Evaluating Tinidazole. J. AOAC Int. 2023, 106, 309–315. [Google Scholar] [CrossRef]
- Karpi, T.M.; Szkaradkiewicz, A.K. Chlorhexidine—Pharmaco-Biological Activity and Application. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1321–1326. [Google Scholar]
- Moon, E.-K.; Choi, H.-S.; Kong, H.-H.; Quan, F.-S. Polyhexamethylene Biguanide and Chloroquine Induce Programmed Cell Death in Acanthamoeba castellanii. Exp. Parasitol. 2018, 191, 31–35. [Google Scholar] [CrossRef]
- Khatter, N.J.; Khan, M.A. Clotrimazole. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Govindarajan, A.; Bistas, K.G.; Ingold, C.J.; Aboeed, A. Fluconazole. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Padda, I.S.; Parmar, M. Flucytosine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Regidor, P.A.; Thamkhantho, M.; Chayachinda, C.; Palacios, S. Miconazole for the Treatment of Vulvovaginal Candidiasis. In vitro, in vivo and Clinical Results. Review of the Literature. J. Obstet. Gynaecol. 2023, 43, 2195001. [Google Scholar] [CrossRef]
- Mooney, R.; Masala, M.; Martial, T.; McGinness, C.; Henriquez, F.L.; Williams, R.A.M. Alkyl-Carbon Chain Length of Two Distinct Compounds and Derivatives Are Key Determinants of Their Anti-Acanthamoeba Activities. Sci. Rep. 2020, 10, 6420. [Google Scholar] [CrossRef]
- Anwar, A.; Siddiqui, R.; Shah, M.; Khan, N. Gold nanoparticles conjugation enhances antiacanthamoebic properties of nystatin, fluconazole and amphotericin B. Mol. Cell. Microbiol. 2019, 29, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkaufer, G.; Li, P.; Stebbins, E.E.; Kangussu-Marcolino, M.M.; Debnath, A.; White, C.V.; Moser, M.S.; DeRisi, J.; Gisselberg, J.; Yeh, E.; et al. Identification of Anisomycin, Prodigiosin and Obatoclax as Compounds with Broad-Spectrum Anti-Parasitic Activity. PLoS Negl. Trop. Dis. 2020, 14, e0008150. [Google Scholar] [CrossRef] [PubMed]
- Kolören, O.; Kolören, Z.; Şekeroğlu, Z.A.; Çolayvaz, M.; Karanis, P. Amoebicidal and Amoebistatic Effects of Artemisia Argyi Methanolic Extracts on Acanthamoeba castellanii Trophozoites and Cysts. Acta Parasitol. 2019, 64, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Sifaoui, I.; Capote Yanes, E.; Reyes-Batlle, M.; Rodríguez-Expósito, R.L.; Piñero, J.E.; Lorenzo-Morales, J. Combined Amoebicidal Effect of Atorvastatin and Commercial Eye Drops against Acanthamoeba castellanii Neff: In vitro Assay Based on Mixture Design. Pathogens 2020, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Loufouma Mbouaka, A.; Leitsch, D.; Koehsler, M.; Walochnik, J. Antimicrobial Effect of Auranofin against Acanthamoeba spp. Int. J. Antimicrob. Agents 2021, 58, 106425. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Akbar, N.; Khatoon, B.; Kawish, M.; Ali, M.S.; Shah, M.R.; Khan, N.A. Novel Plant-Based Metabolites as Disinfectants against Acanthamoeba castellanii. Antibiotics 2022, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Fakae, L.B.; Stevenson, C.W.; Zhu, X.Q.; Elsheika, H. M In Vitro Activity of Camellia sinensis (Green Tea) against Trophozoites and Cysts of Acanthamoeba castellanii. ScienceDirect 2020, 13, 59–72. Available online: https://www.sciencedirect.com/science/article/pii/S2211320720300117?via%3Dihub (accessed on 13 March 2023). [CrossRef] [PubMed]
- Souhaiel, N.; Sifaoui, I.; Ben Hassine, D.; Bleton, J.; Bonose, M.; Moussa, F.; Piñero, J.E.; Lorenzo-Morales, J.; Abderrabba, M. Ammoides Pusilla (Apiaceae) Essential Oil: Activity against Acanthamoeba castellanii Neff. Exp. Parasitol. 2017, 183, 99–103. [Google Scholar] [CrossRef]
- Gulec, B.; Tore, D.; Tepe, A.S.; Kilic, I.H. Investigation of the Anti-Parasitic Effect of the Water Extract of Thymbra Spicata on Acanthamoeba castellanii (L.) Trophozoites and Cysts. Int. J. Plant Based Pharm. 2021, 1, 48–51. [Google Scholar]
- García-Davis, S.; Sifaoui, I.; Reyes-Batlle, M.; Viveros-Valdez, E.; Piñero, J.E.; Lorenzo-Morales, J.; Fernández, J.J.; Díaz-Marrero, A.R. Anti-Acanthamoeba Activity of Brominated Sesquiterpenes from Laurencia Johnstonii. Mar. Drugs 2018, 16, 443. [Google Scholar] [CrossRef]
- Martín-Escolano, R.; Romero, S.M.; Díaz, J.G.; Marín, C.; Sánchez-Moreno, M.; Rosales, M.J. In vitro Anti-Acanthamoeba Activity of Flavonoid Glycosides Isolated from Delphinium Gracile, D. Staphisagria, Consolida Oliveriana and Aconitum Napellus. Parasitology 2021, 148, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Sama-ae, I.; Sangkanu, S.; Siyadatpanah, A.; Norouzi, R.; Chuprom, J.; Mitsuwan, W.; Surinkaew, S.; Boonhok, R.; Paul, A.K.; Mahboob, T.; et al. Targeting Acanthamoeba Proteins Interaction with Flavonoids of Propolis Extract by in vitro and in silico Studies for Promising Therapeutic Effects. F1000Research 2023, 11, 1274. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Siddiqui, R.; Shah, M.R.; Khan, N.A. Antidiabetic Drugs and Their Nanoconjugates Repurposed as Novel Antimicrobial Agents against Acanthamoeba castellanii. J. Microbiol. Biotechnol. 2019, 29, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-A.; Park, S.-M.; Chu, K.-B.; Quan, F.-S.; Kurz, T.; Pflieger, M.; Moon, E.-K. Application of Histone Deacetylase Inhibitors MPK472 and KSK64 as a Potential Treatment Option for Acanthamoeba Keratitis. Antimicrob. Agents Chemother. 2020, 64, e01506-20. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.-B.; Lee, H.-A.; Pflieger, M.; Fischer, F.; Asfaha, Y.; Alves Avelar, L.A.; Skerhut, A.; Kassack, M.U.; Hansen, F.K.; Schöler, A.; et al. Antiproliferation and Antiencystation Effect of Class II Histone Deacetylase Inhibitors on Acanthamoeba castellanii. ACS Infect. Dis. 2022, 8, 271–279. [Google Scholar] [CrossRef]
- Siddiqui, R.; Rawas-Qalaji, M.; El-Gamal, M.I.; Sajeev, S.; Jagal, J.; Zaraei, S.-O.; Sbenati, R.M.; Anbar, H.S.; Dohle, W.; Potter, B.V.L.; et al. Novel Anti-Acanthamoebic Activities of Irosustat and STX140 and Their Nanoformulations. Antibiotics 2023, 12, 561. [Google Scholar] [CrossRef]
- Anwar, A.; Soomaroo, A.; Anwar, A.; Siddiqui, R.; Khan, N.A. Metformin-Coated Silver Nanoparticles Exhibit Anti-Acanthamoebic Activities against Both Trophozoite and Cyst Stages. Exp. Parasitol. 2020, 215, 107915. [Google Scholar] [CrossRef]
- Siddiqui, R.; Makhlouf, Z.; Akbar, N.; Khamis, M.; Ibrahim, T.; Khan, A.S.; Khan, N.A. Antiamoebic Properties of Methyltrioctylammonium Chloride Based Deep Eutectic Solvents. Contact Lens Anterior Eye 2023, 46, 101758. [Google Scholar] [CrossRef]
- Abdelnasir, S.; Mungroo, M.R.; Chew, J.; Siddiqui, R.; Khan, N.A.; Ahmad, I.; Shahabuddin, S.; Anwar, A. Applications of Polyaniline-Based Molybdenum Disulfide Nanoparticles against Brain-Eating Amoebae. ACS Omega 2023, 8, 8237–8247. [Google Scholar] [CrossRef]
- Rosales, M.J.; Ximenis, M.; Costa, A.; Romero, D.; Olmo, F.; Delgado, E.; Clares, M.P.; García-España, e.; Marín, C.; Sánches, M. In Vitro Activity of Squaramides and Acyclic Polyamine Derivatives against Trophozoites and Cysts of Acanthamoeba castellanii. J. Biociences Med. 2018, 6, 1–14. [Google Scholar] [CrossRef][Green Version]
- Ahmed, U.; Manzoor, M.; Qureshi, S.; Mazhar, M.; Fatima, A.; Aurangzeb, S.; Hamid, M.; Khan, K.M.; Khan, N.A.; Rashid, Y.; et al. Anti-Amoebic Effects of Synthetic Acridine-9(10H)-One against Brain-Eating Amoebae. Acta Trop. 2023, 239, 106824. [Google Scholar] [CrossRef] [PubMed]
- Padzik, M.; Hendiger, E.B.; Chomicz, L.; Grodzik, M.; Szmidt, M.; Grobelny, J.; Lorenzo-Morales, J. Tannic Acid-Modified Silver Nanoparticles as a Novel Therapeutic Agent against Acanthamoeba. Parasitol. Res. 2018, 117, 3519–3525. [Google Scholar] [CrossRef] [PubMed]
- Mennai. Chemical Composition and Antioxidant, Antiparasitic, Cytotoxicity and Antimicrobial Potential of the Algerian Limonium Oleifolium Mill. Essential Oil and Organic Extract. Chem. Biodivers. 2021, 18, e2100278. [Google Scholar] [CrossRef]
- Sifaoui, I.; Rodríguez-Expósito, R.L.; Reyes-Batlle, M.; Rizo-Liendo, A.; Piñero, J.E.; Bazzocchi, I.L.; Lorenzo-Morales, J.; Jiménez, I.A. Ursolic Acid Derivatives as Potential Agents Against Acanthamoeba spp. Pathogens 2019, 8, 130. [Google Scholar] [CrossRef]
- Dwia Pertiwi, Y.; Chikama, T.; Sueoka, K.; Ko, J.-A.; Kiuchi, Y.; Onodera, M.; Sakaguchi, T. Efficacy of Photodynamic Anti-Microbial Chemotherapy for Acanthamoeba Keratitisi in vivo. Lasers Surg. Med. 2021, 53, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Koike, N.; Ehara, T.; Hattori, T.; Narimatsu, A.; Kumakura, S.; Goto, H. Corticosteroid Eye Drop Instillation Aggravates the Development of Acanthamoeba Keratitis in Rabbit Corneas Inoculated with Acanthamoeba and Bacteria. Sci. Rep. 2019, 9, 12821. [Google Scholar] [CrossRef] [PubMed]
- Atalay, H.T.; Uysal, B.S.; Sarzhanov, F.; Usluca, S.; Yeşilırmak, N.; Özmen, M.C.; Erganiş, S.; Tefon, A.B.; Dogruman-Al, F.; Bilgihan, K. Rose Bengal-Mediated Photodynamic Antimicrobial Treatment of Acanthamoeba Keratitis. Curr. Eye Res. 2020, 45, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Taravaud, A.; Fechtali-Moute, Z.; Loiseau, P.M.; Pomel, S. Drugs Used for the Treatment of Cerebral and Disseminated Infections Caused by Free-living Amoebae. Clin. Transl. Sci. 2021, 14, 791–805. [Google Scholar] [CrossRef]
- Rosa, J.G.S.; Lopes-Ferreira, M.; Lima, C. An Overview towards Zebrafish Larvae as a Model for Ocular Diseases. Int. J. Mol. Sci. 2023, 24, 5387. [Google Scholar] [CrossRef]
- Schuster, F.L. Cultivation of Pathogenic and Opportunistic Free-Living Amoebas. Clin. Microbiol. Rev. 2002, 15, 342–354. [Google Scholar] [CrossRef]
- Zeouk, I.; Sifaoui, I.; Rizo-Liendo, A.; Arberas-Jiménez, I.; Reyes-Batlle, M.; Bazzocchi, I.L.; Bekhti, K.; Piñero, J.E.; Jiménez, I.A.; Lorenzo-Morales, J. Exploring the Anti-Infective Value of Inuloxin A Isolated from Inula Viscosa against the Brain-Eating Amoeba (Naegleria Fowleri) by Activation of Programmed Cell Death. ACS Chem. Neurosci. 2021, 12, 195–202. [Google Scholar] [CrossRef]
- Shing, B.; Singh, S.; Podust, L.M.; McKerrow, J.H.; Debnath, A. The Antifungal Drug Isavuconazole Is Both Amebicidal and Cysticidal against Acanthamoeba castellanii. Antimicrob. Agents Chemother. 2020, 64, e02223-19. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, J.N.; Nosanchuk, J.D.; Malliaris, S.D.; Casadevall, A. Interaction of Blastomyces Dermatitidis, Sporothrix Schenckii, and Histoplasma Capsulatum with Acanthamoeba castellanii. Infect. Immun. 2004, 72, 3478–3488. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.A.; Colon, B.L.; Chen, E.; Hull, M.V.; Kyle, D.E. Discovery of Repurposing Drug Candidates for the Treatment of Diseases Caused by Pathogenic Free-Living Amoebae. PLoS Negl. Trop. Dis. 2020, 14, e0008353. [Google Scholar] [CrossRef] [PubMed]
- Macías Saint-Gerons, D.; de la Fuente Honrubia, C.; de Andrés Trelles, F.; Catalá-López, F. Perspectiva Futura de La Farmacoepidemiología En La Era Del “Big Data” y La Expansión de Las Fuentes de Información. Rev. Esp. Salud Pública. 2016, 90, e20010. [Google Scholar]
- Mahboob, T.; Nawaz, M.; Tian-Chye, T.; Samudi, C.; Wiart, C.; Nissapatorn, V. Preparation of Poly (Dl-Lactide-Co-Glycolide) Nanoparticles Encapsulated with Periglaucine A and Betulinic Acid for in vitro Anti-Acanthamoeba and Cytotoxicity Activities. Pathogens 2018, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Vieyra, L.G.; la Fuente, I.D.; Mota, S.E.H.D.; Martínez, M.T.Z. Cromoforo fotoactivado con crosslinking como tratamiento para la queratitis por Acanthamoeba. Rev. Cuba. Oftalmol. 2020, 33, 1–8. [Google Scholar]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery Technologies for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Tao, T.; Liu, Y.; Zhang, J.; Huang, L.; Tao, Y. Dynamic Observation: Immune-Privileged Microenvironment Limited the Effectiveness of Immunotherapy in an Intraocular Metastasis Mouse Model. Ophthalmic Res. 2022, 65, 584–594. [Google Scholar] [CrossRef]
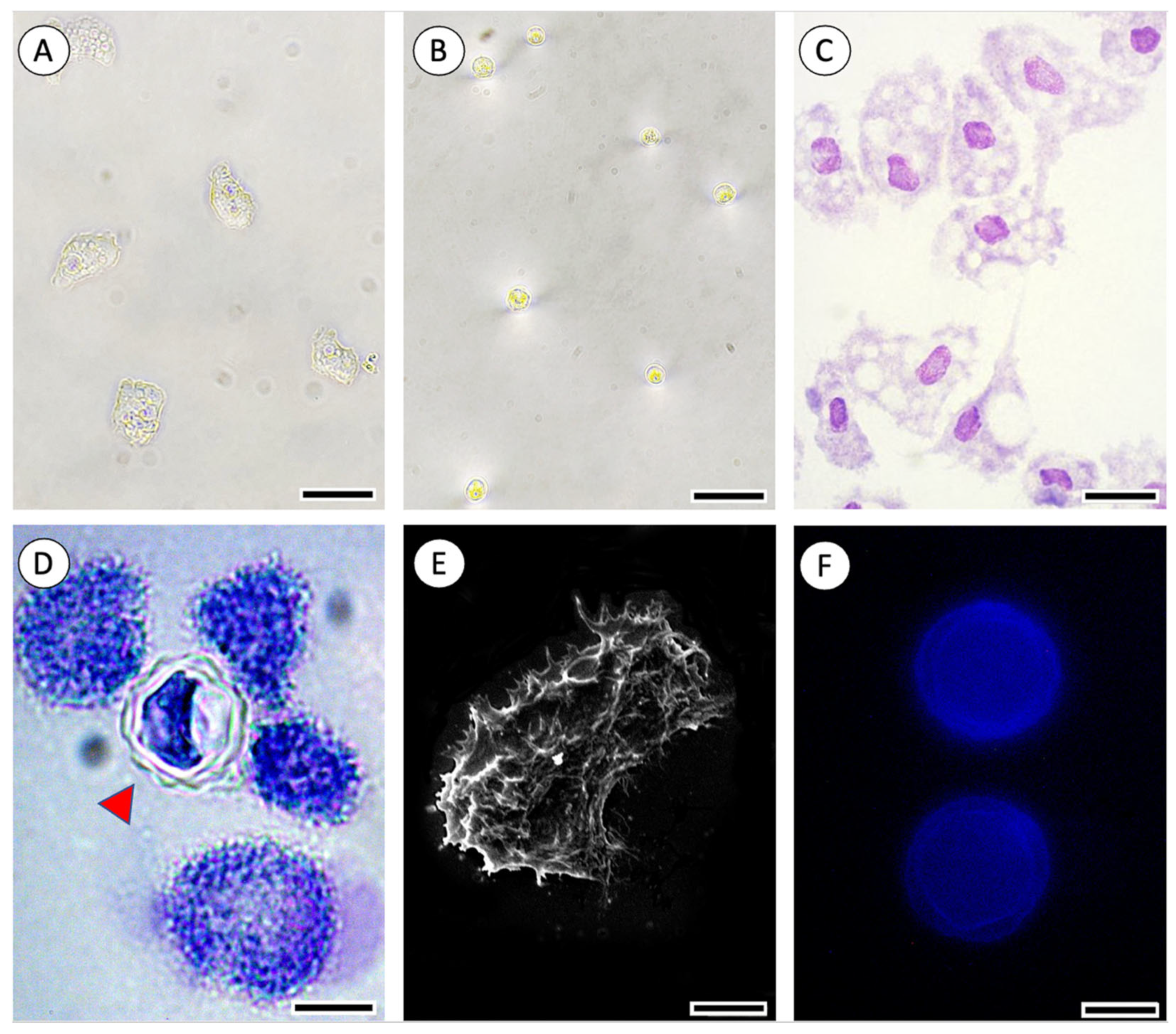
| Clinical Manifestations | Association Drugs for the Treatment |
|---|---|
| Keratitis | Polyhexamethylbiguanide + Propamidine Isethionate + Hexamidine + Antibiotics (Gatifloxacin or Ciprofloxacin) + Antifungal (Clotrimazole or Miconazole) + Miltefosine |
| Chlorhexidine + Propamidine Isethionate + Hexamidine + Antibiotics (Gatifloxacin or Ciprofloxacin) + Antifungal (Clotrimazole or Miconazole) + Miltefosine | |
| Skin ulcers | Miltefosine + Pentamidine + Antifungal (Fluconazole or Itraconazole) + Antibiotics (Azithromycin or Clarithromycin) |
| Amphotericin B + Miltefosine + Sulfamethoxazole + Trimethoprim + Sulfadiazine + Flucytosine | |
| Metronidazole + Paromomycin | |
| Mebendazole + Ivermectin + Praziquantel | |
| Tinidazole or Secnidazole + Nitazoxanide | |
| Granulomatous encephalitis | Miltefosine + Pentamidine + Antifungal (Fluconazole or Itraconazole) + Antibiotics (Azithromycin or Clarithromycin) |
| Amphotericin B + Miltefosine + Sulfamethoxazole + Trimethoprim + Sulfadiazine + Flucytosine | |
| Pneumonia | Antiparasitic (Secnidazole or Tinidazole or Metronidazole + Paramomycin + Nitazoxanide) + Antifungal (Fluconazole or Itraconazole) + Antibiotics (Azithromycin or Moxifloxacin or Levofloxacin or Amoxicillin) + Ceftriaxone |
| Drug of Choice | Mechanism of Action Known for Microrganisms | Descriptions and Effects on A. castellanii | Chemical Structure | Reference |
|---|---|---|---|---|
| Antibiotic | ||||
| Amoxicillin | Inhibits bacterial cell wall synthesis of Proteus mirabilis, Streptococcus pyogenes, and Neisseria gonorrhoeae | Not available |  | [19] |
| Azithromycin | Binds to the 50S ribosomal subunit, inhibiting essential protein synthesis of Legionella pneumophila, Chlamydia ssp., and Fusobacterium ssp. | Effective adjuvant to standard anti-A. castellanii chemotherapy | 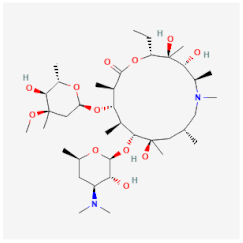 | [20] |
| Ceftriaxone | Inhibits bacterial cell wall synthesis of Escherichia coli, Klebsiella pneumoniae, and Streptococcus pneumoniae | Not available | 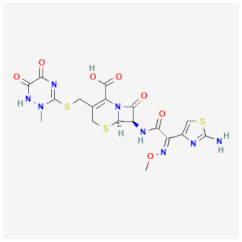 | [21] |
| Ciprofloxacin | Inhibits DNA gyrase and DNA topoisomerase, preventing transcription and replication of the genetic material of Staphylococcus aureus, Salmonella spp., and Campylobacter jejuni | Not available | 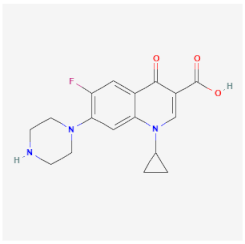 | [22] |
| Clarithromycin | Binds to the 50S ribosomal subunit, inhibiting the protein synthesis of S. pneumoniae, Haemophilus influenzae, and Helicobacter pylori | Not available | 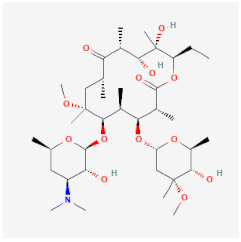 | [23] |
| Gatifloxacin | Inhibits DNA gyrase and DNA topoisomerase, preventing the transcription and replication of S. pneumoniae and Moraxella catarrhalis | Cysticidal capacity | 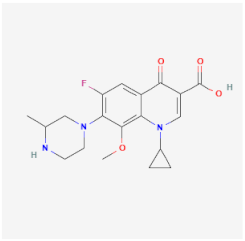 | [24] |
| Moxifloxacin | Acts on topoisomerase enzymes, preventing DNA replication and repair of Enterococcus faecalis, E. coli, and K. pneumoniae | Low effectiveness against granulomatous amoebic encephalitis | 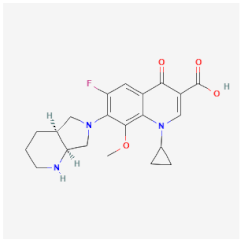 | [25] |
| Sulfadiazine | Inhibits the folic acid synthesis and metabolism of S. pneumoniae, S. aureus, and E. coli | Low effectiveness against cysts | 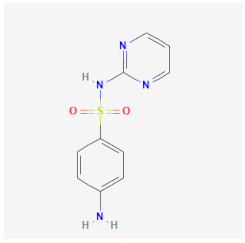 | [26] |
| Sulfamethoxazole-trimethoprim | Inhibits enzymes that are related to the folate biosynthesis of K. pneumoniae, Proteus spp., and Salmonella spp. | Cytotoxic activities against trophozoites and cysts | 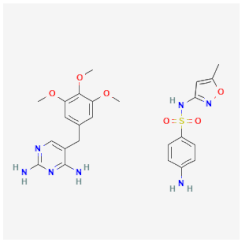 | [27] |
| Antiparasitic | ||||
| Ivermectin | Induces tonic paralysis of the musculature of parasites through the interference with the chloride channels of Sarcoptes scabiei, Pediculus humanus capitis, and Ascaris lumbricoides | Not available | 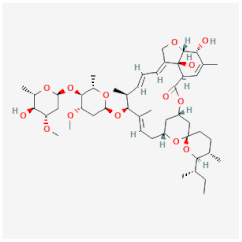 | [28] |
| Mebendazole | Interrupts cell replication, inhibiting microtubule synthesis and the glucose uptake cascade of Enterobius vermicularis, A. lumbricoides, and Ancylostoma duodenale | Not available | 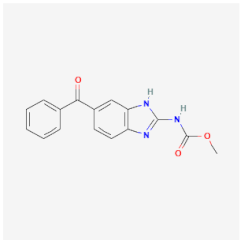 | [29] |
| Metronidazole | Inhibits DNA synthesis of Trichomonas vaginalis, E. histolytica, and Giardia lamblia | Low effectiveness against A. castellanii mainly in trophozoites | 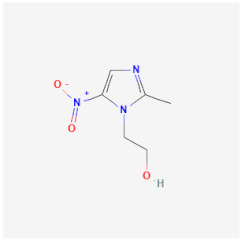 | [30] |
| Miltefosine | Inhibits the synthesis of important glycoproteins in the cell membrane of Trypanosoma brucei and Leishmania spp. | Induces apoptosis-like cell death, inhibiting proteinase kinase B in trophozoites and cysts | [31] | |
| Nitazoxanide | Deprives the parasite of its energy production, inhibiting the enzyme pyruvate ferredoxin oxidoreductase of G. lamblia and Cryptosporidium parvum | Affects cellular differentiation processes, extracellular proteases, and the viability of trophozoites and cysts under microaerophilic conditions | 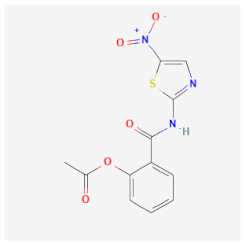 | [32] |
| Paromomycin | Binds to the 16S RNA portion of the ribosome, preventing protein synthesis of E. histolytica and G. lamblia | Cytotoxic activities against trophozoites and cysts | 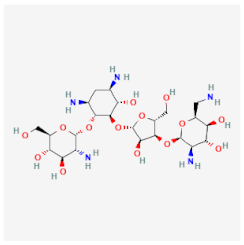 | [33] |
| Praziquantel | Inhibits the Na+ and K+ pumps of schistosomes, increasing the permeability of the cell membrane and causing the weakening of the integument through the contraction and hardening of the integument | Not available |  | [34] |
| Propamidine isethionate | Inhibits metabolic pathways or the synthesis of DNA/RNA linked to metabolic pathways of E. histolytica | Cytotoxic activities against trophozoites and cysts |  | [35] |
| Secnidazole | Interferes with the flow gradient of the cell membrane, altering the ability to absorb some nutrients of E. histolytica and T. vaginalis | Crosses the plasma membrane, interacting with cytoplasmic molecules of amoeba |  | [36] |
| Tinidazole | Interacts with DNA by destroying its chain or inhibiting DNA synthesis of T. vaginalis, E. histolytica, and G. lamblia | Not available | 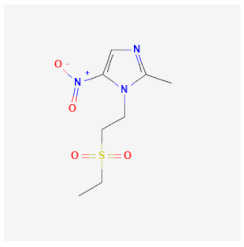 | [37] |
| Antiseptic and germicidal | ||||
| Chlorhexidine | Cationic molecules of chlorhexidine react with cell wall-negative molecules of Streptococcus mutans | Cytotoxic activities against trophozoites and cysts |  | [38] |
| Polyhexamethylene biguanide | Molecules of this germicide interact with the cell membrane causing pores and increasing the membrane permeability of Candida spp. | Impairs membrane integrity of trophozoites and cysts | 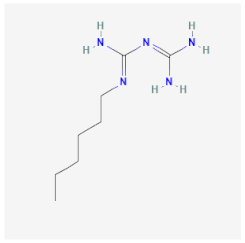 | [39] |
| Antifungal | ||||
| Clotrimazole | Damages and destroys the cell membrane, inhibiting ergosterol synthesis of Candida spp. and Malassezia spp. | Amoebicidal effects are mainly in trophozoites | 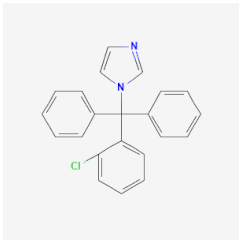 | [40] |
| Fluconazole | Inhibits ergosterol biosynthesis, damaging the cell membrane of Candida albicans and Cryptococcus neoformans | Not available | 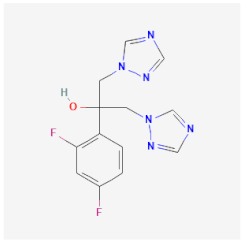 | [41] |
| Flucytosine | Inhibits DNA synthesis and interferes with the RNA transcription of C. neoformans, Fusarium spp., and Aspergillus spp. | Not available | 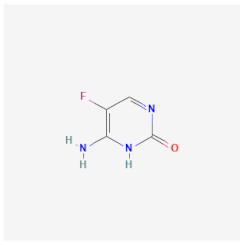 | [42] |
| Itraconazole | Damages the membrane of Blastomyces dermatitidis, C. neoformans, and Histoplasma capsulatum | Cytotoxic activities against trophozoites and cysts | 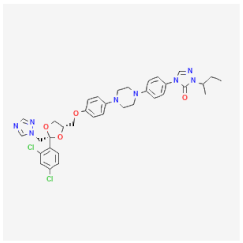 | [33] |
| Miconazole | Damages and destroys the cell membrane, inhibiting the ergosterol synthesis of Candida spp. and Malassezia spp. | Cytotoxic activities against trophozoites and cysts | 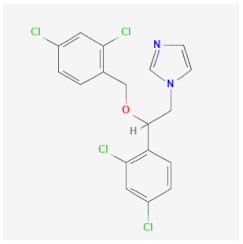 | [43] |
| Compound | Isolate/Stage | Assays | Cell Model for Infection | IC50 | Mechanism of Action and Observation | Reference |
|---|---|---|---|---|---|---|
| Alkyl-carbon, alkylphosphocholines (APCs), and quaternary ammonium compounds (QACs) | Trophozoites and cysts of A. castellanii (ATCC 50370) | The alamar blue assay and count by hemocytometer | Not used | Octadecyltrimethyl ammonium bromide had the best IC50 17.58 µM against trophozoites and 38.21 µM against cysts | Induces membrane permeabilization | [44] |
| Amphotericin B and fluconazole conjugated to silver particles | Trophozoites of A. castellanii (ATCC 50492) | Count by hemocytometer | HeLa cells | Amp-AgNPs and Nys-AgNPs damaged 80% of trophozoites at 10 μM. The compounds had low cytotoxicity | Mechanism of action was not evaluated | [45] |
| Anisomycin, prodigiosin, and obatoclax | Trophozoites of A. castellanii (ATCC 50370) | Count by hemocytometer | Fibroblast cells (hFF-1) | Prodigiosin and obatoclax had IC50 2.2 μM and 0.5 μM against trophozoites, respectively. The compounds had low cytotoxicity against hFF-1 | Mechanism of action was not evaluated | [46] |
| Artemisia argyi | Trophozoites and cysts of A. castellanii (ATCC30010) | Count by hemocytometer | Human bronchial epithelial cells (ATCC CRL-9609) | The extract had IC50 37.4 μg/mL against trophozoites and IC50 74.8 μg/mL against cysts. The extract had high cytotoxic against human bronchial epithelial cells | Mechanism of action was not evaluated | [47] |
| Atorvastatin and commercial eye drops | Trophozoites of A. castellanii (ATCC 30010) | The alamar blue assay | Murine macrophages (ATCC J774-A1) | The best association was atorvastatin (82.4%) plus diclofenaco-lepori (17.6%), which inhibited 100% trophozoite proliferation. The compounds had low cytotoxicity murine macrophages | Mechanism of action was not evaluated | [48] |
| Auranofin | Trophozoites of A. castellanii (ATCC 30010 and JEA19) | Count by hemocytometer | Not used | A. castellanii strains were susceptible to auranofin and had IC50 2.97 μM–3.48 μM against trophozoites | Blocked thioredoxin reductases TrxR within the redox-active domain and disrupted the homeostasis of this system, leading to cellular oxidative stress and intrinsic apoptosis | [49] |
| Benzothiazole | Trophozoites and cysts of A. castellanii (ATCC 30010) | Count by hemocytometer | Fibroblast cell line (ATCC WI-38) | Benzothiazole had IC50 0.02% and damaged 100% trophozoites at 0.04% against trophozoites and cysts. Benzothiazole had low cytotoxicity against human fibroblast cells | Mechanism of action was not evaluated | [5] |
| Betulinic acid and botulin | Trophozoites and cysts of A. castellanii (ATCC 50492) | Count by hemocytometer | HeLa cell | Betulinic acid and botulin damaged 65% of trophozoites and 57% of cysts. Betulinic acid and botulin had low cytotoxicity against HeLa cell | Blocked the amoeba binding to human cells | [50] |
| Camellia sinesis | Trophozoites of A. castellanii (ATCC 30010) | Count by hemocytometer | Murine macrophages (ATCC J774A.1) and HCE | C. sinesis extract was tested against trophozoites in 25%, 50%, 75%, and 100% concentrations. C. sinesis extract damaged 100% of trophozoites in a concentration of 75%. C. sinesis extract had low cytotoxicity against HCE | Mechanism of action was not evaluated | [51] |
| Ammoides pusilla | Trophozoites of A. castellanii (ATCC 30010) | The alamar blue assay | Not used | Leaves and flower essential oil had IC50 5.32 μg/mL and aerial parts had IC50 97.18 μg/mL against trophozoites | Mechanism of action was not evaluated | [52] |
| Thymbra spicata | Trophozoites and cysts of A. castellanii | Count by hemocytometer | Not used | T. spicata extract damaged 100% of trophozoites at 16.0 μg/mL. The extract was not effective against cysts | Mechanism of action was not evaluated | [53] |
| Laurencia johnstonii | Trophozoites of A. castellanii (ATCC 30010) | The alamar blue assay | Murine macrophages (ATCC J774A.1) | 3α-bromojohnstane extract had IC50 41.51 μM against trophozoites. Extract had low cytotoxicity against murine macrophages | Mechanism of action was not evaluated | [54] |
| Delphinium gracile, D. staphisagria, Consolida oliveriana, and Aconitum napellus | Trophozoites and cysts of A. castellanii (ATCC 30010) | The alamar blue assay | Vero cells (ATCC CCL-81) | Four flavonoids were effective against trophozoites and had IC50 3.5, 1.4, 1.4, and 2.3 μM and inhibited excystation. Flavonoids had low cytotoxicity against Vero cells | Mechanism of action was not evaluated | [55] |
| Propolis flavonoids | Trophozoites of A. castellanii (ATCC30010 and ATCC50739) | Count by hemocytometer | Vero cell (ATCC 84113001) | The best minimum inhibitory concentrations of the most active propolis extract against trophozoites were 62.5 μg/mL. Flavonoids had low cytotoxicity against Vero cells | Mechanism of action was not evaluated | [56] |
| Glimepiride, vildagliptin, and repaglinide coated in nanoparticles | Trophozoites and cysts of A. castellanii (ATCC 50492) | Count by hemocytometer | HeLa cells | Vildagliptin coated in silver nanoparticles damaged 80% of trophozoites at 5 μM and inhibited encystation and excystation. Vildagliptin coated in silver nanoparticles had low cytotoxicity against Vero cells | Mechanism of action was not evaluated | [57] |
| Histone deacetylase inhibitors | Trophozoites and cysts of A. castellanii (ATCC 30868) | Count by hemocytometer | HCE cells | FFK29 and MPK576 against trophozoites had IC50 4.8 and 4.7 μM, respectively, and inhibited trophozoite proliferation. Histone deacetylase inhibitors had low cytotoxicity against HCE | Mechanism of action was not evaluated | [58,59] |
| Irosustat and STX140 in nanoformulations | Trophozoites and cysts of A. castellanii (ATCC 50492) | Count by hemocytometer | HeLa cells | Irosustat NP and STX140 NP at 100 μM damaged 20% of trophozoites and inhibited excystation. Nanoformulations had low cytotoxicity against HeLa cells | Mechanism of action was not evaluated | [60] |
| Metformin-coated silver | Trophozoites and cysts of A. castellanii (ATCC 50492) | Count by hemocytometer | HeLa cells | Metformin-coated silver damaged 26.67% of trophozoite proliferation at 10 µM and inhibited encystation and excystation. Metformin-coated silver decreased A. castellanii cytotoxic against HeLa cells | Mechanism of action was not evaluated | [61] |
| Methyltrioctylammonium chloride-based deep eutectic solvents | Trophozoites and cysts of A. castellanii (ATCC 50492) | Count by hemocytometer | HeLa cells | DES-E had the best amoebicidal activity, damaged 85% of trophozoites at 10 µM, and inhibited encystation and excystation | Mechanism of action was not evaluated | [62] |
| Polyaniline-based molybdenum disulfide nanoparticles | Trophozoites and cysts of A. castellanii (ATCC 50492) | Count by hemocytometer | HeLa cells and primary human corneal epithelial cells | PANI/MoS2 had IC50 100 μg/mL against trophozoites and cysts. PANI/MoS2 had low cytotoxicity against HeLa cells and primary human corneal epithelial cells | Mechanism of action was not evaluated | [63] |
| Squaramides and acyclic polyamine derivatives | Trophozoites and cyst of A. castellanii (ATCC 30010) | The alamar blue assay | Vero cells (ATCC CCL-81) | Squaramides and acyclic polyamine had IC50 3.5 µM and 26.7 µM, respectively, and had complete cysticidal activity at 100 µM and 200 µM | Mechanism of action was not evaluated | [64] |
| Synthetic acridine-9(10H)-1 | Trophozoites and cysts of A. castellanii (ATCC 50492) | Count by hemocytometer | Human keratinocyte cells (HaCaT cells) | Synthetic acridine-9(10H)-1 VII had IC50 53.46 μg/mL against trophozoites and inhibited excystation | Interacts with the catalytic residues and causes morphological alterations | [65] |
| Tannic acid-modified silver nanoparticles | Trophozoites and cysts of A. castellanii (P19) | The alamar blue assay | Not used | Tannic acid-modified silver nanoparticles had IC50 14 parts per million (ppm). Tannic acid-modified silver nanoparticles had low cytotoxicity | Causes a disturbance in in the plasmatic membrane, mitochondria, and nucleus | [66] |
| Algerian Limonium oleifolium Mill | Trophozoites of A. castellani strain (ATCC 30010) | The alamar blue assay | Murine macrophage (ATCC J774) | The essential oil had IC50 7.48 μg/mL against trophozoites. The essential oil had low cytotoxicity against murine macrophages | Mechanism of action was not evaluated | [67] |
| Ursolic acid derivatives | Trophozoites and cysts of A. castellanii Neff (ATCC 30010) | The alamar blue assay | Murine macrophage (ATCC J774.A1) | The two ursolic acids derivated had IC50 22.7 µM and 21.4 µM against trophozoites and IC50 18 µM and 17 µM against cysts. Ursolic acid had low cytotoxicity against murine macrophages | Mechanism of action not elucidated | [68] |
| Compound | Isolate/Stage | Model Animal | Methods/Dosage/Route/Time | Results and Observations | Reference |
|---|---|---|---|---|---|
| Cationic chlorin derivative photosensitizer (TONS504) mediated photodynamic antimicrobial chemotherapy | Trophozoites of A. castellanii (ATCC 30868) | Male Japanese white rabbits | TONS504 was administered as eye drops at 1 mg/mL, followed by light-emitting diode irradiation after 7 days of the establishment of keratitis | 58% of the rabbits recovered completely after the treatment, showing that drugs with photodynamic treatment are a good therapy | [69] |
| Corticosteroid eye drop | Trophozoites of A. castellanii (ATCC 50492) | Male Japanese white rabbits | A 26 G needle attached to a microliter syringe was advanced to the center of the cornea, and 30 μL of a suspension containing 1 × 105 trophozoites cells/mL was inoculated in the right eye, and betamethasone sodium phosphate (BSP) eye drops were administered for 5 or 7 days | Topical corticosteroids have the potential to aggravate keratitis when the cornea is infected by A. castellanii | [70] |
| Rose bengal (RB)-mediated photodynamic antimicrobial | Trophozoites of A. castellanii | Male New Zealand white rabbits | Rabbits were divided equally into three groups: group control without treatment and animals treated topically with two concentrations of Rose bengal (0.1% and 0.2%) associated with photodynamic treatment (+518 nm irradiation) for 5 days | RB-mediated photodynamic antimicrobial is effective in decreasing the parasitic load and clinical severity of keratitis, although this study did not perform a control with the drug of choice chlorhexidine; an association of the drug with photodynamic treatment shows good results in ocular lesions | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geres, L.F.; Sartori, E.; Neves, J.M.d.S.; Miguel, D.C.; Giorgio, S. Amebicides against Acanthamoeba castellanii: The Impact of Organism Models Used in Amebicide Assays. Parasitologia 2024, 4, 15-37. https://doi.org/10.3390/parasitologia4010002
Geres LF, Sartori E, Neves JMdS, Miguel DC, Giorgio S. Amebicides against Acanthamoeba castellanii: The Impact of Organism Models Used in Amebicide Assays. Parasitologia. 2024; 4(1):15-37. https://doi.org/10.3390/parasitologia4010002
Chicago/Turabian StyleGeres, Leonardo Fernandes, Elena Sartori, João Marcos dos Santos Neves, Danilo Ciccone Miguel, and Selma Giorgio. 2024. "Amebicides against Acanthamoeba castellanii: The Impact of Organism Models Used in Amebicide Assays" Parasitologia 4, no. 1: 15-37. https://doi.org/10.3390/parasitologia4010002
APA StyleGeres, L. F., Sartori, E., Neves, J. M. d. S., Miguel, D. C., & Giorgio, S. (2024). Amebicides against Acanthamoeba castellanii: The Impact of Organism Models Used in Amebicide Assays. Parasitologia, 4(1), 15-37. https://doi.org/10.3390/parasitologia4010002







