Go West: Hirudins and Decorsin/Ornatin-like Platelet Aggregation Inhibitors in Two Representatives of American Hematophagous Leeches
Abstract
1. Introduction
2. Results
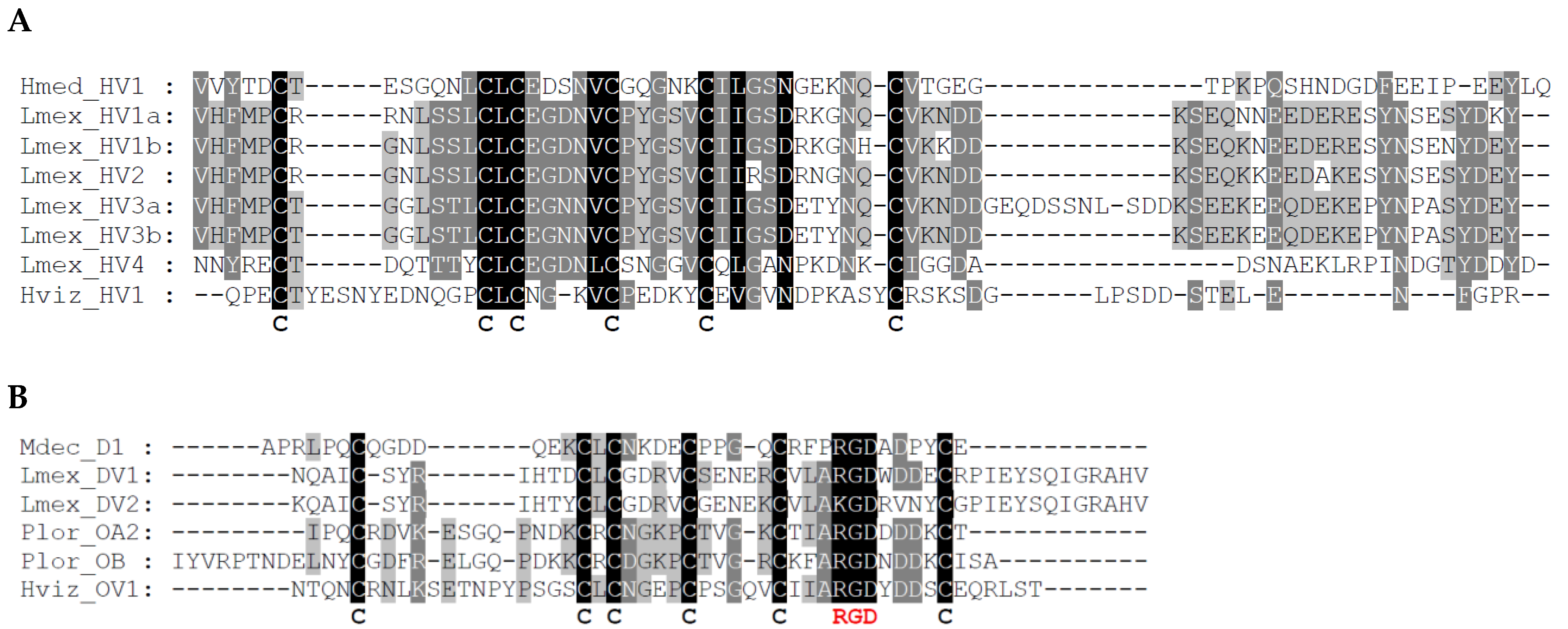
2.1. Identification of Putative Hirudins/HLFs and Decorsins in L. mexicana
2.2. Identification of Putative Hirudins/HLFs and Ornatin in H. vizottoi
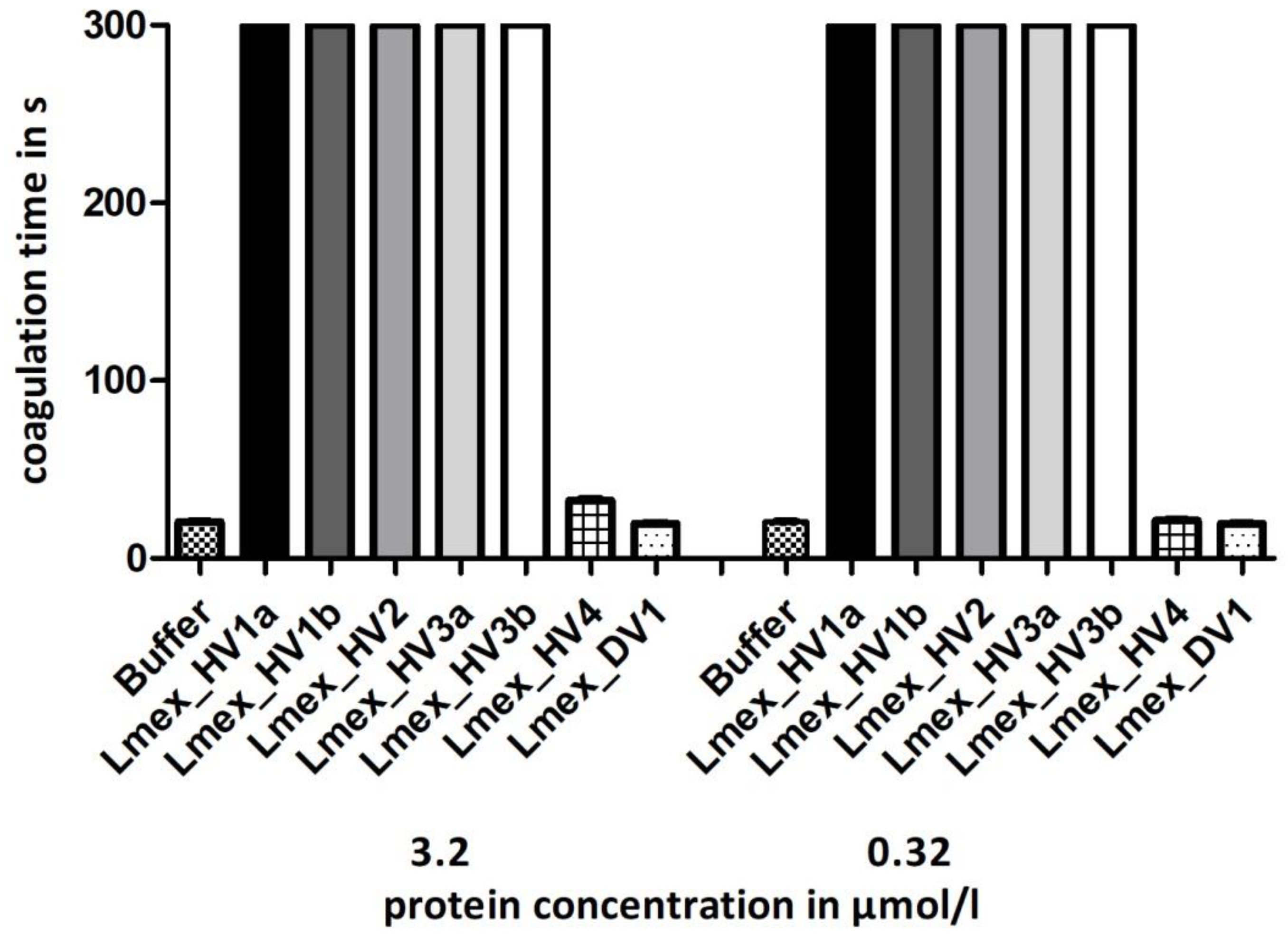
2.3. Functional Characterization I: Thrombin Inhibition
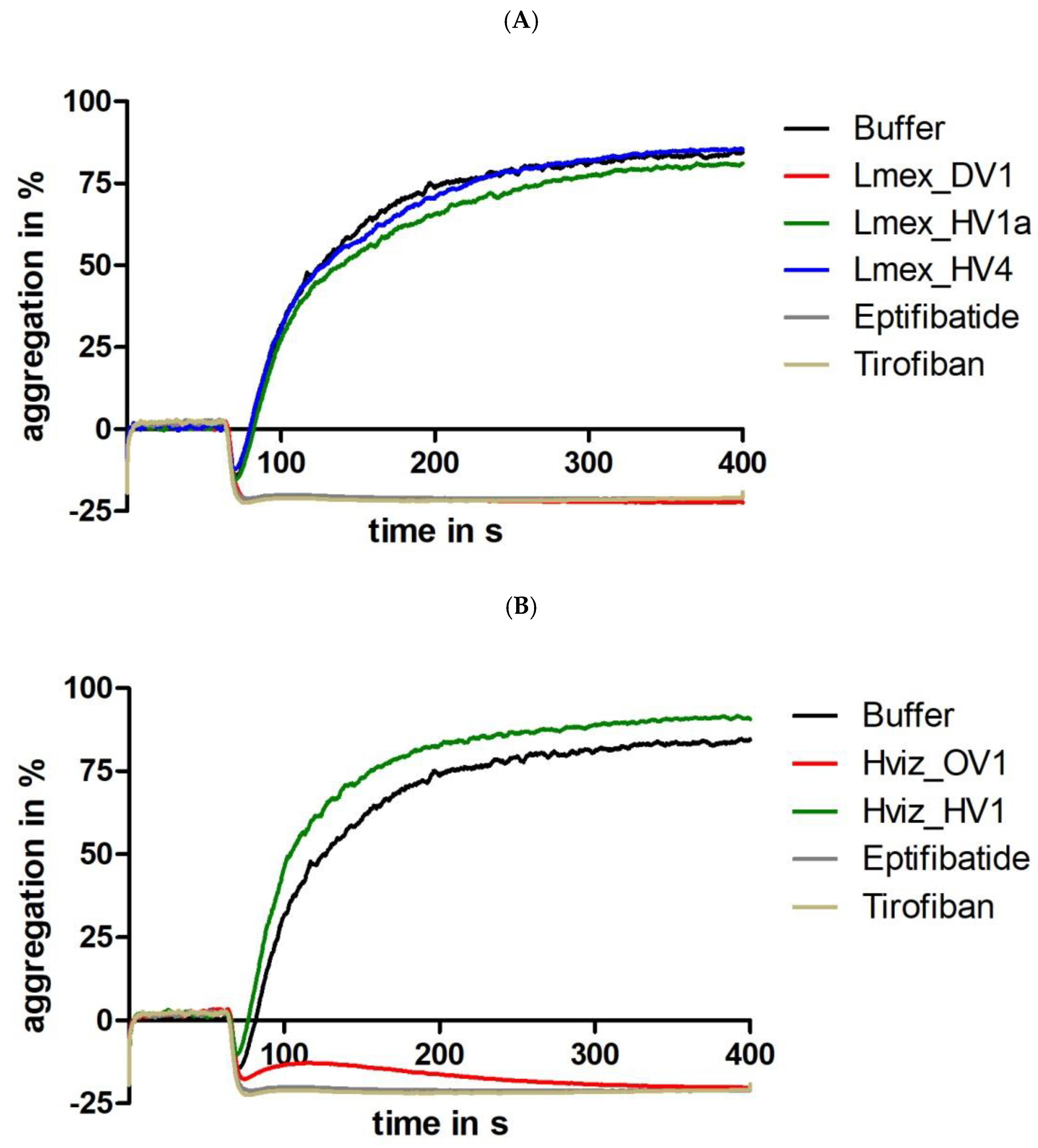
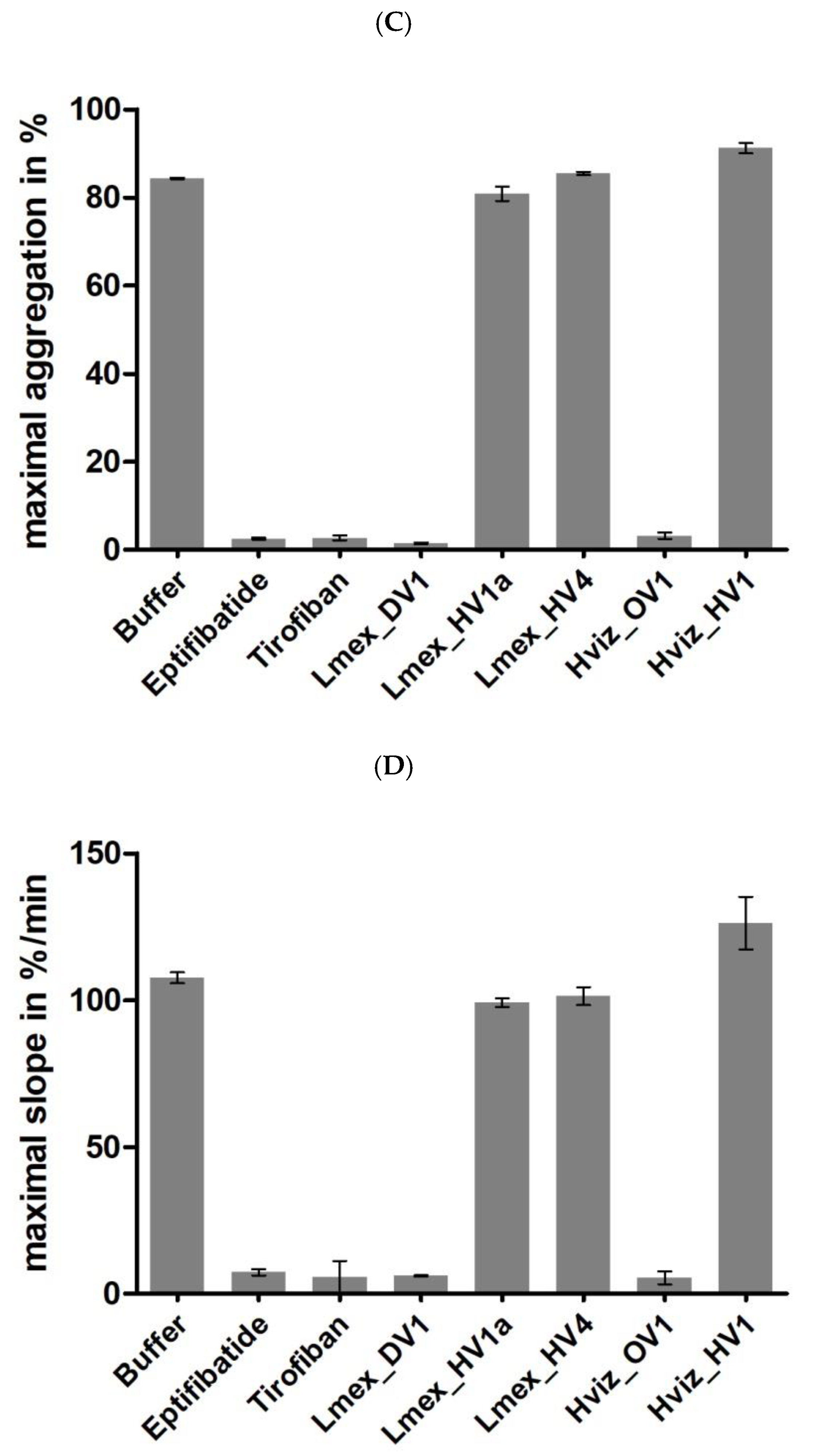
2.4. Functional Characterization II: Platelet Aggregation
3. Discussion
4. Materials and Methods
4.1. Transcriptome Data
4.2. Bioinformatics Tools
4.3. Gene Synthesis
4.4. Amplification and Cloning of Hirudin, Decorsin/Ornatin, and HLF cDNAs
4.5. Expression, Purification, Processing, and Quantification of Putative Hirudins and Decorsins
4.6. Blood Coagulation Assays
4.7. Platelet Aggregation Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boulton, R.A. Hematophagy. In Encyclopedia of Animal Cognition and Behavior; Vonk, J., Shackelford, T.K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Urata, J.; Shojo, H.; Kaneko, Y. Inhibition mechanisms of hematophagous invertebrate compounds acting on the host blood coagulation and platelet aggregation pathways. Biochimie 2003, 85, 493–500. [Google Scholar] [CrossRef]
- Gale, A.J. Continuing education course #2: Current understanding of hemostasis. Toxicol. Pathol. 2011, 39, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Kvist, S.; Brugler, M.R.; Goh, T.G.; Giribet, G.; Siddall, M.E. Pyrosequencing the salivary transcriptome of Haemadipsa interrupta (Annelida: Clitellata: Haemadipsidae) Anticoagulant diversity and insight into the evolution of anticoagulation capabilities in leeches. Invertebr. Biol. 2014, 133, 74–98. [Google Scholar] [CrossRef]
- Siddall, M.E.; Brugler, M.R.; Kvist, S. Comparative transcriptomic analyses of three species of Placobdella (Rhynchobdellida: Glossiphoniidae) confirms a single origin of blood feeding in leeches. J. Parasitol. 2016, 102, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, J.-P.; Lemke, S. Small bite, large impact–saliva and salivary molecules in the medicinal leech. Hirudo medicinalis. Naturwissenschaften 2011, 98, 995–1008. [Google Scholar] [CrossRef]
- Munro, R.; Jones, C.P.; Sawyer, R.T. Calin-a platelet adhesion inhibitor from the saliva of the medicinal leech. Blood Coagul. Fibrinolysis 1991, 2, 179–184. [Google Scholar] [CrossRef]
- Barnes, C.S.; Krafft, B.; Frech, M.; Hofmann, U.R.; Papendieck, A.; Dahlems, U.; Gellissen, G.; Hoylaerts, M.F. Production and characterization of saratin, an inhibitor of von Willebrand factor-dependent platelet adhesion to collagen. Semin. Thromb. Hemost. 2001, 27, 337–348. [Google Scholar] [CrossRef]
- Seymour, J.L.; Henzel, W.J.; Nevins, B.; Stults, J.T.; Lazarus, R.A. Decorsin. A potent glycoprotein IIb-IIIa antagonist and platelet aggregation inhibitor from the leech Macrobdella decora. J. Biol. Chem. 1990, 265, 10143–10147. [Google Scholar] [CrossRef]
- Min, G.-S.; Sarkar, I.N.; Siddall, M.E. Salivary transcriptome of the North American medicinal leech, Macrobdella decora. J. Parasitol. 2010, 96, 1211–1221. [Google Scholar] [CrossRef]
- Müller, C.; Lukas, P.; Lemke, S.; Hildebrandt, J.-P. Hirudin and decorsins of the North American medicinal leech Macrobdella decora: Gene structure reveals homology to hirudins and hirudin-like factors of Eurasian medicinal leeches. J. Parasitol. 2019, 105, 423–431. [Google Scholar] [CrossRef]
- Mazur, P.; Henzel, W.J.; Seymour, J.L.; Lazarus, R.A. Ornatins: Potent glycoprotein IIb-IIIa antagonists and platelet aggregation inhibitors from the leech Placobdella ornata. Eur. J. Biochem. 1991, 202, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P.; Dennis, M.S.; Seymour, J.L.; Lazarus, R.A. Expression, purification, and characterization of recombinant ornatin E, a potent glycoprotein IIb-IIIa antagonist. Protein Expr. Purif. 1993, 4, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Werber, M.M.; Zeelon, E.; Levanon, A.; Goldberg, M.; Greenstein, L.A.; Haim, B.; Ashkenazi, S.; Gelbhart, H.; Rigbi, M.; Ezov, N.; et al. Yagin, a leech derived FXa inhibitor, expressed in and recovered from E. coli: Anti-thrombotic potency in vitro and in vivo. Thromb. Haemost. 1995, 73, 1312. [Google Scholar]
- Tuszynski, G.P.; Gasic, T.B.; Gasic, G.J. Isolation and characterization of antistasin. An inhibitor of metastasis and coagulation. J. Biol. Chem. 1987, 262, 9718–9723. [Google Scholar] [CrossRef]
- Markwardt, F. Past, present and future of hirudin. Haemostasis 1991, 21 (Suppl. 1), 11–26. [Google Scholar] [CrossRef]
- Montinari, M.R.; Minelli, S. From ancient leech to direct thrombin inhibitors and beyond: New from old. Biomed. Pharmacother. 2022, 149, 112878. [Google Scholar] [CrossRef]
- Warkentin, T.E. Bivalent direct thrombin inhibitors: Hirudin and bivalirudin. Best Pract. Res. Clin. Haematol. 2004, 17, 105–125. [Google Scholar] [CrossRef]
- Electricwala, A.; Sawyer, R.T.; Jones, C.P.; Atkinson, T. Isolation of thrombin inhibitor from the leech Hirudinaria manillensis. Blood Coagul. Fibrinolysis 1991, 2, 83–89. [Google Scholar] [CrossRef]
- Steiner, V.; Knecht, R.; Börnsen, K.O.; Gassmann, E.; Stone, S.R.; Raschdorf, F.; Schlaeppi, J.M.; Maschler, R. Primary structure and function of novel O-glycosylated hirudins from the leech Hirudinaria manillensis. Biochemistry 1992, 31, 2294–2298. [Google Scholar] [CrossRef]
- Müller, C.; Mescke, K.; Liebig, S.; Mahfoud, H.; Lemke, S.; Hildebrandt, J.-P. More than just one: Multiplicity of hirudins and hirudin-like factors in the medicinal leech, Hirudo medicinalis. Mol. Genet. Genom. 2016, 291, 227–240. [Google Scholar] [CrossRef]
- Müller, C.; Wang, Z.; Hamann, M.; Sponholz, D.; Hildebrandt, J.-P. Life without blood: Molecular and functional analysis of hirudins and hirudin-like factors of the Asian non-hematophagous leech Whitmania pigra. J. Thromb. Haemost. 2022, 20, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Lukas, P.; Wolf, R.; Rauch, B.H.; Hildebrandt, J.-P.; Müller, C. Hirudins of the Asian medicinal leech, Hirudinaria manillensis: Same same, but different. Parasitol. Res. 2019, 118, 2223–2233. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Lukas, P.; Böhmert, M.; Hildebrandt, J.-P. Hirudin or hirudin-like factor-that is the question: Insights from the analyses of natural and synthetic HLF variants. FEBS Lett. 2020, 594, 841–850. [Google Scholar] [CrossRef]
- Müller, C.; Lukas, P.; Sponholz, D.; Hildebrandt, J.-P. The hirudin-like factors HLF3 and HLF4-hidden hirudins of European medicinal leeches. Parasitol. Res. 2020, 119, 1767–1775. [Google Scholar] [CrossRef]
- Müller, C.; Eickelmann, C.; Sponholz, D.; Hildebrandt, J.-P. Short tail stories: The hirudin-like factors HLF6 and HLF7 of the Asian medicinal leech, Hirudinaria manillensis. Parasitol. Res. 2021, 120, 3761–3769. [Google Scholar] [CrossRef]
- Ma, C.J.; Li, X.; Chen, H. Research progress in the use of leeches for medical purposes. Tradit. Med. Res. 2021, 6, 15. [Google Scholar] [CrossRef]
- Oliveira, D.G.L.; Alvarez-Flores, M.P.; Lopes, A.R.; Chudzinski-Tavassi, A.M. Functional characterisation of vizottin, the first factor Xa inhibitor purified from the leech Haementeria vizottoi. Thromb. Haemost. 2012, 108, 570–578. [Google Scholar] [CrossRef]
- Linhares, D.D.C.; Faria, F.; Kodama, R.T.; Amorim, A.M.X.P.; Portaro, F.C.V.; Trevisan-Silva, D.; Ferraz, K.F.; Chudzinski-Tavassi, A.M. Novel cysteine protease inhibitor derived from the Haementeria vizottoi leech: Recombinant expression, purification, and characterization. Toxins 2021, 13, 857. [Google Scholar] [CrossRef]
- Amorim, A.M.X.P.; de Oliveira, U.C.; Faria, F.; Pasqualoto, K.F.M.; Junqueira-de-Azevedo, I.D.L.M.; Chudzinski-Tavassi, A.M. Transcripts involved in hemostasis: Exploring salivary complexes from Haementeria vizottoi leeches through transcriptomics, phylogenetic studies and structural features. Toxicon 2015, 106, 20–29. [Google Scholar] [CrossRef]
- Phillips, A.J.; Arauco-Brown, R.; Oceguera-Figueroa, A.; Gomez, G.P.; Beltrán, M.; Lai, Y.-T.; Siddall, M.E. Tyrannobdella rex N. Gen. N. Sp. and the evolutionary origins of mucosal leech infestations. PLoS ONE 2010, 5, e10057. [Google Scholar] [CrossRef]
- Iwama, R.E.; Oceguera-Figueroa, A.; Giribet, G.; Kvist, S. The salivary transcriptome of Limnobdella mexicana (Annelida: Clitellata: Praobdellidae) and orthology determination of major leech anticoagulants. Parasitology 2019, 146, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Krezel, A.M.; Wagner, G.; Seymour-Ulmer, J.; Lazarus, R.A. Structure of the RGD protein decorsin: Conserved motif and distinct function in leech proteins that affect blood clotting. Science 1994, 264, 1944–1947. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Steinberg, K.M.; Larson, D.E.; Wilson, R.K.; Mardis, E.R. The next-generation sequencing revolution and its impact on genomics. Cell 2013, 155, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulou, K.; Boti, M.A.; Adamopoulos, P.G.; Skourou, P.C.; Scorilas, A. Third-Generation Sequencing: The spearhead towards the radical transformation of modern genomics. Life 2022, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Wetterstrand, K.A. DNA Sequencing Costs: Data from the NHGRI Genome Sequencing Program (GSP). Available online: www.genome.gov/sequencingcostsdata (accessed on 12 August 2022).
- Blankenship, D.T.; Brankamp, R.G.; Manley, G.D.; Cardin, A.D. Amino acid sequence of ghilanten: Anticoagulant-antimetastatic principle of the South American leech, Haementeria ghilianii. Biochem. Biophys. Res. Commun. 1990, 166, 1384–1389. [Google Scholar] [CrossRef]
- Wallace, A.; Dennis, S.; Hofsteenge, J.; Stone, S.R. Contribution of the N-terminal region of hirudin to its interaction with thrombin. Biochemistry 1989, 28, 10079–10084. [Google Scholar] [CrossRef]
- Betz, A.; Hofsteenge, J.; Stone, S.R. Interaction of the N-terminal region of hirudin with the active-site cleft of thrombin. Biochemistry 1992, 31, 4557–4562. [Google Scholar] [CrossRef]
- Lazar, J.B.; Winant, R.C.; Johnson, P.H. Hirudin: Amino-terminal residues play a major role in the interaction with thrombin. J. Biol. Chem. 1991, 266, 685–688. [Google Scholar] [CrossRef]
- Kvist, S.; Manzano-Marín, A.; de Carle, D.; Trontelj, P.; Siddall, M.E. Draft genome of the European medicinal leech Hirudo medicinalis (Annelida, Clitellata, Hirudiniformes) with emphasis on anticoagulants. Sci. Rep. 2020, 10, 9885. [Google Scholar] [CrossRef]
- Iwama, R.E.; Tessler, M.; Kvist, S. Leech anticoagulants are ancestral and likely to be multifunctional. Zool. J. Linn. Soc. 2022, 196, 137–148. [Google Scholar] [CrossRef]
- Tong, L.; Dai, S.-X.; Kong, D.-J.; Yang, P.-P.; Tong, X.; Tong, X.-R.; Bi, X.-X.; Su, Y.; Zhao, Y.-Q.; Liu, Z.-C. The genome of medicinal leech (Whitmania pigra) and comparative genomic study for exploration of bioactive ingredients. BMC Genom. 2022, 23, 76. [Google Scholar] [CrossRef] [PubMed]
- Kvist, S.; Oceguera-Figueroa, A.; Tessler, M.; Jimenéz-Armenta, J.; Freeman, R.M., Jr.; Giribet, G.; Siddall, M.E. When predator becomes prey: Investigating the salivary transcriptome of the shark-feeding leech Pontobdella macrothela (Hirudinea: Piscicolidae). Zool. J. Linn. Soc. 2016, 179, 725–737. [Google Scholar] [CrossRef]
- de Carle, D.; Oceguera-Figueroa, A.; Tessler, M.; Siddall, M.E.; Kvist, S. Phylogenetic analysis of Placobdella (Hirudinea: Rhynchobdellida: Glossiphoniidae) with consideration of COI variation. Mol. Phylogenet. Evol. 2017, 114, 234–248. [Google Scholar] [CrossRef]
- Borda, E.; Siddal, M.E. Arhynchobdellida (Annelida: Oligochaeta: Hirudinida) Phylogenetic relationships and evolution. Mol. Phylogenet. Evol. 2004, 30, 213–225. [Google Scholar] [CrossRef]
- Phillips, A.J.; Siddall, M.E. Poly-paraphyly of Hirudinidae: Many lineages of medicinal leeches. BMC Evol. Biol. 2009, 9, 246. [Google Scholar] [CrossRef]
- Phillips, A.J.; Dornburg, A.; Zapfe, K.L.; Anderson, F.E.; James, S.W.; Erséus, C.; Lemmon, E.M.; Lemmon, A.R.; Williams, B.W. Phylogenomic analysis of a putative missing link sparks reinterpretation of leech evolution. Genome Biol. Evol. 2019, 11, 3082–3093. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, K.B.; Nicholas, H.B., Jr. GeneDoc: A Tool for Editing and Annotating Multiple Sequence Alignments. Distributed by the Author 1997. Available online: https://nrbsc.org/gfx/genedoc (accessed on 15 September 2022).
- Käll, L.; Krogh, A.; Sonnhammer, E.L.L. Advantages of combined transmembrane topology and signal peptide prediction-the Phobius web server. Nucleic Acids Res. 2007, 35, W429–W432. [Google Scholar] [CrossRef]
- Teufel, F.; Armenteros, J.J.A.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Gill, S.C.; von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989, 182, 319–326. [Google Scholar] [CrossRef]
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995, 4, 2411–2423. [Google Scholar] [CrossRef]
- Zhou, L.; Schmaier, A.H. Platelet aggregation testing in platelet-richplasma: Description of procedures with the aim to develop standards in the field. Am. J. Clin. Pathol. 2005, 123, 172–183. [Google Scholar] [CrossRef] [PubMed]
| Factor | pI | Length in aa | MW in Da |
|---|---|---|---|
| Hmed_HV1 | 4.04 | 65 | 7026.58 |
| Lmex_HV1a | 4.64 | 66 | 7573.23 |
| Lmex_HV1b | 4.69 | 66 | 7539.21 |
| Lmex_HV2 | 4.64 | 66 | 7502.19 |
| Lmex_HV3a | 3.88 | 77 | 8539.1 |
| Lmex_HV3b | 4.07 | 66 | 7391.04 |
| Lmex_HV4 | 4.03 | 63 | 6843.29 |
| Hviz_HV1 | 4.22 | 63 | 6983.51 |
| Mdec_D1 | 4.46 | 39 | 4383.86 |
| Lmex_DV1 | 5.08 | 50 | 5757.4 |
| Lmex_DV2 | 8.3 | 50 | 5621.46 |
| Plor_OA2 | 8.32 | 41 | 4455.03 |
| Plor_OB | 7.75 | 52 | 5872.61 |
| Hviz_OV1 | 4.59 | 50 | 5425.93 |
| Factor | N-Terminus | Source |
|---|---|---|
| Hviz_HV1a | QPEC | native |
| Hviz_HV1b | GQPEC | native |
| Hviz_HV1c | VVYTDC | HV1 of H. medicinalis |
| Hviz_HV1d | VHFPPC | hirudin of Placobdella costata |
| Hviz_HV1e | VHFMPC | Lmex_HV1 of L. mexicana |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfordt, V.; Kalatehjari, P.; Tolksdorf, C.; Rauch, B.H.; Müller, C. Go West: Hirudins and Decorsin/Ornatin-like Platelet Aggregation Inhibitors in Two Representatives of American Hematophagous Leeches. Parasitologia 2022, 2, 313-325. https://doi.org/10.3390/parasitologia2040026
Pfordt V, Kalatehjari P, Tolksdorf C, Rauch BH, Müller C. Go West: Hirudins and Decorsin/Ornatin-like Platelet Aggregation Inhibitors in Two Representatives of American Hematophagous Leeches. Parasitologia. 2022; 2(4):313-325. https://doi.org/10.3390/parasitologia2040026
Chicago/Turabian StylePfordt, Victoria, Pegah Kalatehjari, Céline Tolksdorf, Bernhard H. Rauch, and Christian Müller. 2022. "Go West: Hirudins and Decorsin/Ornatin-like Platelet Aggregation Inhibitors in Two Representatives of American Hematophagous Leeches" Parasitologia 2, no. 4: 313-325. https://doi.org/10.3390/parasitologia2040026
APA StylePfordt, V., Kalatehjari, P., Tolksdorf, C., Rauch, B. H., & Müller, C. (2022). Go West: Hirudins and Decorsin/Ornatin-like Platelet Aggregation Inhibitors in Two Representatives of American Hematophagous Leeches. Parasitologia, 2(4), 313-325. https://doi.org/10.3390/parasitologia2040026







