Infection with Leishmania (Leishmania) infantum Changes the Morphology and Myenteric Neurons of the Jejunum of Golden Hamsters
Abstract
1. Introduction
2. Results
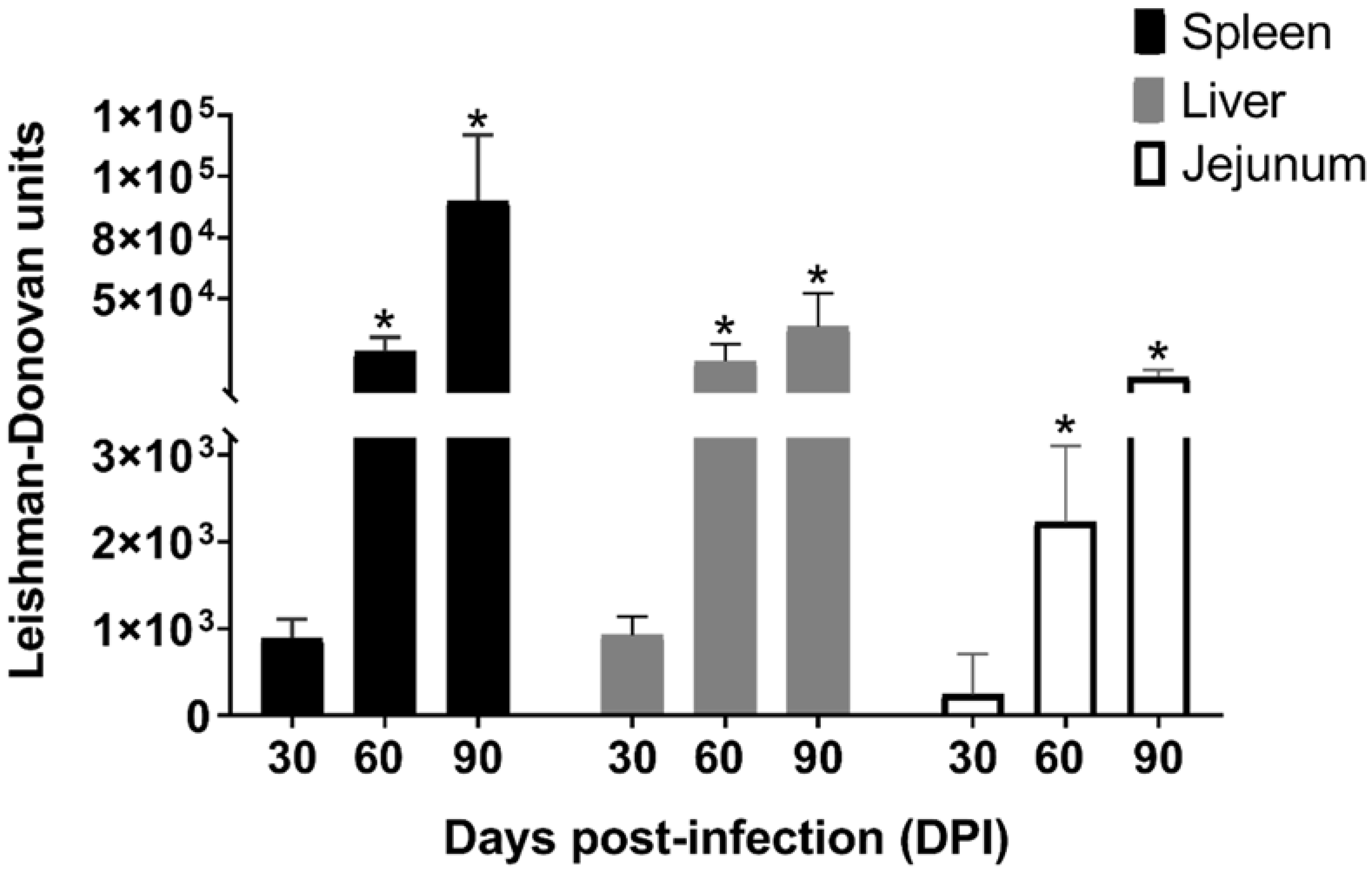
2.1. Parasite Load
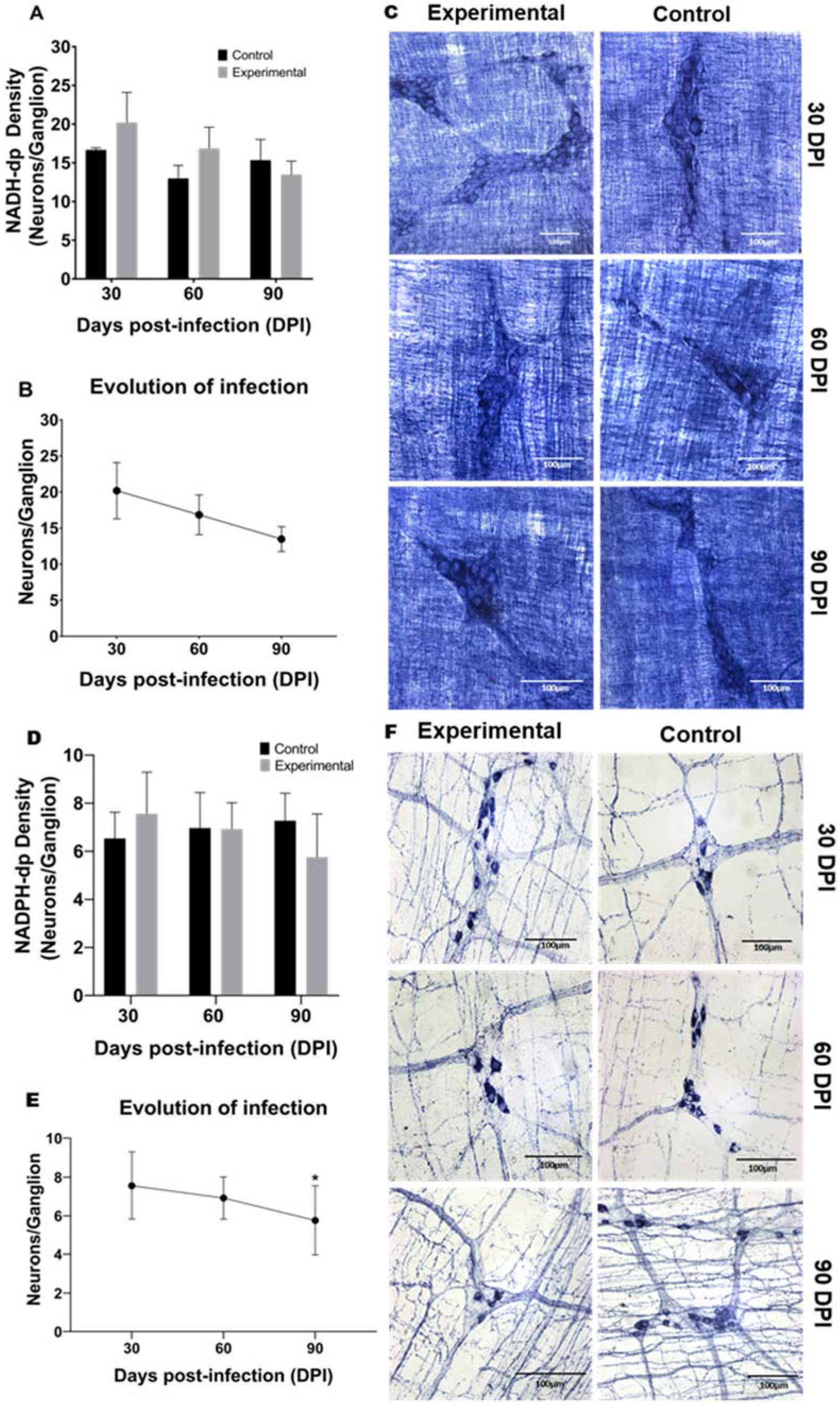
2.2. Neuronal Density and Infection Progression of NADH-dp and NADPH-dp
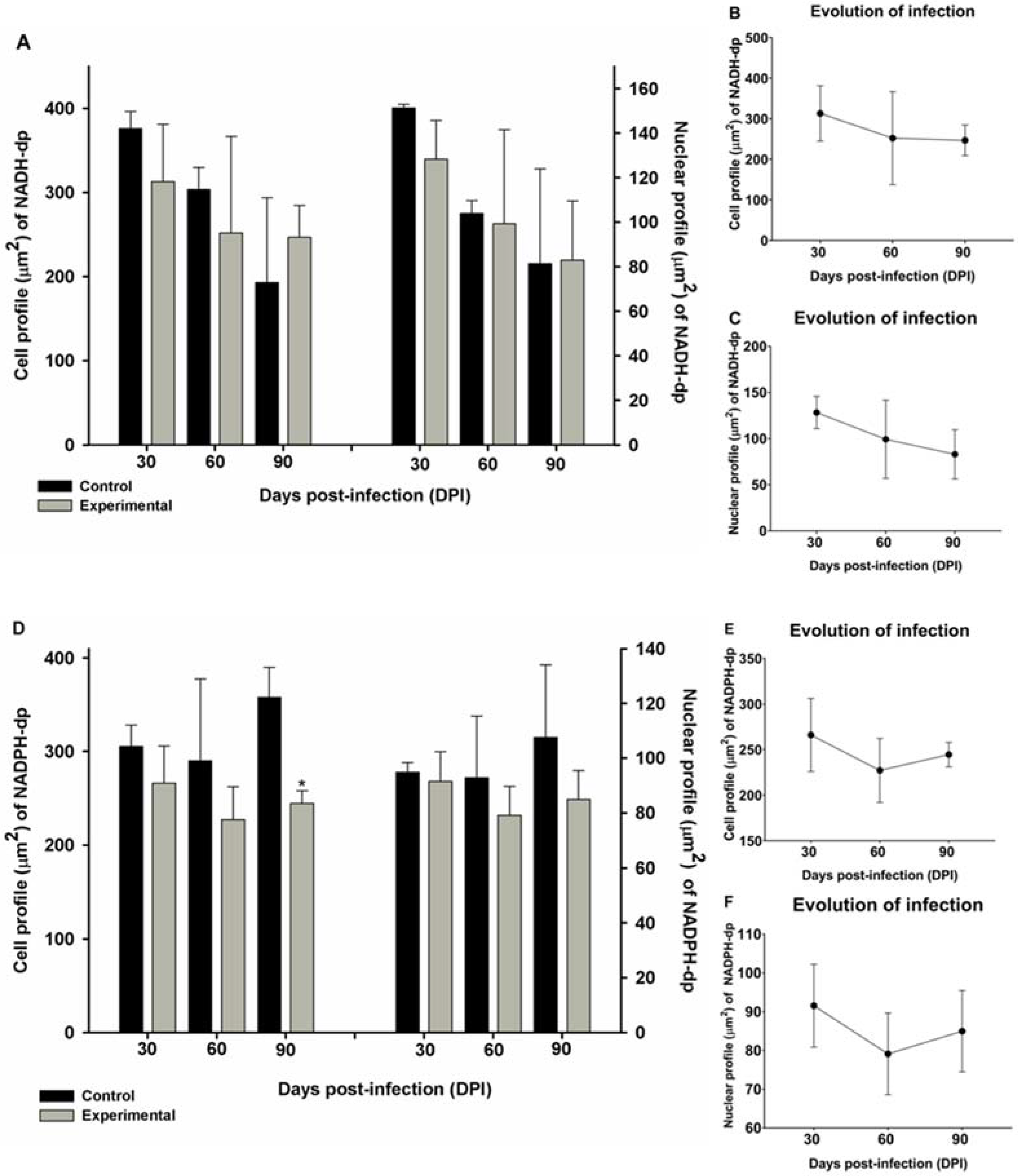
2.3. Morphometry and Infection Progression of NADH-dp and NADPH-dp Neurons
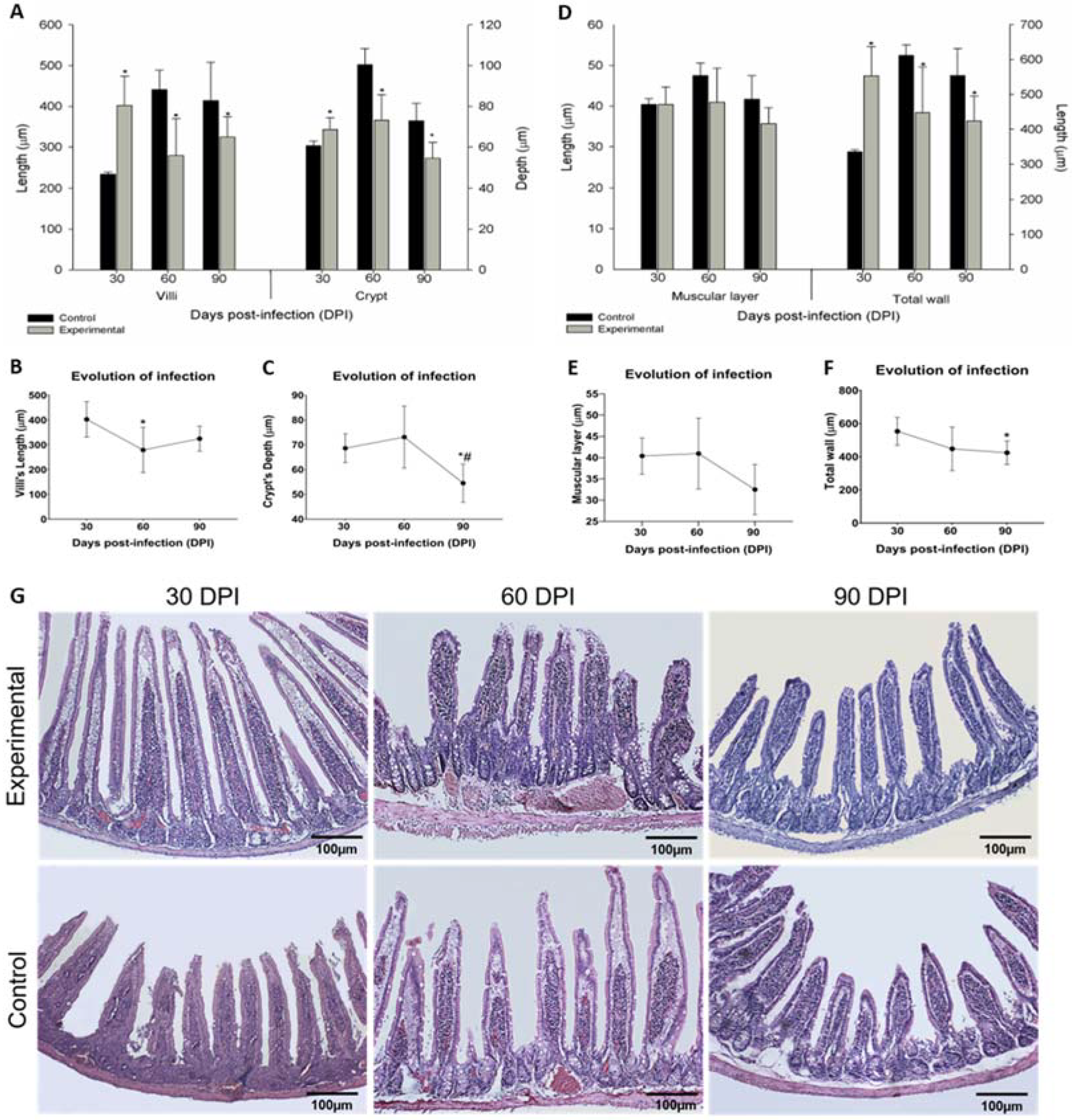
2.4. Stratigraphy Evaluation
2.5. Mucosa Analysis
3. Discussion
4. Materials and Methods
4.1. Animals and Ethical Considerations
4.2. Parasites and Infection
4.3. Parasite Load Analysis
4.4. Neuronal Detection
4.5. Morpho-Quantitative Analysis of Myenteric Neurons
4.6. Morphometric Analysis of Intestinal Stratigraphy and Quantitative Analysis of Mucosal Cells
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef]
- Wilhelm, T.J. Viszerale Leishmaniose. Der Chir. 2019, 90, 833–837. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 25 August 2021).
- Yeshaw, Y.; Tsegaye, A.T.; Nigatu, S.G. Incidence of Mortality and Its Predictors among Adult Visceral Leishmaniasis Patients at the University of Gondar Hospital: A Retrospective Cohort Study. Infect. Drug Resist. 2020, 13, 881. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, P.I.; Da Silva, L.M.; Pessoa, G.T.; De Sá, R.P.R.; Sanches, M.P.; Das Neves, A.D.; Das Chagas, F.A.S.; Guerra, P.C.; Neves, W.C.; Giglio, R.F.; et al. Comparative B-Mode and Doppler Renal Ultrasonography with Histopathological Findings in Dogs Positive for Canine Visceral Leishmaniasis. Microsc. Res. Tech. 2016, 79, 637–645. [Google Scholar] [CrossRef]
- Lisboa, A.R.; Leite, F.C.; Dantas, A.E.F.; Oliveira, I.B.; Evangelista, T.R.; Sousa, J.B.G. Análise Epidemiológica de Leishmaniose Visceral Em Municípios Do Sertão Paraibano. Rev. Bras. Educ. Saúde 2016, 6, 5. [Google Scholar] [CrossRef]
- Marinho, C.P.; Souza, I.M.; Xavier, M.E.B.; Dourisboure, C.J.; Braz, P.H. Achado Citopatológico de Formas Amastigota de Leishmania Spp. Na Língua de Um Canino: Relato de Caso. Pubvet 2017, 11, 1104–1107. [Google Scholar] [CrossRef]
- van Griensven, J.; Diro, E. Visceral Leishmaniasis. Infect. Dis. Clin. N. Am. 2012, 26, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.M.H.S.; Winck, C.A. Leishmaniose Visceral Canina: Revisão de Literatura. Rev. Univ. Val. Rio Verde 2018, 16, 1. [Google Scholar] [CrossRef]
- Silva, D.T.D.; Alves, M.L.; Spada, J.C.P.; Silva, A.C.D.; Silveira, R.D.C.V.D.; Oliveira, T.M.F.D.S.; Starke-Buzetti, W.A. T Lymphocytes and Macrophages in the Intestinal Tissues of Dogs Infected with Leishmania Infantum. Rev. Bras. Parasitol. Vet. 2017, 26, 159–170. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Carneiro, J. Histologia Básica, 12nd ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2013. [Google Scholar]
- Ross, M.H.; Pawlina, W. Histology: A Text and Atlas, with Correlated Cell and Molecular Biology, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2017. [Google Scholar]
- Ahluwalia, B.; Magnusson, M.K.; Öhman, L. Mucosal Immune System of the Gastrointestinal Tract: Maintaining Balance between the Good and the Bad. Scand. J. Gastroenterol. 2017, 52, 1185–1193. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal Epithelial Cells: Regulators of Barrier Function and Immune Homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Romero, E.S.; Cotoner, A.C.; Camacho, C.P.; Bedmar, M.C.; Vicario, M. The Intestinal Barrier Function and Its Involvement in Digestive Disease. Rev. Esp. Enferm. Dig. 2015, 107, 686–696. [Google Scholar] [CrossRef]
- Lema, I.; Araújo, J.R.; Rolhion, N.; Demignot, S. Jejunum: The Understudied Meeting Place of Dietary Lipids and the Microbiota. Biochimie 2020, 178, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Silva, D.; Delbue, D.; Itzlinger, D.; Moerkens, R.; Withoff, S.; Branchi, F.; Schumann, M. Intestinal Barrier Function in Gluten-Related Disorders. Nutrients 2019, 11, 2325. [Google Scholar] [CrossRef] [PubMed]
- Camara-Lemarroy, C.R.; Wee Yong, V. The Intestinal Barrier in Multiple Sclerosis: Implications for Pathophysiology and Therapeutics Development of Treatments for Progressive Multiple Sclerosis View Project Remyelination in MS View Project. Brain 2018, 141, 1900–1916. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Yang, Q.; Chen, D.; Yu, B.; He, J. Benzoic Acid Used as Food and Feed Additives Can Regulate Gut Functions. Biomed Res. Int. 2019, 2019, 5721585. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Kahles, F.; Rattik, S.; Nairz, M.; McAlpine, C.S.; Anzai, A.; Selgrade, D.; Fenn, A.M.; Chan, C.T.; Mindur, J.E.; et al. Gut Intraepithelial T Cells Calibrate Metabolism and Accelerate Cardiovascular Disease. Nature 2019, 566, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.M.; Deoti, B.; Amorim, I.F.; Pinto, A.J.; Moraes, A.; Carvalho, C.S.; da Silva, S.M.; de Faria, A.M.; Tafuri, W.L. Expression of Regulatory T Cells in Jejunum, Colon, and Cervical and Mesenteric Lymph Nodes of Dogs Naturally Infected with Leishmania Infantum. Infect. Immun. 2014, 82, 3704–3712. [Google Scholar] [CrossRef]
- Furness, J.B. The Organisation of the Autonomic Nervous System: Peripheral Connections. Auton. Neurosci. Basic Clin. 2006, 130, 1–5. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H. The Enteric Nervous System and Gastrointestinal Innervation: Integrated Local and Central Control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar] [CrossRef]
- Furness, J.B. The Enteric Nervous System and Neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Boron, W.F.; Boulpaep, E.L. Fisiologia Médica, 4th ed.; Elsevier: Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Lomax, A.E.; Fernández, E.; Sharkey, K.A. Plasticity of the Enteric Nervous System during Intestinal Inflammation. Neurogastroenterol. Motil. 2005, 17, 4–15. [Google Scholar] [CrossRef]
- Palus, K.; Bulc, M.; Całka, J. Changes in VIP-, SP- and CGRP- like Immunoreactivity in Intramural Neurons within the Pig Stomach Following Supplementation with Low and High Doses of Acrylamide. Neurotoxicology 2018, 69, 47–59. [Google Scholar] [CrossRef]
- Machado, C.C.A.; Watanabe, P.S.; Mendes, J.D.L.; Pupim, A.C.E.; Ortigoza, S.M.; Bergoc, H.G.; Nino, B.S.L.; Góis, M.B.; Garcia, J.L.; Blackshaw, L.A.; et al. Toxoplasma Gondii Infection Impairs the Colonic Motility of Rats Due to Loss of Myenteric Neurons. Neurogastroenterol. Motil. 2021, 33, e13967. [Google Scholar] [CrossRef]
- Vicentino-Vieira, S.; Góis, M.B.; Trevizan, A.R.; de Lima, L.L.; Leatte, E.P.; Melo, G.A.N.; Garcia, J.L.; Araújo, E.J.A.; Sant’Ana, D.M.G. Toxoplasma Gondii Infection Causes Structural Changes in the Jejunum of Rats Infected with Different Inoculum Doses. Life Sci. 2017, 191, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Sant’Ana, D.M.G.; Gois, M.B.; Hermes-Uliana, C.; Pereira-Severi, L.S.; Baptista, E.M.; Mantovani, L.C.; da Silva, A.V.; Araújo, E.J.A. Acute Infection with an Avirulent Strain of Toxoplasma Gondii Causes Decreasing and Atrophy of Nitrergic Myenteric Neurons of Rats. Acta Histochem. 2017, 119, 423–427. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.G.A.; da Silva, M.G.L.; Carneiro, E.L.; de Lima, L.L.; Fernandes, A.C.B.S.; Silveira, T.G.V.; Sant’Ana, D.d.M.G.; Nogueira-Melo, G.d.A. A New Target Organ of Leishmania (Viannia) Braziliensis Chronic Infection: The Intestine. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef]
- Santos, A.G.A.D.; Lima, L.L.; Mota, C.A.; Gois, M.B.; Fernandes, A.C.B.S.; Silveira, T.G.V.; Sant’Ana, D.M.G.; de Melo, G.A.N. Insights of Leishmania (Viannia) Braziliensis Infection in Golden Hamster (Mesocricetus Auratus) Intestine. Biomed. Pharmacother. 2018, 106, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.I.; Martín, S.; Marco, A.; Solano-Gallego, L. Detection of Leishmania Spp. Infection by Immunohistochemistry in Archived Biopsy Samples from Dogs with Colitis in an Area Endemic for Leishmaniosis. J. Comp. Pathol. 2019, 167, 12–17. [Google Scholar] [CrossRef]
- Halliez, M.C.M.; Buret, A.G. Gastrointestinal Parasites and the Neural Control of Gut Functions. Front. Cell. Neurosci. 2015, 9, 452. [Google Scholar] [CrossRef]
- Santos, A.G.A.D.; Ferlini, J.P.; Vicentino, S.L.; Lonardoni, M.V.; Sant’Ana, D.M.G.; Melo, G.A.N. Alterations Induced in the Ileum of Mice upon Inoculation with Different Species of Leishmania: A Preliminary Study. Rev. Soc. Bras. Med. Trop. 2018, 51, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.T.D.; Alves, M.L.; Spada, J.C.P.; Silveira, R.C.V.D.; Oliveira, T.M.F.S.; Starke-Buzetti, W.A. Neutrophils, Eosinophils, and Mast Cells in the Intestinal Wall of Dogs Naturally Infected with Leishmania Infantum. Rev. Bras. Parasitol. Vet. 2018, 27, 430–438. [Google Scholar] [CrossRef]
- Lewis, M.D.; Paun, A.; Romano, A.; Langston, H.; Langner, C.A.; Moore, I.N.; Bock, K.W.; Francisco, A.F.; Brenchley, J.M.; Sacks, D.L. Fatal Progression of Experimental Visceral Leishmaniasis Is Associated with Intestinal Parasitism and Secondary Infection by Commensal Bacteria, and Is Delayed by Antibiotic Prophylaxis. PLoS Pathog. 2020, 16, e1008456. [Google Scholar] [CrossRef]
- Hermes-Uliana, C.; Pereira-Severi, L.S.; Luerdes, R.B.; Franco, C.L.M.; da Silva, A.V.; Araújo, E.J.d.A.; Sant’Ana, D.d.M.G. Chronic Infection with Toxoplasma Gondii Causes Myenteric Neuroplasticity of the Jejunum in Rats. Auton. Neurosci. Basic Clin. 2011, 160, 3–8. [Google Scholar] [CrossRef]
- Odorizzi, L.; Moreira, N.M.; Gonçalves, G.F.; da Silva, A.V.; Sant’Ana, D.d.M.G.; Araújo, E.J.d.A. Quantitative and Morphometric Changes of Subpopulations of Myenteric Neurons in Swines with Toxoplasmosis. Auton. Neurosci. Basic Clin. 2010, 155, 68–72. [Google Scholar] [CrossRef]
- Vicentino-Vieira, S.L.; Nogueira de Melo, G.d.A.; Biondaro Góis, M.; Martins Moreira, N.; de Araujo Pereira, L.G.; de Almeida Araújo, E.J.; Garcia, J.L.; de Mello Gonçales Sant’Ana, D. Oral Dependent-Dose Toxoplasmic Infection Model Induced by Oocysts in Rats: Myenteric Plexus and Jejunal Wall Changes. Exp. Parasitol. 2015, 156, 12–18. [Google Scholar] [CrossRef][Green Version]
- Ferezin, R.I.; Vicentino-Vieira, S.L.; Góis, M.B.; Araújo, E.J.D.A.; de Melo, G.D.A.N.; Garcia, J.L.; Sant’Ana, D.D.M.G. Diferentes Inóculos de Toxoplasma Gondii Induzem a Redução de Neurônios Mioentéricos No Cólon de Ratos. Rev. Bras. Parasitol. Vet. 2017, 26, 47–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oda, J.Y.; Belém, M.O.; Carlos, T.M.; Gouveia, R.; Luchetti, B.F.C.; Moreira, N.M.; Massocatto, C.L.; Araújo, S.M.; Sant’Ana, D.M.G.; Buttow, N.C.; et al. Myenteric Neuroprotective Role of Aspirin in Acute and Chronic Experimental Infections with Trypanosoma Cruzi. Neurogastroenterol. Motil. 2017, 29, 1–13. [Google Scholar] [CrossRef]
- Campos, B.L.S.; Silva, T.N.; Ribeiro, S.P.; Carvalho, K.I.L.; Kallás, E.G.; Laurenti, M.D.; Passero, L.F.D. Analysis of Iron Superoxide Dismutase-Encoding DNA Vaccine on the Evolution of the Leishmania Amazonensis Experimental Infection. Parasite Immunol. 2015, 37, 407–416. [Google Scholar] [CrossRef]
- Angiulli, G.; Lantella, A.; Forte, E.; Angelucci, F.; Colotti, G.; Ilari, A.; Malatesta, F. Leishmania Infantum Trypanothione Reductase Is a Promiscuous Enzyme Carrying an NADPH:O2 Oxidoreductase Activity Shared by Glutathione Reductase. Biochim. Biophys. Acta—Gen. Subj. 2015, 1850, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Fialho Junior, L.; da Fonseca Pires, S.; Burchmore, R.; McGill, S.; Weidt, S.; Ruiz, J.C.; Guimarães, F.G.; Chapeourouge, A.; Perales, J.; de Andrade, H.M. Proteomic Analysis Reveals Differentially Abundant Proteins Probably Involved in the Virulence of Amastigote and Promastigote Forms of Leishmania Infantum. Parasitol. Res. 2021, 120, 679–692. [Google Scholar] [CrossRef]
- Liberti, E.A.; Fontes, R.B.V.; Fuggi, V.M.; Maifrino, L.B.M.; Souza, R.R. Effects of Combined Pre- and Post-Natal Protein Deprivation on the Myenteric Plexus of the Esophagus of Weanling Rats: A Histochemical, Quantitative and Ultrastructural Study. World J. Gastroenterol. 2007, 13, 3598. [Google Scholar] [CrossRef]
- Trevizan, A.R.; Schneider, L.C.L.; Araújo, E.J.A.; Garcia, J.L.; Buttow, N.C.; Nogueira-Melo, G.A.; Sant’Ana, D.M.G. Acute Toxoplasma Gondii Infection Alters the Number of Neurons and the Proportion of Enteric Glial Cells in the Duodenum in Wistar Rats. Neurogastroenterol. Motil. 2019, 31, e13523. [Google Scholar] [CrossRef]
- Karaus, M.; Sarna, S.K. Giant Migrating Contractions during Defecation in the Dog Colon. Gastroenterology 1987, 92, 925–933. [Google Scholar] [CrossRef]
- Drugs for Neglected Diseases Initiative Leishmaniasis. Available online: https://dndi.org/diseases/visceral-leishmaniasis/facts/ (accessed on 25 August 2021).
- Costa, C.H.; Werneck, G.L.; Costa, D.L.; Holanda, T.A.; Aguiar, G.B.; Carvalho, A.S.; Cavalcanti, J.C.; Santos, L.S. Is Severe Visceral Leishmaniasis a Systemic Inflammatory Response Syndrome? A Case Control Study. Rev. Soc. Bras. Med. Trop. 2010, 43, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Henke, O.; Mapendo, P.J.; Mremi, A.; Mmbaga, L.G.; Pallangyo, A.E.; Harbaum, T.; Mkwizu, E. Skin Maculae, Chronic Diarrhea, Cachexia, and Splenomegaly—Late Presentation of the First Autochthonous Case of Visceral Leishmaniasis in Tanzania. PLoS Negl. Trop. Dis. 2021, 15, e0008925. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.T.; Neves, M.F.; Queiroz, N.M.G.P.; Spada, J.C.P.; Alves, M.L.; Silva, M.F.; Coelho, W.M.D.; Panosso, A.R.; Noronha, A.C.F.; Starke-Buzetti, W.A. Correlation Study and Histopathological Description of Intestinal Alterations in Dogs Infected with Leishmania Infantum. Rev. Bras. Parasitol. Veterinária 2016, 25, 24–36. [Google Scholar] [CrossRef]
- Trevizan, A.R.; Vicentino-Vieira, S.L.; da Silva, P.W.; Góis, M.B.; de Melo, A.G.; Garcia, J.L.; Araújo, E.J.A.; Sant’Ana, D.M.G. Kinetics of Acute Infection with Toxoplasma Gondii and Histopathological Changes in the Duodenum of Rats. Exp. Parasitol. 2016, 165, 22–29. [Google Scholar] [CrossRef]
- Souza, K.D.; Fernandes, E.P.A.; Dos Santos, A.G.A.; de Lima, L.L.; Gonzaga, W.F.K.M.; Xander, P.; Nogueira-Melo, G.A.; Sant’Ana, D.M.G. Infection by Leishmania (Leishmania) Infantum Chagasi Causes Intestinal Changes B-1 Cells Dependent. Parasite Immunol. 2019, 41, e12661. [Google Scholar] [CrossRef]
- Abbas, A.K.; Kumar, V.; Aster, J.C. Bases Patológicas Das Doenças, 9th ed.; Elsevier: Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Esterházy, D.; Mucida, D. Gut Immune Cells Have a Role in Food Metabolism. Nature 2019, 566, 49–50. [Google Scholar] [CrossRef]
- Farhadi, A.; Banan, A.; Fields, J.; Keshavarzian, A. Intestinal Barrier: An Interface between Health and Disease. J. Gastroenterol. Hepatol. 2003, 18, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Schneeman, B.O. Gastrointestinal Physiology and Functions. Br. J. Nutr. 2002, 88 (Suppl. 2), S159–S163. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.M.; da Silva, A.V.; Araújo, E.J.A.; Sant’Ana, D.M.G. The Effects of the Infection Caused by Toxoplasma Gondii on the Cat Duodenal Wall. Rev. Bras. Parasitol. Vet. 2010, 19, 57–63. [Google Scholar] [CrossRef]
- Shiraishi, C.S.; Azevedo, J.F.; Silva, A.V.; Sant’Ana, D.M.G.; Araújo, E.J.A. Análise Morfométrica Da Parede Intestinal e Dinâmica de Mucinas Secretadas No Íleo de Frangos Infectados Por Toxoplasma Gondii. Ciência Rural 2009, 39, 2146–2153. [Google Scholar] [CrossRef][Green Version]
- Stauber, L. Leishmaniasis in the Hamster. In Some Physiological Aspects and Consequences of Parasitism; Cole, W.H., Ed.; Rutgers University Press: New Brunswisck, NJ, USA, 1955. [Google Scholar]
- Gabella, G. Detection of Nerve Cells by a Histochemical Technique. Experientia 1969, 25, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Scherer-Singler, U.; Vincent, S.R.; Kimura, H.; McGeer, E.G. Demonstration of a Unique Population of Neurons with NADPH-Diaphorase Histochemistry. J. Neurosci. Methods 1983, 9, 229–234. [Google Scholar] [CrossRef]
| Time of Infection | Groups | Ratio Cell Type/Enterocyte (%) | ||
|---|---|---|---|---|
| PAS+/enterocytes | AB+/Enterocytes | IELs/Enterocytes | ||
| 30 DPI | Control | 2.1 ± 1.91 | 7.1 ± 1.15 * | 3.7 ± 0.60 * |
| Experimental | 1.35 ± 0.6 | 3.5 ± 0.7 * | 7.6 ± 1.8 * | |
| 60 DPI | Control | 1.5 ± 0.3 * | 5.0 ± 0.9 * | 2.0 ± 1.1 * |
| Experimental | 1.0 ± 0.6 * | 2.0 ± 1.3 * | 4.0 ± 1.2 * | |
| 90 DPI | Control | 3.8 ± 2.6 * | 3.9 ± 2.7 * | 2.8 ± 1.2 * |
| Experimental | 3.0 ± 1.9 * | 6.0 ± 3.6 * | 3.0 ± 1.9 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lima, S.K.S.; Cavallone, I.N.; Oliveira, K.S.; Passero, L.F.D.; Laurenti, M.D.; Jesus, J.A.; Marinsek, G.P.; Chucri, T.M.; Mari, R.d.B. Infection with Leishmania (Leishmania) infantum Changes the Morphology and Myenteric Neurons of the Jejunum of Golden Hamsters. Parasitologia 2021, 1, 225-237. https://doi.org/10.3390/parasitologia1040024
de Lima SKS, Cavallone IN, Oliveira KS, Passero LFD, Laurenti MD, Jesus JA, Marinsek GP, Chucri TM, Mari RdB. Infection with Leishmania (Leishmania) infantum Changes the Morphology and Myenteric Neurons of the Jejunum of Golden Hamsters. Parasitologia. 2021; 1(4):225-237. https://doi.org/10.3390/parasitologia1040024
Chicago/Turabian Stylede Lima, Sarah Kymberly Santos, Italo Novais Cavallone, Karine Soares Oliveira, Luiz Felipe Domingues Passero, Márcia Dalastra Laurenti, Jéssica Adriana Jesus, Gabriela Pustiglione Marinsek, Thaís Martins Chucri, and Renata de Britto Mari. 2021. "Infection with Leishmania (Leishmania) infantum Changes the Morphology and Myenteric Neurons of the Jejunum of Golden Hamsters" Parasitologia 1, no. 4: 225-237. https://doi.org/10.3390/parasitologia1040024
APA Stylede Lima, S. K. S., Cavallone, I. N., Oliveira, K. S., Passero, L. F. D., Laurenti, M. D., Jesus, J. A., Marinsek, G. P., Chucri, T. M., & Mari, R. d. B. (2021). Infection with Leishmania (Leishmania) infantum Changes the Morphology and Myenteric Neurons of the Jejunum of Golden Hamsters. Parasitologia, 1(4), 225-237. https://doi.org/10.3390/parasitologia1040024








