Abstract
Background: Sex estimation is a basic step of human identification in both legal cases and archeological contexts. The highest accuracy for sex estimation is achieved when a complete skeleton is available, though there are situations, such as cremated, dismembered, and otherwise taphonomically altered skeletal remains, where a complete skeleton is not available. The aim of the present preliminary study was to evaluate the usefulness of four non-metric skull traits that are considered taphonomically resilient for sex estimation and their potential application in forensic cases. Methods: Non-metric skull traits of 100 skulls from the Bass Donated Skeletal Collection were analyzed. These traits included foramen magnum shape, zygomatic arch extension with respect to the external auditory canal, sigmoid notch, and gonial angle muscle attachment. A discriminant function analysis model was used to develop specific formulae for sex estimation. Results: The foramen magnum and sigmoid notch showed no significant differences between males and females. The zygomatic arch extension (ZAE) and gonial angle morphology (GO) showed strong, significant differences between the sexes. However, gonial angle morphology has shown to be affected by edentulism. Based on the ZAE, the function obtained by the discriminant function analysis was sex = 2.469*ZAE − 1.247, with a result of zero pointing to males and result of one pointing to females, which correctly classified 79.8% of the original cases. Conclusions: This study highlights the value of four different skull traits and their potential use in forensic cases. Of all the evaluated traits, zygomatic arch extension was the best indicator for sex estimation. This anatomical region corresponds to a highly resistant skeletal structure.
1. Introduction
Sex estimation is one of the essential building blocks of human identification in that it serves both forensic inquiry and archeological reconstruction. The accurate estimation of sex is one pivotal piece of evidence of the biological profile that drastically reduces the number of possible identities. Qualitatively, sex estimation methodologies divide into two general categories: non-metric and metric. Non-metric techniques consist of the visual estimation of morphological characteristics displaying sexual dimorphism (shape or appearance difference by sex), foremost among them being the pelvis and cranium. Though practical and convenient, these methodologies are by nature observer-dependent and depend upon observer training, experience, and expertise [1]. Metric methodologies employ skeletal landmark measures and tend to command greater objectivity (being less observer-dependent), reproducibility, and statistical strength. They include discriminant function analysis and other multivariate statistical models operating on the dimensions of bones such as the pelvis, cranium, and long bones [2]. More recent developments refine metric methodologies through integration with 3D imaging, geometric morphometrics, and machine learning methodologies such that these methods enhance the dependability of sex estimation in fragmented remains [2,3,4]. Despite these advances through technology, both metric and non-metric indicators are frequently suggested for use in tandem to improve precision, both in cases where reference standards are uncertain or population standardization is questionable (e.g., in cases involving unclear or inappropriate reference standards or where skeletal preservation is badly impaired). Additionally, population-varying standards will always feature because dimorphism expression differs by disparate background ancestry. Consequently, though sex estimation remains fundamental, to that other essential evidence component termed the biological profile, methodological refinements coupled with advanced accumulation tools incrementally improve both forensic and archeological efficiency. However, the utility of such landmark-based measurements is also limited by the incompleteness of remains found in archeological and forensic contexts [5]. Both cranial and postcranial skeletal elements have been tested for sex estimation. It has been established that the pelvis is the most important contributor to sex diagnosis, followed by the skull [6,7]. Even though the advances in machine learning and CT-based methods have further enhanced the accuracy of skull-based sex estimations, the pelvis remains superior in predictive performance [4,8,9,10].
Despite these dimorphic structures, the success of assessing sex in skeletal remains depends on the completeness of the skeleton and the expression of that sexual dimorphism in the recovered structures [11,12]. Still, there are situations, such as partially recovered, cremated, and otherwise taphonomically altered remains, where a complete skeleton is not available. Cases of fragmentary and altered remains often yield a limitation in estimating sex, as no isolated characteristic of any particular bone can perfectly determine the sex of a skeleton. Therefore, it becomes essential to develop new methods to assess sex in fragmented and incomplete bone materials [13].
In these scenarios, only the most resilient parts of the skeleton are preserved for analysis, so sex estimation is a challenging process, especially in those cases. In these situations, the skull, unlike the pelvis or other postcranial elements, is less liable to damage and survives inhumation better than other postcranial structures [14]. Among cranial fragments, vault pieces with suture lines and the petrous part of the temporal bone are especially likely to survive due to their dense structure and protected anatomical location [15]. The petrous bone, in particular, has gained increasing attention in recent years for its exceptional DNA preservation properties, even under poor burial conditions [16]. The postcranial skeletal elements that are most likely to be found are articular portions of the humerus and femur, vertebrae, and certain hand and foot bones [17].
Walker (2008) [5] created discriminant functions for non-metric traits for sexing skulls of different populations. The observed traits were the nuchal crest, mastoid process, supraorbital margin, glabella, and mental eminence. The method consists of the visual and physical evaluation of five skeletal traits against the provided descriptions and illustrations to assign the trait an ordinal score between one (representing minimal trait expression, which is associated with a more feminine appearance) and five (representing maximum trait expression and a more masculine appearance) [18]. An average of 90% of individuals from the modern samples were classified correctly using the five traits [5].
These traits presented by Walker (2008) [5] have been validated for different populations [19,20], so they are considered universally dimorphic [20,21,22].
Subsequent studies have evaluated the performance of these five traits proposed by Walker (2008) [5] based on their accuracy, interobserver reliability, and discriminatory power. Among all of them, glabella showed the highest accuracy and low ambiguity in classification [23,24]. On the contrary, mental eminence was the trait showing the lowest accuracy and observer agreement [23].
In fragmented remain scenarios, the bones that seem to be more resistant to the destruction process are dense and relatively heavy [25]. The most resilient parts of the cranium that can be helpful in sex estimation seem to be the foramen magnum [26,27], occipital condyles [25], and the petrous portion of the temporal bone [23]. These three regions of the skeleton have been suggested to be sexually dimorphic. Towards this line of research, different studies have indicated that the sagittal and transverse dimensions of the foramen magnum are significantly greater in human males than in human female skulls in both archeological and modern samples [26,27,28].
Regarding sex estimation using the zygomatic arch extension with respect to the external auditory canal and sigmoid notch, very few previous studies were found [29,30].
This preliminary study looks at the usefulness of four non-metric skull traits that serve as reliable indicators in sex estimation. It also explores how these traits could be applied in forensic cases, especially when skeletal remains are fragmented or incomplete. The non-metric traits include foramen magnum shape, zygomatic arch extension in relation to the external auditory canal, sigmoid notch shape, and the gonial angle muscle attachment area. These skeletal features are usually available during forensic recovery and tend to be well preserved due to their strong anatomical positions and dense bone structure. These traits are easy to evaluate through visual assessment and need minimal equipment, making them practical for use in both field and lab environments. By concentrating on traits that are typically preserved in forensic and archeological remains, this study helps improve non-metric methods to build biological profiles when metric analysis is not possible or skeletal preservation is lacking.
2. Material and Methods
The non-metric traits of 100 skulls were analyzed. All individuals were white adults from the W. M. Bass Donated Skeletal Collection at the University of Tennessee. The sample of 100 individuals consisted of 45 females and 55 males. Females were aged from 44 to 92 years old (mean age: 60.5 years) and males were aged from 34 to 90 years old (mean age: 59.5); 18 men were dentate, 16 men had significant teeth loss, and 21 men were edentulous, and 14 women were dentate, 12 women had significant teeth loss, and 19 women were edentulous. A Mann–Whitney U test was carried out, and no significant differences in age distribution were found between males and females. Also, as described in this manuscript, the average ages and age ranges were similar between males and females. In all cases, the cranium and mandible were complete or fragmented, but all traits studied could be scored in the specimens.
The following traits were used in this study, with assigned ranks to perform the statistical analysis:
Foramen magnum shape: Foramen magnum shape was registered as angled (0) or round (1) (Figure 1).
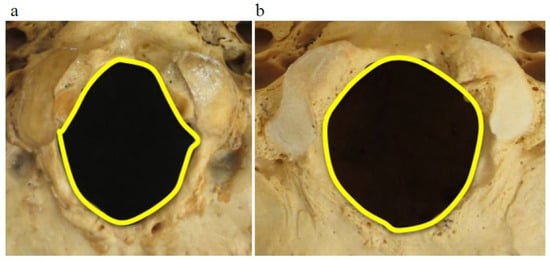
Figure 1.
Foramen magnum morphology is shown as angled (a) and round (b).
Zygomatic arch extension with respect to external auditory canal: zygomatic arch extension (ZAE) was registered with respect to the external auditory canal (EAC) according to whether its end was at the point of EAC (0, extension) or distally to it (1, passing) (Figure 2).
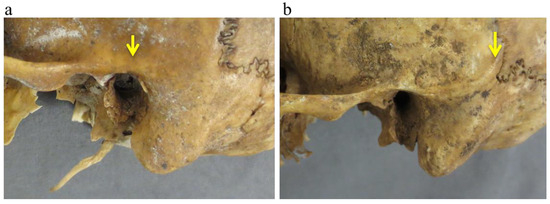
Figure 2.
Zygomatic arch extension was recorded with respect to the external auditory canal (EAC) according to whether its end was anterior or at the point of EAC (a) or distally to it (b). The ending of the zygomatic arch is indicated with the yellow arrow.
Sigmoid notch: Sigmoid notch morphological traits were registered as deep (1) or shallow (0) and wide (1) or narrow (0) (Figure 3).
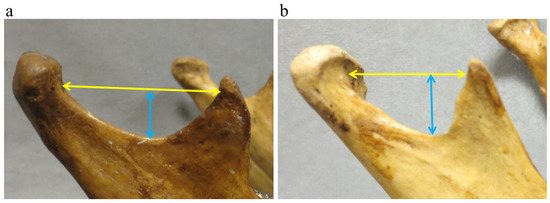
Figure 3.
Sigmoid notch morphological traits were recorded as shallow ((a), blue arrow) or deep ((b), blue arrow) as well as wide ((a), yellow arrow) or narrow ((b), yellow arrow).
Gonial angle area muscle attachment: Gonial angle area (GO) was observed and registered as marked muscle attachment (1) or slightly marked muscle attachment (0) (Figure 4).
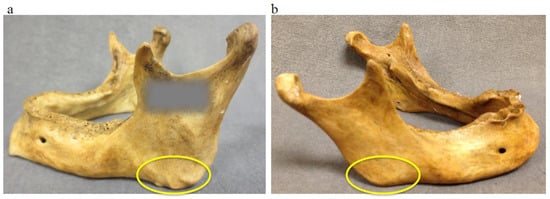
Figure 4.
Gonial angle (indicated with the yellow circles): marked muscle attachment (a) and slightly marked muscle attachment at the gonial angle (b).
Two postdoctoral forensic anthropologists scored the skull traits three times. Neither of the observers were aware of the sex, age, or any other information about the individual’s biological profile at the time of scoring the traits.
Statistical analysis was performed using Statistical Package for Social Science (SPSS) version 15 (IBM, Inc., Armonk, NY, USA). Intra and interobserver errors were assessed by applying a weighted kappa coefficient using linear weights. Comparisons between groups were carried out by applying the Mann–Whitney U test. The Kruskal–Wallis test and Kendall’s Tau were utilized to assess the impact of edentulism on sexual dimorphic traits. Stepwise discriminant function analysis (DFA) leave-one-out modeling was performed to develop specific formulas for sex estimation.
3. Results
The weighted kappa coefficients for intraobserver error for observer 1 were 0.958 with a 95% confidence interval (0.902–1.014) for foramen magnum shape, zygomatic arch extension, and sigmoid notch depth and 0.979 with a 95% confidence interval (0.94–0.973) for sigmoid notch wideness and gonial angle. For observer 2, the weighted kappa coefficients were 0.949 with a 95% confidence interval (0.924–0.966) for foramen magnum shape; 0.948 with a 95% confidence interval (0.877–1.019) for sigmoid notch depth; and 0.979 with a 95% confidence interval (0.94–0.973) for zygomatic arch extension, sigmoid notch wideness, and gonial angle. With respect to interobserver error, the weighted kappa coefficients were 0.958 with a 95% confidence interval (0.902–1.014) for foramen magnum shape, zygomatic arch extension, and sigmoid notch depth and wideness; and 0.948 with a 95% confidence interval (0.877–1.019) for gonial angle. A summary of the values per trait and individual is depicted in Table 1. Table 2 shows the weighted kappa coefficients with confidence intervals for intra and interobserver errors.

Table 1.
Number of individuals per characteristic trait and its presence (1) or absence (0)—please refer to Section 2 Material and Methods Section. FM shape, foramen magnum shape; ZAE, zygomatic arch extension; SN, sigmoid notch depth and wideness; GO, gonial angle.

Table 2.
Weighted kappa coefficients for intra and interobserver errors with confidence intervals. FM shape, foramen magnum shape; ZAE, zygomatic arch extension; SN, sigmoid notch depth and wideness; GO, gonial angle; CI, confidence interval.
The average score of the two observers was used for the following comparison between males and females. Applying Mann–Whitney U tests, there were no significant differences in foramen magnum shape (p = 0.111) and sigmoid notch depth (p = 0.176) and wideness (p = 0.632) between the two groups (males and females). In contrast, strong significant differences were found in zygomatic arch extension and gonial angle (p = 0.008 and p ≤ 0.001). Table 1 indicates that a slightly marked gonial angle and a zygomatic arch extension anteriorly to the external auditory canal (EAC) are more frequent in females.
Considering zygomatic arch extension after the EAC, sex diagnosis, based on discriminant function analysis, was correct in 82.2% of the female individuals and 77.8% of the male individuals when evaluating this trait alone. The formula for sex estimation using this parameter is sex = 2.469*ZAE − 1.247, with a result of zero pointing to males and a result of one pointing to females (Table 3). In contrast, the gonial angle alone discriminates 80% of males and 55.6% females correctly. The formula for sex estimation is sex = 1.326*GO − 1.897, with a result of zero pointing to males and a result of one pointing to females. Based on this data, we assessed the potential influence of edentulism in these traits, and did not find any significant relation with ZAE (Kruskal–Wallis’s test, p = 0.373 and Kendall’s Tau 0.112 with p = 0.246). However, edentulism had a significant impact on GO (Kruskal–Wallis’s test, p = 0.004 and Kendall’s Tau 0.287 with p = 0.003). Further studies, with a greater number of individuals, should be performed to clearly elucidate the influence of edentulism on this trait.

Table 3.
Classification results based on discriminant function using ZAE.
4. Discussion
Based on the results of this work, the shape of the foramen magnum is not an appropriate parameter to discriminate between males and females. In contrast to our findings, different studies have established sexual dimorphism in the foramen magnum, although these studies relied on metric data [23,31,32,33]. While some of these studies were based on archeological or historical remains, their results must be applied in the reference population where data were collected [31,33]. All these works concluded that the foramen magnum area is larger in males than in females. Though one area of the foramen magnum alone is not enough to estimate sex, it could be useful in combination with other sex indicators [26].
The same applies to the sigmoid notch characteristics, which did not present significant differences between the two groups. The role of zygomatic arch extension for sex estimation is in agreement with previous works [34,35], indicating this cranial trait to be a good sex indicator, as well as gonial angle, for which our results are also consistent with other previous studies [36,37]. According to the obtained results, when all these parameters are analyzed through a discriminant function analysis, the best variables to differentiate males from females are the zygomatic arch extension (ZAE) and the gonial angle morphology (GO). However, the use of gonial angle morphology as a sex indicator was shown to be affected by edentulism. Thus, it is not applicable in all cases, and further studies are needed to clearly define the impact of edentulism in this trait for sex estimation.
The results of the present study agree with previous studies, since Krogman and Isçan achieved 92% accuracy in visually assessing sex from the skull and 75% accuracy in visually assessing the postcranial skeleton using the Todd Collection [17]. Konigsberg and Hens (1998) achieved correct classification rates of 83% by evaluating non-metric cranial traits via logistic regression [37,38]. More recently, Walker utilized the same non-metric traits as Konigsberg and Hens (nuchal crest, mastoid process, supraorbital margin, glabella, and mental eminence), although using a quadratic discriminant function, achieving 90% accuracy [5].
Traits that are sexually dimorphic in one population will be much less so in another [5,39]. Hence there is a requirement for a reappraisal of the diagnostic value of sexually dimorphic characters each time a new population is studied. This reassessment ensures that the biological and morphological variation specific to that group is accurately accounted for, reducing the risk of misclassification. Failing to consider population-specific differences can compromise the reliability of sex estimation methods, especially when applying standards derived from reference datasets that are not representative. Therefore, incorporating population variation into methodological frameworks is crucial for enhancing the accuracy and cross-population applicability of forensic and bioarchaeological analyses.
This study evaluated the usefulness of four non-metric skull traits for estimating sex, obtaining a sexual dimorphism value of two in particular: zygomatic arch extension and gonial angle shape. These parts of the skull are resilient areas of the facial skeleton. Their ability to resist taphonomical damage makes them especially useful in forensic contexts and archeological situations, where skeletal remains are often fragmentary or incomplete [39,40]. However, extending the applicability of the traits explored in this paper to archeological remains would require validation on a reference archeological population sample to verify that no secular changes have occurred.
Moreover, these traits are also easy to assess and can usually be evaluated through visual assessment, which makes them practical for both field and laboratory work. The zygomatic arch extension and gonial angle shape have shown noticeable differences in males and females. This sexual dimorphism can be explained by due to differences in muscle mass. However, the use of the gonial angle shape has been shown to be affected by edentulism, so further research is required for its application.
While any non-metric skull feature should not be used in isolation for sex estimation, combining them into multi-trait sex estimation models can greatly improve accuracy, especially when not all traits to be assessed are present. Recent studies have shown that merging qualitative assessments of these traits with more numerical or semi-quantitative scoring can help reduce observer bias and improve consistency [5,7,8]. According to this, the next step of this project is to create a gradient scoring system of the zygomatic arch extension and combine it with other cranial sex indicators.
Additional future directions involve using machine learning methods to improve the accuracy and consistency of skeletal sex estimation. Traditional discriminant function analyses and logistic regression models have established their worth but often depend on linearity and may have difficulties with complex variation. Machine learning methods, on the other hand, cope with the multivariate, non-linear associations of skeletal morphology well. They may bring out patterns that are not recognized by established approaches.
In that sense, efforts will be focused on developing and testing supervised algorithms with the extension of the zygomatic arch for the estimation of skeletal sex. The utilization of such models in forensic anthropology has a variety of benefits. They can create automated as well as reproducible tools that could be validated across populations.
Validation studies using larger samples and different populations would be necessary to make these traits applicable to casework.
Author Contributions
Conceptualization, J.A.-G.; methodology, S.C.Z., J.A.-G. and H.M.; formal analysis, J.A.-G., S.C.Z. and H.M.; resources, J.A.-G. and H.M.; data curation, J.A.-G. and S.C.Z.; writing—original draft preparation, J.A.-G. and S.C.Z.; writing—review and editing, H.M., S.C.Z. and J.A.-G.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The New Jersey Institute of Technology Institutional Review Board (IRB) granted an exemption of these experiments under 45 CFR 46.104(d)(704), category 4 (protocol number: 2205021027) on 7 June 2022.
Informed Consent Statement
Not applicable. The specimens were from a donated skeletal collection, and informed consent is not required based on 45 CFR 46.104(d), category 4.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
All our gratitude to the donated individuals included in this study; without their generous donation to science, this (and many other) research projects wouldn’t be possible.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bruzek, J.; Murail, P. Methodology and reliability of sex determination from the skeleton. In Forensic Anthropology and Medicine: Complementary Sciences from Recovery to Cause of Death; Schmitt, A., Cunha, E., Pinheiro, J., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 225–242. [Google Scholar] [CrossRef]
- Nowaczewska, W.; Kubicka, A.M.; Piontek, J.; Biecek, P. Sex estimation from dimensions of the base of the skull in Black South Africans. Anthropol. Anz. 2022, 79, 153–164. [Google Scholar] [CrossRef]
- Franklin, D.; O’HIggins, P.; Oxnard, C.E.; Dadour, I. Determination of sex in South African blacks by discriminant function analysis of mandibular linear dimensions: A preliminary investigation using the Zulu local population. Forensic Sci. Med. Pathol. 2014, 2, 263–268. [Google Scholar]
- Zhang, M. The application of forensic imaging to sex estimation: Focus on skull and pelvic structures. Perspect. Leg. Forensic Sci. 2024, 1, 10005. Available online: https://www.sciepublish.com/article/pii/186 (accessed on 15 October 2025). [CrossRef]
- Walker, P.L. Sexing skulls using discriminant function analysis of visual assessed traits. Am. J. Phys. Anthr. 2008, 136, 39–50. [Google Scholar] [CrossRef] [PubMed]
- González, P.N.; Bernal, V.; Pérez, S.I. Geometric morphometric approach to sex estimation of human pelvis. Forensic Sci. Int. 2009, 189, 68.e1–68.e8. [Google Scholar] [CrossRef]
- Klales, A.R. Current state of sex estimation in forensic anthropology. Forensic Anthropol. 2021, 4, 219–230. [Google Scholar] [CrossRef]
- Klales, A.R.; Lesciotto, K.M. Reevaluating skeletal sex estimation practices in forensic anthropology. J. Forensic Sci. 2025, 70, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Mello-Gentil, T.; Souza-Mello, V. Contributions of anatomy to forensic sex estimation: Focus on head and neck bones. Forensic Sci. Res. 2021, 7, 11–23. [Google Scholar] [CrossRef]
- Wang, X.; Liu, G.; Wu, Q.; Zheng, Y.; Song, F.; Li, Y. Sex estimation techniques based on skulls in forensic anthropology: A scoping review. PLoS ONE 2024, 19, e0311762. [Google Scholar] [CrossRef]
- Krogman, W.M. The Human Skeleton in Forensic Medicine; Charles C. Thomas: Springfield, MO, USA, 1962. [Google Scholar]
- Barrio, P.A.; Trancho, G.J.; Sánchez, A. Metacarpal sexual determination in a Spanish population. J. Forensic Sci. 2006, 51, 990–995. [Google Scholar] [CrossRef]
- Mastrangelo, P.; de Luca, S.; Sánchez-Mejorada, G. Sex assessment from carpal bones: Discriminant function analysis in a contemporary Mexican sample. Forensic Sci. Int. 2011, 209, 196.e1–196.e15. [Google Scholar] [CrossRef]
- Black, S.; Ferguson, E. Forensic Anthropology 2000 to 2010; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Spence, T.F. The Anatomical Study of Cremated Fragments from Archaeological Sites. Proc. Prehist. Soc. 1967, 5, 70–83. [Google Scholar] [CrossRef]
- Ibrahim, J.; Brumfeld, V.; Addadi, Y.; Rubin, S.; Weiner, S.; Boaretto, E. The petrous bone contains high concentrations of osteocytes: One possible reason why ancient DNA is better preserved in this bone. PLoS ONE 2022, 17, e0269348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dokládal, M. Über die Heutigen Möglichkeiten der Personenidentifikation auf Grund von Verbrannten Knochen. In Aktuelle Kriminologie; Kriminalistik Verlag: Heidelberg, Germany, 1969; pp. 223–246. [Google Scholar]
- Lewis, C.J.; Garvin, H.M. Reliability of the Walker Cranial Nonmetric Method and Implications for Sex Estimation. J. Forensic Sci. 2016, 61, 743–751. [Google Scholar] [CrossRef]
- Krüger, G.C.; L’Abbe, E.N.; Stull, K.E.; Kenyhercz, M.W. Sexual dimorphism in cranial morphology among modern South Africans. Int. J. Leg. Med. 2014, 129, 869–875. [Google Scholar] [CrossRef]
- Oikonomopoulou, E.K.; Valakos, E.; Nikita, E. Population-specificity of sexual dimorphism in cranial and pelvic traits. Int. J. Leg. Med. 2017, 131, 1731–1738. [Google Scholar] [CrossRef]
- Acsádi, G.; Nemeskéri, J. History of Human Life Span and Mortality; Akadémiai Kiado: Budapest, Hungary, 1970. [Google Scholar]
- Garvin, H.M.; Ruff, C.B. Sexual dimorphism in skeletal browridge and chin morphologies determined using a new quantitative method. Am. J. Phys. Anthr. 2012, 147, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Garvin, H.M.; Sholts, S.B.; Mosca, L.A. Sexual dimorphism in human cranial trait scores: Effects of population, age, and body size. Am. J. Phys. Anthropol. 2014, 154, 259–269. [Google Scholar] [CrossRef]
- Stevenson, J.C.; Mahoney, E.R.; Walker, P.L.; Everson, P.M. Technical note: Prediction of sex based on five skull traits using decision analysis (CHAID). Am. J. Phys. Anthr. 2009, 139, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Waldron, T. The relative survival of the human skeleton: Implications for palaeopathology. In Death, Decay and Reconstruction; Manchester University Press: Manchester, UK, 1987; pp. 55–64. [Google Scholar]
- Günay, Y.; Altinkök, M. The value of the size of foramen magnum in sex determination. J. Clin. Forensic Med. 2000, 7, 147–149. [Google Scholar] [CrossRef]
- Uysal, S.; Gokharman, D.; Kacar, M.; Tuncbilek, I.; Kosar, U. Estimation of sex by 3D CT measurements of the foramen magnum. J. Forensic Sci. 2005, 50, 1310–1314. [Google Scholar] [CrossRef]
- Catalina-Herrera, C.J. Study of the anatomic metric values of the foramen magnum and its relation to sex. Acta Anat. 1987, 130, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Basso, I.B.; Freitas, P.F.d.J.; Ferraz, A.X.; Borkovski, A.J.; Santos, R.S.; Rached, R.N.; Küchler, E.C.; Schroder, A.G.D.; de Araujo, C.M.; Guariza-Filho, O. Sex prediction through machine learning utilizing mandibular condyles, coronoid processes, and sigmoid notches features. PLoS ONE 2024, 19, e0312824. [Google Scholar] [CrossRef] [PubMed]
- Langley, N.R.; Dudzik, B.; Cloutier, A. A Decision Tree for Nonmetric Sex Assessment from the Skull. J. Forensic Sci. 2018, 63, 31–37. [Google Scholar] [CrossRef]
- Gruber, P.; Henneberg, M.; Böni, T.; Rühli, F.J. Variability of Human Foramen Magnum Size. Anat. Rec. 2009, 292, 1713–1719. [Google Scholar] [CrossRef]
- Uthman, A.; Al-Rawi, N.; Al-Timimi, J. Evaluation of foramen magnum in gender determination using helical CT. Dentomaxillofacial Radiol. 2012, 41, 197–202. [Google Scholar] [CrossRef]
- Gapert, R.; Black, S.; Last, J. Sex determination from the foramen magnum: Discriminant function analysis. Int. J. Leg. Med. 2009, 123, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.L. Determining the sex of human remains through cranial morphology. J. Forensic Sci. 2005, 50, 493–500. [Google Scholar] [CrossRef]
- Monticelli, F.; Graw, M. Investigation on the reliability of determining sex from the human os zygomaticum. Forensic Sci. Med. Pathol. 2008, 4, 181–186. [Google Scholar] [CrossRef]
- Poongodi, V.; Kanmani, R.; Anandi, M.S.; Krithika, C.L.; Kannan, A.; Raghuram, P.H. Prediction of age and gender using digital radiographic method. J. Pharm. Bioallied Sci. 2015, 7 (Suppl. 2), S504–S508. [Google Scholar] [CrossRef]
- Kharoshah, M.A.; Almadani, O.; Ghaleb, S.S.; Zaki, M.K.; Fattah, Y.A.A. Sexual dimorphism of the mandible in a modern Egyptian population. J. Forensic Leg. Med. 2010, 17, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Konigsberg, L.W.; Hens, S.M. Use of ordinal categorical variables in skeletal assessment of sex from the cranium. Am. J. Phys. Anthropol. 1998, 107, 97–112. [Google Scholar] [CrossRef]
- Steyn, M.; Isçan, M.Y. Sexual dimorphism in the crania and mandibles of South African whites. Forensic Sci. Int. 1998, 98, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Saul, J.M.; Saul, F.P. Forensics, archaeology, and taphonomy: The symbiotic relationship. In Advances in Forensic Taphonomy: Method, Theory, and Archaeological Perspectives; Haglund, W.D., Sorg, M.H., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 63–73. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).