Seroprevalence and Risk Factors Associated with Toxoplasma gondii and Chlamydophila abortus Infection in Domestic Small Ruminants in Cameroon
Abstract
1. Introduction
2. Results
2.1. Seroprevalence of T. gondii and C. abortus at Flock and Individual (Animal) Level
2.2. Risk Factor Analysis
3. Discussion
4. Materials and Methods
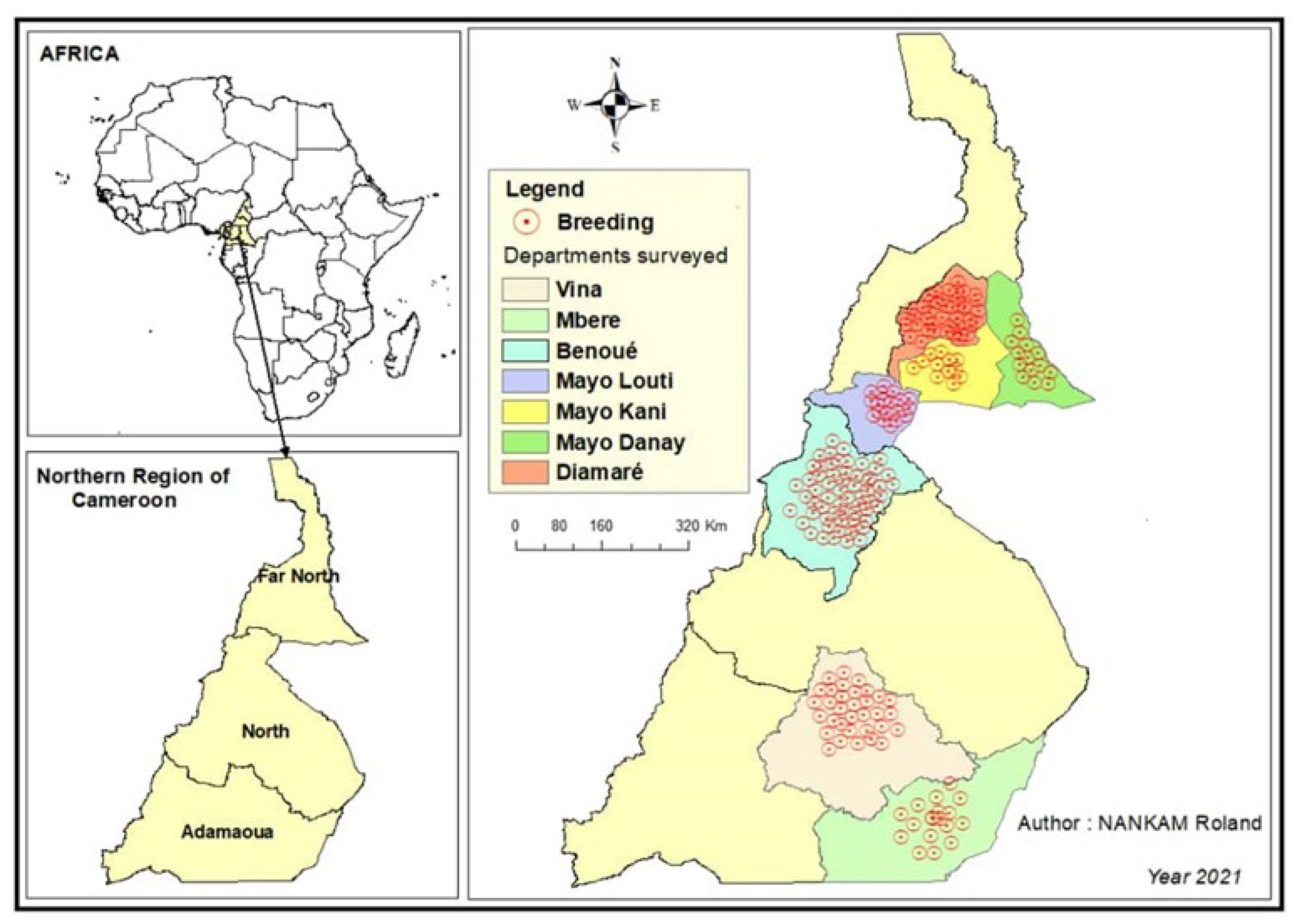
4.1. Study Area
4.2. Study Design, Sample and Data Collection
4.3. Serological Examination
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Njoya, A. Strengthening the Resilience and Adaptive Capacity of Livestock Farmers to Climate Variability and Extreme Events in the Sahel and Savannah Regions of West and Central Africa. 2010, p. 96. Available online: https://www.apess.org/wp-content/uploads/2019/05/Rapport-final-1.pdf (accessed on 17 April 2022).
- MINEPIA. Support for the Improvement of the Control of Transboundary Diseases of Livestock in Trade. 2011, pp. 1–32. Available online: https://www.standardsfacility.org/sites/default/files/STDF_PG_336_Application_May-12.pdf (accessed on 17 April 2022).
- MINEPIA. National Plan for the Control and Eradication of Peste des Petits Ruminants. 2018, p. 57. Available online: https://www.prodel.cm/wp-content/uploads/2019/04/Plan-national-de-contrôle-et-déradication-de-la-PPR-au-Cameroun-Phase-2018-2023.pdf (accessed on 17 April 2022).
- FAO. Support for the Improvement of Transboundary Livestock Disease Control in Cameroon. 2018, pp. 1–46. Available online: http://www.standardsfacility.org/sites/default/files/STDF_PG_336_Evaluation_Report_Final_FR.pdf (accessed on 17 April 2022).
- MINEPIA. Environmental and Social Management Framework (CGES) of the Livestock Development Project (PRODEL) in the Working Group for the Preparation of the Livestock Development Project (PRODEL). 2018, p. 124. Available online: https://documents1.worldbank.org/curated/en/291221472106313475/pdf/SFG2401-EA-FRENCH-P154908-Box396304B–PUBLIC-Disclosed-8-24-2016.pdf (accessed on 17 April 2022).
- Dubey, J.P. Toxoplasmosis of Animals and Humans, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2021; p. 564. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009; p. 336. [Google Scholar] [CrossRef]
- Menzies, P. Control of Important Causes of Infectious Abortion in Sheep and Goats. Vet. Clin. Food Anim. Pract. 2011, 27, 81–93. [Google Scholar] [CrossRef]
- Cremoux, R.; Pouget, C.; Lacz, C. Differential diagnosis of abortions in small ruminants in Midi-Pyrénées. Bull. GTV 2017, 1, 73–82. [Google Scholar]
- Elandalousi, R.B.; Ghram, A.; Maaroufi, A.; Mnif, W. Seroprevalence of zoonotic abortifacient diseases in ruminants in northern Tunisia. Research 2015, 2, 1419. [Google Scholar] [CrossRef]
- Khammassi-Khabou, M.; Hammami, S.; Cherif, A.; Majok, A. Seroprevalence of Major Infectious Diseases Causing Abortion in Small Ruminants. 2009, pp. 5–29. Available online: https://www.researchgate.net/publication/279978031_Seroprevalence_des_majeures_maladies_infectieuses_causant_l’avortement_chez_les_petits_ruminants (accessed on 31 July 2020).
- Qin, S.Y.; Huang, S.Y.; Yin, M.Y.; Tan, Q.D.; Liu, G.X.; Zhou, D.H.; Zhu, X.Q.; Zhou, J.Z.; Qian, A.D. Seroprevalence and risk factors of Chlamydophila abortus infection in free-ranging white yaks in China. BMC Vet. Res. 2015, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, H.L.; Zahida, T. Seroprevalence of Toxoplasmosis in Sheep in Southern Punjab, Pakistan. Pak. Vet. J. 2010, 30, 91–94. [Google Scholar]
- Lahmar, I.; Lachkhem, A.; Slama, D.; Sakly, W.; Haouas, N.; Gorcii, M.; Pfaff, A.W.; Candolfi, E.; Babba, H. Prevalence of Toxoplasmosis in Sheep, Goats and Cattle in Southern Tunisia. J. Bacteriol. Parasitol. 2015, 6, 10–14. [Google Scholar] [CrossRef]
- Galván-Ramírez, M.D.L.L.; Charles-Niño, C.; Pedroza-Roldán, C.; Salazar-Reveles, C.; Ocampo-Figueroa, K.L.; Rodríguez-Pérez, L.R.; Paez-Magallán, V.M. Prevalence of Toxoplasma gondii Measured by Western Blot, ELISA and DNA Analysis, by PCR, in Cats of Western Mexico. Pathogens 2022, 11, 109. [Google Scholar] [CrossRef]
- Li, G.; Zheng, W.; Yang, J.; Qi, T.; He, Y.; Chen, W.; Ma, H.; Sun, Y.; Li, Y.; Kang, M.; et al. Seroprevalence and epidemiology of toxoplasma gondii in animals in the qinghai-tibetan plateau area, china. Pathogens 2021, 10, 432. [Google Scholar] [CrossRef]
- Sidibe, S.; Coulibaly, K.; Sery, A.; Fofana, M.; Sidibe, S.; Kanoute, M. Prevalence of brucellosis, chlamydia and toxoplasmosis in small ruminants in Mali: Results of a sero-epidemiological survey. Rev. Mali. D’infect. Microbiol. 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Malal, M.E.; Karagül, M.S.; Akar, K. Serological investigation of ovine chlamydiosis in small ruminants in Western Turkey. Acta Vet. Brno 2020, 89, 255–261. [Google Scholar] [CrossRef]
- Al-Qudah, K.M.; Sharif, L.A.; Raouf, R.Y.; Hailat, N.Q.; Al-Domy, F.M. Seroprevalence of antibodies to Chlamydophila abortus shown in Awassi sheep and local goats in Jordan. Vet. Med. 2004, 49, 460–466. [Google Scholar] [CrossRef]
- Bamba, S.; Faye, B.; Tarnagda, Z.; Boly, N.; Guiguemdé, T.; Villena, I. Séroprévalence de la toxoplasmose chez les ovins à Bobo-Dioulasso, Burkina Faso. Rev. D’élevage Méd. Vét. Pays Trop. 2012, 65, 63. [Google Scholar] [CrossRef][Green Version]
- Fayez, M.; Elmoslemany, A.; Alorabi, M.; Alkafafy, M.; Qasim, I.; Al-Marri, T.; Elsohaby, I. Seroprevalence and risk factors associated with Chlamydophila abortus infection in sheep and goats in eastern Saudi Arabia. Pathogens 2021, 10, 489. [Google Scholar] [CrossRef]
- Li, M.-H.; Yang, B.-T.; Yin, Z.-W.; Wang, W.; Zhao, Q.; Jiang, J. A Seroepidemiological Survey of Toxoplasma gondii and Chlamydia Infection in Chickens, Ducks, and Geese in Jilin Province, Northeastern China. Vector-Borne Zoonotic Dis. 2020, 20, 825–830. [Google Scholar] [CrossRef]
- Sachse, K.; Hotzel, H.; Slickers, P.; Ellinger, T.; Ehricht, R. DNA microarray-based detection and identification of Chlamydia and Chlamydophila spp. Mol. Cell. Probes 2005, 19, 41–50. [Google Scholar] [CrossRef]
- Todjom, F.G.; Tsapi, E.M.; Gamago, G.; Vignoles, P.; Pone, J.W.; Teukeng, F.D. Seroprevalence of Toxoplasmosis and associated risk factors in pregnant women at the Protestant Hospital, Mbouo-Bandjoun, Cameroon. Afr. J. Clin. Exp. Microbiol. 2019, 20, 221. [Google Scholar] [CrossRef]
- Njunda, A.L.; Assob, J.C.N.; Nsagha, D.S.; Kamga, H.L.; Nde, P.F.; Yugah, V.C. Seroprevalence of toxoplasma gondii infection among pregnant women in Cameroon. J. Public Health Afr. 2011, 2, 98–101. [Google Scholar] [CrossRef]
- Domenech, J.; Trap, D.; Gaumont, R. Cattle in Central Africa: A survey of chlamydia and Q fever. Rev. Elev. Méd. Vét. Pays Trop. 1985, 38, 138–143. [Google Scholar]
- Musallam, I.; Abo-Shehada, M.; Omar, M.; Guitian, J. Cross-sectional study of brucellosis in Jordan: Prevalence, risk factors and spatial distribution in small ruminants and cattle. Prev. Vet. Med. 2015, 118, 387–396. [Google Scholar] [CrossRef]
- Laanen, M.; Beek, J.; Ribbens, S.; Vangroenweghe, F.; Maes, D.; Dewulf, J. Biosecurity on pig herds: Development of an on-line scoring system and the results of the first 99 participating herds. Vlaams Diergeneeskd. Tijdschr. 2010, 79, 302–306. [Google Scholar]
- Duteurtre, G.; Faye, B.; Dutilly-diane, C.; Alary, V. Elevage et dynamique de la pauvreté: L’approche micro-économique. Mémento L’agronome CIRAD GRET Fr Montp. CIRAD 2002, 5, 1–10. [Google Scholar]
- Orskov, E.R. Goat production on a global basis. Small Rumin. Res. 2011, 98, 9–11. [Google Scholar] [CrossRef]
- Iñiguez, L. The challenges of research and development of small ruminant production in dry areas. Small Rumin. Res. 2011, 98, 12–20. [Google Scholar] [CrossRef]
- Dedieu, B.; Aubin, J.; Duteurtre, G.; Alexandre, G.; Vayssieres, J.; Faye, B.; Bommel, P.; Mahieu, A.; Fanchone, A.; Tourrand, J.-F.; et al. Design and Evaluation of Sustainable Livestock Systems in Hot Regions. 2011. Available online: https://agritrop.cirad.fr/560828/1/document_560828.pdf (accessed on 24 March 2020).
- INS. Chapitre 14: Breeding and Fishing. In Annuary Statistics of the Cameroun; National Statistical Institute: Yaounde, Cameroun, 2020; pp. 209–219. Available online: https://ins-cameroun.cm/wp-content/uploads/2021/02/0CHAPITRE-14_PECHE-ET-ELEVAGE.pdf (accessed on 24 February 2022).
- García-Bocanegra, I.; Cabezón, O.; Hernández, E.; Martinez-Cruz, M.S.; Martinez-Moreno, A.; Martinez-Moreno, J. Toxoplasma gondii in ruminant species (cattle, sheep, and goats) from southern Spain. J. Parasitol. 2013, 99, 438–440. [Google Scholar] [CrossRef]
- Kamani, J.; Mani, A.U.; Egwu, G.O. Seroprevalence of Toxoplasma gondii infection in domestic sheep and goats in Borno state, Nigeria. Trop. Anim. Health Prod. 2010, 42, 793–797. [Google Scholar] [CrossRef]
- Sah, R.P.; Talukder, H.; Rahman, A.A.; Alam, M.Z.; Ward, M.P. Seroprevalence of Toxoplasma gondii infection in ruminants in selected districts in Bangladesh. Vet. Parasitol. Reg. Stud. Rep. 2018, 11, 1–5. [Google Scholar] [CrossRef]
- Romano, J.D.; Coppens, I. Host Organelle Hijackers: A similar modus operandi for Toxoplasma gondii and Chlamydia trachomatis: Co-infection model as a tool to investigate pathogenesis. Pathog. Dis. 2013, 69, 72–86. [Google Scholar] [CrossRef][Green Version]
- Données-Mondiale. Climate: Adamawa, Cameroon. World Data. Available online: https://www.worlddata.info/africa/cameroon/index.php (accessed on 24 February 2022).
- Alexander, J.; Stimson, W.H. Sex hormones and the course of parasitic infection. Parasitol. Today 1988, 4, 189–193. [Google Scholar] [CrossRef]
- Meyer, C.; Faye, B.; Karembe, H. Mediterranean and Tropical Sheep Breeding Guide; CIRAD-EMVT: Montpellier, France, 2004; pp. 92–116. [Google Scholar]
- SCAR. Observatory and Monitoring of the Causes of Abortions in Ruminants Report 2019. 2020, p. 80. Available online: https://www.plateforme-esa.fr/sites/default/files/RésultatsOSCAR2019_VF.pdf (accessed on 24 February 2022).
- Guillaume, B.; Maud, G. Form of Resistance in the External Environment. Biología 2017, 11, 3. Available online: https://m.20-bal.com/biolog/16568/index.html (accessed on 24 February 2022).
| Variables | N | T. gondii | C. abortus | ||||
|---|---|---|---|---|---|---|---|
| Prevalence n (%) | 95% CI | p Value | Prevalence n (%) | 95% CI | p Value | ||
| Flock prevalence | 200 | 74 (37) | 30.3–43.7 | 0.0001 ** | 6 (3) | 0.64–5.4 | 0.0001 ** |
| Individual prevalence | 1061 | 329 (31.1) | 28.2–33.8 | 0.0001 ** | 45 (4.2) | 3.0–5.5 | 0.0001 ** |
| Variables | N | C. abortus | ||
|---|---|---|---|---|
| Prevalence n (%) | OR (95% CI) | p Value | ||
| T. gondii | ||||
| Doubtful | 37 | 1 (2.7) | 1.0 (Ref.) | 0.02 * |
| Negative | 695 | 38 (5.5) | 2.1 (3.8–7.2) | 0.46 |
| Positive | 329 | 6 (1.8) | 0.67 (0.37–3.3) | 0.71 |
| Factors | T. gondii (95% CI) | p Value | C. abortus (95% CI) | p Value |
|---|---|---|---|---|
| Flock risk factors association | ||||
| Regions | 0.22 (0,17; 0.26) | 0.00 ** | 0.01 (0.00; 0.02) | 0.02 * |
| Breeding objectives | 0.36 (0,17; 0.56) | 0.00 ** | 0.02 (−0.03; 0.07) | 0.45 |
| Hygiene level | 0.11 (0.01; 0.21) | 0.03 * | 0.009 (0.0008; 0.01) | 0.03 |
| Presence of abortions | 0.55 (0.46; 0.64) | 0.00 ** | −0.26 (−0.39; −0.14) | 0.0001 ** |
| Constant | 0.11 (−0.49; 0.71) | 0.002 ** | 0.30 (0.14; 0.45) | 0.002 ** |
| N = 200 | R2 = 0.08 | R2 = 0.09 | ||
| Animal risk-factors association | ||||
| Region | −0.09 (−0.14; −0.04) | 0.00 ** | 0.02 (0.00; 0.03) | 0.04 * |
| Species | 0.07 (0.01; 0.14) | 0.03 * | 0.008 (−0.01; 0.03) | 0.52 |
| Sex | −0.11 (−0.18; −0.03) | 0.005 ** | 0.01 (−0.01; 0.04) | 0.32 |
| Age | 0.08 (0.04; 0.12) | 0.0001 ** | −0.00 (−0.02; 0.01) | 0.94 |
| Physiological status | 0.21 (0.18; 0.24) | 0.00 ** | 0.01 (−0.01; 0.02) | 0.42 |
| Breed | 0.01 (−0.01; 0.03) | 0.30 | 0.01 (0.01; 0.02) | 0.001 ** |
| Constant | 0.48 (0.26; 0.74) | 0.0001 ** | −0.05 (−0.14; 0.05) | 0.01 * |
| N = 200 | R2 = 0.03 | R2 = 0.02 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nankam, R.C.; Kouamo, J.; Kouengoua, A.P.K.; Tchinze, G.J.T.; Dzousse, M.F.; Gapgueu, S.V.; Guefack Noumedem, R.N.; Ngoula, F. Seroprevalence and Risk Factors Associated with Toxoplasma gondii and Chlamydophila abortus Infection in Domestic Small Ruminants in Cameroon. Parasitologia 2022, 2, 198-205. https://doi.org/10.3390/parasitologia2030017
Nankam RC, Kouamo J, Kouengoua APK, Tchinze GJT, Dzousse MF, Gapgueu SV, Guefack Noumedem RN, Ngoula F. Seroprevalence and Risk Factors Associated with Toxoplasma gondii and Chlamydophila abortus Infection in Domestic Small Ruminants in Cameroon. Parasitologia. 2022; 2(3):198-205. https://doi.org/10.3390/parasitologia2030017
Chicago/Turabian StyleNankam, Roland Chimi, Justin Kouamo, Armelle Prudence Kouengoua Kouengoua, Grace Jedida Toukem Tchinze, Müller Fotsac Dzousse, Sandra Vanessa Gapgueu, Ranyl Nguena Guefack Noumedem, and Ferdinand Ngoula. 2022. "Seroprevalence and Risk Factors Associated with Toxoplasma gondii and Chlamydophila abortus Infection in Domestic Small Ruminants in Cameroon" Parasitologia 2, no. 3: 198-205. https://doi.org/10.3390/parasitologia2030017
APA StyleNankam, R. C., Kouamo, J., Kouengoua, A. P. K., Tchinze, G. J. T., Dzousse, M. F., Gapgueu, S. V., Guefack Noumedem, R. N., & Ngoula, F. (2022). Seroprevalence and Risk Factors Associated with Toxoplasma gondii and Chlamydophila abortus Infection in Domestic Small Ruminants in Cameroon. Parasitologia, 2(3), 198-205. https://doi.org/10.3390/parasitologia2030017






