Scale Sensilla in the Snakes of the Genus Natrix, and in the Old and New World Natricids
Abstract
1. Introduction
2. Materials and Methods
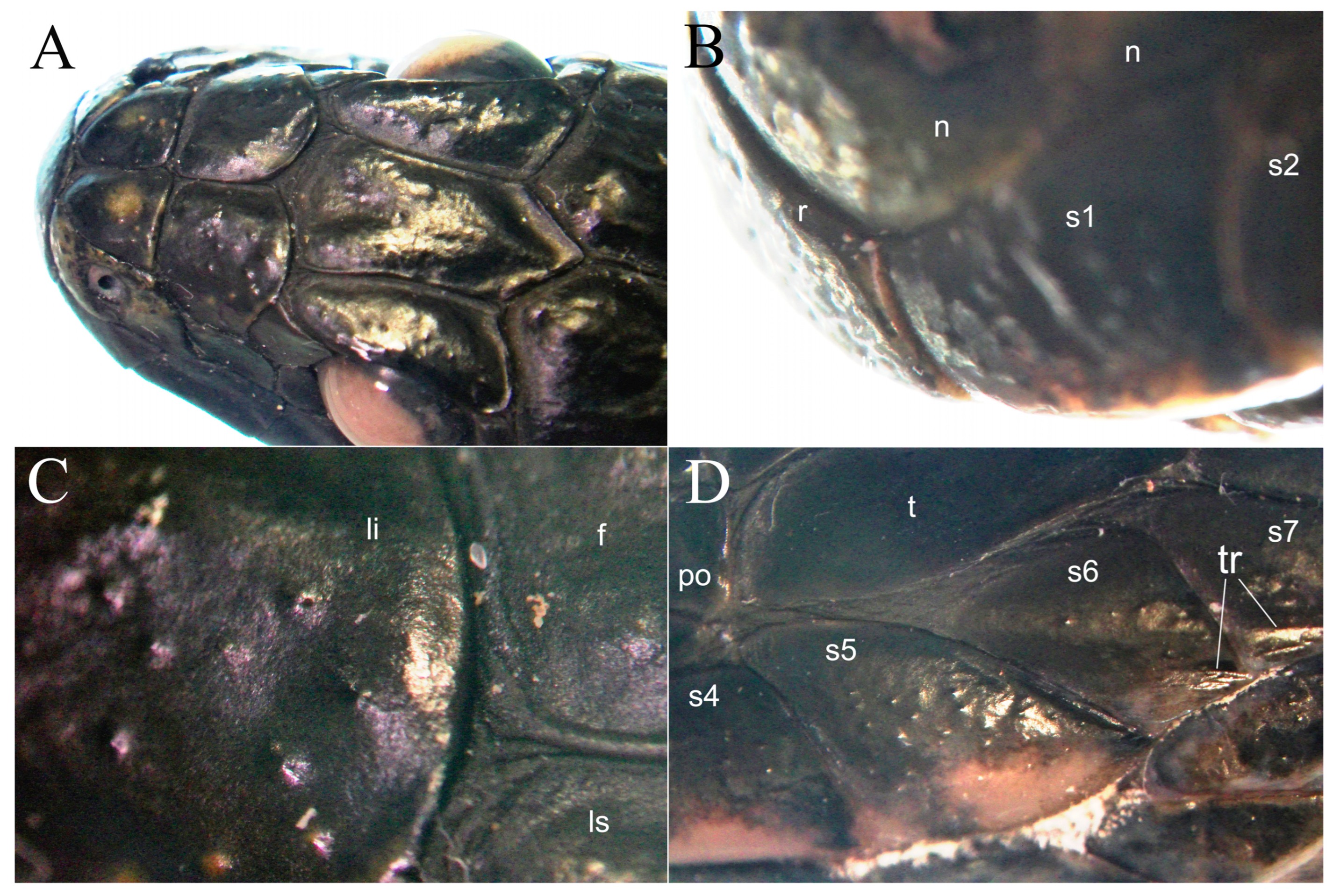
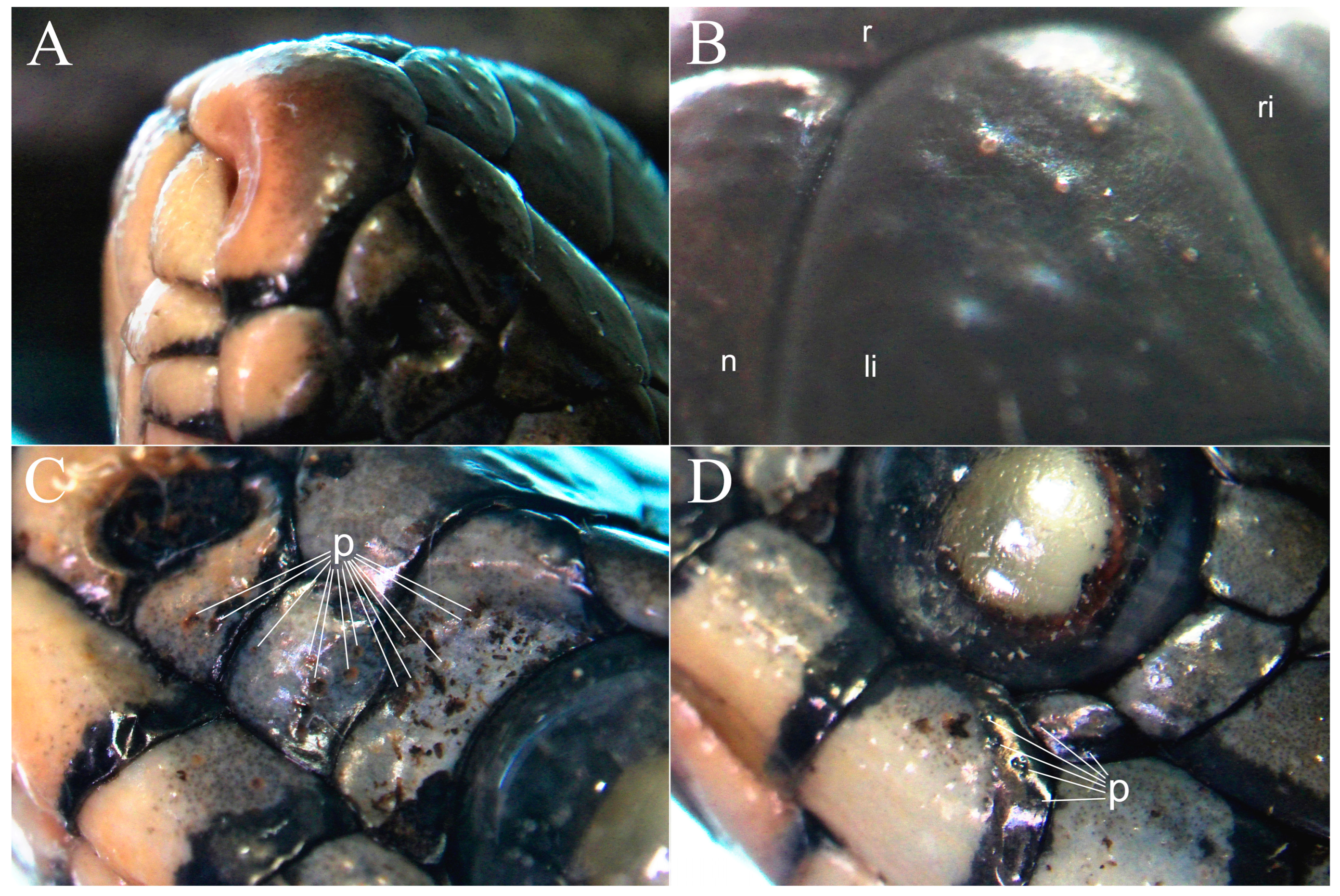
3. Results
4. Discussion
4.1. Protruted Scale Sensilla in European Natricids
4.2. Preliminary Comparative Examination in Extra-European Natricids
4.3. Protruded Scale Sensilla in Non-Aquatic Snakes
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, M.K. Histology and distribution of cutaneous touch corpuscles in some Leptotyphlopid and colubrid snakes (Reptilia, Serpentes). J. Herpetol. 1977, 11, 7–15. [Google Scholar] [CrossRef]
- Jackson, M.K.; Sharawy, M. Scanning electron microscopy and distribution of specialized mechanoreceptors in the Texas rat snake, Elaphe obsoleta lindheimeri (Baird and Girard). J. Morphol. 1980, 163, 59–67. [Google Scholar] [CrossRef]
- Ananjeva, N.B.; Dilmuchamedov, M.E.; Matveyeva, T.N. The skin sense organs of some iguanian lizards. J. Herpetol. 1991, 25, 186–199. [Google Scholar] [CrossRef]
- Bucklitsch, Y.; Böhme, W.; Koch, A. Scanning electron micros copy (SEM) of monitor Lizards’ scale ultrastructure: Systematic implications. Biawak 2012, 6, 57–60. [Google Scholar]
- Gans, C. A Taxonomic Revision of the African Snake Genus Dasypeltis (Reptilia: Serpentes); Carnegie Museum: Tervuren, Belgium; Pittsburg, PA, USA, 1959. [Google Scholar]
- Keathley, V.L. Tactile Discrimination in Three Species of Garter Snake (Thamnophis); University of Texas at Arlington: Arlington, TX, USA, 2004. [Google Scholar]
- Crowe-Riddell, J.M.; Williams, R.; Chapuis, L.; Sanders, K.L. Ultrastructural evidence of a mechanosensory function of scale organs (sensilla) in sea snakes (Hydrophiinae). Roy. Soc. Open Sci. 2019, 6, 182022. [Google Scholar] [CrossRef] [PubMed]
- García-Cobos, D.; Gómez-Sánchez, D.A.; Crowe-Riddell, J.M.; Sanders, K.L.; Molina, J. Ecological and sexual roles of scale mechanoreceptors in two species of Neotropical freshwater snake (Dipsadinae: Helicops). Biol. J. Linn. Soc. 2021, 134, 958–974. [Google Scholar] [CrossRef]
- Noble, G.K. The sense organs involved in the courtship of Storeria, Thamnophis and other snakes. Bull. Am. Mus. Nat. Hist. 1937, 73, 673–725. [Google Scholar]
- Velasquez-Cañon, V.; Bravo-Vega, C.; Galeano, S.P.; Molina, J.; Salazar-Guzman, A.M.; García-Cobos, D. The mechanosensory world in aquatic snakes: Corporal scale sensilla in three species of Neotropical freshwater dipsadine. Front. Amphib. Reptile Sci. 2024, 2, 1412004. [Google Scholar] [CrossRef]
- Crowe-Riddell, J.M.; Snelling, E.P.; Watson, A.P.; Suh, A.K.; Partridge, J.C.; Sanders, K.L. The evolution of scale sensilla in the transition from land to sea in elapid snakes. Open Biol. 2016, 6, 160054. [Google Scholar] [CrossRef]
- Lillywhite, H.B.; Menon, G.K. Structure and function of skin in the pelagic sea snake, Hydrophis platurus. J. Morphol. 2019, 280, 544–554. [Google Scholar] [CrossRef]
- García-Cobos, D.; Vásquez-Restrepo, J.D. Notes on the distribution, morphological variation, and mechanoreceptors of Tretanorhinus nigroluteus (Serpentes: Dipsadidae) in Colombia. Biota Colomb. 2022, 23, e943. [Google Scholar] [CrossRef]
- Wagner, A.; Johnson, C.; Hoon Ha, M.; Sanders, K.L.; Collin, S.P.; Crowe-Riddell, J.M. Morphology and distribution of a new scale mechanoreceptor type in olive-headed sea snakes (Hydrophis major). BioRxviv 2025. preprint. [Google Scholar] [CrossRef]
- Udyawer, V.; Barnes, P.; Bonnet, X.; Brischoux, F.; Crowe-Riddell, J.M.; D’Anastasi, B.; Fry, B.G.; Gillett, A.; Goiran, C.; Guinea, M.L.; et al. Future Directions in the Research and Management of Marine Snakes. Front. Mar. Sci. 2018, 5, 399. [Google Scholar] [CrossRef]
- Schmidt, W. Studien am Integument der Reptilien. VII. Über die Haut der Acrochordinen. Zool. Jahrb. Allg. Zool. 1918, 40, 155–202. [Google Scholar]
- Povel, D.; Van der Kooij, J. Scale sensillae of the file snake (Serpentes: Acrochordidae) and some other aquatic and burrowing snakes. Neth. J. Zool. 1997, 47, 443–456. [Google Scholar] [CrossRef]
- Guicking, D.; Lawson, R.; Joger, U.; Wink, M. Evolution and phylogeny of the genus Natrix (Serpentes: Colubridae). Biol. J. Linn. Soc. 2006, 87, 127–143. [Google Scholar] [CrossRef]
- Kindler, C.; de Pous, P.; Carranza, S.; Beddek, M.; Geniez, P.; Fritz, U. Phylogeography of the Ibero-Maghrebian red-eyed grass snake (Natrix astreptophora). Org. Divers. Evol. 2018, 18, 143–150. [Google Scholar] [CrossRef]
- Figueroa, A.; McKelvy, A.D.; Grismer, L.L.; Bell, C.D.; Lailvaux, S.P. A species-level phylogeny of extant snakes with description of a new colubrid subfamily and genus. PLoS ONE 2016, 11, e0161070. [Google Scholar] [CrossRef]
- von Düring, M. The Ultrastructure of Lamellated Mechanoreceptors in the Skin of Reptiles. Z. Anat. Entwicklungs. 1973, 143, 81–94. [Google Scholar] [CrossRef]
- von Düring, M.; Miller, M. Sensory nerve endings of the skin and deeper structures. In Biology of the Reptilia; Gans, C., Ed.; Academic Press: New York, NY, USA, 1979; pp. 407–441. [Google Scholar]
- Jackson, M.K.; Butler, D.G.; Youson, J.H. Morphology and ultrastructure of possible integumentary sense organs in the estuarine crocodile (Crocodylus porosus). J. Morphol. 1996, 229, 315–324. [Google Scholar] [CrossRef]
- Soares, D. An ancient sensory organ in crocodilians. Nature 2002, 417, 241. [Google Scholar] [CrossRef] [PubMed]
- Leitch, D.B.; Catania, K.C. Structure, innervation and response properties of integumentary sensory organs in crocodilians. The J. Exp. Biol. 2012, 215, 4217–4230. [Google Scholar] [CrossRef] [PubMed]
- Deepak, V.; Cooper, N.; Poyarkov, N.A.; Kraus, F.; Burin, G.; Das, A.; Narayanan, S.; Streicher, J.W.; Smith, S.J.; Gower, D.J. Multilocus phylogeny, natural history traits and classification of natricine snakes (Serpentes: Natricinae). Zool. J. Linn. Soc.-Lond. 2021, 195, 279–298. [Google Scholar] [CrossRef]
- Paterna, A. Reproduction cycle of the Eastern egg-eater snake Dasypeltis medici medici (Bianconi, 1859) in captivity. Russ. J. Herpetol. 2017, 24, 228–234. [Google Scholar] [CrossRef]
- Young, B.A.; Wallach, V. Description of a papillate tactile organ in the Typhlopidae. S. Afr. J. Zool. 1998, 33, 249–253. [Google Scholar] [CrossRef]



| Continent | Genus | Prot. Sensilla | In Vivo Exam. | Photogr. Exam. |
|---|---|---|---|---|
| Asia | Amphiesma | 1 | • | |
| Asia | Aspidura | • | ||
| Asia | Atretium | 1 | • | |
| Asia | Hebius | 1, 2 | • | |
| Asia | Herpetoreas | 1 | • | |
| Asia | Opisthotropis | 1, 2 | • | |
| Asia | Pseudagkistrodon | 2, 3 | • | • |
| Asia | Rhabdophis | 2, 3 | • | • |
| Asia | Sahyadriophis | 2 | • | |
| Asia | Trachischium | • | ||
| Asia | Trimerodytes | 1, 2 | • | |
| Asia | Xenochrophis | 1 | • | |
| Africa | Afronatrix | 1, 2 | • | |
| Africa | Helophis | • | ||
| Africa | Hydraethiops | 1 | • | |
| Africa | Limnophis | 1 | • | |
| Africa | Lycognathophis | 1 | • | |
| Africa | Natriciteres | 1, 2 | • | |
| Europe * | Natrix | 2, 3 | • | • |
| America | Clonophis | • | ||
| America | Liodytes | 1 | • | |
| America | Nerodia | 2, 3 | • | • |
| America | Regina | 1 | • | |
| America | Storeria | • | ||
| America | Thamnophis | 1, 2 | • | • |
| America | Tropidoclonion | • | ||
| America | Virginia | • |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paterna, A. Scale Sensilla in the Snakes of the Genus Natrix, and in the Old and New World Natricids. Taxonomy 2025, 5, 34. https://doi.org/10.3390/taxonomy5030034
Paterna A. Scale Sensilla in the Snakes of the Genus Natrix, and in the Old and New World Natricids. Taxonomy. 2025; 5(3):34. https://doi.org/10.3390/taxonomy5030034
Chicago/Turabian StylePaterna, Alessandro. 2025. "Scale Sensilla in the Snakes of the Genus Natrix, and in the Old and New World Natricids" Taxonomy 5, no. 3: 34. https://doi.org/10.3390/taxonomy5030034
APA StylePaterna, A. (2025). Scale Sensilla in the Snakes of the Genus Natrix, and in the Old and New World Natricids. Taxonomy, 5(3), 34. https://doi.org/10.3390/taxonomy5030034







