Growth and Development of the Cranial Complex and Its Implications for Sex Estimation
Abstract
1. Introduction
1.1. What Does ‘Mature’ Mean in Forensic and Biological Anthropology?
1.2. Why Does the Division Between Subadult and Adult Persist in Anthropological Methods?
1.3. An Ontogenetic Framework
2. Materials and Methods
2.1. Defining Life History Stages for the Sample
2.2. Interlandmark Distances (ILDs)
2.3. Statistical Methodology
3. Results
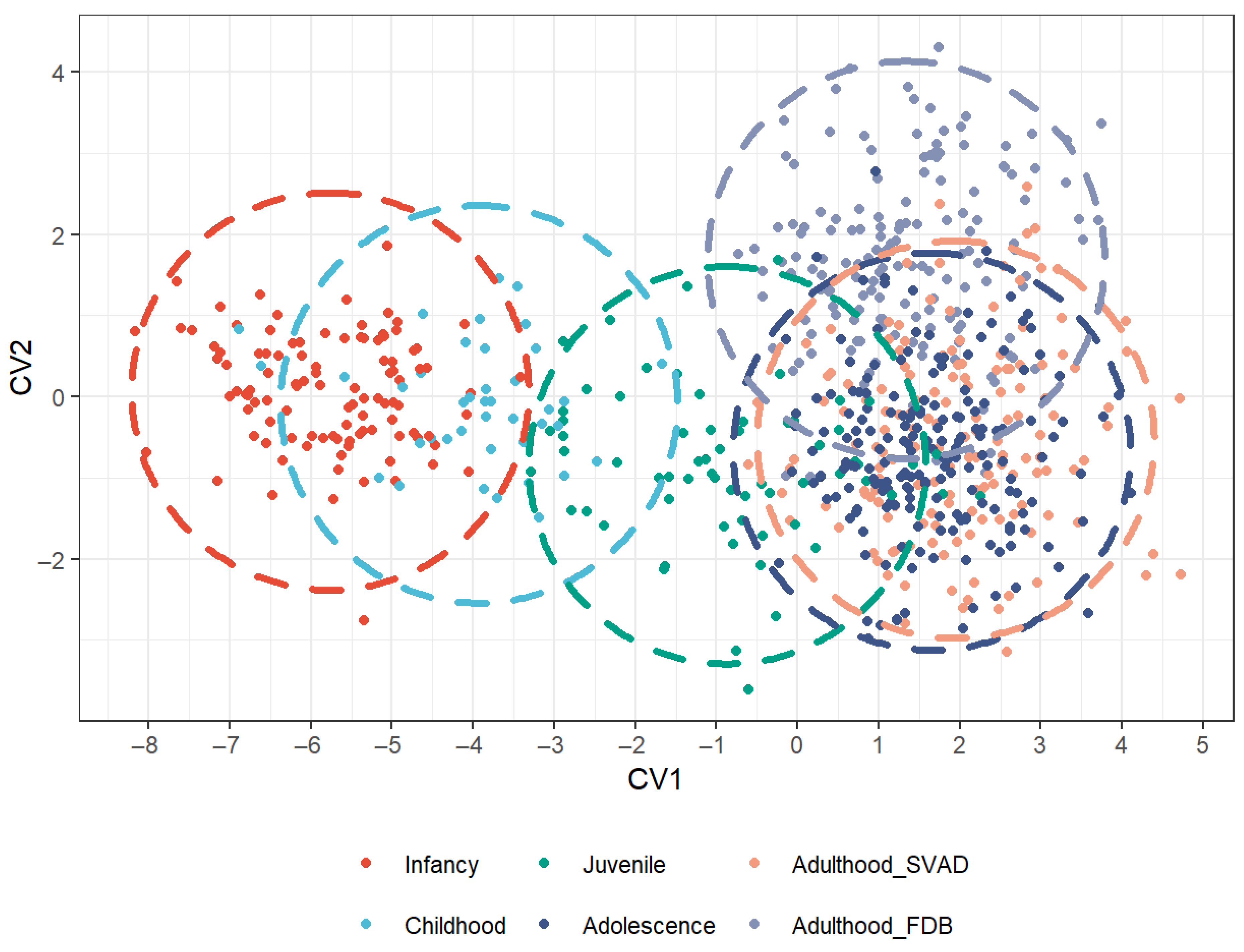
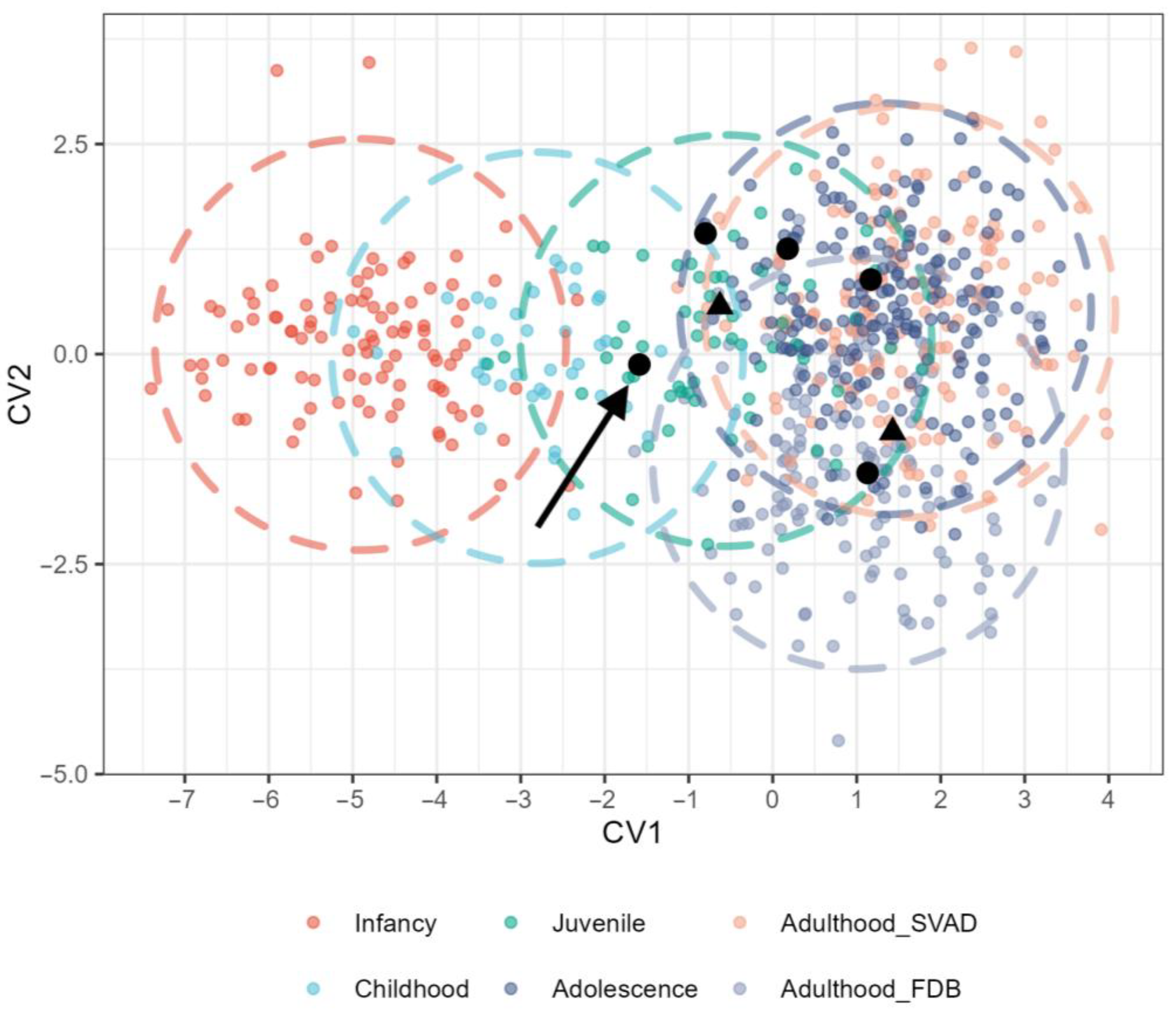
3.1. Cranial Variation Across LH Stages
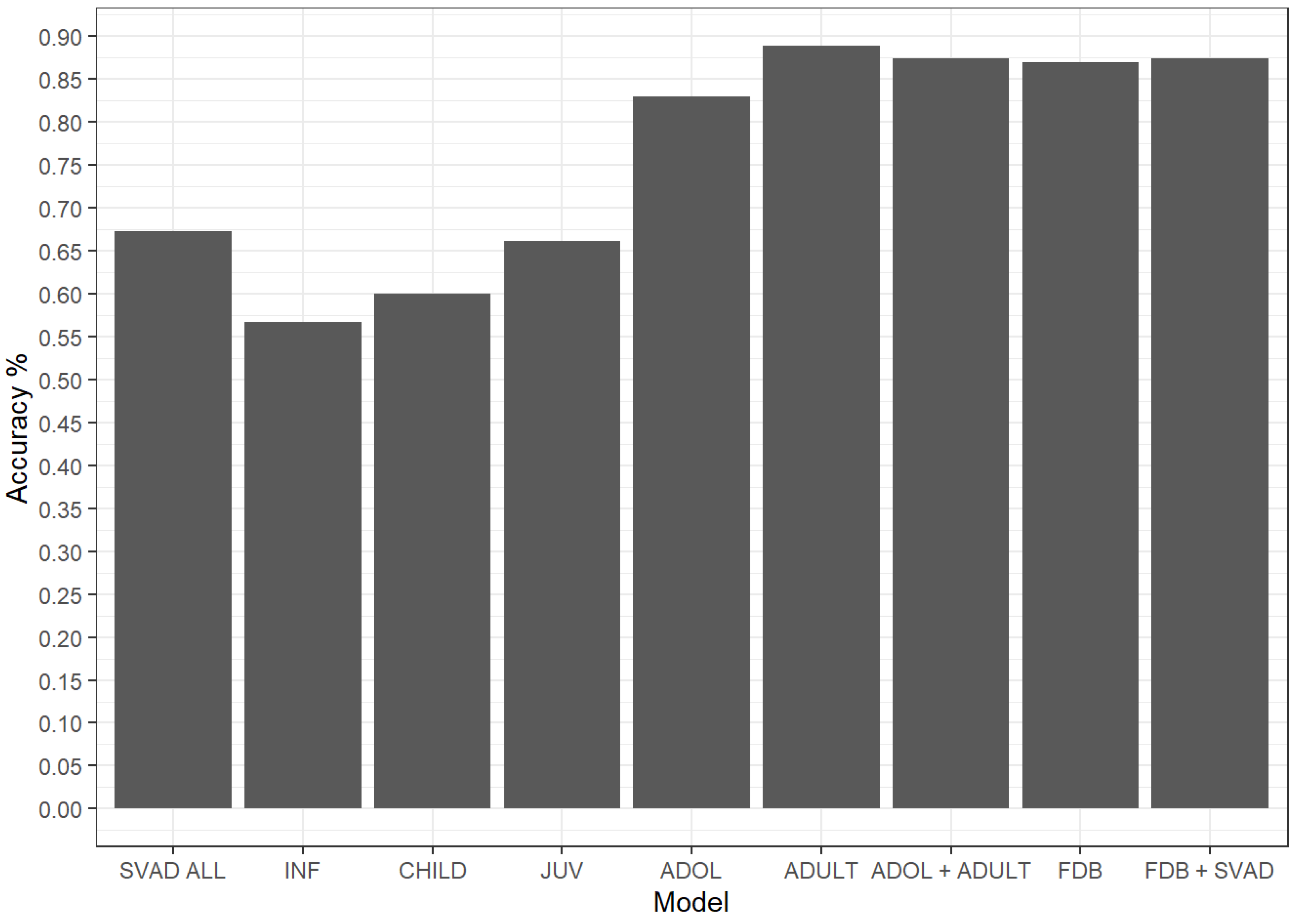
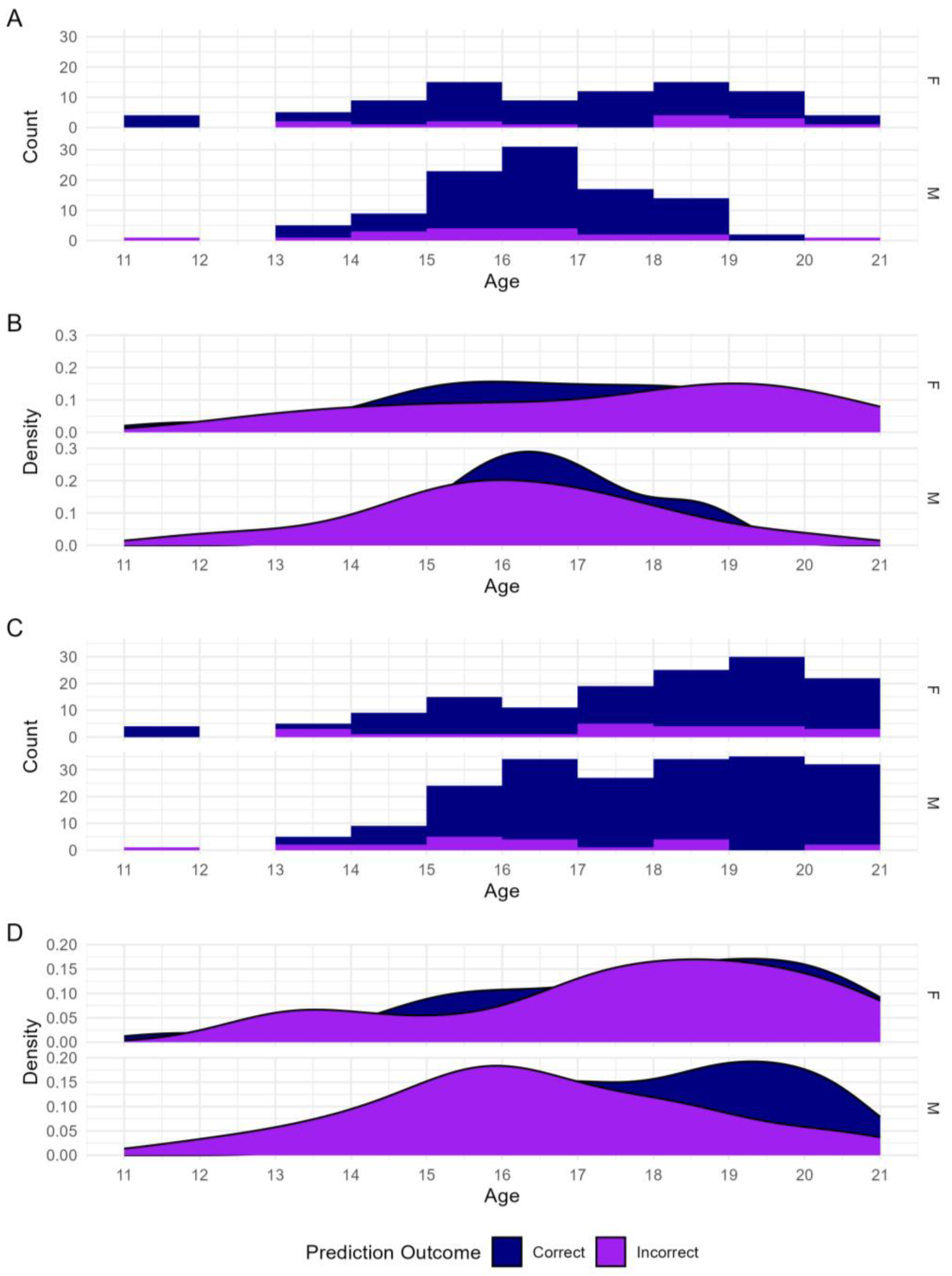
3.2. Sex Estimation
4. Discussion
4.1. How the Cranium Captures Life History Theory
4.2. Impact on Forensic Anthropology: Sex Estimation
Practical Application
4.3. The Relationship Among Skeletal and Sexual Maturity Indicators
4.4. The Second Dimension and Population Variation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huda, T.F.J.; Bowman, J.E. Age Determination from Dental Microstructure in Juveniles. Am. J. Phys. Anthropol. 1995, 97, 135–150. [Google Scholar] [CrossRef]
- Niel, M.; Chaumoître, K.; Adalian, P. Age-at-Death Estimation of Fetuses and Infants in Forensic Anthropology: A New “Coupling” Method to Detect Biases Due to Altered Growth Trajectories. Biology 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Nyström, M.; Peck, L.; Kleemola-Kujala, E.; Evälahti, M.; Kataja, M. Age Estimation in Small Children: Reference Values Based on Counts of Deciduous Teeth in Finns. Forensic Sci. Int. 2000, 110, 179–188. [Google Scholar] [CrossRef]
- Corron, L.; Marchal, F.; Condemi, S.; Telmon, S. Integrating Growth Variability of the Ilium, Fifth Lumbar Vertebra, and Clavicle with Multivariate Adaptive Regression Splines Models for Subadult Age Estimation. J. Forensic Sci. 2019, 64, 34. [Google Scholar] [CrossRef]
- Stull, K.E.; L’Abbé, E.N.; Ousley, S.D. Using Multivariate Adaptive Regression Splines to Estimate Subadult Age from Diaphyseal Dimensions. Am. J. Phys. Anthr. 2014, 154, 376–386. [Google Scholar] [CrossRef] [PubMed]
- De Tobel, J.; Fieuws, S.; Hillewig, E.; Phlypo, I.; van Wijk, M.; de Haas, M.B.; Politis, C.; Verstraete, K.L.; Thevissen, P.W. Multi-Factorial Age Estimation: A Bayesian Approach Combining Dental and Skeletal Magnetic Resonance Imaging. Forensic Sci. Int. 2020, 306, 110054. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.S.; Humphreys, C.; Manson, A. A Human Craniofacial Life-Course: Cross-Sectional Morphological Covariations during Postnatal Growth, Adolescence, and Aging. Anat. Rec. 2022, 305, 81–99. [Google Scholar] [CrossRef]
- Viðarsdóttir, U.S.; O’Higgins, P.; Stringer, C. A Geometric Morphometric Study of Regional Differences in the Ontogeny of the Modern Human Facial Skeleton. J. Anat. 2002, 201, 211–229. [Google Scholar] [CrossRef]
- Bilfeld, M.F.; Dedouit, F.; Sans, N.; Rousseau, H.; Rougé, D.; Telmon, N. Ontogeny of Size and Shape Sexual Dimorphism in the Pubis: A Multislice Computed Tomography Study by Geometric Morphometry. J. Forensic Sci. 2015, 60, 1121–1128. [Google Scholar] [CrossRef]
- Cole, S.J. Developing Subadult Sex Estimation Standards Using Adult Morphological Sex Traits and an Ontogenetic Approach. Ph.D. Thesis, University of Nevada, Reno, NV, USA, 2022. [Google Scholar]
- Stock, M.K. A Preliminary Analysis of the Age of Full Expression of Sexually Dimorphic Cranial Traits. J. Forensic Sci. 2018, 63, 1802–1808. [Google Scholar] [CrossRef]
- New, B.T.; Stull, K.E.; Corron, L.K.; Wolfe, C.A. Exploring Cranial Growth Patterns from Birth to Adulthood for Forensic Research and Practice. Forensic Sci. 2025, 5, 32. [Google Scholar] [CrossRef]
- Wilson, L.A.; Ives, R.; Cardoso, H.F.; Humphrey, L.T. Shape, Size, and Maturity Trajectories of the Human Ilium. Am. J. Phys. Anthropol. 2015, 156, 19–34. [Google Scholar] [CrossRef]
- Matthews, H.S.; Penington, A.J.; Hardiman, R.; Fan, Y.; Clement, J.G.; Kilpatrick, N.M.; Claes, P.D. Modelling 3D Craniofacial Growth Trajectories for Population Comparison and Classification Illustrated Using Sex-Differences. Sci. Rep. 2018, 8, 4771. [Google Scholar] [CrossRef]
- Liang, C.; Profico, A.; Buzi, C.; Khonsari, R.H.; Johnson, D.; O’Higgins, P.; Moazen, M. Normal Human Craniofacial Growth and Development from 0 to 4 Years. Sci. Rep. 2023, 13, 9641. [Google Scholar] [CrossRef]
- Smith, O.A.M.; Nashed, Y.S.G.; Duncan, C.; Pears, N.; Profico, A.; O’Higgins, P. 3D Modeling of Craniofacial Ontogeny and Sexual Dimorphism in Children. Anat. Rec. 2021, 304, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Bilfeld, M.F.; Dedouit, F.; Sans, N.; Rousseau, H.; Rougé, D.; Telmon, N. Ontogeny of Size and Shape Sexual Dimorphism in the Ilium: A Multislice Computed Tomography Study by Geometric Morphometry. J. Forensic Sci. 2013, 58, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Corron, L.K.; Santos, F.; Adalian, P.; Chaumoitre, K.; Guyomarc’h, P.; Marchal, F.; Brůžek, J. How Low Can We Go? A Skeletal Maturity Threshold for Probabilistic Visual Sex Estimation from Immature Human Os Coxae. Forensic Sci. Int. 2021, 325, 110854. [Google Scholar] [CrossRef]
- Noble, J.; Cardini, A.; Flavel, A.; Franklin, D. Geometric Morphometrics on Juvenile Crania: Exploring Age and Sex Variation in an Australian Population. Forensic Sci. Int. 2019, 294, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Bastir, M.; Rosas, A.; O’Higgins, P. Craniofacial Levels and the Morphological Maturation of the Human Skull. J. Anat. 2006, 209, 637–654. [Google Scholar] [CrossRef]
- Kesterke, M.J.; Raffensperger, Z.D.; Heike, C.L.; Cunningham, M.L.; Hecht, J.T.; Kau, C.H.; Nidey, N.L.; Moreno, L.M.; Wehby, G.L.; Marazita, M.L.; et al. Using the 3D Facial Norms Database to Investigate Craniofacial Sexual Dimorphism in Healthy Children, Adolescents, and Adults. Biol. Sex. Differ. 2016, 7, 23. [Google Scholar] [CrossRef]
- Ferrario, V.F.; Sforza, C.; Poggio, C.E.; Schmitz, J.H. Craniofacial Growth: A Three-Dimensional Soft-Tissue Study from 6 Years to Adulthood. J. Craniofac. Genet. Dev. Biol. 1998, 18, 138–149. [Google Scholar]
- Koudelová, J.; Brůžek, J.; Cagáňová, V.; Krajíček, V.; Velemínská, J. Development of Facial Sexual Dimorphism in Children Aged between 12 and 15 Years: A Three-Dimensional Longitudinal Study. Orthod. Craniofac. Res. 2015, 18, 175–184. [Google Scholar] [CrossRef] [PubMed]
- ANSI/ASB Standard 0090; Standard for Sex Estimation in Forensic Anthropology. AFS Standards Board, LLC: Colorado Springs, CO, USA, 2019.
- ANSI/ASB Standard 132; Standard for Population Affinity Estimation in Forensic Anthropology. AFS Standards Board, LLC: Colorado Springs, CO, USA, 2023.
- Cameron, N. Assessment of Maturation. In Human Growth and Development, 2nd ed.; Cameron, N., Bogin, B., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 515–535. ISBN 978-0-12-383882-7. [Google Scholar]
- Christensen, A.M.; Passalacqua, N.V.; Bartelink, E.J. Age Estimation. In Forensic Anthropology, 2nd ed.; Christensen, A.M., Passalacqua, N.V., Bartelink, E.J., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 307–349. ISBN 978-0-12-815734-3. [Google Scholar]
- Beunen, G.P.; Rogol, A.D.; Malina, R.M. Indicators of Biological Maturation and Secular Changes in Biological Maturation. Food Nutr. Bull. 2006, 27, S244–S256. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in Pattern of Pubertal Changes in Girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Tanner, J.M. Variations in the Pattern of Pubertal Changes in Boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N. Can Maturity Indicators Be Used to Estimate Chronological Age in Children? Ann. Hum. Biol. 2015, 42, 302–307. [Google Scholar] [CrossRef]
- Ellis, B.J. Timing of Pubertal Maturation in Girls: An Integrated Life History Approach. Psychol. Bull. 2004, 130, 920–958. [Google Scholar] [CrossRef]
- Demirjian, A.; Goldstein, H.; Tanner, J.M. A New System of Dental Age Assessment. Hum. Biol. 1973, 45, 211–227. [Google Scholar]
- Langley-Shirley, N.; Jantz, R.L. A Bayesian Approach to Age Estimation in Modern Americans from the Clavicle. J. Forensic Sci. 2010, 55, 571–583. [Google Scholar] [CrossRef]
- Asad, A.L.; Anteby, M.; Garip, F. Who Donates Their Bodies to Science? The Combined Role of Gender and Migration Status among California Whole-Body Donors. Soc. Sci. Med. 2014, 106, 53–58. [Google Scholar] [CrossRef]
- Boulware, L.E.; Ratner, L.E.; Cooper, L.A.; LaVeist, T.A.; Powe, N.R. Whole Body Donation for Medical Science: A Population-Based Study. Clin. Anat. 2004, 17, 570–577. [Google Scholar] [CrossRef]
- Go, M.; Yukyi, N.; Chu, E. On WEIRD Anthropologists and Their White Skeletons. Forensic Anthropol. 2021, 4, 283–298. [Google Scholar] [CrossRef]
- Hughes, C.E.; Juarez, C.; Yim, A.-D. Forensic Anthropology Casework Performance: Assessing Accuracy and Trends for Biological Profile Estimates on a Comprehensive Sample of Identified Decedent Cases. J. Forensic Sci. 2021, 66, 1602–1616. [Google Scholar] [CrossRef]
- Winburn, A.P.; Algee-Hewitt, B. Evaluating Population Affinity Estimates in Forensic Anthropology: Insights from the Forensic Anthropology Database for Assessing Methods Accuracy (FADAMA). J. Forensic Sci. 2021, 66, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Albanese, J. Identified Skeletal Reference Collections and the Study of Human Variation. Ph.D. Dissertation, McMaster University, Hamilton, ON, Canada, 2003. [Google Scholar]
- Stull, K.E.; Corron, L.K. The Subadult Virtual Anthropology Database (SVAD): An Accessible Repository of Contemporary Subadult Reference Data. Forensic Sci. 2022, 2, 20–36. [Google Scholar] [CrossRef]
- Alonzo, S.H.; Kindsvater, H.K. Life-History Patterns. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 2175–2180. ISBN 978-0-08-045405-4. [Google Scholar]
- Hill, K. Life History Theory and Evolutionary Anthropology. Evol. Anthropol. Issues News Rev. 1993, 2, 78–88. [Google Scholar] [CrossRef]
- Kuzawa, C.W.; Bragg, J.M. Plasticity in Human Life History Strategy: Implications for Contemporary Human Variation and the Evolution of Genus Homo. Curr. Anthropol. 2012, 53, s369–s382. [Google Scholar] [CrossRef]
- Mace, R. Evolutionary Ecology of Human Life History. Anim. Behav. 2000, 59, 1–10. [Google Scholar] [CrossRef]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992; ISBN 978-0-19-857741-6. [Google Scholar]
- Gadgil, M.; Bossert, W.H. Life Historical Consequences of Natural Selection. Am. Nat. 1970, 104, 1–24. [Google Scholar] [CrossRef]
- Bogin, B. Patterns of Human Growth, 3rd ed.; Cambridge Studies in Biological and Evolutionary Anthropology; Cambridge University Press: Cambridge, UK, 2020; ISBN 978-1-108-43448-5. [Google Scholar]
- Ellison, P.T.; Reiches, M.W.; Shattuck-Faegre, H.; Breakey, A.; Konecna, M.; Urlacher, S.; Wobber, V. Puberty as a Life History Transition. Ann. Hum. Biol. 2012, 39, 352–360. [Google Scholar] [CrossRef]
- Tanner, J.M. Growth and Maturation during Adolescence. Nutr. Rev. 1981, 39, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Abbassi, V. Growth and Normal Puberty. Pediatrics 1998, 102, 507–511. [Google Scholar] [CrossRef]
- Gårdstedt-Berghog, J.; Niklasson, A.; Sjöberg, A.; Aronson, A.S.; Pivodic, A.; Nierop, A.F.M.; Albertsson-Wikland, K.; Holmgren, A. Timing of Menarche and Pubertal Growth Patterns Using the QEPS Growth Model. Front. Pediatr. 2024, 12, 1438042. [Google Scholar] [CrossRef]
- Stull, K.E.; Wolfe, C.A.; Corron, L.K.; Heim, K.; Hulse, C.N.; Pilloud, M.A. A Comparison of Subadult Skeletal and Dental Development Based on Living and Deceased Samples. Am. J. Phys. Anthropol. 2021, 175, 36–58. [Google Scholar] [CrossRef]
- Berry, S.D.; Edgar, H.J. Announcement: The New Mexico Decedent Image Database. Forensic Imaging 2021, 24, 200436. [Google Scholar] [CrossRef]
- Stull, K.; Corron, L. Subadult Virtual Anthropology Database (SVAD) Data Collection Protocol: Amira; Zenodo: Geneva, Switzerland, 2021. [Google Scholar] [CrossRef]
- Stock, M.K.; Garvin, H.M.; Corron, L.K.; Hulse, C.N.; Cirillo, L.E.; Klales, A.R.; Colman, K.L.; Stull, K.E. The Importance of Processing Procedures and Threshold Values in CT Scan Segmentation of Skeletal Elements: An Example Using the Immature Os Coxa. Forensic Sci. Int. 2020, 309, 110232. [Google Scholar] [CrossRef] [PubMed]
- Sellen, D.W. Evolution of Infant and Young Child Feeding: Implications for Contemporary Public Health. Annu. Rev. Nutr. 2007, 27, 123–148. [Google Scholar] [CrossRef]
- AlQahtani, S.J.; Hector, M.P.; Liversidge, H.M. Brief Communication: The London Atlas of Human Tooth Development and Eruption. Am. J. Phys. Anthr. 2010, 142, 481–490. [Google Scholar] [CrossRef]
- Hägg, U.; Taranger, J. Skeletal Stages of the Hand and Wrist as Indicators of the Pubertal Growth Spurt. Acta Odontol. Scand. 1980, 38, 187–200. [Google Scholar] [CrossRef]
- Buehl, C.C.; Pyle, S.I. The Use of Age at First Appearance of Three Ossification Centers in Determining the Skeletal Status of Children. J. Pediatr. 1942, 21, 335–342. [Google Scholar] [CrossRef]
- Wheeler, M.D. Physical Changes of Puberty. Endocrinol. Metab. Clin. N. Am. 1991, 20, 1–14. [Google Scholar] [CrossRef]
- Stull, K.; Chu, E.; Wolfe, C.; Corron, L. Application of Subadult Age Estimation. In A Visual Atlas of Skeletal Growth and Development; Stull, K., Garvin, H., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2025; ISBN 978-1-032-75485-7. [Google Scholar]
- Cardoso, H.F.V. Epiphyseal Union at the Innominate and Lower Limb in a Modern Portuguese Skeletal Sample, and Age Estimation in Adolescent and Young Adult Male and Female Skeletons. Am. J. Phys. Anthropol. 2008, 135, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Coqueugniot, H.; Weaver, T.; Houët, F. Brief Communication: A Probabilistic Approach to Age Estimation from Infracranial Sequences of Maturation. Am. J. Phys. Anthropol. 2010, 142, 655–664. [Google Scholar] [CrossRef]
- Schaefer, M.C. A Summary of Epiphyseal Union Timings in Bosnian Males. Int. J. Osteoarchaeol. 2008, 18, 536–545. [Google Scholar] [CrossRef]
- Webb, P.A.O.; Suchey, J.M. Epiphyseal Union of the Anterior Iliac Crest and Medial Clavicle in a Modern Multiracial Sample of American Males and Females. Am. J. Phys. Anthropol. 1985, 68, 457–466. [Google Scholar] [CrossRef]
- Thabtah, F.; Hammoud, S.; Kamalov, F.; Gonsalves, A. Data Imbalance in Classification: Experimental Evaluation. Inf. Sci. 2020, 513, 429–441. [Google Scholar] [CrossRef]
- Spradley, M.K.; Wolfe, C.A.; Stull, K.E.; Chu, E.Y.; Broehl, K.A.; Vlemincq-Mendieta, T.; Pilloud, M.A.; Scott, G.R.; Corron, L.K. Subadult Virtual Anthropology Database (SVAD) Data Collection Protocol: Cranial Landmarks and Craniometrics; Zenodo: Geneva, Switzerland, 2021. [Google Scholar]
- Langley, N.; Jantz, L.; Ousley, S.; Jantz, R.; Milner, G. Data Collection Procedures for Forensic Skeletal Material 2.0; Department of Anthropology, The University of Tennessee: Knoxville, TN, USA, 2016. [Google Scholar]
- Moore-Jansen, P.; Ousley, S.; Jantz, R. Data Collection Procedures for Forensic Skeletal Material; Department of Anthropology, The University of Tennessee: Knoxville, TN, USA, 1994. [Google Scholar]
- Corron, L.K.; Broehl, K.A.; Chu, E.Y.; Vlemincq-Mendieta, T.; Wolfe, C.A.; Pilloud, M.A.; Scott, G.R.; Spradley, M.K.; Stull, K.E. Agreement and Error Rates Associated with Standardized Data Collection Protocols for Skeletal and Dental Data on 3D Virtual Subadult Crania. Forensic Sci. Int. 2022, 334, 111272. [Google Scholar] [CrossRef]
- Barbeito-Andrés, J.; Anzelmo, M.; Ventrice, F.; Sardi, M.L. Measurement Error of 3D Cranial Landmarks of an Ontogenetic Sample Using Computed Tomography. J. Oral. Biol. Craniofacial Res. 2012, 2, 77–82. [Google Scholar] [CrossRef]
- Colman, K.L.; de Boer, H.H.; Dobbe, J.G.G.; Liberton, N.P.T.J.; Stull, K.E.; van Eijnatten, M.; Streekstra, G.J.; Oostra, R.-J.; van Rijn, R.R.; van der Merwe, A.E. Virtual Forensic Anthropology: The Accuracy of Osteometric Analysis of 3D Bone Models Derived from Clinical Computed Tomography (CT) Scans. Forensic Sci. Int. 2019, 304, 109963. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, R version 4.5.0 (2025-04-11); R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Roever, C.; Raabe, N.; Leubke, K.; Ligges, U.; Szepannek, G.; Zentgraf, M. klaR: Classification and Visualization 2013. Version 1.7-3. Available online: http://cran.r-project.org/web/packages/klaR/klaR.pdf (accessed on 13 August 2025).
- Wolfe, C.A.; Stull, K.E. ChristopherAWolfe/Stulletal2025_ForensicScience: Code Compendium (Version v1.0.0). 2025. Available online: https://zenodo.org/records/15741672 (accessed on 13 August 2025). [CrossRef]
- Baughan, B.; Demirjian, A. Sexual Dimorphism in the Growth of the Cranium. Am. J. Phys. Anthropol. 1978, 49, 383–390. [Google Scholar] [CrossRef]
- Hardin, A.M.; Knigge, R.P.; Oh, H.S.; Valiathan, M.; Duren, D.L.; McNulty, K.P.; Middleton, K.M.; Sherwood, R.J. Estimating Craniofacial Growth Cessation: Comparison of Asymptote- and Rate-Based Methods. Cleft Palate. Craniofac. J. 2022, 59, 230–238. [Google Scholar] [CrossRef]
- Humphrey, L.T. Growth Patterns in the Modern Human Skeleton. Am. J. Phys. Anthropol. 1998, 105, 57–72. [Google Scholar] [CrossRef]
- Bulygina, E.; Mitteroecker, P.; Aiello, L. Ontogeny of Facial Dimorphism and Patterns of Individual Development within One Human Population. Am. J. Phys. Anthropol. 2006, 131, 432–443. [Google Scholar] [CrossRef]
- Enlow, D. Facial Growth, 3rd ed.; W.B. Saunders: Philadelphia, PA, USA, 1990. [Google Scholar]
- Forsberg, C.-M.; Eliasson, S.; Westergren, H. Face Height and Tooth Eruption in Adults—A 20-Year Follow-up Investigation. Eur. J. Orthod. 1991, 13, 249–254. [Google Scholar] [CrossRef]
- Ross, A.H.; Williams, S.E. Craniofacial Growth, Maturation, and Change: Teens to Midadulthood. J. Craniofac. Surg. 2010, 21, 458. [Google Scholar] [CrossRef] [PubMed]
- Bogin, B. Patterns of Human Growth; Cambridge University Press: Cambridge, MA, USA, 1999; Volume 2. [Google Scholar]
- Kuzawa, C.W.; Chugani, H.T.; Grossman, L.I.; Lipovich, L.; Muzik, O.; Hof, P.R.; Wildman, D.E.; Sherwood, C.C.; Leonard, W.R.; Lange, N. Metabolic Costs and Evolutionary Implications of Human Brain Development. Proc. Natl. Acad. Sci. USA 2014, 111, 13010–13015. [Google Scholar] [CrossRef] [PubMed]
- Spradley, M.; Jantz, R. Sex Estimation in Forensic Anthropology: Skull versus Postcranial Elements. J. Forensic Sci. 2011, 56, 289–296. [Google Scholar] [CrossRef]
- Konigsberg, L.W.; Hens, S.M. Use of Ordinal Categorical Variables in Skeletal Assessment of Sex from the Cranium. Am. J. Phys. Anthropol. 1998, 107, 97–112. [Google Scholar] [CrossRef]
- Pilmann Kotěrová, A.; Santos, F.; Bejdová, Š.; Rmoutilová, R.; Attia, M.H.; Habiba, A.; Velemínská, J.; Brůžek, J. Prioritizing a High Posterior Probability Threshold Leading to Low Error Rate over High Classification Accuracy: The Validity of MorphoPASSE Software for Cranial Morphological Sex Estimation in a Contemporary Population. Int. J. Legal Med. 2024, 138, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.L. Sexing Skulls Using Discriminant Function Analysis of Visually Assessed Traits. Am. J. Phys. Anthropol. 2008, 136, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Auchter, L.E.; Stull, K. Development and Validation of a Subadult Sex Estimation Method Using Pelvic Metrics. Forensic Anthropol. 2025, 8, 19–35. [Google Scholar] [CrossRef]
- Sanchez, J.; Hoppa, R. Is Adulthood Required? Examining the Accuracy of Pelvic Sex Estimation Throughout Pubertal Growth. Bioarchaeology Int. 2022, 7, 173–188. [Google Scholar] [CrossRef]
- Eveleth, P.; Tanner, J. Worldwide Variation in Human Growth, 2nd ed.; Cambridge University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Del Bove, A.; Menéndez, L.; Manzi, G.; Moggi-Cecchi, J.; Lorenzo, C.; Profico, A. Mapping Sexual Dimorphism Signal in the Human Cranium. Sci. Rep. 2023, 13, 16847. [Google Scholar] [CrossRef]
- Jantz, R.L.; Ousley, S.D. FORDISC 3: Computerized Forensic Discriminant Functions. Version 3.1; The University of Tennessee: Knoxville, TN, USA, 2005. [Google Scholar]
- Scott, S.; Jantz, R.L. Survivability versus Rate of Recovery for Skeletal Elements in Forensic Anthropology. J. Forensic Sci. 2022, 67, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Biro, F.M.; Pajak, A.; Wolff, M.S.; Pinney, S.M.; Windham, G.C.; Galvez, M.P.; Greenspan, L.C.; Kushi, L.H.; Teitelbaum, S.L. Age of Menarche in a Longitudinal US Cohort. J. Pediatr. Adolesc. Gynecol. 2018, 31, 339–345. [Google Scholar] [CrossRef]
- Marshall, W.A. Interrelationships of Skeletal Maturation, Sexual Development and Somatic Growth in Man. Ann. Hum. Biol. 1974, 1, 29–40. [Google Scholar] [CrossRef]
- Sanders, J.O.; Qiu, X.; Lu, X.; Duren, D.L.; Liu, R.W.; Dang, D.; Menendez, M.E.; Hans, S.D.; Weber, D.R.; Cooperman, D.R. The Uniform Pattern of Growth and Skeletal Maturation during the Human Adolescent Growth Spurt. Sci. Rep. 2017, 7, 16705. [Google Scholar] [CrossRef]
- Gonzalez, P.N.; Perez, S.I.; Bernal, V. Ontogeny of Robusticity of Craniofacial Traits in Modern Humans: A Study of South American Populations. Am. J. Phys. Anthr. 2010, 142, 367–379. [Google Scholar] [CrossRef]
- Ackermann, R.R.; Krovitz, G.E. Common Patterns of Facial Ontogeny in the Hominid Lineage. Anat. Rec. 2002, 269, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Stull, K.; Chu, E.; Corron, L. From Subadult to Adult: The Expression of Cranial Macromorphoscopic Traits through Ontogeny. In Proceedings of the 71st Annual Meeting of the American Academy of Forensic Sciences, Seattle, WA, USA, 21–25 February 2022. [Google Scholar]
- Wood, C. The Influence of Growth and Development in the Expression of Human Morphological Variation. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2013. [Google Scholar]
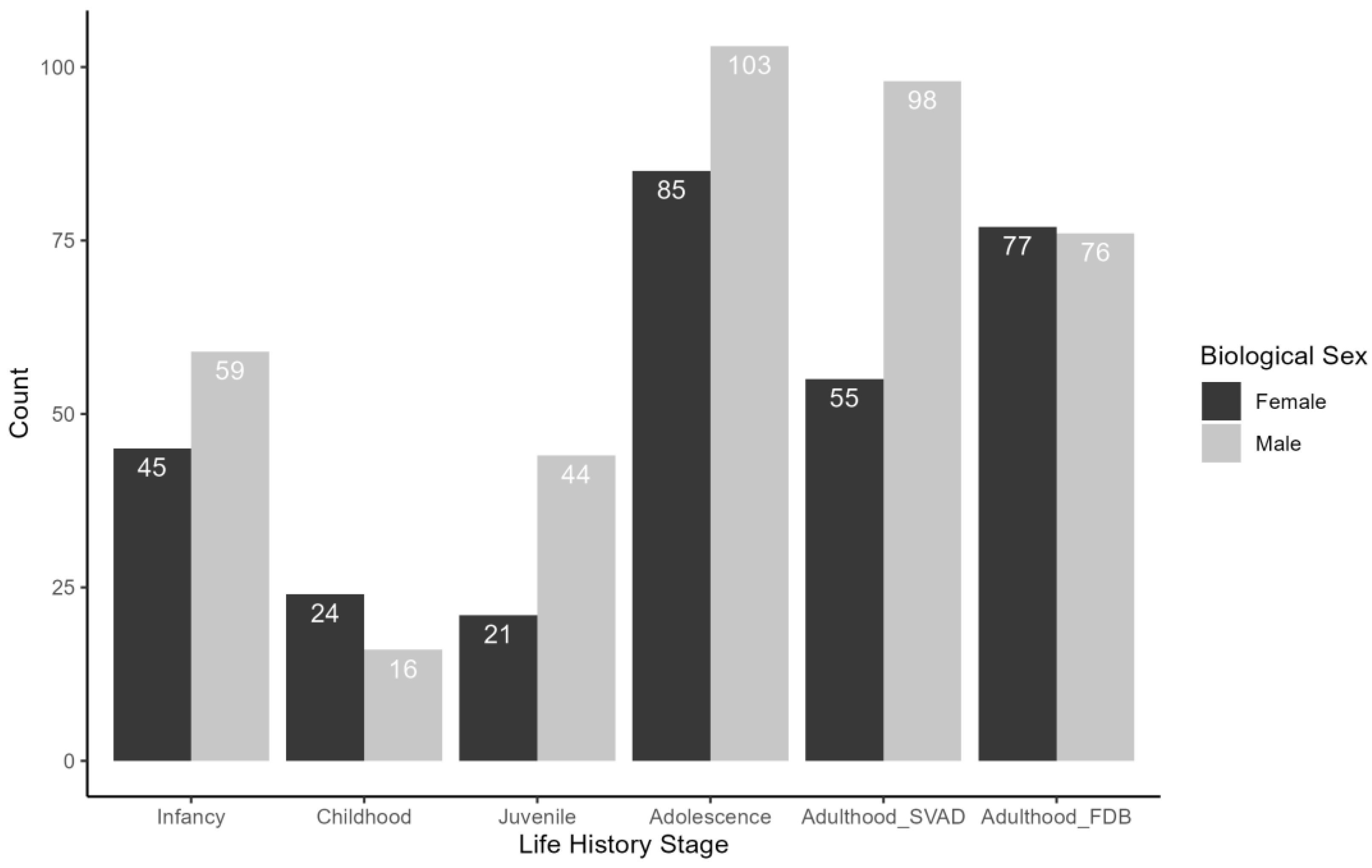



| Chronologically Derived Age Ranges | Developmental Criteria | Developmentally Derived Age Ranges (with Means per Sex) | |
|---|---|---|---|
| Infancy | <3 years | First molars ≤ crown complete | 0–3.45 years mean F = 1.32 years mean M = 1.56 years |
| Childhood | 3–6.99 years | First molars > crown complete and First molars ≤ root 1/2 | 2–6 years mean F = 4.24 years mean M = 4.26 years |
| Juvenile | 7–10.99 years (females); 7–12.99 years (males | First molars ≥ root 3/4 and Iliac crest epiphysis is absent | 5 to 15 years mean F = 9.14 years mean M = 10.6 years |
| Adolescence | 11–17.99 years (females); 13–17.99 years (males) | Iliac crest epiphysis is present and unfused or fusing | 11–20 years mean F = 16.8 years mean M = 16.5 years |
| Adulthood—SVAD | 18+ | Iliac crest is fused or the proximal humerus epiphysis is fused | 15–20 years mean F = 19.3 years mean M = 19.3 years |
| Adulthood—FDB | 18+ | No developmental data available | 20–96 years mean F = 58.0 years mean M = 56.2 years |
| ILD | Abbreviation | Definitions |
|---|---|---|
| Nasal Height | NLH | Nasion to most inferior nasal border (L) |
| Nasion-Prosthion Height | NPH | Nasion to prosthion |
| Nasal Breadth | NLB | Alare (L) to alare (R) |
| Orbital Height | OBH | Orbit height inferior (R) to orbit height superior (R) |
| Orbital Breadth | OBB | Dacryon (R) to ectoconchion (R) |
| Interorbital Breadth | DKB | Dacryon (L) to dacryon (R) |
| Biorbital Breadth | EKB | Ectoconchion (L) to ectoconchion (R) |
| Bifrontal Breadth | FMB | Frontomalare anterior (L) to frontomalare anterior (R) |
| Bizygomatic Breadth | ZYB | Zygion (L) to zygion (R) |
| Bijugal Breadth | JUB | Jugale (L) to jugale (R) |
| Maximum Cranial Length | GOL | Glabella to opisthocranion |
| Nasio-occipital Length | NOL | Nasion to opisthocranion |
| Mastoid Height | MDH | Porion (R) to mastoideale (R) |
| Maximum Cranial Breadth | XCB | Euryon (L) to euryon (R) |
| Minimum Frontal Breadth | WFB | Frontotemporale (L) to frontotemporale (R) |
| Frontal Chord | FRC | Nasion to bregma |
| Parietal Chord | PAC | Bregma to lambda |
| Occipital Chord | OCC | Lambda to opisthion |
| Biauricular Breadth | AUB | Radiculare (L) to radiculare (R) |
| Biasterionic Breadth | ASB | Asterion (L) to asterion (R) |
| Foramen Magnum Length | FOL | Basion to opisthion |
| Foramen Magnum Breadth | FOB | Foramen magnum breadth (L) to foramen magnum breadth (R) |
| Cranial Base Length | BNL | Basion to nasion |
| Basion-Bregma Height | BBH | Basion to bregma |
| Basion-Prosthion Length | BPL | Basion to prosthion |
| Infancy | Childhood | Juvenile | Adolescence | Adulthood SVAD | |
|---|---|---|---|---|---|
| Infancy | 0.000 | 3.078 | 5.073 | 5.559 | 5.958 |
| Childhood | 3.078 | 0.000 | 3.735 | 5.032 | 5.156 |
| Juvenile | 5.073 | 3.735 | 0.000 | 2.330 | 3.584 |
| Adolescence | 5.559 | 5.032 | 2.330 | 0.000 | 0.489 |
| Adulthood SVAD | 5.958 | 5.156 | 3.584 | 0.489 | 0.000 |
| Actual | |||||
|---|---|---|---|---|---|
| Predicted | Infancy | Childhood | Juvenile | Adolescence | Adulthood SVAD |
| Infancy | 87 | 8 | 0 | 0 | 0 |
| Childhood | 17 | 31 | 8 | 0 | 0 |
| Juvenile | 0 | 1 | 45 | 3 | 3 |
| Adolescence | 0 | 0 | 11 | 128 | 81 |
| Adulthood SVAD | 0 | 0 | 1 | 57 | 69 |
| Infancy | Childhood | Juvenile | Adolescence | Adulthood SVAD | Adulthood FDB | |
|---|---|---|---|---|---|---|
| Infancy | 0.000 | 3.275 | 5.715 | 6.757 | 7.195 | 7.472 |
| Childhood | 3.275 | 0.000 | 3.945 | 5.687 | 5.880 | 6.431 |
| Juvenile | 5.715 | 3.945 | 0.000 | 2.388 | 3.634 | 5.172 |
| Adolescence | 6.757 | 5.687 | 2.388 | 0.000 | 0.469 | 3.091 |
| Adulthood SVAD | 7.195 | 5.880 | 3.634 | 0.469 | 0.000 | 3.054 |
| Adulthood FDB | 7.472 | 6.431 | 5.172 | 3.091 | 3.054 | 0.000 |
| Actual | ||||||
|---|---|---|---|---|---|---|
| Predicted | Infancy | Childhood | Juvenile | Adolescence | Adulthood SVAD | Adulthood FDB |
| Infancy | 90 | 7 | 0 | 0 | 0 | 0 |
| Childhood | 14 | 32 | 8 | 0 | 0 | 0 |
| Juvenile | 0 | 1 | 41 | 1 | 4 | 1 |
| Adolescence | 0 | 0 | 14 | 119 | 74 | 19 |
| Adulthood SVAD | 0 | 0 | 1 | 49 | 54 | 10 |
| Adulthood FDB | 0 | 0 | 1 | 19 | 21 | 123 |
| Models | Best Performing Model | Leave-One-Out Classification Accuracy | 95% CI |
|---|---|---|---|
| Infancy | y ~ WFB + AUB + ASB + FRC + PAC | 0.567 | 0.467–0.664 |
| Childhood | y ~ GOL + NOL + BNL + BBH + XCB + ZYB + BPL + NLB + OCC + FOL | 0.600 | 0.433–0.751 |
| Juvenile | y ~ BNL + ZYB + AUB + BPL | 0.662 | 0.534–0.774 |
| Adolescence | y ~ BNL + XCB + WFB + ZYB + ASB + OBH_L + DKB + FRC + FOL | 0.830 | 0.768–0.881 |
| Adulthood SVAD | y ~ GOL + BNL + BBH + ZYB + ASB + DKB | 0.889 | 0.828–0.934 |
| Adulthood FDB | y ~ GOL + NOL + BNL + BBH + WFB + ZYB + DKB + FOL | 0.869 | 0.805–0.918 |
| Actual | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predicted | Infancy | Childhood | Juvenile | Adolescence | Adulthood SVAD | Adulthood FDB | ||||||
| Sex | F | M | F | M | F | F | F | M | F | M | F | M |
| F | 16 | 16 | 16 | 8 | 6 | 7 | 71 | 18 | 46 | 8 | 67 | 10 |
| M | 29 | 43 | 8 | 8 | 15 | 37 | 14 | 85 | 9 | 90 | 10 | 66 |
| Actual | ||||
|---|---|---|---|---|
| Predicted | Adolescence and Adulthood SVAD | Adolescence (SVAD) and Adulthood (SVAD and FDB) | ||
| Sex | F | M | F | M |
| F | 118 | 21 | 186 | 31 |
| M | 22 | 180 | 31 | 246 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stull, K.E.; Wolfe, C.A.; New, B.T.; Corron, L.K.; Spradley, K. Growth and Development of the Cranial Complex and Its Implications for Sex Estimation. Forensic Sci. 2025, 5, 43. https://doi.org/10.3390/forensicsci5030043
Stull KE, Wolfe CA, New BT, Corron LK, Spradley K. Growth and Development of the Cranial Complex and Its Implications for Sex Estimation. Forensic Sciences. 2025; 5(3):43. https://doi.org/10.3390/forensicsci5030043
Chicago/Turabian StyleStull, Kyra E., Christopher A. Wolfe, Briana T. New, Louise K. Corron, and Kate Spradley. 2025. "Growth and Development of the Cranial Complex and Its Implications for Sex Estimation" Forensic Sciences 5, no. 3: 43. https://doi.org/10.3390/forensicsci5030043
APA StyleStull, K. E., Wolfe, C. A., New, B. T., Corron, L. K., & Spradley, K. (2025). Growth and Development of the Cranial Complex and Its Implications for Sex Estimation. Forensic Sciences, 5(3), 43. https://doi.org/10.3390/forensicsci5030043






