Two New Species of Miniature Tetras of the Genus Priocharax (Teleostei: Characiformes: Acestrorhamphidae) from the Rio Purus and Solimões Drainages, Amazonas, Brazil †
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological Analysis
2.2. Molecular Analysis
3. Results
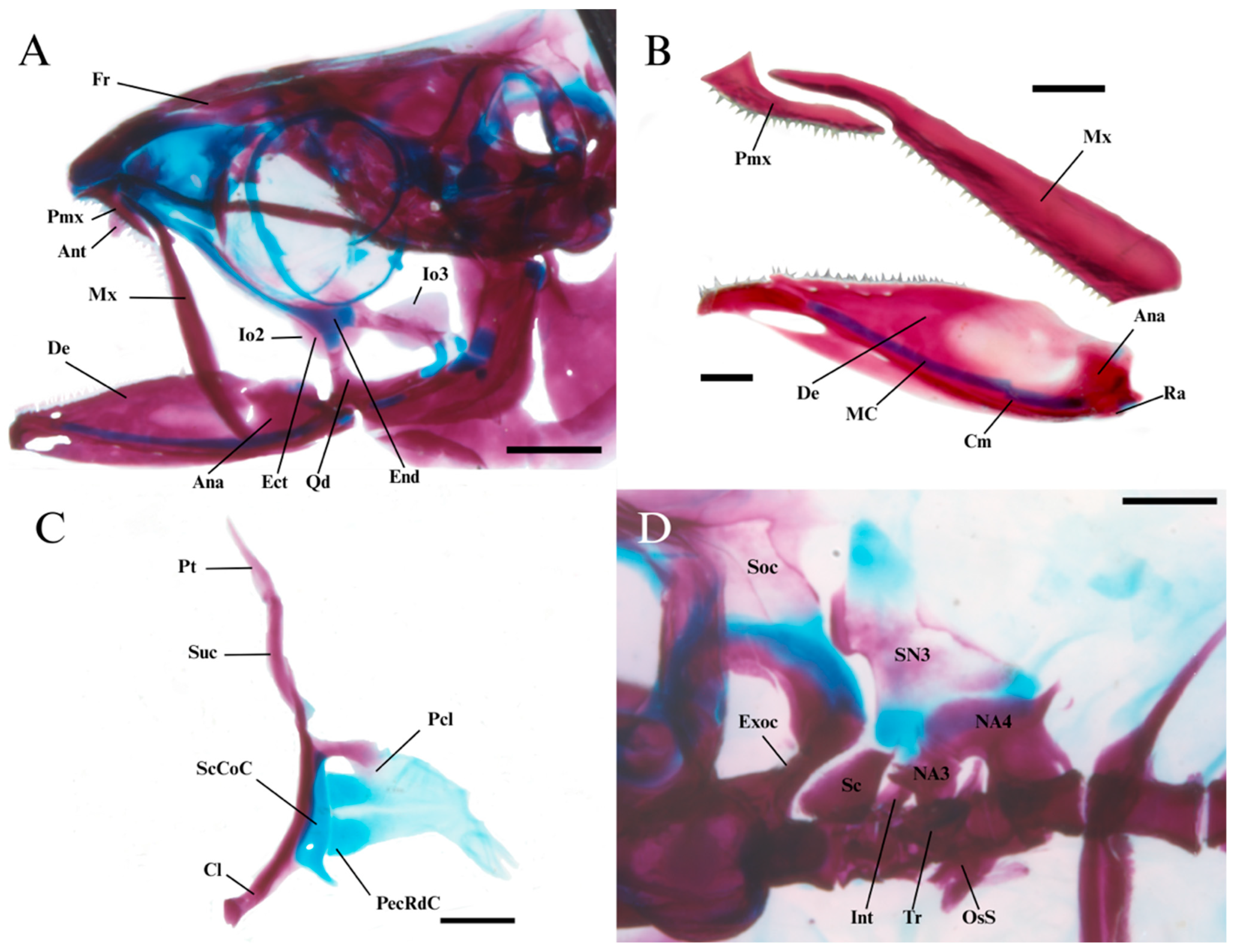
3.1. Species Description
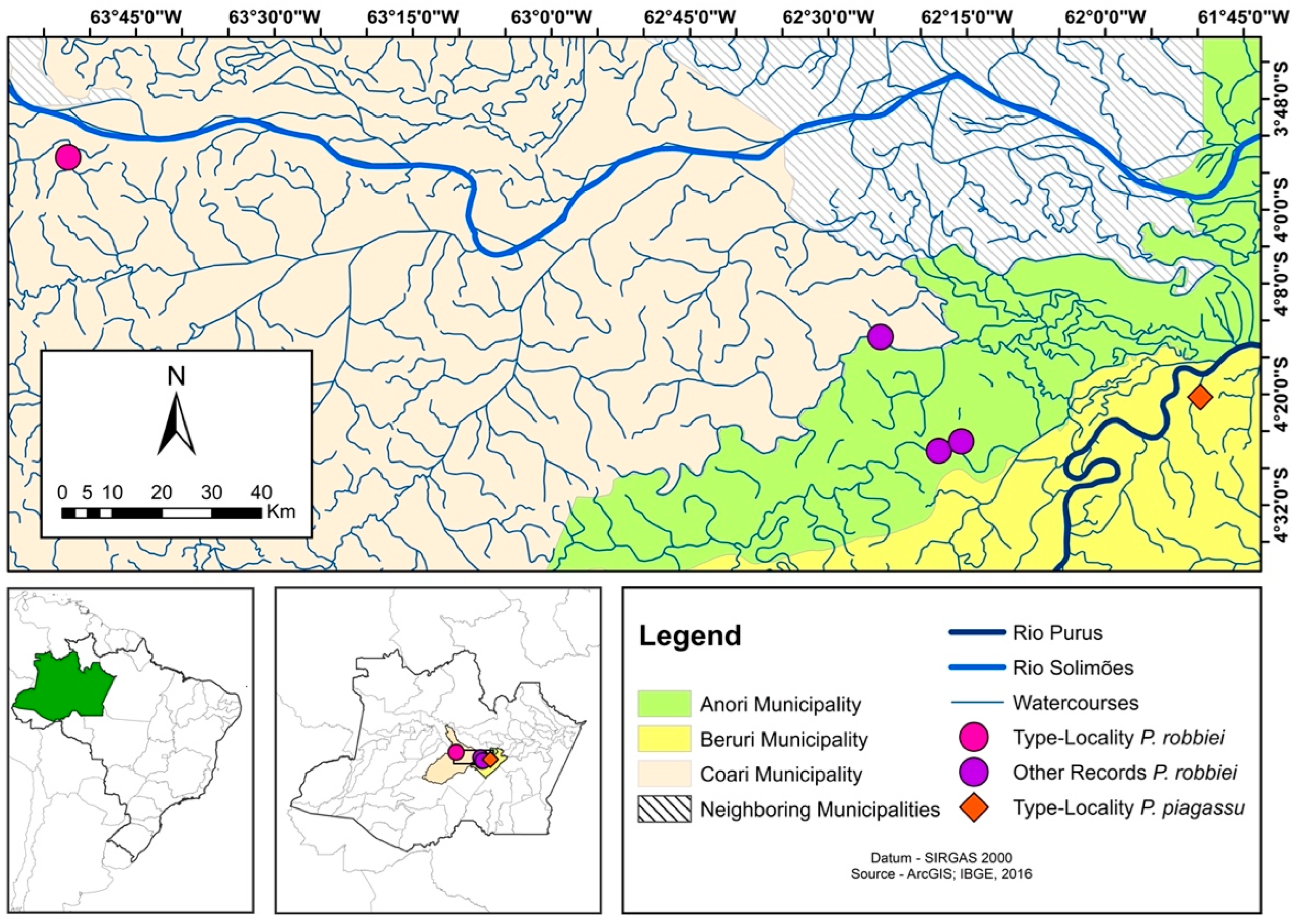
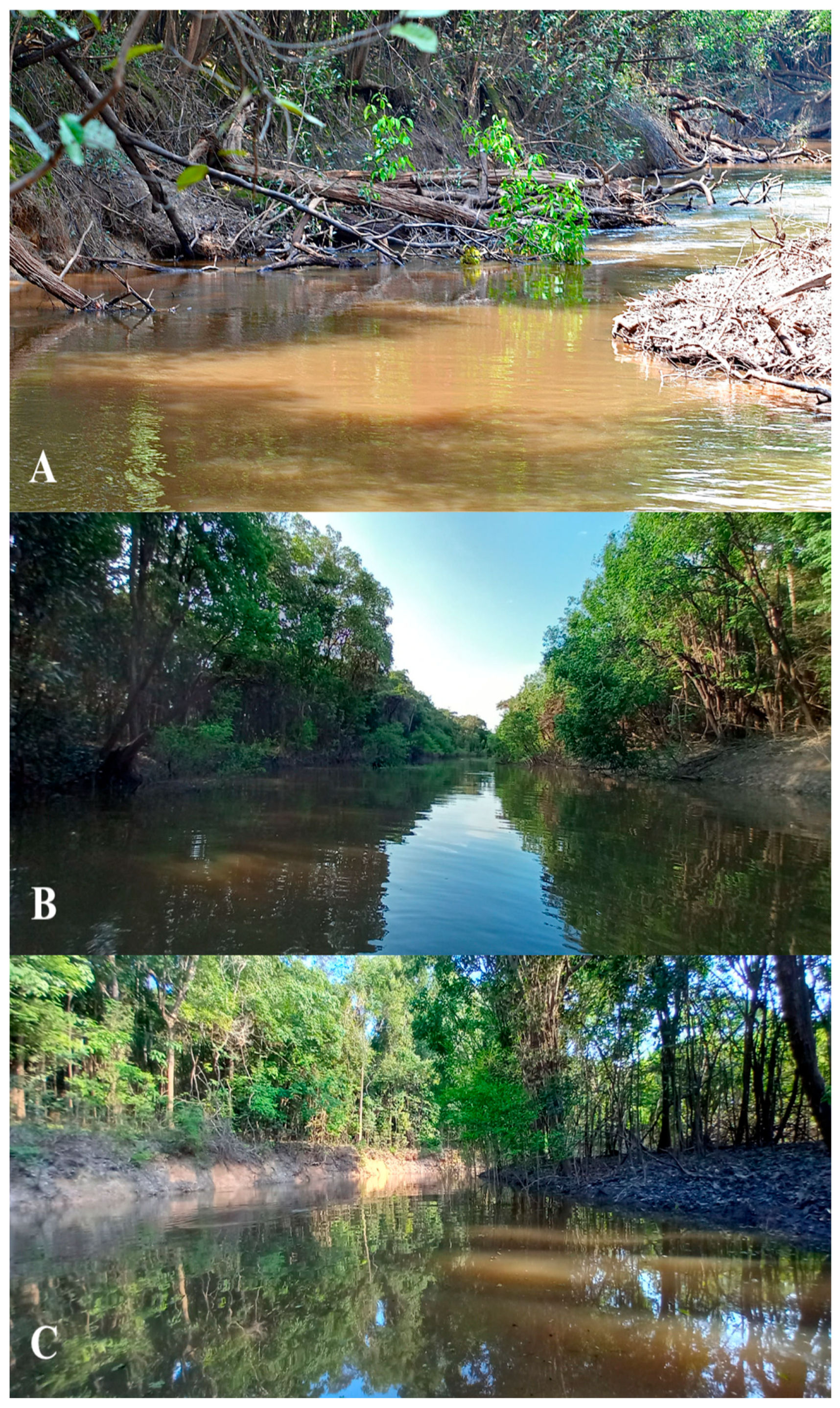
3.1.1. Priocharax robbiei sp. nov.
3.1.2. Priocharax piagassu sp. nov.
3.2. PCA and LDA
3.3. Molecular Data Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Lot | Voucher | Basin | Locality | Coordinates | GenBank n. |
|---|---|---|---|---|---|---|
| Priocharax robbiei | LBP 36836 | 114736 | Rio Solimões | “Igarapezinho” upstream from the São Sebastião da Água Branca community, a tributary of the Água Branca stream, affluent of Ipixuna stream, São Francisco, Coari, AM, Brazil | 3°54′20.7″ S 63°52′20.5″ W | PV698181 |
| Priocharax robbiei | LBP 36836 | 114737 | Rio Solimões | “Igarapezinho” upstream from the São Sebastião da Água Branca community, a tributary of the Água Branca stream, affluent of Ipixuna stream, São Francisco, Coari, AM, Brazil | 3°54′20.7″ S 63°52′20.5″ W | PV698181 |
| Priocharax piagassu | LBP 31205 | 108403 | Rio Purus | Lago Xaviana, Rio Purus, Beruri, AM, Brazil | 4°20′18.70″ S 61°49′43.20″ W | PV698179 |
| Priocharax piagassu | LBP 31205 | 108404 | Rio Purus | Lago Xaviana, Rio Purus, Beruri, AM, Brazil | 4°20′18.70″ S 61°49′43.20″ W | PV698180 |
| Priocharax conwayi | LBP 31740 | 108405 | Rio Tapajós | Igarapé do Henrique, Rio Maró, affluent of Rio Arapiuns, Santarém, PA, Brazil | 02°41′9.30″ S 55°40′32.97″ W | PP902468 |
| Priocharax conwayi | LBP 31740 | 108406 | Rio Tapajós | Igarapé do Henrique, Rio Maró, affluent of Rio Arapiuns, Santarém, PA, Brazil | 02°41′9.30″ S 55°40′32.97″ W | PP902469 |
| Priocharax conwayi | LBP 31740 | 108407 | Rio Tapajós | Igarapé do Henrique, Rio Maró, affluent of Rio Arapiuns, Santarém, PA, Brazil | 02°41′9.30″ S 55°40′32.97″ W | PP902470 |
| Priocharax conwayi | LBP 31741 | 108408 | Rio Tapajós | Igarapé do Henrique, Rio Maró, affluent of Rio Arapiuns, Santarém, PA, Brazil | 02°41′9.30″ S 55°40′32.97″ W | PP902471 |
| Priocharax conwayi | LBP 31741 | 108409 | Rio Tapajós | Rio Mentaí, in the vicinity of the community at mouth of Rio Mentaí, affluent of Rio Arapiuns, Santarém, PA, Brazil | 02°37′52.51″ S 55°34′40.98″ W | PP902472 |
| Priocharax conwayi | LBP 31741 | 108410 | Rio Tapajós | Rio Mentaí, in the vicinity of the community at mouth of Rio Mentaí, affluent of Rio Arapiuns, Santarém, PA, Brazil | 02°37′52.51″ S 55°34′40.98″ W | PP902473 |
| Priocharax conwayi | LBP 31741 | 108411 | Rio Tapajós | Rio Mentaí, in the vicinity of the community at mouth of Rio Mentaí, affluent of Rio Arapiuns, Santarém, PA, Brazil | 02°37′52.51″ S 55°34′40.98″ W | PP902474 |
| Priocharax conwayi | LBP 31741 | 108412 | Rio Tapajós | Rio Mentaí, in the vicinity of the community at mouth of Rio Mentaí, affluent of Rio Arapiuns, Santarém, PA, Brazil | 02°37′52.51″ S 55°34′40.98″ W | PP902475 |
| Priocharax phasma | LBP 31742 | 108418 | Rio Amazonas | Lago Santana, Ilha de Marimarituba, Rio Amazonas, Santarém, PA, Brazil | 02°11′12.31″ S 55°02′12.00″ W | PP902476 |
| Priocharax phasma | LBP 31742 | 108419 | Rio Amazonas | Lago Santana, Ilha de Marimarituba, Rio Amazonas, Santarém, PA, Brazil | 02°11′12.31″ S 55°02′12.00″ W | PP902477 |
| Priocharax phasma | LBP 31742 | 108420 | Rio Amazonas | Lago Santana, Ilha de Marimarituba, Rio Amazonas, Santarém, PA, Brazil | 02°11′12.31″ S 55°02′12.00″ W | PP902478 |
| Priocharax phasma | LBP 31742 | 108421 | Rio Amazonas | Lago Santana, Ilha de Marimarituba, Rio Amazonas, Santarém, PA, Brazil | 02°11′12.31″ S 55°02′12.00″ W | PP902479 |
| Priocharax phasma | LBP 31743 | 108428 | Rio Amazonas | Lago Pajaú, Ilha Nazareth, Rio Amazonas, Santarém, PA, Brazil | 02°11′28.53″ S 54°51′27.93″ W | PP902480 |
| Priocharax phasma | LBP 31743 | 108429 | Rio Amazonas | Lago Pajaú, Ilha Nazareth, Rio Amazonas, Santarém, PA, Brazil | 02°11′28.53″ S 54°51′27.93″ W | PP902481 |
| Priocharax phasma | LBP 31743 | 108430 | Rio Amazonas | Lago Pajaú, Ilha Nazareth, Rio Amazonas, Santarém, PA, Brazil | 02°11′28.53″ S 54°51′27.93″ W | PP902482 |
| Priocharax toledopizae | LBP 31744 | 108432 | Rio Juruá | Igarapé Preto, tributary of Rio Moa, Cruzeiro do Sul, AC, Brazil | 07°35′11″ S 72°45′20″ W | OP257279 |
| Priocharax toledopizae | LBP 31744 | 108435 | Rio Juruá | Igarapé Preto, tributary of Rio Moa, Cruzeiro do Sul, AC, Brazil | 07°35′11″ S 72°45′20″ W | OP257280 |
| Priocharax toledopizae | LBP 31745 | 108439 | Rio Juruá | Balneário at Igarapé Preto near road BR-307, Cruzeiro do Sul, AC, Brazil | 07°35′45″ S 72°45′16″ W | OP257278 |
| Priocharax toledopizae | LBP 31745 | 108440 | Rio Juruá | Balneário at Igarapé Preto near road BR-307, Cruzeiro do Sul, AC, Brazil | 07°35′45″ S 72°45′16″ W | OP257282 |
| Priocharax toledopizae | LBP 31745 | 108441 | Rio Juruá | Balneário at Igarapé Preto near road BR-307, Cruzeiro do Sul, AC, Brazil | 07°35′45″ S 72°45′16″ W | OP257281 |
| Priocharax toledopizae | LBP 31746 | 108443 | Rio Juruá | Igarapé das Piabas, tributary of Rio Moa, Cruzeiro do Sul, AC, Brazil | 07°31′15″ S 72°53′48″ W | OP257283 |
| Priocharax marupiara | LBP 31747 | 108445 | Rio Juruá | Igarapé Canela Fina, Cruzeiro do Sul, AC, Brazil | 07°34′02″ S 72°39′40″ W | OP257285 |
| Priocharax marupiara | LBP 31747 | 108446 | Rio Juruá | Igarapé Canela Fina, Cruzeiro do Sul, AC, Brazil | 07°34′02″ S 72°39′40″ W | OP257286 |
| Priocharax marupiara | LBP 31747 | 108447 | Rio Juruá | Igarapé Canela Fina, Cruzeiro do Sul, AC, Brazil | 07°34′02″ S 72°39′40″ W | OP257284 |
| Priocharax varii | LBP 28495 | 96981 | Rio Madeira | Rio Preto, affluent of Rio Jamari, Candeias do Jamari, RO, Brazil | 08°52′53.5″ S 63°37′50.8″ W | MT754786 |
| Priocharax varii | LBP 28495 | 96982 | Rio Madeira | Rio Preto, affluent of Rio Jamari, Candeias do Jamari, RO, Brazil | 08°52′53.5″ S 63°37′50.8″ W | MT754785 |
| Priocharax varii | LBP 28495 | 96984 | Rio Madeira | Rio Preto, affluent of Rio Jamari, Candeias do Jamari, RO, Brazil | 08°52′53.5″ S 63°37′50.8″ W | MT754783 |
| Priocharax varii | LBP 28495 | 96985 | Rio Madeira | Rio Preto, affluent of Rio Jamari, Candeias do Jamari, RO, Brazil | 08°52′53.5″ S 63°37′50.8″ W | MT754784 |
| Priocharax ariel | LBP 28442 | 98284 | Rio Negro | Igarapé Tibarrá on left side of Rio Negro, Santa Isabel do Rio Negro, AM, Brazil | 00°26′28.1″ S 64°56′57.5″ W | MT754780 |
| Priocharax ariel | LBP 28442 | 98285 | Rio Negro | Igarapé Tibarrá on left side of Rio Negro, Santa Isabel do Rio Negro, AM, Brazil | 00°26′28.1″ S 64°56′57.5″ W | MT754781 |
| Priocharax ariel | LBP 27704 | 98286 | Rio Negro | Igarapé Tapage, Rio Urubaxi, approximately 1 h from mouth of river, S. I. Rio Negro, AM, Brazil | 00°30′05.3″ S 64°49′11.7″ W | MT754778 |
| Priocharax ariel | LBP 27704 | 98287 | Rio Negro | Igarapé Tapage, Rio Urubaxi, approximately 1 h from mouth of river, S. I. Rio Negro, AM, Brazil | 00°30′05.3″ S 64°49′11.7″ W | MT754782 |
| Priocharax ariel | LBP 27704 | 98288 | Rio Negro | Igarapé Tapage, Rio Urubaxi, approximately 1 h from mouth of river, S. I. Rio Negro, AM, Brazil | 00°30′05.3″ S 64°49′11.7″ W | MT754779 |
| Priocharax ariel | LBP 25858 | 96383 | Rio Negro | Igarapé Uacatuna, São Gabriel da Cachoeira, AM, Brazil | 00°03′38.0″ S 67°05′45.0″ W | MT754777 |
| Priocharax nanus | LBP 28490 | 98283 | Rio Negro | Igarapé Tibarrá on left side of Rio Negro, Santa Isabel do Rio Negro, AM, Brazil | 00°26′28.1″ S 64°56′57.5″ W | MT754766 |
| Priocharax pygmaeus | LBP 22464 | 96986 | Rio Amazonas | Quebrada La Ponderosa, Letícia, Colombia | 04°08′24.4″ S 69°56′53.4″ W | MT754771 |
| Priocharax pygmaeus | LBP 22464 | 96987 | Rio Amazonas | Quebrada La Ponderosa, Letícia, Colombia | 04°08′24.4″ S 69°56′53.4″ W | MT754774 |
| Priocharax pygmaeus | LBP 22464 | 96988 | Rio Amazonas | Quebrada La Ponderosa, Letícia, Colombia | 04°08′24.4″ S 69°56′53.4″ W | MT754769 |
| Priocharax pygmaeus | LBP 22464 | 96998 | Rio Amazonas | Quebrada La Ponderosa, Letícia, Colombia | 04°08′24.4″ S 69°56′53.4″ W | MT754768 |
| Priocharax pygmaeus | LBP 22739 | 96989 | Rio Amazonas | Quebrada Pichuna, Letícia, Colombia | 04°07′33.8″ S 70°00′28.9″ W | MT754772 |
| Priocharax pygmaeus | LBP 22739 | 96990 | Rio Amazonas | Quebrada Pichuna, Letícia, Colombia | 04°07′33.8″ S 70°00′28.9″ W | MT754773 |
| Priocharax pygmaeus | LBP 22739 | 96991 | Rio Amazonas | Quebrada Pichuna, Letícia, Colombia | 04°07′33.8″ S 70°00′28.9″ W | MT754776 |
| Priocharax pygmaeus | LBP 22739 | 96992 | Rio Amazonas | Quebrada Pichuna, Letícia, Colombia | 04°07′33.8″ S 70°00′28.9″ W | MT754770 |
| Priocharax pygmaeus | LBP 22739 | 96993 | Rio Amazonas | Quebrada Pichuna, Letícia, Colombia | 04°07′33.8″ S 70°00′28.9″ W | MT754775 |
| Priocharax britzi | LBP 28493 | 98295 | Rio Purus | Marginal lake to Rio Ipixuna, Canutama, AM, Brazil | 07°31′11.5″ S 63°20′59.6″ W | MW374298 |
| Priocharax britzi | LBP 28493 | 98296 | Rio Purus | Marginal lake to Rio Ipixuna, Canutama, AM, Brazil | 07°31′11.5″ S 63°20′59.6″ W | MW374297 |
| Priocharax britzi | LBP 28493 | 98297 | Rio Purus | Marginal lake to Rio Ipixuna, Canutama, AM, Brazil | 07°31′11.5″ S 63°20′59.6″ W | MW374296 |
| Priocharax britzi | LBP 28493 | 98298 | Rio Purus | Marginal lake to Rio Ipixuna, Canutama, AM, Brazil | 07°31′11.5″ S 63°20′59.6″ W | MW374300 |
| Priocharax britzi | LBP 28493 | 98299 | Rio Purus | Marginal lake to Rio Ipixuna, Canutama, AM, Brazil | 07°31′11.5″ S 63°20′59.6″ W | MW374299 |
References
- Weitzman, S.H.; Vari, R.P. Miniaturization in South American freshwater fishes: An overview and discussion. Proc. Biol. Soc. Wash. 1988, 101, 444–465. [Google Scholar]
- Weitzman, S.H.; Vari, R.P. Two new species and a new genus of miniature characid fishes (Teleostei: Characiformes) from northern South America. Proc. Biol. Soc. Wash. 1987, 100, 640–652. [Google Scholar] [CrossRef]
- Toledo-Piza, M.; Mattox, G.M.T.; Britz, R. Priocharax nanus, a new miniature characid from the rio Negro, Amazon basin (Ostariophysi: Characiformes), with an updated list of miniature Neotropical freshwater fishes. Neotrop. Ichthyol. 2014, 12, 229–246. [Google Scholar] [CrossRef]
- Mattox, G.M.T.; Souza, C.S.; Toledo-Piza, M.; Britz, R.; Oliveira, C. A new miniature species of Priocharax (Teleostei: Characiformes: Characidae) from the Rio Madeira drainage, Brazil, with comments on the adipose fin in characiforms. Vertebr. Zool. 2020, 70, 417–433. [Google Scholar] [CrossRef]
- Mattox, G.M.T.; Souza, C.S.; Toledo-Piza, M.; Oliveira, C. A new miniature species of Priocharax (Characiformes: Characidae) from the upper rio Ipixuna, Purus drainage, Brazil. Neotrop. Ichthyol. 2021, 19, e210048. [Google Scholar] [CrossRef]
- Mattox, G.M.T.; Britz, R.; Souza, C.S.; Casas, A.L.S.; Lima, F.C.T.; Oliveira, C. Two new species of the miniature tetras Priocharax from the Rio Juruá drainage, Acre, Brazil (Teleostei: Characiformes: Characidae). Can. J. Zool. 2023, 101, 248–266. [Google Scholar] [CrossRef]
- Mattox, G.M.T.; Lima, F.C.T.; Britz, R.; Souza, C.S.; Oliveira, C. Two new miniature species of the fish genus Priocharax from the Rio Tapajós and Amazonas drainages, Pará, Brazil (Teleostei: Characiformes: Characidae). Vertebr. Zool. 2024, 74, 533–550. [Google Scholar] [CrossRef]
- Mattox, G.M.T.; Acosta-Santos, A.; Bogotá-Gregory, J.; Agudelo, E.; Lima, F.C.T. A new miniature Priocharax from the río Putumayo drainage, Amazonas State, Colombia (Teleostei: Characiformes: Characidae) with an unusual skin flap between pelvic fins. Zootaxa 2025, 5575, 545–554. [Google Scholar] [CrossRef]
- Fink, W.L.; Weitzman, S.H. The so-called cheirodontin fishes of Central America with descriptions of two new species (Pisces: Characidae). Smithson. Contrib. Zool. 1974, 172, 1–46. [Google Scholar] [CrossRef]
- Menezes, N.A.; Weitzman, S.H. Two new species of Mimagoniates (Teleostei: Characidae: Glandulocaudinae), their phylogeny and biogeography and a key to the glandulocaudin fishes of Brazil and Paraguay. Proc. Biol. Soc. Wash. 1990, 103, 380–426. [Google Scholar]
- Taylor, W.R.; Van Dyke, G.C. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium 1985, 9, 107–109. [Google Scholar]
- Weitzman, S.H. The osteology of Brycon meeki, a generalized characid fish, with an osteological definition of the family. Stanford Ichthyol. Bull. 1962, 8, 1–77. [Google Scholar]
- Mattox, G.M.T.; Britz, R.; Toledo-Piza, M. Skeletal development and ossification sequence of the characiform Salminus brasiliensis (Teleostei: Ostariophysi: Characidae). Ichthyol. Explor. Freshwaters 2014, 25, 103–158. [Google Scholar]
- Mattox, G.M.T.; Britz, R.; Toledo-Piza, M. Osteology of Priocharax and remarkable developmental truncation in a miniature Amazonian fish (Teleostei: Characiformes: Characidae). J. Morphol. 2016, 277, 65–85. [Google Scholar] [CrossRef]
- Mattox, G.M.T.; Kubicek, K.M.; Britz, R. Notes on the skeletal anatomy of Priocharax ariel Weitzman & Vari, 1987 with implications for its taxonomy (Teleostei: Characiformes). Zootaxa 2022, 5138, 597–599. [Google Scholar] [CrossRef]
- Manly, B.F.J. Métodos Estatísticos Multivariados—Uma Introdução, 3rd ed.; Bookman: Porto Alegre, Brazil, 2008; 229p. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
- Javonillo, R.; Malabarba, L.R.; Weitzman, S.H.; Burns, J.R. Relationships among major lineages of characid fishes (Teleostei: Ostariophysi: Characiformes), based on molecular sequence data. Mol. Phylogenet. Evol. 2010, 54, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D. DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. B 2005, 360, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Xia, X.; Xie, Z.; Salemi, M.; Chen, L.; Wang, Y. An index of substitution saturation and its application. Mol. Phylogenet. Evol. 2003, 26, 1–7. [Google Scholar] [CrossRef]
- Xia, X. DAMBE6: New tools for microbial genomics, phylogenetics and molecular evolution. J. Hered. 2017, 108, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.R.; Rocha, M.S.; Anjos, M.B.; Zuanon, J.; Py-Daniel, L.H.R. Fish fauna of small streams of the Catua-Ipixuna Extractive Reserve, State of Amazonas, Brazil. Check List 2009, 5, 154–172. [Google Scholar] [CrossRef]
- Venticinque, E.; Forsberg, B.; Barthem, R.; Petry, P.; Hess, L.; Mercado, A.; Canas, C.; Montoya, M.; Durigan, C.; Goulding, M. An explicit GIS-based river basin framework for aquatic ecosystem conservation in the Amazon. Earth Syst. Sci. Data 2016, 8, 651–661. [Google Scholar] [CrossRef]
- Pelicice, F.M.; Bialetzki, A.; Camelier, P.; Carvalho, F.R.; García-Berthou, E.; Pompeu, P.S.; Mello, F.T.; Pavanelli, C.S. Human impacts and the loss of Neotropical freshwater fish diversity. Neotrop. Ichthyol. 2021, 19, e210134. [Google Scholar] [CrossRef]
- Boehm, M.M.A.; Cronk, Q.C.B. Dark extinction: The problem of unknown historical extinctions. Philos. Trans. R. Soc. B 2021, 376, 20200376. [Google Scholar] [CrossRef]


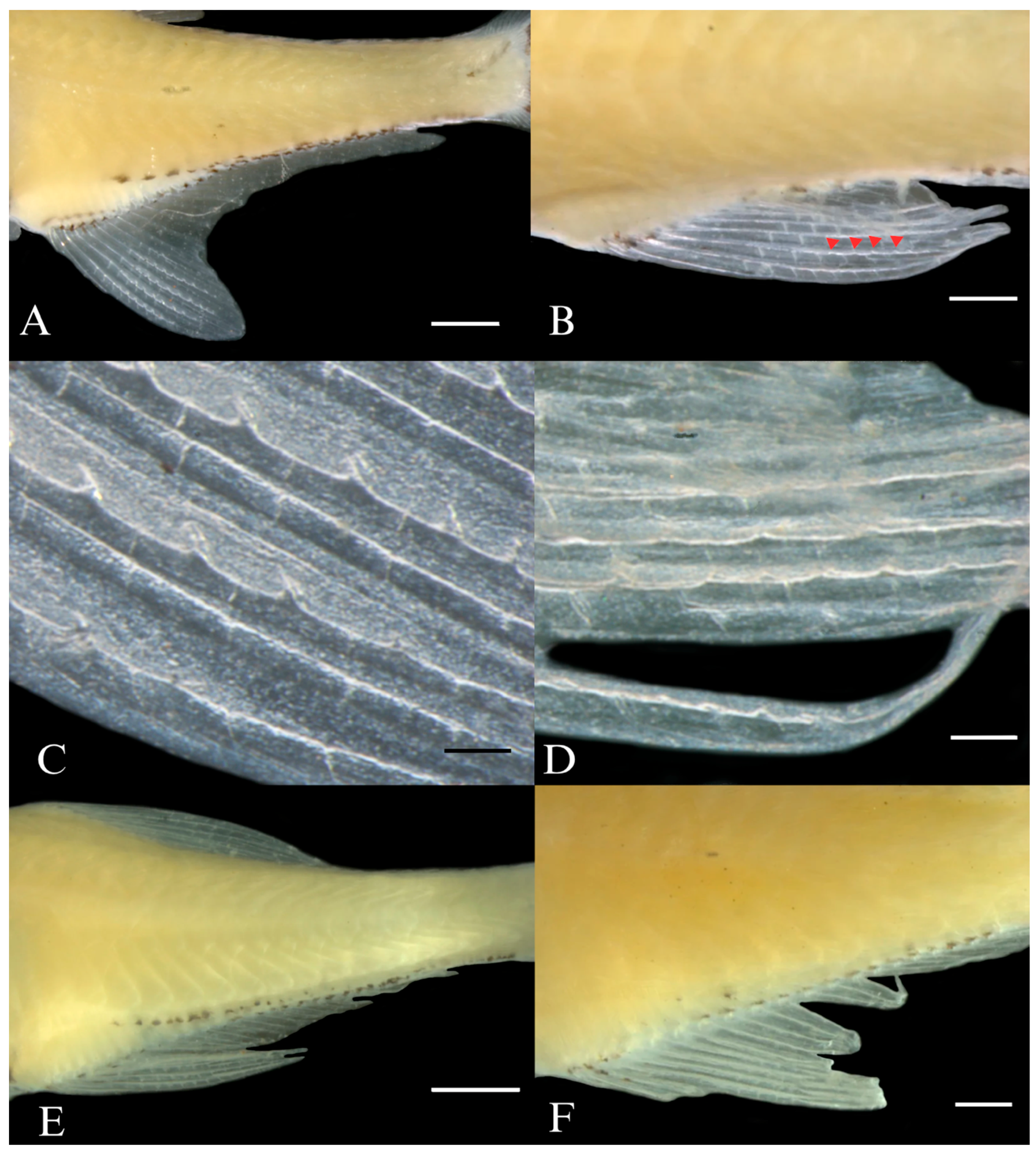
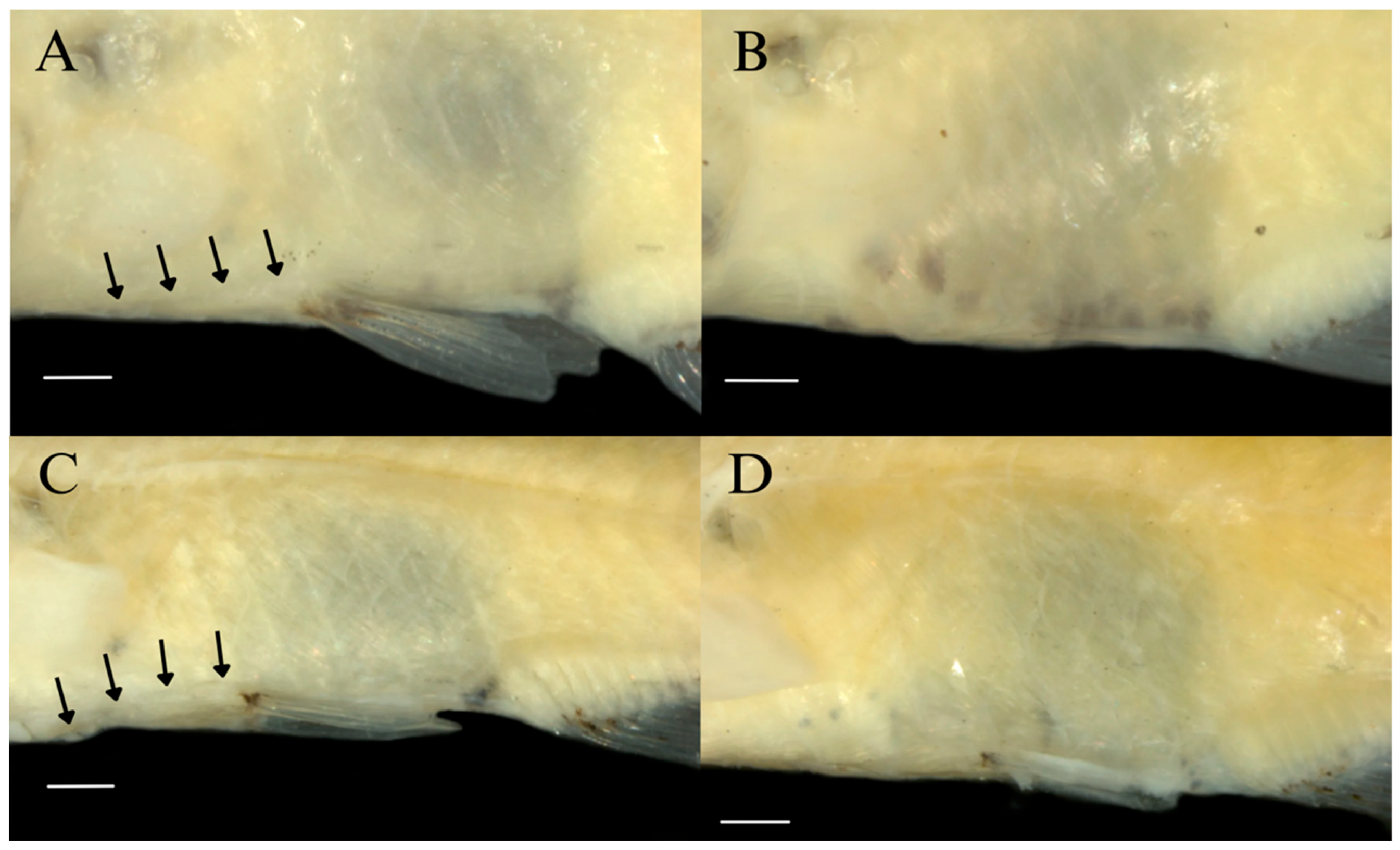


| Holotype | N | Range | Mean | SD | |
|---|---|---|---|---|---|
| Standard length (SL) (mm) | 13.2 | 146 * | 10.2–15.9 | 12.5 | - |
| Percentages of Standard Length | |||||
| Depth at dorsal-fin origin | 25.2 | 138 | 19–28 | 23 | 1.5 |
| Snout to dorsal-fin origin | 53.7 | 140 | 50–58 | 54 | 1.4 |
| Snout to pelvic-fin origin | 41.4 | 139 | 37–45 | 41 | 1.2 |
| Snout to anal-fin origin | 53.4 | 139 | 50–57 | 57 | 1.2 |
| Dorsal-fin length | 26.2 | 107 | 23–29 | 26 | 1.2 |
| Dorsal-fin base | 12.1 | 139 | 11–15 | 13 | 0,6 |
| Pelvic-fin length | 8.9 | 134 | 8–13 | 10 | 0.8 |
| Anal-fin length | 23.9 | 89 | 19–26 | 23 | 1.3 |
| Anal-fin base | 37.6 | 130 | 30–38 | 34 | 1.2 |
| Caudal-peduncle depth | 7.9 | 140 | 6–10 | 7 | 0.6 |
| Caudal-peduncle length | 12.1 | 131 | 12–19 | 14 | 1.2 |
| Percentages of head length (HL) | |||||
| Head length | 24.1 | 139 | 19–29 | 26 | 1.2 |
| Orbital diameter | 34.4 | 128 | 30–38 | 34 | 1.6 |
| Interorbital distance | 29.8 | 129 | 23–33 | 27 | 1.9 |
| Snout length | 20.9 | 125 | 16–26 | 20 | 2.0 |
| Upper jaw length | 55.4 | 119 | 42–57 | 49 | 2.8 |
| Percentages of caudal-peduncle length (CPL) | |||||
| Caudal-peduncle depth | 65.5 | 134 | 37–67 | 50 | 5.0 |
| Percentages of orbital diameter (OD) | |||||
| Snout length | 60.7 | 134 | 39–87 | 59 | 9.3 |
| Holotype | N | Range | Mean | SD | |
|---|---|---|---|---|---|
| Standard length (SL) (mm) | 13.8 | 17 | 11.5–16.1 | 13.7 | - |
| Percentages of Standard Length | |||||
| Depth at dorsal-fin origin | 21.1 | 15 | 20–25 | 22 | 1.3 |
| Snout to dorsal-fin origin | 52.9 | 15 | 53–56 | 54 | 0.9 |
| Snout to pelvic-fin origin | 39.1 | 15 | 39–43 | 41 | 1.3 |
| Snout to anal-fin origin | 56.9 | 15 | 54–58 | 57 | 1.2 |
| Dorsal-fin length | 24.7 | 8 | 23–26 | 24 | 0.7 |
| Dorsal-fin base | 11.3 | 15 | 11–13 | 12 | 0.8 |
| Pelvic-fin length | 12.0 | 14 | 11–12 | 12 | 0.4 |
| Anal-fin length | - | 7 | 22–25 | 23 | 1.0 |
| Anal-fin base | 30.0 | 10 | 28–32 | 30 | 1.4 |
| Caudal-peduncle depth | 7.3 | 15 | 7–9 | 8 | 0.4 |
| Caudal-peduncle length | 17.2 | 10 | 16–20 | 18 | 1.3 |
| Percentages of head length (HL) | |||||
| Head length | 23.1 | 15 | 23–29 | 25 | 1.5 |
| Orbital diameter | 38.4 | 10 | 33–38 | 35 | 2.1 |
| Interorbital distance | 30.5 | 11 | 25–31 | 28 | 1.5 |
| Snout length | 15.4 | 12 | 13–19 | 16 | 2.1 |
| Upper jaw length | 51.7 | 12 | 43–52 | 48 | 2.5 |
| Percentages of caudal-peduncle length (CPL) | |||||
| Caudal-peduncle depth | 42.8 | 10 | 41–52 | 47 | 3.7 |
| Percentages of orbital diameter (OD) | |||||
| Snout length | 40.1 | 9 | 33–58 | 46 | 8.6 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. P. ariel | - | ||||||||||
| 2. P. varii | 22.9 | 0.1 | |||||||||
| 3. P. britzi | 26.1 | 26.1 | 0.1 | ||||||||
| 4. P. nanus | 21.8 | 21.1 | 22.1 | - | |||||||
| 5. P. phasma | 28.5 | 21 | 18.9 | 22 | 0.2 | ||||||
| 6. P. conwayi | 24.1 | 18.3 | 26.9 | 22.4 | 26.3 | 0.2 | |||||
| 7. P. marupiara | 18.5 | 21.3 | 19.4 | 18.7 | 26.2 | 20.5 | 0 | ||||
| 8. P. pygmaeus | 31.7 | 23.2 | 24.5 | 22 | 25.6 | 30.7 | 22.1 | 0 | |||
| 9. P. toledopizae | 29.5 | 23 | 22.9 | 20.7 | 23.5 | 25.5 | 22 | 8.8 | 0.1 | ||
| 10. P. robbiei | 24.9 | 24.6 | 4.8 | 21.8 | 18.8 | 23.1 | 20 | 25 | 24.3 | 0 | |
| 11. P. piagassu | 19.5 | 19.9 | 20.8 | 22.2 | 25 | 21.5 | 8.5 | 24.7 | 24.4 | 18.5 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, G.G.S.; Souza, C.S.; Reia, L.; Mantuaneli, L.A.; Morales, B.F.; Lima, F.C.T.; Oliveira, C.; Mattox, G.M.T. Two New Species of Miniature Tetras of the Genus Priocharax (Teleostei: Characiformes: Acestrorhamphidae) from the Rio Purus and Solimões Drainages, Amazonas, Brazil. Taxonomy 2025, 5, 36. https://doi.org/10.3390/taxonomy5030036
Lopez GGS, Souza CS, Reia L, Mantuaneli LA, Morales BF, Lima FCT, Oliveira C, Mattox GMT. Two New Species of Miniature Tetras of the Genus Priocharax (Teleostei: Characiformes: Acestrorhamphidae) from the Rio Purus and Solimões Drainages, Amazonas, Brazil. Taxonomy. 2025; 5(3):36. https://doi.org/10.3390/taxonomy5030036
Chicago/Turabian StyleLopez, Giovanna Guimarães Silva, Camila Silva Souza, Lais Reia, Larissa Arruda Mantuaneli, Bruno Ferezim Morales, Flávio Cesar Thadeo Lima, Claudio Oliveira, and George Mendes Taliaferro Mattox. 2025. "Two New Species of Miniature Tetras of the Genus Priocharax (Teleostei: Characiformes: Acestrorhamphidae) from the Rio Purus and Solimões Drainages, Amazonas, Brazil" Taxonomy 5, no. 3: 36. https://doi.org/10.3390/taxonomy5030036
APA StyleLopez, G. G. S., Souza, C. S., Reia, L., Mantuaneli, L. A., Morales, B. F., Lima, F. C. T., Oliveira, C., & Mattox, G. M. T. (2025). Two New Species of Miniature Tetras of the Genus Priocharax (Teleostei: Characiformes: Acestrorhamphidae) from the Rio Purus and Solimões Drainages, Amazonas, Brazil. Taxonomy, 5(3), 36. https://doi.org/10.3390/taxonomy5030036






