3.1. Taxonomy
3.1.1. Family Actinopodidae Simon, 1892
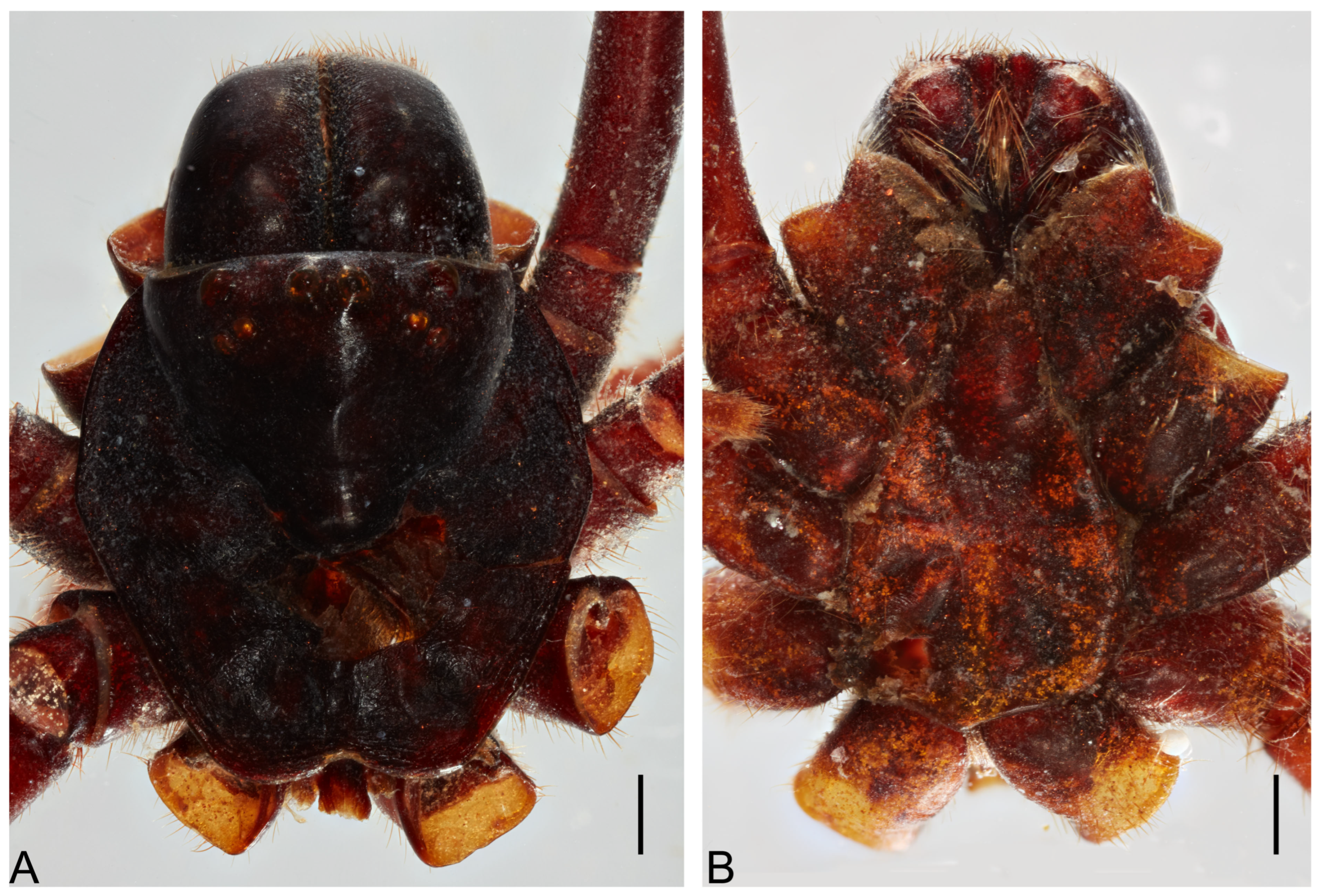
Diagnosis. Actinopodidae can be easily distinguished by their strongly elevated pars cephalica [
24] (see Jocqué & Dippenaar-Shoeman 2006: fig. 6c), fovea strongly procurved (Figure 6A), and elongated labium (Figure 6B).
Composition in South America. Actinopus Perty, 1833, Missulena Walckenaer, 1805, and Plesiolena Goloboff & Platnick, 1987.
3.1.2. Genus Actinopus Perty, 1833
Type species. Actinopus tarsalis Perty, 1833.
Diagnosis. See Miglio et al., 2020: 8 [
8].
Composition. See Miglio et al., 2020: 8 [
8] and WSC 2025 [
7].
Distribution. Panama, Saint-Vincent, Trinidad, Colombia, Venezuela, Brazil, Bolivia, Ecuador, Paraguay, Uruguay, Chile, and Argentina.
3.1.3. Actinopus nattereri (Doleschall, 1871)
Pachyloscelis nattereri Doleschall, in Ausserer, 1871a: 139 (Description female).
Actinopus nattereri F.O. Pickard-Cambridge, 1896: 730.
Figure 1.
Actinopus nattereri (Doleschall, 1871) female holotype. (A) Habitus, dorsal view. (B) Habitus, ventral view. Scale bars: 5.0 mm.
Figure 1.
Actinopus nattereri (Doleschall, 1871) female holotype. (A) Habitus, dorsal view. (B) Habitus, ventral view. Scale bars: 5.0 mm.
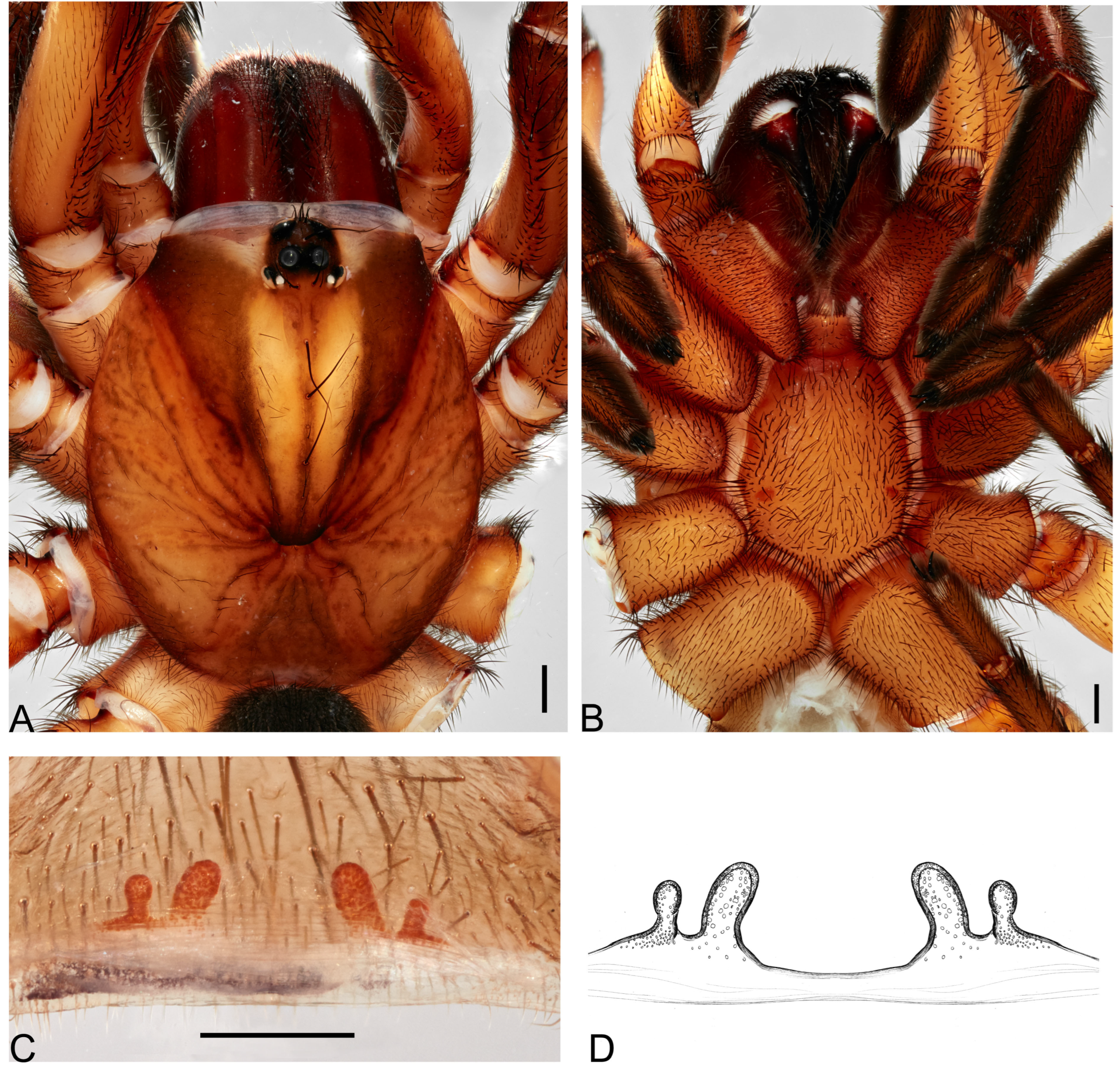
Figure 2.
Actinopus nattereri (Doleschall, 1871) female holotype. (A) Sternum, ventral view. (B) Labium and endites, ventral view. (C) Internal genitalia, dorsal view. Abbreviation: exl, external lobe; inl, internal lobe; st, spermathecae stalk; sr, sclerotized rim. Scale bars: A–C = 1.0 mm, C = 5.0 mm.
Figure 2.
Actinopus nattereri (Doleschall, 1871) female holotype. (A) Sternum, ventral view. (B) Labium and endites, ventral view. (C) Internal genitalia, dorsal view. Abbreviation: exl, external lobe; inl, internal lobe; st, spermathecae stalk; sr, sclerotized rim. Scale bars: A–C = 1.0 mm, C = 5.0 mm.
Type material.
Holotype: ♀, Pachyloscelis nattereri Rio Negro (Brazil). Natterer. Type (NHMW I.N. 42). Acquisition datum 1818.
Diagnosis. Females
A. nattereri closely resembles
A. ipioca Miglio et al., 2020, and
A. gerschiapelliarum Ríos-Tamayo & Goloboff, 2018, but are distinguished by the cuspules on the labium and endites, labium with 9 cuspules and endites with 60 cuspules in
A. nattereri, whereas 22 cuspules on labium and 123–140 cupules on endites in
A. ipioca [
8] from
A. gerschiapelliarum by the spermathecae external lobe larger and rounded (
Figure 2C) while smaller and elongated in the latter [
10] (see Ríos-Tamayo & Goloboff 2018: fig. 19C,D).
Description. Female (holotype). Total length: 13.71; carapace length: 5.81; carapace width: 4.85; abdomen length: 7.90.
Cephalothorax. Carapace orange, pars cephalica highly elevated; pars thoracica flat; fovea deep, procurved (
Figure 1A). Eyes faded. Chelicerae orange; with five retromarginal teeth, seven promarginal ones, and eight denticles in the furrow; rastellum on rectangular mound with nine marginal, blunt cusps, and five small dorsal ones (
Figure 2B). Labium elongated with 9 anterior cuspules; endites with small anterior lobe, with ~60 cuspules (
Figure 2B). Sternum longer than wide (3.91 length, 3.27 width) with slight depression (
Figure 2A); postlabial sigillum deep; posterior sigilla shallow and not well defined; not reaching the center of the sternum (
Figure 2A).
Abdomen. Uniformly light beige, oval (
Figure 1A,B); spinnerets: PMS 0.63; PLS 1.80.
Legs. Uniformly orange. Leg measurements: I 10.72 (3.81/1.69/2.13/1.92/1.17); II 9.92 (3.57/1.33/2.06/1.85/1.11); III 10.35 (3.92/2.26/1.80/1.07/1.30); IV 13.71 (4.66/2.09/3.07/2.29/1.60); leg formula 4132. Total number of retrolateral spines on tibiae I and II, 15 and 55, respectively. Spination not observed, specimen too fragile. Filiform trichobothria not observed. STC I-IV with one basal tooth, ITC present; palpal claw without tooth; claw tufts absent.
Genitalia. Spermathecae on wide base with sclerotized inverted V-shape rim; spermathecae stalks short; spermathecae with rounded external and small internal lobes (
Figure 2C).
Distribution. Only known from Brazil.
Note. The female specimen was redescribed by Doleschall in 1871 [
25], based on a specimen from the Rio Negro in Brazil. The Rio Negro flows for approximately 2250 km through the Amazon region, making it difficult to identify a specific locality for the type specimen. Miglio et al. [
8] (2020) conducted a further redescription of the species, examining both males and females from the same region. However, their observations of the female genitalia [
8] (see Miglio et al., 2020: fig. 67D) revealed differences: they described the spermathecae as unilobed and uniformly triangular, while the type specimen’s spermathecae is considered bilobed, with a larger external lobe (
Figure 2C). As a result, the specimen described by Miglio et al. [
8] (2020) is regarded as a possible misidentification.
3.1.4. Actinopus piceus (Ausserer, 1871)
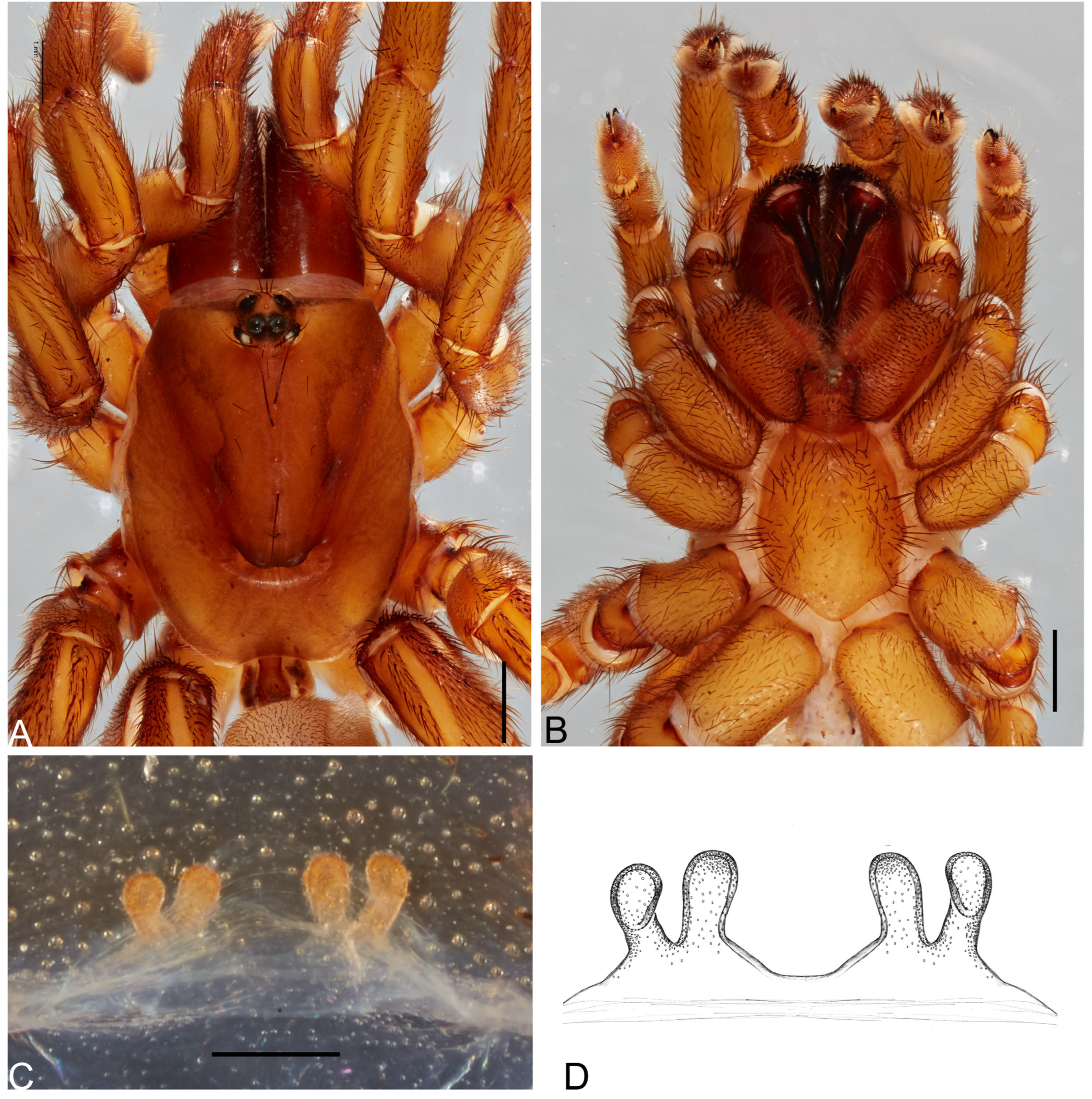
Figure 3.
Actinopus piceus (Ausserer, 1871) male holotype. (A) Carapace, dorsal view. (B) Sternum, ventral view. Scale bars: 1.0 mm.
Figure 3.
Actinopus piceus (Ausserer, 1871) male holotype. (A) Carapace, dorsal view. (B) Sternum, ventral view. Scale bars: 1.0 mm.
Figure 4.
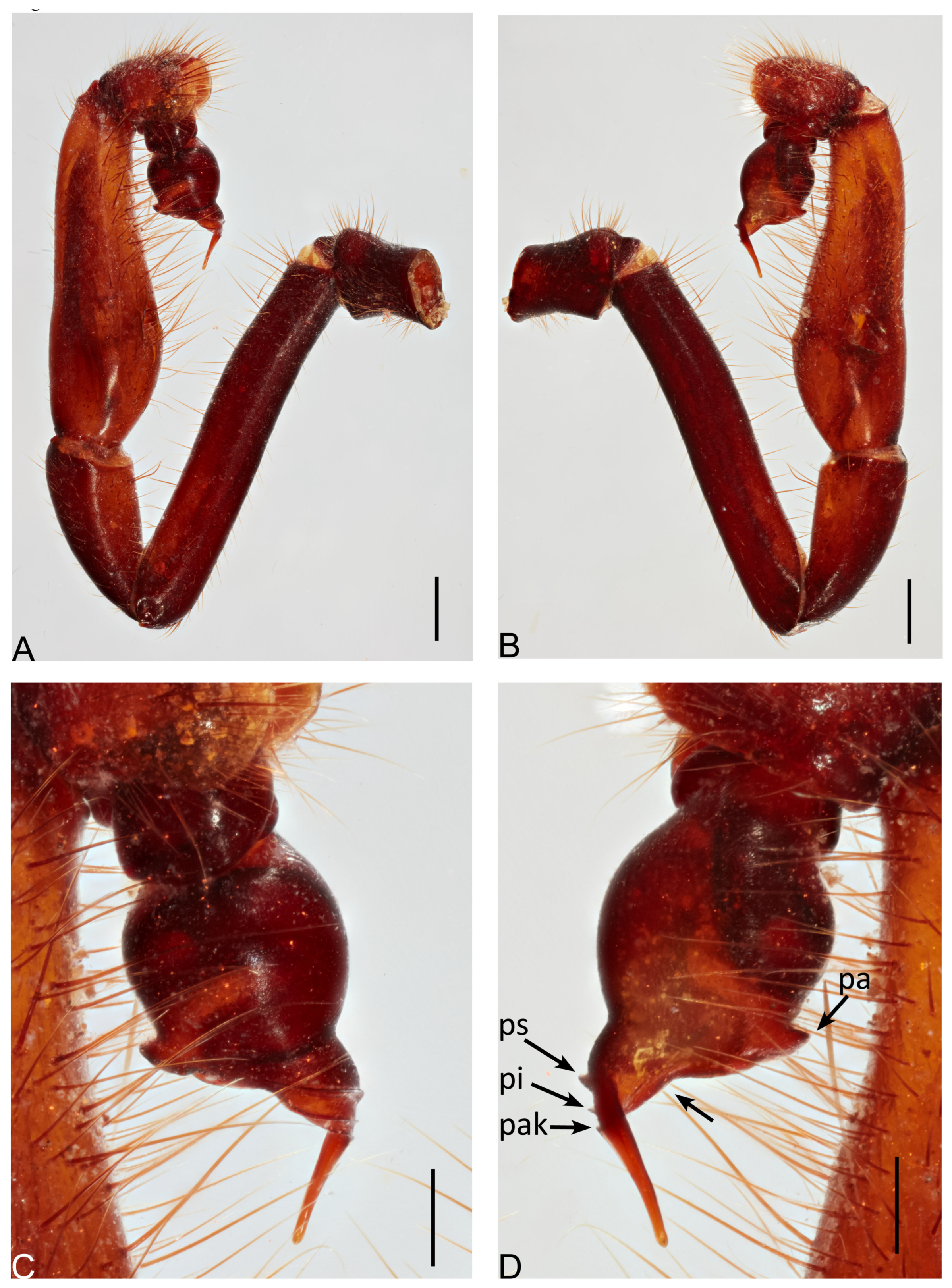
Actinopus piceus (Ausserer, 1871) male holotype. (A) Palp, prolateral view. (B) Palp, retrolateral view. (C) Bulb, prolateral view. (D) Bulb, retrolateral view. Abbreviations: pa, paraembolic apophysis; pak, prolateral accessory keel; pi, prolateral inferior keel; ps, prolateral superior keel. Scale bars: A, B = 1.0 mm; C, D = 0.5 mm.
Figure 4.
Actinopus piceus (Ausserer, 1871) male holotype. (A) Palp, prolateral view. (B) Palp, retrolateral view. (C) Bulb, prolateral view. (D) Bulb, retrolateral view. Abbreviations: pa, paraembolic apophysis; pak, prolateral accessory keel; pi, prolateral inferior keel; ps, prolateral superior keel. Scale bars: A, B = 1.0 mm; C, D = 0.5 mm.
Type material.
Holotype: ♂, Pachyloscelis piceus Brasilien. Acquisition datum 1818. Type (NHMW I.N. 44).
Diagnosis. Males most resemble
A. confusus Miglio et al., 2020, and
A. osbournei Miglio et al., 2020, based on their pointed paraembolic apophysis and the presence of prolateral superior keel, prolateral inferior keel, and prolateral accessory keel (
Figure 4C,D), but are distinguished by their triangular paraembolic apophysis and excavated base of embolus without serration (
Figure 4D, arrow), while paraembolic apophysis oval or rounded and base of embolus not excavated and with serrated area in the two latter species [
8] (see Miglio et al., 2020: figs 44, 135).
Description. Male (holotype). Carapace length: 6.58; carapace width: 6.23; abdomen missing.
Cephalothorax. Carapace dark red-brown; pars cephalica highly elevated; pars thoracica flat; fovea wide, procurved (
Figure 3A). Labium and endites cuspules
unobservable (
Figure 3B). Eyes, eight in two rows; AME 0.38; ALE 0.52; PME 0.30; PLE 0.29; PME-PME 1.98; ocular quadrangle, anterior 3.24; posterior 3.03, height 1.23. Chelicerae dark red-brown; teeth unobservable; rastellum damaged (
Figure 3B). Sternum longer than wide (4.25 length; 3.59 width); postlabial sigillum deep, posterior sigilla shallow and not visible (
Figure 3B).
Abdomen. Missing.
Legs. Uniformly reddish-brown, most detached from body. Leg measurements: II 14.93 (5.37/1.34/2.69/3.00/2.53); III 18.76 (5.25/2.07/2.87/4.64/3.93). Leg spination not observed, too fragile, and damaged.
Genitalia. Palpal tibia (5.20 length; 1.75 width); bulb with triangular paraembolic apophysis; embolus with three keels (ps, pi and pak); serrated area developed at base of embolus absent (
Figure 4C,D).
Distribution. Brazil, no specific locality.
Note. Unfortunately, there are no data except being labelled as Brasilien; therefore, the exact locality where the type specimen was collected remains unknown.
3.1.5. Actinopus saraguro New Species
Type material.
Holotype: ♀ from Ecuador, Loja, E. Witt l.d. 10.10.1899 (ZMH-A0009208). Paratype:1♀, same data as holotype (ZMH-A0029201).
Etymology. The specific name is a noun in apposition, invariable in honor of the Saraguro indigenous tribe established in the region of Loja for more than 500 years.
Diagnosis. Adult females resemble females of type II morphology [
10] by their size (15 to 26 mm), number of retrolateral spines on tibia II of females from 50 to 160, and postlabial sigillum triangular (
Figure 6C). They are differentiated from
A. coylei Ríos-Tamayo & Goloboff, 2018;
A. gerschiapelliarum Ríos-Tamayo & Goloboff, 2018; and
A. casuhati Ríos-Tamayo & Goloboff, 2018, by the numbers of retrolateral spines on tibia II, 77 spines in
A. saraguro n. sp., while 68, 48, and 89 spines in
A. coylei,
A. gerschiapelliarum, and
A. casuhati, respectively. Furthermore,
A. saraguro n. sp. is distinguished from the three species by their spermathecae with external lobe short and directed diagonally, outwardly arising from a shallow constriction and short triangular internal lobe (
Figure 6D), while
A. casuhati external lobes are long, projecting diagonally and arising after a well-marked constriction [
10] (see Ríos-Tamayo & Golobff 2018: fig. 14D);
A. coylei has long external lobe, straight or curved inward [
10] (see Ramos & Goloboff 2018: fig. 17D,E), and
A. gerschiapelliarum has with short external lobes projecting perpendicularly [
10] (see Ríos-Tamayo & Goloboff 2018: fig. 19C,D). Females also closely resemble
A. ipioca Miglio et al., 2020;
A. itacolomi Miglio et al., 2020;
A. pampulha Miglio et al., 2020; and
A. princeps Chamberlin, 1917, in the shape of the internal genitalia but are distinguished by their well-developed external lobe (
Figure 6D), whereas external lobe reduced or absent in the latter species [
8] (see Miglio et al., 2020: figs 49D, 88D, 153D, 271A–D).
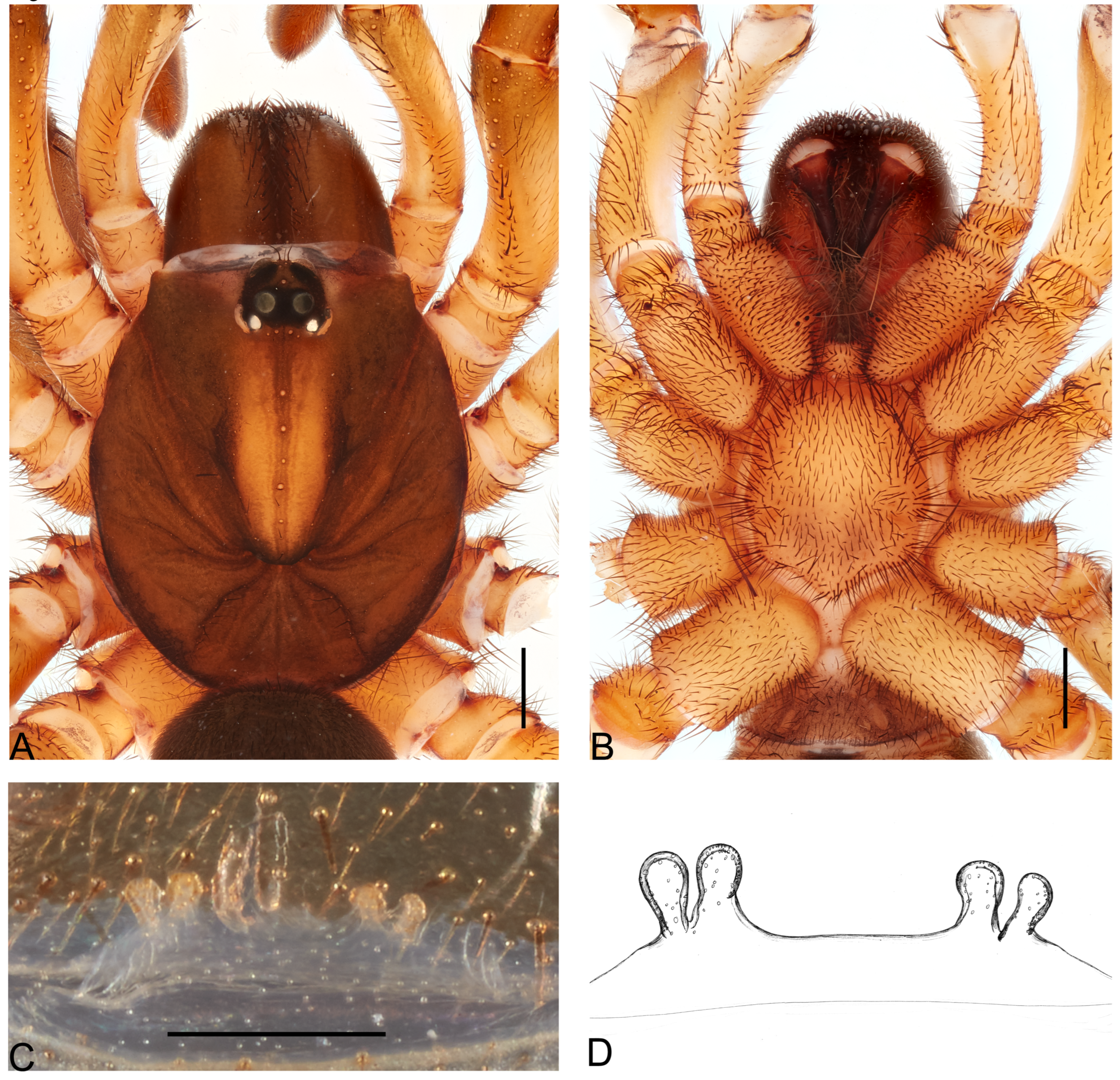
Description. Female (holotype). Total length: 17.71; carapace length: 7.23; carapace width: 7.65; abdomen length: 11.48; abdomen width 8.86.
Cephalothorax. Pars cephalica highly elevated, dark reddish-brown; pars thoracica flat, lighter reddish-brown, posteriorly yellow; fovea deep, procurved (
Figure 6A). Eyes, eight in two rows; AME 0.17; ALE 0.4; PME 0.27; PLE 0.38; AME-ALE 1.09; PME-PLE 0.23; AME-PME 0.94; ALE-PLE 0.72; AME-AME 0.43; ocular quadrangle, anterior 3.51, posterior 3.31, 1.19 height. Chelicerae dark reddish-brown; with eight retromarginal teeth, seven promarginal ones, and eight intermarginal denticles; rastellum on rectangular mound, with 11–12 marginal, blunt cusps, and 8–9 small dorsal ones (
Figure 6B). Labium elongated with 9 anterior cuspules; endites with small anterior lobe, with ~80 cuspules (
Figure 6B). Sternum longer than wide (5.68 length, 4.6 maximum width); postlabial sigillum deep; posterior sigilla shallow and elongated oval (
Figure 6B).
Abdomen. Uniformly light beige (
Figure 5A,B); spinnerets: PMS 1.00; PLS 2.19.
Legs. Uniformly reddish-brown; ascopulate. Leg measurements: I 14.60 (4.89/2.81/2.93/2.56/1.41); II 13.87 (4.57/2.09/2.81/2.85/1.55); III 13.63 (4.73/2.57/1.94/3.1/1.29); IV 18.39 (6.28/2.96/4.09/3.32/1.74); palp 13.64 (4.64/2.36/3.47/3.15); leg formula: 4123. Leg spination, all segments with spines except femora: I pa 0, ti p3, 12 R INF; mt 16 P INF, 14 R INF, 1-1-1 V POST, ta 8 P INF, 10 R INF, 2V A; II pa 0, ti r77, 1-1 V POST; mt 15 P INF, 14 R INF, 1-1-2 V POST, ta 12 P INF, 8 R INF, 2-1 V A; III pa 3p + 6 (apical), d2 + 6 (apical), r8 + 11 (apical), ti, 1 D B, a crown of 19 spines, r35, mt 4/2 D B, 45r, 3 P SUP, 1-1 V ANT, ta 14r, 11p, 10V A; IV pa ~34 P SUP-D ANT (some broken off); ti (not observed, too damaged), mt p8, 2 P A INF, 1 D A, ta 16P, 14V A. Total number of retrolateral spines on tibiae I and II, 15 and 77, respectively (
Figure 6C). Filiform trichobothria present on all tarsi. STC I-IV with one basal tooth; ITC present; claw tufts absent.
Genitalia. Spermathecae on narrow base, with W-shaped sclerotized rim, spermathecae on short stalks, external lobes asymmetrical, internal lobe triangular shorter than external ones (
Figure 6D).
Distribution. Only known from the type locality, Loja, Ecuador.
Note. The specimen was collected by Ernesto Witt Jordan in 1899. Born in Germany, he settled in Loja, Ecuador. He worked with Franz Theodor Wolff, a German naturalist, geologist, and botanist who conducted the first geological survey of mainland Ecuador. Wolff mentioned Ernesto’s help in writing his monograph and collecting material [
26,
27].
3.1.6. Family Barychelidae Simon, 1889
Diagnosis. Barychelidae most resemble Theraphosidae but can be recognized by the combination of the following characters: scopulae on legs I–II; maxillary anterior lobe small (Figure 10B); front eye row strongly procurved, divided into two or three rows (Figures 8A and 10A); short, domed distal segment of PLS (Figure 38B) [
1,
11,
20,
28].
Composition in South America. Cosmopelma Simon, 1889; Idiophthalma O. Pickard- Cambridge, 1877; Neodiplothele Mello-Leitão, 1917; Paracenobiopelma Feio, 1952; Strophaeus Ausserer, 1875; and Troglothele Fage, 1929.
3.1.7. Genus Paracenobiopelma Feio, 1952
Type species. Paracenobiopelma gerecormophilum Feio, 1952.
Diagnosis. Modified from Feio (1952) [
29].
Paracenobiopelma is distinguished from other South American Barychelidae based on the combination of the following characters: small mygalomorph (5.0–8.25), ocular rectangle 2× wider than high, rastellum reduced to 3–6 min spines, labium with 10–50 cuspules, and with 5–12 spatulate setae on all tarsi.
Composition. Paracenobiopelma vesca n. sp., P. gerecormophilum Feio, 1952.
Distribution. Brazil and Ecuador.
3.1.8. Paracenobiopelma vesca New Species
Type material.
Holotype: ♀ from Ecuador, Cotopaxi Province, San Francisco de las Pampas, Pristirana Reserve (−00.42414°, −78.95719°) 1485 m, 10.vi.2014, hand-collected, E. Tapia, N. Dupérré, family Tapia-Caisaguano, ECFN 11078 (QCAZ).
Etymology. The specific epithet is a Latin adjective meaning “thin” or “attenuated”.
Diagnosis. Females most resemble
P. gerecormophilum but are easily distinguished by the presence of 46 cuspules on labium and 71 cuspules on endites (
Figure 8B) and 10 cheliceral prolateral teeth, whereas the latter has 12 cuspules on labium, 3 cuspules on the endites [
29] (see Feio 1952: fig. 3), and 4–5 cheliceral prolateral teeth [
29].
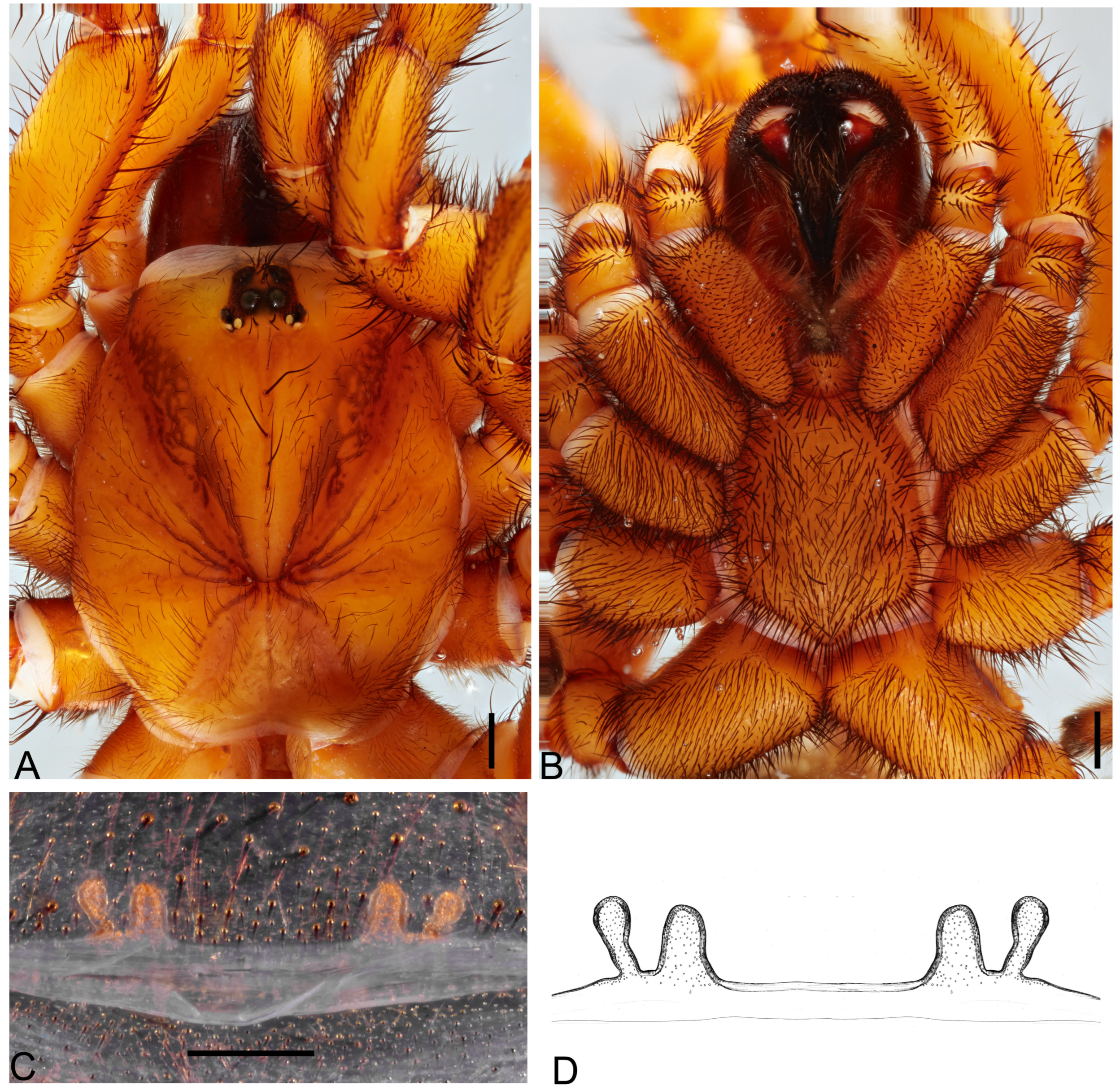
Description. Female (holotype). Total length: 9.30; carapace length: 4.01; carapace width: 3.61; abdomen length: 5.29.
Cephalothorax. Carapace rectangular, dark orange-brown with dark brown meshed pattern, with few pale setae, smooth; cephalica slightly elevated; fovea straight (
Figure 7A and
Figure 8A). Chelicerae with 10 prolateral teeth, 14 basal intermarginal denticles; rastellum present with five weak small spines. Labium quadrangle with 46 cuspules; endites elongated with weak anterior lobe; with 71 cuspules; serrula not observed (
Figure 8B). Sternum wider than long (length 1.84, width 2.04); covered with dark setae; three pairs of rounded sigilla close to the margin (
Figure 8B). Eight eyes on a raised ovoid tubercle, arranged in two rows; anterior eye row procurved, posterior eye row straight (
Figure 8A); AME 0.18, ALE 0.24, PME 0.09, PLE 0.16, PME-PME 0.42; ocular quadrangle, anterior row 0.89, posterior row 0.93, height 0.41.
Abdomen. Oval, dorsally dark brown with pale markings (
Figure 7A); ventrally mostly light beige (
Figure 7B); spinnerets: PMS 0.32, PLS 1.10.
Legs. Femora dark orange-brown, patella to tarsi yellow-orange with darker markings basally and apically on tibia and basally on metatarsi. Tarsi and metatarsi I–II scopulae complete but divided; tarsi III–IV scopulae present, weak and divide; metatarsi III scopulae present, weak and divide apically; metatarsi IV scopulae absent; palpal tarsi with scopulae divided. All tarsi with claw tufts; all tarsi with filiform trichobothria; spatulate trichobothria (8/6/10/12); palpal tarsi with 10 spatulate trichobothria. Leg measurements: I 9.72 (2.92/1.71/2.32/1.50/1.27); II 9.21 (2.76/1.64/2.09/1.55/1.17); III 8.01 (2.17/1.22/1.42/1.78/1.42); IV 12.07 (3.15/1.68/2.47/3.17/1.60); leg formula 4123. STC uniseriate with 4–5 teeth, ITC absent; palpal claw without teeth. Leg spination, spines absent on femora, patella, tibia I, II, and all tarsi: leg III ti v3(apical), d1, p1, r1; leg IV ti v1-1-3(apical), d1-1, p1; mt I-II v1-1, III v3(apical), d1, p1, IV v1-1-1-3(apical), d1-1, p1.
Genitalia. Internal genital with two rounded to oval spermathecae on short stalks (
Figure 8C).
Male. Unknown.
Distribution. Only found at the type locality in Cotopaxi Province.
Natural History. The only female specimen was hand-collected in an evergreen foothill forest on the western side of the Andes (BsPn01) [
30].
3.1.9. Genus Strophaeus Ausserer, 1875
Type species. Strophaeus kochi (O. Pickard-Cambridge, 1870).
Composition. S. austeni (F. O. Pickard-Cambridge, 1896), S. kawsay n. sp., S. elongata n. sp., S. josefita n. sp., S. kaiae n. sp., S. kochi (O. Pickard-Cambridge, 1870), S. pacificanus n. sp., S. pentodon (Simon, 1892), S. peronii n. sp., S. quillacinga Ríos-Tamayo, 2023, S. real n. sp., S. sebastiani Miranda & Bermúdez, 2010, S. spiculum n. sp., and S. subterraneus n. sp.
Distribution. Panama, Colombia, Ecuador, Brazil, and Peru.
Diagnosis.
Strophaeus differs from other South American Barychelidae (
Paracenobiopelma,
Cosmopelma, and
Neodiplothele) by the combination of the following characters: eye arrangement in quadrangle (three rows) (Figure 10A); endites with fewer than 10 cuspules (Figure 10B) and fovea strongly procurved (Figures 10A and 13A), whereas
Paracenobiopelma,
Cosmopelma, and
Neodiplothele eye arrangement in two rows (
Figure 8A; fig. 14 [
12]; fig. 24 [
13]); endites with more than 10 cuspules in
Paracenobiopelma (
Figure 8B);
Cosmopelma and
Neodiplothele fovea straight to slightly procurved (fig. 20 [
12]; fig. 39 [
13]). Currently, there are no contemporary descriptions or images of any species from the genus
Idiophthalma O. Pickard-Cambridge, 1877, making it difficult to differentiate the genus from
Strophaeus.
3.1.10. Strophaeus pacificanus New Species
Type material.
Holotype: ♀ from Ecuador, Manabí Province, via Machalilla—Puerto Cayo, en carretera hacia el gaseoducto (−01.442919° −80.753069°) 9.x.2021, 115 m, collected inside burrows in dry forest, N. Dupérré, E. Tapia, A. Tapia, ECFN 8025 (QCAZ). Paratypes: same data as holotype: 1♀, ECFN 8019 (ZMH-A0029188); 1♀, ECFN 8016 (ZIMG); 1♀, ECFN 8014 (QCAZ); 1♀, ECFN 8021 (ZIMG); 1♀, ECFN 8022 (QCAZ); 1♀, ECFN 8020 (DTC); 1♀, ECFN 8024 (DTC); 1♀, ECFN 8023 (ZMH-A0029189); 1♀, ECFN 8018 (ZMH-A0029187); 1♀, ECFN 11663 (QCAZ).
Other material examined: Same data as holotype: 20juv., ECFN 8015 (DTC); 20juv., ECFN 8017 (DTC).
Etymology. The specific epithet is an adjective, invariable, derived from the ecoregion where the species occurs.
Diagnosis. Females most resemble
S. josefita n. sp. and
S. quillacinga Ríos-Tamayo, 2023 but can be distinguished by their internal spermathecae separated by 3× their diameter, internal and external spermathecae separated by 1× their diameter (
Figure 10C–E), whereas internal spermathecae separated by 2.4× their diameter and internal and external spermathecae touching in
S. josefita n. sp. (Figure 23C), and internal spermathecae separated by 5× their diameter and internal and external spermathecae touching in
S. quillacinga [
31] (see Ríos-Tamayo 2023: fig. 1I).
Description. Female (holotype). Total length: 14.30; carapace length: 6.70; carapace width: 4.99; abdomen length: 7.60.
Cephalothorax. Carapace elongated rectangular; pars cephalica orange-brown and light yellow along midline; pars thoracica yellow, radiating lines orange-brown, margin with some black; with stiff setae along midline; fovea dark, deep and recurved (
Figure 10A). Chelicerae dark reddish-brown; promargin with nine teeth and ~25 intermarginal denticles; rastellum with 5–7 spines (Figure 59A); serrula absent (Figure 59B). Labium orange without cuspule; endites yellow-orange with three cuspules (
Figure 10B). Sternum yellow-orange, longer than wide (3.47 length, 2.69 width); three oval sigilla, anterior and middle one close to margin, posterior one submarginal (
Figure 10B). Eyes, eight in three rows; AME 0.25, ALE 0.30; PLE 0.36, PME 0.14, PME-PME 0.53; ocular quadrangle, anterior 0.87, posterior 1.17, height 0.86.
Abdomen. Oval, light yellow-beige with dark gray mesh pattern (
Figure 9A), ventrally mostly light yellow-beige (
Figure 9B); spinnerets: PMS 0.40, PLS 1.88.
Legs. Light yellow-brown; femora III–IV with longitudinal brown band retrolaterally; complete divided scopulae on tarsi I–II; complete undivided scopulae on metatarsi I–II; complete sparse, divide scopulae on tarsi III–IV; sparse apical scopulae on metatarsi III, scopulae on metatarsi IV absent; palpal tarsus with complete, divided scopulae; filiform trichobothria present on tarsi. Preening combs present, metatarsi III p5, r5; metatarsi IV p4, 3r (
Figure 9C,D). Leg measurements: I 13.32 (4.07/2.67/2.97/2.19/1.42); II 11.80 (3.59/2.27/2.39/1.99/1.56); III 10.46 (3.07/1.65/1.50/2.53/1.71); IV 16.87 (4.92/2.16/3.17/4.54/2.08); leg formula: 4123. STC I–II with 2–3 teeth; III-IV without teeth; palpal claw with 1 tooth. Leg spination, all segments with spines except tarsi: I fe d1-1-1-2-1(apical); pa 0; ti v1(apical); mt v2(apical); II fe d1-1-1-1(apical); pa 0; ti v2(apical); mt v1(apical); III fe d1-1-1-1-1(apical); pa p1-1-1; ti d12, p1-1-1, r1-1,v1(apical); mt v2-2-3(apical), p1-1-1-1, r1-1-1-1, d1; IV fe d1-1(apical); pa p1-1-1; ti p1-1-1, r1-1,v1-2-2(apical); mt v1-1-1-1-2(apical), p1-1-1-1-1, r1-1-1-1, d1.
Genitalia. Internal spermathecae slightly constricted, separated by 3× their diameter, internal and external spermathecae separated by 1× their diameter, external spermathecae slightly constricted (
Figure 10C–F).
Male. Unknown.
Variation. Female carapace length: 6.45–7.04, 6.82, n = 5.
Distribution. Only found at the type locality in Manabí Province.
Natural history. Specimens were collected in burrows, in a dry, low deciduous arbustive forest of the Jama-Zapotillo sector (Figure 65A) (BdTc02) [
32].
3.1.11. Strophaeus real New Species
Type material.
Holotype: ♀ from Ecuador, Cañar Province, Manta Real (−02.54863° −79.36391°) 17.vii.2016, 235 m, N. Dupérré, E. Tapia, A. Tapia, ECFN 916 (QCAZ).
Etymology. The specific epithet is taken from the type locality, Manta Real.
Diagnosis. Females most resemble
S. kaiae n. sp. and
S. quillacinga Ríos-Tamayo, 2023, but can be distinguished from
S. kaiae n. sp. by their external spermathecae much longer than internal spermathecae (
Figure 13C), whereas about equal height in the latter (Figure 30C), and from
S. quillacinga by the absence of scopulae on metatarsi IV, present in the latter [
31].
Description. Female (holotype). Live coloration. Carapace dark brown with black coloration along lateral side of pars cephalica, radiating lines, and margin; chelicerae dark brown; abdomen dorsally gray with pale spots; leg femora orange basally, dark chocolate brown apically, patella to tarsi dark chocolate brown (
Figure 11). Total length: 18.24; carapace length: 8.73; carapace width: 7.21; abdomen length: 9.51.
Cephalothorax. Carapace oval; pars cephalica orange-brown with black meshy markings; pars orange-brown, radiating lines and margin with some black; with stiff setae along midline; fovea, deep and recurved (
Figure 13A). Chelicerae dark reddish-brown; promargin with nine teeth and ~20 intermarginal denticles; rastellum with 5–7 spines. Labium orange without cuspule; endites yellow-orange with three cuspules (
Figure 13B). Sternum yellow-orange, longer than wide (4.72 length, 3.64 width); anterior and median sigilla rounded, marginal; posterior one submarginal and oval (
Figure 13B). Eyes, eight in three rows; AME 0.39, ALE 0.44; PLE 0.47, PME 0.19, PME-PME 0.78; ocular quadrangle, anterior 1.10, posterior 1.62, height 1.26.
Abdomen. Oval, dorsally dark gray with few pale spots (
Figure 12A), ventrally mostly light yellow-beige, except apically with dark gray meshy pattern (
Figure 12B); spinnerets: PMS 0.75, PLS 2.08.
Legs. Yellow-orange; patella to tarsi dark brown; complete divided scopulae on tarsi I–II; complete undivided scopulae on metatarsi I–II; complete sparse, divide scopulae on tarsi III–IV; sparse apical scopulae on metatarsi III, scopulae on metatarsi IV absent; palpal tarsus with complete, divided scopulae; filiform trichobothria present on tarsi. Preening combs present, metatarsi III 3pl, 3rl; metatarsi IV 4pl, 2rl. Leg measurements: I 17.62 (6.24/2.33/3.70/3.14/2.21); II 16.25 (5.19/2.36/3.56/3.17/1.97); III 14.59 (4.08/2.30/2.38/3.89/1.94); IV 20.72 (6.45/3.16/3.64/5.06/2.41); leg formula 4123. STC I–II with four teeth; III with two teeth; IV without tooth; palpal claw with four teeth. Leg spination, all segments with spines except tarsi: I fe d1-1-1-1(apical); pa 0; ti v1(apical); mt v1-1(apical); II fe d1-1-1-1(apical); pa 0; ti v1-1-3(apical); mt v1-1(apical); III fe d1-1(apical); pa p1-1-1; ti d8, p1-1, r1-1, v1-1-3(apical); mt v2-2-3(apical), p1-1-1, r1-1-1, d1-2-2(apical); IV fe d1(apical); pa 0; ti p1-1, r1, v1-1-2(apical); mt v2-1-2-3(apical), p1-1-1-1-1, r1, d1.
Genitalia. Internal spermathecae straight, separated by 5× their diameter, internal and external spermathecae close, separated by 0.5× their diameter, external spermathecae sinuous (
Figure 13C,D).
Male. Unknown.
Distribution. Only found at the type locality in Cañar Province.
Natural history. The female was collected at 235 m in the Jama-Zapotillo region, in lowland semi-deciduous forest (BmTc01) [
33].
3.1.12. Strophaeus elongata New Species
Type material.
Holotype: ♂ from Pichincha Province, La Unión del Toachi, Otongachi Natural Reserve (−00.321295° −78.95163°), 835 m, 21 February 2019, E.E. Tapia, I.G. Tapia, ECFN 6184 (QCAZ). Paratypes: same data as holotype, 1♀, ECFN 1052 (QCAZ). Santo Domingo de los Tsáchilas: Sapo Parque La Florida (−00.248529° −79.026887°) 868 m, 20 February 2020, 1♂, pitfall, I. Tapia, N. Dupérré, E. Tapia, ECFN 5551 (QCAZ); 27 February 2020, 1♂, pitfall, I. Tapia, N. Dupérré, E. Tapia, ECFN 6803 (ZMH-A0029190).
Etymology. The specific epithet is a Latin adjective meaning “elongated” or “stretched out,” referring to the elongated male carapace.
Diagnosis.
S. elongata n. sp. most resemble
S. peronii n. sp. and
S. josefita n. sp. but are distinguished from
S. peronii n. sp. by their carapace elongated oval (
Figure 15A), circular in the latter (Figure 25A), embolus slightly bent and straight (
Figure 16E), whereas strongly bent and sinuous in the latter (Figure 26E); from
S. josefita n. sp. by their larger size (carapace length =
6.88), presence of a much denser scopulae on legs I–II (
Figure 15C), palpal tibia with five prolateral spines (
Figure 16A), whereas
S. josefita n. sp. smaller in size (carapace length =
4.04), scopulae weak on legs I–II (Figure 20C), and palpal tibia with three prolateral spines (Figure 21A). Females most resemble
S. peronii n. sp. but are distinguished by their elongated, unconstricted internal spermathecae and much smaller external spermathecae (
Figure 18C), whereas internal spermathecae and external spermathecae of similar size in the latter (Figure 28C).
Description. Male (holotype). Total length: 12.86; carapace length: 7.27; carapace width: 5.42; abdomen length: 5.59.
Cephalothorax. Carapace: elongated oval, pars cephalica dark orange-brown with two dark gray bands, pars thoracica dark orange-brown with black along radiating lines and margin; cover with stiff setae along midline, dark gray bands, radiating lines and margin; fovea recurved (
Figure 15A). Chelicerae dark reddish-brown; promargin with nine teeth, no intermarginal denticles; rastellum with four spines. Labium yellow-orange without cuspule; endites yellow-orange with four cuspules; anterior lobe not well developed (
Figure 15B). Sternum yellow-orange, longer than wide (3.16 length, 2.27 width) with posterior oval sigilla, median and anterior inconspicuous (
Figure 15B). Eyes, eight in three rows; AME 0.27, ALE 0.37, PLE 0.33, PME 0.14, PME-PME 0.63; ocular quadrangle, anterior: 0.97; posterior: 1.26; height: 0.95.
Abdomen. Oval, dorsally dark gray (
Figure 14A), ventrally dark gray except epigastric region light yellow (
Figure 14B); spinnerets: PMS 0.22; PLS 0.75.
Legs. Orange-brown, femora lighter; leg I with prolateral tibial mating spur composed of a large spur on a large tubercle and one curved large spine (
Figure 15C); sparse complete scopulae tarsi I–II undivided; sparse scopulae on 50% of metatarsi I–II; very sparse scopulae on tarsi III–IV; scopulae absent on metatarsi III–IV; preening comb absent. Leg measurements: I 21.44 (6.14/2.84/4.86/4.60/3.00); II 20.11 (5.68/2.21/4.87/4.58/2.77); III 17.37 (4.94/1.74/3.53/4.46/2.70); IV 25.89 (6.60/2.44/5.58/8.23/3.04); leg formula: 4123. STC claw teeth: claw I with 3–4, claw II with 3–4, claw III–IV with 2–3 teeth. Leg spination, all segments except tarsi with spines: I fe d1-1-1-1-1; pa 0; tibia v1-1-1(apical); mt v1; II fe d1-1-2-2; pa 0; tibia v2-1-2(apical), p1; mt v1-2(apical); III fe d1-1-1-1, p1-1-1, r1-1-1, pa 1-1-1; tibia d2-2-1-1, p1-1-1-1, r1-1, v2-2-2(apical); mt v2-2-3(apical), p1-1-1, r1, d1-1-2; IV fe d1-1-1-2; pa p1-1; ti d1, p1-1-1, r1-1, v2-2-3(apical); mt v1-2-1-2-3(apical), p1-1-1-1-1, r1-1-1-1, d1-1-1.
Genitalia. Palpal tibia (2.56 length, 1.12 width) with five spines prolaterally (
Figure 16A); bulb globular; embolus short, dark, and slightly bent (
Figure 16C–E).
Female (paratype). Total length: 16.83; carapace length: 8.89; carapace width: 7.37; abdomen length: 7.94.
Cephalothorax. Carapace: elongated oval, pars cephalica orange-brown with black marking along midline radiating line and pars cephalica; cover with long setae along midline, radiating lines and margin; fovea recurved (
Figure 18A). Chelicerae dark reddish-brown; promargin with 10 teeth, and ~30 intermarginal denticles; rastellum with ~10 spines. Labium yellow without cuspule; endites yellow with three to four cuspules (
Figure 18B). Sternum longer than wide (4.99 length, 3.89 width); with posterior oval, orange sigilla, median rounded and anterior one inconspicuous (
Figure 18B). Eyes, eight in three rows; AME 0.30, ALE 0.48, PLE 0.46, PME 0.14, PME-PME 0.82; ocular quadrangle, anterior 0.87; posterior: 1.60; height: 1.33.
Abdomen. Oval, dorsally uniformly dark gray (
Figure 17A), ventrally uniformly dark gray except epigastric region (
Figure 17B); spinnerets: PMS 0.73; PLS 2.30.
Legs. Coloration as in male; with thick complete scopulae on tarsi I–II and metatarsi I–II, with sparse scopulae, divided on tarsi III–IV; scopulae restricted apically to few setae on metatarsi III, scopulae absent on tarsi IV; preening comb present: metatarsi III, spines 3pl, 5rl: metatarsi IV, spines 3pl, 7rl. Leg measurements: 20.25 (6.63/3.04/4.73/3.54/2.31); II 18.17 (5.69/2.82/4.14/3.39/2.13); III 14.91 (4.22/2.26/2.45/3.59/2.39); IV 22.45 (6.14/3.30/4.36/6.00/2.65); leg formula: 4123. STC claw teeth; I–II with 4 teeth, claw III with 2–4, claw IV with 1–4; palp claw with 4 teeth; ITC absent. Leg spination, spines present on all segments except tarsi: I fe d1(apical); pa 0; tibia p1-1-1, r1-1, v1-2-3(apical); mt v1(apical); II fe d1(apical); pa 0; tibia p1-1-1, r1, v3-1-1-2(apical); mt v1(basal); III fe d1-1-1-1(apical); pa d2; tibia d2, p7, r12, v1-3(apical); mt v2-2-3(apical), p1-1-1-1-1, r1-1-1-1, d1; IV fe d1(apical); pa 0, tibia p1-1-1, r1-1, v1-1-2(apical); mt v1-1-1-2-3(apical), p1-1-1-1-1-1, r1, d1.
Genitalia. Internal genitalia with elongated, unconstricted internal spermathecae separated by 4× their diameter, external spermathecae smaller (
Figure 18C,D).
Distribution. Santo Domingo de los Tsáchilas and Pichincha Provinces.
Variation. Male carapace length: 6.62–7.27, 6.88, n = 3.
Natural history. Specimens were collected between 835 and 869 m in elevation in the evergreen foothill forest of the western side of the Andes (Figure 65B) (BsPn01) [
30].
3.1.13. Strophaeus josefita New Species
Etymology. The specific epithet is a patronym in honor of María José Gil Larroda, author of the book Etnografia del Mestizo pampeño, a devoted nun of the Acción Misionera Franciscana, for sharing the love for nature.
Type material.
Holotype: ♂ from Ecuador, Cotopaxi Province, OTONGA Biological Reserve (−00.41433° −79.00035°) 1888 m, 24 May–8 June 2014, pitfall trap, E.E. Tapia, C. Tapia & N. Dupérré, ECFN 11652 (QCAZ). Paratypes: Cotopaxi Province: OTONGA Biological Reserve (−00.41994° −79.000623°) 1997 m, 8–21 June 2014, 1♂, pitfall trap, E. Tapia, C. Tapia & N. Dupérré, ECFN 11653 (ZMH-A0029191); 5–19 September 2014, 1♂, pitfall trap, ECFN 11654 (QCAZ); 19 September–2 October 2014, 1♂, pitfall trap, E. Tapia, C. Tapia & N. Dupérré, ECFN 11909 (DTC); 3–16 August 2014, 1♂, pitfall trap, ECFN 11657 (DTC); 16 August–5 September 2014, 1♀, pitfall trap, E. Tapia, C. Tapia & N. Dupérré, ECFN 11908 (QCAZ); OTONGA Biological Reserve (−00.41941° −78.99660°) 1717 m, 16 August–5 September 2014, 1♂, pitfall trap, E. Tapia, C. Tapia & N. Dupérré, ECFN 11655 (ZIMG); 19 September–2 October 2014, 1♂, ECFN 11656 (ZMH-A0029192).
Diagnosis. Males most resemble
S. sebastiani Miranda & Bermúdez, 2010, and
S. elongata n. sp. but can be as such: from
S. sebastiani by reduced number of setae on carapace (
Figure 20A) and absence of apical scopulae on metatarsus IV, while carapace with numerous setae, and scopulae present on metatarsus IV in the latter [
34] (see Miranda & Bermúdez 2010: fig. 4); and from
S. elongata n. sp., by their smaller size (carapace length =
4.04), sparse scopulae on legs I–II (
Figure 20C), palpal tibia with three spines prolaterally (
Figure 21A), while in
S. elongata n. sp., males of larger size (carapace length =
6.88), presence of dense scopulae on legs I–II (
Figure 15C), palpal tibia with five prolateral spines (
Figure 16A). Females most resemble
S. pacificanus n. sp. but can be distinguished by their internal spermathecae separated 2.4× their diameter and internal and external spermathecae touching (
Figure 23C) while internal spermathecae separated by 3× their diameter, internal and external spermathecae separated by 1× their diameter in the latter (
Figure 10C–E).
Description. Male (holotype). Total length: 7.78; carapace length: 4.12; carapace width: 3.21; abdomen length: 3.66.
Cephalothorax. Carapace elongated oval, pars cephalica dark brown with two dark gray bands, pars thoracica yellow; lightly cover with stiff setae along midline, along dark gray bands, radiating lines and margin; fovea dark and recurved (
Figure 20A). Chelicerae dark reddish-brown; promargin with eight teeth, ~15 intercheliceral denticles; rastellum with five spines. Labium yellow without cuspule; endites yellow with three cuspules; anterior lobe not well developed (
Figure 20B). Sternum yellow with three oval sigilla, posterior one submarginal, anterior and median one marginal (
Figure 20B). Eyes, eight in three rows; AME 0.16; ALE 0.29, PLE 0.24; PME 0.09; PME-PME 0.39; ocular quadrangle, 0.63 anterior, 0.83 posterior; 0.58 height.
Abdomen. Oval, dorsally dark gray with mesh pattern (
Figure 19A), ventrally dark gray with mesh pattern lower half, upper half uniformly light beige (
Figure 19B); spinnerets: PMS 0.26; PLS 1.02.
Legs. Uniformly light yellow-brown; leg I with prolateral tibial mating spur composed of a large spur on a large tubercle and one curved large spine (
Figure 20C); legs I–II, tarsi with complete, sparse, divided scopulae; metatarsi with sparse scopulae on apical third; legs III–IV, tarsi with sparse scopulae, metatarsi without scopulae. Leg measurements: I 9.97 (3.16/1.31/2.42/1.72/1.36); II 9.62 (3.06/1.21/2.22/1.79/1.34); III 7.68 (2.43/0.71/1.47/1.78/1.29); IV 12.41 (3.77/1.24/2.60/3.49/1.31); leg formula: 4123. STC I–II with 2–3 teeth; III–IV without teeth. Leg spination, all segments except tarsi with spines: I fe d1-1-1; pa 0; tibia v1-1-2(apical); mt v1-1(apical); II fed1-1; pa 0; tibia v1-1-3(apical), p1; mt v1-1(apical); III fe d2-2-1; pa 1-1-1-1-1; tibia d2-1-1, p1-1-1-1, r1-1,v3(apical); mt v1-1-3(apical), p1-1-1-1, r1-1, d1-1-2; IV fe d1-1-1; pa p1-1-1; tibia p1-1-1, r1-1, v1-1-2(apical); mt v2-1-1-3(apical), p1-1-1-1, r1, d1.
Genitalia. Palpal tibia (1.43 length, 0.67) with three spines prolaterally (
Figure 21A); bulb globular; embolus short, dark, and smoothly bent (
Figure 21C–E).
Female (paratype). Total length: 10.46; carapace length: 4.91; carapace width: 3.66; abdomen length: 5.55.
Cephalothorax. Carapace rectangular, pars cephalica orange-brown, with two longitudinal black marking, pars cephalica orange-brown, sloping steeply; fovea W-shaped recurved (
Figure 23A). Chelicerae dark reddish-brown; promargin eight teeth, and ~15 intermarginal denticles; rastellum with ~six spines. Labium orange without cuspule; endites orange with three cuspules; anterior lobe not well developed (
Figure 23B). Sternum as in male (2.55 length, 1.97 width); sigilla marginal; posterior and anterior sigilla oval, median sigilla rounded (
Figure 23B). Eyes, eight in three rows; AME 0.19, ALE 0.29, PLE 0.25, PME 0.10, PME-PME 0.45; ocular quadrangle, anterior 0.79; posterior: 0.89; height: 0.72.
Abdomen. Oval, dorsally and ventrally uniformly light beige (
Figure 22A,B); spinnerets: PMS 0.39; PLS 1.68.
Legs. Coloration as in male; with thick complete, divided scopulae on tarsi and metatarsi I–II, sparse scopulae on tarsi III–IV, and metatarsi III–IV ascopulate. Leg measurements: I 9.32 (2.77/1.80/2.26/1.18/1.31); II 8.59 (2.52/1.43/2.13/1.21/1.30); III 7.47 (2.09/1.09/1.30/1.66/1.33); IV 11.56 (3.57/1.29/2.22/2.74/1.74); leg formula: 4123. STC claw teeth; I–II with 2–3 teeth; claw III–IV without teeth; palp claw with 2 teeth; ITC absent. Leg spination, spines present on all segments except tarsi: I fe 0; pa 0; ti 0; mt v1-1(apical); II fe 0; pa 0; ti 0; mt v1-2(apical); III fe d1; pa p10; ti d1-1, p2, r5, v1(apical); mt v2-3(apical), p1-1-1-1, d1-1-1; IV fe0; pa p1-1-1, ti p1-1, r1, v2(apical); mt v1-2-3(apical), p1-1-1-1, r1, d1. Metatarsal preening combs present, III 7rl, 6 pl; IV 7rl, 7pl.
Genitalia. Internal genital with elongated, unconstricted internal spermathecae 2.4× their diameter, internal and external spermathecae touching (
Figure 23C,D).
Distribution. Only known from the type locality.
Variation. Male carapace length: 4.03–4.12, 4.04, n = 5.
Natural history. Specimens were collected by pitfall trap between 1717 and 1997 m in the low montane evergreen forest of the western side of the Andes (Figure 66A) (BsBn04) [
35].
3.1.14. Strophaeus peronii New Species
Etymology. The specific epithet is a patronym honoring Padre Giorgio Peroni for his more than 20 years of support for the community in San Francisco de las Pampas, as well as his dedication to the conservation and protection of Ecuador’s forests.
Type material.
Holotype: ♂ from Ecuador, Cotopaxi Province, San Francisco de las Pampas, Pristirana Natural Reserve (−00.424351° −78.959237°) 1521 m, hand-collected, 27 February 2020, family Tapia Caisaguano, ECFN 5543 (QCAZ). Paratypes: 1♀, San Francisco de las Pampas, Pristirana Natural Reserve (−00.42492° −78.95708°) 1480 m, November 2022, hand-collected, family Tapia Caisaguano, ECFN 10361 (QCAZ), 1♂, 1449 m, 15 February 2019, hand-collected, family Tapia Caisaguano, ECFN 1402 (DTC); Santo Domingo de los Tsáchilas Province, OTONGA Biological Reserve (−00.41564° −79.00642°) 2105 m, pitfall trap, 19 September–2 October 2014, E. Tapia, C. Tapia, N. Dupérré 1♂, pitfall trap, ECFN 11660 (QCAZ); 1♂ (−00.42189° −79.01325°) 2225 m, 08–21 June 2014, pitfall trap, ECFN 11661 (ZMH-A0029193); 1♂ (−00.41564° −79.006425°) 2105 m, 3–16 August 2014, pitfall trap, E.E. Tapia, C. Tapia & N. Dupérré, ECFN 11658 (QCAZ); 1♂ (−00.39506° −78.98100°) 1290 m, pitfall trap, 23 July–5 August 2014, E. Tapia, I. Tapia, N. Dupérré, ECFN 11662 (DTC).
Diagnosis. Males most resemble
S. elongata n. sp. but can be distinguished as such: carapace circular (
Figure 25A), embolus strongly bent (
Figure 26C–D), while carapace elongated oval (
Figure 15A) and embolus not strongly bent in the latter (
Figure 16C–D). Females most resemble
S. elongata n. sp. but are distinguished by internal spermathecae and external spermathecae of similar size (
Figure 28C), while external spermathecae much smaller than internal spermathecae in the latter (
Figure 18C).
Description. Male (holotype). Total length: 11.88; carapace length: 6.25; carapace width: 5.28; abdomen length: 5.63.
Cephalothorax. Carapace oval, orange with black marking along midline; heavily cover with stiff setae along midline, radiating lines and margin; fovea dark, straight (
Figure 24A and
Figure 25A). Chelicerae dark reddish-brown; promargin with 10 teeth, and no intermarginal denticle; rastellum with six spines. Labium yellow without cuspule; endites yellow with three to four cuspules; anterior lobe not well developed (
Figure 25B). Sternum yellow with marginal sigilla, anterior sigilla small, median sigilla large and triangular, posterior sigilla elongated oval (
Figure 25B). Eyes, eight in three rows; AME 0.29; ALE 0.34; PLE 0.43; PME 0.19; PME-PME 0.54; ocular anterior 0.86; posterior 1.23; height: 0.94.
Abdomen. Oval, dorsally uniformly dark gray (
Figure 24A), ventrally dark gray except epigastric region light beige (
Figure 24B); spinnerets: PMS 0.42; PLS 1.84.
Legs. Femora and tarsi light brown; patella, tibia, and metatarsi dark brown; leg I with prolateral tibial mating spur composed of a large spur on a large tubercle and one curved large spine (
Figure 25C); thick complete scopulae on tarsi I–II; thick scopulae on apical half of metatarsi I–II; complete thick scopulae on metatarsi I–II; scopulae absent on metatarsi III–IV; sparse scopulae on tarsi III–IV. Leg measurements: I 20.21 (6.33/2.71/4.59/4.07/2.51); II 18.38 (5.75/2.31/3.84/4.17/2.31); III 15.17 (4.38/1.38/2.86/4.44/2.11); IV 21.76 (6.66/1.73/4.69/6.51/2.17); leg formula: 4123. STC I with 5–6 teeth; II with 5–7 teeth; III 3–4 teeth; IV with 3–5 teeth. Tarsal organ low with concentric ridges (Figure 59C,D). Leg spination, spines present on all segments except tarsi: I fe 1-1-1; pa 0; ti v2-1-2, p1-1; mt v1(apical); II fe 1-1-1; pa 0; ti 1-1-3(apical), p1-1; mt v1-1(apical); III fe d1-2-2; pa p4; ti d1-1-1, p1-1-1-1, r1-1-1, v2-1-1(apical); mt v1-1-3(apical), p1-1, d1-1-2; IV fe 1-1-1; pa p1, ti p1-1, r1-1, v2-1-3(apical); mt v2-1-2-3(apical), p1-1, r1, d1-1.
Genitalia. Palpal tibia (1.22 length, 0.40 width), with five spines prolaterally (
Figure 26A); bulb pyriform; embolus short and strongly bent (
Figure 26C–E).
Female (paratype ECFN 10361). Total length: 11.13; carapace length: 5.58; carapace width: 4.54; abdomen length: 5.55.
Cephalothorax. Carapace oval, pars cephalica arched, dark brown with pale region along midline, pars thoracica sloping, dark brown; fovea dark, recurved (
Figure 27A and
Figure 28A). Chelicerae dark brown; promargin with nine teeth, and ~17 intermarginal denticles; rastellum with five spines. Labium yellow-orange without cuspule; endites yellow-orange with three cuspules; anterior lobe not well-developed (
Figure 28B). Sternum yellow, longer than wide (2.76 length, 2.45 width); marginal sigilla, posterior sigilla elongated oval, median and anterior one rounded (
Figure 28B). Eyes, eight in three rows; AME 0.25; ALE 0.27; PLE 0.34; PME 0.11, PME-PME 0.54; ocular quadrangle, anterior 0.80, posterior 1.19, height 0.88.
Abdomen. Oval, dorsally gray (
Figure 27A); ventrally light beige with dark gray meshy pattern (
Figure 27B); spinnerets PMS 0.44; PLS 1.48.
Legs. Femora light orange, patella to tarsi brown; tarsi I–II with dense, complete divided scopulae; metatarsi I–II with dense, complete undivided scopulae; tarsi III–IV with sparse divided scopulae; metatarsi III with few apical setae, metatarsi IV ascopulate. Leg measurements: I 11.77 (3.98/1.93/2.43/1.89/1.54); II 10.16 (3.20/1.60/2.21/1.69/1.46); III 9.19 (2.85/1.35/1.58/1.77/1.64); IV 13.11(3.99/1.97/2.29/3.21/1.65); leg formula 4123. Preening combs present, metatarsi III p6, r7; metatarsi IV p2, r8. STC I-II with 2–3 claw teeth; III with 2 teeth; IV with 3 teeth. Leg spination, spines present on all segments except tarsi: I fe 0; pa 0; ti v1-1-3(apical), p1-1; mt v1-1(apical); II fe 0; pa 0; ti v1-1-3(apical), p1-1-1; mt v1-a(apical); III fe d1-1; pa p3; ti d2-1-1-1, p1-1-1, r1-1-1, v1-1-3(apical); mt v2-2-3(apical), p1-1-1, d1-1-1; IV fe d1-1-1; pa p1, ti p1-1-1, r1, v1-1-3(apical); mt v2-1-2-3(apical), p1-1-1-1, r1, d1-1.
Genitalia. Internal genitalia with short internal and external spermathecae, internal spermathecae separated by 5.4× their diameter (
Figure 28C,D).
Distribution. Cotopaxi and Santo Domingo de los Tsáchilas Provinces.
Natural history. Males were collected between 1290 and 2225 m in evergreen foothill forest up to evergreen mountain forest of the western side of the Andes (BsPn01) (BsMn03) [
30,
36].
Variation. Male carapace length: 5.68–6.25, 5.80, n = 5.
3.1.15. Strophaeus kaiae New Species
Type material.
Holotype: ♀ from Ecuador, Carchi Province, Pimampiro (00 24 20N, 77 56 10 W) 2038 m, 04.08.2003, Monseratt (QCAZ).
Etymology. The specific epithet is a matronym in honor of Kaia Ambrose for her efforts in promoting the sustainable use of natural resources across agroecology systems in close collaboration with local communities in the Ecuadorian Andes.
Diagnosis. Females most resemble
S. pacificanus n. sp. and
S. real n. sp. but are distinguished by their large PLE 0.54 and external spermathecae stalks sinuous (
Figure 30D), whereas PLE 0.36 and straight external spermathecae stalks in
S. pacificanus n. sp. (
Figure 10C–D) and external spermathecae and internal spermathecae of equal length in
S. pacificanus n. sp., while external spermathecae much longer than internal spermathecae in
S. real n. sp. (
Figure 13C).
Description. Female (holotype). Total length: 20.86; carapace length: 9.19; carapace width: 7.33; abdomen length: 11.67.
Cephalothorax. Carapace elongated rectangular, pars cephalica orange-brown, with dark gray meshy bands, pars thoracica dark orange, radiating lines dark brown; fovea recurved (
Figure 29A and
Figure 30A). Chelicerae orange-brown; promargin with eight teeth and ~17 intermarginal denticles; rastellum with six spines. Labium orange without cuspule; endites orange with three cuspules; anterior lobe not well-developed (
Figure 30B). Sternum orange, longer than wide (4.89 length, 3.84 width) with three sigilla, posterior one large and submarginal, anterior and median one marginal (
Figure 30B). Eyes, eight in three rows; AME 0.31, ALE 0.43, PLE 0.54, PME 0.18; PME-PME 0.79; ocular quadrangle anterior 1.07; posterior 1.70; height 1.20.
Abdomen. Oval, light yellow with dark gray meshed pattern (
Figure 29), ventrally damaged; spinnerets: PMS 0.78; PLS 2.85.
Legs. Yellow-orange, metatarsi and tarsi slightly darker; tarsi I–II with dense complete, divided scopulae; metatarsi I–II with dense complete, undivided scopulae; sparse, apical scopulae on metatarsi III–IV; complete sparse scopulae on tarsi III–IV, palp tarsus with complete, dense, divided scopulae. Leg measurements: I 19.11 (6.32/3.44/4.02/3.06/2.27); II 17.38 5.73/2.74/3.51/3.14/2.26); III 14.70 (4.03/2.51/2.24/3.58/2.34); IV 22.55 (6.73/3.65/3.93/5.91/2.33); leg formula: 4123. STC claw teeth: I–II with 3–4; claw III–IV with 1–3 teeth, palp claw with 4 teeth. Preening combs present, metatarsi III, p9, r10; metatarsi IV p2, r5. Leg spination, all segments with spines except tarsi: I fe d2-1; pa 1p, 1v; ti v1-3(apical), p 1-1-1, r 1-1-1; mt v1-1(apical); II fe d2; pa 1p, 1v; ti v1-1-3(apical), p 1-1-1, r 1-1-1; mt v1-1(apical); III fe d1-1-1-1-1(apical); pa d14, p1; ti v2-1-2 (apical), p1-1-1, r1-1, d6; mt v1-2-3(apical), p1-1-1, r1-1-1, d1; IV fe d1(apical); pa p1-1-2-1-1; ti p1-1-1, r1-1,v 2-2-3(apical); mt v2-1-2-3(apical), p1-1-1-1-1-1, r1, d1.
Genitalia. Internal genital with smaller internal spermathecae separated by 6x their diameter, external spermathecae with sinuous stalks (
Figure 30C,D).
Male. Unknown.
Distribution. Only known from the type locality.
Natural History. The only female specimen was collected at 2038 m in semi-deciduous forest and shrubland of the northern valleys (BmMn01) [
37].
3.1.16. Strophaeus subterraneus New Species
Type material.
Holotype: ♂ from Ecuador, Napo, Misahualli, Comunidad Miraflores, Caverna Sr. Paredes (−00.958370° −77.645851°) 854 m, inside cave, hand-collected, 24 February 2018, E. Tapia, ECFN 527 (QCAZ).
Etymology. The specific epithet is a Latin adjective in apposition meaning “underground,” which refers to the species cave affinities.
Diagnosis. Males most resemble
S. spiculum n. sp. but are distinguished by their large tibial mating spur composed of a large spur on a small tubercle and one long spine, strongly bent apically (
Figure 32C) and embolus smoothly curved (
Figure 33C–E), while prolateral tibial mating spur composed of a short spur on a large tubercle and one uniformly curved large spine (Figure 35D) and embolus bent in the latter (Figure 36C–E).
Description. Male (holotype). Total length: 5.91; carapace length: 3.31; carapace width: 2.60; abdomen length: 3.66.
Cephalothorax. Carapace elongated oval, pars cephalica orange-brown, with dark gray bands, pars thoracica dark orange-brown, radiating lines dark brown, margin with some black, with stiff setae along midline and radiating lines; fovea recurved (
Figure 31A and
Figure 32A). Chelicerae orange-brown; promargin with eight teeth and no intermarginal denticles; rastellum with four spines. Labium orange-brown without cuspule; endites orange-brown with three cuspules (
Figure 32A). Sternum yellow-orange, longer than wide (1.68 length, 1.34 width); three small, marginal sigilla (
Figure 32A). Eyes, eight in three rows, AME 0.14; ALE 0.16; PLE 0.16; PME 0.06; PME-PME 0.34; ocular quadrangle, anterior: 0.62; posterior: 0.76; height: 0.59.
Abdomen. Oval, light yellow with dark gray meshed pattern (
Figure 31A); ventrally light yellow with dark gray meshed pattern basally (
Figure 31B); PMS 0.23; PLS 0.90.
Legs. Uniformly light yellow-brown; leg I with prolateral tibial mating spur composed of a large spur on a small tubercle and one long spine, strongly bent apically (
Figure 32C); legs I–II, tarsi with sparse complete, divided scopulae, metatarsi with sparse, divided complete scopulae; legs III–IV, tarsi with very sparse scopulae; metatarsi III–IV without scopulae. Leg measurements: I 11.50 (3.52/1.43/2.85/2.21/1.49); II 10.65 (3.18/1.24/2.48/2.27/1.48); III 8.96 (2.49/1.04/1.64/2.45/1.34); IV 14.12 (4.01/1.30/3.20/4.12/1.49); leg formula: 4123. STC claw teeth: I–II with 2-3 teeth; claws III–IV without teeth. Leg spination, all segments with spines except tarsi: I fe d1-1-1-1(apical); pa 0; ti v1-2(apical); mt v1-1(apical); leg II, fe d1-1-1-1(apical); pa0; ti v1-2(apical); mt v1-1(apical); III fe d1-1-1(apical); pa 1-1; ti d2-1-1, p1-1-1, r1-1, v1-3(apical); mt v1-1-3(apical), p1-1, r1-1, d1-1-1; IV fe d1-2-2; pa p0; ti p1-1, r1, v1-2-2(apical); mt v1-1-3(apical), p1-1-1-1, r1-1-1, d1-1.
Genitalia. Palpal tibia 1.42 length, 0.46 width, with four prolateral spines (
Figure 33A); bulb pear-shaped; embolus short, smoothly curved apically (
Figure 33C,D).
Female. Unknown.
Distribution. Only known from the type locality in Napo Province.
Natural history. The only male was collected in a karstic cave situated in the Napo–Curaray lowland evergreen forest (BsTa02) [
38].
3.1.17. Strophaeus spiculum New Species
Type material.
Holotype: ♂ from Ecuador, Napo, Colonso Chalupas Natural Reserve (−00.90884° −77.88586°), 1057 m, pitfall trap, 29 March 2020, E. Tapia, N. Dupérré, A. Tapia, ECFN 5768 (QCAZ).
Etymology. The specific epithet is a Latin noun, meaning “sharp point” or “sting” in reference to the sharp cuspules found on the endites.
Diagnosis. Males most resemble
S. subterraneus n. sp. but are distinguished by their prolateral tibial mating spur composed of a short spur on a large tubercle and one uniformly curved large spine (
Figure 35D) and embolus bent (
Figure 36C–E), while tibial mating spur composed of a large spur on a small tubercle and one long spine, strongly bent apically (
Figure 32C) and embolus smoothly curved in the latter (
Figure 33C–E).
Description. Male (holotype). Total length: 11.28; carapace length: 6.11; carapace width: 4.49; abdomen length: 5.17.
Cephalothorax. Carapace elongated oval, pars cephalica orange-brown, with dark gray bands, pars thoracica dark orange-brown, radiating lines dark brown, margin with some black; with stiff setae along midline and radiating lines; fovea recurved (
Figure 34A and
Figure 35A). Chelicerae orange-brown; promargin with eight teeth, intermarginal ~15 denticles; rastellum with six spines. Labium orange-brown without cuspule; endites orange-brown with two cuspules; endites with two cuspules and long spicule (
Figure 35C, arrow). Sternum yellow-orange (3.02 length, 2.28 width) with three oval, marginal sigilla (
Figure 35B). Eyes, eight in three rows, AME 0.28; ALE 0.30; PLE 0.37; PME 0.14; PME-PME 0.55; ocular quadrangle, anterior 0.78; posterior 1.13; height: 0.90.
Abdomen. Oval, light yellow with dark gray meshed pattern (
Figure 34A); ventrally light yellow with dark gray meshed pattern basally (
Figure 34B); spinnerets: PMS 0.41; PLS 1.48.
Legs. Uniformly brown, metatarsi and tarsi darker brown; leg I with prolateral tibial mating spur composed of a short spur on a large tubercle and one uniformly curved large spine (
Figure 35D); tarsi I–II with sparse complete, divided scopulae; metatarsi I–II with thin, complete scopulae; sparse scopulae apically on metatarsi III–IV; sparse complete, divided scopulae on tarsi III–IV. Leg measurements: I 19.81 (5.71/2.57/4.23/4.47/2.83); II 18.32 (5.03/2.32/4.29/3.94/2.74); III 16.11 (4.10/1.86/3.15/4.30/2.70); IV 24.00 (6.31/2.37/5.33/7.05/2.94); leg formula: 4123. STC I–II with 4–5 teeth; III–IV without teeth. Leg spination, all segments except tarsi with spine: I fe d1-1-2-1-2(apical); pa0; ti v3-1-1(apical) plus apical group7, p1, r1; mt v1-1(apical); II fe d1-1-1-2(apical); pa 0; ti v3-1-3(apical), p2; mt v1-1(apical); III fe d2-1-3-3-1(apical); pa1-1-1, p1; ti d1-1-1, p1-1-1, r1-1-1, v2-2-3(apical); mt v2-2-3(apical), p1-1-1, r1-1, d1-2-2; IV fe d1-2-3-3; pa p1-1-1; ti p1-1-1, r1-1, v3-2-3(apical); mt v2-1-1-1-3(apical), p1-1-1-1, r1-1-1, d1-1-2.
Genitalia. Palpal tibia (2.07 length, width 0.91) with four spines prolaterally (
Figure 36A); bulb elongated pear-shaped; embolus short, strongly bent apically (
Figure 36C–E).
Female. Unknown.
Distribution. Only known from the type locality, in Napo Province.
Natural History. The male was collected by pitfall trap in a low montane evergreen forest of the eastern Andes (BsBn01) [
39].
3.1.18. Strophaeus kawsay New Species
Type material.
Holotype: ♀ from Ecuador, Napo Province, Ahuano, Via Misahualli-Ahuano, sector Jatun Sacha, poblado Ñucanchi Kawsay (−01.064397° −77.612393°), 387 m, 27.xii.2022, in between an old wood piece, base of a branch from a tree at 5 m from ground in virgin forest at dry season, E. Tapia, I. Tapia, ECFN 9371 (QCAZ).
Etymology. The specific epithet is taken from the type locality.
Diagnosis. Females most resemble
S. pacifica n. sp. and
S. kaiae n. sp. but can be distinguished by the combination of the following characters: abdomen uniformly colored (
Figure 38A) with mesh pattern in
S. pacifica n. sp. and
S. kaiae n. sp. (
Figure 9A and
Figure 29A) and internal genitalia with triangular internal spermathecae separated by 4.3× their diameter, and external spermathecae with straight stalks (
Figure 39C), while in
S. pacifica n. sp., internal spermathecae rounded, separated by 3× their diameter (
Figure 10C–F), and in
S. kaiae n. sp., internal spermathecae separated by 6x their diameter, and external spermathecae with sinuous stalks (
Figure 30C).
Description. Female (holotype). Total length: 16.54; carapace length: 8.03; carapace width: 6.77; abdomen length: 8.51.
Cephalothorax. Carapace oval, pars cephalica dark orange-brown and yellow medially, pars thoracica dark orange-brown, radiating lines dark brown, margin with some black; with stiff setae along midline; fovea recurved (
Figure 38A and
Figure 39A). Chelicerae dark reddish-brown; promargin with eight teeth, 28 intermarginal denticles; rastellum with five spines. Labium dark orange-brown without cuspule; endites dark orange-brown with four cuspules, anterior lobe not well developed (
Figure 39A). Sternum dark orange-brown with posterior elongate oval sigilla touching margin, median and posterior sigilla marginal and rounded (
Figure 39A). Eyes, eight in three rows; AME 0.36; ALE 0.42; PLE 0.44; PME 0.19; PME-PME 0.84; ocular quadrangle, anterior: 0.99; posterior 1.65; height: 1.24.
Abdomen. Oval, dorsally and ventrally uniformly dark gray (
Figure 38A,B); spinnerets: PMS 0.61; PLS 2.51.
Legs. Uniformly brown; tarsi I–II with dense, complete and divided scopulae; metatarsi I–II with dense complete, undivided scopulae; metatarsi III with sparse, divided apical scopulae, sparse scopulae (a few setae); apical on metatarsi IV; complete, divided scopulae on tarsi III–IV. Leg measurements: I 17.28 (5.77/2.91/3.72/2.93/1.95); II 16.13 (5.02/2.57/3.62/2.86/2.06); III 13.46 (3.87/2.30/2.07/3.28/1.94); IV 20.24 (6.31/2.81/3.73/5.24/2.15); leg formula: 4123. Preening combs present on metatarsi III, p8, r9, metatarsi IV p3, r6. STC teeth: I–II with 2–3 teeth; III–IV with 0–1 tooth; palpal tarsal claw with 3 teeth. Leg spination, all segments with spines except tarsi: I fe d1-1; pa 0; ti v1-3, p1-1-1, r1; mt v1(apical); fe II d1-1; pa 0; ti v1-3, p1-1; mt v1-1(apical); III fe d1-1; pa 1-1-1; ti d1-2-2,-2, p1-1-1-1, r1-1, v3(apical); mt v1-2-3(apical), p1-1-1, r1, d1-2-2(apical); IV fe d1; pa p0; ti p1-1-1, r1-1, v1-1-3(apical); mt v1-3(apical), p1-1-1-1, r1, d1-1-2 (apical).
Genitalia. Internal genitalia with internal spermathecae triangular, external spermathecae of equal height, internal spermathecae separated by 4.3× their diameter (
Figure 39C,D).
Male. Unknown.
Distribution. Only found at the type locality in Napo Province.
Natural history. The female was collected at 5 m high in the detritus of an old tree; it is possible the species is arboreal. The locality is located in the Napo–Curaray lowland evergreen forest (BsTa02) [
38].
3.1.19. Family Halonoproctidae Pocock, 1901
Diagnosis. Pars cephalica gradually sloping or rising steeply with posterior region of carapace sloping; fovea deep and strongly procurved (Figure 41A); possessing rastellum with large teeth on a mound; posterior lateral spinnerets (PLS) relatively short with apical segment shortest and domed to triangulate; females, legs I and II with uniquely curved thorn-like spines on the lateral faces of the distal segments of legs I and II [
40].
Composition in South America. Ummidia Thorell, 1875.
3.1.20. Genus Ummidia Thorell, 1875
Type species. Ummidia picea Thorell, 1875
Diagnosis. See Godwin & Bond, 2021 [
14].
Composition. 57 species [
7], only 6 species in South America:
Ummidia asperula (Simon, 1889);
U. neblina Godwin & Bond, 2021;
U. pupulae n. sp.;
U. quijichacaca Godwin & Bond, 2021;
U. solana Echeverri et al., 2023; and
U. tibacuy Godwin & Bond, 2021.
Distribution in the Americas. USA, Mexico, Central America, Caribbean, Venezuela, Colombia, and Ecuador.
3.1.21. Ummidia pupulae New Species
Type material.
Holotype: ♂ from Ecuador, Pichincha, La union del Toachi, Otongachi, 850 m (−00.321207° −78.950968°), hand-collected, G. Onore, 09.08.2020, ECFN 8765 (QCAZ). Paratype: same data as holotype, 1♂, 10.11.2022, ECFN 11910 (QCAZ).
Etymology. The specific epithet is a Latin noun meaning “pupil (of the eye)” in reference to the large rounded anterior median eyes.
Diagnosis. Males resemble from
U. solana Echeverri et al., 2023, and
U. asperula (Simon, 1889) but are differentiated by the combination of the following characters: rastellum present, absence of spine on tarsi I, four spatulate trichobothria on tarsi I, embolus sinuous (
Figure 42C–E), while rastellum absent, with numerous spines on tarsi I [
41] (see Echeverri et al., 2023: fig. 6C); one to three spatulate trichobothria on tarsi and embolus straight [
41] (Echeverri et al., 2023: fig. 7B,C) in the latter; from
U. asperula by the absence of spines on tarsi I (
Figure 41C) and the absence of prolateral spines on tibia I, whereas one retrolateral spines on tarsi I and three prolateral spines on tibia I spines in the latter [
14] (see Godwin & Bond 2021: fig. 69A,B).
Description. Male (holotype): Total length: 5.53; carapace length: 2.93; carapace width: 2.86; abdomen length: 2.60.
Cephalothorax. Carapace circular, glabrous, and reticulate; pars cephalica slightly elevated, pars thoracica sloping; fovea transverse, strongly procurved absent (
Figure 41A). Chelicerae with three prolateral and six retrolateral teeth; rastellum present on small mound with five short spines. Labium with 11 cuspules; endites rectangular, with 17–19 cuspules; anterior lobe not well-developed (
Figure 31B). Sternum oval (length 1.73, width 1.62); smooth, with few setae mainly on the edge; lacking sigilla (
Figure 31B). Eight eyes on a raised ovoid tubercle, arranged in two rows; anterior eye row procurved, posterior eye row straight; AME 0.24, ALE 0.27, PLE 0.17, PME 0.12, PME-PME 0.36; ocular tubercle: anterior 0.93, posterior 0.89, height 0.47.
Abdomen. Oval, dorsally uniformly dark gray (
Figure 40A), ventrally meshy gray pattern (
Figure 40B); spinnerets: PMS 0.20, PLS 0.50.
Legs. Uniformly dark brown and striated except tarsi light yellow; tarsi I–II swollen (
Figure 41C); tibia of leg III saddle-like. Pseudoscopulae present on tarsi I–II, III–IV absent; metatarsi I–II present apically, III–IV absent. Leg measurements: I 7.90 (2.83/1.30/1.73/1.31/0.73); II 7.35 (2.61/1.1 1/1.65/1.15/0.83); III 6.46 (2.23/0.78/1.31/1.16/0.98); IV 8.06 (2.82/1.05/1.76/1.40/1.03); leg formula 4123. STC uniseriate with 3–4 teeth, ITC short and steeply curved, absent on tarsi IV; claw tufts absent; tarsi with filiform trichobothria present; four spatulate trichobothria on tarsi I–II, two spatulate trichobothria on tarsi III–IV. Leg spination, spines absent on all femora: I ti v1-1-3-3(apical), mt v1-1-1-2(apical); II ti v1-1-2 (apical), mt v1-1-2 (apical); III pa pd 5, ti v3(apical), mt v1-3(apical), p2, d 1-4 (apical), ta v1; IV ti v2 (apical) mt v1-2(apical), ta v1, p1.
Genitalia. Palp tibia swollen basally (1.67 length, 0.61 width) without spines (
Figure 42A,B); bulb pyriform, subtegulum small; embolus thin, sinuous (
Figure 42C–E).
Female. Unknown.
Distribution. Only known from the type locality in Pichincha Province.
Natural history. Specimens were collected at 850 m in an evergreen foothill forest on the western side of the Andes (BsPn01) [
30].
3.1.22. Family Idiopidae Simon, 1889
Diagnosis. Anterior lateral eyes projected in front of the others, close to the anterior margin of the carapace (Figure 44A); pars cephalica arched; distal segments of the front legs with numerous lateral spines; the tibia of leg I of males with the tibial apophysis with one or two apical branches [
18] (see Fonseca-Ferreira et al., 2021; fig. 15G) [
18,
20,
24].
Composition in South America. Idiops Perty, 1833, and Neocteniza Pocock, 1895.
3.1.23. Genus Idiops Perty, 1833
Type species. Idiops fuscus Perty, 1833.
Diagnosis.
Idiops is easily distinguished from the only other American Idiopidae,
Neocteniza, by the strongly procurved fovea, U-shaped (
Figure 43A and
Figure 44A), while fovea recurved and T-shaped in the latter [
19] (see Platnick & Shadad 1976: figs 2, 3).
Composition. 80 species [
7], 25 species in South America:
Idiops argus Simon, 1889;
I. bonapartei van Hasselt, 1888;
I. cambridgei Ausserer, 1875;
I. camelus (Mello-Leitão, 1937);
I. carajas Fonseca-Ferreira et al., 2017;
I. clarus (Mello-Leitão, 1946);
I. clepsydra n. sp.;
I. dilatatus Gomes et al., 2024;
I. duocordibus Fonseca-Ferreira et al., 2017;
I. fuscus Perty, 1833;
I. germaini Simon, 1892;
I. guri Fonseca-Ferreira et al., 2021;
I. hirsutipedis Mello-Leitão, 1941;
I. minguito Ferretti, 2017;
I. mocambo Fonseca-Ferreira et al., 2021;
I. nilopolensis Fonseca-Ferreira et al., 2021;
I. opifex (Simon, 1889);
I. petiti (Guérin, 1838);
I. piluso Ferretti et al., 2017;
I. pirassununguensis Fukami & Lucas, 2005;
I. rastratus (O. Pickard-Cambridge, 1889);
I. rohdei Karsch, 1886;
I. sertania Fonseca-Ferreira et al., 2021;
I. siolii (Bücherl, 1953); and
I. tolengo Ferretti, 2017.
Distribution in the Americas. Venezuela, Suriname, French Guiana, Colombia, Ecuador, Brazil, Paraguay, Uruguay, and Argentina.
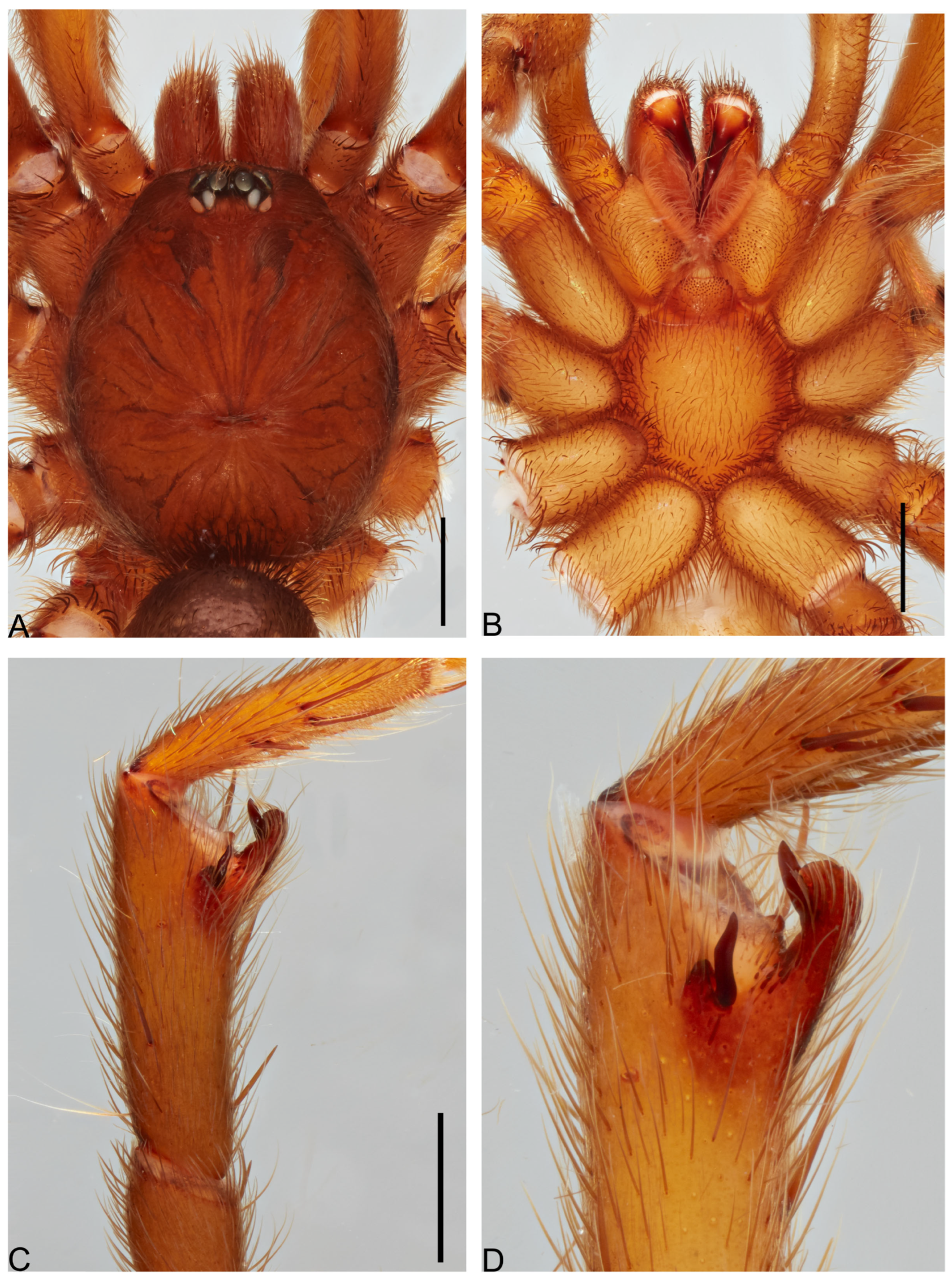
3.1.24. Idiops clepsydra New Species
Figure 43.
Idiops clepsydra n. sp., female holotype. (A) Habitus, dorsal view. (B) Habitus, ventral view. Scale bars: 1.0 mm.
Figure 43.
Idiops clepsydra n. sp., female holotype. (A) Habitus, dorsal view. (B) Habitus, ventral view. Scale bars: 1.0 mm.
Figure 44.
Idiops clepsydra n. sp., female holotype. (A) Carapace, dorsal view. (B) Sternum, ventral view. (C) Internal genitalia, dorsal view. (D) Illustration of internal genitalia, dorsal view. Scale bars: A, B = 1.0 mm; C = 0.5 mm.
Figure 44.
Idiops clepsydra n. sp., female holotype. (A) Carapace, dorsal view. (B) Sternum, ventral view. (C) Internal genitalia, dorsal view. (D) Illustration of internal genitalia, dorsal view. Scale bars: A, B = 1.0 mm; C = 0.5 mm.
Type material.
Holotype: ♀ from Ecuador, Napo Province, Ahuano, Via Misahualli-Ahuano, sector Jatun Sacha, Poblado Ñucanchi Kawsay (−01.064397° −77.612393°), 387 m, 26.xii.2022, shaking small trees and bushes in virgin forest at the end of the dry season, E. Tapia, I. Tapia, ECFN 10214 (QCAZ).
Etymology. The specific epithet is a Latin noun, invariable, meaning “hourglass”, in reference to the dark hourglass marking around the eyes.
Diagnosis. The species most resembles
I. cambridgei and
I. dilatatus, but is distinguished by the black hourglass mark around the eyes without bristle (
Figure 44A) black hourglass mark around absent in the other species; 62 triangular cuspules on the entire surface of endites and rastellum with spines, while endites with 114 cuspules in
I. cambridgei [
18] and 86 cuspules in
I. dilatatus and ocular region with bristle [
42] (see Gomes et al., 2024: fig. 1C).
Description. Female (holotype). Total length: 5.77; carapace length: 2.84; carapace width: 2.42; abdomen length: 2.93.
Cephalothorax. Carapace light orange-brown with black mesh pattern on pars thoracica, elongated oval, reticulate; cephalic area strongly domed; pars thoracica sloping steeply; fovea transverse, strongly procurved (
Figure 44A). Chelicerae orange, with seven teeth; rastellum with eight spines on mound. Labium basally brown, apically yellow, with nine triangular cuspules; endites yellow-orange with 62 triangular cuspules (
Figure 44B). Sternum rectangular, length 1.51, width 1.40; sigilla absent (
Figure 44B). Eight eyes arranged in three rows; surrounded with black hourglass mark; AME 0.14, ALE 0.16, PME 0.08, PLE 0.16, PME-PME 0.21; ocular rectangle 0.40 anterior, 0.73 posterior, 0.81 high.
Abdomen. Oval, dorsally dark gray (
Figure 43A), ventrally with dark gray meshy pattern, epigastric region light beige (
Figure 43B); spinnerets: PMS 0.31, PLS 0.99.
Legs. Yellow-orange, patellae III–IV, femora and metatarsi III–IV with black marks apically (
Figure 43A). Leg measurements: I 5.77 (1.53/0.78/1.07/0.82/1.57); II missing; III 4.48 (1.48/0.59/0.81/0.87/0.73); IV 6.89 (2.20/0.98/1.61/1.26/0.84); leg formula: 41-3. STC uniseriated with 1–3 teeth, ITC present on all tarsi IV; palpal claw with one tooth; claw tufts absent; filiform trichobothria present, clavate trichobothria absent. Leg spination, all segments with spines: I fe v1-1, pa 0, ti r1-1-1-1, p1-1, mt p1-2-1-2, r2-2-3, ta p1-1-2-1, r1-2-1-2; II missing; III ti p1-1-2, r1-1-1-2-2, mt v2(apical), p 1-1-1, r1-1, ta 1p; IV ti v2(apical), r1-1, mt v1-3(apical), p1, ta p1-1-1, r1.
Genitalia. Visible through integument (
Figure 43B). Internal genitalia with spermathecae with short stalks, spermathecal head oval, separated in V-shaped from (
Figure 44C,D).
Male. Unknown.
Distribution. Only known from the type locality.
Natural History. The female was collected by shaking trees in the Napo–Curaray lowland evergreen forest (BsTa02) [
38].
Note.
Idiops fulvipes Simon, 1889 was synonymized under
Idiops argus Simon, 1889, by Fonseca-Ferreira et al. [
18] (2021) based on the observation that the holotype female was a pre-adult female. We argue that even though the holotype might be a pre-adult, the internal genitalia shows well-sclerotized spermathecae and well-sclerotized membranic plates [
43] (see Dupérré & Tapia 2021: fig. 15C). Furthermore, the internal genitalia is completely different from
I. argus, which has strongly twisted stalks [
43] (see Dupérré & Tapia 2021: fig. 14C), while the latter has straight stalks [
43] (see Dupérré & Tapia 2021: fig. 15C). The two specimens were collected ~195 km away from each other, specimens from the type locality should be collected and compared before synonymization; hence, the synonym is rejected here.
3.1.25. Family Theraphosidae Thorell, 1869
Diagnosis. Small to very large-sized spider; with or without urticating or stridulating setae (Figure 61D); claw tufts present together with tarsal scopulae (Figure 57C); anterior projection of endites small to large (Figure 47B); filiform and spatulate trichobothria present on tarsi in rows (Figure 62A) [
11].
Composition in South America. 81 genera [
7].
3.1.26. Subfamilies. Ischnocolinae Simon, 1892, and Schismatothelinae Guadanucci, 2014
Diagnosis. Modified from Guadanucci 2020 [
44]. Small to medium-sized; without urticating or stridulating setae, male palpal bulb simple, without great modifications (Figures 48 and 53; see Guadanucci 2020: figs 3.2 C, H, 3.3 C, H) [
44].
Composition in South America. 12 genera [
44].
3.1.27. Genus Pululahua New Genus
Type species. Pululahua kunukyaku New Genus, New Species.
Etymology. The generic name is taken from the type locality Pululahua Geobotanical Reserve, where the type species was collected. The gender is neutral.
Diagnosis. Species of
Pululahua gen. n. most resembles species from the genus
Holothele Karsch, 1879, and
Schismatothele Karsch, 1879, but is distinguished by the combination of the following characters: male leg I with large tibial apophysis composed of two ramifications; prolateral branch short with large curved megaspine; ventral branch wide with large, thick megaspine; furrow of branches with small spinules (Figures 47D and 52D, arrows), while leg I tibial apophysis furrow without small spinules in the latter [
45,
46] (see Moeller et al., 2023: fig. 37; Guadanucci et al., 2017: fig. 1E); male palpal tibia not strongly swollen, not bearing several short and thick spines (Figures 48A and 52A), whereas swollen bearing several short and thick spines in
Schismatothele [
46] (see Moeller et al., 2023: fig. 30), and twisted palpal bulb with conspicuous keels and short embolus (Figures 47D and 53D,G), whereas palpal bulb without keels and long embolus in
Holothele [
45] (see Guadanucci et al., 2017: fig. 1B–D). Females are distinguished by unique, oval to rectangular spermathecae with sclerotized plates (Figure 50C,D and Figure 55A,B); dual spermathecae in
Holothele [
45] (see Guadanucci et al., 2017: fig. 1F, G) and
Schismatothele [
46] (see Moeller et al., 2023: fig. 40).
Description. Small spider (8.48–11.43).
Cephalothorax. Carapace elongated oval; pars cephalica flat, pars thoracica slightly sloping; fovea straight (Figures 47A and 50A). Chelicerae with 8–9 promarginal teeth and numerous intermarginal denticles (22–25); rastellum absent (Figure 61A,B). Sternum slightly longer than wide, with three oval, marginal sigilla (Figures 47B, 50B and 60A). Labium rectangular, wider than long, margin straight, with more than 30 cuspules (Figures 47B and 60C,D); endites with small anterior lobe; more than 30 cuspules (Figures 47B and 60C,D); serrula present with a few triangular projections (Figure 60F). Eight eyes in two row, ocular region rectangular (1.8–2.0x wider than long) (Figure 60B).
Abdomen. Oval, covered with setae, without urticating hairs (Figure 61D); four spinnerets; PMS short, PLS long, last segment elongated (Figure 61E,F).
Legs. Spines present except on tarsi; leg formula 4123. Complete divided scopulae on all tarsi, reduce to apical region in metatarsi, sparser in males than females. Spatulate trichobothria present on all tarsi in two lines (6–12) with corrugated socket; filiform trichobothria present, with corrugated socket (Figure 62A–E). Tarsal organ apical and centrally positioned, low with a few concentric circles (Figure 62F). Male leg I with large tibial apophysis composed of two ramifications; prolateral branch short with large curved megaspine; ventral branch wide with large, thick megaspine; furrow of branches with small spinules (Figures 47D and 52D, arrows).
Genitalia. Palpal tibia wider basally, with spines prolaterally, absent retrolaterally; bulb with keels, embolus twisted (Figure 48C–E). Female internal genitalia with unique spermathecae oval to rectangular (Figures 50C,D and 55A,B).
Distribution. Ecuador.
Composition. Pululahua kunukyaku n. sp. and Pululahua winku n. sp.
3.1.28. Pululahua kunukyaku New Species
Figure 45A,B,
Figure 46A,B,
Figure 47A–D,
Figure 48A–E,
Figure 49A,B,
Figure 50A–D, Figure 60A–F, Figure 61A–F, Figure 62A–F, Figure 64 and Figure 66B.
Type material.
Holotype: ♂ from Ecuador, Pichincha, Termas de Pululahua (00.059720° −78.509230°), 2128 m, 4 November 2016, E. Tapia, N. Dupérré, A. Tapia, ECFN 11668 (QCAZ).
Paratypes: same data as the holotype: 1♂, ECFN 11669 (ZMH-A0029199), 1♀ female, ECFN 11670 (QCAZ); 1♀, ECFN 11671 (DTC); 1♀, ECFN 11672 (ZMH-A0029200).
Etymology. The specific epithet is an invariable noun, taken from the Kichwa language meaning thermal springs, in reference to habitat in which the species was collected.
Diagnosis. The species most resemble
P. winku n. sp. but is distinguished by the presence of one prolateral keel, less pronounce striation and bulb without angular constriction, rounded (
Figure 48C–E), while two prolateral keels and pronounced striation and bulb with angular constriction (Figure 53C–H). Females are distinguished by their angular to oval spermathecae and straight posterior region of pore plate (
Figure 50C,D), while spermathecae oval with heart-shaped posterior pore plate region in the latter (Figure 55A,B).
Description. Male (holotype). Total length: 8.60; carapace length: 4.21; carapace width: 3.48; abdomen length: 4.39.
Live coloration. Carapace dark chocolate brown densely covered with golden setae, abdomen and leg dark chocolate brown densely covered with brown setae (
Figure 45A,B).
Cephalothorax. Carapace oval-shaped, orange-brown, pars cephalica flat, with black marking, pars thoracica sloping gradually, with black markings along radiating line; fovea straight (
Figure 46A and
Figure 47A). Chelicerae orange-brown, rastellum absent; with nine promarginal teeth and 23 intermarginal denticles (
Figure 47A). Labium orange, wider than long, with 77 cuspules; endites orange with 79 cuspules; anterior lobe small; serrula present. Sternum orange, slightly longer than wide (2.09 length, 1.73 width); three small rounded sigilla along margin (
Figure 47A). Eyes, eight in two rows, no tubercle; AME 0.18, ALE 0.28, PLE 0.20, PME 0.20, PME-PME 0.38; ocular rectangle, anterior 0.89, posterior 0.90, height 0.49.
Abdomen. Elongated oval, dorsally dark brown covered with setae; without urticating setae (
Figure 46A); ventrally dark brown, epigastric region light beige (
Figure 46B); spinnerets, PMS 0.45, PLS 2.46.
Legs. Uniformly orange-brown; leg tarsi I–II with complete, divided scopulae, metatarsi I-II with 50%, divided scopulae, tarsi III–IV with complete, divided sparse scopulae, metatarsi III–IV with apical sparse scopulae; filiform trichobothria present on all tarsi; spatulate trichobothria on tarsi (6/5/7/6). Leg measurements: I 12.45 (3.63/1.78/3.03/2.31/1.70); II 11.04 (3.23/1.56/2.52/2.04/1.72); III 10.34 (2.92/1.46/1.93/2.41/1.62); IV 14.54 (3.81/1.67/3.24/3.94/1.88); leg formula 4123. Leg I with large tibial apophysis with two ramifications, prolateral branch short with large curved megaspine, ventral branch wide with large, thick megaspine, furrow of branches with eight spinules (
Figure 47C,D). STC claw biseriate, with 5–6 teeth, ITC absent; claw tufts present. Leg spinations, spines present all segment except tarsi: I fe d1-1-1-1-1-2, pa 0, ti v2-1-1-1(apical), p1-1, mt v3-3-1(apical), p1; II fe d1-1-1-1-2, pa 0, ti v1-1-1-3(apical), p1-1, mt v1-2-1(apical), p1; III fe d1-2-3-3-3, pat 1p, ti p1-1, r1-1, v1-2-2(apical), mt v1-2-3(apical), p1-1-1, r1-1, d2; IV fe d1-1-1-2-3-1, pa 1p, ti v1-1-2-2(apical), p1-1, r1-1-1, mt v1-1-1-3(apical), r1-1-1, p1-1-1, d2-2.
Genitalia. Palpal tibia enlarged basally (2.34 length, 0.91 width) with one prolateral spine (
Figure 48A); cymbium with seven spatulate trichobothria; bulb with one keel, numerous weak striations, without angular constriction; embolus short, twisted (
Figure 48C–E).
Female (paratype). Total length: 11.43; carapace length: 5.49; carapace width: 4.51; abdomen length: 5.94.
Cephalothorax. Carapace as in male (
Figure 50A and Figure 60A). Chelicerae as in male, with nine promarginal teeth and 25 intermarginal denticles (Figure 61A,B); rastellum absent. Labium orange, wider than long, with 77 cuspules; endites orange, with 120 cuspules (Figure 61D); anterior lobe small, serrula present (
Figure 50B and Figure 61E,F). Sternum orange, slightly longer than wide (2.60 length, 2.44 width); three rounded sigilla along margin (
Figure 50B and Figure 61C). Eyes as in male; AME 0.24, ALE 0.28, PLE 0.23, PME 0.15, PME-PME 0.42; ocular quadrangle 1.13 anterior, 1.18 posterior, 0.59 height (Figure 61B).
Abdomen. As in male (
Figure 49A,B); spinnerets: PMS 0.56, PLS 3.47 (Figure 61E,F).
Legs. As in male. Leg tarsi I–II with complete, divided scopulae, metatarsi I–II with 75%, divided scopulae, tarsi III–IV with complete, divided sparse scopulae, metatarsi III–IV with apical sparse scopulae; filiform trichobothria present on all tarsi; spatulate tarsi (9/8/8/7); palpal tarsi with 10 spatulate trichobothria; tarsal organ apical and centrally positioned, low with a few concentric circles (Figure 62F). Leg measurements: I 13.11 (3.79/2.21/3.08/2.23/1.80); II 11.90 (3.48/1.85/2.50/2.21/1.86); III 11.45 (3.40/1.85/1.85/2.77/1.58); IV 14.66 (4.55/2.00/3.06/3.28/1.77); leg formula 4123. STC claw biseriate, with 5–6 teeth, palpal claw without teeth, ITC absent. Leg spination, spines present all segment except tarsi: I fe d1-1-1, pa 0, ti v1-1-1(apical), p1-1, mt v1-1-1(apical); II fe d1-1-1-1-1, pa 0, ti v1-1-1(apical), p1, mt v1-1-1(apical); III fe d1-2-1, pa r1, ti p2-2, r1-2, v1-2-3(apical), mt v2-2-3(apical), p1-1-1, r1-1-1, d2; IV fe d1-1-1, pa r1, ti v2-1-1-1-2(apical), p1-1-1, r1-1-1, mt v2-1-1-3(apical), r1-1-1, p1-1-1, d2-2.
Genitalia. Internal genitalia with unique spermathecae (
Figure 50C–D); margin sinuous, with numerous pores.
Distribution. Only found at the type locality in Pichincha Province.
Natural history. Males and females were collected at 2128 m in an evergreen mountain forest of the Western Cordillera (Figure 66B) (BsMn03) [
36], on the calcareous wall of a thermal spring together with
Linothele yunguilla Dupérré & Tapia, 2023.
3.1.29. Pululahua winku New Species
Type material.
Holotype: ♂ from Cotopaxi Province, San Francisco de las Pampas, OTONGA (−00.41994° −79.00623°) 1997 m, 04-07.ix.2014, sifting litter and Berlese extraction, E. Tapia, N. Dupérré, C. Tapia, ECFN 11240 (QCAZ). Paratypes: same data as holotype, 1♀, hand collected, ECFN 11911 (QCAZ); 2♂, 1♀, Pichicha, Via Tandapi-Sarapullo (−00° 27.206″, −78° 50.768″) 1702 m, 15.viii.2001, collected searching for Lucanidae in epiphytes, I. Tapia, Q. Tapia, ECFN 8773 (QCAZ).
Etymology. The specific epithet is a noun in apposition taken from the Kichwa language meaning twisted, in the reference to the twisted palpal bulb.
Diagnosis. The species most resemble
P. kunukyaku n. sp. but are distinguished by the presence absence of prolateral keel present and pronounced striae and bulb with angular constriction (
Figure 53C–H), while the latter with absence prolateral keel absent, less pronounce striae and bulb without angular constriction, rounded (
Figure 48C–E); females are distinguished by their spermathecae with posterior region of pore plate heart-shaped (
Figure 55A,B), while straight in the latter (
Figure 50C,D).
Description. Male (holotype). Male (holotype). Total length: 8.48; carapace length: 4.22; carapace width: 3.11; abdomen length: 4.26.
Cephalothorax. Carapace oval-shaped, orange-brown, pars cephalica flat, with dark brown markings, pars thoracica slightly sloping with dark gray markings along radiating line; fovea straight (
Figure 51A and
Figure 52A). Chelicerae orange-brown; rastellum absent, with eight promarginal teeth and 22 intermarginal denticles. Labium orange, wider than long, with 80 cuspules; endites orange, with 82 cuspules; anterior lobe small (
Figure 52B). Sternum orange, slightly longer than wide (2.14 length, 1.99 width); with three small rounded sigilla along margin. Eyes, eight in two rows, no tubercle; AME 0.17, ALE 0.23, PLE 0.16, PME 0.11, PME-PME 0.37; ocular rectangular, anterior 0.84, posterior 0.88, height 0.42.
Abdomen. Elongated oval, dorsally light brown covered with setae (
Figure 51A); without urticating setae; ventrally light brown, epigastric region light beige (
Figure 51B); spinnerets, PMS 0.55, PLS 2.41.
Legs. Uniformly orange-brown; tarsi I–II with complete, divided scopulae; metatarsi I–II with 50% apical, divided scopulae; tarsi III with sparse, complete, divided scopulae; metatarsi III with sparse, 50% apical, divided scopulae; tarsi IV with sparse, complete, divided scopulae; metatarsi IV with sparse, 25% apical, divided scopulae. Leg measurements: I 12.21 (3.70/1.70/2.84/2.22/1.75); II 10.68 (2.65/1.57/2.51/2.17/1.78); III 10.63 (2.85/1.35/2.18/2.44/1.81); IV 15.08 (3.79/1.77/3.34/4.13/2.05); leg formula 4123. Leg I with large tibial apophysis with two ramifications, prolateral branch short with large curved megaspine, ventral branch wide with large, thick megaspine, furrow of branches with seven spinules (
Figure 52C,D). Leg spination, spines present all segment except tarsi: I fe d1-1-1-1, pa 0, ti v2-2-1(apical), p1-1, mt v1-2-1(apical), p1; II fe d1-1-1, pa 0, ti v1-1-3(apical), p1, r1, mt v1-2-1(apical), p1; III fe d3-3, pa r1, p1-1, ti v2-2-3, p1-1, r1-1, mt p1-1-1, r1-1-, v2-2-3(apical), d1; IV fe d1-2-2, pa r1, ti d2-2-3, r1-1, p1-1, mt d1, p1-1-1, r1-1, v2-1-2-3(apical). Filiform trichobothria present on tarsi; spatulate trichobothria on tarsi (8/6/7/5). STC with I–II with 3–7; III–IV with 5–6, ITC absent, claw tuft present.
Genitalia. Palpal tibia (2.24 length, 0.96 width) with three prolateral spines (
Figure 53A); cymbium with eight spatulate trichobothria; bulb with two keels, numerous strong striations, with angular constriction; embolus short, twisted (
Figure 53C–H).
Female (paratype). Total length: 8.49; carapace length: 4.17; carapace width: 3.12; abdomen length: 4.32.
Cephalothorax. Carapace as in male (
Figure 54A). Chelicerae as in male, with eight promarginal teeth and 17 intermarginal denticles. Labium orange, wider than long, margin straight, with 84 cuspules; endites orange, with 88 cuspules; anterior lobe small. Sternum orange, longer than wide (2.10 length, 1.57 width); three small rounded sigilla along margin. Eyes, eight in two rows, no tubercle; AME 0.14, ALE 0.21, PLE 0.21, PME 0.1, PME-PME 0.42; ocular rectangle anterior 0.84, posterior 0.90, height 0.43.
Abdomen. As in male (
Figure 54A,B); spinnerets, PMS, 0.36, PLS 2.43.
Legs. As in male; tarsi I–II with complete, divided scopulae; metatarsi I–II with 50% apical, divided scopulae; tarsi III with sparse, complete, divided scopulae; metatarsi III with sparse, 50% apical, divided scopulae; tarsi IV with sparse, complete, divided scopulae; metatarsi IV with sparse, 25% apical, divided scopulae. Leg measurements: I 11.09 (3.02/1.79/2.74/1.80/1.74); II 10.17 (2.89/1.32/2.36/1.87/1.73); III 9.81 (2.71/1.25/1.96/1.90/1.99); IV 13.25 (3.65/1.53/3.12/3.15/1.80); 4123. Leg spination, spines present all segment except tarsi: I fe d1-1-1-1, pa 0, ti v1-1-1(apical), mt v1-1(apical); II fe d1-1-1, pa 0, ti v1-1-1(apical), mt v1-1-1(apical); III fe d1-2-1, pa r1, p1, ti v1-1-2 (apical), p1-1, r1-1, mt p1-1-1, r1-1-1, v2-2-3(apical), d1; IV fe d1-1-2-1, pa r1, ti d1-1, r1, p1, mt d1, p1-1-1, r1-1, v1-1-2(apical). Filiform trichobothria present on tarsi, spatulate trichobothria on tarsi (9/9/9/9); palpal spatulate trichobothria 12. STC biseriate, I–II with 3–6, III–IV with 4–6, palpal claw with 4 teeth.
Genitalia. Internal genitalia with unique spermathecae, anterior margin oval with heart-shaped posterior pore plate region (
Figure 55A,B).
Distribution. Known from Cotopaxi and Pichincha Provinces.
Natural History. Specimens were collected between 1702 and 1997 m in low montane evergreen forest of the western side of the Andes (BsBn04) [
35].
3.1.30. Subfamily Trichopelmatinae Raven, 1985
Diagnosis. Eyes arrangement in two rows (Figure 57A); male with biserially dentate STC; distal article of PLS triangular [
47] (see Mori & Bertani 2020: fig. 106)
Composition in South America. Three genera [
7,
48].
3.1.31. Thalerommata Auserer, 1871
Type species. Thalerommata gracilis Ausserer, 1875.
Diagnosis. See Bertani & Raven 2023 [
48].
Composition. Thalerommata anae Ríos-Tamayo, 2024; T. caudicula (Simon, 1886); T. gertschi Bertani & Raven, 2023; T. gracilis Ausserer, 1875; T. huila Bertani & Raven, 2023; T. kogui Osorio, et al., 2024; T. maculata Bertani & Raven, 2023; T. margarita Osorio, et al., 2024; T. pecki Bertani & Raven, 2023; T. splendens Bertani & Raven, 2023; T. squamea Bertani & Raven, 2023; T. yukpa Osorio, et al., 2024.
Distribution. Cuba, Bahamas, Jamaica, Venezuela, Colombia, Ecuador, and Argentina.
Note.
Thalerommata was recently transferred from Barychelidae to Theraphosidae by Bertani & Raven (2023) [
48]. The authors considered the genus closely related to
Trichopelma, subfamily Trichopelmatinae.
3.1.32. Thalerommata yasuni New Species
Type material.
Holotype: ♂ from Ecuador, Orellana, Yasuni National Park, PUCE scientific station (−00.672376° −76.398725°), 218 m, 22–25 July 2024, pitfall, E. Tapia, N. Dupérré, A. Tapia, ECFN 11730 (QCAZ). Paratypes: same data as holotype, 1♂, ECFN 11912 (QCAZ), 1♂, (ZMH-A0029194).
Etymology. The specific epithet is taken from the locality.
Diagnosis. Males most resemble
T. huila,
T. margarita, and
T. squamea but are distinguished from
T. huila by their gradually curving embolus (
Figure 58C–E), whereas embolus sinuous in the latter [
48] (see Bertani & Raven 2023: figs 41, 42); from
T. margarita by their straight metatarsus I without distal bulge (
Figure 57C), whereas metatarsus I with distal bulge in the latter [
49] (see Osorio et al., 2024: fig 9D,E); and from
T. squamea by their tibial apophysis without upper process (
Figure 57C), whereas tibial apophysis with short upper process present in
T. squamea [
48] (see Bertani & Raven 2023: fig. 23).
Description. Male (holotype). Total length: 4.18; carapace length: 2.04; carapace width: 1.72; abdomen length: 2.14.
Cephalothorax. Carapace oval-shaped, orange-brown, pars cephalica flat, pars thoracica gradually sloping; fovea straight (
Figure 56A and
Figure 57A). Chelicerae orange-brown, rastellum absent, 11 promarginal teeth and 12 intercheliceral denticles. Labium orange, margin V-shaped, with 7 cuspules; endites orange, with 12 cuspules; anterior lobe not well-developed (
Figure 57B). Sternum orange, slightly longer than wide (1.12 length, 0.98 width); three pairs of small, marginal sigilla (
Figure 57B). Eyes, eight in two rows on small tubercle; AME 0.08, ALE 0.12, PLE 0.09, PME 0.06, PME-PME 0.18; ocular rectangular 0.43 anterior, 0.44 posterior, 0.23 height.
Abdomen. Elongated oval, dorsally and ventrally light brown covered with flattened setae (
Figure 56A,B); spinnerets: PMS 0.14, PLS 0.58.
Legs. Uniformly light brown; scopulae sparse on tarsi I–II, absent on III–IV, all metatarsi ascopulate. Tibial apophysis without upper process with single sinuous, curved spine prolaterally (
Figure 57C). Leg measurements: I 5.17 (1.49/0.71/1.31/0.92/0.74); II 4.63 (1.27/0.73/0.97/0.92/0.74); III 4.49 (1.22/0.57/0.84/1.12/0.74); IV 6.70 (1.69/0.73/1.55/1.80/0.93); leg formula 4123. Leg spination, spine present all segments except tarsi: I fe d1-1-1, p0-0-1, pa 0, ti v1-2-1(apical), p1 (plus clasping spur), r1, mt v1-3(apical), p1; II fe d1-1, pa 0, tib v1-1-3(apical), p1-1, mt v1-1-3(apical), p1; III fe d1-2, pat d1, ti p1-1, r1-1, v2-1-3(apical), mt v3-2-3(apical), p1-1-1, r1-1; IV fe d1-1-1-2-1, pa 1p, ti v2-2-3(apical), p1-1-1, r1-1-1, mt d1-1, p1-1, r1-1, v2-2-3(apical). Clavate trichobothria on tarsi in two rows: 5/5/5/6, cymbium: 4. STC biseriate, legs I–II with 5–6 teeth, leg III with 3–4 teeth, leg IV with 2–3; ITC absent; claw tufts present.
Genitalia. Palpal tibia (0.82 length, 0.42 width) with 5 prolateral spines and 1 retrolateral spine (
Figure 58A,B); cymbium with 4–5 spines apically (
Figure 58A,B); bulb pyriform with embolus tapered in middle portion, gradually curving (
Figure 58C–E).
Female. Unknown.
Distribution. Only known from the type locality.
Natural History. Males were collected by pitfall trap at 218 m in Amazonian region, in a lowland evergreen forest of the Napo–Curaray (BsTa02) [
38].
Figure 59.
SEM. (A) Strophaeus pacificanus n. sp., rastellum. (B) Strophaeus pacificanus n. sp., endites. (C) Strophaeus peronii n. sp., tarsus I. (D) Strophaeus peronii n. sp. tarsal organ I.
Figure 59.
SEM. (A) Strophaeus pacificanus n. sp., rastellum. (B) Strophaeus pacificanus n. sp., endites. (C) Strophaeus peronii n. sp., tarsus I. (D) Strophaeus peronii n. sp. tarsal organ I.
Figure 60.
SEM. Pululahua kunukyaku n. sp., female. (A) Carapace, dorsal view. (B) Ocular region, dorsal view. (C) Sternum, ventral view. (D) Endites and labium, ventral view. (E) Endites, dorsal view. (F) Serrula, dorsal view.
Figure 60.
SEM. Pululahua kunukyaku n. sp., female. (A) Carapace, dorsal view. (B) Ocular region, dorsal view. (C) Sternum, ventral view. (D) Endites and labium, ventral view. (E) Endites, dorsal view. (F) Serrula, dorsal view.
Figure 61.
SEM. Pululahua kunukyaku n. sp., female. (A) Chelicerae, internal view. (B) Chelicerae denticles. (C) Epigastric region, ventral view. (D) Abdominal setae dorsal view. (E) PLS, lateral view. (F) Apical segment of PLS, lateral view.
Figure 61.
SEM. Pululahua kunukyaku n. sp., female. (A) Chelicerae, internal view. (B) Chelicerae denticles. (C) Epigastric region, ventral view. (D) Abdominal setae dorsal view. (E) PLS, lateral view. (F) Apical segment of PLS, lateral view.
Figure 62.
SEM. Pululahua kunukyaku n. sp., female. (A) Tarsus I, dorsal view. (B) Tarsal organ leg I, dorsal view. (C) Spatulate trichobothria tarsus IV, dorsal view. (D) Tarsus III, dorsal view. (E) Tarsus IV, dorsal view. (F) Tarsal organ leg IV, dorsal view.
Figure 62.
SEM. Pululahua kunukyaku n. sp., female. (A) Tarsus I, dorsal view. (B) Tarsal organ leg I, dorsal view. (C) Spatulate trichobothria tarsus IV, dorsal view. (D) Tarsus III, dorsal view. (E) Tarsus IV, dorsal view. (F) Tarsal organ leg IV, dorsal view.
Figure 63.
Distribution map of new species of Actinopodidae and Barychelidae in Ecuador. Actinopus saraguro n. sp. (green circle), Paracenobiopelma vesca n. sp. (orange triangle), Strophaeus pacificanus n. sp. (turquoise circle), Strophaeus elongata n. sp. (purple circle), Strophaeus josefita n. sp. (yellow star), Strophaeus peronii n. sp. (dark blue circle), Strophaeus kaiae n. sp. (red star), Strophaeus real (red square), Strophaeus subterraneus n. sp. (red triangle), Strophaeus spiculum n. sp. (black star), and Strophaeus kawsay n. sp. (yellow circle).
Figure 63.
Distribution map of new species of Actinopodidae and Barychelidae in Ecuador. Actinopus saraguro n. sp. (green circle), Paracenobiopelma vesca n. sp. (orange triangle), Strophaeus pacificanus n. sp. (turquoise circle), Strophaeus elongata n. sp. (purple circle), Strophaeus josefita n. sp. (yellow star), Strophaeus peronii n. sp. (dark blue circle), Strophaeus kaiae n. sp. (red star), Strophaeus real (red square), Strophaeus subterraneus n. sp. (red triangle), Strophaeus spiculum n. sp. (black star), and Strophaeus kawsay n. sp. (yellow circle).
Figure 64.
Distribution map of new species of Halonoproctidae, Idiopidae, and Theraphosidae in Ecuador. Ummidia pupulae n. sp. (turquoise circle), Idiops clepsydra n. sp. (yellow star), Pululahua kunukyaku n. sp. (purple circle), Pululahua winku n. sp. (red triangle), and Thalerommata yasuni n. sp. (green circle).
Figure 64.
Distribution map of new species of Halonoproctidae, Idiopidae, and Theraphosidae in Ecuador. Ummidia pupulae n. sp. (turquoise circle), Idiops clepsydra n. sp. (yellow star), Pululahua kunukyaku n. sp. (purple circle), Pululahua winku n. sp. (red triangle), and Thalerommata yasuni n. sp. (green circle).
Figure 65.
Habitats. (A) Type locality of Strophaeus pacificanus n. sp. (B) Type locality of Strophaeus elongata n. sp.
Figure 65.
Habitats. (A) Type locality of Strophaeus pacificanus n. sp. (B) Type locality of Strophaeus elongata n. sp.
Figure 66.
(A) Type locality of Strophaeus josefita n. sp., OTONGA reserve. (B) Type locality of Pululahua kunukyaku n. sp., Termas de Pululahua.
Figure 66.
(A) Type locality of Strophaeus josefita n. sp., OTONGA reserve. (B) Type locality of Pululahua kunukyaku n. sp., Termas de Pululahua.