3. Results
Eugnathogobius ganuensis n. sp.
[New English name: High-fin Brackish Goby; new standard Malay name: Belodok Ganu]
Holotype. UMTF 13296, male, 30.0 mm SL, drainage ditch at Jalan Permint Perdana, Seberang Takir, Kuala Terengganu, Terengganu, Malaysia, 5 cm depth, 18 July 2025.
Paratypes. Four specimens (27.5–31.5 mm SL: 3 males and 1 female). KAUM–I. 219716, male, 31.5 mm SL, KAUM–I. 219718, male, 27.0 mm SL, UMTF 13297, male, 30.3 mm SL, KAUM–I. 219721, female, 27.5 mm SL, same data as holotype.
Diagnosis. A species of Eugnathogobius distinguished from all other congeners by the following combination of characters: 16 segmented caudal-fin rays; 30 or 31 longitudinal scale rows; upper jaw short, not well extended in both sexes (upper-jaw length 14.8–18.1% of SL); high first dorsal fin, especially in males; first dorsal-fin first spine length 13.0–18.1% of SL; first dorsal-fin second spine length 16.8–36.4% in SL; no head pores; shoulder with oblique black band; transverse black markings on each scale; paired black blotches on caudal-fin base; distinct black dots on upper caudal fin; throat yellowish in fresh specimens; and second dorsal fin yellowish in fresh specimens.
Description. Counts and measurements shown in
Table 1. Data for holotype first, then data for paratypes in parentheses (if different). Body elongated, compressed posteriorly, maximum height at pelvic-fin origin (
Figure 1 and
Figure 2). Head large, slightly compressed. Snout rounded, almost same length as eye. Eye located anteriorly on dorsolateral aspect of head. Interorbital space wide. Anterior nostril just behind upper lip, with a short tube, tip not extending over upper lip. Posterior nostril in front of anterior margin of eye, its diameter less than half that of pupil. Mouth terminal, forming angle of 45° with body axis, gape relatively wide; end of maxilla extending beyond midpoint of eye. Gill opening narrow, with smooth margin, extending from uppermost point of pectoral-fin base to slightly below pectoral-fin base (
Figure 3). Anus located slightly posterior to ventral midpoint of body. Urogenital papilla elongated, pointed (rounded in female).
First dorsal fin high (
Figure 1 and
Figure 2); second and third spines especially elongated; second spines with short filament; tip of depressed first dorsal fin over second dorsal-fin base (first dorsal fin rounded without elongated spine in female; spines relatively shorter in smaller male than in holotype). Second dorsal fin lower than first dorsal fin, not connected to first dorsal fin (almost same height in females); origin posterior to anus; spine slightly lower than first soft ray; sixth ray longest. Anal fin slightly lower than second dorsal fin; origin just below second dorsal-fin origin; spine shorter than soft rays; posteriormost ray longest; end of base slightly behind end of second dorsal-fin base. Pectoral fin rounded; fin base just behind gill opening; tip not quite extending to above anus. Pelvic fin connecting membranes forming disc; origin below pectoral-fin base; fifth soft ray longest; depressed fin tip not reaching anus. Caudal fin large, rounded.
Predorsal scales cycloid; anterior part of scales larger; anteriormost scale reaching interorbital space. Opercle (except lower part) with cycloid scales. Cheek completely naked. Anterior and posterior part of body with cycloid and ctenoid scales, respectively; boundary between ctenoid and cycloid scales from below posterior part of first dorsal-fin base to slightly above anus. Pectoral-fin base and prepelvic-fin area with cycloid scales, shallowly embedded under thin skin. All fins scaleless, except for basal part of caudal fin with several ctenoid and a few cycloid scales.
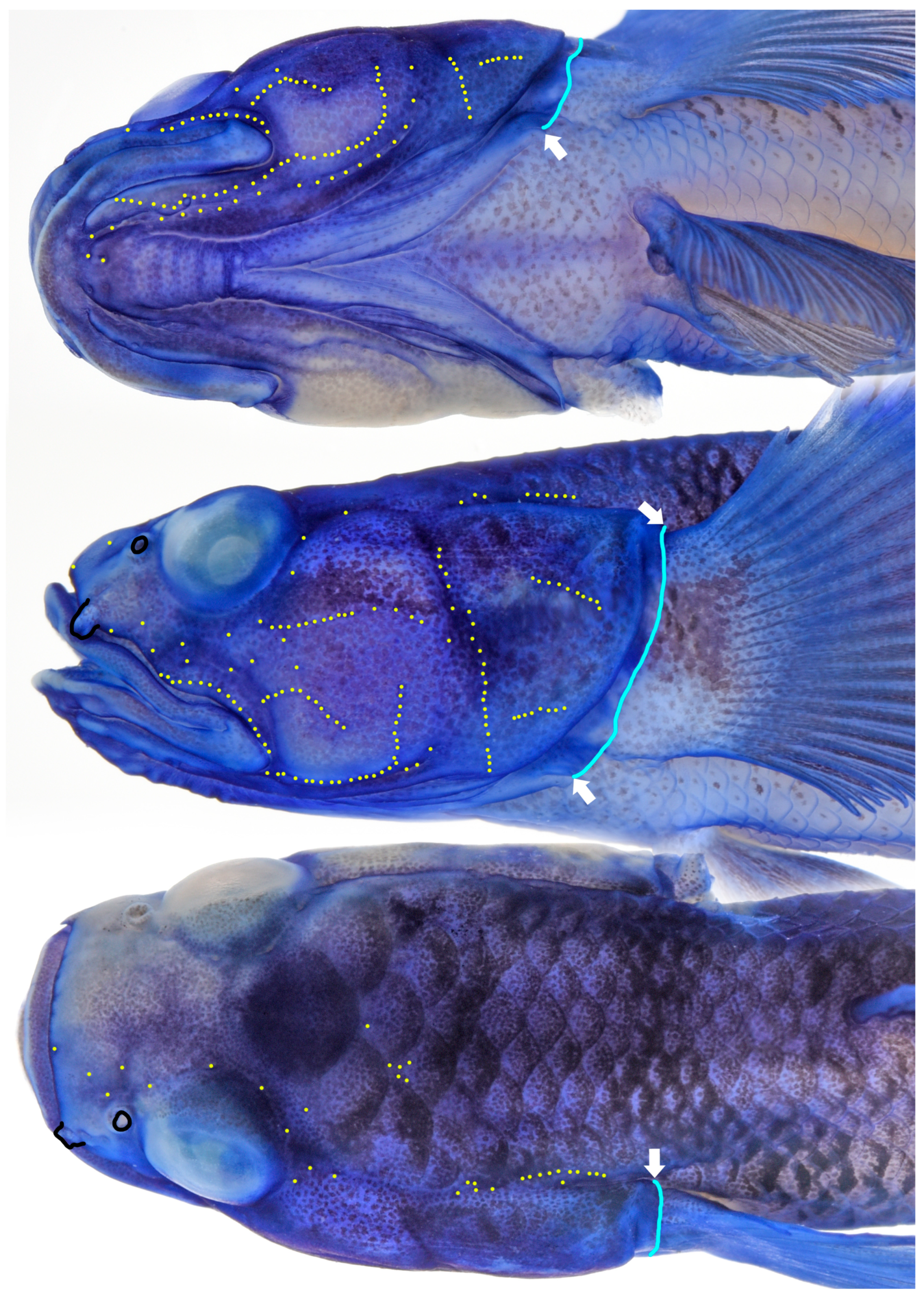
Sensory system (Figure 3). Head without canals and pores. Preopercle with a papillae row extending partly along lower margin, upturned posteriorly.
Color in live/fresh specimens (Figure 1 and
Figure 2). Ground color relatively pale brown. Transverse narrow pattern on each body scale. Somewhat indistinct oblique black band from nape to just behind pectoral-fin base; left and right sides of band barely connecting at mid-dorsum. Two longitudinal black bands posterodorsal and posteroventral to eye. Oblique black band from anteroventral to eye to just behind upper lip. Lower part of opercle black; black area of opercle anteriorly barely connecting with lower bar from eye. Upper lip slightly yellowish. Ventral head yellow, partially reddish. Dorsal fins yellowish to orange. Distinct dots on second dorsal-fin elements, absent from first dorsal-fin spines. Distal margin of second dorsal fin with narrow white margin. Anal fin orange with white distal margin, black submarginally. Pectoral fin almost semi-translucent whitish, yellowish basally, blackish ventrally. Pectoral-fin base with longitudinal black band. Caudal fin orange, yellowish dorsally, and slightly reddish ventrally; each ray (except for lower part) with black dots; thin white ventral margin. Caudal-fin base with distinct black blotches, a single narrow transverse black stripe just behind paired blotches.
Color after preservation (Figure 4). Body grayish-brown. Black pattern persisting in fresh specimens, other colors lost.
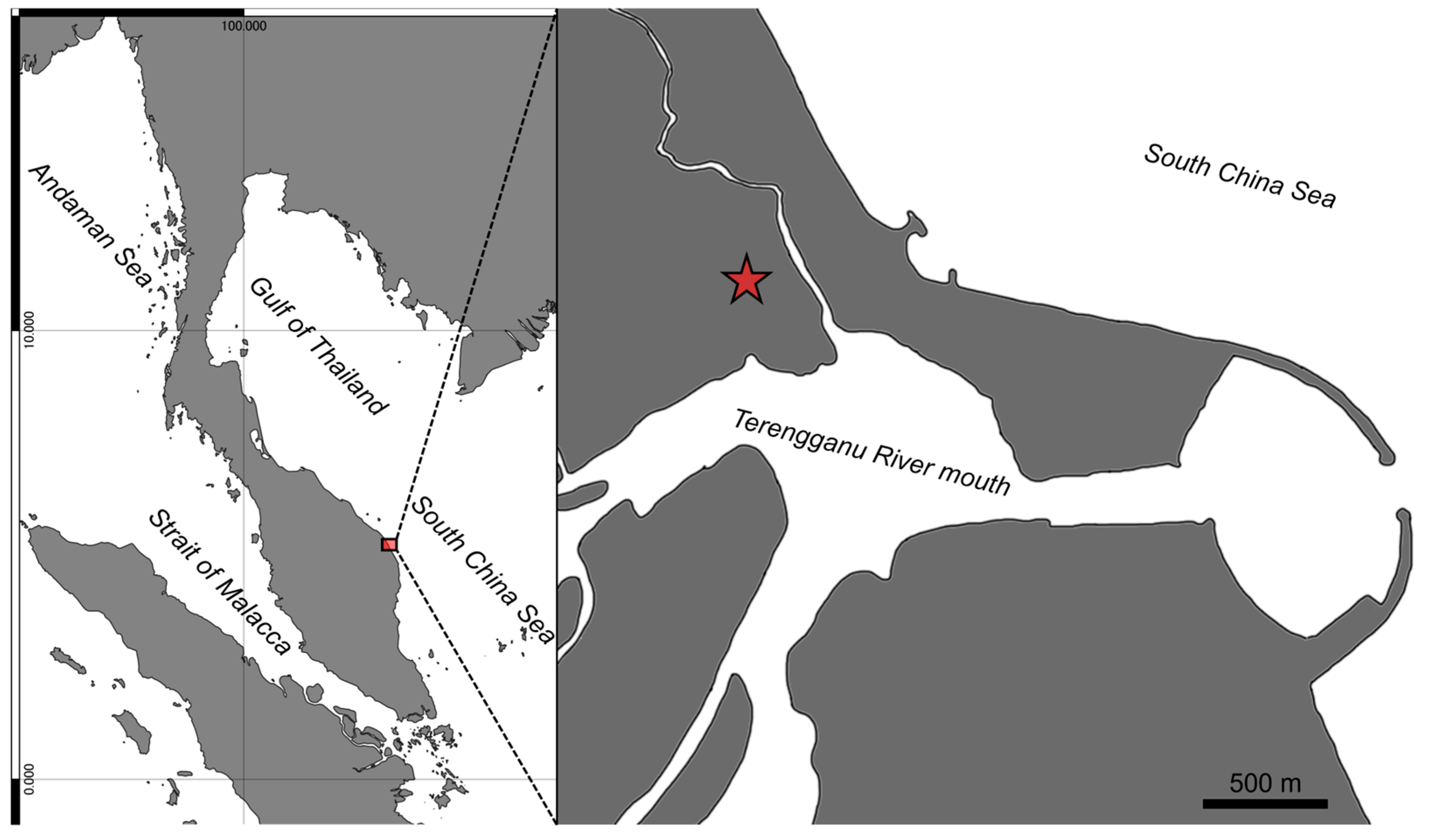
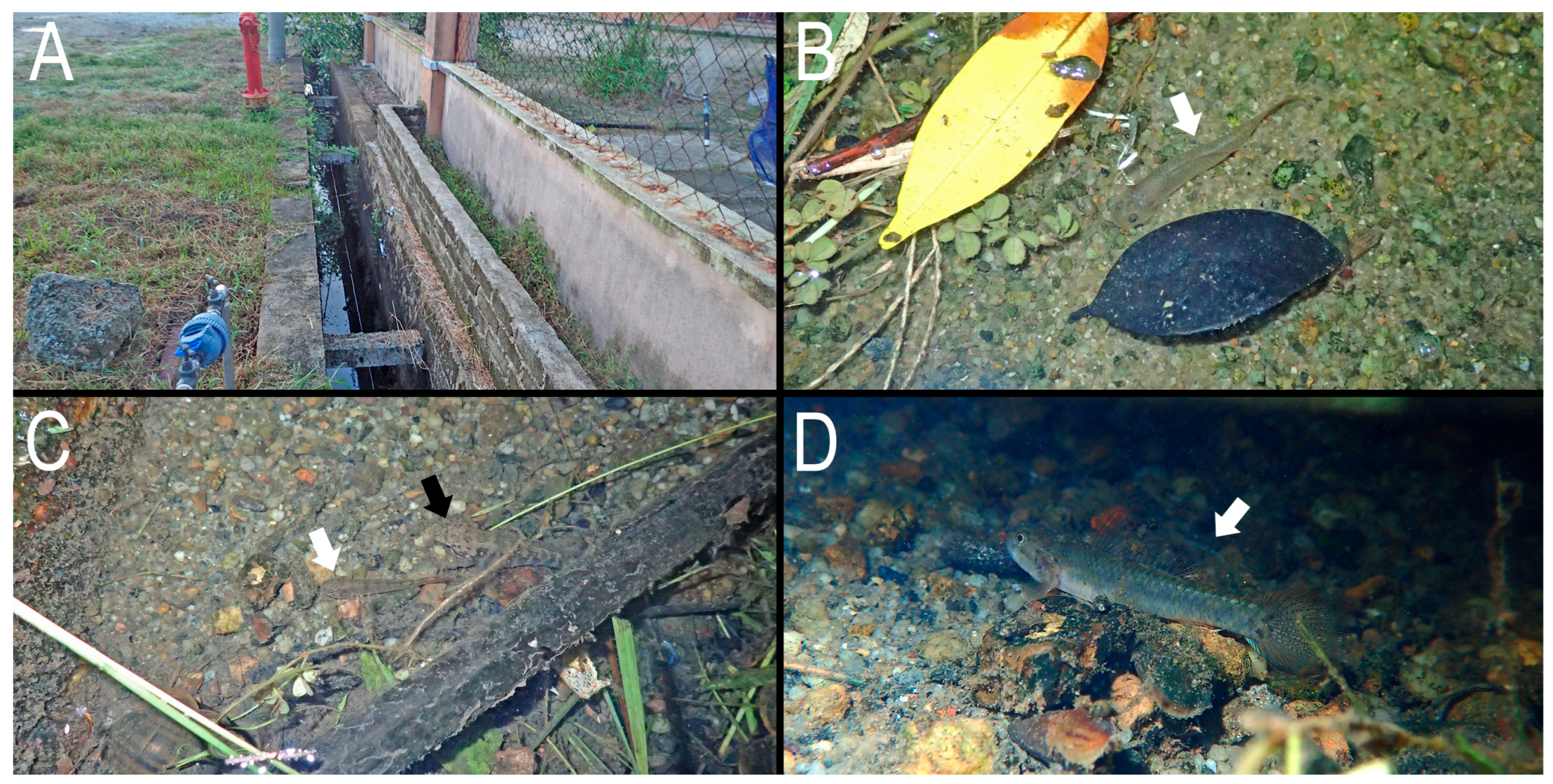
Distribution and ecological notes. Eugnathogobius ganuensis sp. nov. is currently known only from the type locality, a drainage ditch, comprising concrete blocks, on the lower reach of the Terengganu River basin (
Figure 5 and
Figure 6). A shallow layer of sandy sediment covered the bottom surface. The ditch contained fresh water (0‰) at low tide, although included in a tidal area, the water level changed with incoming/outgoing tide.
Mugilogobius chulae,
Mugilogobius rambaiae, and
Oxyeleotris urophthalmus were observed together with
Eugnathogobius ganuensis (
Figure 6C).
Etymology. The specific name “ganuensis” refers to the local dialect of the Malaysian state of Terengganu, where the species was found.
Table 1.
Counts and measurements of Eugnathogobius ganuensis n. sp.
Table 1.
Counts and measurements of Eugnathogobius ganuensis n. sp.
| | Holotype | Paratypes |
|---|
| | UMTF | KAUM–I. | KAUM–I. | UMTF | KAUM–I. |
| | 13296 | 219716 | 219718 | 13297 | 219721 |
| Standard length (mm) | 30.0 | 31.5 | 27.0 | 30.3 | 27.5 |
| Counts | | | | | |
| Dorsal-fin rays | VI-I, 7 | VI-I, 7 | VI-I, 7 | VI-I, 7 | VI-I, 7 |
| Anal-fin rays | I, 7 | I, 7 | I, 8 | I, 8 | I, 7 |
| Pectoral-fin rays | 18 | 18 | 18 | 18 | 18 |
| Pelvic-fin rays | I, 5 | I, 5 | I, 5 | I, 5 | I,5 |
| Caudal-fin segmented rays | 16 | 16 | 16 | 16 | 16 |
| Longitudinal scale rows | 31 | 30 | 30 | 31 | 31 |
| TRF | 12 | 11 | 12 | 12 | 11 |
| TRB-1 | 10 | 9 | 9 | 10 | 8 |
| TRB-2 | 10 | 8 | 8 | 9 | 8 |
| Pre-dorsal-fin scale rows | 15 | 11 | 12 | 11 | 12 |
| Pre-pelvic-fin scale rows | 10 | 9 | 8 | 8 | 8 |
| Circumpeduncular scales | 12 | 12 | 12 | 12 | 12 |
| Vertebrae | 10 + 16 | 10 + 16 | 10 + 16 | 10 + 16 | 10 + 16 |
| P-V pattern | 3/12210/9 | 3/12210/9 | 3/12210/9 | 3/12210/9 | 3/12210/9 |
| AP | 2 | 2 | 2 | 2 | 2 |
| Measurements (%SL) | | | | | |
| Head length | 30.4 | 28.4 | 29.0 | 29.0 | 28.9 |
| Head depth | 16.6 | 15.0 | 15.3 | 16.3 | 15.2 |
| Head width | 19.9 | 19.1 | 17.4 | 19.0 | 19.3 |
| Body depth at pelvic-fin origin | 19.0 | 18.6 | 17.7 | 19.8 | 19.2 |
| Body depth at anal-fin origin | 20.1 | 18.6 | 18.3 | 20.5 | 19.7 |
| Caudal-peduncle length | 27.7 | 28.7 | 23.5 | 30.1 | 28.4 |
| Caudal-peduncle depth | 14.2 | 14.0 | 14.8 | 14.5 | 13.6 |
| Snout length | 7.1 | 6.7 | 6.5 | 6.3 | 6.9 |
| Eye diameter | 6.8 | 7.1 | 7.1 | 6.9 | 7.4 |
| Upper-jaw length | 14.8 | 14.2 | 13.8 | 14.2 | 11.3 |
| Pre-first-dorsal-fin length | 38.7 | 38.1 | 38.0 | 37.6 | 38.4 |
| Pre-second-dorsal-fin length | 58.5 | 57.8 | 56.6 | 58.1 | 57.1 |
| D1-D2 | 20.2 | 20.2 | 20.3 | 21.2 | 20.7 |
| Pre-anal-fin length | 58.6 | 57.6 | 58.3 | 55.9 | 56.9 |
| Pre-pelvic-fin length | 30.6 | 29.3 | 30.1 | 29.2 | 29.4 |
| First dorsal-fin 1st spine length | 18.1 | 17.3 | 17.3 | 16.6 | 13.0 |
| First dorsal-fin 2nd spine length | 36.4 | 31.5 | 21.7 | 22.7 | 16.8 |
| Second dorsal-fin spine length | 12.2 | 11.9 | 12.7 | 11.2 | 10.9 |
| Second dorsal-fin longest soft ray length | 17.3 | 17.3 | 16.0 | 17.6 | 13.0 |
| Anal-fin spine length | 7.1 | 7.8 | 7.5 | 6.2 | 7.0 |
| Anal-fin longest soft ray length | 19.7 | 17.8 | 15.8 | 16.9 | 15.1 |
| Pectoral-fin length | 22.8 | 22.4 | 23.9 | 22.5 | 22.7 |
| Pelvic-fin length | 18.5 | 17.2 | 16.2 | 16.7 | 16.0 |
| Caudal-fin length | 29.6 | 28.0 | 28.3 | 30.2 | 24.0 |
Figure 1.
Fresh holotype (male) of Eugnathogobius ganuensis n. sp. (UMTF 13296, 30.0 mm SL).
Figure 1.
Fresh holotype (male) of Eugnathogobius ganuensis n. sp. (UMTF 13296, 30.0 mm SL).
Figure 2.
Fresh paratypes of Eugnathogobius ganuensis n. sp. ((A): KAUM–I. 219716, male; (B): KAUM–I. 219718, male; (C): UMTF 13297, male; (D): KAUM–I. 219721, female).
Figure 2.
Fresh paratypes of Eugnathogobius ganuensis n. sp. ((A): KAUM–I. 219716, male; (B): KAUM–I. 219718, male; (C): UMTF 13297, male; (D): KAUM–I. 219721, female).
Figure 3.
Head of preserved holotype of Eugnathogobius ganuensis n. sp. (UMTF 13296: temporarily stained with cyanine blue), showing head sensory system (yellow dots: sensory papillae; arrows: upper and lower end of gill opening).
Figure 3.
Head of preserved holotype of Eugnathogobius ganuensis n. sp. (UMTF 13296: temporarily stained with cyanine blue), showing head sensory system (yellow dots: sensory papillae; arrows: upper and lower end of gill opening).
Figure 4.
Preserved specimens of Eugnathogobius ganuensis n. sp. ((A): UMTF 13296, male; (B): KAUM–I. 219716, male; (C): KAUM–I. 219718, male; (D): UMTF 13297, male; (E): KAUM–I. 219721, female).
Figure 4.
Preserved specimens of Eugnathogobius ganuensis n. sp. ((A): UMTF 13296, male; (B): KAUM–I. 219716, male; (C): KAUM–I. 219718, male; (D): UMTF 13297, male; (E): KAUM–I. 219721, female).
Figure 5.
Map of the type locality of Eugnathogobius ganuensis n. sp. (Red star: sampling point), prepared using QGIS v. 3.40.2.
Figure 5.
Map of the type locality of Eugnathogobius ganuensis n. sp. (Red star: sampling point), prepared using QGIS v. 3.40.2.
Figure 6.
Type locality (A) and live individuals (B–D) of Eugnathogobius ganuensis n. sp., Terengganu, Peninsular Malaysia. White and black arrows indicate E. ganuensis n. sp. and Oxyeleotris urophthalmus, respectively.
Figure 6.
Type locality (A) and live individuals (B–D) of Eugnathogobius ganuensis n. sp., Terengganu, Peninsular Malaysia. White and black arrows indicate E. ganuensis n. sp. and Oxyeleotris urophthalmus, respectively.
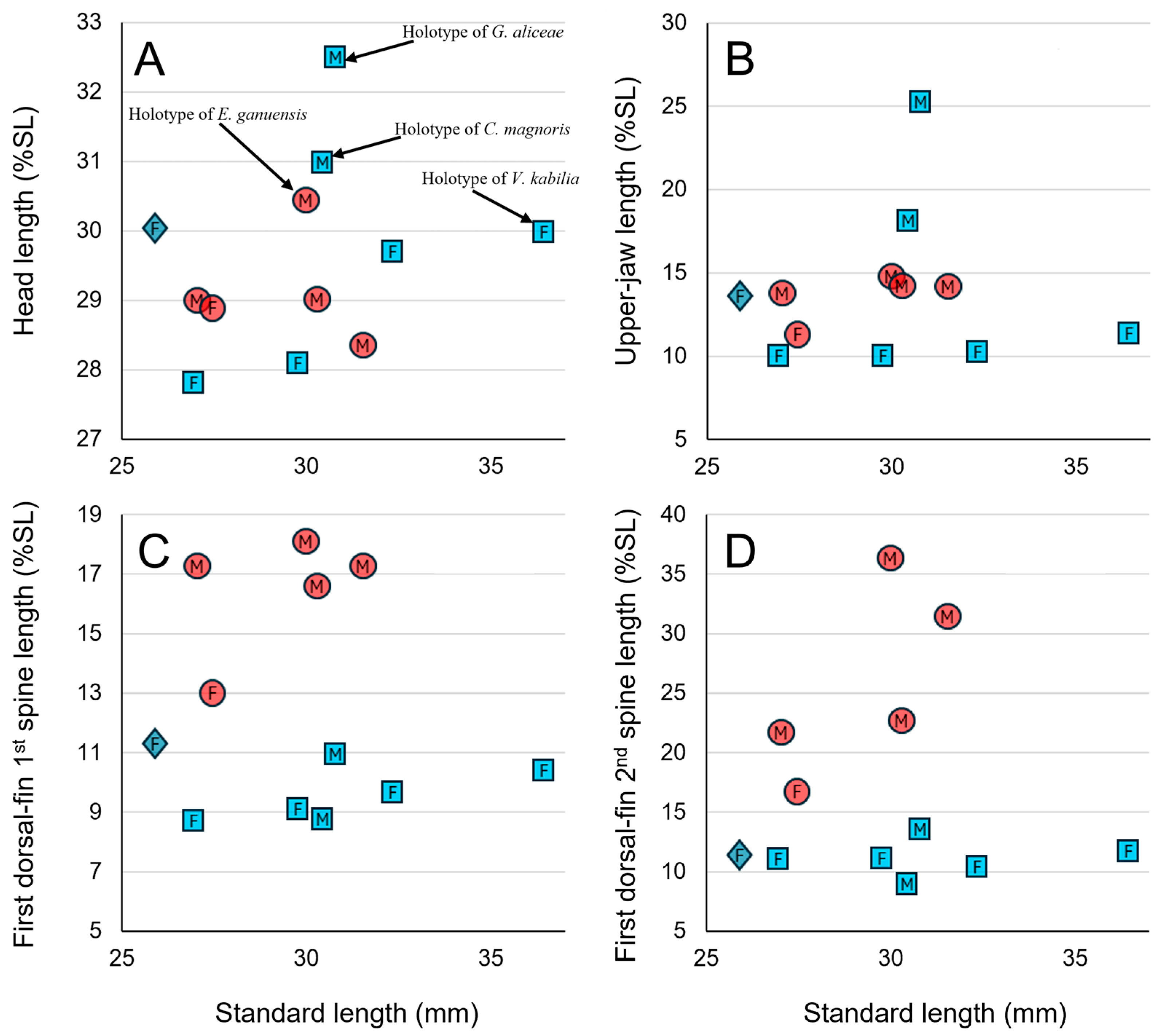
Figure 7.
Relationships of head length as % SL (A), upper-jaw length (B), and lengths of first and second spines of first dorsal fin (C,D) with standard length (mm) of Eugnathogobius ganuensis n. sp. (circles); E. kabilia (squares); E. cf. kabilia (diamond). M and F indicating meles and females of each species, respectively.
Figure 7.
Relationships of head length as % SL (A), upper-jaw length (B), and lengths of first and second spines of first dorsal fin (C,D) with standard length (mm) of Eugnathogobius ganuensis n. sp. (circles); E. kabilia (squares); E. cf. kabilia (diamond). M and F indicating meles and females of each species, respectively.
4. Discussion
Eugnathogobius Smith, 1931 is a small estuarine gobiid genus, later revised by Larson (2009) [
1], who recognized nine valid species:
E. variegatus (Peters, 1869);
E. microps Smith, 1931;
E. siamensis (Fowler, 1934);
E. kabilia (Herre, 1940);
E. mindora (Herre, 1945);
E. polylepis (Wu and Ni, 1985);
E. illotus (Larson, 1999);
E. indicus Larson, 2009; and
E. stictos Larson, 2009, and later (Larson et al., 2022) [
4] resurrected
E. mas (Hora, 1923) as a senior synonym of
E. microps. However, recent studies have proposed splitting the genus. Huang et al. (2013) [
5] utilized morphological and molecular approaches to
Eugnathogobius sensu Larson (2009), established
Wuhanlinigobius Huang, Jaafar and Chen, 2013 based on
Mugilogobius polylepis (=
E. polylepis), newly described
Wuhanlinigobius malayensis Huang, Jaafar and Chen (2013), and regarded
Calamiana Herre, 1945 as a valid genus, including
E. mindora,
E. illotus, and
E. variegatus as species of the latter, based on the presence of head pores and the medium-sized mouth in adult males. However, the nominal name “
Calamiana” is not considered to be suitable for the taxon for
E. mindora,
E. illotus, and
E. variegatus, because their
Calamiana did not include
Calamiana magnoris Herre, 1945 type species of
Calamiana [
1,
6,
7], and recognized by Larson (2009) [
1] as
Eugnathogobius kabilia, having a large mouth in adult males and lacking head pores. Subsequently,
Calamiana taiwanensis Chen, Shao and Huang, 2024 was described, being closely similar to
E. mindora [
8]. Several studies failed to follow Huang et al.’s (2013) [
5] suggestion [
9,
10] due to the latter’s analysis lacking several valid species of
Eugnathogobius sensu Larson (2009). Chen et al. (2024) [
8] also proposed a key to species of
Calamiana, but did not include
C. magnoris (=
E. kabilia), the type species of
Calamiana [
1,
7]. Clearly, several taxonomic problems of
Eugnathogobius and related genera still persist. Although further study of the generic status of
Eugnathogobius and related genera is required, a total of 11 species [9 valid species recognized by Larson (2009) [
1] and Larson (2022) [
4],
W. malayensis, and
C. taiwanensis] can be recognized as members of
Eugnathogobius sensu lato.
According to Larson (2009) [
1], Huang et al. (2013) [
5], Larson et al. (2022) [
4], and Chen et al. (2024) [
8], and confirmed by the present study,
Eugnathogobius ganuensis is easily distinguished from all other species of
Eugnathogobius sensu lato (see above) by having 16 segmented caudal-fin rays, 30 or 31 longitudinal scale rows, a high first dorsal fin (especially in males), the absence of head pores, oblique black bands on the shoulder, transverse black markings on each scale, paired black blotches on the caudal-fin base, and distinct black dots on the upper caudal fin.
Eugnathogobius kabilia is most similar to
E. ganuensis, having similar meristic counts, two distinct brown stripes extending posteriorly from the eye, an oblique band on the shoulder, and transverse markings on each scale, plus similar overall fresh coloration, although the latter differs as follows (Larson 2009 [
1]; Nagao Natural Environment Foundation 2021 [
9]; this study:
Figure 1,
Figure 2,
Figure 4 and
Figure 7): short upper jaw in males, the length < 14.8–18.1% of SL (well extended in males of
E. kabilia:
Figure 6B); first dorsal fin relatively higher, especially in males (low, rounded: lower than second dorsal fin:
Figure 7C,D); yellow throat (whiteish), and yellowish second dorsal fin when fresh (reddish in males). Although few specimens of
E. kabilia were available, males of the species had a greater head length (31.0–32.5% of SL) than females (27.8–30.0% of SL), compared with no difference between the sexes of
E. ganuensis (28.4–30.4% of SL:
Figure 7A).
The two species,
Eugnathogobius taiwanensis and
E. mindora, are similar to
E. ganuensis in having the high first dorsal fin and the pore less head. However, the former two species are distinguished from the new species by the following characteristics [
1,
7], especially in color patterns: narrow oblique stripes on cheek (absent in
E. ganuensis); no distinct pattern on lateral scales (transverse pattern on most lateral scales); pectoral-fin rays 15–18 in
E. taiwanensis and 10–17 in
E. mindora (18); longitudinal scale rows 34–37 in
E. taiwanensis and 27–39 in
E. mindora (30 or 31); TRB-2 12 or 13 in
E. taiwanensis and 9–14 in
E. mindora (8–10). The necessity of further study on
E. mindora was suggested by Larson [
7]. Although
E. mindora exhibits relatively wide variations in meristics and the head pore patterns, it may be due to geographic variation or the presence of cryptic species included within
E. mindora.
Remarks. During a survey of USNM specimens, it became clear that one of two paratypes of
Mugilogobius rambaiae (USNM 359628, 29.8 mm SL:
Figure 8E), now recognized as
E. kabilia (Larson 2009) [
11], had 38 longitudinal scale rows, considerably more than the range of longitudinal scale row numbers for that species (25–34: Larson 2009 [
1]). Moreover, one specimen from Myanmar (USNM 372148, 25.9 mm SL:
Figure 9) also had high scale count numbers (39 longitudinal scale rows; 18 TRF; 11 TRB-1; and 12 TRB-2). Because the paratype (above) of
M. rambaiae matched the diagnosis for
E. kabilia (except for longitudinal scale counts), the difference in the latter number is regarded as intraspecific variation in the species. On the other hand, the Myanmar specimen (above) also differed in transverse scale number from the anal fin origin to the first dorsal-fin base (18 vs. 9–15: this study). Therefore, it is here tentatively regarded as
E. cf.
kabilia, further study being necessary to reveal if the difference indicates intraspecific variation within
E. kabilia or the existence of a cryptic species.
Tamanka umbra Herre, 1927 was also regarded as belonging to
Eugnathogobius by Larson (2001) [
9] and Kottelat (2013) [
12]. If such is the case, the species is similar to
E. ganuensis in having a high first dorsal fin [
13,
14]. However,
T. umbra differs from the latter in having numerous longitudinal scale rows (38 in
T. umbra vs. 30 or 31 in
E. ganuensis) and irregular blotches on the first dorsal fin [present (especially between the third and fourth spines, and posteriorly) vs. absent] (Herre, 1927 [
13]; Koumans, 1940 [
14]; this study). Unfortunately, the syntypes of
T. umbra have been destroyed (Larson, 2001) [
11].
The type locality of E. ganuensis is not a natural environmental feature. Because the ditch contained only fresh water (0‰) at low tide, despite being included in the tidal area, the true habitat of the species is suggested as being the upper reaches of the Terengganu River estuary. This suggests a limited habitat, with high habitat preference by the species.
Comparative materials. Eugnathogobius kabilia (6 specimens: 26.9–36.4 mm SL): CAS-SU 32978, holotype of
Vaimosa kabilia, female, 36.4 mm SL (
Figure 8A); CAS-SU 32979, paratype of
Vaimosa kabilia, female, 32.3 mm SL (
Figure 8B); USNM 119605, paratype of
Gnathogobius aliceae, male, 30.8 mm SL (
Figure 8C); CAS-SU 39881, holotype of
Calamiana magnoris, male, 30.4 mm SL (
Figure 8D); USNM 372148, paratypes of
Vaimosa rambaiae, females, 29.8 mm SL (
Figure 8E) and 26.9 mm SL (
Figure 8F).
Eugnathogobius cf.
kabilia: USNM 372148, female, 25.9 mm SL.