A New Species of Pareas Wagler, 1830 (Squamata, Pareidae) from Northwestern Yunnan, China †
Abstract
1. Introduction
2. Materials and Methods
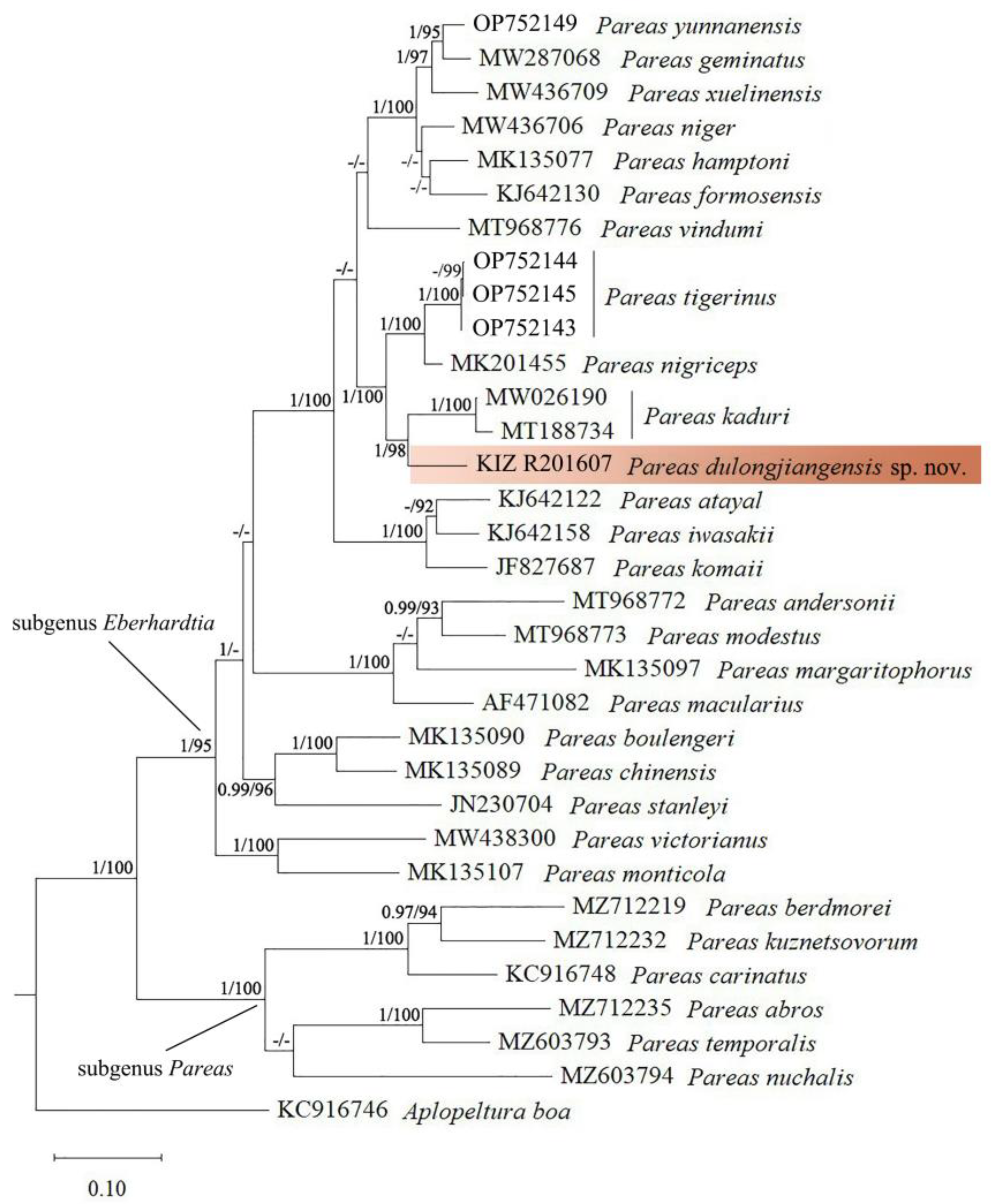
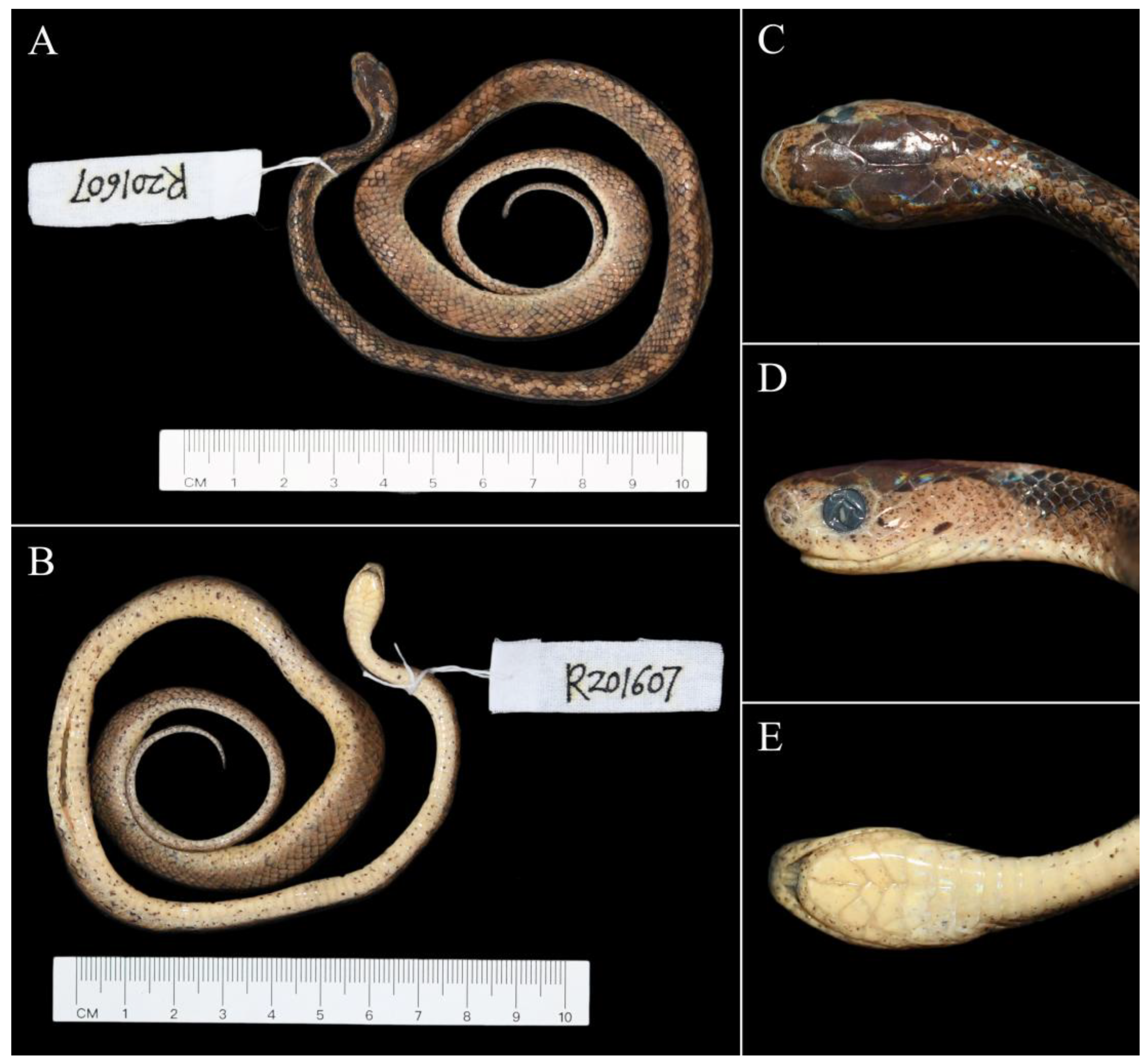
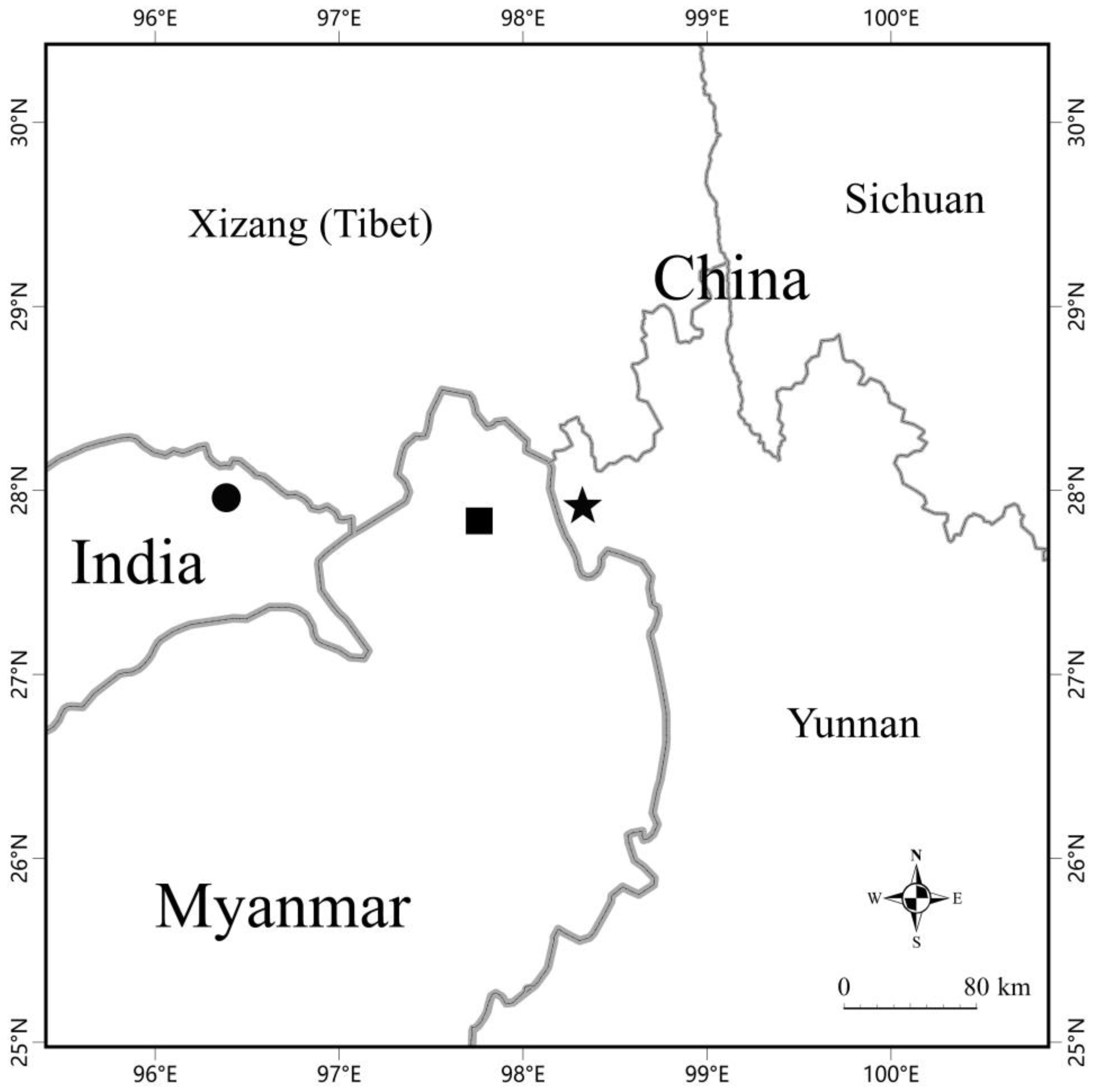
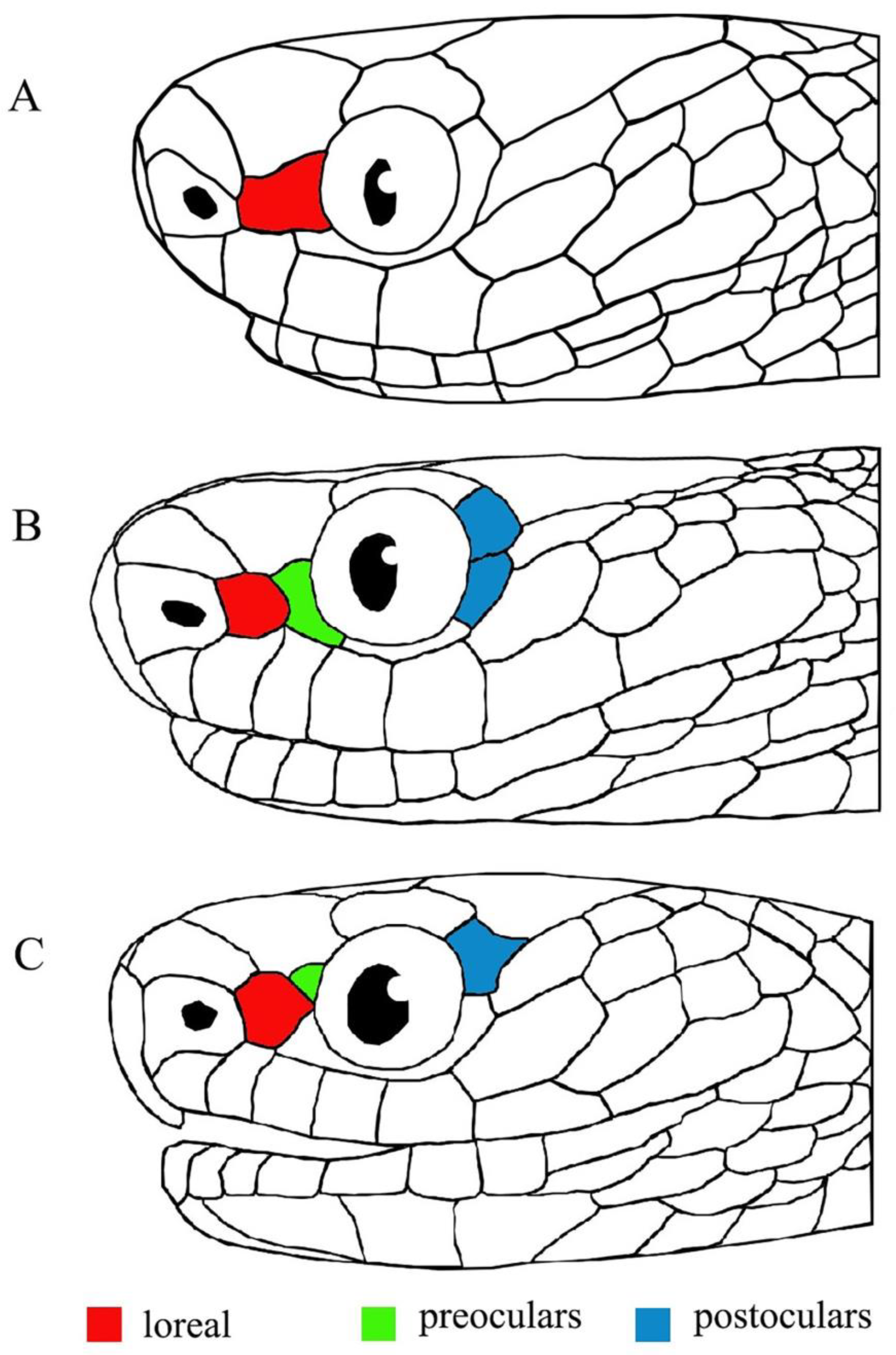
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, M.A. The Fauna of British India, Ceylon and Burma, including the Whole of the Indo-Chinese Subregion. Reptilia and Amphibia. Vol. III. Serpentes; Taylor and Francis: London, UK, 1943. [Google Scholar]
- Zhao, E.M.; Huang, M.H.; Zong, Y.; Jiang, Y.M.; Huang, Q.Y.; Zhao, H.; Ma, J.F.; Huang, Z.J.; Wei, G.; Yang, D.T.; et al. Fauna Sinica, Reptilia, Squamata. Serpentes; Science Press: Beijing, China, 1998. [Google Scholar]
- Zhao, E.M. Snakes of China; Anhui Science Technology Publishing House: Hefei, China, 2006; Volume 1. [Google Scholar]
- Wagler, J.G. Natürliches System der Amphibien, mit Vorangehender Classification der Säugthiere und Vogel: Ein Neitrag zur Vergleichenden Zoologie; J.G. Cottasche Buchhandlung Nachfolger: Stuttgart, Germany, 1830. [Google Scholar]
- Deepak, V.; Ruane, S.; Gower, D.J. A New Subfamily of Fossorial Colubroid Snakes from the Western Ghats of Peninsular India. J. Nat. Hist. 2019, 52, 2919–2934. [Google Scholar] [CrossRef]
- Wang, P.; Che, J.; Liu, Q.; Li, K.; Jin, J.Q.; Jiang, K.; Shi, L.; Guo, P. A Revised Taxonomy of Asian Snail-Eating Snakes Pareas (Squamata, Pareidae): Evidence from Morphological Comparison and Molecular Phylogeny. ZooKeys 2020, 939, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Uetz, P.; Freed, P.; Aguilar, R.; Hošek, J. (Eds.) The Reptile Database. Available online: http://www.reptile-database.org (accessed on 16 January 2023).
- Poyarkov, N.A.; Nguyen, T.V.; Pawangkhanant, P.; Yushchenko, P.V.; Brakels, P.; Nguyen, L.H.; Nguyen, H.N.; Suwannapoom, C.; Orlov, N.; Vogel, G. An Integrative Taxonomic Revision of Slug-Eating Snakes (Squamata: Pareidae: Pareineae) Reveals Unprecedented Diversity in Indochina. PeerJ 2022, 10, e12713. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.T.; Georgalis, G.L. The Diversity and Distribution of Palaeogene Snakes—A Review, with Comments on Vertebral Sufficiency. In The Origin and Early Evolution of Snakes; Gower, D., Zaher, H., Eds.; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Huang, Q.Y. Pareas macularius Theobald, 1868 should be a Junior Synonym of Pareas margaritophorus (Jan, 1866). Sichuan J. Zool. 2004, 23, 207–208. [Google Scholar]
- Hauser, S. On the Validity of Pareas macularius Theobald, 1868 (Squamata: Pareidae) as a Species Distinct from Pareas margaritophorus (Jan in Bocourt, 1866). Trop. Nat. Hist. 2017, 17, 25–52. [Google Scholar]
- Guo, K.J.; Deng, X.J. A New Species of Pareas (Serpentes: Colubridae: Pareatinae) from the Gaoligong Mountains, Southwestern China. Zootaxa 2009, 2008, 53–60. [Google Scholar] [CrossRef]
- Vogel, G. A New Montane Species of the Genus Pareas Wagler, 1830 (Squamata: Pareatidae) from Northern Myanmar. Taprobanica 2015, 7, 1–7. [Google Scholar] [CrossRef]
- You, C.W.; Poyarkov, N.A.; Lin, S.M. Diversity of the Snail-Eating Snakes Pareas (Serpentes, Pareatidae) from Taiwan. Zool. Scr. 2015, 44, 349–361. [Google Scholar] [CrossRef]
- Ding, L.; Chen, Z.N.; Suwannapoom, C.; Nguyen, T.V.; Poyarkov, N.A.; Vogel, G. A New Species of the Pareas hamptoni Complex (Squamata: Serpentes: Pareidae) from the Golden Triangle. Taprobanica 2020, 9, 174–193. [Google Scholar] [CrossRef]
- Bhosale, H.; Phansalkar, P.; Sawant, M.; Gowande, G.; Patel, H.; Mirza, Z.A. A New Species of Snail-Eating Snakes of the Genus Pareas Wagler, 1830 (Reptilia: Serpentes) from Eastern Himalayas, India. Eur. J. Taxon. 2020, 729, 54–73. [Google Scholar] [CrossRef]
- Vogel, G.; Nguyen, T.V.; Lalremsanga, H.T.; Biakzuala, L.; Hrima, V.; Poyarkov, N.A. Taxonomic Reassessment of the Pareas margaritophorus-macularius Species Complex (Squamata, Pareidae). Vertebr. Zool. 2020, 70, 547–569. [Google Scholar] [CrossRef]
- Liu, S.; Rao, D.Q. A New Species of the Genus Pareas (Squamata, Pareidae) from Yunnan, China. Zookeys 2021, 1011, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Vogel, G.; Nguyen, T.V.; Zaw, T.; Poyarkov, N.A. A New Species of the Pareas monticola Complex (Squamata: Serpentes: Pareidae) from Chin Mountains with Additions to the Pareas Fauna of Myanmar. J. Nat. Hist. 2021, 54, 2577–2612. [Google Scholar] [CrossRef]
- Le, D.T.T.; Tran, T.G.; Hoang, H.D.; Stuart, B.L. A New Species of Pareas (Squamata, Pareidae) from Southern Vietnam. Vertebr. Zool. 2021, 71, 439–451. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, D.R.; Poyarkov, N.A.; Hou, M.; Wu, L.; Rao, D.Q.; Nguyen, T.V.; Vogel, G. Resurrection of Pareas yunnanensis (Vogt, 1922) with Description of a New Species of Pareas from Yunnan Province, China (Squamata, Pareidae). Eur. J. Taxon. 2023, 860, 1–26. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Marchese, C. Biodiversity hotspots: A Shortcut for a More Complicated Concept. Glob. Ecol. Conserv. 2015, 3, 297–309. [Google Scholar] [CrossRef]
- Xue, J.R.; Tang, J.S.; Xu, Z.H.; Yang, Y.M.; Chen, Y.S.; Wang, J.H. Goligongshan National Nature Reserve; China Forestry Press: Beijing, China, 1995. [Google Scholar]
- Boulenger, G.A. Descriptions of New Reptiles and Batrachians from Borneo. Proc. Zool. Soc. London 1900, 69, 182–187. [Google Scholar] [CrossRef]
- Boulenger, G.A. Descriptions of Two New Snakes from Upper Burma. Bombay Nat. Hist. Soc. 1905, 16, 235–236. [Google Scholar] [CrossRef]
- Vogt, T. Zur Reptilien-und Amphibienfauna Südchinas. Arch. Für Nat. 1992, 88, 135–146. [Google Scholar]
- Pope, C.H. The Reptiles of China. Turtles, Crocodilians, Snakes, Lizards, Natural History of Central Asia; The American Museum of Natural History: New York, NY, USA, 1935; Volume X. [Google Scholar]
- Grossmann, W.; Tillack, F. On the Taxonomic Status of Asthenodipsas tropidonotus (Van Lidth de Jeude, 1923) and Pareas vertebralis (Boulenger, 1900) (Serpentes: Colubridae: Pareatinae). Russ. J. Herpetol. 2003, 10, 175–190. [Google Scholar]
- Guo, Y.H.; Wu, Y.K.; He, S.P.; Shi, H.T.; Zhao, E.M. Systematics and Molecular Phylogenetics of Asian Snail-Eating Snakes (Pareatidae). Zootaxa 2011, 3001, 57–64. [Google Scholar] [CrossRef]
- Loredo, A.I.; Wood, P.L., Jr.; Quah, E.S.; Anuar, S.; Greer, L.; Norhayati, A.; Grismer, L.L. Cryptic Speciation within Asthenodipsas vertebralis (Boulenger, 1900) (Squamata: Pareatidae), the Description of a New Species from Peninsular Malaysia, and the Resurrection of A. tropidonotus (Lidth de Jude, 1923) from Sumatra: An Integrative Taxonomic Analysis. Zootaxa 2013, 3664, 505–524. [Google Scholar] [CrossRef]
- Yang, J.H.; Yeung, H.Y.; Huang, X.Y.; Yang, S.P. First Record of Pareas vindumi Vogel, 2015 (Reptilia: Pareidae) from China with a Revision to Morphology. Taprobanica 2021, 10, 39–46. [Google Scholar] [CrossRef]
- David, P.; Deuti, K. On the Type Specimens of Pareas macularius Theobald, 1868 and Pareas berdmorei Theobald, 1868 with the Designation of a Lectotype for Pareas macularius (Squamata: Serpentes: Pareidae). Zootaxa 2022, 5105, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Lawson, R.; Slowinski, J.B.; Crother, B.I.; Burbrink, F.T. Phylogeny of the Colubroidea (Serpentes): New evidence from Mitochondrial and Nuclear Genes. Mol. Phylogenetics Evol. 2005, 37, 581–601. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment Through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F.; Nielsen, R.; Bollback, J.P. Bayesian Inference of Phylogeny and its Impact on Evolutionary Biology. Science 2001, 294, 2310–2314. [Google Scholar] [CrossRef]
- Wilcox, T.P.; Zwickl, D.J.; Heath, T.A.; Hillis, D.M. Phylogenetic Relationships of the Dwarf Boas and a Comparison of Bayesian and Bootstrap Measures of Phylogenetic Support. Mol. Phylogenetics Evol. 2002, 25, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, M.E.; Zoller, S.; Lutzoni, F. Bayes or Bootstrap? A Simulation Study Comparing the Performance of Bayesian Markov chain Monte Carlo Sampling and Bootstrapping in Assessing Phylogenetic Confidence. Mol. Biol. Evol. 2003, 20, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast Approximation for Phylogenetic Bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Che, J.; Jiang, K.; Yan, F.; Zhang, Y.P. Amphibians and Reptiles in Tibet-Diversity and Evolution; Science Press: Beijing, China, 2020. [Google Scholar]


| Species | Locality | Voucher No. | GenBank No. |
|---|---|---|---|
| Pareas abros | Song Thanh, Quang Nam, Vietnam | ZMMU R-16393 | MZ712235 |
| Pareas andersonii | Mt. Natmataung, Chin, Myanmar | CAS 235359 | MT968772 |
| Pareas atayal | N. Cross Is. Hw., Taiwan, China | NMNS 05594 | KJ642122 |
| Pareas berdmorei | Kin Pon Chaung, Mon, Myanmar | CAS 240362 | MZ712219 |
| Pareas boulengeri | Jiangkou, Guizhou, China | GP 2923 | MK135090 |
| Pareas carinatus | Sungai Sedim, Kedah, Malaysia | LSUHC10604 | KC916748 |
| Pareas chinensis | Hongya, Sichuan, China | GP 2383 | MK135089 |
| Pareas formosensis | N. Cross Is. Hw., Taiwan, China | NMNS 05632 | KJ642130 |
| Pareas geminatus | Jiangcheng, Yunnan, China | CIB 118021 | MW287068 |
| Pareas hamptoni | Kachin, Myanmar | YPX 18219 | MK135077 |
| Pareas iwasakii | Ishigaki Is., S. Ryukyu, Japan | I03-ISG1 | KJ642158 |
| Pareas kaduri | Lohit, Arunachal, India | BNHS 3574 | MT188734 |
| Pareas kaduri | Lohit, Arunachal, India | BNHS 3575 | MW026190 |
| Pareas komaii | Taitung, Taiwan, China | HC 000669 | JF827687 |
| Pareas kuznetsovorum | Song Hinh, Phu Yen, Vietnam | ZMMU R-16802 | MZ712232 |
| Pareas macularius | Bago, Myanmar | CAS 206620 | AF471082 |
| Pareas margaritophorus | Cangwu, Guangxi, China | YBU 16061 | MK135097 |
| Pareas modestus | Aizawl, Mizoram, India | MZMU 1293 | MT968773 |
| Pareas monticola | Medog, Tibet, China | GP 2027 | MK135107 |
| Pareas niger | Kunming, Yunnan, China | KIZ 059339 | MW436706 |
| Pareas nigriceps | Mt. Gaoligong, Yunnan, China | SYSr001222 | MK201455 |
| Pareas nuchalis | Belait, Brunei | FK 2626 | MZ603794 |
| Pareas stanleyi | Guilin, Guangxi, China | HM 2007-S001 | JN230704 |
| Pareas temporalis | Da Huoai, Lam Dong, Vietnam | UNS 09992 | MZ603793 |
| Pareas tigerinus | Menghai, Yunnan, China | KIZ 20210703 | OP752143 |
| Pareas tigerinus | Menghai, Yunnan, China | KIZ 20210704 | OP752144 |
| Pareas tigerinus | Menghai, Yunnan, China | KIZ 20210705 | OP752145 |
| Pareas victorianus | Mt. Natmataung, Chin, Myanmar | CAS 235254 | MW438300 |
| Pareas vindumi | Lukpwir, Kachin, Myanmar | CAS 248147 | MT968776 |
| Pareas xuelinensis | Lancang, Yunnan, China | KIZ XL1 | MW436709 |
| Pareas yunnanensis | Dali, Yunnan, China | KIZ 2022036 | OP752149 |
| Pareas dulongjiangensis sp. nov. | Gongshan, Yunnan, China | KIZ R201607 | OQ718498 |
| Aplopeltura boa | Malaysia | LSUHC 7248 | KC916746 |
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | (13) | (14) | (15) | (16) | (17) | (18) | (19) | (20) | (21) | (22) | (23) | (24) | (25) | (26) | (27) | (28) | |
| (1) | ||||||||||||||||||||||||||||
| (2) | 23.5 | |||||||||||||||||||||||||||
| (3) | 22.8 | 20.2 | ||||||||||||||||||||||||||
| (4) | 21.3 | 23.8 | 23.5 | |||||||||||||||||||||||||
| (5) | 23.2 | 19.7 | 18.3 | 23.7 | ||||||||||||||||||||||||
| (6) | 21.8 | 22.9 | 22.6 | 13.8 | 22.2 | |||||||||||||||||||||||
| (7) | 23.7 | 19.1 | 18.4 | 24.7 | 9.0 | 22.6 | ||||||||||||||||||||||
| (8) | 22.9 | 21.7 | 15.1 | 24.9 | 17.2 | 23.9 | 17.4 | |||||||||||||||||||||
| (9) | 22.9 | 22.2 | 14.2 | 23.0 | 17.4 | 23.1 | 18.8 | 8.3 | ||||||||||||||||||||
| (10) | 23.6 | 21.5 | 14.2 | 23.7 | 17.2 | 23.6 | 18.3 | 7.2 | 7.3 | |||||||||||||||||||
| (11) | 23.4 | 20.4 | 7.2 | 23.7 | 16.8 | 23.3 | 17.9 | 14.4 | 14.4 | 13.5 | ||||||||||||||||||
| (12) | 25.0 | 20.7 | 15.6 | 24.9 | 19.8 | 22.6 | 19.3 | 13.3 | 13.7 | 13.0 | 15.2 | |||||||||||||||||
| (13) | 23.3 | 19.5 | 8.5 | 23.9 | 18.1 | 23.9 | 18.3 | 14.7 | 14.9 | 14.5 | 7.9 | 16.1 | ||||||||||||||||
| (14) | 20.9 | 23.8 | 22.9 | 13.0 | 22.6 | 13.0 | 23.0 | 23.7 | 22.8 | 23.3 | 23.9 | 23.1 | 24.2 | |||||||||||||||
| (15) | 23.0 | 13.9 | 19.2 | 22.7 | 17.8 | 22.1 | 17.4 | 19.0 | 20.4 | 19.7 | 18.9 | 19.8 | 18.3 | 22.8 | ||||||||||||||
| (16) | 25.8 | 15.3 | 19.1 | 24.7 | 19.2 | 23.5 | 18.3 | 20.5 | 21.8 | 20.5 | 18.8 | 20.8 | 19.5 | 23.7 | 14.8 | |||||||||||||
| (17) | 23.5 | 12.0 | 18.7 | 24.4 | 19.2 | 24.0 | 18.7 | 20.7 | 19.9 | 19.6 | 19.3 | 19.4 | 17.8 | 24.4 | 11.0 | 13.9 | ||||||||||||
| (18) | 22.6 | 18.9 | 17.3 | 22.0 | 18.7 | 22.8 | 18.1 | 18.9 | 19.7 | 19.0 | 17.8 | 19.0 | 17.9 | 22.5 | 18.1 | 19.7 | 18.2 | |||||||||||
| (19) | 22.7 | 20.4 | 14.3 | 23.8 | 17.5 | 23.0 | 17.8 | 7.2 | 6.8 | 5.6 | 13.7 | 12.5 | 14.9 | 22.7 | 18.9 | 20.1 | 18.9 | 18.5 | ||||||||||
| (20) | 23.6 | 18.8 | 16.2 | 22.9 | 16.9 | 22.6 | 16.2 | 12.6 | 13.5 | 12.6 | 16.1 | 10.1 | 16.2 | 23.9 | 17.8 | 17.9 | 16.4 | 19.1 | 12.5 | |||||||||
| (21) | 21.1 | 24.3 | 23.7 | 21.5 | 24.3 | 21.6 | 24.0 | 24.4 | 25.1 | 24.8 | 24.5 | 25.6 | 23.5 | 20.4 | 23.1 | 26.1 | 24.5 | 21.4 | 25.2 | 23.8 | ||||||||
| (22) | 25.7 | 20.4 | 19.2 | 25.0 | 15.7 | 24.9 | 15.4 | 19.6 | 19.4 | 18.7 | 18.2 | 20.6 | 17.4 | 24.9 | 19.9 | 19.5 | 19.4 | 19.2 | 19.5 | 19.0 | 24.0 | |||||||
| (23) | 12.3 | 23.6 | 23.1 | 20.6 | 22.1 | 19.9 | 21.5 | 24.3 | 23.6 | 23.4 | 23.1 | 24.8 | 23.8 | 20.1 | 24.4 | 24.0 | 23.2 | 21.3 | 23.3 | 23.8 | 19.8 | 23.4 | ||||||
| (24) | 23.1 | 19.4 | 14.7 | 24.2 | 19.0 | 23.3 | 18.6 | 12.3 | 12.3 | 11.8 | 14.1 | 11.2 | 14.0 | 24.3 | 18.6 | 20.4 | 18.1 | 18.9 | 11.4 | 4.3 | 25.2 | 19.4 | 24.6 | |||||
| (25) | 24.3 | 20.6 | 19.6 | 22.8 | 19.1 | 22.8 | 17.4 | 17.8 | 18.4 | 18.6 | 19.7 | 18.9 | 19.4 | 22.9 | 19.1 | 21.5 | 19.3 | 15.1 | 17.9 | 19.1 | 24.7 | 19.0 | 24.2 | 18.1 | ||||
| (26) | 24.5 | 20.8 | 14.9 | 24.7 | 18.4 | 23.8 | 17.5 | 12.1 | 12.4 | 11.4 | 14.7 | 12.9 | 15.2 | 23.7 | 19.3 | 20.5 | 19.9 | 18.3 | 10.8 | 12.3 | 24.7 | 19.4 | 24.9 | 12.0 | 17.8 | |||
| (27) | 23.1 | 21.2 | 13.8 | 25.1 | 16.9 | 24.3 | 18.6 | 8.0 | 5.9 | 8.1 | 13.7 | 13.6 | 14.8 | 24.5 | 19.2 | 21.3 | 20.2 | 19.8 | 7.2 | 12.5 | 25.9 | 19.4 | 24.4 | 12.1 | 18.8 | 12.6 | ||
| (28) | 23.2 | 22.1 | 14.6 | 24.6 | 16.7 | 23.4 | 18.0 | 7.9 | 4.0 | 6.1 | 14.0 | 12.9 | 14.7 | 23.7 | 19.7 | 21.5 | 20.7 | 20.0 | 6.3 | 12.8 | 24.8 | 19.5 | 23.7 | 11.7 | 18.7 | 11.4 | 6.1 | |
| (29) | 24.0 | 19.6 | 14.0 | 24.4 | 18.0 | 23.3 | 17.6 | 12.9 | 12.7 | 12.4 | 13.9 | 9.4 | 13.8 | 24.2 | 19.4 | 18.4 | 18.6 | 16.5 | 12.3 | 10.3 | 24.6 | 19.2 | 25.0 | 10.3 | 18.0 | 12.6 | 13.2 | 12.9 |
| Pareas dulongjiangensis sp. nov. Holotype KIZ R201607 | Pareas kaduri n = 4 3, 1 | |
|---|---|---|
| SVL | 373 | 455–550 |
| TaL | 115 | 113–144 |
| TL | 488 | 571–694 |
| TaL/SVL | 0.308 | 0.226–0.262 |
| TaL/TL | 0.236 | 0.184–0.207 |
| HL | 14.0 | 10.4–14.6 |
| CL | 15.4 | 14.3–18.8 |
| HW | 8.1 | 7.1–8.8 |
| ED | 2.6 | 2.7–3.6 |
| El | 1.7 | 1.5–1.8 |
| ES | 4.1 | 3.7–4.3 |
| EN | 1.8 | 2.3–2.7 |
| NW | 3.6 | 3.5–5.8 |
| PrFBO | Yes | Yes |
| PreO | 0 | 1 |
| PosO | Fused | 2 |
| SubO | Fused | 1 |
| SPOF | Yes | No |
| ATem | 2/2 | 2 |
| PTem | 3/2 | 3 |
| SupL | 6/7 | 7 |
| InfL | 7/9 | 7 |
| LoBO | Yes | No |
| Vs | 182 | 160–183 |
| Prec | Undivided | Undivided |
| Sc | 76 | 52–70 |
| Ds | 15-15-15 | 15-15-15 |
| NED | 3 | 1 |
| NKD | 5 | 8 in ♂, 0 in ♀ |
| Max | 5/4 | 6–7 |
| DNB | Yes | Yes |
| VBTr | 52 | 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Yang, M.; Rao, J.; Guo, Y.; Rao, D. A New Species of Pareas Wagler, 1830 (Squamata, Pareidae) from Northwestern Yunnan, China. Taxonomy 2023, 3, 169-182. https://doi.org/10.3390/taxonomy3020013
Liu S, Yang M, Rao J, Guo Y, Rao D. A New Species of Pareas Wagler, 1830 (Squamata, Pareidae) from Northwestern Yunnan, China. Taxonomy. 2023; 3(2):169-182. https://doi.org/10.3390/taxonomy3020013
Chicago/Turabian StyleLiu, Shuo, Mingjing Yang, Jingqiu Rao, Yuhong Guo, and Dingqi Rao. 2023. "A New Species of Pareas Wagler, 1830 (Squamata, Pareidae) from Northwestern Yunnan, China" Taxonomy 3, no. 2: 169-182. https://doi.org/10.3390/taxonomy3020013
APA StyleLiu, S., Yang, M., Rao, J., Guo, Y., & Rao, D. (2023). A New Species of Pareas Wagler, 1830 (Squamata, Pareidae) from Northwestern Yunnan, China. Taxonomy, 3(2), 169-182. https://doi.org/10.3390/taxonomy3020013






