Four New Caddisfly Species of Marilia Müller, 1880 (Trichoptera: Odontoceridae) from a Tailings Dam Disaster Area, Rio Doce basin, Brazil †
Abstract
1. Introduction
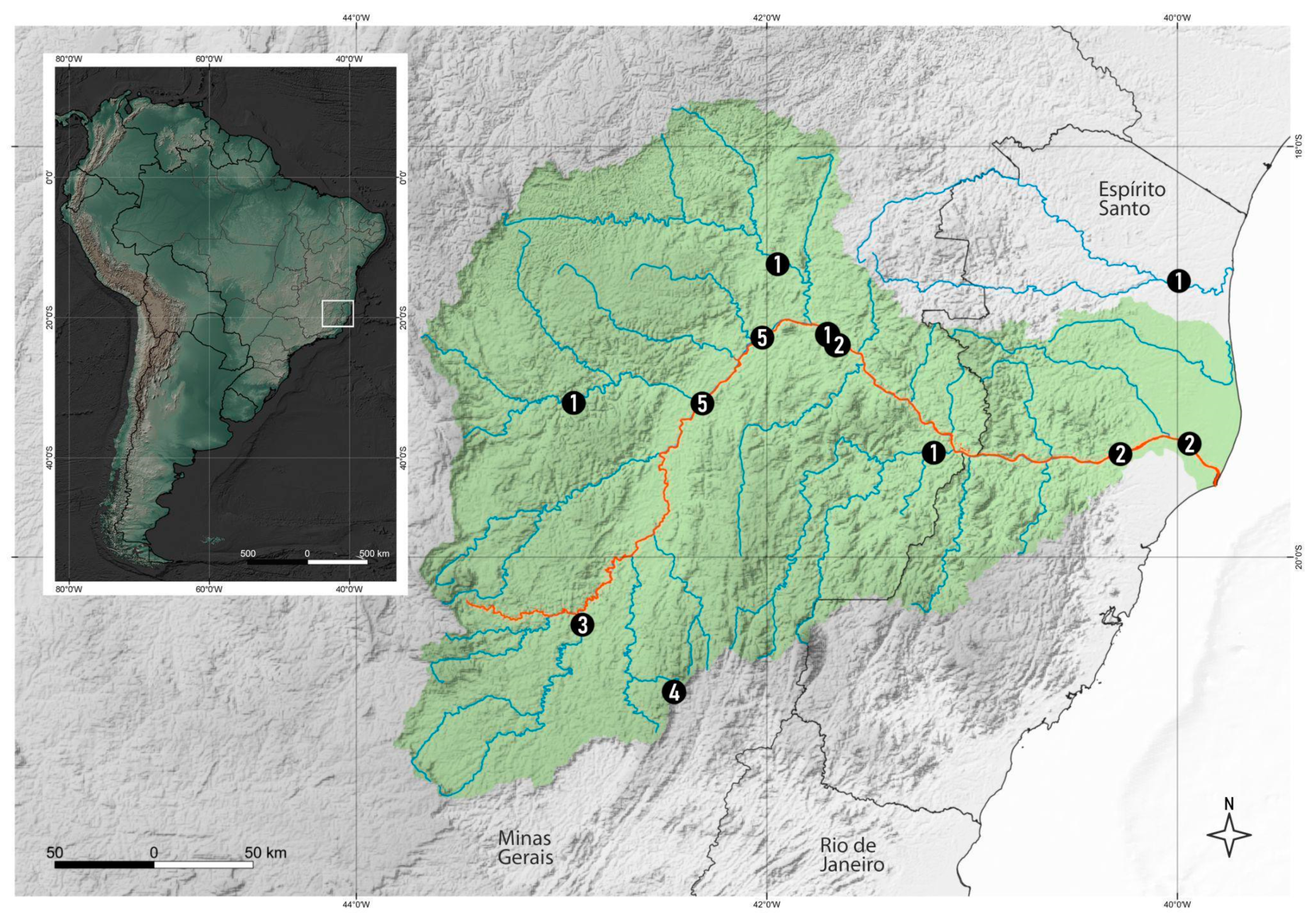
2. Materials and Methods
3. Results
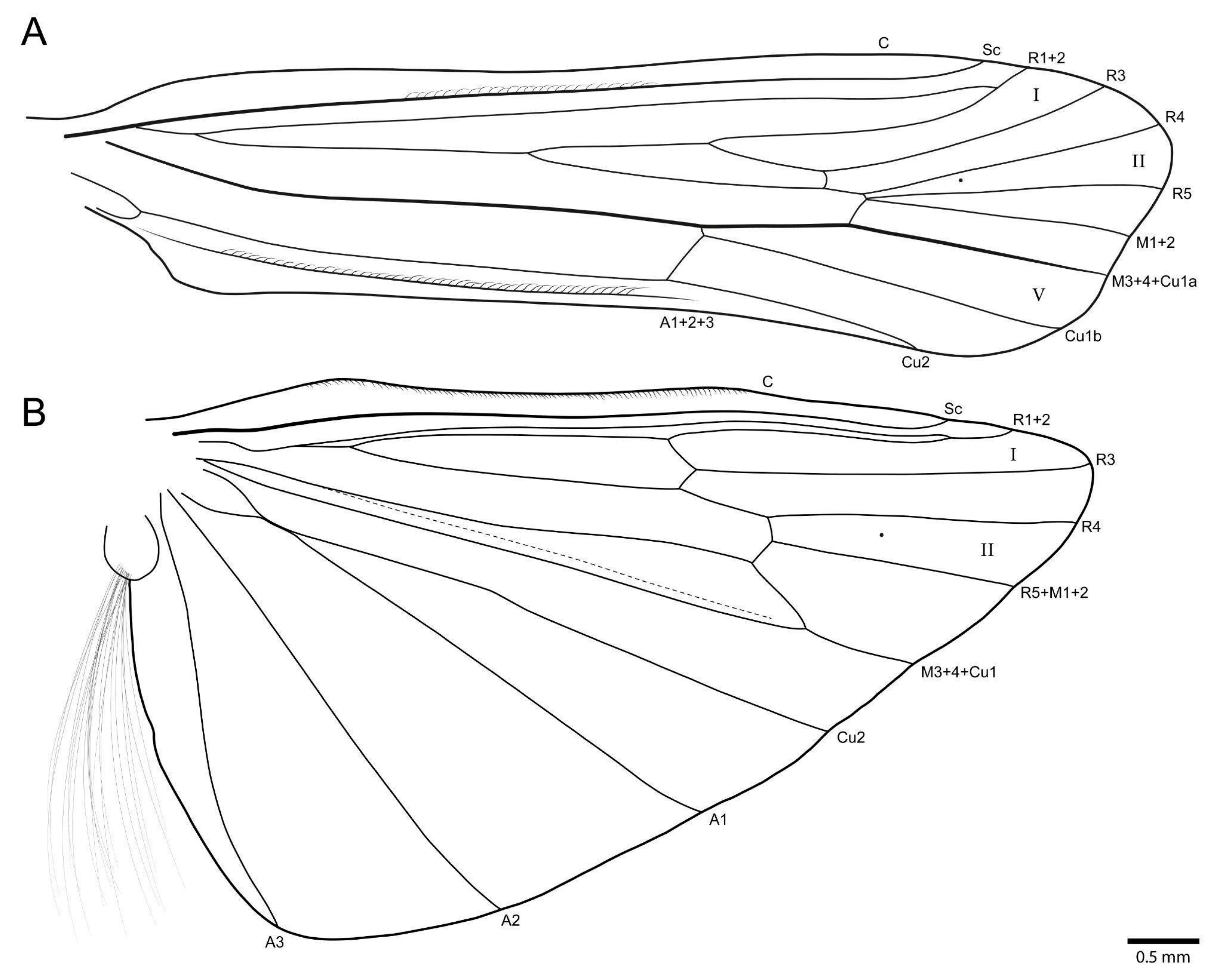
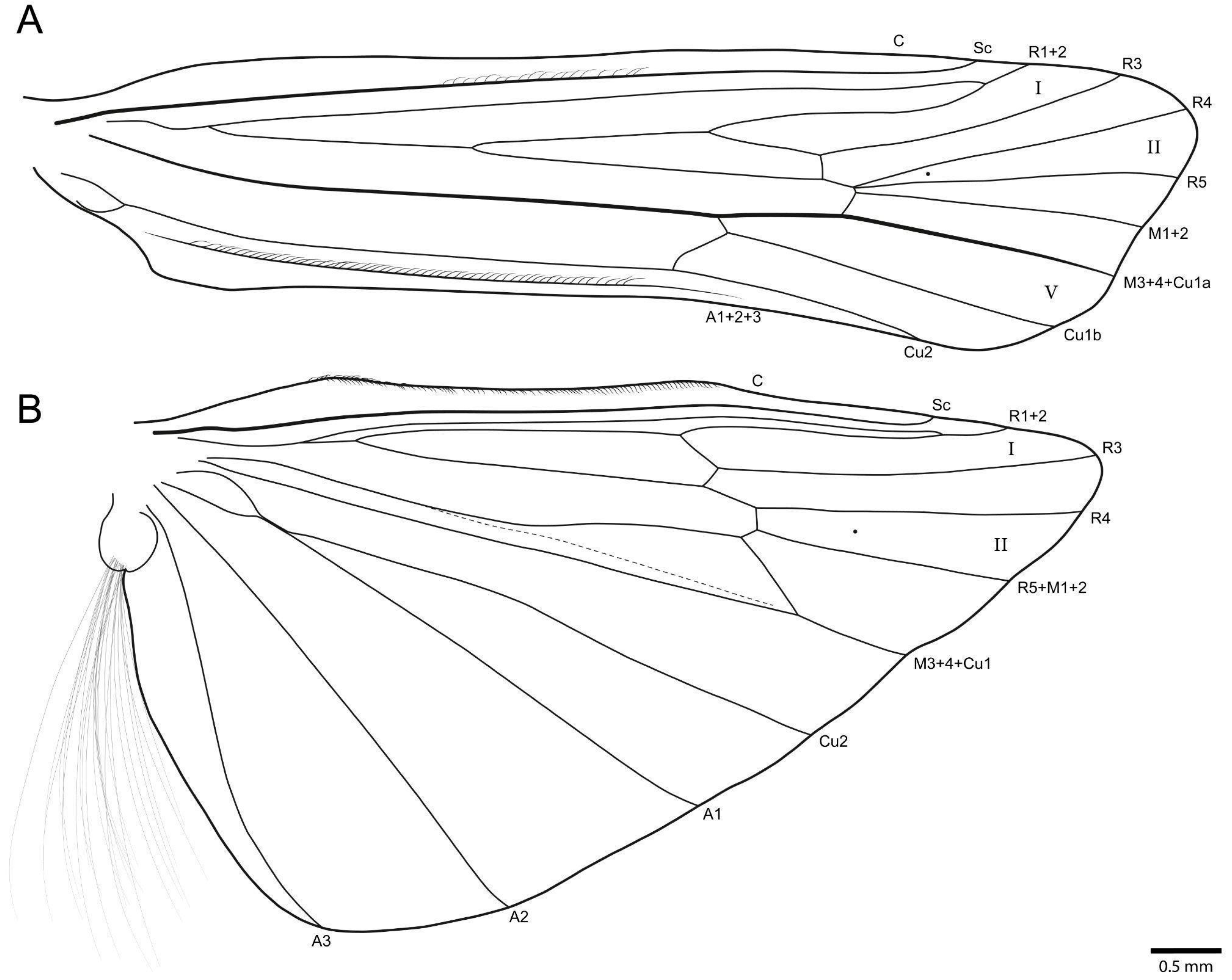
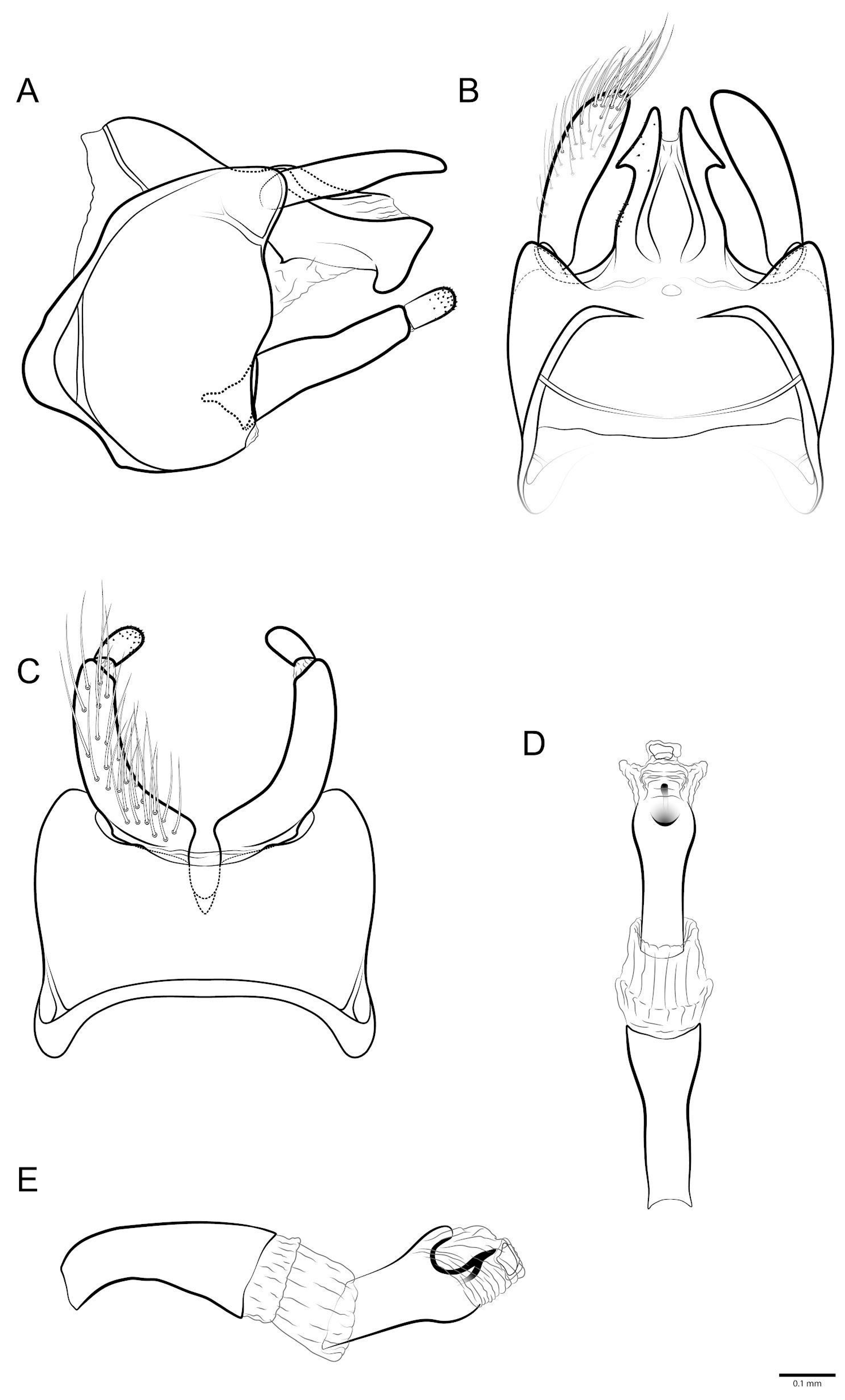
3.1. Taxonomy
- Genus Marilia Müller, 1880
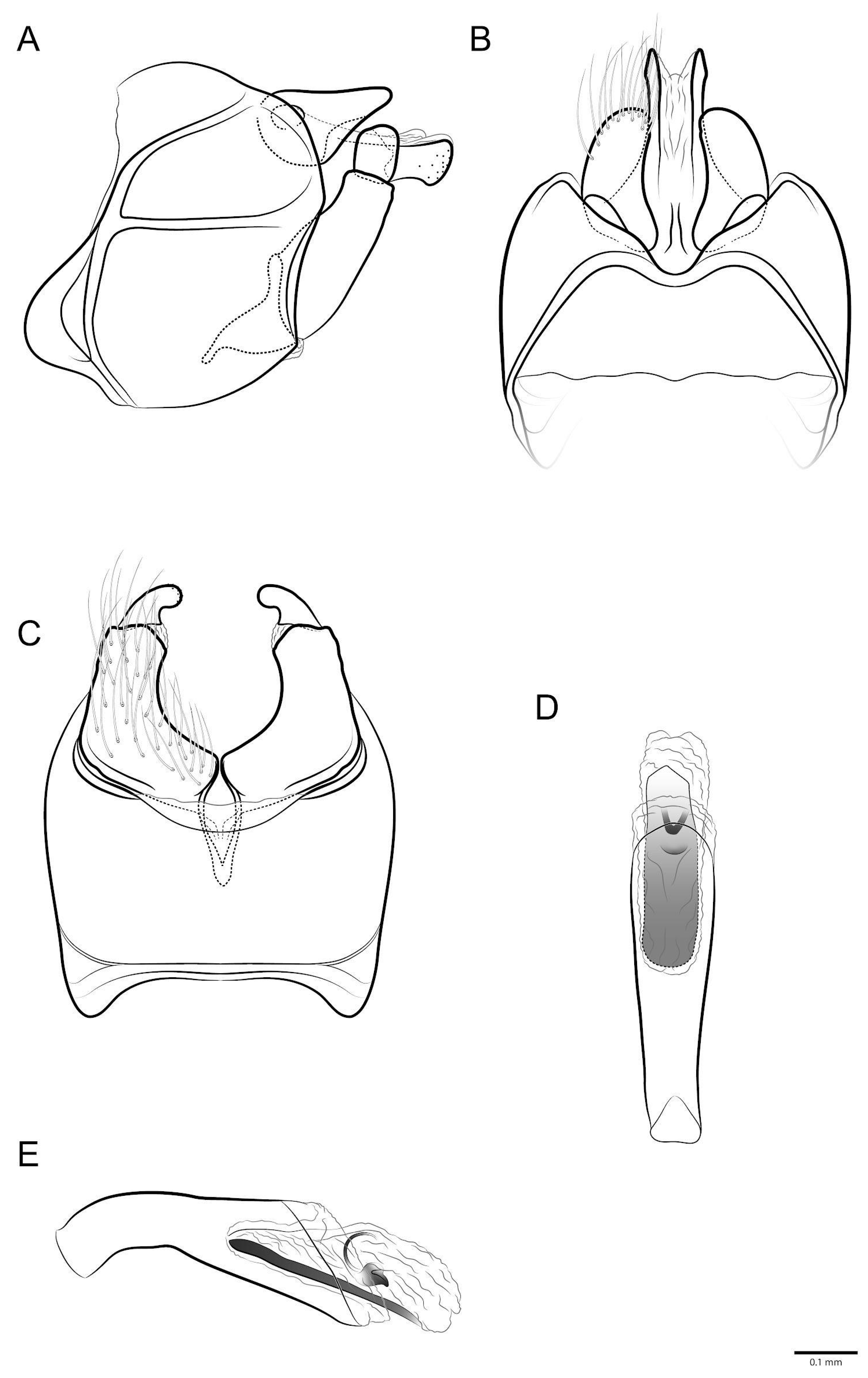
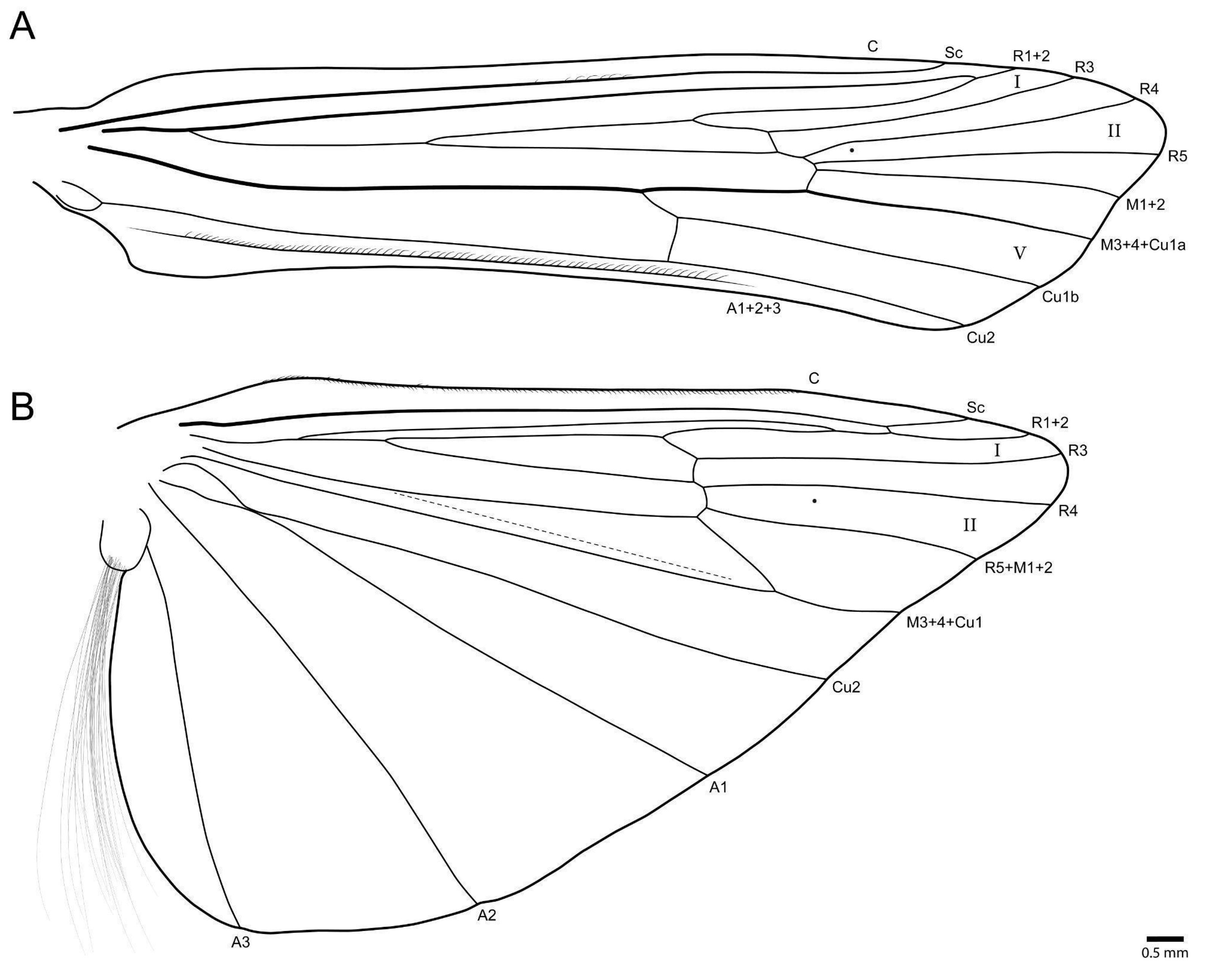
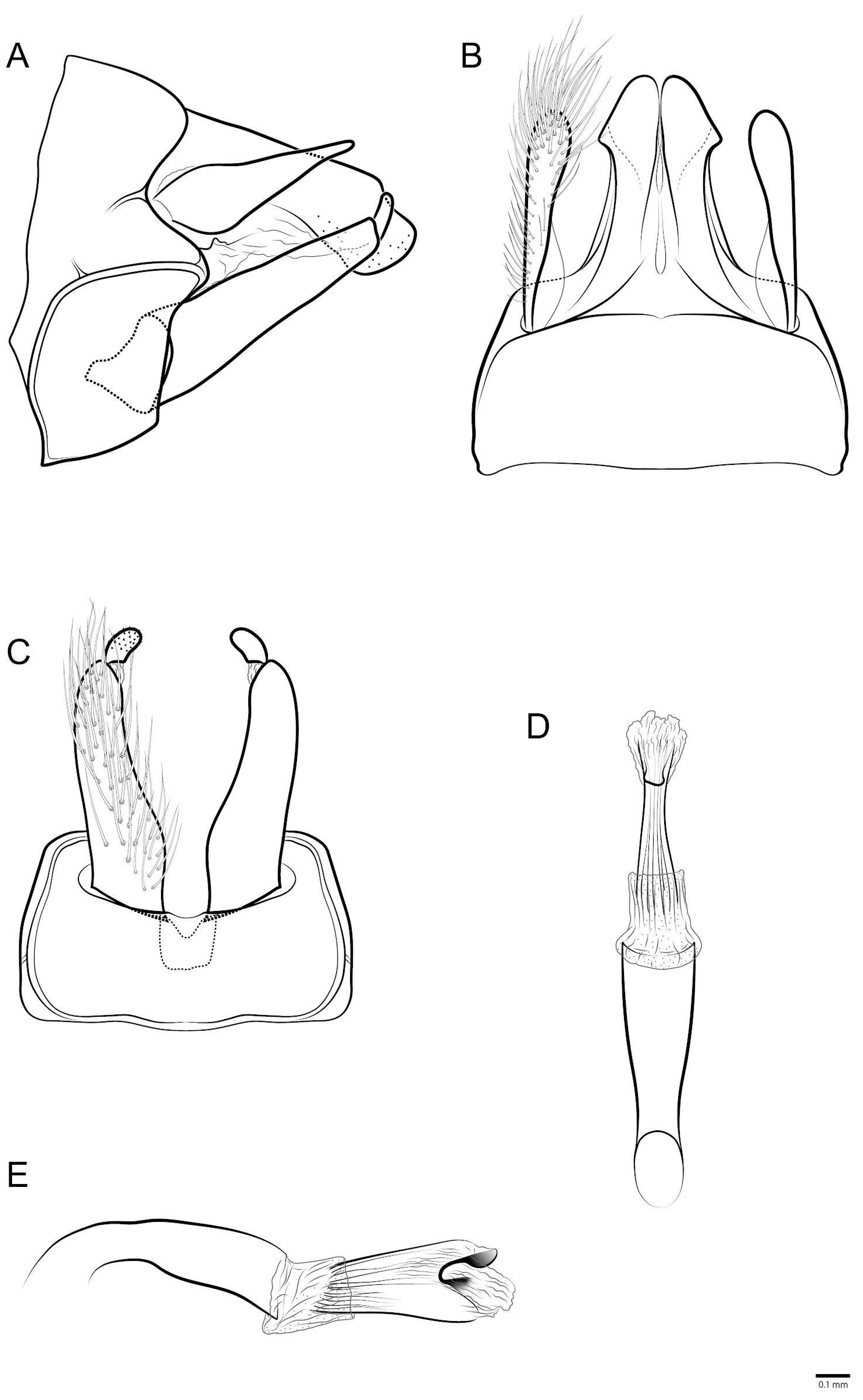
- Marilia aranan sp. nov.
- Marilia krenak sp. nov.
- Marilia maxakali sp. nov.
- Marilia mukurin sp. nov.
3.2. New Records
- Marilia guaira Flint, 1983
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morse, J.C.; Frandsen, P.B.; Graf, W.; Thomas, J.A. Diversity and ecosystem services of Trichoptera. Insects 2019, 10, 125. [Google Scholar] [CrossRef]
- Morse, J.C. Trichoptera World Checklist. 2023. Available online: https://trichopt.app.clemson.edu/search.php (accessed on 15 May 2023).
- Huamantinco, A.A.; Nessimian, J.L. A new Neotropical genus and species of Odontocerinae (Trichoptera: Odontoceridae) from southeastern Brazil. Aquat. Insects 2004, 26, 281–288. [Google Scholar] [CrossRef]
- Burmeister, H. Handbuch der Entomologie, Zweiter Band, Zweite Ubtheilung. Theod. Chr. Friedr. Enslin Berl. 1839, 12, 397–1050. [Google Scholar] [CrossRef]
- Müller, F. Sôbre as casas construídas pelas larvas de insectos Trichopteros da Província de Santa Catharina. Arch. Mus. Nac. Rio De Jan. 1880, 3, 99–134. [Google Scholar]
- Calor, A.R.; Santos, A.P.M. Trichoptera in: Catálogo Taxonômico da Fauna do Brazil. PNUD. 2023. Available online: http://fauna.jbrj.gov.br/ (accessed on 15 May 2023).
- Wiggins, G.B. Larvae of the North American Caddisfly Genera (trichoptera); University of Toronto Press: Toronto, ON, Canada, 1996; 400p. [Google Scholar] [CrossRef]
- Holzenthal, R.W.; Blahnik, R.J.; Prather, A.P.; Kjer, K.M. Order Trichoptera Kirby, 1813 (Insecta), Caddisflies. Zootaxa 2007, 1668, 639–698. [Google Scholar] [CrossRef]
- Camargos, L.M.; Pes, A.M.; Hamada, N. New Neotropical species of Marilia Müller (Trichoptera: Odontoceridae). Zootaxa 2020, 4853, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Schmid, F. The Insects and Arachnids of Canada, Part 7. Genera of the Trichoptera of Canada and Adjoining or Adjacent United States; NRC Research Press: Ottawa, ON, Canada, 1998; 319 pp. [Google Scholar]
- Pes, A.M.; Holzenthal, R.W.; Sganga, J.V.; Santos, A.P.M.; Barcelos-Silva, P.; Camargos, L.M. Order Trichoptera. In Keys to Neotropical Hexapoda. Vol III. Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Hamada, N., Thorp., J.H., Rogers, D.C., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 237–323. [Google Scholar] [CrossRef]
- Pes, A.M.; Santos, A.P.M.; Barcelos-Silva, P.; Camargos, L.M. Ordem Trichoptera, In Insetos Aquáticos na Amazônia Brasileira: Taxonomia, Biologia e Ecologia; Hamada, N., Nessimian, J., Querino, R.B., Eds.; Editora do Instituto Nacional de Pesquisas da Amazônia: Manaus, Brazil, 2019; pp. 387–429.
- Dumas, L.L.; Nessimian, J.L. Description of two new species of Marilia Müller (Trichoptera, Odontoceridae) from southeastern Brazil, including the description of the female of Marilia major Müller. Rev. Bras. Entomol. 2009, 53, 344–348. [Google Scholar] [CrossRef][Green Version]
- Flint, O.S., Jr. Studies of Neotropical Caddisflies, XXXIII: New species from austral South America (Trichoptera). Smithson. Contrib. Zool. 1983, 377, 1–100. [Google Scholar] [CrossRef]
- Costa, A.M. Revisão taxonômica e filogenia das espécies de Marilia Müller, 1880 (Trichoptera: Odontoceridae) da Região Neotopical. Ph.D. Thesis, Universidade de São Paulo, Ribeirão Preto, Brazil, 2016; 157p. [Google Scholar]
- Oláh, J.; Johanson, K.A. Description of 33 new species of Calamoceratidae, Molannidae, Odontoceridae and Philorheithridae (Trichoptera), with detailed presentation of their cephalic setal warts and grooves. Zootaxa 2010, 2457, 1–128. [Google Scholar] [CrossRef]
- Yang, L.F.; Yuan, H.Y.; Morse, J.C. Lannapsyche and Marilia species of China (Trichoptera: Odontoceridae). Zootaxa 2017, 4320, 81–99. [Google Scholar] [CrossRef]
- Flint, O.S., Jr. Studies of Neotropical caddisflies, XLV: The taxonomy, phenology, and faunistics of Trichoptera of Antioquia, Colombia. Smithson. Contrib. Zool. 1991, 520, 1–113. [Google Scholar] [CrossRef]
- Bueno-Soria, J.; Rojas-Ascencio, A. New species and distribution of the genus Marilia Muller (Trichoptera; Odontoceridae) in Mexico and Central America. Proc. Entomol. Soc. Wash. 2004, 106, 679–696. [Google Scholar]
- Coelho, A.L.N. Bacia hidrográfica do Rio Doce (MG/ES): Uma análise socioambiental integrada. Rev. Geogr. 2009, 7, 131–146. [Google Scholar] [CrossRef]
- Segura, F.R.; Nunes, E.A.; Panis, F.P.; Paulelli, A.C.C.; Rodrigues, G.B.; Braga, G.U.L.; Filho, W.R.P.; Barbosa, F., Jr.; Cerchiaro, G.; Silva, F.F.; et al. Potential risks of the residue from Samarco’s mine dam burst (Bento Rodrigues, Brazil). Environ. Pollut. 2016, 218, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Carmo, F.F.; Kamino, L.H.Y.; Junior, R.T.; Campos, I.C.; Carmo, F.F.; Silvino, G.; Castro, K.J.S.X.; Mauro, M.L.; Rodrigues, N.U.A.; Miranda, M.P.S.; et al. Fundão tailings dam failures: The environment tragedy of the largest technological disaster of Brazilian mining in global context. Perspect. Ecol. Conserv. 2017, 15, 145–151. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Nobrega, G.N.; Ferreira, T.O.; Almeida, L.S.; Romero, T.B.; Santaella, S.T.; Bernardino, A.F.; Otero, X.L. The Samarco mine tailing disaster: A possible time-bomb for heavy metals contamination? Sci. Total Environ. 2018, 637–638, 498–506. [Google Scholar] [CrossRef]
- Rudorff, N.; Rudorff, C.M.; Kampel, M.; Ortiz, G. Remote sensing monitoring of the impact of a major mining wastewater disaster on the turbidity of the Doce River plume off the eastern Brazilian coast. ISPRS J. Photogramm. Remote Sens. 2018, 145, 349–361. [Google Scholar] [CrossRef]
- CEPTA [Centro Nacional de Pesquisa e Conservação da Biodiversidade Aquática Continental] Nota técnica Nº. 24, de 24 de Novembro de 2015. Consequências Parciais na Biodiversidade Aquática da bacia do rio doce, Provocadas pelo Rompimento da Barragem de Rejeitos de Mineração da Samarco Mineradora S.A. no município de Mariana, MG. Available online: https://www.icmbio.gov.br/portal/publicacoes?id=7862:documentos-rio-doce (accessed on 18 April 2022).
- Lopes, L.M.N. O rompimento da barragem de Mariana e seus impactos socioambientais. Sinapse Múltipla 2016, 5, 1. [Google Scholar]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish, 2nd ed.; EPA 841-B-99-002; U.S. Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999. Available online: https://www3.epa.gov/region1/npdes/merrimackstation/pdfs/ar/AR-1164.pdf (accessed on 31 January 2022).
- Frost, S.W. Light traps for insect collection, survey and control. Bull. Pa. State Coll. Agric. Exp. Stn. 1952, 550, 1–32. [Google Scholar]
- Rocha, I.C.; Souza, W.R.M. Marilia caramuru sp. nov. and Marilia paraguassu sp. nov. (Trichoptera: Odontoceridae), two new species from Maranhão State, Northeast Brazil. Zootaxa 2018, 4425, 393–400. [Google Scholar] [CrossRef]
- Blahnik, R.J.; Holzenthal, R.W. Collection and curation of Trichoptera, with an emphasis on pinned material. Nectopsyche Neotrop. Trichoptera Newsl. 2004, 1, 8–20. [Google Scholar]
- Mosely, M.E.; Kimmins, D.E. The trichoptera (Caddis-Flies) of Australia and New Zealand; British Museum (Natural History): London, UK, 1953; 550p. [Google Scholar] [CrossRef]
- Cordeiro, M.C.; Garcia, G.D.; Rocha, A.M.; Tschoeke, D.A.; Campeão, M.E.; Appolinario, L.R.; Soares, A.C.; Leomil, L.; Froes, A.; Bahiense, L.; et al. Insights on the freshwater microbiomes metabolic changes associated with the world’s largest mining disaster. Sci. Total Environ. 2019, 654, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Duarte, E.B.; Neves, M.A.; de Oliveira, F.B.; Martins, M.E.; de Oliveira, C.H.R.; Burak, D.L.; Orlando, M.T.D.; Rangel, C.V.G.T. Trace metals in Rio Doce sediments before and after the collapse of the Fundão iron ore tailing dam, Southeastern Brazil. Chemosphere 2021, 262, 127879. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, F.Â.; Ferreira, A.D.; Queiroz, H.M.; Vasconcelos, A.L.S.; Ferreira, T.O.; Bernardino, A.F. Long-term contamination of the Rio Doce estuary as a result of Brazil’s largest environmental disaster. Perspect. Ecol. Conserv. 2021, 19, 417–428. [Google Scholar] [CrossRef]
- Gomes, L.E.O.; Correa, L.B.; Sá, F.; Neto, R.R.; Bernardino, A.F. The impacts of the Samarco mine tailing spill on the Rio Doce estuary, Eastern Brazil. Mar. Pollut. Bull. 2017, 120, 28–36. [Google Scholar] [CrossRef]
- Vergilio, C.D.S.; Lacerda, D.; Souza, T.D.S.; de Oliveira, B.C.V.; Fioresi, V.S.; de Souza, V.V.; Rodrigues, G.D.R.; Barbosa, M.K.D.A.M.; Sartori, E.; Rangel, T.P.; et al. Immediate and long-term impacts of one of the worst mining tailing dam failure worldwide (Bento Rodrigues, Minas Gerais, Brazil). Sci. Total Environ. 2021, 756, 143697. [Google Scholar] [CrossRef]
- Wild, M.; Rouhani, S.; Oehrig, J.; Alves, P.H.G.; Odle, W.; Gaspar, D.F.A. Using Spatiotemporal Ratio Analyses to Quantitatively Estimate Water Quality Recovery of the Rio Doce. Integr. Environ. Assess. Manag. 2023. [Google Scholar] [CrossRef]
- Yamamoto, F.; Onishi, K.; Ralha, T.; Silva, L.; Deda, B.; Pessali, T.; Souza, C.; Ribeiro, C.O.; Abessa, D. Earlier biomarkers in fish evidencing stress responses to metal and organic pollution along the Doce River Basin. Environ. Pollut. 2023, 329, 121720. [Google Scholar] [CrossRef]
- Aguila, G.E.N.; Pujoni, D.G.F.; Marques, M.M.; Santos, L.G.C.; Dornelas, N.M.d.L.; Andrade, K.; Monteiro, I.M.; Maia-Barbosa, P.M.; Barbosa, F.A.R. Benthic macroinvertebrate diversity in the Middle Doce River Basin, Brazil. Data 2018, 3, 17. [Google Scholar] [CrossRef]
- Cota, L.; Goulart, M.; Moreno, P.; Callisto, M. Rapid assessment of river water quality using an adapted BMWP index: A practical tool to evaluate ecosystem health. Int. Ver. Für Theor. Und Angew. Limnol. Verhandlungen 2002, 28, 1713–1716. [Google Scholar] [CrossRef]
- Marques, M.G.S.M.; Ferreira, R.L.; Barbosa, F.A.R. A comunidade de macroinvertebrados aquáticos e características limnológicas das lagoas Carioca e da Barra, Parque Estadual do Rio Doce, MG. Rev. Bras. Biol. 1999, 59, 203–210. [Google Scholar] [CrossRef]
- Marques, M.M.; Barbosa, F. Biological quality of waters from an impacted tropical watershed (middle Rio Doce basin, southeast Brazil), using benthic macroinvertebrate communities as an indicator. Hydrobiologia 2021, 457, 69–76. [Google Scholar] [CrossRef]
- Moretti, M.S.; Callisto, M. Biomonitoring of benthic macroinvertebrates in the middle Doce River watershed. Acta Limnol. Bras. 2005, 17, 267–281. [Google Scholar]
- Gammon, J.R. The Effect of Inorganic Sediment on Stream Biota; U.S. Environmental Protection Agency, Water Quality Office 18050 DWC: Washington, DC, USA, 1970; 108–113p. [Google Scholar]
- White, D.S. The effect of inorganic limestone sediment on the macroinvertebrates of Deer Creek. Master’s Thesis, DePauw University, Greencastle, IN, USA, 1970; 135p. [Google Scholar]
- Herbert, D.W.; Alabaster, J.S.; Dart, M.C.; Lloyd, R. The effect of chinaclay wastes on trout streams. Int. J. Air Watsr Poll. 1961, 5, 56–74. [Google Scholar]
- Kaufmann, P.R.; Larsen, D.P.; Faustini, J.M. Bed stability and sedimentation associated with human disturbances in Pacific Northwest streams. J. Am. Water Resour. Assoc. 2009, 45, 434–459. [Google Scholar]
- Williams, D.D.; Smith, M.R. Colonization dynamics of river benthos in response to local changes in bed characteristics. Freshw. Biol. 1996, 36, 237–248. [Google Scholar]
- Sarriquet, P.E.; Bordenave, P.; Marmonier, P. Effects of bottom sediment restoration on interstitial habitat characteristics and benthic macro-invertebrate assemblages in a headwater stream. River Res. Appl. 2007, 23, 815–828. [Google Scholar] [CrossRef]
- Collier, K.J.; Wilcock, R.J.; Meredith, A.S. Influence of substrate type and physico-chemical conditions on macroinvertebrate faunas and biotic indices of some lowland Waikato, New Zealand, streams. N. Z. J. Mar. Freshw. Res. 1998, 32, 1–19. [Google Scholar]
- Angradi, T.R. Fine sediment and macroinvertebrate assemblages in Appalachian streams: A field experiment with biomonitoring applications. J. N. Am. Benthol. Soc. 1999, 18, 49–66. [Google Scholar] [CrossRef]
- Armitage, P.D.; Cannan, C.E. Annual changes in summer patterns of mesohabitat distribution and associated macroinvertebrate assemblages. Hydrol. Process. 2000, 14, 3161–3179. [Google Scholar] [CrossRef]
- Buffagni, A.; Crosa, G.A.; Harper, D.M.; Kemp, J.L. Using macroinvertebrate species assemblages to identify river channel habitat units: An application of the functional habitats concept to a large, unpolluted Italian river (River Ticino, northern Italy). Hydrobiologia 2000, 435, 213–225. [Google Scholar]
- Lancaster, J. Scaling the effects of predation and disturbance in a patchy environment. Oecologia 1996, 107, 321–331. [Google Scholar] [PubMed]
- Swan, C.M.; Palmer, M.A. What drives small—Scale spatial patterns in lotic meiofauna communities? Freshw. Biol. 2000, 44, 109–121. [Google Scholar]
- Gjerløv, C.; Hildrew, A.G.; Jones, J.I. Mobility of stream invertebrates in relation to disturbance and refugia: A test of habitat templet theory. J. N. Am. Benthol. Soc. 2003, 22, 207–223. [Google Scholar] [CrossRef]
- Armitage, P.D.; Moss, D.; Wright, J.F.; Furse, M.T. The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running-water sites. Water Res. 1983, 17, 333–347. [Google Scholar]
- Junqueira, M.V.; Amarante, M.; Dias, C.; França, E. Biomonitoramento de qualidade das águas da Bacia do Rio das Velhas (MG/Brasil) através de macroinvertebrados. Acta Limnol. Bras. 2000, 12, 7387. [Google Scholar]
- Muller, K. Investigations on the organic drift in north swedish streams. Rep. Inst. Freshwat. Res. Drottningholm 1954, 35, 133–148. [Google Scholar]
- Waters, T.F. Interpretation of invertebrate drift in streams. Ecology 1965, 46, 327–334. [Google Scholar] [CrossRef]
- Gray, L.J.; Ward, J.V. Effects of sediment releases from a reservoir on stream macroinvertebrates. Hydrobiologia 1982, 96, 177–184. [Google Scholar]
- Cairns, J.J.; Cıtossman, J.S.; Dıckson, K.L.; Herricks, E.E. The recovery of damaged Streams. Ass. South-East. Biol. Bull. 1971, 18, 79–106. [Google Scholar]
- Williams, D.D.; Hynes, H.B.N. The recolonization mechanisms of stream benthos. Oikos 1976, 27, 265–272. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonfá Neto, P.; Salles, F.F.; Vilarino, A. Four New Caddisfly Species of Marilia Müller, 1880 (Trichoptera: Odontoceridae) from a Tailings Dam Disaster Area, Rio Doce basin, Brazil. Taxonomy 2023, 3, 381-400. https://doi.org/10.3390/taxonomy3030022
Bonfá Neto P, Salles FF, Vilarino A. Four New Caddisfly Species of Marilia Müller, 1880 (Trichoptera: Odontoceridae) from a Tailings Dam Disaster Area, Rio Doce basin, Brazil. Taxonomy. 2023; 3(3):381-400. https://doi.org/10.3390/taxonomy3030022
Chicago/Turabian StyleBonfá Neto, Pedro, Frederico Falcão Salles, and Albane Vilarino. 2023. "Four New Caddisfly Species of Marilia Müller, 1880 (Trichoptera: Odontoceridae) from a Tailings Dam Disaster Area, Rio Doce basin, Brazil" Taxonomy 3, no. 3: 381-400. https://doi.org/10.3390/taxonomy3030022
APA StyleBonfá Neto, P., Salles, F. F., & Vilarino, A. (2023). Four New Caddisfly Species of Marilia Müller, 1880 (Trichoptera: Odontoceridae) from a Tailings Dam Disaster Area, Rio Doce basin, Brazil. Taxonomy, 3(3), 381-400. https://doi.org/10.3390/taxonomy3030022






