3. Taxonomy
Order Amphipoda Latreille, 1816
Suborder Senticaudata Lowry and Myers, 2013
Superfamily Bogidielloidea Hertzog, 1936
Family Artesiidae Holsinger, 1980
Genus Spelaeogammarus da Silva Brum, 1975
Emended diagnosis (adapted from Koenemann and Holsinger, 2000 [4]): Eyes absent. Body smooth and slender, unpigmented. Flagellum of antenna 1 longer than peduncle by 11–20 articles. Accessory flagellum 3–6-articulate. Antenna 2 flagellum bearing 5–10 segments. Mandibular palp 3-segmented. Maxilla 1 inner plate with 3 setae, with symmetrical 2-segmented palp. Inner plate of maxilliped bearing apically 2 bifid (y-shaped) setae. Propodus of gnathopod 1 stronger than propodus of gnathopod 2. Pereopods without any trace of lenticular organs, pereopods 5–7 bases broad, propodus and/or carpus with long, thin, bifurcate setae. Pleopods biramous with 3-segmented outer ramus and 1-segmented inner ramus. Uropods biramous: uropod 3 with subequal, 1-segmented rami, outer ramus bearing a row of long, bifurcate setae along dorsal margin. Telson typically longer than wide, apex with shallow excavation, bearing apical and subapical spines. Presence of 3 pairs of coxal gills, on pereopods 4–6. Presence of 4 pairs of oostegites, linear and elongate, on coxal plates 2–5 (in females).
Spelaeogammarus rafaelae sp. nov.
urn:lsid:zoobank.org:act:D27E65F8-A7CE-439C-B034-F8174D722BA6
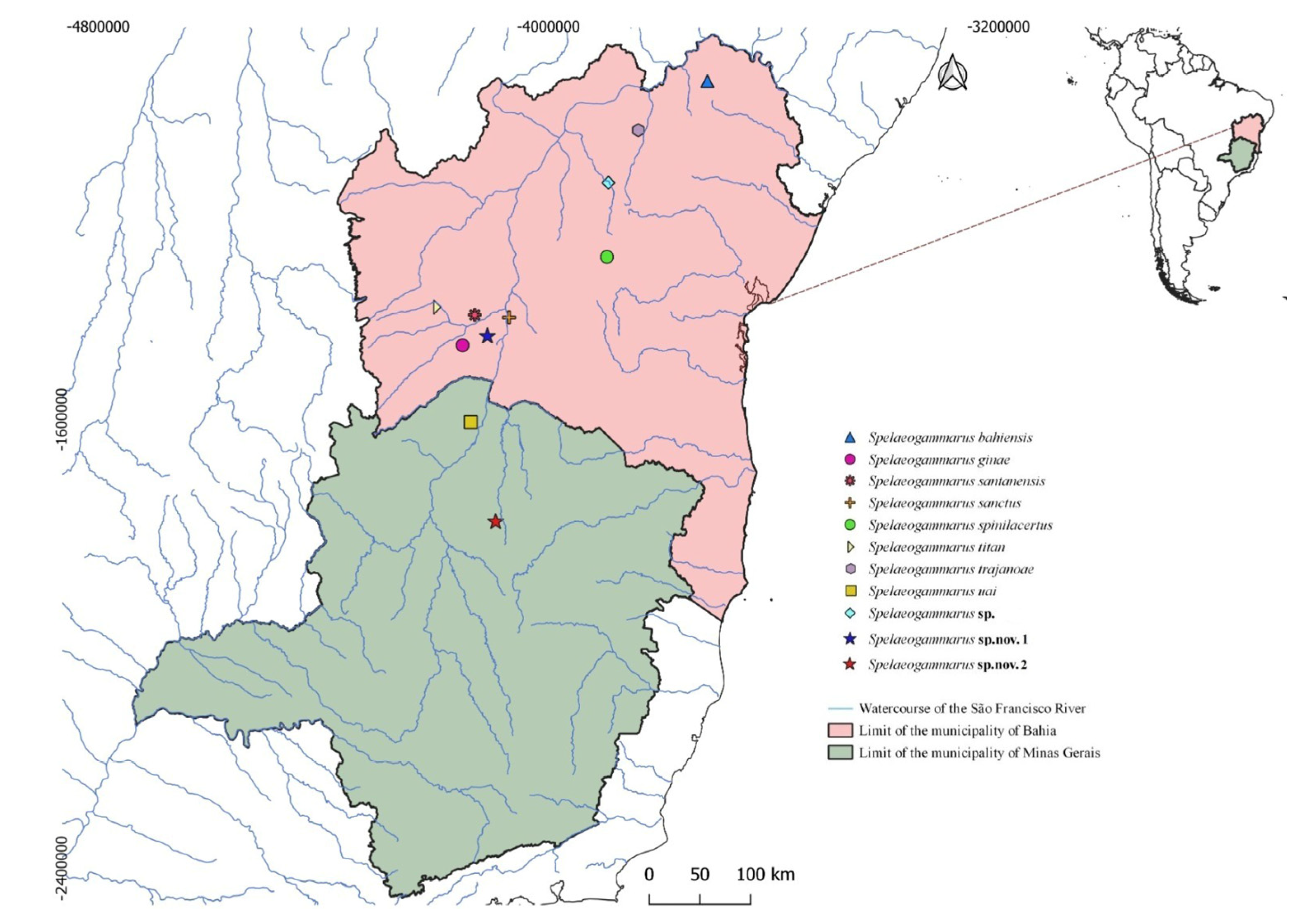
Type locality: Gruna do Zoológico, Serra do Ramalho.
Type material: Holotype:• ♀ (in slide), 4.64 mm; Brazil, Bahia state, municipality of Serra do Ramalho, Gruna do Zoológico; −13,57058° −43,844028°;5 June 2024; R.L. Ferreira leg; ISLA97010.
Diagnosis. Antenna 1 flagellum with 10 articles; accessory flagellum 4-articulate. Antenna 2 flagellum with five articles. Maxilla 1 outer lobe apical margin with seven simple robust setae, palp 2-articulate with three long simple setae. Maxilliped inner plate apical margin with 2 Y-shaped stout setae, without plumose setae, palp article 3, apical margin with row of long setae. Gnathopod 1 basis anterior margin with two small setae, posterior margin with two simple long setae; propodus palm acute, 1.6× longer than posterior margin. Gnathopod 2 basis rectangular, 2.9× longer than wide, posterior margin with three long slender setae. Pereopod 5 propodus 7.3× longer than wide. Pleopods inner ramus with 4–6 setae. Pleopod 2 peduncle 4.4× longer than wide. Uropod 3 outer ramus with 7–8 dorsal bifid setae and 4 apical stout setae. Telson with one apical and three subapical stout setae.
Etymology. The specific epithet of the species is dedicated to the biospeleologist and carcinologist Rafaela Bastos Pereira Pietrobon, whose dedication to the study of Spelaeogammarus species (and other crustaceans) has been exceptional. The specific epithet is expressed as a Latinized adjective, paying homage to her significant contributions.
Description.
Female (holotype (ISLA97010))
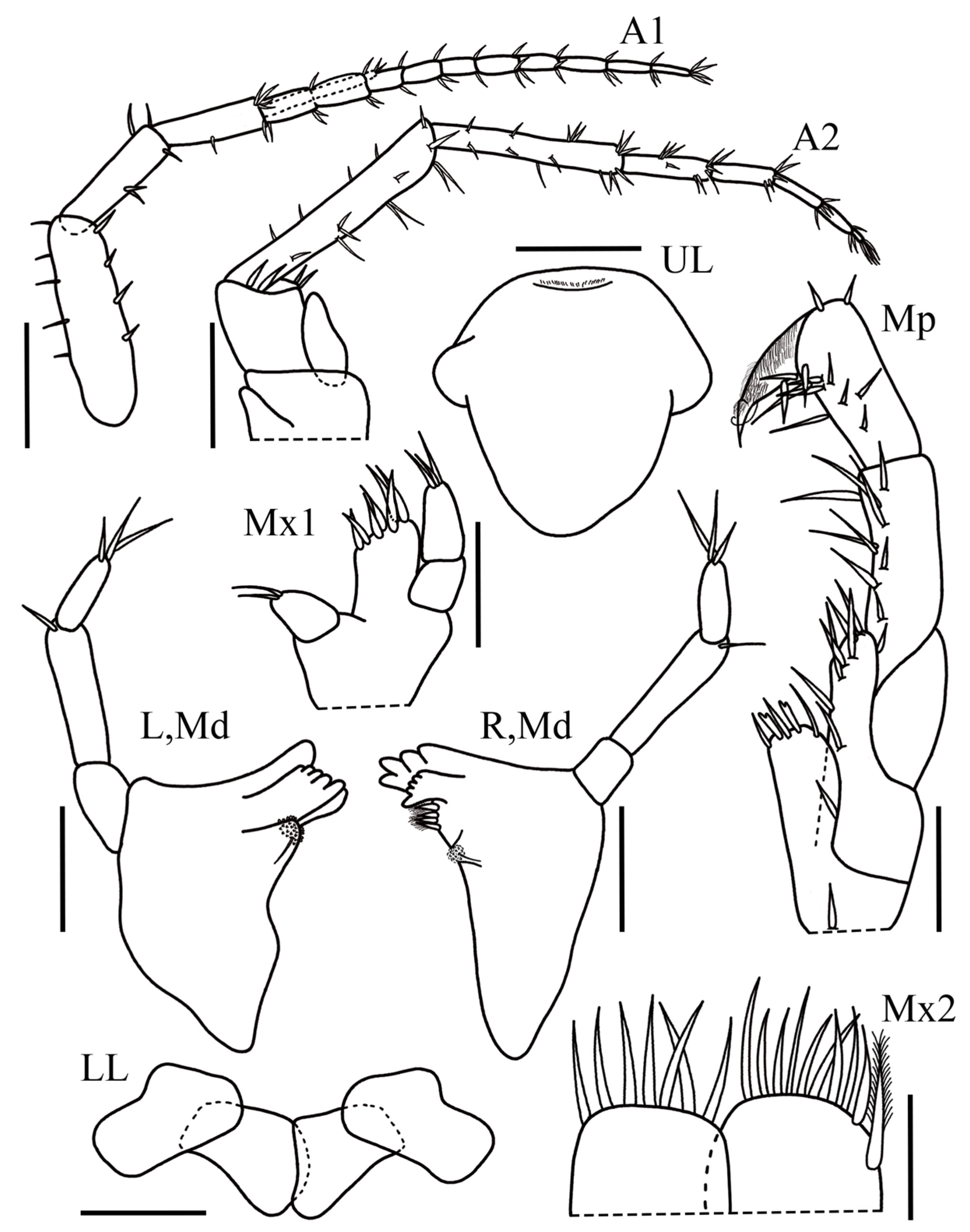
Body slender and unpigmented. Head without eyes. Antenna 1 reduced in size, less than half of body length; flagellum 1.07× longer than peduncle; peduncular article 1 1.7× as long as article 2 and 2.6× as long as article 3; flagellum with 10 articles; accessory flagellum 4-articulate; article 4 reduced. Tip of accessory flagellum reaching up to the third article of flagellum. Antenna 2 short, 0.76× as long as antenna 1; peduncle 1.87× longer than flagellum; article 4 1.2× the length of article 5; flagellum with five articles. Upper lip subtriangular and smooth, with few setules apically. Lower lip inner lobe suboval; outer lobe slightly rounded. Left mandible with molar broad, semi-triturative, subcircular; accessory setal row not visible; lacinia mobilis present, well developed, and apically multi-cuspidate; incisor process multi-cuspidate. Palp 3-articulate, article 1 1.1× longer than wide; article 25.3× longer than wide, ventral margin with one simple seta; article 3 2.7× longer than wide, tapering distally, apex with three slender setae. Right mandible subequal in size with left mandible; molar reduced, semi-triturative, subcircular; accessory setal row with 4 plumose setae; lacinia mobilis present and multi-cuspidate; incisor process multi-cuspidate. Palp 3-articulate, article 1 1.3× longer than wide; article 2 slightly robust,5.7× longer than wide, ventral margin with one long seta; article 3 2.8× longer than wide, tapering distally, with three slender setae inserted apically. Maxilla 1 inner plate rounded, with two simple setae on apical margin; outer plate rectangular with seven simple robust setae on apical margin; sparse setules on medial margin not visible; palp 2-articulate with article 2 tapering distally, apex with three slender setae. Maxilla 2 outer plate as long as inner plate, apical margin with seven simple stout setae; inner plate with nine simple stout setae with one stout plumose seta. Maxilliped inner plate suboval, inner margin with two Y-shaped stout setae and three long simple setae; outer plate rounded, with five stout setae apically and three long simple setae on dorsal margin; palp 4-articulate, article 1 1.4× longer than wide; article 2, the largest, 2.1× longer than wide and 1.2× longer than article 3, row of long setae on apical margin; article 4 subrectangular, inner margin setose, claw present distally.
Figure 1.
Spelaeogammarus rafaelae sp. nov., ♀, holotype (ISLA97010). Scale bar: 1 mm.
Figure 1.
Spelaeogammarus rafaelae sp. nov., ♀, holotype (ISLA97010). Scale bar: 1 mm.
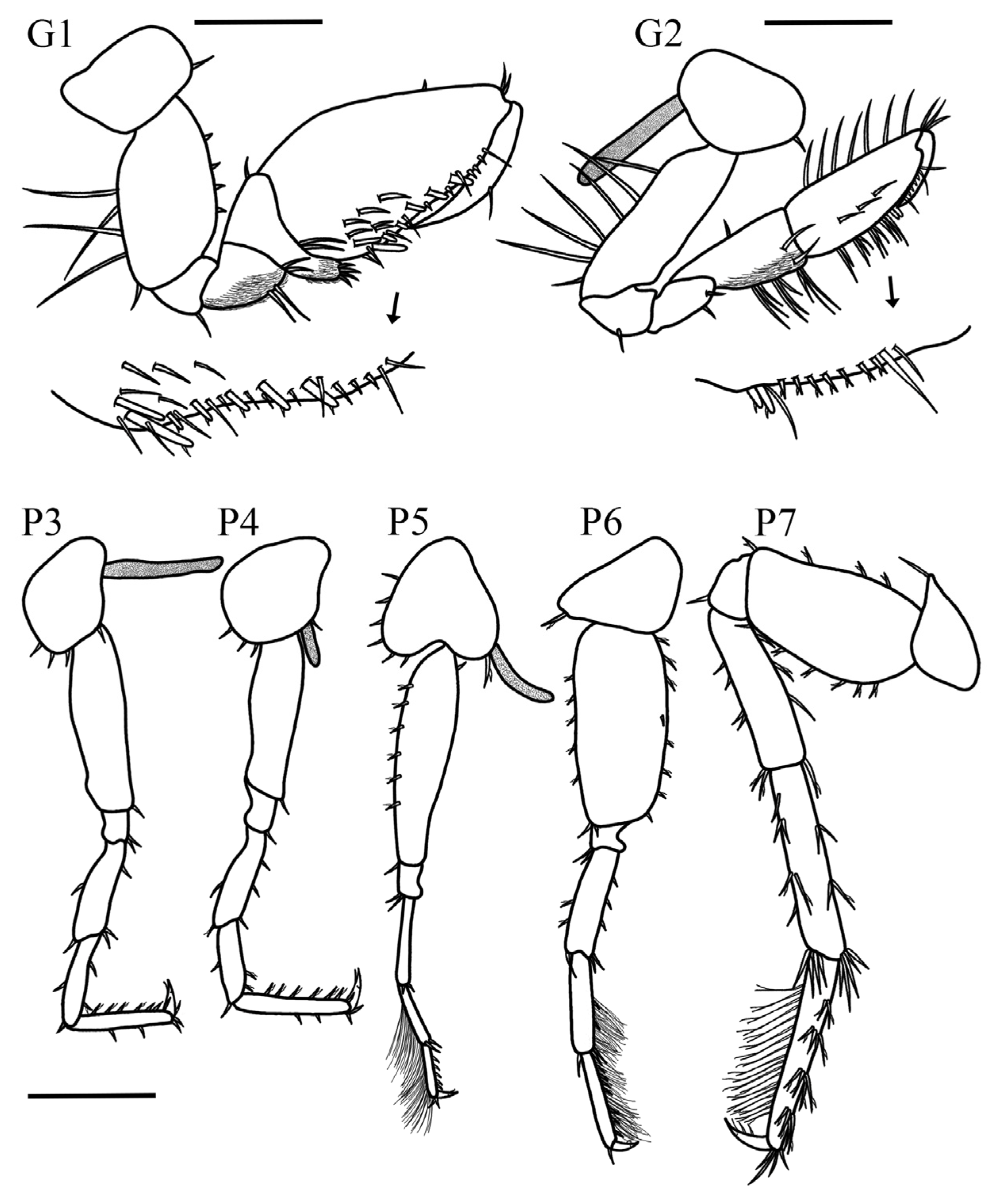
Gnathopod 1 coxa rounded with one seta on posterodistal corner, 1.6× longer than wide; basis short and stout, 1.4× longer than wide, anterior margin with two small setae, posterior margin with two long setae, and one small seta on distal corner; ischium subrectangular, naked with one long seta on posterodistal corner; merus, 1.3× longer than wide, ventral margin setose with two slender setae inserted apically; carpus triangular, 1.7× wider than long, posterodistal corner short and produced, posterodistal margin with fringe of plumose setae and four slender setae on anterior margin; propodus suboval 2.4× longer than wide, palm acute, 1.6× longer than posterior margin, covered by fringe of small setae, with eight stout setae and six slender setae; dactylus long, naked and curved reaching the palmar corner. Gnathopod 2 coxa suboval with one small seta on distal margin, 1.4× wider than long; basis rectangular, 2.9× longer than wide, anterior margin naked, posterior margin with three long slender setae; ischium posterior margin with one long seta; merus suboval, distoventral corner with two simple setae; carpus elongated, subtrapezoidal, 2.5× longer than wide, posterior margin with five sets (2-2-2-2-1) of simple long setae, anterior margin with two small simple setae; propodus subrectangular, slightly elongated and tapering distally, 2.8× longer than wide, anterior margin with four long setae, posterior ventral margin with seven sets of (2-2-2-3-1-1-1) long setae, two stout setae, and few small; posterior dorsal margin with fringe of small and long simple setae and eight stout setae; palm acute, about 0.3× shorter than posterior margin; dactylus short, curved, naked, not reaching the palmar corner.
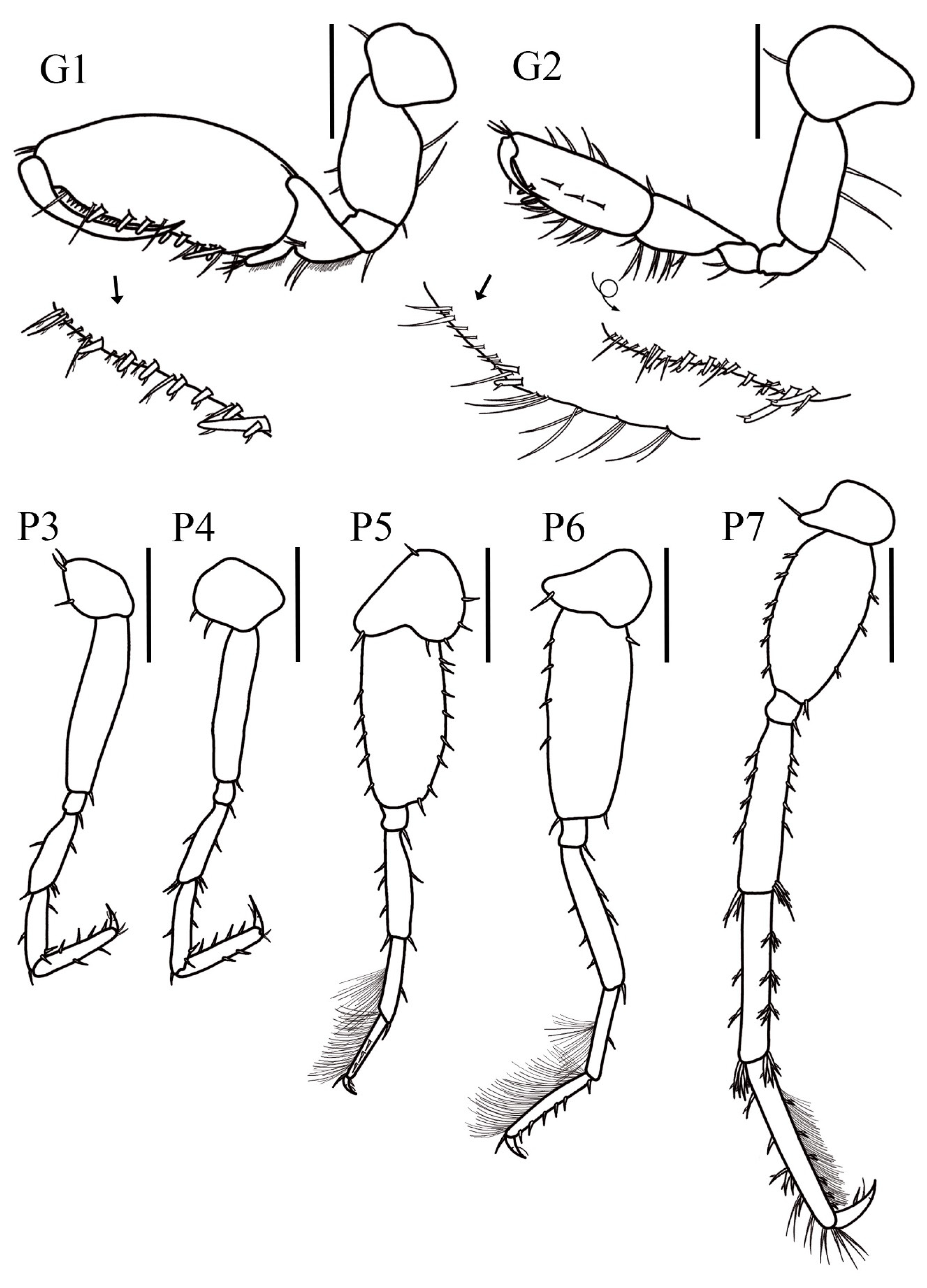
Pereopod 3, coxa suboval, 1.2× longer than wide, distal margin with three short setae; basis elongated, 6.5× longer than wide, anterior and posterior margin without setae, posteroproximal corner with one small seta; merus 4.1× longer than wide, anterior margin with one short seta, anterodistal corner with one small seta, posterior margin with one small seta, posterodistal corner with two small setae; carpus, 3.6× longer than wide, anterior and posterior margin with one small setae each, anterodistal corner with one slender seta, posteroproximal corner with two small setae; propodus 7× longer than wide, subequal in length with carpus, anterior margin with two short setae, posterior margin with four short setae; dactylus slightly curved with two small simple setae on dorsal margin, apical nail present. Pereopod 4 coxa rounded with two small setae on distal margin, 1.4× longer than wide; subequal in size with pereopod 3. Pereopod 5 slightly shorter than pereopod 6, coxa bilobate, 1.5× longer than wide, posterior lobe more developed than anterior, posterior margin with one stout seta, anterior margin with five slender setae; basis suboval, 2.1× longer than wide, anterior margin with five small setae, posterior margin with eight small setae; merus 4.3× longer than wide, posterior margin with one seta, anterior margin with three setae, posterodistal and anteroproximal corner with one seta each; carpus short, 5× longer than wide, anterior margin with dense fringe of thin slender setae, posterior margin bearing one seta and posterodistal corner with one stout seta; propodus 7.3× longer than wide, anterior margin with dense fringe of thin slender setae, anterodistal corner with one stout seta, three short setae on ventral margin; dactylus short slightly curved, apical nail present. Pereopod 6 coxa small and bilobate, anterior lobe slightly developed, as wide as long, with one stout seta, posterior lobe suboval; basis elongate, 2.2× longer than wide, with four setae on anterior margin, one seta on posterior margin, posterodistal and anterodistal corner with one seta each; merus 4.3× longer than wide, three setae on anterior margin, two setae on posterior margin, and one seta on posterodistal corner; carpus 0.7× smaller than merus, 6.3× longer than wide, anterior margin covered by dense fringe of slender setae, posterior margin with one seta; propodus 7.1× longer than wide, 0.9× the carpus length, anterior margin with dense fringe of slender setae, posterior margin with six small setae; dactylus slightly curved, apical nail present. Pereopod 7 coxa subtriangular, anterior lobe well developed with one long seta on distal corner, 1.9× longer than wide; basis short and stout, 1.8× longer than wide, anterior margin with six small setae, posterior margin with three small setae, posterodistal corner with one seta; merus elongated, 3.9× longer than wide, anterior margin with four setae, anterodistal corner with two long setae, posterior margin with five simple setae, posterodistal corner with three long setae; carpus rectangular, 5.7× longer than wide, anterior margin with two setae, anterodistal corner with five long setae, posterior margin with three sets of (3-3-3) setae, posterodistal corner with three long setae; propodus elongated, 7.3× longer than wide, 1.1× longer than carpus, anterior margin with four sets of (1-1-3-1) setae, with few long setae on apical margin, posterior margin with dense fringe of thin elongated setae and six short setae; dactylus long and slightly curved, apical nail present.
Figure 2.
Spelaeogammarus rafaelae sp. nov., ♀, holotype (ISLA97010). Scale bar: 1 mm.
Figure 2.
Spelaeogammarus rafaelae sp. nov., ♀, holotype (ISLA97010). Scale bar: 1 mm.
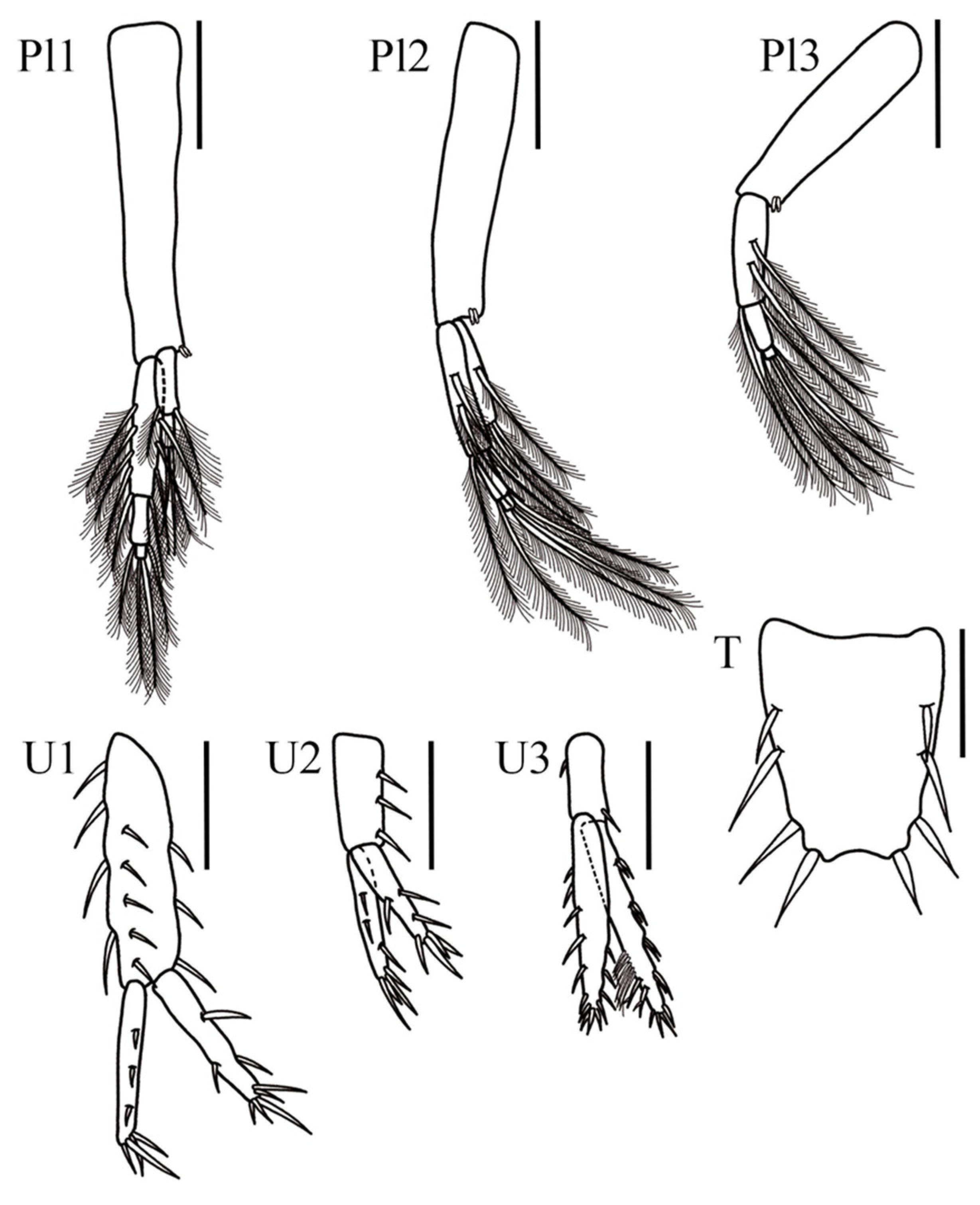
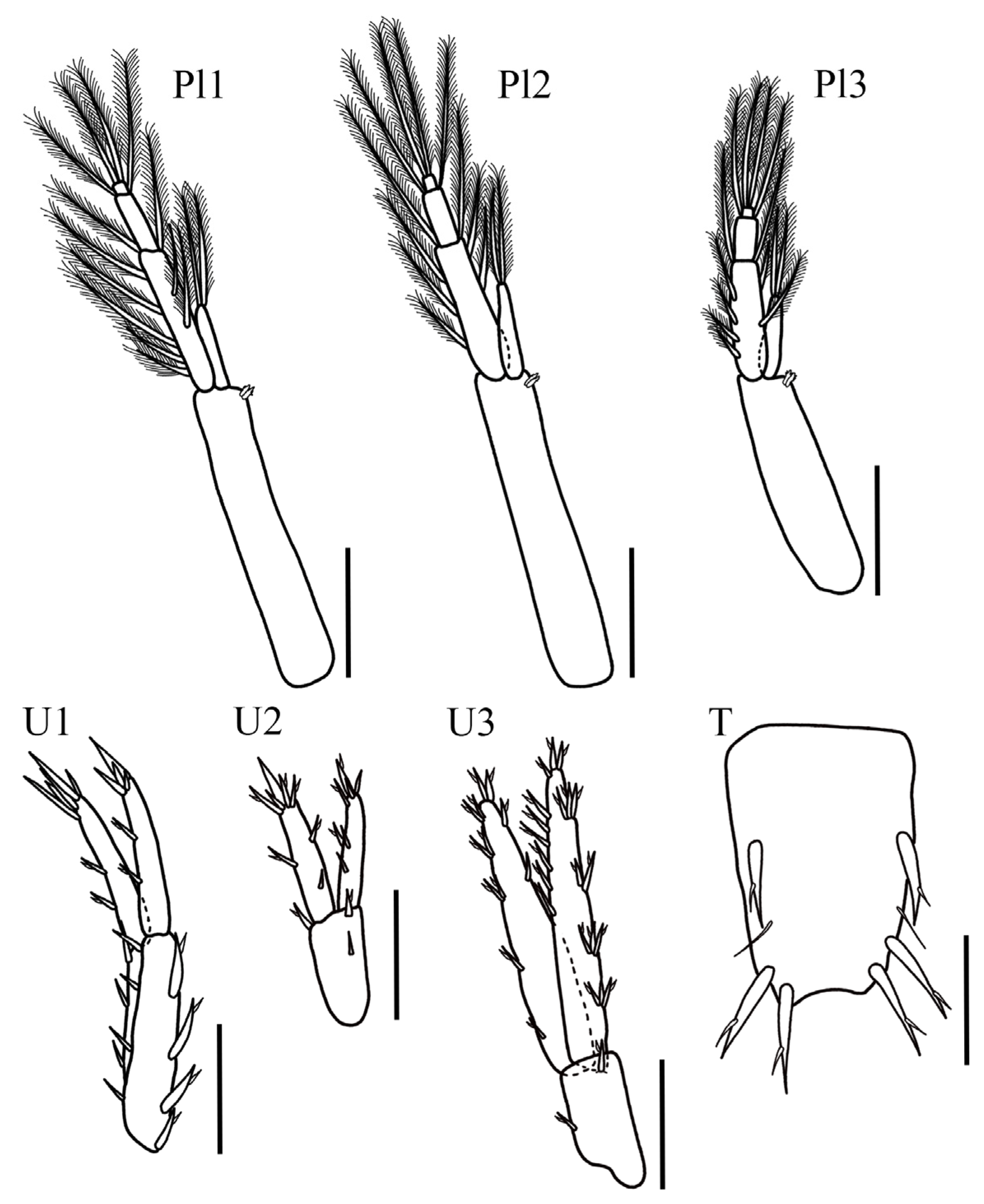
Coxal gills present, ventrally inserted on coxal plates of pereopods 4–6. Pleopod 1, peduncle elongated and rectangular, 6× longer than wide, with one pair of coupling spines; inner ramus 1-articulate, with four slender plumose setae; outer ramus 3-articulate, 2.1× longer than inner ramus; article 1 inner margin with four plumose setae, outer margin with five plumose setae; article 2 with two plumose setae, article 3, smallest, with two apical plumose setae. Pleopod 2 peduncle rectangular, 4.4× longer than wide, with one pair of coupling spines; inner ramus 1-articulate bearing four plumose slender setae; outer ramus 3-articulate, 1.7× longer than inner ramus; article 1 inner margin and outer margin with four plumose slender setae each, article 2 with two plumose setae, article 3, smallest, with two apical plumose setae. Pleopod 3 smallest, peduncle rectangular, 5.8× longer than wide, with one pair of coupling spines; inner ramus 1-articulate with six plumose slender setae; outer ramus 3-articulate, as long as inner ramus; article 1 inner and outer margin with three slender plumose setae each, article 2 with two plumose setae, article 3, smallest, with two apical plumose setae.
Uropod 1 peduncle elongated, 3.6× longer than wide with five ventral stout setae, four dorsal stout setae and three lateral setae; inner ramus slightly longer than outer ramus, both lanceolate; inner ramus 0.6× the peduncle length with three dorsal setae and five apical stout setae; outer ramus with three dorsal setae and four stout apical setae. Uropod 2 shortest, peduncle 2.4× longer than wide, with three dorsolateral stout setae; inner ramus 1.15× shorter than outer ramus, both lanceolate; inner ramus 1.2× the peduncle length, with three dorsal setae and four apical stout setae; outer ramus with two ventral, one dorsal, and four apical stout setae. Uropod 3 peduncle short, 2.2× longer than wide, with one distolateral stout seta and one ventrolateral stout seta; rami elongated, subequal, both lanceolate; inner ramus with four lateral sets of stout setae (2-2-2-2), seven–eight long bifid setae on dorsal margin and four apical setae; outer ramus with five stout setae on dorsal margin, three ventrodistal stout setae, and six apical stout setae. Telson 1.4× longer than wide, apical margin with a slightly shallow U-shaped excavation with one apical and three subapical stout setae in each lobe.
Female: Oostegites long and naked inserted on coxal plates 2–5.
Character states of the congeneric species with the new species are summarized in
Table 1.
Figure 3.
Spelaeogammarus rafaelae sp. nov. ♀, holotype (ISLA97010). Scale bar: 1 mm.
Figure 3.
Spelaeogammarus rafaelae sp. nov. ♀, holotype (ISLA97010). Scale bar: 1 mm.
Morphological remarks.
Spelaeogammarus rafaelae sp. nov. is similar to
S. spinilacertus and
S. bahiensis because of the 4-articulate accessory flagellum. It resembles
S. santanensis,
S. bahiensis,
S uai, and
S. sanctus via the seven multi-cuspidate stout setae on the outer plate of maxilla 1. It resembles
S. bahiensis due to the absence of plumose setae on the inner plate of maxilliped. It resembles
S. spinilacertus and
S. trajanoae via the one stout seta on coxa 6. Finally, through the one apical + three subapical stout setae per lobe on telson,
Spelaeogammarus rafaelae sp. nov. resembles
S. uai and
S. titan but differs due to the 4-articulate accessory flagellum; flagellum of antenna 2 5-articulate; the number of setae on anterior and posterior margin of basis 1; three setae on posterior margin of basis 2; and seven bifid setae on dorsal margin of outer ramus of uropod 3 (
Table 1).
Habitat and threats.
Specimens of
Spelaeogammarus rafaelae sp. nov. were found exclusively in Gruna do Zoológico, located in the municipality of Serra do Ramalho, western Bahia state (
Figure 4A). Gruna do Zoológico has two entrances: a larger one (
Figure 4B) at the base of a small outcrop, which serves as an intermittent sinkhole, and a smaller one (
Figure 4C) at the bottom of a doline, also functioning as an intermittent sinkhole. The smaller entrance leads to a narrow passage that connects to the main conduct, which is more extensive and linked to the primary entrance.
Since both entrances act as sinkholes, the cave accumulates significant plant-derived organic matter along the sediment banks. During our initial visit, we encountered abundant water, initially assumed to be residual pools from the previous rainy season. However, we discovered that this water originates from a spring within the cave, flowing along the main conduit before disappearing into a sinkhole about 200 m from the main entrance. During a subsequent visit at the peak of the dry season, the lotic watercourse remained active, confirming its perennial nature.
Wall markings and large accumulations of organic matter suggest seasonal flooding events that transport debris to deeper sections of the cave. The main conduit, where the perennial watercourse flows, features travertine dams (
Figure 4D) that host other stygobitic organisms, such as isopods and planarians.
Spelaeogammarus rafaelae sp. nov. (
Figure 4E) were observed only in a deeper pool near the cave’s final section. Despite extensive searches, only one specimen was recorded, indicating an extremely low population density. Notably, the specimens were confined to this specific pool, even though the perennial watercourse was thoroughly inspected on both visits. Surveys of other regional caves with perennial water bodies did not yield additional
Spelaeogammarus rafaelae sp. nov. specimens.
Access to Gruna do Zoológico is challenging due to poorly maintained roads and a lengthy hike. Consequently, there are no signs of regular human visitation, and the cave remains well preserved. However, seasonal flooding pulses from the external environment impact the cave, likely prompting cave-restricted fauna to seek sheltered microhabitats that protect them from being washed away.
Despite the ongoing deforestation in the Serra do Ramalho region, the forest surrounding the cave is relatively intact, though selective logging is evident. While the cave is currently in a good state of conservation, it is crucial for Brazilian environmental authorities to regularly monitor the conservation status of this species, given the rapid environmental changes affecting the region.
Figure 4.
(A). Karst area surround Gruna do Zoológico. (B). Larger cave entrance. (C). Smaller cave entrance. (D). Continuous watercourse flows. (E). Live specimen of Spelaeogammarus rafaelae sp. nov.
Figure 4.
(A). Karst area surround Gruna do Zoológico. (B). Larger cave entrance. (C). Smaller cave entrance. (D). Continuous watercourse flows. (E). Live specimen of Spelaeogammarus rafaelae sp. nov.
Spelaeogammarus lundi sp. nov.
urn:lsid:zoobank.org:act:4E4994CE-1DF7-4CF9-9B4F-15F7A4F2CE45
Type locality: Lapa da Lagoinha cave, municipality of Montes Claros.
Type material: Holotype:• ♀ (in slide), 4.78 mm; Brazil, state of Minas Gerais, municipality of Montes Claros, Lapa da Lagoinha cave; −23.5444° −46.8181°;19 March 2023; R.L. Ferreira leg.; ISLA97006.
Paratypes. 1 ♂ (in slide); same date as holotype; ISLA97007. 1 ♀ (in slide); same date as holotype, ISLA97008. 1 ♀; 6 ♂; same data as holotype; ISLA97009.
Diagnosis. Antenna 1 flagellum with eleven articles; accessory flagellum 3-articulate. Antenna 2 flagellum with five articles. Maxilla 1 outer lobe apical margin with six simple robust setae and one plumose seta, inner lobe with three simple setae. Maxilliped inner plate apical margin with two Y-shaped stout setae and three plumose setae. Gnathopod 1 basis anterior margin with four small setae, posterior margin with four simple long setae and one on anterior margin; propodus palm acute, 1.6× longer than posterior margin. Gnathopod 2, basis rectangular, 2.8× longer than wide, anterior margin naked, and posterior margin with six long slender setae and one on anterior margin. Pleopod 2 peduncle 5.8× longer than wide. Uropod 3 outer ramus with 2–4dorsal bifid setae. Telson elongated with one apical and two subapical stout setae.
Etymology. The specific epithet honors the Danish naturalist Peter Wilhelm Lund, widely regarded as the founder of speleology as a scientific discipline in Brazil. Lund dedicated decades of research to the caves of Minas Gerais, making outstanding contributions to Brazilian paleontology. Additionally, this name also pays tribute to the caving group Espeleo Grupo Peter Lund for their valuable contributions to the exploration and study of caves in northern Minas Gerais, as well as for the support they provided during our fieldwork in the region.
Description.
Female holotype (ISLA97006).
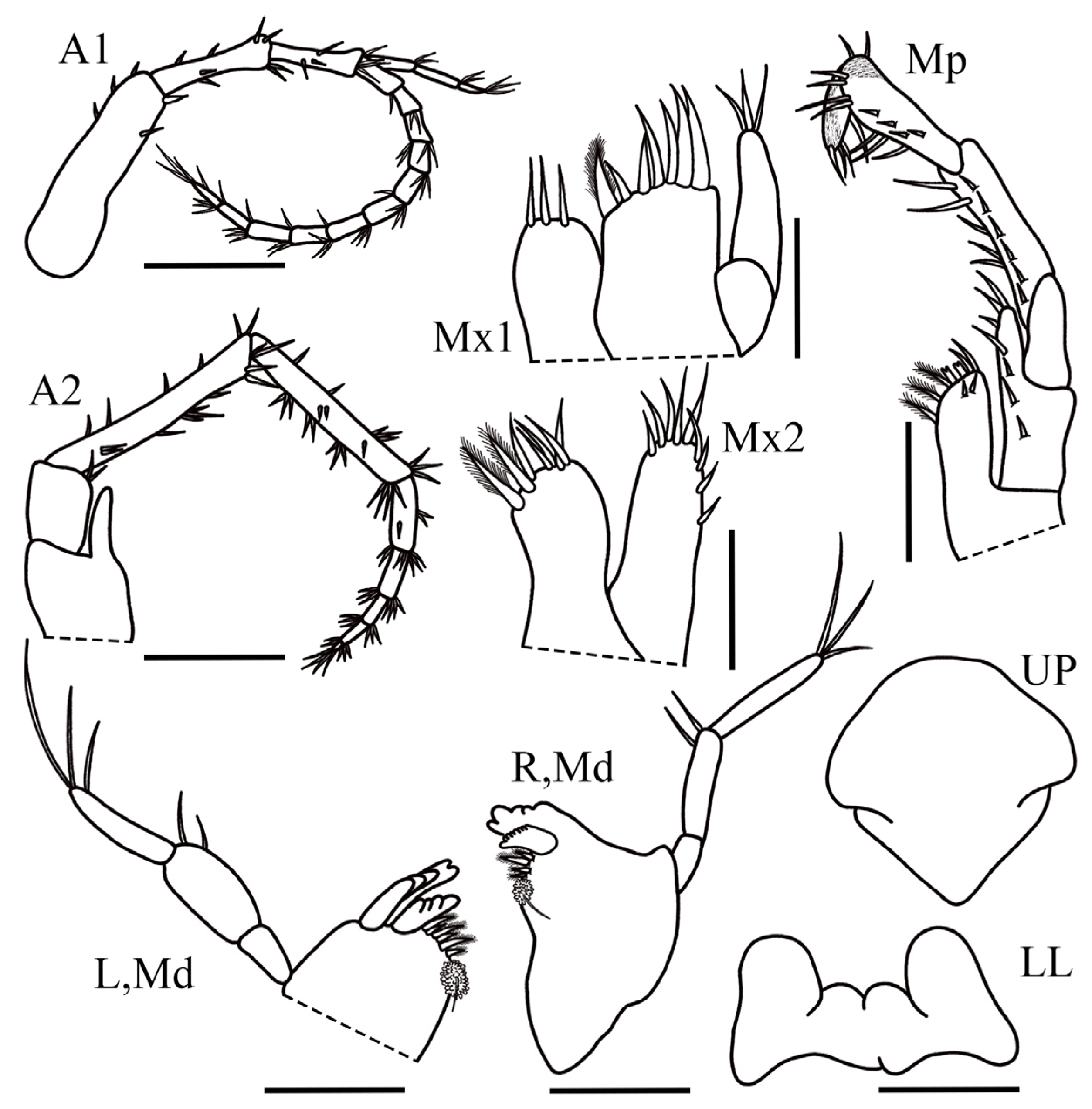
Body slender and unpigmented. Head without eyes. Antenna 1 reduced in size, less than half of body length, flagellum 0.7× shorter than peduncle, peduncular article 1 1.6× as long as article 2, 2.2× as long as article 3; flagellum with eleven articles; accessory flagellum 3-articulate, article 3 reduced; accessory flagellum reaching up to the third article of flagellum. Antenna 2 1.02× as long as antenna 1; peduncle 2.8× longer than flagellum; peduncular article 4 1.05× the length of article 5; flagellum with five articles. Upper lip triangular and smooth. Lower lip inner lobe round, outer lobe slightly rounded. Left mandible molar broad, semi-triturative, subcircular, with one simple seta; accessory setal row with six curved plumose setae; lacinia mobilis present, apically multi-cuspidate; incisor process multi-cuspidate; palp 3-articulate, article 1 1.9× longer than wide; article 2 2.2× longer than wide, ventral margin with two slender setae; article 3 3× longer than wide, tapering distally, with three apical long setae. Right mandible subequal to left mandible, molar broad, semi-triturative with one simple seta; accessory setal row with four plumose setae; lacinia mobilis well-marked and multi-cuspidate apically; incisor process multi-cuspidate; palp 3-articulate; article 1 1.7× longer than wide; article 2 3.2× longer than wide, ventral margin with two simple setae; article 3 5.8× longer than wide, tapering distally with three slender setae apically. Maxilla 1 inner plate rounded, with three simple setae on apical margin; outer plate rectangular with six simple robust setae and one plumose seta on apical margin, medial margin without sparse setules apically or not visible; palp 2-articulate, article 2 tapering distally, with three slender apical setae. Maxilla 2 outer plate subequal in size to inner plate, apical margin with nine simple setae; inner plate with five simple setae and two stout plumose setae. Maxilliped inner plate suboval, apical margin with 2-Y shaped stout setae, three plumose setae and two simple setae, medial margin with two short setae; outer plate rounded, with two stout setae apically and five simple setae on dorsal margin; palp 4-articulate, article 12× longer than wide; article 2, 1.6× longer than wide and subequal in size with article 3, with one row of long setae; article 4 tapering distally, inner margin setose, claw present distally.
Figure 5.
Spelaeogammarus lundi sp. nov., ♀, holotype (ISLA97006). Scale bar: 1 mm.
Figure 5.
Spelaeogammarus lundi sp. nov., ♀, holotype (ISLA97006). Scale bar: 1 mm.
Gnathopod 1 coxa quadrate, 1.6× wider than long, with one seta on anterior margin; basis stout and rectangular, 1.5× longer than wide, anterior margin with four small setae, posterior margin with four slender setae, distoventral corner with one small seta; ischium short subtriangular with one seta on posterodistal margin; merus ventral margin setose with four slender setae; carpus posterodistal corner produced, without comb-scales, posterodistal margin with fringe of plumose setae; propodus stout, suboval, 1.8× longer than basis, palm acute, 1.6× longer than posterior margin, covered by few small setae, with six stout setae; dactylus long, curved, with one small simple seta dorsally, almost reaching the palmar corner. Gnathopod 2 coxa oval, 1.16× wider than long; basis subrectangular, 2.8× longer than wide, posterior margin with six slender setae and one small on posterodistal margin; ischium subquadrate with one seta on posterior margin, 1.6× longer than wide; merus slightly oval with two small setae, 1.5× longer than wide; carpus subtrapezoidal, 1.5× longer than wide, posterior margin setose with three sets of (3-3-3) slender setae, two setae on distal margin and one small seta on anterior margin; propodus slightly reduced, 2.3× longer than wide, anterior margin with seven slender setae; posterior margin with four sets of (2-3-3-3) slender setae; palm acute, 0.6× longer than posterior margin, covered by fringe of small setae, with two stout setae and three slender setae; dactylus small, curved, naked, not reaching the palmar corner. Pereopod 3 coxa suboval, 1.1× longer than wide, ventral margin with four setae; basis subrectangular, 3.2× longer than wide, anterior and posterior margins without setae, posterodistal corner with one small seta; merus, 3.1× longer than wide, slightly longer than carpus, anterior margin with two stout setae, anterodistal corner with two stout setae, posterior margin with one seta; carpus elongate, 3.8× longer than wide, slightly shorter than merus, anterior margin and posterior margin with two setae each; propodus elongate, 8.8× longer than wide, slightly shorter than carpus, anterior margin with four setae, posterior margin with seven small setae with accessory seta; dactylus slightly curved with one small seta on dorsal margin, apical nail present. Pereopod 4 subequal in length to pereopod 3. Pereopod 5 coxa bilobate, anterior lobe well developed, 1.4× wider than long, with six slender setae, posterior margin of posterior lobe slightly concave, with one stout seta; basis short, 2× longer than wide, anterior margin with six stout setae and one distal stout setae, anterior margin with one marginal stout seta; merus 4.1× longer than wide, anterior margin and posterior margin naked, two distal stout setae; carpus short, 4.8× longer than wide, posterior margin naked, anterodistal corner with two stout setae, anterior margin covered by fringe of slender setae; propodus short, 16× longer than wide, as long as carpus, with five lateral short setae, anterior margin with dense fringe of slender setae; dactylus slightly curved, apical nail present. Pereopod 6 longer and wider than pereopod 5, coxa bilobate, anterior lobe slightly more developed than posterior, posterior margin with one stout seta; basis suboval, 2× longer than wide, anterior margin with five stout setae, anterodistal corner with two stout setae, posterior margin with eight stout setae; merus slightly elongate, 4× longer than wide, anterior margin with three stout setae and two on the anterodistal corner, posterior margin with two stout setae and one stout seta on posterodistal corner; carpus slightly elongate, 5× longer than wide, 1.1× longer than merus, anterior margin with a dense fringe of slender setae and two small setae, anterodistal corner with two stout setae, posterior margin naked, posterodistal corner with one stout seta; propodus elongate, 12× longer than wide, 0.9× the length of carpus, with dense fringe of setae on anterior margin and 15 lateral sets of small setae (1-1-1-1-2-1-1-2-1-1-2-1-2-1-2) underneath, posterior margin naked, one stout seta on posterodistal margin; dactylus slightly curved, apical nail present. Pereopod 7 coxa subtriangular, anterior lobe well developed with one long seta on distal corner, 1.7× longer than wide; basis short and stout, 1.7× longer than wide, anterior margin with four small setae, posterior margin with five small setae, posterodistal corner with one seta; merus elongated, 3.9× longer than wide, anterior margin with three long setae, anterodistal corner with one long seta, posterior margin with four simple setae, posterodistal corner with two long setae; carpus rectangular, 4.9X×longer than wide, anterior margin with two sets of (2-2) setae, anterodistal corner with five long setae, posterior margin with four sets of (1-2-2-1) setae, posterodistal corner with four long setae; propodus elongated, 8.1× longer than wide, 1.06× longer than carpus, anterior margin with four sets of (2-3-4-4) setae, with few long setae on apical margin, posterior margin with dense fringe of thin elongated setae; dactylus long and slightly curved, apical nail present.
Figure 6.
Spelaeogammarus lundi sp. nov., ♀, holotype (ISLA97006). Scale bar: 1 mm.
Figure 6.
Spelaeogammarus lundi sp. nov., ♀, holotype (ISLA97006). Scale bar: 1 mm.
Coxal gills present, ventrally inserted on coxal plates of pereopods 4–6. Pleopod 1 peduncle elongated and rectangular, 5.1× longer than wide, with one pair of coupling spines; inner ramus 1-articulate, with four slender plumose setae; outer ramus 3-articulate, 2.1× longer than inner ramus; article 1 inner margin with four plumose setae, outer margin with five plumose setae; article 2 with two plumose setae, article 3, smallest, with two apical plumose setae. Pleopod 2 smallest, peduncle rectangular, 5.8× longer than wide, with one pair of coupling spines; inner ramus 1-articulate with four plumose slender setae; outer ramus 3-articulate, 1.5× longer than inner ramus; article 1 inner margin and outer margin with four plumose slender setae each, article 2 with two plumose setae, article 3, smallest, with two apical plumose setae. Pleopod 3 peduncle rectangular, 7.3× longer than wide, with one pair of coupling spines; inner ramus 1-articulate with six plumose slender setae; outer ramus 3-articulate, 2.4× longer than inner ramus; article 1 inner and outer margin with three slender plumose setae each, article 2 with two plumose setae, article 3, smallest, with two apical plumose setae.
Uropod 1 peduncle elongated, 3.6× longer than wide, with five ventral stout setae, four dorsal stout setae and three lateral setae; inner ramus slightly longer than outer ramus, both lanceolate; inner ramus 0.6× the peduncle length, with three dorsal setae and five apical stout setae; outer ramus with three dorsal setae and four stout apical setae. Uropod 2 the shortest, peduncle 2× longer than wide, with three distolateral stout setae; inner ramus 0.8× shorter than outer ramus, both lanceolate; inner ramus 0.9× the peduncle length, with three dorsal setae and four apical stout setae; outer ramus with two ventral, one dorsal and four apical stout setae. Uropod 3peduncle short, 2× longer than wide, with one distolateral stout seta and one ventrolateral stout seta; rami elongated, subequal, both lanceolate; inner ramus with five lateral sets of stout setae (1-1-2-2-2), two setae on dorsal margin and two apical setae, inner ramus slightly shorter than outer ramus; outer ramus with four ventral sets of stout setae (3-3-2-3); dorsal margin with six simple setae with accessory setae, with two–three bifid setae and three apical setae. Telson 1.4× longer than wide, apical margin with a slightly shallow U-shaped excavation bearing in each lobe one apical and two subapical stout setae.
Female: Oostegites long and naked inserted on coxal plates 2–5.
Figure 7.
Spelaeogammarus lundi sp. nov., ♀, holotype (ISLA97006). Scale bar: 1 mm.
Figure 7.
Spelaeogammarus lundi sp. nov., ♀, holotype (ISLA97006). Scale bar: 1 mm.
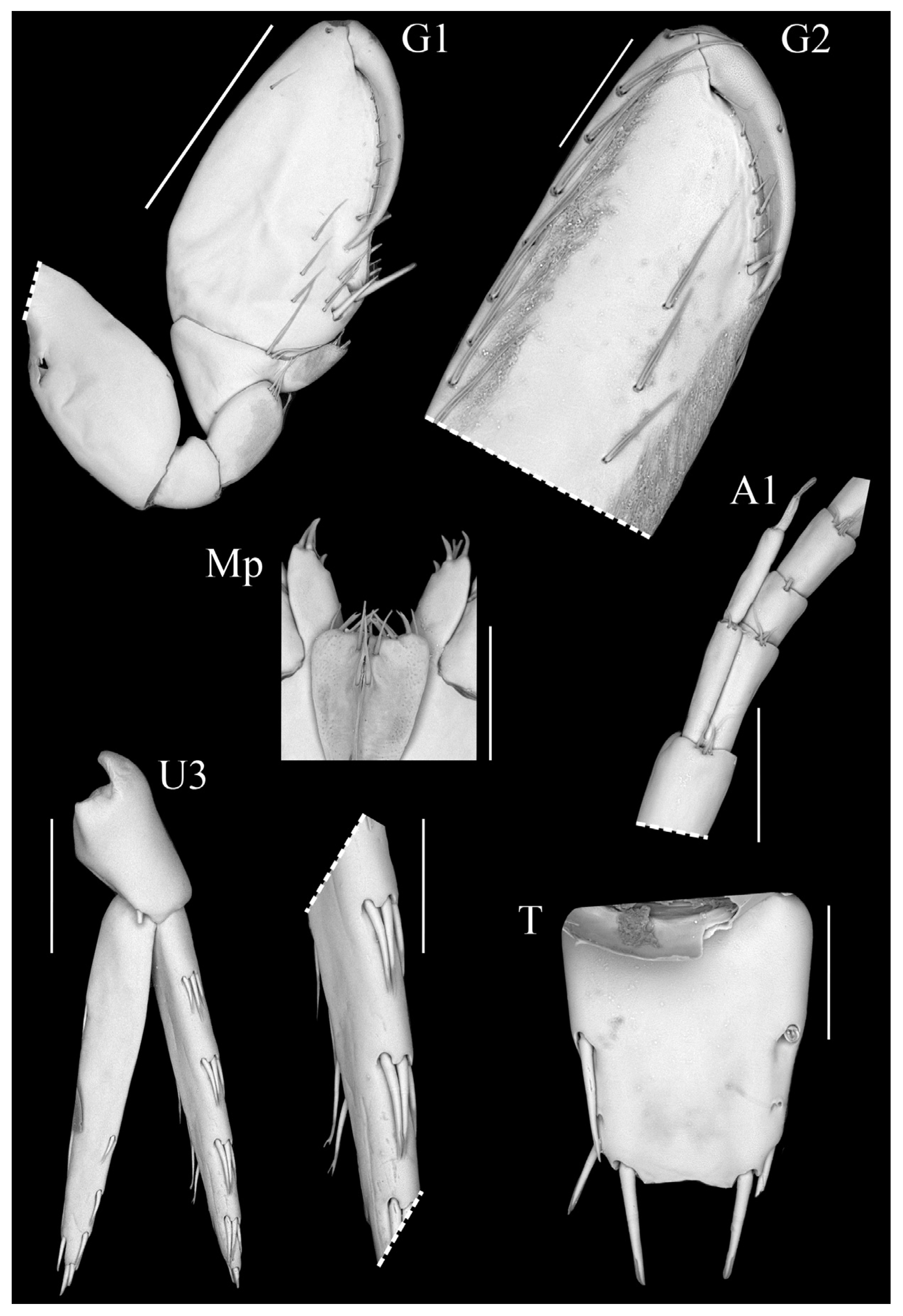
Figure 8.
Scanning electron micrographs of Spelaeogammarus lundi sp. nov., ♂, paratype (ISLA97009). Scale bars: 100 μm; gnathopod 1 = 500 μm.
Figure 8.
Scanning electron micrographs of Spelaeogammarus lundi sp. nov., ♂, paratype (ISLA97009). Scale bars: 100 μm; gnathopod 1 = 500 μm.
Morphological remarks
Spelaeogammarus lundi sp. nov. resembles
S. sanctus due to one apical + two subapical stout setae per lobe on telson. By the number of plumose setae on the apical margin of the inner plate of maxilliped, it resembles
S. uai and
S. ginae. By the number of setae on anterior margin of gnathopod 1 basis, it resembles
S. ginae and
S. santanensis. Finally, it resembles
S. spinilacertus and
S. trajanoae according to the one stout seta on coxa 6 (
Table 1).
Although Spelaeogammarus lundi sp. nov. shares similarities with Spelaeogammarus rafaelae sp. nov. such as 5-articulate antenna 2 flagellum and one stout seta on coxa 6, it is characterized by the presence of only two–four bifid setae on the dorsal margin of the uropod 3 outer ramus as well as through being the second species of the genus that presents a 3-articulate accessory flagellum.
Habitat and threats
Specimens of
S. lundi sp. nov. were found exclusively in Lagoinha cave, located on the outskirts of Montes Claros city, Minas Gerais state, southeastern Brazil. Despite sampling at numerous other caves in the region with perennial water bodies, no specimens of this new species were detected elsewhere. Lagoinha cave features two entrances: a larger one (
Figure 9A), leading to a spacious entry chamber (
Figure 9B), and a smaller one, providing access to a narrow conduit. The cave extends for 1080 m, with predominantly dry passages. In its deeper sections, the water table surfaces, forming pools of varying size and depth (
Figure 9C). These pools show fluctuating water levels, as evidenced by watermarks along the conduit edges, likely due to seasonal variations. Nevertheless, the environment maintains a degree of stability, indicated by the presence of “rafts” (speleothems on the pool surface—
Figure 9C), observed consistently during all visits, suggesting slow water level changes.
Specimens of
Spelaeogammarus lundi sp. nov. were observed in these pools (
Figure 9E), either swimming freely in the water column or moving along submerged walls. They share their habitat with stygobitic isopods of the genus
Xangoniscus (a species currently under description by the authors). The population density of
Spelaeogammarus lundi sp. nov. is relatively low compared to the isopods, which are abundant. Some pools contain submerged deposits of bat guano that attract large numbers of
Xangoniscus. However,
Spelaeogammarus lundi sp. nov. was not observed on these guano deposits.
Although the cave is surrounded by a sizable patch of secondary forest, its proximity to the city leads to occasional visits by curious explorers, who leave graffiti and litter behind. Additionally, evidence of historical saltpeter extraction is visible in several parts of the cave (
Figure 9D). Saltpeter, used in the 19th century for gunpowder production, was extensively mined from caves in various regions of Brazil. Despite these historical and recent anthropogenic impacts, the areas where
Spelaeogammarus lundi sp. nov. occur are relatively well preserved due to their inaccessibility, requiring navigation through very narrow passages that deter human intrusion.
Given the cave’s proximity to urban areas, concerns for the species’ conservation remain. Regular monitoring by environmental authorities is strongly recommended to assess the conservation status of the cave and the Spelaeogammarus lundi sp. nov. population.
Figure 9.
(A). The smaller entrance of Lapa da Lagoinha cave. (B). The larger entrance of Lapa da Lagoinha cave. (C). Pools inside the cave. (D). Collection of specimens. (E). Live specimens of Spelaeogammarus lundi sp. nov.
Figure 9.
(A). The smaller entrance of Lapa da Lagoinha cave. (B). The larger entrance of Lapa da Lagoinha cave. (C). Pools inside the cave. (D). Collection of specimens. (E). Live specimens of Spelaeogammarus lundi sp. nov.
Identification key for the genus Spelaeogammarus:
Accessory flagellum of antenna 1 with 4 articles; flagellum of antenna 2 with 5–7 articles; propodus of gnathopod 1 slightly longer than basis.......................................................................................................2.
- -
Accessory flagellum of antenna 1 with 5 or more articles; flagellum of antenna 2 with 8 or more articles; propodus of gnathopod 1 much longer or slightly longer than basis................................................................4.
Coxal plate 6 bearing 1 stout seta, slender setae absent; outer plate of maxilla 1 with 6 multi-cuspidate setae and 1 plumose seta.......................................3.
- -
Coxal plate 6 bearing 1 stout seta and >20 setae; outer plate of maxilla 1 with 7 multi-cuspidate setae......................... Spelaeogammarus bahiensis.
Inner plate of maxilliped with 4 plumose setae...................................... Spelaeogammarus trajanoae.
- -
Inner plate of maxilliped with 2–4 stout setae and 1 small seta; coxal plate 5 bearing 1 stout seta and >9 short setae.......................................................... Spelaeogammarus spinilacertus.
Accessory flagellum of antenna 1 with 4 articles; antenna 2 flagellum with 5 articles....................................................................5.
- -
Accessory flagellum of antenna 1 with 5 articles; coxa 5 with posterior lobe round..............................................................................6.
Anterior margin of gnathopod 1 basis with 2 small setae; outer ramus of uropod 3 with 7–8 bifid setae.............................. Spelaeogammarus rafaelae sp. nov.
- -
Anterior margin of gnathopod 1 basis with 4 small setae; outer ramus of uropod 3 with 2–4 bifid setae; accessory flagellum of antenna 1 with 3 articles........................................................ Spelaeogammarus lundi sp. nov.
Posterior margin of gnathopod 1 basis with bifid setae; propodus of gnathopod 1 slightly longer than basis...................................Spelaeogammarus ginae.
- -
Posterior margin of gnathopod 1 basis with simple setae; propodus of gnathopod 1 longer than basis.........................................7.
Inner ramus of uropod 3 with 8–18 dorsal bifid setae. .......................................................8.
- -
Inner ramus of uropod 3 with more than 18 dorsal bifid setae.
...................................................................................... Spelaeogammarus sanctus.
Inner plate of maxilliped with 3 plumose setae; anterior margin of gnathopod 1 basis with 3–5 setae; posterior margin of gnathopod 2 basis with 4–6 setae................................................ Spelaeogammarus uai.
- -
Inner plate of maxilliped with 2 plumose setae or fewer; anterior margin of gnathopod 1 basis with less than 3 setae..............................................................................9.
Accessory flagellum of antenna 1 with 6 articles; propodus of gnathopod 1 1.8× longer than basis; body length up to 18.3 mm..................................................................... Spelaeogammarus titan.
- -
Accessory flagellum of antenna 1 with 5 articles; propodus of gnathopod 1 slightly longer than basis........................................................ Spelaeogammarus santanensis.