Dermacentor variabilis Does Not Transstadially Transmit the U.S. Isolate of Theileria orientalis Ikeda: A Controlled Acquisition and Transmission Study
Abstract
:1. Introduction
2. Results
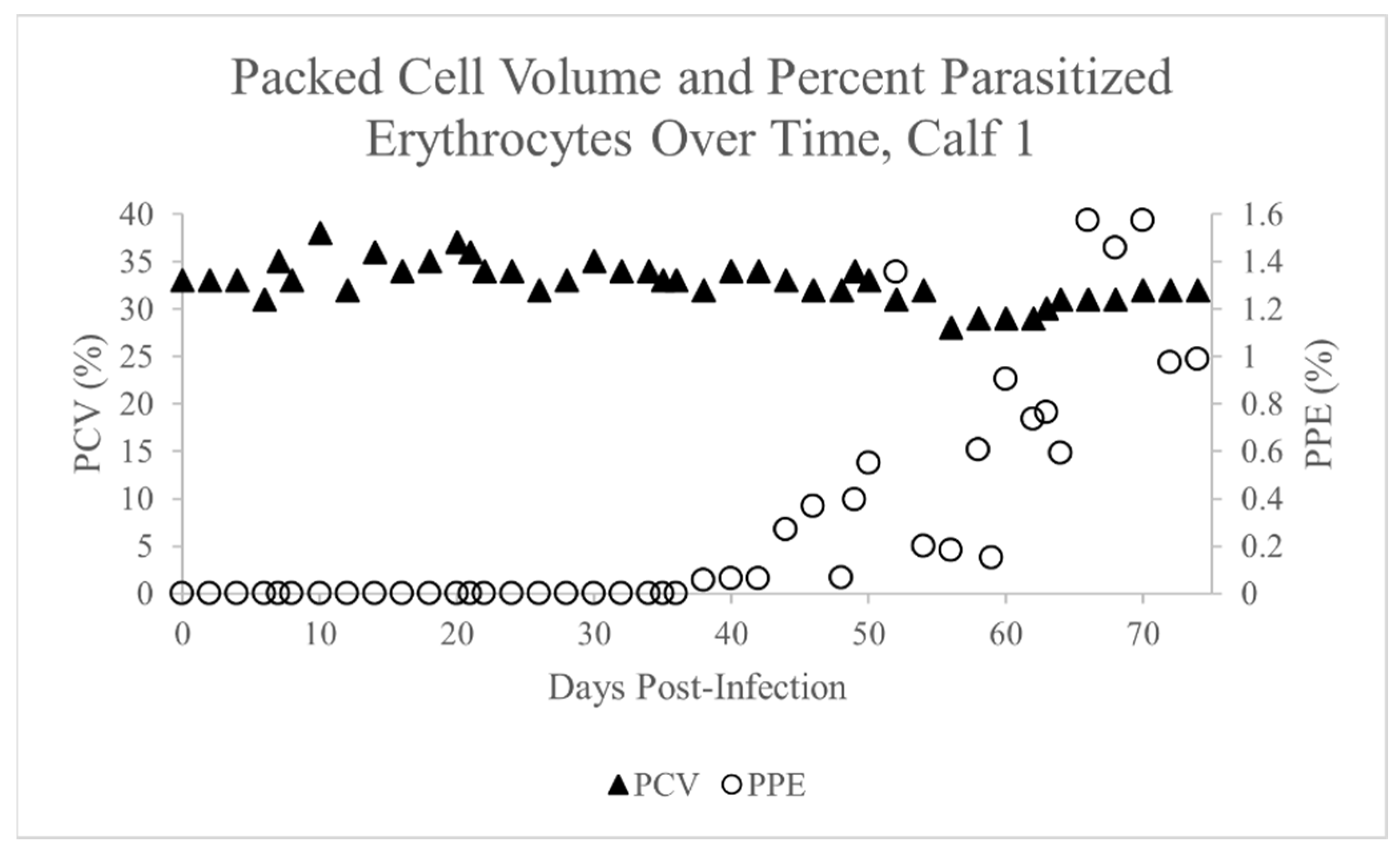
2.1. Theileria Orientalis Infection of Calf 1
2.2. Dermacentor variabilis Acquisition Feed on Calf 1
2.3. Failure of Adult D. variabilis Ticks to Acquire and Transmit T. orientalis to Calves 2 and 3
3. Discussion
4. Materials and Methods
4.1. Cattle
4.2. Infection of Calf 1 and Tick Acquisition Feeding
4.3. Tick Transmission Feeding on Calves 2 and 3
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watts, J.G.; Playford, M.C.; Hickey, K.L. Theileria orientalis: A review. N. Z. Vet. J. 2016, 64, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, T.; Hayashida, K.; Sugimoto, C.; Yokoyama, N. Evolution and genetic diversity of Theileria. Infect. Genet. Evol. 2014, 27, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Hara, Y.; Abe, T.; Yamasaki, C.; Toyoda, A.; Kosuge, T.; Suzuki, Y.; Sato, Y.; Kawashima, S.; Katayama, T.; et al. Comparative genome analysis of three eukaryotic parasites with differing abilities to transform leukocytes reveals key mediators of Theileria-induced leukocyte transformation. mBio 2012, 3, e00204-12. [Google Scholar] [CrossRef]
- Oakes, V.J.; Yabsley, M.J.; Schwartz, D.; LeRoith, T.; Bissett, C.; Broaddus, C.; Schlater, J.L.; Todd, S.M.; Boes, K.M.; Brookhart, M.; et al. Theileria orientalis Ikeda Genotype in Cattle, Virginia, USA. Emerg. Infect. Dis. 2019, 25, 1653–1659. [Google Scholar] [CrossRef]
- Kakuda, T.; Shiki, M.; Kubota, S.; Sugimoto, C.; Brown, W.C.; Kosum, C.; Nopporn, S.; Onuma, M. Phylogeny of benign Theileria species from cattle in Thailand, China and the U.S.A. based on the major piroplasm surface protein and small subunit ribosomal RNA genes. Int. J. Parasitol. 1998, 28, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Kamau, J.; de Vos, A.J.; Playford, M.; Salim, B.; Kinyanjui, P.; Sugimoto, C. Emergence of new types of Theileria orientalis in Australian cattle and possible cause of theileriosis outbreaks. Parasit. Vectors 2011, 4, 22. [Google Scholar] [CrossRef]
- Gubbels, M.J.; Hong, Y.; van der Weide, M.; Qi, B.; Nijman, I.J.; Guangyuan, L.; Jongejan, F. Molecular characterisation of the Theileria buffeli/orientalis group. Int. J. Parasitol. 2000, 30, 943–952. [Google Scholar] [CrossRef]
- Khukhuu, A.; Lan, D.T.B.; Long, P.T.; Ueno, A.; Li, Y.; Luo, Y.; de Macedo, A.C.C.; Matsumoto, K.; Inokuma, H.; Kawazu, S.-I.; et al. Molecular epidemiological survey of Theileria orientalis in Thua Thien Hue Province, Vietnam. J. Vet. Med. Sci. 2011, 73, 701–705. [Google Scholar] [CrossRef]
- Jeong, W.; Yoon, S.H.; An, D.J.; Cho, S.-H.; Lee, K.-K.; Kim, J.-Y. A molecular phylogeny of the benign Theileria parasites based on major piroplasm surface protein (MPSP) gene sequences. Parasitology 2010, 137, 241–249. [Google Scholar] [CrossRef]
- Fujisaki, K.; Kawazu, S.; Kamio, T. The taxonomy of the bovine Theileria spp. Parasitol. Today 1994, 10, 31–33. [Google Scholar] [CrossRef]
- Ota, N.; Mizuno, D.; Kuboki, N.; Igarashi, I.; Nakamura, Y.; Yamashina, H.; Hanzaike, T.; Fujii, K.; Onoe, S.; Hata, H.; et al. Epidemiological survey of Theileria orientalis infection in grazing cattle in the eastern part of Hokkaido, Japan. J. Vet. Med. Sci. 2009, 71, 937–944. [Google Scholar] [CrossRef] [PubMed]
- McFadden, A.M.; Rawdon, T.G.; Meyer, J.; Makin, J.; Morley, C.M.; Clough, R.R.; Tham, K.; Mullner, P.; Geysen, D. An outbreak of haemolytic anaemia associated with infection of Theileria orientalis in naive cattle. N. Z. Vet. J. 2011, 59, 79–85. [Google Scholar] [CrossRef]
- McFadden, A.M.; Vink, D.; Pulford, D.; Lawrence, K.; Gias, E.; Heath, A.; McFadden, C.; Bingham, P. Monitoring an epidemic of Theileria-associated bovine anaemia (Ikeda) in cattle herds in New Zealand. Prev. Vet. Med. 2016, 125, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Eamens, G.J.; Bailey, G.; Jenkins, C.; Gonsalves, J.R. Significance of Theileria orientalis types in individual affected beef herds in New South Wales based on clinical, smear and PCR findings. Vet. Parasitol. 2013, 196, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Eamens, G.J.; Gonsalves, J.R.; Jenkins, C.; Collins, D.; Bailey, G. Theileria orientalis MPSP types in Australian cattle herds associated with outbreaks of clinical disease and their association with clinical pathology findings. Vet. Parasitol. 2013, 191, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Jabbar, A.; Campbell, B.E.; Cantacessi, C.; Gasser, R.B. Bovine theileriosis—An emerging problem in south-eastern Australia? Infect. Genet. Evol. 2011, 11, 2095–2097. [Google Scholar] [CrossRef]
- Lawrence, K.E.; Forsyth, S.; Vaatstra, B.; McFadden, A.; Pulford, D.; Govindaraju, K.; Pomroy, W. Clinical haematology and biochemistry profiles of cattle naturally infected with Theileria orientalis Ikeda type in New Zealand. N. Z. Vet. J. 2018, 66, 21–29. [Google Scholar] [CrossRef]
- McFadden, A.; Hart, M.; Bueno, I.; Ha, H.; Heath, A.; Pulford, D. Monitoring Theileria orientalis (Ikeda)-associated bovine anaemia in affected cattle over time. Vet. Parasitol. 2017, 245, 29–33. [Google Scholar] [CrossRef]
- Vink, W.D.; Lawrence, K.; McFadden, A.; Bingham, P. An assessment of the herd-level impact of the Theileria orientalis (Ikeda) epidemic of cattle in New Zealand, 2012–2013: A mixed methods approach. N. Z. Vet. J. 2016, 64, 48–54. [Google Scholar] [CrossRef]
- Swilks, E.; Fell, S.A.; Hammer, J.F.; Sales, N.; Krebs, G.L.; Jenkins, C. Transplacental transmission of Theileria orientalis occurs at a low rate in field-affected cattle: Infection in utero does not appear to be a major cause of abortion. Parasit. Vectors 2017, 10, 227. [Google Scholar] [CrossRef]
- Mekata, H.; Minamino, T.; Mikurino, Y.; Yamamoto, M.; Yoshida, A.; Nonaka, N.; Horii, Y. Evaluation of the natural vertical transmission of Theileria orientalis. Vet. Parasitol. 2018, 263, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.K.; Gasser, R.B.; Anderson, G.A.; Jeffers, M.; Bell, C.M.; Jabbar, A. Epidemiological survey following oriental theileriosis outbreaks in Victoria, Australia, on selected cattle farms. Vet. Parasitol. 2013, 197, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.K.; Gasser, R.B.; Firestone, S.M.; Anderson, G.A.; Malmo, J.; Davis, G.; Beggs, D.S.; Jabbar, A. Oriental theileriosis in dairy cows causes a significant milk production loss. Parasit. Vectors 2014, 7, 73. [Google Scholar] [CrossRef]
- Lawrence, K.E.; Gedye, K.; Pomroy, W.E. A longitudinal study of the effect of Theileria orientalis Ikeda type infection on three New Zealand dairy farms naturally infected at pasture. Vet. Parasitol. 2019, 276, 108977. [Google Scholar] [CrossRef] [PubMed]
- Beard, C.B.; Occi, J.; Bonilla, D.L.; Egizi, A.M.; Fonseca, D.M.; Mertins, J.W.; Backenson, B.P.; Bajwa, W.I.; Barbarin, A.M.; Bertone, M.A.; et al. Multistate Infestation with the Exotic Disease-Vector Tick Haemaphysalis longicornis—United States, August 2017-September 2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1310–1313. [Google Scholar] [CrossRef]
- Dinkel, K.D.; Herndon, D.R.; Noh, S.M.; Lahmers, K.K.; Todd, S.M.; Ueti, M.W.; Scoles, G.A.; Mason, K.L.; Fry, L.M. A U.S. isolate of Theileria orientalis, Ikeda genotype, is transmitted to cattle by the invasive Asian longhorned tick, Haemaphysalis longicornis. Parasit. Vectors 2021, 14, 157. [Google Scholar] [CrossRef]
- Rainey, T.; Occi, J.L.; Robbins, R.G.; Egizi, A. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) Parasitizing a Sheep in New Jersey, United States. J. Med. Entomol. 2018, 55, 757–759. [Google Scholar] [CrossRef]
- Thompson, A.T.; White, S.; Shaw, D.; Egizi, A.; Lahmers, K.; Ruder, M.G.; Yabsley, M.J. Theileria orientalis Ikeda in host-seeking Haemaphysalis longicornis in Virginia, U. S.A. Ticks Tick Borne Dis. 2020, 11, 101450. [Google Scholar] [CrossRef]
- Tufts, D.M.; VanAcker, M.C.; Fernandez, M.P.; DeNicola, A.; Egizi, A.; Diuk-Wasser, M.A. Distribution, Host-Seeking Phenology, and Host and Habitat Associations of Haemaphysalis longicornis Ticks, Staten Island, New York, USA. Emerg. Infect. Dis. 2019, 25, 792–796. [Google Scholar] [CrossRef]
- Bonilla, D.L. National Haemaphysalis longicornis (Asian longhorned tick) Situation Report; United State Department of Argriculture, USDA: Washington, DC, USA, 2023.
- Heath, A. Biology, ecology and distribution of the tick, Haemaphysalis longicornis Neumann (Acari: Ixodidae) in New Zealand. N. Z. Vet. J. 2016, 64, 10–20. [Google Scholar] [CrossRef]
- Yabsley, M.J.; Thompson, A.T. Haemaphysalis longicornis (Asian longhorned tick). Trends Parasitol. 2023, 39, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Marendy, D.; Baker, K.; Emery, D.; Rolls, P.; Stutchbury, R. Haemaphysalis longicornis: The life-cycle on dogs and cattle, with confirmation of its vector status for Theileria orientalis in Australia. Vet. Parasitol. 2020, 277S, 100022. [Google Scholar] [CrossRef]
- Alkishe, A.; Raghavan, R.K.; Peterson, A.T. Likely Geographic Distributional Shifts among Medically Important Tick Species and Tick-Associated Diseases under Climate Change in North America: A Review. Insects 2021, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Onzere, C.K.; Herndon, D.R.; Hassan, A.; Oyen, K.; Poh, K.C.; Scoles, G.A.; Fry, L.M. A U.S. Isolate of Theileria orientalis Ikeda Is Not Transstadially Transmitted to Cattle by Rhipicephalus microplus. Pathogens 2023, 12, 559. [Google Scholar]
- Kaufman, E.L.; Stone, N.E.; Scoles, G.A.; Hepp, C.M.; Busch, J.D.; Wagner, D.M. Range-wide genetic analysis of Dermacentor variabilis and its Francisella-like endosymbionts demonstrates phylogeographic concordance between both taxa. Parasit. Vectors 2018, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Sonenshine, D.E.; Stout, I.J. Ticks infesting medium-sized wild mammals in two forest localities in Virginia (Acarina: Ixodidae). J. Med. Entomol. 1971, 8, 217–227. [Google Scholar] [CrossRef]
- Scoles, G.A.; Hutcheson, H.J.; Schlater, J.L.; Hennager, S.G.; Pelzel, A.M.; Knowles, D.P. Equine piroplasmosis associated with Amblyomma cajennense Ticks, Texas, USA. Emerg. Infect. Dis. 2011, 17, 1903–1905. [Google Scholar] [CrossRef]
- Scoles, G.A.; Ueti, M.W. Amblyomma cajennense is an intrastadial biological vector of Theileria equi. Parasit. Vectors 2013, 6, 306. [Google Scholar] [CrossRef]
- Stiller, D.; Goff, W.L.; Johnson, L.W.; Knowles, D.P. Dermacentor variabilis and boophilus microplus (Acari: Ixodidae): Experimental vectors of Babesia equi to equids. J. Med. Entomol. 2002, 39, 667–670. [Google Scholar] [CrossRef]
- Wang, H.; Henbest, P.J.; Nuttall, P.A. Successful interrupted feeding of adult Rhipicephalus appendiculatus (Ixodidae) is accompanied by reprogramming of salivary gland protein expression. Parasitology 1999, 119 Pt 2, 143–149. [Google Scholar] [CrossRef]
- Lysyk, T.J. Movement of male Dermacentor andersoni (Acari: Ixodidae) among cattle. J. Med. Entomol. 2013, 50, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Little, S.E.; Hostetler, J.; Kocan, K.M. Movement of Rhipicephalus sanguineus adults between co-housed dogs during active feeding. Vet. Parasitol. 2007, 150, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco Panizza, M.N.; Rossner, M.V.; Signorini, M.L.; Nava, S. Migration of Rhipicephalus microplus ticks among cattle. Med. Vet. Entomol. 2023, 37, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Scoles, G.A.; Broce, A.B.; Lysyk, T.J.; Palmer, G.H. Relative efficiency of biological transmission of Anaplasma marginale (Rickettsiales: Anaplasmataceae) by Dermacentor andersoni (Acari: Ixodidae) compared with mechanical transmission by Stomoxys calcitrans (Diptera: Muscidae). J. Med. Entomol. 2005, 42, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Stiller, D.; Coan, M.E. Recent developments in elucidating tick vector relationships for anaplasmosis and equine piroplasmosis. Vet. Parasitol. 1995, 57, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Scoles, G.A.; Ueti, M.W. Vector ecology of equine piroplasmosis. Annu. Rev. Entomol. 2015, 60, 561–580. [Google Scholar] [CrossRef]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef]
- McDade, J.E.; Newhouse, V.F. Natural history of Rickettsia rickettsii. Annu. Rev. Microbiol. 1986, 40, 287–309. [Google Scholar] [CrossRef]
- Hopla, C.E. The ecology of tularemia. Adv. Vet. Sci. Comp. Med. 1974, 18, 25–53. [Google Scholar]
- Heinzen, R.A.; Grieshaber, S.S.; Van Kirk, L.S.; Devin, C.J. Dynamics of actin-based movement by Rickettsia rickettsii in vero cells. Infect. Immun. 1999, 67, 4201–4207. [Google Scholar] [CrossRef]
- Mullen, G.R.; Durden, L.A. (Eds.) Medical and Veterinary Entomology; Academic Press: London, UK, 2019; pp. 603–672. [Google Scholar]
- Saleh, M.N.; Allen, K.E.; Lineberry, M.W.; Little, S.E.; Reichard, M.V. Ticks infesting dogs and cats in North America: Biology, geographic distribution, and pathogen transmission. Vet. Parasitol. 2021, 294, 109392. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.N.; Sundstrom, K.D.; Duncan, K.T.; Ientile, M.M.; Jordy, J.; Ghosh, P.; Little, S.E. Show us your ticks: A survey of ticks infesting dogs and cats across the USA. Parasit. Vectors 2019, 12, 595. [Google Scholar] [CrossRef] [PubMed]
- Little, S.E.; Barrett, A.W.; Nagamori, Y.; Herrin, B.H.; Normile, D.; Heaney, K.; Armstrong, R. Ticks from cats in the United States: Patterns of infestation and infection with pathogens. Vet. Parasitol. 2018, 257, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Kollars, T.M., Jr.; Oliver, J.H., Jr.; Masters, E.J.; Kollars, P.G.; Durden, L.A. Host utilization and seasonal occurrence of Dermacentor species (Acari:Ixodidae) in Missouri, USA. Exp. Appl. Acarol. 2000, 24, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Dergousoff, S.J.; Galloway, T.D.; Lindsay, L.R.; Curry, P.S.; Chilton, N.B. Range expansion of Dermacentor variabilis and Dermacentor andersoni (Acari: Ixodidae) near their northern distributional limits. J. Med. Entomol. 2013, 50, 510–520. [Google Scholar] [CrossRef]

| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Calf 1 | − | − | + | + | + | + | + | + | + | + | + |
| Calf 2 | − | − | − | − | − | − | − | − | − | N/A | N/A |
| Calf 3 | − | − | − | − | − | − | − | − | − | N/A | N/A |
| Tick Batch | Females Tested | Males Tested | Number Positive |
|---|---|---|---|
| Group 1 Adults | 5 | 5 | 0 |
| Group 2 Adults | 5 | 5 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onzere, C.K.; Hassan, A.; Herndon, D.R.; Oyen, K.; Poh, K.C.; Scoles, G.A.; Fry, L.M. Dermacentor variabilis Does Not Transstadially Transmit the U.S. Isolate of Theileria orientalis Ikeda: A Controlled Acquisition and Transmission Study. Parasitologia 2023, 3, 284-292. https://doi.org/10.3390/parasitologia3030029
Onzere CK, Hassan A, Herndon DR, Oyen K, Poh KC, Scoles GA, Fry LM. Dermacentor variabilis Does Not Transstadially Transmit the U.S. Isolate of Theileria orientalis Ikeda: A Controlled Acquisition and Transmission Study. Parasitologia. 2023; 3(3):284-292. https://doi.org/10.3390/parasitologia3030029
Chicago/Turabian StyleOnzere, Cynthia K., Amany Hassan, David R. Herndon, Kennan Oyen, Karen C. Poh, Glen A. Scoles, and Lindsay M. Fry. 2023. "Dermacentor variabilis Does Not Transstadially Transmit the U.S. Isolate of Theileria orientalis Ikeda: A Controlled Acquisition and Transmission Study" Parasitologia 3, no. 3: 284-292. https://doi.org/10.3390/parasitologia3030029
APA StyleOnzere, C. K., Hassan, A., Herndon, D. R., Oyen, K., Poh, K. C., Scoles, G. A., & Fry, L. M. (2023). Dermacentor variabilis Does Not Transstadially Transmit the U.S. Isolate of Theileria orientalis Ikeda: A Controlled Acquisition and Transmission Study. Parasitologia, 3(3), 284-292. https://doi.org/10.3390/parasitologia3030029






