Prevalence and Seasonal Variation of Trichuris Worms Infection in Mastomys natalensis in Morogoro and Iringa Regions, Tanzania
Abstract
:1. Introduction
2. Materials and Methods
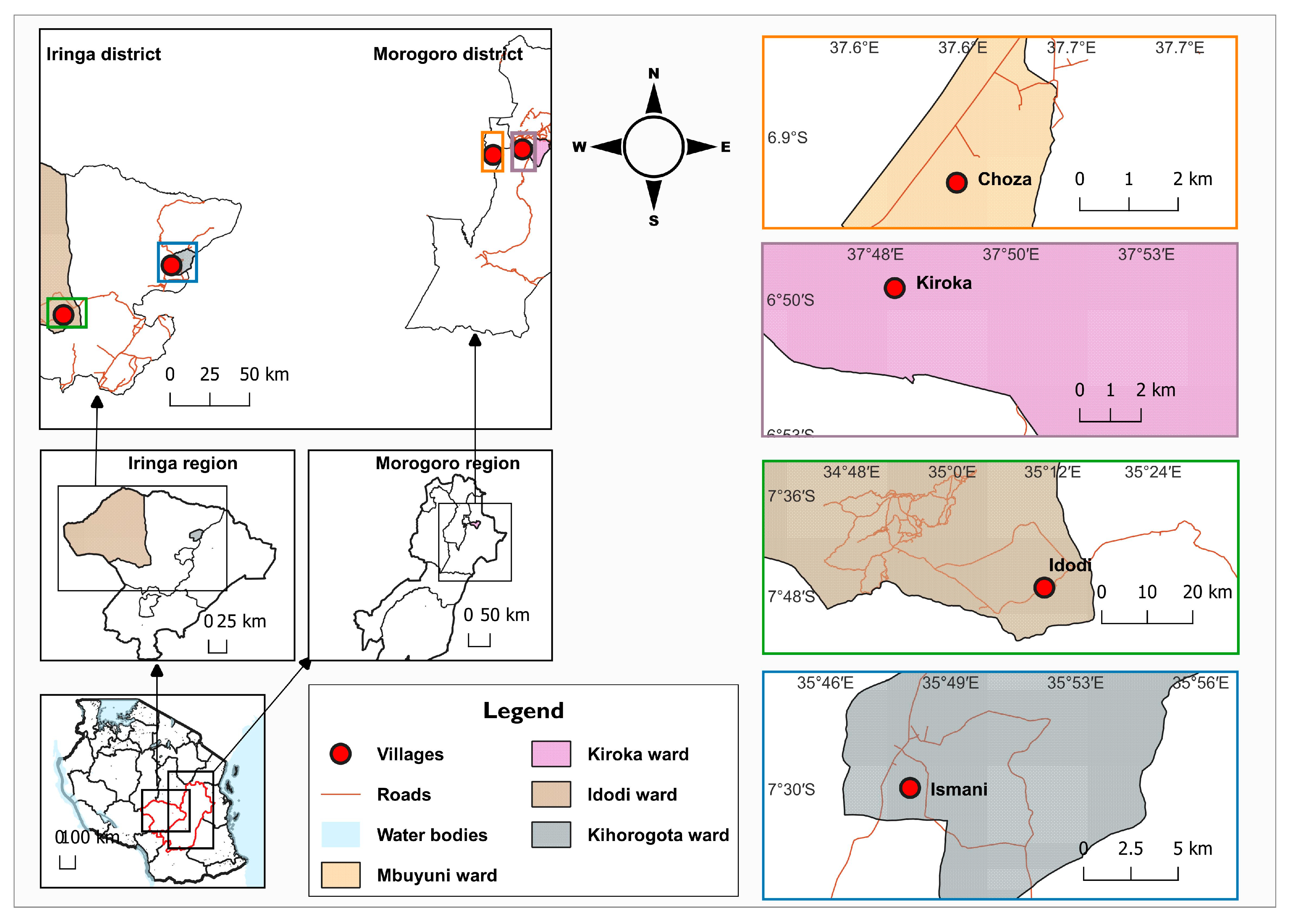
2.1. Study Sites and Design
2.2. Climatic Conditions of Morogoro and Iringa
2.3. Trapping of Rodents
2.4. Animal Processing and Parasitological Screening for Trichuris Worms
2.5. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribas, A.; López, S.; Makundi, R.H.; Leirs, H.; de Bellocq, J.G. Trichuris spp.(Nematoda: Trichuridae) from two rodents, Mastomys natalensis and Gerbilliscus vicinus in Tanzania. J. Parasitol. 2013, 99, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Wang, X.L.; Wang, X.L.; Wang, S.; An, C.L. Trichuriasis diagnosed by colonoscopy: Case report and review of the literature spanning 22 years in mainland China. Int. J. Infect. Dis. 2013, 17, e1073–e1075. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.R. Trichuris spp. in animals, with specific reference to neo-tropical rodents. Vet. Sci. 2021, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Gul, N.; Tak, H. Prevalence of Trichuris spp. in small ruminants slaughtered in Srinagar District (J&K). J. Parasit. Dis. 2016, 40, 741–744. [Google Scholar] [PubMed]
- Xie, Y.; Zhao, B.; Hoberg, E.P.; Li, M.; Zhou, X.; Gu, X.; Lai, W.; Peng, X.; Yang, G. Genetic characterisation and phylogenetic status of whipworms (Trichuris spp.) from captive non-human primates in China, determined by nuclear and mitochondrial sequencing. Parasit. Vectors 2018, 11, 516. [Google Scholar] [CrossRef]
- Rinderknecht, A.; Blanco, R.E. The largest fossil rodent. Proc. R. Soc. B Biol. Sci. 2008, 275, 923–928. [Google Scholar] [CrossRef]
- Mlyashimbi, E.C.; Mariën, J.; Kimaro, D.N.; Tarimo, A.J.; Machang’u, R.S.; Makundi, R.H.; Isabirye, M.; Massawe, A.W.; Leirs, H.; Mdangi, M.E.; et al. Home ranges, sex ratio and recruitment of the multimammate rat (Mastomys natalensis) in semi-arid areas in Tanzania. Mammalia 2020, 84, 336–343. [Google Scholar] [CrossRef]
- Makundi, R.H.; Massawe, A.W.; Mulungu, L.S. Reproduction and population dynamics of Mastomys natalensis Smith, 1834 in an agricultural landscape in the Western Usambara Mountains, Tanzania. Integr. Zool. 2007, 2, 233–238. [Google Scholar] [CrossRef]
- Mariën, J.; Vanden Broecke, B.; Tafompa, P.J.; Bernaerts, L.; Ribas, A.; Mnyone, L.L.; Mulugu, L.S.; Leirs, H. Host related factors determine co-occurrence patterns between pathogenic bacteria, protozoa, and helminths in populations of the multimammate mouse, Mastomys natalensis. bioRxiv 2022. [Google Scholar] [CrossRef]
- Mlyashimbi, E.C.; Mariën, J.; Kimaro, D.N.; Tarimo, A.J.; Isabirye, M.; Makundi, R.H.; Massawe, A.W.; Mdangi, M.E.; Kifumba, D.; Nakiyemba, A.; et al. Relationships between seasonal changes in diet of Multimammate rat (Mastomys natalensis) and its breeding patterns in semi-arid areas in Tanzania. Cogent Food Agric. 2018, 4, 1507509. [Google Scholar] [CrossRef]
- Mehraj, V.; Hatcher, J.; Akhtar, S.; Rafique, G.; Beg, M.A. Prevalence and factors associated with intestinal parasitic infection among children in an urban slum of Karachi. PLoS ONE 2008, 3, e3680. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Aslam, N.; Zainab, R.; Aziz-Ur-Rehman; Rasool, G.; Ullah, M.I.; Daniyal, M.; Akram, M. Prevalence, risk factors, challenges, and the currently available diagnostic tools for the determination of helminths infections in human. Eur. J. Inflamm. 2020, 18. [Google Scholar] [CrossRef]
- Chen, H.; Mozzicafreddo, M.; Pierella, E.; Carletti, V.; Piersanti, A.; Ali, S.M.; Ame, S.M.; Wang, C.; Miceli, C. Dissection of the gut microbiota in mothers and children with chronic Trichuris trichiura infection in Pemba Island, Tanzania. Parasit. Vectors 2021, 14, 62. [Google Scholar] [CrossRef]
- Vanden Broecke, B.; Bernaerts, L.; Ribas, A.; Sluydts, V.; Mnyone, L.; Matthysen, E.; Leirs, H. Linking behavior, co-infection patterns, and viral infection risk with the whole gastrointestinal helminth community structure in Mastomys natalensis. Front. Vet. Sci. 2021, 8, 669058. [Google Scholar] [CrossRef] [PubMed]
- Kanojiya, D.; Shanker, D.; Sudan, V.; Jaiswal, A.K. Prevalence and seasonal variation of trichurid worm infections of small ruminants of Mathura, India. J. Parasit. Dis. 2016, 40, 199–202. [Google Scholar] [CrossRef]
- Van Aelst, K.; Holvoet, N. Climate change adaptation in the Morogoro Region of Tanzania: Women’s decision-making participation in small-scale farm households. Clim. Dev. 2018, 10, 495–508. [Google Scholar] [CrossRef]
- Mbululo, Y.; Nyihirani, F. Climate Characteristics over Southern Highlands Tanzania. Atmos. Clim. Sci. 2012, 2, 454–463. [Google Scholar] [CrossRef]
- Kassian, L.M.; Tenywa, M.; Liwenga, E.T.; Dyer, K.W.; Bamutaze, Y. Implication of climate change and variability on stream flow in Iringa region, Tanzania. J. Water Clim. Change 2017, 8, 336–347. [Google Scholar] [CrossRef]
- Claus, A.T.; Abdul, A.S.; Rhodes, H.M.; Robert, S.; Stella, T.K. Prevalence of Rickettsia typhi in rodent fleas from areas with and without previous history of plague in Mbulu district, Tanzania. Afr. J. Microbiol. Res. 2020, 14, 65–70. [Google Scholar] [CrossRef]
- Aplin, K.P.; Brown, P.R.; Jacob, J.; Krebs, C.J.; Singleton, G.R. Field Methods for Rodent Studies in Asia and the Indo-Pacific; Australian Centre for International Agricultural: Canberra, Austrialia, 2003. [Google Scholar]
- Tun, S.; Ithoi, I.; Mahmud, R.; Samsudin, N.I.; Kek Heng, C.; Ling, L.Y. Detection of helminth eggs and identification of hookworm species in stray cats, dogs and soil from Klang Valley, Malaysia. PLoS ONE 2015, 10, e0142231. [Google Scholar] [CrossRef]
- Moravec, F. Review of Keys to the Nematode Parasites of Vertebrates. Parasit. Vectors 2009, 2, 42. [Google Scholar] [CrossRef]
- Hansen, J.; Perry, B.D. The Epidemiology, Diagnosis and Control of Gastro-Intestinal Parasites of Ruminants in Africa: A Handbook; ILRI (aka ILCA and ILRAD): Nairobi, Kenya, 1990. [Google Scholar]
- Ghai, R.R.; Simons, N.D.; Chapman, C.A.; Omeja, P.A.; Davies, T.J.; Ting, N.; Goldberg, T.L. Hidden population structure and cross-species transmission of whipworms (Trichuris sp.) in humans and non-human primates in Uganda. PLoS Neglected Trop. Dis. 2014, 8, e3256. [Google Scholar] [CrossRef]
- Haydée Sardella, N.; Horacio Fugassa, M. Parasites in rodent coprolites from the historical archaeological site Alero Mazquiarán, Chubut Province, Argentina. Memórias Inst. Oswaldo Cruz 2009, 104, 37–42. [Google Scholar] [CrossRef]
- Jaran, A.S. Prevalence and seasonal variation of human intestinal parasites in patients attending hospital with abdominal symptoms in northern Jordan. EMHJ-East. Mediterr. Health J. 2016, 22, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Kataranovski, M.; Mirkov, I.; Belij, S.; Popov, A.; Petrović, Z.; Gačić, Z.; Kataranovski, D. Intestinal helminths infection of rats (Ratus norvegicus) in the Belgrade area (Serbia): The effect of sex, age and habitat. Parasite J. Société Française Parasitol. 2011, 18, 189. [Google Scholar] [CrossRef]
- Malsawmtluangi, C.; Tandon, V. Helminth parasite spectrum in rodent hosts from bamboo growing areas of Mizoram, North-east India. J. Parasit. Dis. 2009, 33, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Yevstafieva, V.A.; Kravchenko, S.O.; Gutyj, B.V.; Melnychuk, V.V.; Kovalenko, P.N.; Volovyk, L.B. Morphobiological analysis of Trichuris vulpis (Nematoda, Trichuridae), obtained from domestic dogs. Regul. Mech. Biosyst. 2019, 10, 165–176. [Google Scholar] [CrossRef]
- Pakdel, N.; Naem, S.; Rezaei, F.; Chalehchaleh, A.A. A survey on helminthic infection in mice (Mus musculus) and rats (Rattus norvegicus and Rattus rattus) in Kermanshah, Iran. In Veterinary Research Forum; Faculty of Veterinary Medicine, Urmia University: Urmia, Iran, 2013; Volume 4, p. 105. [Google Scholar]
- Mafiana, C.F.; Osho, M.B.; Sam-Wobo, S. Gastrointestinal helminth parasites of the black rat (Rattus rattus) in Abeokuta, southwest Nigeria. J. Helminthol. 1997, 71, 217–220. [Google Scholar] [CrossRef]

| Rainy Season | Dry Season | |||||
|---|---|---|---|---|---|---|
| Helminths Detected | Total Prevalence (n = 100) | Male | Female | Total Prevalence (n = 100) | Male | Female |
| Trichuris spp. | 22% | 12% | 10% | 30% | 16% | 14% |
| Anoplocephalid species. * | 10% | 4% | 6% | 22% | 15% | 7% |
| Strongyloides spp. | 0% | 0% | 0% | 50% | 22% | 28% |
| Capillaria spp. | 0% | 0% | 0% | 14% | 6% | 8% |
| Hymenolepis spp. | 0% | 0% | 0% | 55% | 27% | 28% |
| Rainy Season | Dry Season | |||||
|---|---|---|---|---|---|---|
| Helminths Detected | Total Prevalence (n = 100) | Male | Female | Total Prevalence (n = 100) | Male | Female |
| Trichuris spp. | 80% | 44% | 36% | 50% | 20% | 30% |
| Anoplocephalid species. * | 20% | 12% | 8% | 28% | 15% | 13% |
| Strongyloides spp. | 90% | 56% | 34% | 50% | 39% | 21% |
| Capillaria spp. | 0% | 0% | 0% | 16% | 6% | 10% |
| Hymenolepis spp. | 0% | 0% | 0% | 6% | 2% | 4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, C.; Msoffe, V.; Van Houtte, N.; Mhamphi, G.; Mariën, J.; Sabuni, C.; Makundi, I.; Nzalawahe, J.; Machang’u, R.; Leirs, H. Prevalence and Seasonal Variation of Trichuris Worms Infection in Mastomys natalensis in Morogoro and Iringa Regions, Tanzania. Parasitologia 2023, 3, 293-299. https://doi.org/10.3390/parasitologia3030030
Thomas C, Msoffe V, Van Houtte N, Mhamphi G, Mariën J, Sabuni C, Makundi I, Nzalawahe J, Machang’u R, Leirs H. Prevalence and Seasonal Variation of Trichuris Worms Infection in Mastomys natalensis in Morogoro and Iringa Regions, Tanzania. Parasitologia. 2023; 3(3):293-299. https://doi.org/10.3390/parasitologia3030030
Chicago/Turabian StyleThomas, Claus, Venance Msoffe, Natalie Van Houtte, Ginethon Mhamphi, Joachim Mariën, Christopher Sabuni, Isaac Makundi, Jahashi Nzalawahe, Robert Machang’u, and Herwig Leirs. 2023. "Prevalence and Seasonal Variation of Trichuris Worms Infection in Mastomys natalensis in Morogoro and Iringa Regions, Tanzania" Parasitologia 3, no. 3: 293-299. https://doi.org/10.3390/parasitologia3030030
APA StyleThomas, C., Msoffe, V., Van Houtte, N., Mhamphi, G., Mariën, J., Sabuni, C., Makundi, I., Nzalawahe, J., Machang’u, R., & Leirs, H. (2023). Prevalence and Seasonal Variation of Trichuris Worms Infection in Mastomys natalensis in Morogoro and Iringa Regions, Tanzania. Parasitologia, 3(3), 293-299. https://doi.org/10.3390/parasitologia3030030








