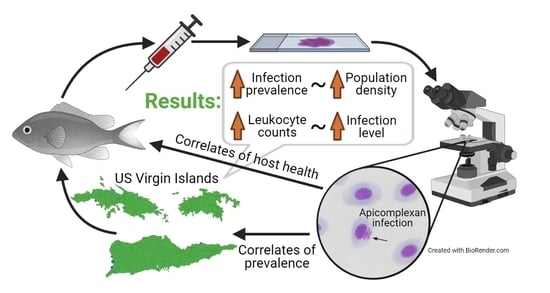
Environmental Correlates of Prevalence of an Intraerythrocytic Apicomplexan Infecting Caribbean Damselfish
Abstract
1. Introduction
2. Results
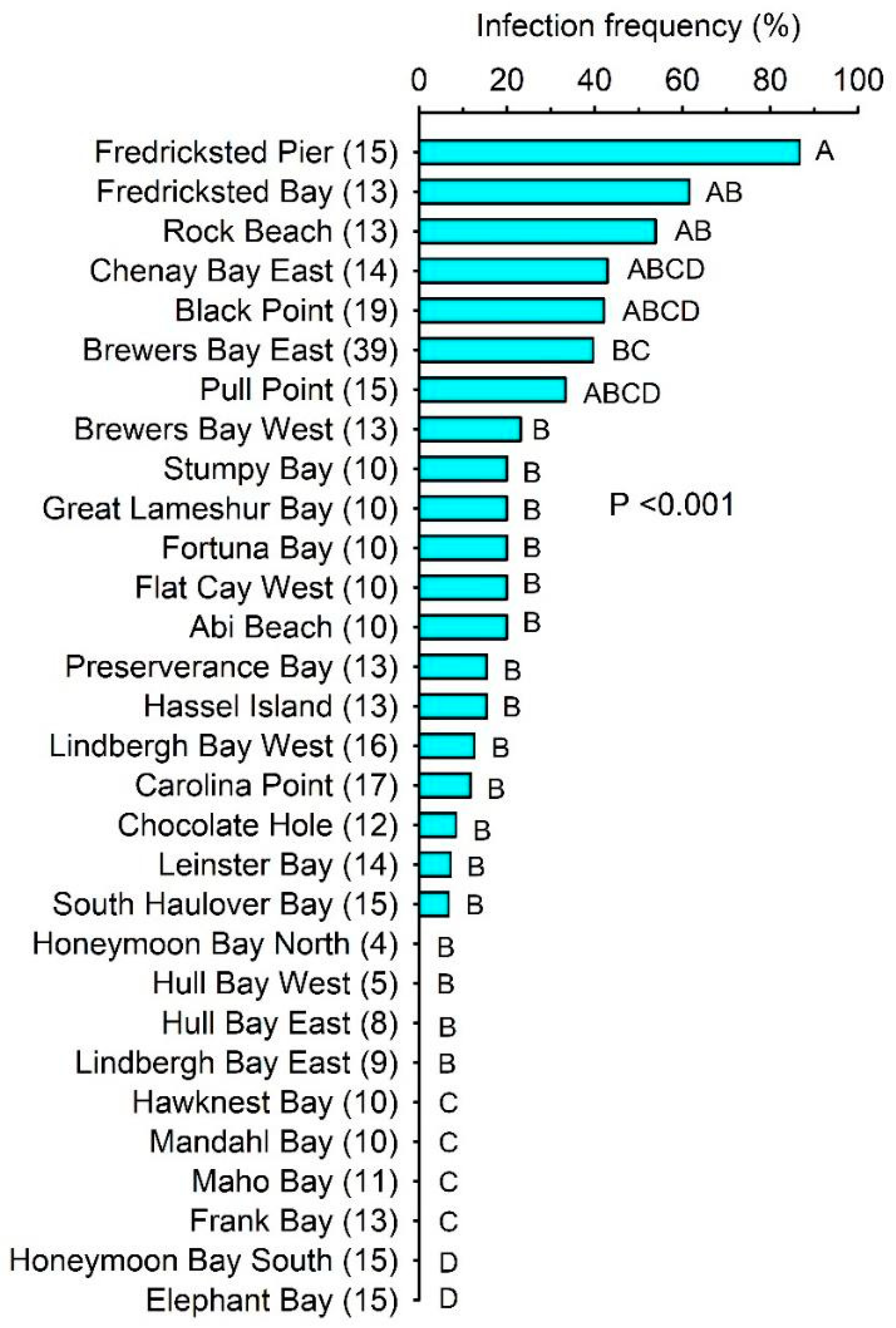
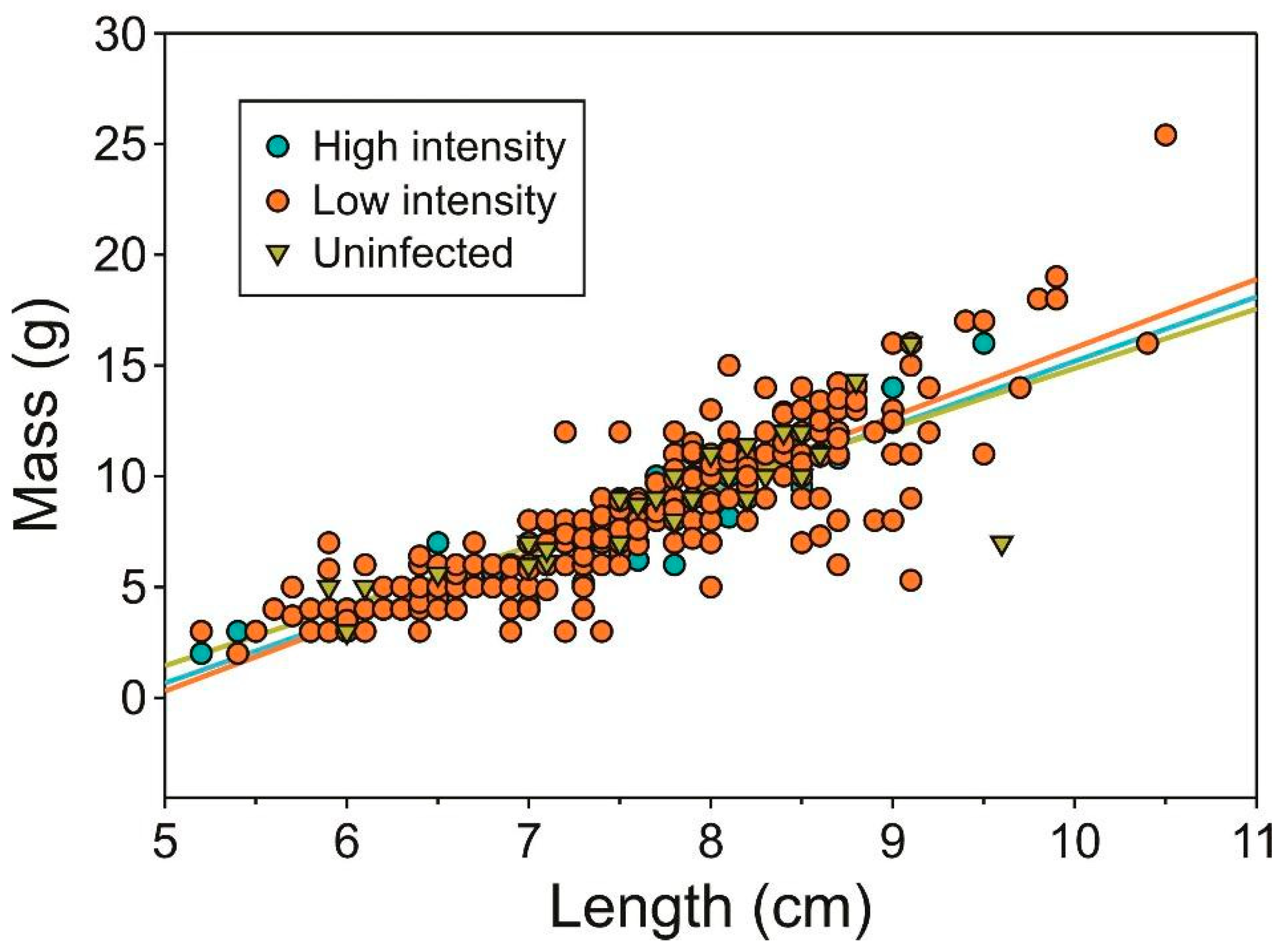
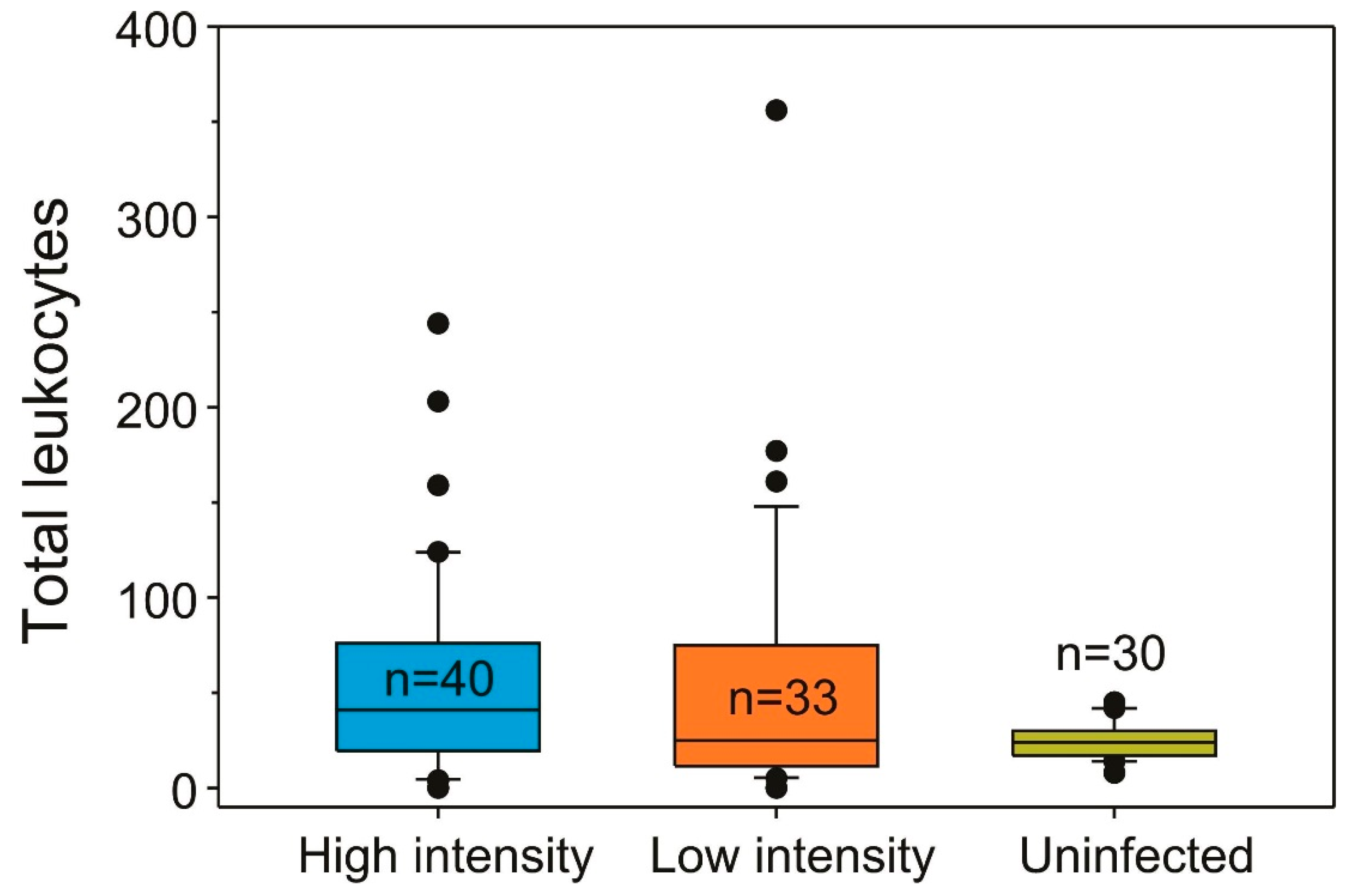
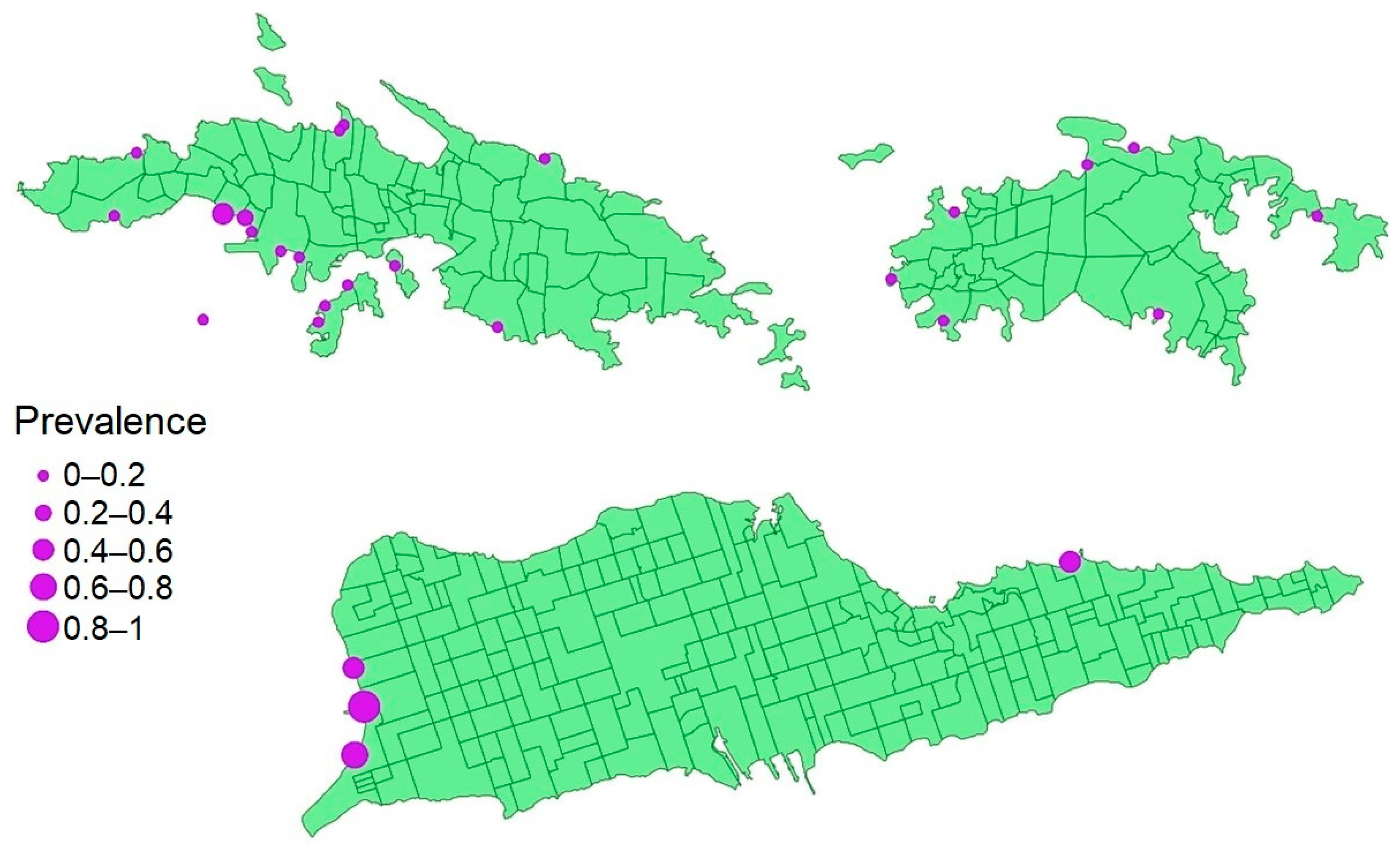
2.1. Infection Prevalence and Host Condition
2.2. Factors That Predict Prevalence
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Kuris, A.M.; Hechinger, R.F.; Shaw, J.C.; Whitney, K.L.; Aguirre-Macedo, L.; Boch, C.A.; Dobson, A.P.; Dunham, E.J.; Fredensborg, B.L.; Huspeni, T.C.; et al. Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature 2008, 454, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Rohde, K. Ecology and biogeography of marine parasites. Adv. Mar. Biol. 2002, 43, 1–83. [Google Scholar] [PubMed]
- Marcogliese, D.J. Food webs and biodiversity: Are parasites the missing link. J. Parasitol. 2003, 89, 106–113. [Google Scholar]
- Wood, C.L.; Byers, J.E.; Cottingham, K.L.; Altman, I.; Donahue, M.J.; Blakeslee, A.M. Parasites alter community structure. Proc. Natl. Acad. Sci. USA 2007, 104, 9335–9339. [Google Scholar] [CrossRef]
- Johnson, P.T.; Hartson, R.B.; Larson, D.J.; Sutherland, D.R. Diversity and disease: Community structure drives parasite transmission and host fitness. Ecol. Lett. 2008, 11, 1017–1026. [Google Scholar] [CrossRef]
- Hudson, P.J.; Dobson, A.P.; Lafferty, K.D. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 2006, 21, 381–385. [Google Scholar] [CrossRef]
- Frost, C.M.; Peralta, G.; Rand, T.A.; Didham, R.K.; Varsani, A.; Tylianakis, J.M. Apparent competition drives community-wide parasitism rates and changes in host abundance across ecosystem boundaries. Nat. Commun. 2016, 7, 12644. [Google Scholar] [CrossRef]
- Price, P.W.; Westoby, M.; Rice, B.; Atsatt, P.R.; Fritz, R.S.; Thompson, J.N.; Mobley, K. Parasite mediation in ecological interactions. Ann. Rev. Ecol. Syst. 1986, 17, 487–505. [Google Scholar] [CrossRef]
- Price, P.; Westoby, M.; Rice, B. Parasite-mediated competition: Some predictions and tests. Am. Nat. 1988, 131, 544–555. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Dobson, A.P.; Kuris, A.M. Parasites dominate food web links. Proc. Natl. Acad. Sci. USA 2006, 103, 11211–11216. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, K.D.; Allesina, S.; Arim, M.; Briggs, C.J.; De Leo, G.; Dobson, A.P.; Thieltges, D.W. Parasites in food webs: The ultimate missing links. Ecol. Lett. 2008, 11, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, M.J.; Dunn, A.M. Parasites in Ecological Communities: From Interactions to Ecosystems; Cambridge University Press: Cambridge, UK, 2011; pp. 265–318. [Google Scholar]
- Hatcher, M.J.; Dick, J.T.A.; Dunn, A.M. Diverse effects of parasites in ecosystems: Linking interdependent processes. Front. Ecol. Environ. 2012, 10, 186–194. [Google Scholar] [CrossRef]
- Sures, B. Host-parasite interactions in polluted environments. J. Fish. Biol. 2008, 73, 2133–2142. [Google Scholar] [CrossRef]
- Blanar, C.A.; Munkittrick, K.R.; Houlahan, J.; MacLatchy, D.L.; Marcogliese, D.J. Pollution and parasitism in aquatic animals: A meta-analysis of effect size. Aquat. Toxicol. 2009, 93, 18–28. [Google Scholar] [CrossRef]
- Vidal-Martínez, V.M.; Pech, D.; Sures, B.; Purucker, S.T.; Poulin, R. Can parasites really reveal environmental impact? Trends Parasitol. 2010, 26, 44–51. [Google Scholar] [CrossRef]
- Gilbert, B.M.; Avenant-Oldewage, A. Parasites and pollution: The effectiveness of tiny organisms in assessing the quality of aquatic ecosystems, with a focus on Africa. Environ. Sci. Pollut. R 2017, 24, 18742–18769. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P. Immune defence, parasite evasion strategies and their relevance for ‘macroscopic phenomena’ such as virulence. Philos. T Roy. Soc. B 2008, 364, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Rohde, K. Zoogeography of marine parasites. Helgolander. Meeresun. 1984, 37, 35–52. [Google Scholar] [CrossRef]
- Morrissette, N.S.; Sibley, L.D. Cytoskeleton of apicomplexan parasites. Microbiol. Mol. Biol. R 2002, 66, 21–38. [Google Scholar] [CrossRef] [PubMed]
- McFadden, G.I. Apicoplast. Curr. Biol. 2014, 24, R262–R263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kopečná, J.; Jirků, M.; Oborník, M.; Tokarev, Y.S.; Lukeš, J.; Modrý, D. Phylogenetic analysis of coccidian parasites from invertebrates: Search for missing links. Protist 2006, 157, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.; Sikkel, P.; Renoux, L.; Smit, N. Blood parasite biodiversity of reef-associated fishes of the eastern Caribbean. Mar. Ecol. Prog. Ser. 2015, 533, 1–13. [Google Scholar] [CrossRef]
- Ogedengbe, M.E.; El-Sherry, S.; Ogedengbe, J.D.; Chapman, H.D.; Barta, J.R. Phylogenies based on combined mitochondrial and nuclear sequences conflict with morphologically defined genera in the eimeriid coccidia (Apicomplexa). Int. J. Parasitol. 2018, 48, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.; Severino, R.; Perez-Losada, M.; Gestal, C.; Freitas, R.; Harris, D.J.; Verissimo, A.; Rosado, D.; Cable, J. Phylogenetic analysis of apicomplexan parasites infecting commercially valuable species from the North-East Atlantic reveals high levels of diversity and insights into the evolution of the group. Parasite Vector 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Hayes, P.M.; Smit, N.J. Molecular insights into the identification and phylogenetics of the cosmopolitan marine fish blood parasite, Haemogregarina bigemina (Adeleorina: Haemogregarinidae). Int. J. Parasitol. 2019, 8, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.J.; Smit, N.J.; Hayes, P.M.; Seddon, A.M.; Wertheim, D. Haemogregarina bigemina (Protozoa: Apicomplexa: Adeleorina)—Past, present and future. Folia Parasit. 2004, 51, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.C. A survey of the blood parasites in the fishes of the Red Sea. T. Am. Microsc. Soc. 1960, 79, 239–252. [Google Scholar] [CrossRef]
- Allen, G.R. Damselfishes of the World; Mergus Press: Melle, Germany, 1991. [Google Scholar]
- Helfman, G.; Collette, B.B.; Facey, D.E.; Bowen, B.W. The Diversity of Fishes: Biology, Evolution and Ecology, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2009. [Google Scholar]
- Greenfield, D.W.; Johnson, R.K. Community structure of western Caribbean blennioid fishes. Copeia 1990, 1990, 433–448. [Google Scholar] [CrossRef]
- Wilson, D.T.; Meekan, M.G. Growth-related advantages for survival to the point of replenishment in the coral reef fish Stegastes partitus (Pomacentridae). Mar. Ecol. Prog. Ser. 2002, 231, 247–260. [Google Scholar] [CrossRef]
- Mumby, P.J.; Steneck, R.S.; Edwards, A.J.; Ferrari, R.; Coleman, R.; Harborne, A.R.; Gibson, J.P. Fishing down a Caribbean food web relaxes trophic cascades. Mar. Ecol. Prog. Ser. 2012, 445, 13–24. [Google Scholar] [CrossRef]
- Hixon, M.A.; Brostoff, W.N. Fish grazing and community structure of Hawaiian reef algae. In Proceedings of the 4th International Coral Reef Symposium, Manila, Philippines, 18–22 May 1981; Volume 2, pp. 507–514. [Google Scholar]
- Sammarco, P.W. Effects of fish grazing and damselfish territoriality on coral reef algae. I. Algal community structure. Mar. Ecol. Prog. Ser. 1983, 13, 1–14. [Google Scholar] [CrossRef]
- Hoey, A.S.; Bellwood, D.R. Damselfish territories as a refuge for macroalgae on coral reefs. Coral Reefs 2010, 29, 107–118. [Google Scholar] [CrossRef]
- Sikkel, P.C.; Cook, C.A.; Renoux, L.P.; Bennett, C.L.; Tuttle, L.J.; Smit, N.J. The distribution and host-association of a haemoparasite of damselfishes (Pomacentridae) from the eastern Caribbean based on a combination of morphology and 18S rDNA sequences. Int. J. Parasitol. 2018, 7, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.J.; Smit, N.J. The life cycle of Haemogregarina bigemina (Adeleina: Haemogregarinidae) in South African hosts. Folia Parasitol. 2001, 48, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.M.; Smit, N.J.; Seddon, A.M.; Wertheim, D.F.; Davies, A.J. A new fish haemogregarine and its suspected dual transmission with trypanosomes by a marine leech. Folia Parasitol. 2006, 53, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Curtis, L.M.; Grutter, A.S.; Smit, N.J.; Davies, A.J. Gnathia aureamaculosa, a likely definitive host of Haemogregarina balistapi and potential vector for Haemogregarina bigemina between fishes of the Great Barrier Reef, Australia. Int. J. Parasitol. 2013, 43, 361–370. [Google Scholar] [CrossRef]
- Sikkel, P.C.; Pagan, J.A.; Santos, J.L.; Hendrick, G.C.; Nicholson, M.D.; Xavier, R. Molecular detection of apicomplexan blood parasites of coral reef fishes from free-living stages of ectoparasitic gnathiid isopods. Parasitol. Res. 2020, 119, 1975–1980. [Google Scholar] [CrossRef]
- Sikkel, P.C.; Cheney, K.L.; Côté, I.M. In situ evidence for ectoparasites as a proximate cause of cleaning interactions in reef fish. Anim. Behav. 2004, 68, 241–247. [Google Scholar] [CrossRef]
- Sikkel, P.C.; Schaumburg, C.S.; Mathenia, J.K. Diel infestation dynamics of gnathiid isopod larvae parasitic on Caribbean reef fish. Coral Reefs 2006, 25, 683–689. [Google Scholar] [CrossRef]
- Coile, A.M.; Sikkel, P.C. An experimental field test of susceptibility to ectoparasitic gnathiid isopods among Caribbean reef fishes. Parasitology 2013, 140, 888. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.R. Fish feces as fish food on a Pacific coral reef. Mar. Ecol. Prog. Ser. 1982, 7, 253–265. [Google Scholar] [CrossRef]
- Nicholson, M.D.; Sikkel, P.C. Localized defecation in territorial herbivorous fishes. Copeia 2018, 106, 532–538. [Google Scholar] [CrossRef]
- Stiven, A.E. Experimental studies on the epidemiology of the host-parasite system Hydra and Hydramoeba hydroxena (Entz). II. the components of a simple epidemic. Ecol. Monogr. 1964, 34, 119–142. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. The invasion, persistence and spread of infectious diseases within animal and plant communities. Philos. T R Soc. B 1986, 314, 533–570. [Google Scholar]
- McCallum, H.; Barlow, N.; Hone, J. How should pathogen transmission be modelled? Trends Ecol. Evol. 2001, 16, 295–300. [Google Scholar] [CrossRef]
- Murray, A.G. Using simple models to review the application and implications of different approaches used to simulate transmission of pathogens among aquatic animals. Prev. Vet. Med. 2009, 88, 167–177. [Google Scholar] [CrossRef]
- Blackwood, J.C.; Childs, L.M. An introduction to compartmental modeling for the budding infectious disease modeler. Lett. Biomath. 2018, 5, 195–221. [Google Scholar] [CrossRef]
- Hopkins, S.R.; Fleming-Davies, A.E.; Belden, L.K.; Wojdak, J.M. Systematic review of modelling assumptions and empirical evidence: Does parasite transmission increase nonlinearly with host density? Methods Ecol. Evol. 2020, 11, 476–486. [Google Scholar] [CrossRef]
- Burdon, J.; Chilvers, G.A. Host density as a factor in plant disease ecology. Annu. Rev. Phytopathol. 1982, 20, 143–166. [Google Scholar] [CrossRef]
- Arneberg, P.; Skorping, A.; Grenfell, B.; Read, A.F. Host densities as determinants of abundance in parasite communities. Proc. Roy. Soc. Lond. Ser. B Biol. 1998, 265, 1283–1289. [Google Scholar] [CrossRef]
- Ramsey, D.; Spencer, N.; Caley, P.; Efford, M.; Hansen, K.; Lam, M.; Cooper, D. The effects of reducing population density on contact rates between brushtail possums: Implications for transmission of bovine tuberculosis. J. Appl Ecol. 2002, 39, 806–818. [Google Scholar] [CrossRef]
- Brown, C.R.; Brown, M.B. Empirical measurement of parasite transmission between groups in a colonial bird. Ecology 2004, 85, 1619–1626. [Google Scholar] [CrossRef]
- Vicente, J.; Höfle, U.; Fernández-De-Mera, I.G.; Gortazar, C. The importance of parasite life history and host density in predicting the impact of infections in red deer. Oecologia 2007, 152, 655–664. [Google Scholar] [CrossRef]
- Smith, M.J.; Telfer, S.; Kallio, E.R.; Burthe, S.; Cook, A.R.; Lambin, X.; Begon, M. Host–pathogen time series data in wildlife support a transmission function between density and frequency dependence. Proc. Natl. Acad. Sci. USA 2009, 106, 7905–7909. [Google Scholar] [CrossRef]
- Johnson, M.B.; Lafferty, K.D.; Van Oosterhout, C.; Cable, J. Parasite transmission in social interacting hosts: Monogenean epidemics in guppies. PLoS ONE 2011, 6, e22634. [Google Scholar] [CrossRef] [PubMed]
- Bagamian, K.H.; Douglass, R.J.; Alvarado, A.; Kuenzi, A.J.; Amman, B.R.; Waller, L.A.; Mills, J.N. Population density and seasonality effects on Sin Nombre virus transmission in North American deermice (Peromyscus maniculatus) in outdoor enclosures. PLoS ONE 2012, 7, e37254. [Google Scholar] [CrossRef]
- Booth, D.J.; Beretta, G.A. Seasonal recruitment, habitat associations and survival of pomacentrid reef fish in the US Virgin Islands. Coral Reefs 1994, 13, 81–89. [Google Scholar] [CrossRef]
- Little, K.; Draud, M.; Itzkowitz, M. Interspecific aggression in two highly similar Stegastes damselfish. Ethol. Ecol. Evol. 2013, 25, 227–242. [Google Scholar] [CrossRef]
- Black, A.; Draud, M.; Richter, M.; Itzkowitz, M. Are conspecific and heterospecific opponents assessed similarly? A test in two species of territorial damselfish (Pomacentridae). Behav. Process. 2014, 106, 107–110. [Google Scholar] [CrossRef]
- Pilosof, S.; Morand, S.; Krasnov, B.R.; Nunn, C.L. Potential parasite transmission in multi-host networks based on parasite sharing. PLoS ONE 2015, 10, e0117909. [Google Scholar] [CrossRef]
- McLeish, M.; Sacristán, S.; Fraile, A.; Garcia-Arenal, F. Scale dependencies and generalism in host use shape virus prevalence. Proc. Roy. Soc. Lond. Ser. B Biol. 2017, 284, 2017–2066. [Google Scholar] [CrossRef]
- Salama, N.K.G.; Murray, A.G. A comparison of modelling approaches to assess the transmission of pathogens between Scottish fish farms: The role of hydrodynamics and site biomass. Prev. Vet. Med. 2013, 108, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Alaliyat, S.; Yndestad, H.; Davidsen, P.I. An agent-based approach for predicting patterns of pathogen transmission between aquaculture sites in the Norwegian fjords. Aquaculture 2019, 505, 98–111. [Google Scholar] [CrossRef]
- Samsing, F.; Solstorm, D.; Oppedal, F.; Solstorm, F.; Dempster, T. Gone with the flow: Current velocities mediate parasitic infestation of an aquatic host. Int. J. Parasitol. 2015, 45, 559–565. [Google Scholar] [CrossRef]
- Fingerut, J.T.; Ann Zimmer, C.; Zimmer, R.K. Larval swimming overpowers turbulent mixing and facilitates transmission of a marine parasite. Ecology 2003, 84, 2502–2515. [Google Scholar] [CrossRef]
- Sabater, S.; Navarro, E.; Guasch, H. Effects of copper on algal communities at different current velocities. J. Appl. Phycol. 2002, 14, 391–398. [Google Scholar] [CrossRef]
- Polst, B.H.; Anlanger, C.; Risse-Buhl, U.; Larras, F.; Hein, T.; Weitere, M.; Schmitt-Jansen, M. Hydrodynamics alter the tolerance of autotrophic biofilm communities toward herbicides. Front. Microbiol. 2018, 9, 2884. [Google Scholar] [CrossRef] [PubMed]
- Marcogliese, D.J.; Pietrock, M. Combined effects of parasites and contaminants on animal health: Parasites do matter. Trends Parasitol. 2011, 27, 123–130. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Porter, J.W.; Ford, S.E. Are diseases increasing in the ocean? Annu. Rev. Ecol. Evol. Syst. 2004, 35, 31–54. [Google Scholar] [CrossRef]
- Krkošek, M. Host density thresholds and disease control for fisheries and aquaculture. Aquacult. Environ. Interac. 2010, 1, 21–32. [Google Scholar] [CrossRef]
- Cantrell, D.L.; Groner, M.L.; Ben-Horin, T.; Grant, J.; Revie, C.W. Modeling pathogen dispersal in marine fish and shellfish. Trends Parasitol. 2020, 36, 239–249. [Google Scholar] [CrossRef]
- Budischak, S.A.; O’Neal, D.; Jolles, A.E.; Ezenwa, V.O. Differential host responses to parasitism shape divergent fitness costs of infection. Funct. Ecol. 2018, 32, 324–333. [Google Scholar] [CrossRef]
- Pennycuick, L. Quantitative effects of three species of parasites on a population of three-spined sticklebacks, Gasterosteus aculeatus. J. Zool 1971, 165, 143–162. [Google Scholar] [CrossRef]
- Martin-Smith, K.M. Length/weight relationships of fishes in a diverse tropical freshwater community, Sabah, Malaysia. J. Fish. Biol. 1996, 49, 731–734. [Google Scholar] [CrossRef]
- Ak, O.; Kutlu, S.; Aydin, I. Length-weight relationship for 16 fish species from the Easter Black Sea, Turkey. Turk. J. Fish. Aquat. Sci. 2009, 9, 125–126. [Google Scholar]
- Faradonbeh, M.Z.; Eagderi, S.; Ghojoghi, F. Length-weight relationship and condition factor of seven fish species of Totkabon River (south Caspian Sea basin), Guilan, Iran. Int. J. Aquat. Biol. 2015, 3, 172–176. [Google Scholar]
- Jog, M.M.; Watve, M.G. Sarcocystosis of chital-dhole: Conditions for evolutionary stability of a predator parasite mutualism. BMC Ecol. 2005, 5, 1–4. [Google Scholar] [CrossRef]
- Saffo, M.B.; McCoy, A.M.; Rieken, C.; Slamovits, C.H. Nephromyces, a beneficial apicomplexan symbiont in marine animals. Proc. Natl. Acad. Sci. USA 2010, 107, 16190–16195. [Google Scholar] [CrossRef]
- Cumbo, V.R.; Baird, A.H.; Moore, R.B.; Negri, A.P.; Neilan, B.A.; Salih, A.; van Oppen, M.J.H.; Wang, Y.; Marquis, C.P. Chromera velia is endosymbiotic in larvae of the reef corals Acropora digitifera and A. tenuis. Protist 2013, 164, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.B.; Oborník, M.; Janouškovec, J.; Chrudimský, T.; Vancová, M.; Green, D.H.; Wright, S.W.; Davies, N.W.; Bolch, C.J.S.; Heimann, K.; et al. A photosynthetic alveolate closely related to apicomplexan parasites. Nature 2008, 451, 959–963. [Google Scholar] [CrossRef]
- Barber, I.; Hoare, D.; Krause, J. Effects of parasites on fish behaviour: A review and evolutionary perspective. Rev. Fish. Biol. Fish. 2000, 10, 131–165. [Google Scholar] [CrossRef]
- Carlton, J.T.; Blakeslee, A.M.; Fowler, A.E. Accidental associates are not symbionts: The absence of a non-parasitic endosymbiotic community inside the common periwinkle Littorina littorea (Mollusca: Gastropoda). Mar. Biol. 2020, 167, 97. [Google Scholar] [CrossRef]
- Chapman, G. Not simply accept relationships: Editorial comment on the article “Accidental associates are not symbionts: The absence of a non-parasitic endosymbiotic community inside the common periwinkle Littorina littorea (Mollusca: Gastropoda)” by J. T. Carlton et al. (2020). Mar. Biol. 2020, 167, 98. [Google Scholar] [CrossRef]
- Renoux, L.P.; Dolan, M.; Cook, C.A.; Smit, N.J.; Sikkel, P.C. Developing an Apicomplexan DNA Barcoding System to Detect Blood Parasites of Small Coral Reef Fishes. J. Parasitol. 2017, 103, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Length-Weight Relationships of Selected Marine Reef Fishes from the Southeastern United States and the Caribbean. Available online: https://repository.library.noaa.gov/view/noaa/3027 (accessed on 24 April 2021).
- Thrush, M.A.; Murray, A.G.; Brun, E.; Wallace, S.; Peeler, E.J. The application of risk and disease modelling to emerging freshwater diseases in wild aquatic animals. Freshw. Biol. 2011, 56, 658–675. [Google Scholar] [CrossRef]
- Foreman, M.G.G.; Guo, M.; Garver, K.A.; Stucchi, D.; Chandler, P.; Wan, D.; Tuele, D. Modelling infectious hematopoietic necrosis virus dispersion from marine salmon farms in the Discovery Islands, British Columbia, Canada. PLoS ONE 2015, 10, 1–25. [Google Scholar] [CrossRef]
- Escobar, L.E.; Escobar-Dodero, J.; Phelps, N.B. Infectious disease in fish: Global risk of viral hemorrhagic septicemia virus. Rev. Fish. Biol. Fish. 2018, 28, 637–655. [Google Scholar] [CrossRef]
| Model | Linear Mixed Effects R2/R2 Adjusted = 0.499/0.236 Prevalence | Selected Best Fit R2/R2 Adjusted = 0.399/0.329 Prevalence | ||||
|---|---|---|---|---|---|---|
| Predictors | Estimates | CI | p | Estimates | CI | p |
| (Intercept) | 0.97 | −0.71–2.65 | 0.241 | −0.03 | −0.30–0.24 | 0.835 |
| S. adustus population density | 0.01 | −0.08–0.09 | 0.864 | 0.01 | 0.00–0.03 | 0.048 |
| Stegastes spp. population density | 0 | −0.03–0.02 | 0.833 | |||
| Habitat complexity | −0.43 | −2.01–1.16 | 0.580 | |||
| Average zonal velocity | 8.46 | −8.33–25.25 | 0.305 | |||
| Average meridional velocity | −10.49 | −34.97–13.98 | 0.381 | 3.36 | 1.24–5.49 | 0.003 |
| Distance from nearest watershed | −15.93 | −44.65–12.79 | 0.260 | −8.12 | −19.78–3.55 | 0.165 |
| Average zonal velocity × average meridional velocity | −172.65 | −502.87–157.57 | 0.288 | |||
| Average meridional velocity × distance from nearest watershed | 89.50 | −298.56–477.56 | 0.635 | |||
| S. adustus population density × Stegastes spp. population density | 0 | −0.00–0.00 | 0.918 | |||
| S. adustus population density × habitat complexity | 0.02 | −0.08–0.11 | 0.711 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halliday-Isaac, A.K.; Robinson, J.B.; Cruz-Rivera, E.; Campbell, A.G.; Sikkel, P.C. Environmental Correlates of Prevalence of an Intraerythrocytic Apicomplexan Infecting Caribbean Damselfish. Parasitologia 2021, 1, 69-82. https://doi.org/10.3390/parasitologia1020009
Halliday-Isaac AK, Robinson JB, Cruz-Rivera E, Campbell AG, Sikkel PC. Environmental Correlates of Prevalence of an Intraerythrocytic Apicomplexan Infecting Caribbean Damselfish. Parasitologia. 2021; 1(2):69-82. https://doi.org/10.3390/parasitologia1020009
Chicago/Turabian StyleHalliday-Isaac, Akacia K., Jennilee B. Robinson, Edwin Cruz-Rivera, Andrew G. Campbell, and Paul C. Sikkel. 2021. "Environmental Correlates of Prevalence of an Intraerythrocytic Apicomplexan Infecting Caribbean Damselfish" Parasitologia 1, no. 2: 69-82. https://doi.org/10.3390/parasitologia1020009
APA StyleHalliday-Isaac, A. K., Robinson, J. B., Cruz-Rivera, E., Campbell, A. G., & Sikkel, P. C. (2021). Environmental Correlates of Prevalence of an Intraerythrocytic Apicomplexan Infecting Caribbean Damselfish. Parasitologia, 1(2), 69-82. https://doi.org/10.3390/parasitologia1020009