Abstract
Cystic echinococcosis (CE) is endemic in Argentina, and approximately 30% of the national territory has characteristics appropriate for the development of the zoonotic domestic cycle of this disease. This community-wide study was implemented in rural areas of Añatuya, Santiago del Estero (northern Argentina) to determine the presence of Echinococcus granulosus sensu lato in the definitive host (dogs) and the presence of CE in humans. Infection data from dogs were obtained through the collection and analysis of fecal samples; these were processed through sedimentation/flotation techniques and PCR. The presence in humans was determined by ultrasound (US) and serology (ELISA confirmed by Western Blot—WB) in the Miel de Palo settlement. A standardized questionnaire was used to investigate potential risk factors for CE; more than half of the studied population carried out activities that favor the maintenance of the cycle. The prevalence of E. granulosus s.l. in dogs from 10 rural settlements, confirmed by PCR, was 4.7% (32/678). The results of the US and serology screening showed a human prevalence of 0.55% (1/183) in Miel de Palo. This prevalence increased to 4.9% (9/183) if imaging-negative but serology-positive (ELISA+WB) individuals are included, as per national guidelines. One of the participants with CE, confirmed by US, was less than 15 years old, which evidences the presence of active transmission. A comprehensive multidisciplinary approach, taking into consideration social, behavioral, sanitary, and environmental aspects intimately tied to the parasite cycle, is needed.
1. Introduction
Cystic echinococcosis (CE), a zoonosis associated with dogs and livestock, is a parasitic disease whose current global burden in humans is hard to estimate due to the lack of systematic surveillance programs [1,2,3,4]. CE is caused by the larval stage of cestodes from the Family Taeniidae, genus Echinococcus, where dogs act as definitive hosts and the intermediate hosts are bovine, ovine, and caprine ruminants, and to a lesser extent, pigs, equines, American camelids [5] and accidentally humans (dead-end hosts for this parasite). Human diseases caused by parasites of the genus Echinococcus include cystic (CE), alveolar (AE) and neotropical (NE) echinococcosis. These parasitic diseases are caused by the etiologic agents Echinococcus granulosus sensu lato (CE), which have a worldwide distribution, Echinococcus multilocularis (AE), present in the Northern hemisphere, and both E. vogeli (NE) and E. oligarthra (NE), which are restricted to Mexico, Central America, and South America [6,7]. Echinococcus granulosus s.l. is a complex of species; within these species, the most important for humans is E. granulosus sensu stricto., which is the most widely spread worldwide and is estimated to be responsible for 80% of human cases [8,9,10,11].
The World Health Organization (WHO) has estimated a global yearly burden of 871,000 disability-adjusted life years (DALYs) [12], and recent reports have estimated 322,400 DALYs only for China, making it the country with the highest prevalence rate for CE in the world [13]. Given the importance of this Neglected Tropical Disease (NTD) in South America; Argentina, Brazil, Chile, Paraguay, Peru, and Uruguay have formed a “South American initiative for the control and surveillance of cystic echinococcosis” spearheaded by the Pan American Health Organization (PAHO) [12,14]. These five countries together (data from Paraguay is lacking) reported 29,556 cases of human CE between January 2009 and December 2014, of which 15% were in children under the age of 15 years. In this period (2009 to 2014), CE caused more than 800 deaths [14]. It is important to note that the number of registered cases and deaths are under-reported, even though it is a nationally notifiable disease in many countries.
Early diagnosis of CE is crucial, since if it goes inadvertent for years, it can lead to disability and even death. According to the WHO, the post-operative death rate for surgical patients is 2.2% worldwide and approximately 6.5% relapse after intervention [5]. Studies performed in different endemic areas reported that the median age at which detection occurs is usually quite late and ranges from 21 to 45 years of age, depending on the country [15]. At the global level, annual costs associated with the treatment of CE cases in humans, together with losses in the livestock industry, are estimated to be US$3 billion [12]. Diagnosis of CE in humans is usually based on clinical presentation, epidemiological data, ultrasound (US) or X-ray imaging and serology. US is a crucial tool for the diagnosis of this disease, and there is an internationally validated classification key to identify the stage of the cysts [16].
In Argentina, CE is endemic, and approximately 30% of the national territory has characteristics appropriate for the development of the zoonotic domestic cycle of this parasite, with large extensions of rural areas with free-ranging animals (livestock and dogs), home slaughtering of livestock and feeding dogs with raw viscera [17]. Given these characteristics, there are important foci in most of the areas of Patagonia, the Humid Pampa, Mesopotamia, Cuyo, and the high mountains of the northeast [18]. As a nationally notifiable disease since 1936 (Law 12317), in the year 2018, 697 cases were officially notified in Argentina, most of them from the Central Region of the country (Buenos Aires City, Buenos Aires Province, Cordoba, Entre Rios and Santa Fe); in 2019, 617 cases were officially notified, again mostly again from the Central Region [19]. Argentinian national guidelines indicate that the treatment of humans should be based on albendazole (10–15 mg/kg/day divided into two doses) for a minimum of three months in asymptomatic individuals [20]. In some cases, cysts need to be surgically removed [20].
Argentina has a National Program for the Control of Zoonotic Diseases within the Epidemiology Section of the National Ministry of Health [21], and CE is included. Through this program, US machines were distributed to each of the 24 provinces or Argentina for surveillance of CE in school-aged children (SAC), albendazole was distributed for the treatment of humans and praziquantel for the treatment of dogs. Surveillance and control are based on national guidelines elaborated prior to the program itself, and given that Argentina is a Federal Government, it is up to each province to plan and execute the activities described within [22]. The Province of Santiago del Estero adhered to this program in 2013, and historical data shows an increase in the number of notified cases of CE from four in 2004 to 26 in 2012 [23], although surveillance and control actions have not been uniformly carried out and neither has active case findings through US in SAC.
Given that CE is a notifiable disease in Argentina, some data from humans have been reported from this province; all departments notified at least one case during 2011 and 2012 [23]. The current study was carried out in rural areas of Añatuya, Department of General Taboada in Santiago del Estero (Argentina), as part of a project to determine the presence of E. granulosus in dogs and humans living in the area and thus guide public policy and determine the need to implement an early detection program for CE in the area, following national guidelines [22].
2. Results
2.1. Community Survey
Out of a total of 398 registered households, 214 agreed to participate (53.8%) and were visited as part of the survey (Table 1). Through a household questionnaire, most dog owners declared deworming their dogs, except in Lote 47 (44.1%), Lote 28 Grande (47.4%) and Lote 46 (44.4%), where more than half of the households stated they did not deworm their dogs. On the other hand, 85.0% of the households practiced slaughtering at home, with the highest percentages found in Lote 59 (100%), Miel de Palo (98.3%) and Lote 47 (91.2%). The only settlement whose percentage was less than 50% was Lote 28 (42.9%). This is a regular practice in rural settlements, given they rely on subsistence living and the only slaughterhouse in the area is in Añatuya. From the total amount of households that declared slaughtering animals in their home, 54.4% of them declared that they use the raw viscera to feed their dogs. El Malacara (83.3%) and Lote 28 Grande (82.4%) were the settlements with the highest percentages for this activity, while Lote 28 (33.3%) presented the lowest percentage.

Table 1.
The dog fecal samples collected from different settlements close to Añatuya, Santiago del Estero province (Argentina). Characteristics on dog anti-parasitic treatment practice of slaughtering at home and elimination of viscera are also detailed.
2.2. Dog Fecal Samples
The total number of fecal samples collected was 682 (Table 1) and 678 of these were completely analyzed (Table 2) through both microscopic observation (MO) and PCR. All the localities showed at least one parasitic species, including amebas, Ancylostoma caninum, Dypilidium caninum, coccidians, Macracanthorhynchus spp., Taenia spp., Toxascaris spp., Toxocara canis and Trichuris vulpis. Taenid eggs were identified through MO in the settlements of El Malacara, Miel de Palo, Lote 47, Pozo Herrera, Lote 27, Lote 28, Lote 28 Grande and Lote 46. Confirmation of the identification of E. granulosus through PCR was also achieved; a total of 32 samples from the 678 samples analyzed were confirmed as E. granulosus s.l. (4.7%).

Table 2.
Results of dog fecal samples analyzed through standard coprological techniques for the presence of intestinal parasites and PCR specific for Echinococcus granulosus s.l. from different rural settlements near the City of Añatuya, Santiago del Estero (Argentina).
The comparison of the test results using both standard coprological techniques, including one sedimentation and two flotation techniques, with direct observation using a microscope and a specific PCR for Echinococcus granulosus s.l. are detailed in Table 3. As observed, not all samples with Taenia spp. eggs present, were confirmed as E. granulosus, which is expected since there are other Taeniid species that can infect dogs; 13 of the 26 (50%) MO-positive samples were confirmed as E. granulosus s.l. positive. Additionally, 19 of the 652 (2.9%) samples that were negative for Taenia spp eggs, using standard coprological techniques, were identified as E. granulosus s.l. positive through PCR.

Table 3.
Comparison of the results obtained through processing of dog fecal samples by both standard coprological techniques and PCR specific for Echinococcus granulosus s.l. from different rural settlements near the City of Añatuya, Santiago del Estero.
The coprological results of the dogs were given to the owners, and massive deworming was performed in all the analyzed settlements. A total of 427 dogs were dewormed since some were not present during the deworming visit. Another community meeting was held in each settlement to explain the results and give information on the transmission, prevention and control of CE and other parasites found (Figure 1A). Finally, sanitary wells were built to create a barrier between dogs and raw viscera and thus interrupt the CE domestic cycle (Figure 1B). These were built in El Malacara and Miel de Palo, with the participation of 15 and 41 individuals, respectively.

Figure 1.
Images of fieldwork in the Department of General Taboada, Santiago del Estero, Argentina. (A) Community meeting to explain the cycle of the parasite. (B) Sanitary well, built in the Miel de Palo settlement.
2.3. Ultrasound (US) and Serology Testing
The settlement of Miel de Palo was selected for abdominal-US and serology screening of the population due to the presence of E. granulosus s.l. (confirmed by PCR) in dogs. In total, 183 abdominal US were performed, and blood samples from 181 of the same participants were collected in serum tubes for processing through a specific indirect ELISA for E. granulosus s.l.; positive results were confirmed by Western Blot (WB). The average age of the 181 participants, for both the US and serum data was 24.2 years (range from 3 to 79 years), while 65.2% were female (n = 118) and 34.8% were male (n = 63).
Eight participants with positive results through ELISA and WB did not show hydatid cysts in their abdominal US. These participants were referred for chest X-rays to discard the possibility of pulmonary cysts, and the results for all participants were negative. Another 25 participants were only ELISA positive, two of which were owners of E. granulosus s.l.-positive dogs. Although the main objective of the use of US was to find hydatid cysts, other findings were observed, including pregnancies, splenomegaly, steatosis, kidney cysts, and vesicular lithiasis, among others. Two participants showed compatible lesions with hydatid cysts. The first case was a 7-year-old child who presented a compatible lesion (CL) in the liver (size 14 mm). The second case was a 47-year-old woman who presented three CL in the liver (sizes 8 mm, 35 × 32 mm and 41 × 29 mm). In both cases, the ELISA screening was positive, but only the child´s case was confirmed by WB. The second case could not be confirmed, given that US monitoring of the cyst, after treatment with albendazole, was not possible.
3. Discussion
Argentina is among the most endemic areas for CE in South America [24]. Most human cases are found in rural areas where basic needs are unsatisfied [25], probably because all the necessary factors for the establishment of CE transmission cycles are present in these areas, such as free-roaming livestock and dogs, home slaughtering and feeding of dogs with raw viscera [15]. The Department of General Taboada, located in the Province of Santiago del Estero, is within an endemic area of CE in northeastern Argentina. In this study, 214 households from 10 rural settlements close to Añatuya were surveyed. Given that there are approximately 398 households in total in these 10 settlements, a coverage of 53.8% was achieved. Although half of the households (n = 120; 50.1%) state their dogs were dewormed, most households (n = 182; 85.0%) declared slaughtering animals at home, with half of them feeding raw viscera to their dogs (n = 99; 54.4%). This scenario thus confirms that the factors necessary for the transmission of CE are present in this area of the province.
In all the settlements, dogs were found to be infected with different intestinal parasites, especially Pozo Herrera, with a presence of 54.2% (45/83); despite the results from the survey, in which 69.6% of the dog owners stated deworming their animals. Overall, the presence of intestinal parasites was 30.7% and 50.1% of dog owners stated deworming their dogs, thus, deworming is not being performed regularly or they are not using an appropriate deworming drug (although a question about the drug used was not included in the questionnaire). Most of the households (85.0%) declared the practice of informal slaughtering, and 54.4% of these individuals admitted feeding their dogs with raw viscera. This is confirmed by the presence of Taenid spp. eggs in dog fecal samples and the confirmation of the presence of E. granusolus s.l. through PCR in Miel de Palo, Lote 47, Pozo Herrera, Lote 27, Lote 28, Lote 28 Grande and Lote 46. The overall presence of E. granulosus s.l., as confirmed by PCR, was 4.7%, with the greatest contamination occurring in Pozo Herrera and Lote 28 Grande with a prevalence of 9.6% (8/83) and 9.8% (6/61), respectively.
This overall presence of 4.7% for E. granulosus s.l. in dogs is lower than the one reported in other provinces of northern Argentina. For example, in a study from La Rioja, the presence ranged between 11 and 30% [26], while in Jujuy Province it ranged between 7 and 30% [27,28]. In Rio Negro Province, located in the South and where a CE control program is implemented, the value of its presence was reported as 6.5% in dogs [29]. The difference in values observed with the current study could be due to the number of samples analyzed, different characteristics between the provinces and the difference in sensitivity and specificity of methods employed (Copro-PCR/Copro-ELISA) [14,17].
The results of the US and serology screening in inhabitants of Miel de Palo confirmed the presence of individuals with CE. Given the cysts found were in the early stages, both individuals were treated with albendazole [24]. One of the individuals with CE was less than 15 years old, which evidences the presence of active transmission of E. granulosus in this settlement. Moreover, positive results for the ELISA test, while negative for WB and US, could indicate infection with other parasites, given the ELISA test can cross-react with other parasites, including other cestodes, nematodes and trematodes [30]. Additionally, it is possible that participants with positive results for the ELISA and WB tests, but without symptoms and negative in both the US and X-ray analysis, could have cysts in other atypical locations, although this is unlikely.
With respect to the US surveys conducted in humans, a presence of 0.55% (1/183) was observed. Four children under 15 years old were studied by US; one had a CL lesion compatible with CE. In Rio Negro Province, the initial prevalence of infection in children under 14 years old was 5.6% in 1986. After the implementation of the control program to CE, it persistently decreased until reaching 0.3% in 2008 [31]. The situation reported in this study needs to be taken into consideration, given that all the components for the maintenance of the CE cycle are present and the guidelines and actions detailed in the national control program are not being implemented. In this study, a single settlement was selected for serology and ultrasonography in humans. Therefore, the expansion of screening in SAC could be worth performing. The presence of infection in dogs could be used to guide the implementation of screening in humans. For example, in this area, the settlement of Lote 28 Grande would be worth screening next.
The interventions conducted in the Miel de Palo settlement, including health education and the construction of sanitary wells, could be useful for the interruption of the E. granulosus transmission cycle. The results obtained in this study show that CE in rural areas needs a comprehensive approach at different healthcare levels [31]. The implementation of a “one health” approach to this zoonosis should have a positive impact. This has already been demonstrated in the provinces of the Argentine Patagonian region, with successful results [32]. Future studies could evaluate the result of the interventions carried out in the current study. Also, the performance of other interventions, such as vaccination of intermediate hosts, could be envisaged. CE in rural areas from the Argentinean north needs a comprehensive multidisciplinary approach, taking into consideration social, behavioral, sanitary, and environmental aspects, which are intimately tied to the parasite cycle.
4. Materials and Methods
4.1. Study Area
Añatuya is located 184 km from the Capital of Santiago del Estero, Northeast Region of Argentina. This city has an extensive rural area with different rural settlements, and they are all located within the Department of General Taboada. This area is part of the semi-arid sub-region of the Great Chaco [33], and the climate is usually divided into two main seasons; dry and mild from April to September and hot and rainy from October to March [33]. The study described here was carried out in 10 rural settlements close to Añatuya: El Malacara, El Desvío, Lote 27, Lote 28, Lote 28 Grande, Lote 46, Lote 47, Lote 59, Miel de Palo, and Pozo Herrera (Figure 2). There is a low population density in the province, and the Department of General Taboada has approximately 38,000 inhabitants living in 9073 households, with most of the inhabitants, 23,286, living in the city of Añatuya [34]. In the rural settlements included in this study, there are approximately 398 households. The most common livelihood in this area is animal farming (sheep and goat) and day laborers, although most inhabitants do not have formal jobs and many adults receive government pensions, retirement allowances or child subsidies [35].
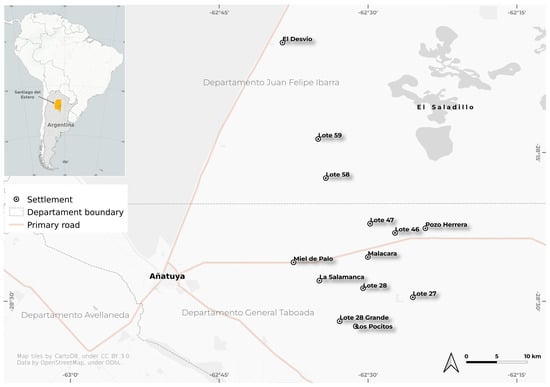
Figure 2.
Map of the study area showing the location of Santiago del Estero Province in Argentina and an insert with the city of Añatuya and the points which represent the households where the dog samples were collected in each of the 10 settlements included in the study: El Malacara, El Desvío, Lote 27, Lote 28 Grande, Lote 28, Lote 46, Lote 47, Lote 59, Miel de Palo, and Pozo Herrera.
4.2. Ethical Considerations
The ethical committee of the Ministry of Health of Santiago del Estero Province (protocol N° 050/2017) approved this protocol. Written informed consent was obtained from all participating adults and from a parent or guardian of every child under the age of 13 years. Moreover, written assent was obtained from children between the ages of 6 and 13, inclusive and finally, written informed consent was obtained from 14 and 15-year-old children with informed assent from their parents or guardians.
4.3. Community Survey
The study was designed as a community-based study. After meetings with each community, explaining the objective of the study, the day of collection of samples was announced. All the households were visited and invited to participate in the study. On that given day, the research team visited the community house-by-house, they georeferenced the household and performed a standardized questionnaire to a responsible adult to collect data on the dogs and the samples themselves. The questionnaire included questions about the frequency of anti-parasitic treatment of dogs as well as the presence of farm animals, the practice of slaughtering at home and the elimination of viscera (Supplementary Data S1. Household questionnaire).
4.4. Dog Fecal Samples Collection and Processing
Samples were collected following national technical guidelines [21], one fecal sample from each household dog was collected, and two samples were collected if the household had only one dog. Fecal samples were collected from the peridomicile of the house and placed in properly labelled bags, one for each sample. The material was transported in a refrigerated container to the laboratory in Añatuya and kept refrigerated until its transport to the Department of Parasitology of the national reference laboratory (Instituto Nacional de Enfermedades Infecciosas (INEI), Agencia Nacional de Laboratorios e Institutos de Salud (ANLIS) “Carlos G. Malbran”). Once in the reference laboratory, the samples were inactivated for one week at −70 °C. Each sample was homogenized, and 10 g were used and processed using three standard coprological techniques, one sedimentation and two flotations, Telemann [36], Sheather [37] and Willis [38] with microscopic observation; the specific procedures have been previously described [35]. All the samples were further processed by molecular biology techniques using chloroform: isoamyl alcohol [39] for DNA extraction, while PCR was implemented to amplify a 285 bp fragment from the cytochrome c oxidase subunit 1 (cox-1) [40] specific for the detection of E. granulosus s.l.
A broad-spectrum anthelminthic, including albendazole and praziquantel, was provided by the project. Sanitary wells were built for the slaughtering of animals to create a barrier between dogs and raw viscera. The sanitary well is a closed-out area where slaughtering is performed using a crossbar. It contains drainpipes for the blood and a pit in which to place the viscera. A fire hearth is also built to be able to boil the viscera. The sanitary wells were constructed by the community, and the project provided the technical advice of a foreman and the materials. Two different models for the sanitary wells were offered (Figure 3).
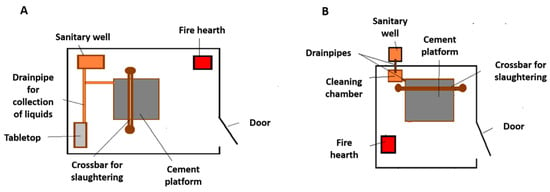
Figure 3.
Sanitary well models constructed by the community with supervision from the project´s foreman in the rural settlement of Miel de Palo, General Taboada, Santiago del Estero (Argentina). (A) Model with the sanitary well located withing the closed structure and with a tabletop for processing of the slaughtered animal. (B) Model with the sanitary well located outside the closed structure and with a simple cleaning chamber.
4.5. Ultrasound and Serology Testing
According to national guidelines [17], the diagnosis of CE is based on epidemiological data, clinical manifestations, complementary imaging methods and serological tests (ELISA/WB); ELISA-positive tests must be confirmed by WB [27]. The definition of a confirmed case is based on imaging results (US, X-ray, or tomography) and/or serological diagnosis through ELISA and WB, or through direct visualization through microscopy of protoscoleces, cestode hooks or membranes, or the histopathological study of surgically removed cysts [41]. Herein, we report the presence of CE based on visualization of a hydatid cyst which could be confirmed as CE according to international consensus (1 case) [41] and if serology is considered (ELISA confirmed by WB) 9 cases, as per Argentinean guidelines [20]. Based on the results of the E. granulosus s.l. contamination of dog fecal samples, communities were selected for testing humans in order to determine the presence of asymptomatic infected individuals. Inhabitants older than 2 years of age were invited to participate in the study. Given the chronology of settlements included in the study, the first settlement to be selected for ultrasound and ELISA screening was Miel de Palo. Due to the pandemic, the field interventions were interrupted in 2020 and no other settlements were selected for screening of the human population.
The school of Miel de Palo, N° 228 “Hernando de Magallanes”, was used as a base to perform the US and collection of blood for serology assays. An abdominal US was performed by a medical doctor with a specialization in clinical US for infectious diseases using a portable DP10 machine (Mindray, Shenzhen, China); all the organs of the abdominal area were observed for the presence of cysts [17,19]. The activity was coordinated with the local hospital in Añatuya and the local health authorities, in order to inform of any CE case and derive any participant that might need medical attention, either for CE or for any other medical finding. Blood samples were drawn by a specialized nursing team prior to the US for performing an indirect in-house ELISA, specific to E. granulosus s.l. [42], with confirmation through WB [21].
The antigen used in the in-house ELISA was obtained from a pool of ovine hydatid cyst fluid (HCF), acquired through puncture and aspiration of cysts from the viscera of infected animals. Each lot of OHL produced was tested for the presence/absence of specific immunogenic bands using WB. The qualified lots were lyophilized, fractioned, and stored at −70 °C. This same OHL is used in the WB for the detection of specific IgG. A test serum was considered positive for E. granulosus s.l. if the presence of specific bands for antigen 5 were detected (55 KDa and 65 KDa), while specific bands for antigen B (8, 16 and 32 KDa) could be present or absent.
For the ELISA, 50 µL of the HCF was added to each flat-bottomed Immulon II well (Immunochemistry Technologies, CA, USA) for a final concentration of 0.5 µg/mL [43,44]. The wells were incubated at 4 °C for 18 h and then rinsed three times with phosphate-buffered saline (PBS) +0.1% Tween 20 for 5 min and then blocked with PBS +1.5% skimmed milk for 1 h at 37 °C. The wells were then incubated with 50 µL of each serum sample diluted at 1:50 for 30 min at 37° in a humid incubator; positive, negative, and blank controls were also included. The wells were then rewashed as previously and incubated with 50 µL of anti-human-IgG-Peroxidase antibody (Merck KGaA, Darmstadt, Germany) diluted 1:5000. The wells were once again washed as previously, and then 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)—ABTS—substrate (Merck KGaA) was added and incubated for 10 min; the reaction was stopped with 100 µL of 0.1N hydrofluoric acid, pH 3.2. The wells were read at 410 nm using the Dynatech MR 4100 reader (Dynatech Laboratories Inc., VA, USA). The cut-off values used for the interpretation of the ELISA were as follows: negative (OD < 0.289), indeterminate (0.289 > OD < 0.422) and positive (OD > 0.423).
For the WB, specific E. granulosus s.l. bands were transferred to a nitrocellulose membrane for one hour at 250 mA (Trans-Blot cell, Bio-Rad, CA, USA). The membrane was blocked with PBS +0.1% Tween 20 +5.0% skimmed milk for one hour with agitation at room temperature (RT). The membrane was then washed three times with PBS +0.1% Tween 20. The nitrocellulose membranes were then incubated with the serum samples at 1:50 dilution and incubated for one hour at RT. The membranes were washed as previously described and incubated in agitation with a 1:5000 dilution of anti-human IgG marked with peroxidase (Gibco, Life Technology Corporation, NY, USA) for one hour at RT. The membranes were then incubated with 3,3′-Diaminobenzidine (DAB) at RT for 5 min and then washed several times with water.
The inhabitants with positive results for ELISA and WB assays, but negative results for US, were derived to the hospital in Añatuya to perform an X-Ray of the thorax to discard the presence of cysts in the lung [7,41].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/parasitologia2040027/s1. Supplementary Data S1. Household questionnaire.
Author Contributions
Conceptualization, H.G.A., G.I.S., M.G.C. and M.V.P.; methodology, M.V.P., G.I.S., M.G.C. and M.S.; formal analysis, H.G.A. and M.V.P.; investigation, M.S., R.G.C. and M.G.C.; data curation, H.G.A., M.G.C., R.G.C. and M.V.P.; writing—original draft preparation, H.G.A. and M.G.C.; writing—review and editing, M.V.P.; supervision, G.I.S. and M.V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundación Mundo Sano and Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET).
Institutional Review Board Statement
The study protocol was approved by the ethical committee of the Ministry of Health of Santiago del Estero Province (protocol N° 050/2017).
Informed Consent Statement
Written informed consent was obtained from all participating adults and from a parent or guardian of every child under the age of 13 years. Moreover, written assent was obtained from children between the ages of 6 and 13, inclusive and finally, written informed consent was obtained from 14 and 15-year-old children with informed assent from their parents or guardians.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References and Note
- Ali, V.; Martinez, E.; Duran, P.; Villena, E.; Deplazes, P.; Rojas, C.A.A. Past and present of cystic echinococcosis in bolivia. PLoS Negl. Trop. Dis. 2021, 15, e0009426. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Jamett, G.; Hernández, F.A.; Castro, N.; Tamarozzi, F.; Uchiumi, L.; Salvitti, J.C.; Cueva, M.; Casulli, A. Prevalence rate and risk factors of human cystic echinococcosis: A cross-sectional, community-based, abdominal ultrasound study in rural and urban north-central Chile. PLoS Negl. Trop. Dis. 2022, 16, e0010280. [Google Scholar] [CrossRef] [PubMed]
- Joanny, G.; Cappai, M.G.; Nonnis, F.; Tamponi, C.; Dessì, G.; Mehmood, N.; Dahdah, J.; Hosri, C.; Scala, A.; Varcasia, A. Human Cystic Echinococcosis in Lebanon: A Retrospective Study and Molecular Epidemiology. Acta Parasitol. 2022, 67, 186–195. [Google Scholar] [CrossRef]
- Rossi, P.; Tamarozzi, F.; Galati, F.; Akhan, O.; Cretu, C.M.; Vutova, K.; Siles-Lucas, M.; Brunetti, E.; Casulli, A.; Angheben, A.; et al. The European Register of Cystic Echinococcosis, ERCE: State-of-the-art five years after its launch. Parasites Vectors 2020, 13, 236. [Google Scholar] [CrossRef]
- Acha, P.; Szyfres, B. Zoonosis y Enfermedades Transmisibles Comunes al Hombre y a Los Animales; Organización Panamericana de la Salud: Washington, DC, USA, 2003; Volume III. [Google Scholar]
- Vuitton, D.A.; McManus, D.P.; Rogan, M.T.; Romig, T.; Gottstein, B.; Naidich, A.; Tuxun, T.; Wen, H.; Menezes da Silva, A. Consensus international sur la terminologie à utiliser dans le domaine des échinococcoses. Parasite 2020, 27, 41. [Google Scholar] [CrossRef]
- Ito, A.; Nakao, M.; Lavikainen, A.; Hoberg, E. Cystic echinococcosis: Future perspectives of molecular epidemiology. Acta Trop. 2017, 165, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.A.A.; Romig, T.; Lightowlers, M.W. Echinococcus granulosus sensu lato genotypes infecting humans—Review of current knowledge. Int. J. Parasitol. 2014, 44, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Cucher, M.A.; Macchiaroli, N.; Baldi, G.; Camicia, F.; Prada, L.; Maldonado, L.; Avila, H.G.; Fox, A.; Gutiérrez, A.; Negro, P.; et al. Cystic echinococcosis in South America: Systematic review of species and genotypes of Echinococcus granulosus sensu lato in humans and natural domestic hosts. Trop. Med. Int. Health 2016, 21, 166–175. [Google Scholar] [CrossRef]
- Debiaggi, M.F.; Soriano, S.V.; Pierangeli, N.B.; Lazzarini, L.E.; Pianciola, L.A.; Mazzeo, M.L.; Moguillansky, S.; Farjat, J.A.B. Genetic characterization of human hydatid cysts shows coinfection by Echinococcus canadensis G7 and Echinococcus granulosus sensu stricto G1 in Argentina. Parasitol. Res. 2017, 116, 2599–2604. [Google Scholar] [CrossRef]
- Restrepo, A.M.C.; Yang, Y.R.; McManus, D.P.; Gray, D.J.; Giraudoux, P.; Barnes, T.S.; Williams, G.M.; Soares Magalhães, R.J.; Hamm, N.A.S.; Clements, A.C.A. The landscape epidemiology of echinococcoses. Infect. Dis. Poverty 2016, 5, 13. [Google Scholar] [CrossRef]
- World Health Organization Echinococcosis. Available online: https://www.who.int/news-room/fact-sheets/detail/echinococcosis (accessed on 3 June 2021).
- Wang, L.Y.; Qin, M.; Liu, Z.H.; Wu, W.P.; Xiao, N.; Zhou, X.N.; Manguin, S.; Gavotte, L.; Frutos, R. Prevalence and spatial distribution characteristics of human echinococcosis in China. PLoS Negl. Trop. Dis. 2021, 15, e0009996. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud. Prevención y Control de la Hidatidosis en el Nivel Local. In Prevención y Control la Hidatidosis en el Nivel Local Iniciativa Sudamericana para el Control y Vigilancia de la Equinococosis Quística/Hidatidosis; Organización Mundial de la Salud: Geneva, Switzerland, 2017. [Google Scholar]
- Budke, C.M.; Deplazes, P.; Torgerson, P.R. Global socioeconomic impact of cystic echinococcosis. Emerg. Infect Dis. 2006, 12, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.; Mastin, A.; van Kesteren, F.; Boufana, B. Echinococcus granulosus: Epidemiology and state-of-the-art of diagnostics in animals. Vet. Parasitol. 2015, 213, 132–148. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, N.; Benitez, M.; Vizcay, R.; Bolpe, J. Hidatidosis en la Provincia de Buenos Aires: Áreas endémicas y estimación de costos por tratamiento. Rev. Argent. Zoonosis Enferm. Infecc. Emerg. 2012, II, 5–7. [Google Scholar]
- Ministerio de Salud Argentina. Dirección Nacional de Epidemiología y Análisis de la Información. In Boletín Integrado de Vigilancia, Versión Ampliada; No°481; Ministerio de Salud Argentina: Buenos Aires, Argentina, 2020. [Google Scholar]
- Ministerio de Salud de la Nación. Enfermedades Infecciosas, Hidatidosis, Diagnóstico de Hidatidosis, Guía para el Equipo de Salud; Moral, M., Ed.; Dirección de Epidemiología; Ministerio de Salud de la Nación: Buenos Aires, Argentina, 2012. [Google Scholar]
- Ministerio de Justicia y Derechos Humanos Argentina. Creación del Programa Nacional de Control de Enfermedades Zoonóticas. Available online: http://servicios.infoleg.gob.ar/infolegInternet/anexos/185000-189999/189688/norma.htm (accessed on 4 June 2021).
- Ministerio de Salud de la Nación. República Argentina Norma Técnica y Manual de Procedimientos para el Control de la Hidatidosis; Ministerio de Salud de la Nación: Buenos Aires, Argentina, 2009. [Google Scholar]
- Ministerio de Salud y Desarrollo Social de la Provincia de Santiago del Estero Resolution Ministerial No. 1651. Available online: https://e-legis-ar.msal.gov.ar/htdocs/legisalud/migration/html/26259.html (accessed on 19 April 2022).
- Panaftosa OPS/OMS Equinococosis. Informe Epidemiológico en la Región de América del Sur 2018. Vet. Public Health—Panaftosa Paho/Who 2020, 3, 4. [Google Scholar]
- Organización Panamericana de la Salud. Prevención y Control de la Hidatidosis en el Nivel Local: Iniciativa Sudamericana para el Control y Vigilancia de la Equinococosis Quística/Hidatidosis; Larrieu, E., Ed.; Organización Panamericana de la Salud: Río de Janeiro, Brazil, 2017. [Google Scholar]
- Amaya, J.C.; Moreno, N.; Salmaso, N.; Bazan, E.; Ricoy, G.; Córdoba, P.; Santillan, G.I. Estudio de infestación de caninos con Echinococcus granulosus en la provincia de La Rioja, Argentina. Rev. Argent. Microbiol. 2016, 48, 38–42. [Google Scholar] [CrossRef]
- De Costas, S.F.; Matas, N.R.; Ricoy, G.; Sosa, S.; Santillan, G. Diagnóstico de situación de la equinococosis quística en heces dispersas en las zonas de Quebrada y Puna, provincia de Jujuy, Argentina. Rev. Argent. Microbiol. 2014, 46, 80–84. [Google Scholar] [CrossRef]
- Casas, N.; Otero, S.C.; Céspedes, G.; Sosa, S.; Santillán, G. Detección de coproantígenos para el diagnóstico de echinococosis canina en la zona fronteriza de La Quiaca-Villazón. Rev. Argent. Microbiol. 2013, 45, 154–159. [Google Scholar] [CrossRef][Green Version]
- Larrieu, E.; Seleiman, M.; Herrero, E.; Mujica, G.; Labanchi, J.L.; Araya, D.; Grizmado, C.; Sepúlveda, L.; Calabro, A.; Talmón, G.; et al. Vigilancia de la equinococosis quística en perros y niños en la provincia de Río Negro, Argentina. Rev. Argent. Microbiol. 2014, 46, 91–97. [Google Scholar] [CrossRef]
- Carmena, D.; Benito, A.; Eraso, E. Antigens for the immunodiagnosis of Echinococcus granulosus infection: An update. Acta Trop. 2006, 98, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Mujica, G.; Uchiumi, L.; Araya, D.; Salvitti, J.C.; Labanchi, J.L.; Sobrino, M.; Herrero, E.; Panomarenko, O.; Blanco, P.; Talmon, G.; et al. The Diagnosis, Treatment, Surveillance and Control of Cystic Echinococcosis in the Province of Rio Negro: The “One-Health” Model. Parasitologia 2021, 1, 177–187. [Google Scholar] [CrossRef]
- Larrieu, E.; Gavidia, C.M.; Lightowlers, M.W. Control of cystic echinococcosis: Background and prospects. Zoonoses Public Health 2019, 66, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Patricia Maldonado and Evelyn Hohne. Atlas del Gran Chaco Americano; Agencia Alemana de Cooperacion Tecnica: Buenos Aires, Argentina, 2006; ISBN 987-21202-2-6. [Google Scholar]
- Instituto Nacional de Estadística y Censos—INDEC Argentina. Censo Nacional de Población, Hogares y Viviendas 2010: Censo del Bicentenario: Resultados Definitivos; INDEC: Buenos Aires, Argentina, 2012; ISBN 978-950-896-421-2. [Google Scholar]
- Periago, M.V.; García, R.; Astudillo, O.G.; Cabrera, M.; Abril, M.C. Prevalence of intestinal parasites and the absence of soil-transmitted helminths in Añatuya, Santiago del Estero, Argentina. Parasites Vectors 2018, 14, 638. [Google Scholar] [CrossRef] [PubMed]
- Telemann, W. Eine Methode zur Erleichterung der Auffindung von Parasiteneiern in den Faeces. Dtsch. Med. Wochenschr. 1908, 35, 1510–1511. [Google Scholar] [CrossRef]
- Sheather, L. The Detection of Intestinal Protozoa and Mange Parasites by a Floatation Technique. J. Pathol. Ther. 1923, 36, 266–275. [Google Scholar] [CrossRef]
- Willis, H. A Simple Levitation Method for the Detection of Hookworm Ova. Med. J. Aust. 1921, 2, 375–376. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning—Sambrook & Russel; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; Volume 18, ISBN 0879695773. [Google Scholar]
- Cabrera, M.; Canova, S.; Rosenzvit, M.; Guarnera, E. Identification of Echinococcus granulosus eggs. Diagn. Microbiol. Infect. Dis. 2002, 44, 29–34. [Google Scholar] [CrossRef]
- World Health Organization. Meeting of the WHO Informal Working Group on Echinococcosis (WHO-IWGE); WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Coltorti, E.A. Standardization and evaluation of an enzyme immunoassay as a screening test for the seroepidemiology of human hydatidosis. Am. J. Trop. Med. Hyg. 1986, 35, 1000–1005. [Google Scholar] [CrossRef]
- Oriol, C.; Oriol, R. Physicochemical properties of a lipoprotein antigen of Echinococcus granulosus. Am. J. Trop. Med. Hyg. 1975, 24, 96–100. [Google Scholar] [CrossRef]
- Centro Panamericano de Zoonosis (OPS/OMS). Diagnóstico inmunológico de la hidatidosis humana mediante la prueba de doble infusión arco 5. Ramos Mejía Buenos Aires Cent. Panam. Zoonosis 1979. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).