Abstract
Age-at-death is one of the most valuable pieces of information in a biological profile, and is an important step in identifying remains. Age-at-death estimation by dental means is performed by forensic odontologists and forensic anthropologists in their daily casework tasks. Both forensic odontologists and forensic anthropologists must be aware of all of the age indicators and of all of the methods that can offer the sufficient scientific robusticity that forensic cases require. Osteological and dental methods of age estimation rely on developmental changes in younger individuals and on degenerative changes in older individuals. Skeletal methods based on developmental changes are highly reliable, while methods based on degenerative or post-formation changes show higher variability. From all skeletal methods, those relying on tooth formation and development are the most accurate to assess an individual’s age. Dental methods of age estimation can be implemented in the skeletal analysis of juvenile and adult remains, representing an additional indicator of age. The aim of this review paper is to provide a practical reference for applying dental age estimation to human remains as a part of skeletal analysis.
1. Introduction
One of the main priorities in the investigation of cases involving unknown human remains is ascertaining the deceased’s identity. The process of identification starts with the reconstruction of the biological profile, which consists of the assessment of ancestry, sex, age and stature. Combined, these represent a broad description of the individual.
Of all the components within the biological profile, sex and age are key contributors to the individual’s identification. Thus, age-at-death is one of the most valuable pieces of information in the biological profile and is an important step in identifying remains. Anthropological analysis can offer a complete assessment of the biological profile; while some dental traits can be informative of the individual’s ancestry, the main contribution of dental analysis to the biological profile is age estimation.
Age-at-death estimation by dental means is performed by forensic odontologists and forensic anthropologists in their daily casework tasks. Both forensic odontologists and forensic anthropologists must be aware of all of the age indicators and of all of the methods that can offer the sufficient scientific robusticity that forensic cases require. They must provide their conclusions, stating the level of uncertainty (the corresponding error associated to the method) and the estimated age interval [1]. Dental methods of age estimation can be implemented in skeletal analysis, and are of particular value in cases involving juvenile remains, as well as cases involving adults, as a complement to osteological methods.
The aim of this review paper is to provide practitioners with useful information to apply in casework and to integrate within their analysis.
2. Tooth Formation and Eruption
The developmental program of teeth is determined by genes, while environmental factors play practically no role [2]. As of today, over 3000 genes have been identified to play a role in tooth development. Most of them are associated with the conserved signaling pathways mediating cellular communication between, in particular, epithelial and mesenchymal tissues [2].
This tide of genetic regulation makes tooth formation a series of biological events occurring in the same fashion, and with predictable timing, with very low environmental impact. For these reasons, tooth formation is the most accurate and precise skeletal age indicator. The regulation mechanisms of tooth development consist of a chain of reciprocal interactions between the epithelium and the mesenchyme, from the initiation of the tooth formation until its completeness. These epithelial–mesenchymal interactions are mediated by signaling molecules, such as bone morphogenic protein (BMP), fibroblast growth factor (FGF), sonic hedgehog (SHH) and ectodysplasin (EDA) [3,4].
Tooth development (or odontogenesis) consist of two main processes: tooth formation and tooth eruption. Tooth formation starts with the thickening of the oral epithelium, also called dental lamina. All teeth are formed within the dental lamina strip, so the dental lamina indicates the position of the future dentition in the maxillary and mandibular arches; this leads to the sequential appearance of localized epithelial swellings along its margin. Therefore, the dental lamina marks the initiation of single teeth within the dentition, which are referred to as placodes. The epithelium continues to proliferate and elongate around the mesenchyme, and several morphological and structural changes will define the different stages of tooth formation referred to as the bud, cap and bell stages [5,6].
After the appearance of the dental placode, around the eighth week of gestation, the epithelium forms a bud which grows and undergoes morphogenesis that will determine the shape of the crown. The cap stage starts around the 10th week; histological differentiation takes place, the dental epithelium invaginates and the enamel organ is formed. The bell stage is characterized by the deepening of the undersurface of the epithelial cap. In the late bell stage, hard dental tissues form through the processes of amelogenesis for the enamel, dentinogenesis for the dentine and cementogenesis for the cementum, which, in essence, consists of the deposition of cell layers and their posterior mineralization [5]. The crown then completes its morphodifferentiation and attains its full size [6].
Once the crown is formed, the root will start its development, which (as the crown) will entail cell proliferation and differentiation. Root formation is a more variable process than crown formation and depends on the root’s overall length and the dentine apposition rate [5]. The dental tissues will elongate from the cementoenamel junction towards the apical, and the pulp will be progressively surrounded by the root dentine and cementum. Soon after the initiation of root formation, the tooth starts its eruption, which consists of essentially axial movements from the bony crypt, until it achieves its final position in the dental arch, with its occlusal surface in the occlusal plane [6]. Classically, root formation and tooth eruption were considered two separate processes. However, recent research demonstrates that root formation and tooth eruption are intertwined [7,8]. The final step to achieve tooth development completeness is the closure of the apex.
The duration of the crown formation depends on the size of the crown and the enamel deposition rate and, typically, this takes 7 to 14 months for deciduous teeth and 3–6 years for permanent teeth. The duration of the root formation is more variable and is a longer process than crown completion; typically, this takes 1.5 to 2 years for deciduous teeth and 5–7 years for permanent teeth. Eruption is longer and presents more variation than tooth formation [5,9].
Deciduous maxillary and mandibular incisors are the first teeth to start their formation, around the 5th–6th week, and the first permanent teeth start around the 9th–10th week of gestation. However, the first stages of tooth formation consist of changes at the cellular level, that are not visible by the naked eye or in radiographs. As mentioned earlier in this article, the late bell stage of tooth formation, which occurs around the 13th–14th weeks in uterus for the first deciduous teeth, is characterized by the mineralization of the dental structures. These mineralized hard tissues can be easily viewed in radiographic images; thus, mineralization is a key event for the application of dental age estimation methods. By the 19th week in utero, all deciduous teeth show some degree of mineralization. The first permanent molar is the earliest tooth of the permanent dentition to form, and its mineralization starts around birth [5,9,10].
The beginning of tooth development will be different for different teeth such that, for deciduous dentition, central incisors are the first to develop, followed by the first molars, then lateral incisors, canines and second molars. Following this fashion, the mandibular tooth is the first to initiate its development and, slightly after, the maxillary tooth will initiate its development [11].
The most relevant events in tooth development for age estimation are mineralization, crown formation, root formation and apex closure. All these events occur at different times and rates for the different teeth of the deciduous and permanent dentition. Thus, the oral cavity shows multiple elements at different stages of development that can be used to accurately estimate the age of the individual. Table 1 provides the approximate times of the start of mineralization, crown and root completion and eruption, for the different teeth of the deciduous and permanent dentition [12,13].

Table 1.
Approximate timing of the start of mineralization, crown completion, root completion and eruption for deciduous and permanent dentition. The times for the start of mineralization are given in weeks in utero; the times for the mineralization of the permanent dentition, crown and root completion and eruption for the deciduous and permanent dentition are given in months and years after birth (iu: in utero) [12,13].
3. Dental Age Estimation Methods Based on Developmental Changes
All dental age estimation methods based on developmental changes rely on the above-described processes of mineralization, crown and root formation and eruption. Since the mineralization of the deciduous incisors occurs at approximately the 14th week in utero, and the closure of the third molars apex occurs at approximately 18 years of age, dental age assessment can be applied from fetal remains to those of young adults [5,10].
Only intact teeth should be assessed for age estimation; teeth exhibiting extensive caries or restorations, periapical pathology or any morphologic abnormality should not be used for age estimation purposes [5].
Dental age estimation methods based on developmental changes can be classified into two main categories: incremental stages scoring methods and atlas style methods.
The most relevant scoring methods are Morrees, Fanning and Hunt (1963) and Demirjian (1976). Morrees, Fanning and Hunt developed two studies of radiological age assessment. One focused on the root resorption and exfoliation of the deciduous mandibular canines and molars, where four stages were presented with different data according to the individual’s sex [14]. Their second study focused on the developmental changes of the permanent dentition, where tooth development is divided into 13 stages for single-rooted teeth and in 14 stages for multi-rooted teeth. Separate data tables are presented for males and females [14,15]. The best contribution of this method is the classification of the different developmental stages for crown and root development and their descriptions. In fact, this system of the stages of tooth development has been the basis for a number of dental age estimation methods relying on developmental changes. Table 2 presents the approximate timings of root resorption and exfoliation for the deciduous dentition according to Moorrees, Fanning and Hunt (1963).

Table 2.
Approximate timing of root resorption and exfoliation for deciduous dentition. The times are expressed in years (Modified from Moorrees, Fanning and Hunt, 1963).
The Demirjian method consists of the radiological assessment of the mandibular left quadrant, in eight stages of tooth development (A through H). Missing, malformed, rotated, or teeth difficult to stage for any reason from one side can be substituted by the same tooth type from the other side of the jaw, and a missing first molar can be substituted with a central incisor. Separate data tables are provided for males and females. The limitations of this method are that it can only be applied on mandibular teeth, has no scores for third molars, and does not account for missing teeth or fragmented remains [16,17].
The atlas style methods for dental age estimation consist of a series of illustrations representing the overall dental development for all teeth for a certain age cohort. The assessment is carried out by assigning the illustration that best represents the case.
Schour and Masler created the first well-known atlas of dental development for age estimation purposes in 1941; however, their study presented some limitations. Several age cohorts were not represented in the sample; additionally, the different age groups did not have a balanced representation in the reference sample. Ubelaker applied the same principals, in 1989, from a sample that included the corresponding age cohorts and included archaeological and modern individuals. Ubelaker’s atlas focuses on tooth emergence through the gingiva [10].
Dr. Sakher AlQahtani created, in 2010, the London Atlas, from a well-represented and balanced sample, including archaeological and modern individuals. AlQahtani’s atlas focuses on teeth eruption through the alveolar bone. The age cohorts illustrated in the London Atlas range from 30 weeks in utero to 23.5 years of age. However, it is best used while the developmental process is occurring in all or some of the teeth present. The London Atlas has been validated for a number of populations, so it can be applied to different ancestries, as wells as modern or ancient remains [18,19,20,21,22,23].
Atlas style methods do not differentiate between sexes. Even though slight differences between sexes were found in the AlQahtani study, they were not statistically significant [5,9]. Between the ages of approximately 14 and 21 years, age assessment is conducted by evaluating the development of the third molars. The third molars are the most developmentally variable tooth; sex and ancestry have a great impact on their developmental rates [22,23,24,25,26,27]. Nevertheless, the third molars are the only teeth undergoing morphologic development at these ages. Mincer, in 1993, conducted a study applying the Demirjian developmental stages (A-H) to the third molars. This is one of the most widely used methods for age assessment, and relying on the third molars is the application. UT-age is a Microsoft application that uses the Mincer approach to estimate age considering the sex and ancestry of the individual. It provides mean age, standard deviation and the empirical probability that an individual had attained the age of 18 years [28].
All the above-mentioned methods are based on 2D conventional radiological images. However, with the advancement of 3D imaging techniques and their application, new methods based on 3D morphological analysis have been developed for juvenile age assessment. Computerized tomography has been implemented for the age estimation of developing teeth. Different authors have applied staging criteria, as well as metric and volumetric analysis, for age estimation using cone-beam computerized tomography (CBCT) [29,30]
Additionally, age-estimation studies have been performed using magnetic resonance imaging (MRI) on third molars [31]. Since the appearance of MRI molars differs greatly from that on radiographs, a specific staging technique is required to classify the maturity of the third molars in MRI. However, unlike radiograph and CBTC image generation, MRI does not require ionizing radiation emission; thus, the use of MRI techniques offers benefits when dealing with living individuals, but not when dealing with the deceased.
4. Post-Formation Changes in the Dental Structures
Age estimation methods based on developmental changes offer higher accuracy and precision than age estimation methods based on the degenerative changes of the skeleton, and this applies to bones and teeth. Several post-formation changes have been explored for age estimation purposes such as dental wear, root resorption, cementum apposition, dental coloration, periodontal recession, secondary dentine apposition and root translucency.
Dental wear methods have shown to be reliable in archaeological samples, but they have not shown an acceptable correlation with chronological age in modern individuals, therefore it is not used in forensic cases. Root resorption and cementum apposition have shown a low correlation with chronological age; thus, the post-formation changes that are of use in the forensic context are tooth coloration, periodontal recession (in combination with other age-related dental changes), secondary dentin formation and root translucency.
The color of dental tissues has been explored for age estimation purposes. The age-related color of enamel is caused by increased nitrogen content and surface cracking, which leads to light refraction changes. However, enamel is highly exposed to external factors that can impact its coloration. Dentin age-related changes are caused by modifications in mineral and organic composition. In general terms, dental crowns are more frequently affected by pathology than roots; thus, the best correlation of tooth color and chronological age is obtained from the dentin color taken at the proximal third of root dentin after the elimination of the cementum, which involves tooth extraction [32,33,34]. Nevertheless, tooth color evaluation from the dentin is a destructive analysis.
Periodontal recession is defined as the maximum distance between the cementoenamel junction and the soft tissue attachment, measured in its buccal aspect. Periodontal recession increases with aging. However, periodontal recession is not only related to aging, and it can be caused by periodontal disease. An important limitation of the periodontal recession measurement is that soft tissue attachment is not visible in a number of cases involving skeletonized remains, damaged alveolar bones or fragmentary remains, and it is often taken with high subjectivity. Periodontal recession is not considered, by itself, to estimate age, but it is included in formulas in conjunction with other parameters [35].
Secondary dentin is formed after the tooth completion, and it starts in the coronal portion of the pulp and progresses through the root canal towards the apex. As a consequence of the accumulation of secondary dentin, the pulp cavity progressively reduces its size, which can be observed in radiographic images [35,36].
Root translucency is produced by the increase in peritubular dentin deposits of hydroxyapatite, which changes the refractive index of root dentin. This physiological change is evident from 20 years of age, and it starts in the dentin closest to the apex and it progressively extends towards the crown [35,37,38].
5. Dental Age Estimation Methods Based on Post-Formation Changes
The extensively referenced study by Gustafson (1950) evaluates six post-formation changes: dental wear, secondary dentin formation, periodontal recession, cementum apposition, root resorption and root translucency. These variables are assessed on tooth sections [39].
The significant limitations of this method are the assumption that all variables are equally effective in age assessment, the suggestion that the staging methodology was equal among the six variables, and the statistical independence of each of the variables [10,40]. Nevertheless, Gustafson’s study had a major impact on adults’ dental age estimation and formed the basis for the development of posterior relevant research, some of which are used in the present day. Johanson, in 1971, modified Gustafson’s method by applying a 7-grade score (instead of the 3-grade score used by Gustafson) to assess the same six post-formation changes and attributed different weights for the different variables in his formula. Johanson’s method presents an error of 5 years when considering multiple teeth and 8 years when considering a single tooth. This methods does not consider the type of tooth or the sex of the individual [41].
Lamendin developed a method of age estimation considering root translucency, periodontal recession and root length measured in the buccal surface of the tooth [35]. Prince and Ubelaker modified this method, creating different formulas according to the sex and ancestry of the individual [42]. Root translucency should be measured by applying 1600 lux [43]. The overall error rate associated with these methods is around 10 years for Lamendin and around 8 years for Prince and Ubelaker. Even though root translucency shows a high correlation with chronological age, these methods should not be applied to individuals under 40 years of age due to its inaccuracy in younger ages.
Martin de las Heras explored, in her study, the correlation between dentin color measured through spectroradiometry and chronological age. The study shows that different criteria must be applied according to the individual’s sex and the stage of decomposition of the body (fresh vs. skeletal remains). The error associated with this method is within the range of 11.7 and 15.2 years [34].
Under certain circumstances tooth extraction is not possible, therefore it is possible to apply any method mentioned above. The reduction in the pulp cavity due to secondary dentine formation can be observed through radiographical images, therefore tooth extraction is not required to assess this age-related change. The Kvaal method of age estimation relies on the assessment of pulp wear. The ratios of pulp/root length, pulp/tooth length and pulp/root widths at three different levels, are used in the regression formula to estimate the age of the individual; only single-rooted teeth presenting normal occlusion and free of trauma, including active caries, dental restorations, erosion or abrasion, should be utilized in this method. The use of ratios instead of direct measurements of the pulp cavity compensates for any potential radiographic magnification and angulation distortions. This method can be applied to mandibular lateral incisors, canines and first premolars and, for the maxillary central and lateral incisors and second premolars, the sex of the individual is considered. The overall error associated with this method is around 10 years (between 8.6 and 11.5 years, depending upon the tooth evaluated) [36,44]. Camariere applied the same principals but using pulp and root area instead of length [45,46].
Three-dimensional imaging techniques can be applied to dental age estimation in adults. In essence, in order to assess secondary dentin formation, metric and volumetric analyses are carried out through CBCT images of teeth to quantify the pulp chamber size changes and correlate them to the individual’s chronological age [47,48]. Table 3 summarizes the most significant dental estimation methods used in contemporary forensic casework.

Table 3.
Dental age estimation methods available for forensic casework based on tooth formation and development and post-formation changes, where the published study is indicated (Method), as well as the dental changes that the method relies on (Dental changes), the methodology of assessment (Methodology) and whether the method is destructive, or the tooth is preserved after the analysis (Sample destruction).
6. Integrating Dental Age Estimation in the Skeletal Analysis
Age estimation is (along with sex estimation) the most critical piece of information in the reconstruction a biological profile.
The bases of age estimation methods using bone and those using teeth follow the same rationale: the goal is to estimate the biological age and correlate it with the chronological age. Both (osteological and dental methods) rely on growth and developmental changes in younger individuals and on degenerative changes in older individuals.
However, dental formation and development are physiological processes that are highly genetically controlled, leaving practically no role to environmental factors. Dental formation and development are less affected by environmental factors than bone formation and development; therefore, dental age estimation methods that rely on formation and development offer more accurate and precise results than osteological ones. Moreover, in cases where there is a disagreement between the osteological and dental results for age estimation, the fact that the environmental factors will impact bone development before impacting teeth development must be considered.
It has been well stablished that combining different age indicators increases the accuracy of the estimation. Depending on the age group where the individual falls, some elements will be more informative than others. This should be considered at the time of selecting the methods that the practitioner will combine in order to obtain the age estimation of the individual.
In the anthropological analysis of remains, dental age estimation is often applied only to juvenile remains. However, dental age estimation can be implemented in other group ages, representing an additional indicator of age that may contribute significantly to the biological profile, especially in cases of highly damaged and fragmentary, where some of the osteological age indicator cannot be assessed.
Osteological methods for the age estimation of fetal and young children remains are based on the appearance and union of the primary centers of ossification, as well as the diaphyseal and crown-heel length of the individual [49,50,51,52,53]. The analysis can be complemented with a dental assessment, including the mineralization and the initial stages of tooth formation and development, which offer very accurate results.
In older children, the osteological age estimation is based on the appearance of some late centers of ossification, the diaphyseal length and the fusion of the epiphysis to the diaphysis [53,54,55,56]. In late adolescents and young adults, the age estimation, in essence, relies on the late fusing epiphyses, such as the sternal end of the clavicle, the iliac crest, the vertebral ring or the spheno-occipitalis synchondrosis [53,55,56]. In this age range, the third molars’ developmental assessment can contribute to the age estimation, especially if one was to set the lower limit of the estimation at approximately 18 years, when the third molars have already closed their apex. The osteological methods for adult age estimation are based on the degenerative changes of the skeletal elements in the form of changes in the articular surfaces, osteophytes formation, cartilage ossification and sutures closure [57,58,59,60,61,62]. Dental age methods based on post-formation changes, although not as accurate and precise as those based on developmental changes, can contribute to a better estimate in adults.
However, most of the dental post-formation changes are not significantly evident nor strongly correlated to chronological age in individuals under approximately 35–40 years of age. Thus, dental age estimation can contribute to the biological profile in adults, but there is a gap for those aged between approximately 20–40 years, where dental methods for age estimation show a lower correlation with the chronological age, therefore osteological methods are more reliable in this age range. Both osteological and dental age estimation methods show a lower accuracy in the elderly, from approximately 70 years of age.
As previously mentioned in this article, 3D-imaging techniques are reliable and reproducible in estimating the age of juveniles and adults. Most of the studies rely on metric and volumetric data, which have shown a higher accuracy than staging methods. Nevertheless, further research in the application of 3D imaging for age estimations is needed in order to explore the population-specific variability with metric and volumetric analysis, as well as to adapt some of the two-dimensional methods to three-dimensional ones.
However, CBCT imaging is not always available for forensic analyses, due to its economic cost or a lack of accessibility to a CT-scanner. Artificial intelligence is the next step in age assessment. Even though this age estimation approach is still in need of further research, recent research studies show promising results increasing the objectivity of the estimations by the application of machine learning algorithms to age estimation methods [63,64,65]. Figure 1 presents the different osteological and dental age-related changes that may be used for age estimation throughout the lifespan.
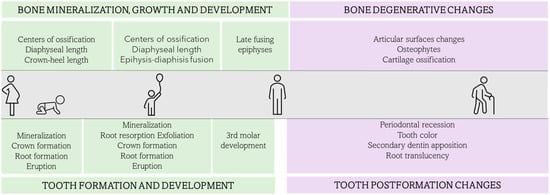
Figure 1.
Osteological and dental age-related changes through lifespan that can be used for age estimation in skeletal analysis.
7. Conclusions
Forensic anthropologist often apply dental age estimation methods in juvenile remains, since dental age estimation based on tooth formation and development is the most reliable means of skeletal age assessment. Dental age estimation based on post-formation changes can offer informative data that complement osteological analysis.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- ADA Technical Report No. 1077–2020. Available online: https://www.nist.gov/system/files/documents/2021/02/23/ADA%20Technical%20Report%20No.%201077_July_2020.pdf (accessed on 15 January 2023).
- Thesleff, I. The genetic basis of tooth development and dental defects. Am. J. Med. Genet. Part A 2006, 140, 2530–2535. [Google Scholar] [CrossRef]
- Thesleff, I. From understanding tooth development to bioengineering of teeth. Eur. J. Oral Sci. 2018, 126 (Suppl. 1), 67–71. [Google Scholar] [CrossRef]
- Yu, T.; Klein, O.D. Molecular and cellular mechanisms of tooth development, homeostasis and repair. Development 2020, 147, dev184754. [Google Scholar] [CrossRef]
- AlQahtani, S.J. Dental age estimation in fetal and children. In Age Estimation. A Multidisciplinary Approach; Adserias-Garriga, J., Ed.; Elsevier: London, UK, 2019; pp. 89–106. [Google Scholar]
- Nanci, A. Chapter 5: Development of the Tooth and Its Supporting Tissues. In Ten Cate’s Oral Histology: Development, Structure and Function, 9th ed.; Nanci, A., Ed.; Elsevier: St. Louis, MO, USA, 2018; pp. 68–90. [Google Scholar]
- Ono, W.; Sakagami, N.; Nishimori, S.; Ono, N.; Kronenberg, H.M. Parathyroid hormone receptor signalling in osterix-expressing mesenchymal progenitors is essential for tooth root formation. Nat. Commun. 2016, 7, 11277. [Google Scholar] [CrossRef]
- Takahashi, A.; Nagata, M.; Gupta, A.; Matsushita, Y.; Yamaguchi, T.; Mizuhashi, K.; Maki, K.; Ruellas, A.C.; Cevidanes, L.S.; Kronenberg, H.M.; et al. Autocrine regulation of mesenchymal progenitor cell fates orchestrates tooth eruption. Proc. Natl. Acad. Sci. USA 2019, 116, 575–580. [Google Scholar] [CrossRef]
- AlQahtani, S.J.; Hector, M.P.; Liversidge, H.M. Brief communication: The London atlas of human tooth development and eruption. Am. J. Phys. Anthropol. 2010, 142, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.M.; Senn, D.R. Dental age estimation. In Manual of forensic odontology, 5th ed.; Senn, D.R., Weems, R.A., Eds.; Taylor and Francis Group: Boca Raton, FL, USA, 2013; pp. 221–255. [Google Scholar]
- Adserias-Garriga, J.; Visnapuu, V. The neonatal line as evidence of live birth. Dental age estimation in fetal and children. In Age Estimation. A Multidisciplinary Approach; Adserias-Garriga, J., Ed.; Elsevier: London, UK, 2019; pp. 161–167. [Google Scholar]
- Nelson, S.J.; Ash, M.M. Development and eruption of the teeth. In Wheeler’s Dental Anatomy, Physiology, and Occlusion, 9th ed.; Saunders Elsevier: St. Louis, MO, USA, 2010; pp. 21–44. [Google Scholar]
- Scheid, R.C.; Weiss, G.; Woelfel, J.B. Primary (and Mixed) Dentition. In Woelfel’s Dental Anatomy, 8th ed.; Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 164–193. [Google Scholar]
- Moorrees, C.; Fanning, E.; Hunt, E. Formation and resorption of three deciduous teeth in children. Am. J. Phys. Anthropol. 1963, 21, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Moorrees, C.; Fanning, E.; Hunt, E. Age variation of formation stages for ten permanent teeth. J. Dent. Res. 1963, 42, 1490–1502. [Google Scholar] [CrossRef] [PubMed]
- Demirjian, A.; Goldstein, H.; Tanner, J.M. A new system of dental age assessment. Hum. Biol. 1973, 45, 211–227. [Google Scholar] [PubMed]
- Demirjian, A.; Goldstein, H. New systems for dental maturity based on seven and four teeth. Ann. Hum. Biol. 1976, 3, 411–421. [Google Scholar] [CrossRef] [PubMed]
- AlQahtani, S.; Nuzzolese, E.; Adserias-Garriga, J. The accuracy of the London Atlas of Human Tooth Development and Eruption in dental age estimations of Saudi, Spanish, and Italian children. J. Forensic Odonto-Stomatol. 2017, 1, 62. [Google Scholar]
- Ishwarkumar, S.; Pillay, P.; Chetty, M.; Satyapal, K.S. Employing the London Atlas in the Age Estimation of a Select South African Population. Dent. J. 2022, 10, 171. [Google Scholar] [CrossRef]
- Namwong, W.; Mânica, S. Testing the London atlas for age estimation in Thai population. Acta Odontol. Scand. 2020, 78, 161–164. [Google Scholar] [CrossRef]
- McCloe, D.; Marion, I.; da Fonseca, M.A.; Colvard, M.; AlQahtani, S. Age estimation of Hispanic children using the London Atlas. Forensic Sci. Int. 2018, 288, 332.e1–332.e6. [Google Scholar] [CrossRef]
- Jayaraman, J.; Roberts, G.J.; Wong, H.M.; King, N.M. Dental age estimation in southern Chinese population using panoramic radiographs: Validation of three population specific reference datasets. BMC Med. Imaging 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- AlQahtani, S.J.; Hector, M.P.; Liversidge, H.M. Accuracy of dental age estimation charts: Schour and Massler, Ubelaker and the London Atlas. Am. J. Phys. Anthropol. 2014, 154, 70–78. [Google Scholar] [CrossRef]
- Harris, E.F. Mineralization of the mandibular third molar: A study of American blacks and whites. Am. J. Phys. Anthropol. 2007, 132, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Mincer, H.H.; Harris, E.F.; Berryman, H.E. The ABFO study of the third molar development and it use as an estimator of chronological age. J. Forensic Sci. 1993, 38, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Solari, A.C.; Abramovitch, K. The accuracy and precision of third molar development as an indicator of chronological age in Hispanics. J. Forensic Sci. 2002, 47, 531–535. [Google Scholar] [CrossRef]
- Blankenship, J.A.; Mincer, H.H.; Anderson, K.M.; Woods, M.A.; Burton, E.L. Third molar development in the estimation of chronologic age in American Blacks as compared with Whites. J. Forensic Sci. 2007, 52, 428–433. [Google Scholar] [CrossRef]
- UT-Age; Lewis, J.; Senn, D.; Silvaggi, J. Forensic Odontology at UT Health San Antonio School of Dentistry. Available online: www.utforensic.org (accessed on 15 January 2023).
- Graham, J.P.; O’Donnell, C.J.; Craig, P.J.; Walker, G.L.; Hill, A.J.; Cirillo, G.N.; Clark, R.M.; Gledhill, S.R.; Schneider-Kolsky, M.E. The application of computerized tomography (CT) to the dental ageing of children and adolescents. Forensic Sci. Int. 2010, 195, 58–62. [Google Scholar] [CrossRef]
- Marquez-Ruiz, A.B.; Treviño-Tijerina, M.C.; Gonzalez-Herrera, L.; Sanchez, B.; Gonzalez-Ramirez, A.R.; Valenzuela, A. Three-dimensional analysis of third molar development to estimate age of majority. Sci. Justice 2017, 57, 376–383. [Google Scholar] [CrossRef]
- De Tobel, J.; Phlypo, I.; Fieuws, S.; Politis, C.; Verstraete, K.L.; Thevissen, P.W. Forensic age estimation based on development of third molars: A staging technique for magnetic resonance imaging. J. Forensic Odontostomatol. 2017, 35, 117–140. [Google Scholar]
- Ten Cate, A.R.; Thompson, G.W.; Dickinson, J.B.; Hunter, H.A. The estimation of age of skeletal remains from the colour of roots of teeth. Dent. J. 1977, 43, 83–86. [Google Scholar]
- Solheim, T. Dental color as an indicator of age. Gerodontics 1988, 4, 114–118. [Google Scholar]
- Martin-de las Heras, S.; Valenzuela, A.; Bellini, R.; Salas, C.; Rubiño, M.; Garcia, J.A. Objective measurement of dental color for age estimation by spectroradiometry. Forensic Sci. Int. 2003, 132, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Martin de las Heras, E. Dental Age Estimation in Adults. Dental age estimation in fetal and children. In Age Estimation. A Multidisciplinary Approach; Adserias-Garriga, J., Ed.; Elsevier: London, UK, 2019; pp. 125–142. [Google Scholar]
- Kvaal, S.I.; Kolltveit, K.M.; Thomsen, I.O.; Solheim, T. Age estimation of adults from dental radiographs. Forensic Sci. Int. 1995, 74, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Solheim, T. Dental root translucency as an indicator of age. Eur. J. Oral Sci. 1989, 97, 189–197. [Google Scholar] [CrossRef]
- Lamendin, H.; Baccino, E.; Humbert, J.F.; Tavernier, J.C.; Nossintchouk, R.M.; Zerilli, A. A simple technique for age estimation in adult corpses the 2 criteria dental method. J. Forensic Sci. 1992, 37, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, G. Age determination on teeth. J. Am. Dent. Assoc. 1950, 41, 45–54. [Google Scholar] [CrossRef]
- Harris, E.F.; Mincer, H.H.; Anderson, K.M.; Senn, D.R. Age estimation from oral and dental structures. In Forensic Dentistry, 2nd ed.; Senn, D.R., Stimson, P.G., Eds.; Taylor & Frances Group: Boca Raton, FL, USA, 2010; pp. 263–303. [Google Scholar]
- Johanson, G. Age determinations from human teeth: A critical evaluation with special consideration of changes after fourteen years of age. Odontol. Rev. 1971, 22, 1–12. [Google Scholar]
- Prince, D.A.; DH Ubelaker. Application of Lamendin’s adult ageing technique to a diverse skeletal sample. J. Forensic Sci. 2002, 47, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Adserias-Garriga, J.; Nogué-Navarro, L.; Zapico, S.C.; Ubelaker, D.H. Setting the light conditions for measuring root transparency for age-at-death estimation methods. Int. J. Leg. Med. 2018, 132, 637–641. [Google Scholar] [CrossRef]
- Kvaal, S.I.; Solheim, T. A non-destructive dental method for age estimation. J. Forensic Odontostomatol. 1994, 12, 6–11. [Google Scholar]
- Cameriere, R.; Ferrante, L.; Belcastro, M.G.; Bonfiglioli, B.; Rastelli, E.; Cingolani, M. Age estimation by pulp/tooth ratio in canines by peri-apical X-rays. J. Forensic Sci. 2007, 52, 166–170. [Google Scholar] [CrossRef]
- Cameriere, R.; De Luca, S.; Aleman, I.; Ferrante, L.; Cingolani, M. Age estimation by pulp/tooth ratio in lower premolars by orthopantomography. Forensic Sci. Int. 2012, 214, 105–112. [Google Scholar] [CrossRef]
- Molina, A.; Bravo, M.; Fonseca, G.M.; Márquez-Grant, N.; Martín-de-Las-Heras, S. Dental age estimation based on pulp chamber/crown volume ratio measured on CBCT images in a Spanish population. Int. J. Leg. Med. 2021, 135, 359–364. [Google Scholar] [CrossRef]
- Merdietio Boedi, R.; Shepherd, S.; Oscandar, F.; Mânica, S.; Franco, A. 3D segmentation of dental crown for volumetric age estimation with CBCT imaging. Int. J. Leg. Med. 2023, 137, 123–130. [Google Scholar] [CrossRef]
- Fazekas, I.G.Y.; Kosa, F. Forensic Fetal Osteology; Akademiai Kaido: Budapest, Hungary, 1978. [Google Scholar]
- Scheuer, J.L.; Musgrave, J.H.; Evans, S.P. The estimation of late fetal and perinatal age from limb bone length by linear and logarithmic regression. Ann. Hum. Biol. 1980, 7, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Greulich, W.W.; Pyle, S.I. Radiographic Atlas of Skeletal Development of the Hand and Wrist; Stanford University Press: Redwood City, CA, USA, 1959. [Google Scholar]
- Warren, M.W. Radiographic determination of development age in fetuses and stillborns. J. For. Sci. 1999, 44, 708–712. [Google Scholar]
- Scheuer, L.; Black, S. Developmental Juvenile Osteology; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Gaskin, C.M.; Kahn, S.L.; Bertozzi, J.C.; Bunch, P.M. Skeletal Development of the Hand and Wrist: A Radiographic Atlas and Digital Bone Age Companion; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Langley-Shirley, N.; Jantz, R.L. A Bayesian approach to age estimation in modern Americans from the clavicle. J. Forensic Sci. 2010, 55, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Shirley, N.R.; Jantz, R.L. Spheno-occipital synchondrosis fusion in modern Americans. J. Forensic Sci. 2011, 56, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.; Suchey, J.M. Skeletal age determination based on the Os pubis: A comparison of the Acsdai-Nemeskeri and Suchey-Brooks methods. Hum. Evol. 1990, 5, 227–238. [Google Scholar] [CrossRef]
- Lovejoy, C.O.; Meindl, R.S.; Pryzbeck, T.R.; Mensforth, R.P. Chronological metamorphosis of the auricular surface of the ilium: A new method for the determination of adult skeletal age at death. Am. J. Phys. Anthropol. 1985, 68, 15–28. [Google Scholar] [CrossRef]
- Meindl, R.S.; Lovejoy, C.O. Ectocranial suture closure: A revised method for the determination of skeletal age at death based on the lateral-anterior sutures. Am. J. Phys. Anthropol. 1985, 68, 57–66. [Google Scholar] [CrossRef]
- Rouge-Maillart, C.; Jousset, N.; Vielle, B.; Gaudin, A.; Telmon, N. Contribution of the study of acetabulum for the estimation of adult subjects. Forensic Sci. Int. 2006, 171, 103–110. [Google Scholar] [CrossRef]
- Hartnett, K.M. Analysis of age-at-death estimation using data from a new, modern autopsy sample—Part II: Sternal end of the fourth rib. J. Forensic Sci. 2010, 55, 1152–1156. [Google Scholar] [CrossRef]
- Falys, C.G.; Prangle, D. Estimating age of mature adults from the degeneration of the sternal end of the clavicle. Am. J. Phys. Anthropol. 2015, 156, 203–214. [Google Scholar] [CrossRef]
- Shen, S.; Liu, Z.; Wang, J.; Fan, L.; Ji, F.; Tao, J. Machine learning assisted Cameriere method for dental age estimation. BMC Oral Health 2021, 21, 641. [Google Scholar] [CrossRef]
- Saric, R.; Kevric, J.; Hadziabdic, N.; Osmanovic, A.; Kadic, M.; Saracevic, M.; Jokic, D.; Rajs, V. Dental age assessment based on CBCT images using machine learning algorithms. Forensic Sci. Int. 2022, 334, 111245. [Google Scholar] [CrossRef]
- Zaborowicz, K.; Garbowski, T.; Biedziak, B.; Zaborowicz, M. Robust Estimation of the Chronological Age of Children and Adolescents Using Tooth Geometry Indicators and POD-GP. Int. J. Environ. Res. Public Health 2022, 19, 2952. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).