The Impact of Entomological Sample Handling Techniques on a Single Larva Odor Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Solid-Phase Microextraction (SPME) Headspace Sampling
2.3. Fiber Chemistry Optimization
2.4. Extraction Time Optimization
2.5. Population Sample Set
2.6. Gas Chromatography–Mass Spectrometry
2.7. Data Analysis
3. Results
3.1. SPME Fiber Chemistry Optimization
3.2. Extraction Time Optimization
3.3. Population Sample Set
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matuszewski, S. Post-Mortem Interval Estimation Based on Insect Evidence: Current Challenges. Insects 2021, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Briones, M.d.L.; Hernández-Cortés, R.; Díaz-Torres, P.; Niderhauser-García, A.; Ancer-Rodríguez, J.; Jaramillo-Rangel, G.; Ortega-Martínez, M. Identification of Human Remains by DNA Analysis of the Gastrointestinal Contents of Fly Larvae. J. Forensic Sci. 2013, 58, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S.; Szafałowicz, M.; Jarmusz, M. Insects colonising carcasses in open and forest habitats of Central Europe: Search for indicators of corpse relocation. Forensic Sci. Int. 2013, 231, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.J.; Wells, J.D. The population genetic structure of North American Lucilia sericata (Diptera: Calliphoridae), and the utility of genetic assignment methods for reconstruction of postmortem corpse relocation. Forensic Sci. Int. 2010, 195, 63–67. [Google Scholar] [CrossRef]
- Catts, E.P.; Goff, M.L. Forensic Entomology in Criminal Investigations. Annu. Rev. Entomol. 1992, 37, 253–272. [Google Scholar] [CrossRef]
- Villet, M.H.; Richards, C.S.; Midgley, J.M. Contemporary Precision, Bias and Accuracy of Minimum Post-Mortem Intervals Estimated Using Development of Carrion-Feeding Insects. In Current Concepts in Forensic Entomology; Amendt, J., Campobasso, C.P., Goff, M.L., Grassberger, M., Eds.; Springer: Dordrecht, Germany, 2010; pp. 109–137. [Google Scholar]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M.J.R. Best practice in forensic entomology—Standards and guidelines. Int. J. Leg. Med. 2006, 121, 90–104. [Google Scholar] [CrossRef]
- Tarone, A.M.; Sanford, M.R. Is PMI the Hypothesis or the Null Hypothesis? J. Med. Entomol. 2017, 54, 1109–1115. [Google Scholar] [CrossRef]
- Moffatt, C.; Heaton, V.; De Haan, D. The distribution of blow fly (Diptera: Calliphoridae) larval lengths and its implications for estimating post mortem intervals. Int. J. Leg. Med. 2015, 130, 287–297. [Google Scholar] [CrossRef]
- Goff, M.L.; Omori, A.I.; Gunatilake, K. Estimation of Postmortem Interval by Arthropod Succession. Forensic Sci. Rev. 1993, 5, 81–93. [Google Scholar] [CrossRef]
- Frederickx, C.; Dekeirsschieter, J.; Brostaux, Y.; Wathelet, J.-P.; Verheggen, F.; Haubruge, E. Volatile organic compounds released by blowfly larvae and pupae: New perspectives in forensic entomology. Forensic Sci. Int. 2012, 219, 215–220. [Google Scholar] [CrossRef]
- Moore, H.E.; Adam, C.D.; Drijfhout, F.P. Potential Use of Hydrocarbons for Aging Lucilia sericata Blowfly Larvae to Establish the Postmortem Interval. J. Forensic Sci. 2013, 58, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.H.; Ye, G.Y.; Hu, C.; Xu, X.H.; Li, K. Development changes of cuticular hydrocarbons in Chrysomya rufifacies larvae: Potential for determining larval age. Med. Vet. Entomol. 2006, 20, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Frere, B.; Suchaud, F.; Bernier, G.; Cottin, F.; Vincent, B.; Dourel, L.; Lelong, A.; Arpino, P. GC-MS analysis of cuticular lipids in recent and older scavenger insect puparia. An approach to estimate the postmortem interval (PMI). Anal. Bioanal. Chem. 2014, 406, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Lunas, B.M.; de Paula, M.C.; Michelutti, K.B.; Lima-Junior, S.E.; Antonialli-Junior, W.F.; Cardoso, C.A.L. Hydrocarbon and Fatty Acid Composition from Blowfly Eggs Represents a Potential Complementary Taxonomic Tool of Forensic Importance. J. Forensic Sci. 2019, 64, 1720–1725. [Google Scholar] [CrossRef]
- Roux, O.; Gers, C.; Legal, L. Ontogenetic study of three Calliphoridae of forensic importance through cuticular hydrocarbon analysis. Med. Vet. Entomol. 2008, 22, 309–317. [Google Scholar] [CrossRef]
- Ye, G.; Li, K.; Zhu, J.; Zhu, G.; Hu, C. Cuticular Hydrocarbon Composition in Pupal Exuviae for Taxonomic Differentiation of Six Necrophagous Flies. J. Med. Entomol. 2007, 44, 450–456. [Google Scholar] [CrossRef]
- Drijfhout, F.P. Cuticular Hydrocarbons: A New Tool in Forensic Entomology? In Current Concepts in Forensic Entomology; Amendt, J., Goff, M.L., Campobasso, C.P., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 179–203. [Google Scholar]
- Zhu, G.H.; Xu, X.H.; Yu, X.J.; Zhang, Y.; Wang, J.F. Puparial case hydrocarbons of Chrysomya megacephala as an indicator of the postmortem interval. Forensic Sci. Int. 2007, 169, 1–5. [Google Scholar] [CrossRef]
- Lockey, K.H. Lipids of the insect cuticle: Origin, composition and function. Comp. Biochem. Physiol. Part B Comp. Biochem. 1988, 89, 595–645. [Google Scholar] [CrossRef]
- Ebbs, M.L.; Amrein, H. Taste and pheromone perception in the fruit fly Drosophila melanogaster. Pflug. Arch. Eur. J. Physiol. 2007, 454, 735–747. [Google Scholar] [CrossRef]
- Giffen-Lemieux, J.E.; Okuda, K.; Rosati, J.Y.; Musah, R.A. Characterization of the Volatiles’ Profiles of the Eggs of Forensically Relevant Lucilia sericata and Phormia regina (Diptera: Calliphoridae) Blow Flies by SPME-Facilitated GC-MS. J. Med. Entomol. 2020, 57, 994–1005. [Google Scholar] [CrossRef]
- Kotzé, Z.; Delclos, P.J.; Knap, A.H.; Wade, T.L.; Tomberlin, J.K. Volatile organic compounds in variably aged carrion impacted by the presence of the primary colonizer, Cochliomyia macellaria (Diptera: Calliphoridae). Int. J. Leg. Med. 2021, 135, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Blanar, K.; Prada-Tiedemann, P.A. Characterization of the volatile odor profile from larval masses in a field decomposition setting. Forensic Chem. 2020, 21, 100288. [Google Scholar] [CrossRef]
- Byrd, J.; Sutton, L. Forensic entomology for the investigator. WIREs Forensic Sci. 2020, 2, e1370. [Google Scholar] [CrossRef]
- Hall, M.J.R. The Relationship between Research and Casework in Forensic Entomology. Insects 2021, 12, 174. [Google Scholar] [CrossRef]
- Smith, J.L. Evaluating the impact of hot water killing larvae on gene expression using the transformer gene in Cochliomyia macellaria (Diptera: Calliphoridae). J. Forensic Sci. 2024, 69, 1467–1472. [Google Scholar] [CrossRef]
- Tarone, A.M.; Foran, D.R. Gene Expression During Blow Fly Development: Improving the Precision of Age Estimates in Forensic Entomology. J. Forensic Sci. 2011, 56, S112–S122. [Google Scholar] [CrossRef]
- Holman, A.P.; Pickett, D.N.; Orr, A.E.; Tarone, A.M.; Kurouski, D. A nondestructive technique for the sex identification of third instar Cochliomyia macellaria larvae. J. Forensic Sci. 2024, 69, 2075–2081. [Google Scholar] [CrossRef]
- Picard, C.J.; Deblois, K.; Tovar, F.; Bradley, J.L.; Johnston, J.S.; Tarone, A.M. Increasing Precision in Development-Based Postmortem Interval Estimates: What’s Sex Got to Do With It? J. Med. Entomol. 2013, 50, 425–431. [Google Scholar] [CrossRef]
- Prada, P.A.; Curran, A.M.; Furton, K.G. Comparison of extraction methods for the removal of volatile organic compounds (VOCs) present in sorbents used for human scent evidence collection. Anal. Methods 2010, 2, 470–478. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Armstrong, P.; Nizio, K.; Perrault, K.; Forbes, S. Establishing the volatile profile of pig carcasses as analogues for human decomposition during the early postmortem period. Heliyon 2016, 2, e00070. [Google Scholar] [CrossRef]
- Brasseur, C.; Dekeirsschieter, J.; Schotsmans, E.M.; de Koning, S.; Wilson, A.S.; Haubruge, E.; Focant, J.-F. Comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry for the forensic study of cadaveric volatile organic compounds released in soil by buried decaying pig carcasses. J. Chromatogr. A 2012, 1255, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Cablk, M.E.; Szelagowski, E.E.; Sagebiel, J.C. Characterization of the volatile organic compounds present in the headspace of decomposing animal remains, and compared with human remains. Forensic Sci. Int. 2012, 220, 118–125. [Google Scholar] [CrossRef]
- Cerkowniak, M.; Boguś, M.I.; Stepnowski, P.; Gołębiowski, M. Comparison of the volatile compounds of Dermestes maculatus and Dermestes ater pupae: Application of headspace solid-phase microextraction-gas chromatography-mass spectrometry (HS-SPME-GC/MS). Invertebr. Surviv. J. 2017, 14, 303–311. [Google Scholar]
- Dekeirsschieter, J.; Stefanuto, P.-H.; Brasseur, C.; Haubruge, E.; Focant, J.-F. Enhanced Characterization of the Smell of Death by Comprehensive Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry (GCxGC-TOFMS). PLoS ONE 2012, 7, e39005. [Google Scholar] [CrossRef]
- Dekeirsschieter, J.; Frederickx, C.; Lognay, G.; Brostaux, Y.; Verheggen, F.J.; Haubruge, E. Electrophysiological and Behavioral Responses of Thanatophilus sinuatus Fabricius (Coleoptera: Silphidae) to Selected Cadaveric Volatile Organic Compounds. J. Forensic Sci. 2013, 58, 917–923. [Google Scholar] [CrossRef]
- Focant, J.-F.; Stefanuto, P.; Brasseur, C.; Dekeirsschieter, J.; Haubruge, E.; Schotsmans, E.; Wilson, A.; Stadler, S.; Forbes, S. Forensic cadaveric decomposition profiling by GC × GC-TOFMS analysis of VOCS. Chem. Bull. Kazakh Natl. Univ. 2013, 4, 177–186. [Google Scholar] [CrossRef]
- Forbes, S.L.; Perrault, K.A.; Stefanuto, P.-H.; Nizio, K.D.; Focant, J.-F. Comparison of the Decomposition VOC Profile during Winter and Summer in a Moist, Mid-Latitude (Cfb) Climate. PLoS ONE 2014, 9, e113681. [Google Scholar] [CrossRef]
- Forbes, S.; Troobnikoff, A.; Ueland, M.; Nizio, K.; Perrault, K. Profiling the decomposition odour at the grave surface before and after probing. Forensic Sci. Int. 2016, 259, 193–199. [Google Scholar] [CrossRef]
- Hoffman, E.M.; Curran, A.M.; Dulgerian, N.; Stockham, R.A.; Eckenrode, B.A. Characterization of the volatile organic compounds present in the headspace of decomposing human remains. Forensic Sci. Int. 2009, 186, 6–13. [Google Scholar] [CrossRef]
- Ki, B.-M.; Ryu, H.W.; Cho, K.-S. Extended local similarity analysis (eLSA) reveals unique associations between bacterial community structure and odor emission during pig carcasses decomposition. J. Environ. Sci. Health Part A 2018, 53, 718–727. [Google Scholar] [CrossRef]
- Martin, C.; Verheggen, F. Behavioural response of Lucilia sericata to a decaying body infested by necrophagous insects. Physiol. Entomol. 2018, 43, 188–195. [Google Scholar] [CrossRef]
- Paczkowski, S.; Nicke, S.; Ziegenhagen, H.; Schütz, S. Volatile Emission of Decomposing Pig Carcasses (Sus scrofa domesticus L.) as an Indicator for the Postmortem Interval. J. Forensic Sci. 2015, 60, S130–S137. [Google Scholar] [CrossRef] [PubMed]
- Perrault, K.A.; Rai, T.; Stuart, B.H.; Forbes, S.L. Seasonal comparison of carrion volatiles in decomposition soil using comprehensive two-dimensional gas chromatography-time of flight mass spectrometry. Anal. Methods 2015, 7, 690–698. [Google Scholar] [CrossRef]
- Rosier, E.; Loix, S.; Develter, W.; Van de Voorde, W.; Cuypers, E.; Tytgat, J. Differentiation between decomposed remains of human origin and bigger mammals. J. Forensic Leg. Med. 2017, 50, 28–35. [Google Scholar] [CrossRef]
- Stadler, S.; Stefanuto, P.-H.; Brokl, M.; Forbes, S.L.; Focant, J.-F. Characterization of Volatile Organic Compounds from Human Analogue Decomposition Using Thermal Desorption Coupled to Comprehensive Two-Dimensional Gas Chromatography–Time-of-Flight Mass Spectrometry. Anal. Chem. 2013, 85, 998–1005. [Google Scholar] [CrossRef]
- Stadler, S.; Desaulniers, J.-P.; Forbes, S.L. Inter-year repeatability study of volatile organic compounds from surface decomposition of human analogues. Int. J. Leg. Med. 2015, 129, 641–650. [Google Scholar] [CrossRef]
- Statheropoulos, M.; Spiliopoulou, C.; Agapiou, A. A study of volatile organic compounds evolved from the decaying human body. Forensic Sci. Int. 2005, 153, 147–155. [Google Scholar] [CrossRef]
- Statheropoulos, M.; Agapiou, A.; Spiliopoulou, C.; Pallis, G.; Sianos, E. Environmental aspects of VOCs evolved in the early stages of human decomposition. Sci. Total. Environ. 2007, 385, 221–227. [Google Scholar] [CrossRef]
- Statheropoulos, M.; Agapiou, A.; Zorba, E.; Mikedi, K.; Karma, S.; Pallis, G.; Eliopoulos, C.; Spiliopoulou, C. Combined chemical and optical methods for monitoring the early decay stages of surrogate human models. Forensic Sci. Int. 2011, 210, 154–163. [Google Scholar] [CrossRef]
- Stefanuto, P.-H.; Perrault, K.A.; Stadler, S.; Pesesse, R.; LeBlanc, H.N.; Forbes, S.L.; Focant, J.-F. GC × GC–TOFMS and supervised multivariate approaches to study human cadaveric decomposition olfactive signatures. Anal. Bioanal. Chem. 2015, 407, 4767–4778. [Google Scholar] [CrossRef]
- Vass, A.; Smith, R.; Thompson, C.; Burnett, M.; Wolf, D.; Synstelien, J.; Dulgerian, N.; Eckenrode, B. Decompositional Odor Analysis Database. J. Forensic Sci. 2004, 49, 1–10. [Google Scholar] [CrossRef]
- Vass, A.A. Odor mortis. Forensic Sci. Int. 2012, 222, 234–241. [Google Scholar] [CrossRef]
- Brodie, B.; Gries, R.; Martins, A.; VanLaerhoven, S.; Gries, G. Bimodal cue complex signifies suitable oviposition sites to gravid females of the common green bottle fly. Entomol. Exp. Appl. 2014, 153, 114–127. [Google Scholar] [CrossRef]
- Brodie, B.S.; Babcock, T.; Gries, R.; Benn, A.; Gries, G. Acquired Smell? Mature Females of the Common Green Bottle Fly Shift Semiochemical Preferences from Feces Feeding Sites to Carrion Oviposition Sites. J. Chem. Ecol. 2016, 42, 40–50. [Google Scholar] [CrossRef] [PubMed]
- DeGreeff, L.E.; Furton, K.G. Collection and identification of human remains volatiles by non-contact, dynamic airflow sampling and SPME-GC/MS using various sorbent materials. Anal. Bioanal. Chem. 2011, 401, 1295–1307. [Google Scholar] [CrossRef]
- Kasper, J.; Mumm, R.; Ruther, J. The composition of carcass volatile profiles in relation to storage time and climate conditions. Forensic Sci. Int. 2012, 223, 64–71. [Google Scholar] [CrossRef]
- Rosier, E.; Loix, S.; Develter, W.; Van de Voorde, W.; Tytgat, J.; Cuypers, E. Time-dependent VOC-profile of decomposed human and animal remains in laboratory environment. Forensic Sci. Int. 2016, 266, 164–169. [Google Scholar] [CrossRef]
- Stefanuto, P.-H.; Perrault, K.A.; Lloyd, R.M.; Stuart, B.; Rai, T.; Forbes, S.L.; Focant, J.-F. Exploring new dimensions in cadaveric decomposition odour analysis. Anal. Methods 2015, 7, 2287–2294. [Google Scholar] [CrossRef]
- Dekeirsschieter, J.; Verheggen, F.; Gohy, M.; Hubrecht, F.; Bourguignon, L.; Lognay, G.; Haubruge, E. Cadaveric volatile organic compounds released by decaying pig carcasses (Sus domesticus L.) in different biotopes. Forensic Sci. Int. 2009, 189, 46–53. [Google Scholar] [CrossRef]
- Pascual, J.; von Hoermann, C.; Rottler-Hoermann, A.; Nevo, O.; Geppert, A.; Sikorski, J.; Huber, K.J.; Steiger, S.; Ayasse, M.; Overmann, J. Function of bacterial community dynamics in the formation of cadaveric semiochemicals during in situ carcass decomposition. Environ. Microbiol. 2017, 19, 3310–3322. [Google Scholar] [CrossRef]
- Rosier, E.; Loix, S.; Develter, W.; Van de Voorde, W.; Tytgat, J.; Cuypers, E. The Search for a Volatile Human Specific Marker in the Decomposition Process. PLoS ONE 2015, 10, e0137341. [Google Scholar] [CrossRef] [PubMed]
- Curran, A.M.; Prada, P.A.; Furton, K.G. The Differentiation of the Volatile Organic Signatures of Individuals Through SPME-GC/MS of Characteristic Human Scent Compounds. J. Forensic Sci. 2010, 55, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Yang, F.; Ngando, F.J.; Zhang, X.; Feng, Y.; Ren, L.; Guo, Y. Development of Forensically Important Sarcophaga peregrina (Diptera: Sarcophagidae) and Intra-Puparial Age Estimation Utilizing Multiple Methods at Constant and Fluctuating Temperatures. Animals 2023, 13, 1607. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-H.; Yu, X.-J.; Xie, L.-X.; Luo, H.; Wang, D.; Lv, J.-Y.; Xu, X.-H. Time of Death Revealed by Hydrocarbons of Empty Puparia of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae): A Field Experiment. PLoS ONE 2013, 8, e73043. [Google Scholar] [CrossRef]
- Zhu, G.-H.; Jia, Z.-J.; Yu, X.-J.; Wu, K.-S.; Chen, L.-S.; Lv, J.-Y.; Benbow, M.E. Predictable weathering of puparial hydrocarbons of necrophagous flies for determining the postmortem interval: A field experiment using Chrysomya rufifacies. Int. J. Leg. Med. 2017, 131, 885–894. [Google Scholar] [CrossRef]
- Titus, K.C.; Gallegos, S.F.; Prada-Tiedemann, P.A. Forensic Odor Analysis: Current Application in Postmortem Examinations. Res. Rep. Forensic Med. Sci. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Kalinová, B.; Podskalská, H.; Růžička, J.; Hoskovec, M. Irresistible bouquet of death—How are burying beetles (Coleoptera: Silphidae: Nicrophorus) attracted by carcasses. Naturwissenschaften 2009, 96, 889–899. [Google Scholar] [CrossRef]
- Nilssen, A.C.; Tømmerås, B.Å.; Schmid, R.; Evensen, S.B. Dimethyl trisulphide is a strong attractant for some calliphorids and a muscid but not for the reindeer oestrids Hypoderma tarandi and Cephenemyia trompe. Entomol. Exp. Appl. 1996, 79, 211–218. [Google Scholar] [CrossRef]
- Podskalská, H.; Růžička, J.; Hoskovec, M.; Šálek, M. Use of infochemicals to attract carrion beetles into pitfall traps. Entomol. Exp. Appl. 2009, 132, 59–64. [Google Scholar] [CrossRef]
- Adams, Z.J.; Hall, M.J. Methods used for the killing and preservation of blowfly larvae, and their effect on post-mortem larval length. Forensic Sci. Int. 2003, 138, 50–61. [Google Scholar] [CrossRef]
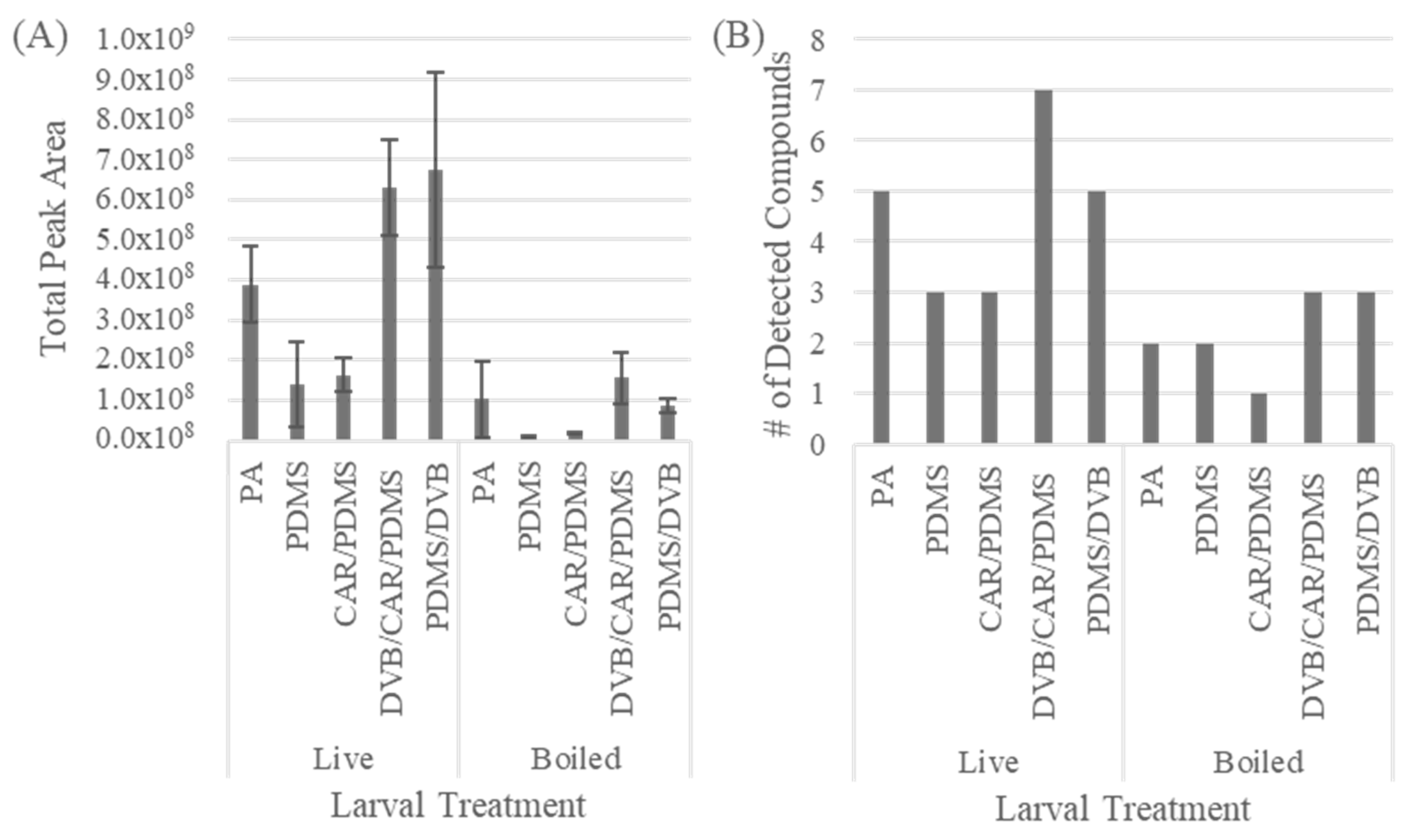
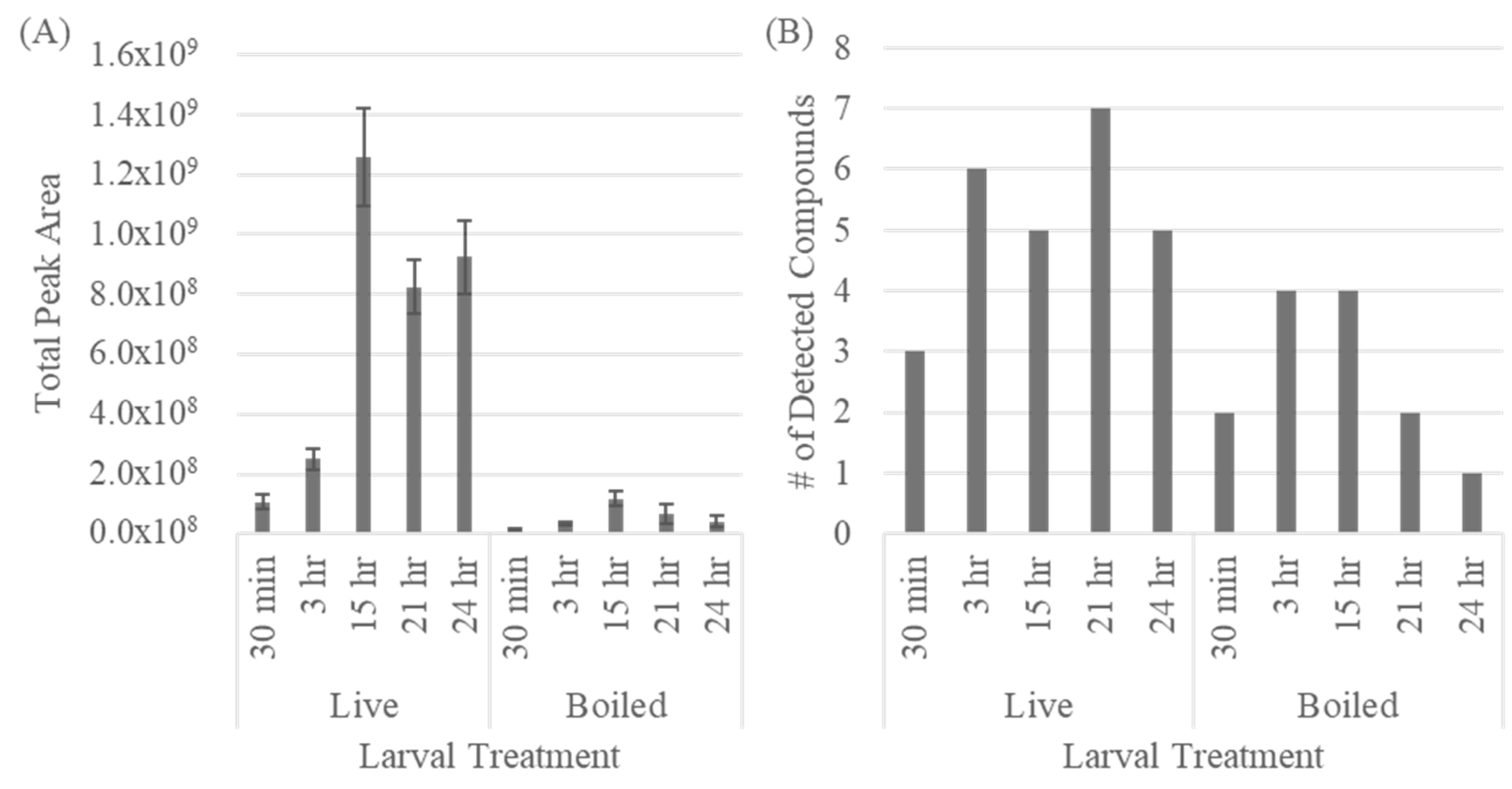
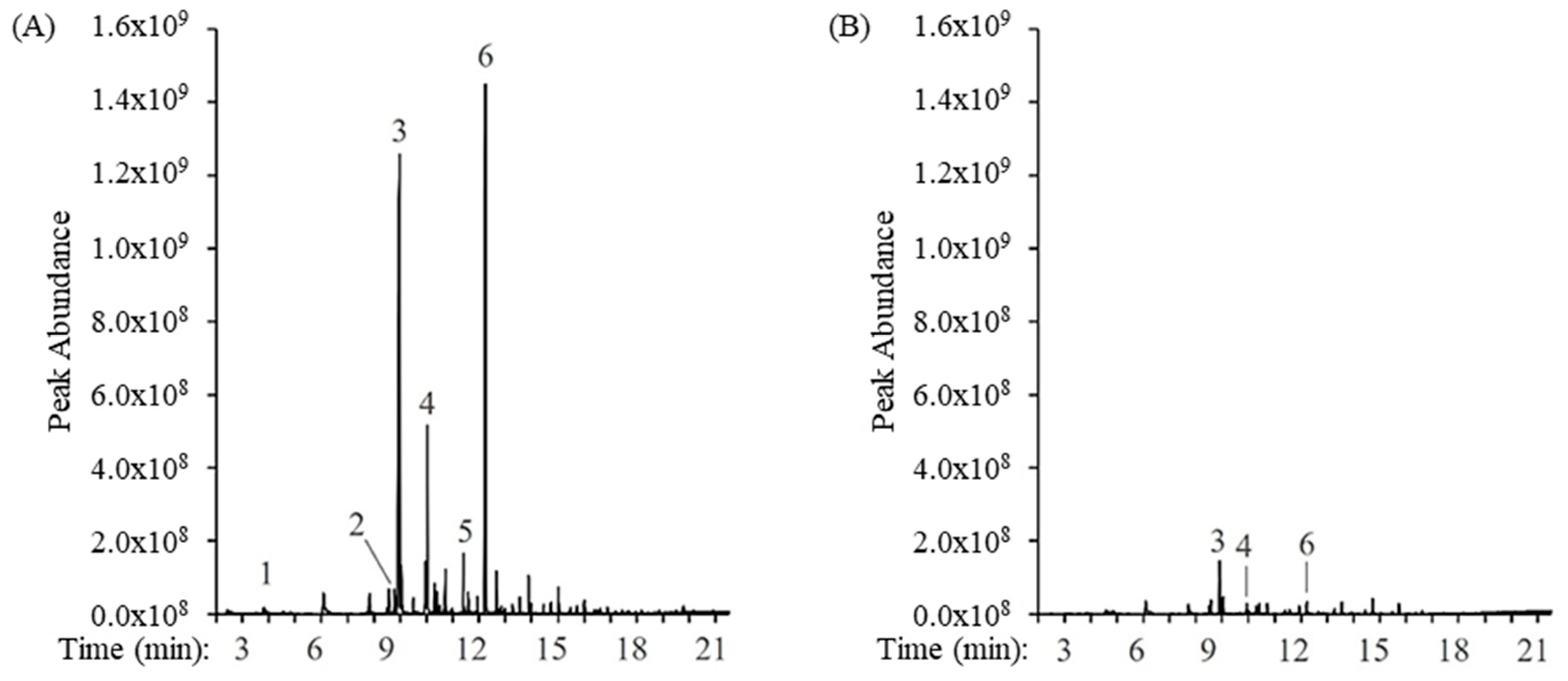


| Frequency of Occurrence | ||||||
|---|---|---|---|---|---|---|
| Compound | Live | Percent | Boiled | Percent | Total | Previously Reported in the Literature |
| Dimethyl disulfide | 20 | 80% | 18 | 72% | 38 | [22,23,24,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55] |
| Dimethyl trisulfide | 24 | 96% | 13 | 52% | 37 | [22,23,24,33,34,37,39,44,45,47,48,49,51,52,53,54,55,56,57,58,59,60,61] |
| Indole | 25 | 100% | 19 | 76% | 44 | [11,23,24,34,35,37,38,45,48,49,57,59,62,63,64] |
| p-Cresol | 23 | 92% | 21 | 84% | 44 | [24,57] |
| Phenol | 25 | 100% | 25 | 100% | 50 | [11,22,24,34,37,38,45,48,49,51,52,57,58,59,62,63,65] |
| Phenylethyl alcohol | 22 | 88% | 10 | 40% | 32 | [22,23,24,59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monogan, A.Z.; Smith, J.L.; Prada-Tiedemann, P.A. The Impact of Entomological Sample Handling Techniques on a Single Larva Odor Profile. Forensic Sci. 2025, 5, 21. https://doi.org/10.3390/forensicsci5020021
Monogan AZ, Smith JL, Prada-Tiedemann PA. The Impact of Entomological Sample Handling Techniques on a Single Larva Odor Profile. Forensic Sciences. 2025; 5(2):21. https://doi.org/10.3390/forensicsci5020021
Chicago/Turabian StyleMonogan, Ana Zoe, Joshua L. Smith, and Paola A. Prada-Tiedemann. 2025. "The Impact of Entomological Sample Handling Techniques on a Single Larva Odor Profile" Forensic Sciences 5, no. 2: 21. https://doi.org/10.3390/forensicsci5020021
APA StyleMonogan, A. Z., Smith, J. L., & Prada-Tiedemann, P. A. (2025). The Impact of Entomological Sample Handling Techniques on a Single Larva Odor Profile. Forensic Sciences, 5(2), 21. https://doi.org/10.3390/forensicsci5020021






