2. Materials and Methods
The specimens were deposited in the collections of the Instituto Butantan, São Paulo (IBSP, curator A.D. Brescovit) and Museu Paraense Emilio Goeldi, Belém (MPEG, curator A.B. Bonaldo).
The morphological terms follow those used by Polotow and Brescovit, 2009 [
1]. The descriptions and measurements were performed using a LEICA 165C stereomicroscope. Photographs were taken with a Leica DFC 500 digital camera on a Leica MZ16A stereomicroscope. Focal-range images were made using Leica Application Suite software, version 2.5.0. All measurements are in millimeters. Female genitalia were excised with a sharp needle, digested using one tablet of enzymatic eye lens cleaner (Ultrazyme enzymatic cleaner) into 5 mL distilled water for 24 h, and photographs were taken using Hoyer’s microscope slides (Krantz and Walter, 2009 [
4]). For scanning electron microscopy (SEM) images, body parts were dehydrated in a graded series of ethanol washes (80% to 100%), dried by critical point, mounted on metal stubs using adhesive copper tape and nail polish for fixation, and covered with gold. SEM images were taken with a FEI Quanta 250 scanning electron microscope at the Laboratório de Biologia Celular of Instituto Butantan, São Paulo, Brazil. Maps were produced using GPS Track Maker-PRO and edited in GIMP v2.8.14 and Inkscape v0.48.4. Graphics and tables were made in Microsoft Office Excel 2013.
The following abbreviations were used in the description and legends: ALE, anterior lateral eyes; AME, anterior median eyes; C, conductor; CD, copulatory ducts; CI, cymbium; E, embolus; FD, fertilization ducts; LP, lateral projection; MA, median apophysis; MS, median sector of the epigynum; PLE, posterior lateral eyes; PLP, posterior lateral projection of the epigyne; PME, posterior median eyes; RCP, retrolateral cymbial process; RTA, retrolateral tibial apophysis; S, spermathecae; ST, subtegulum; T, tegulum; TI, tibia; VTP, ventral tibial process.
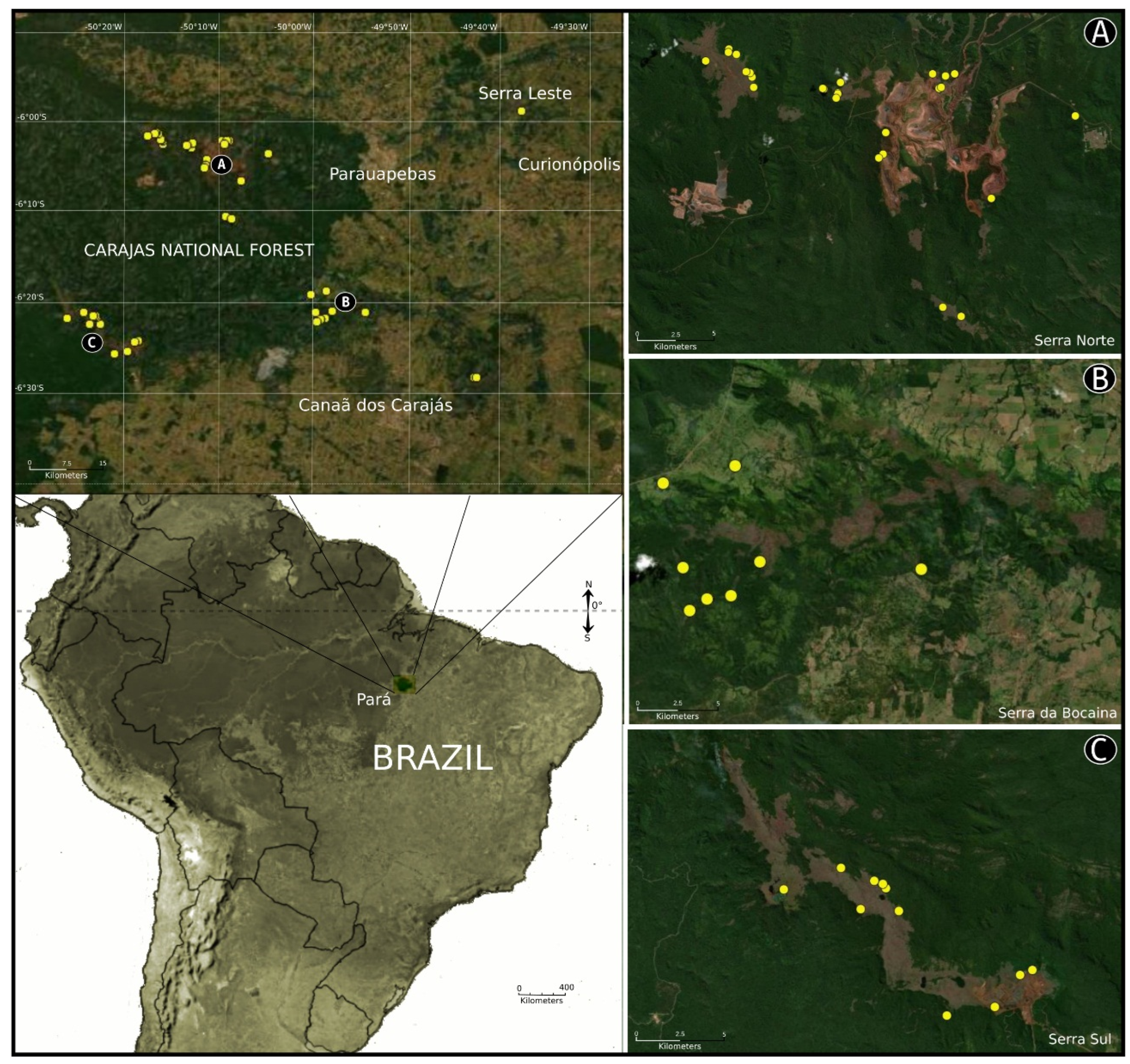
Study area: The caves where the spiders were found are located in the ferruginous outcrops in the Carajás region in southeastern Pará state, in the eastern region of the Amazon Forest in Brazil. These caves are in and around the National Carajas Forest (FLONA de Carajás), which covers approximately 411,000 hectares. This region consists of a mosaic of protected areas forming a continuous area of 1.31 million hectares of preserved forest (Rolim et al. 2006 [
5]), surrounded by pastures that replaced the original forest (Martins et al. 2012 [
6]; Carmo and Jacobi 2013 [
7]). The material cited here was collected in caves that are located in areas of mining interest in the municipalities of Parauapebas, Canaã dos Carajás, and Curionópolis.
3. Results
Ctenidae Keyserling, 1877
Cteninae Keyserling, 1877
Parabatinga Polotow and Brescovit, 2009. Type species: Parabatinga brevipes (Keyserling, 1891).
Diagnosis (modified from Polotow and Brescovit [
1]):
Parabatinga is distinguished from other Ctenidae genera by an elongated RTA, a cymbium with a ventral tegular projection, a wide embolus with a strong z-shaped probasal projection (in ventral view), a subtegulum with a projection and excavation that locks with the z-shaped probasal projection of the embolus, and an elongated cup-shaped median apophysis (Polotow and Brescovit, [
1], figs. 17A,B, 1E, 3D–F and 5A). Females of
Parabatinga are distinguished by a median sector of the epigyne wider than long with two posterior projections, lateral projections pointing posteriorly, and a spermathecae that is oval and dorsally projected (Polotow and Brescovit, [
1], figs. 17C,D, 2B,C, 4F and 5C,D).
Composition: Parabatinga brevipes (Keyserling, 1891) and P. danielae sp. n.
Distribution: Colombia, Brazil, Bolivia, Paraguay, Argentina, and Uruguay.
Parabatinga brevipes (Keyserling, 1891)
Diagnosis:
Parabatinga brevipes can be distinguished from
P. danielae sp. n. by the dorsal branch of the RTA with a hyaline tip (Polotow and Brescovit [
1]: fig. 17A,B), an embolus with a distal hyaline projection (Polotow and Brescovit [
1]: fig. 16C), and a median apophysis with a prolateral laminar process (Polotow and Brescovit [
1]: fig. 16D). Females of
Parabatinga brevipes are distinguished from
P. danielae sp. n. by the subhexagonal median sector and smaller spermathecae (Polotow and Brescovit [
1]: fig. 17C,D).
Description: See Polotow and Brescovit. [
1]: 603, figs. 16, 17 and 20C.
Type material: Male holotype from Cave CAV_0027 (6°24′58″ S 50°21′33″ W), FLONA de Carajás, Canaã dos Carajás, Pará, Brazil, 22–31/V/2010, R. Andrade and I. Cizauskas et al. coll., deposited in IBSP 175354. Paratypes: 1 ♀ from same locality, Cave SB_0231 (6°20′32″ S 49°9′44″ W), 12−22/X/2013, C.A.R. Souza et. al. coll. (IBSP 195244); 1 ♂ from Cave SB_0214 (6°18′12″ S 49°58′32″ W), 08−22/V/2013, C.A.R. Souza et. al. coll. (IBSP 195238); 1 ♀ from Cave CAV_0001 (6°24′42″ S 50°20′05″ W), 22-31/V/2010, R. Andrade and I. Cizauskas et al. coll. (IBSP 175353); Canaã dos Carajás, Cave SB_0170 (6°18′37″ S 50°00′10″ W), 08−22/V/2013, 1 ♂ (MPEG 37256; ex IBSP 195237); Parauapebas, Cave N4WS_0025 (GEM-1131) (6°03′58″ S 50°11′29″ W), 18/XI−01/XII/2010, 1 ♀ (MPEG 37257, ex IBSP 174011).
Diagnosis: Males of
Parabatinga danielae sp. n. can be distinguished from
P. brevipes by the cymbium with a large retrolateral cymbial process (
Figure 1E,
Figure 4B and
Figure 5A), the RTA excavated prolaterally and acute apically, and the ventral projection on coxa IV (
Figure 1B,C). Females of
Parabatinga danielae sp. n. are distinguished from
P. brevipes by the longer median sector and larger spermathecae (
Figure 2B,
Figure 4F and
Figure 5B).
Etymology: The specific name is a patronym in honor of the first author’s daughter, Daniela Ohlweiler Brescovit.
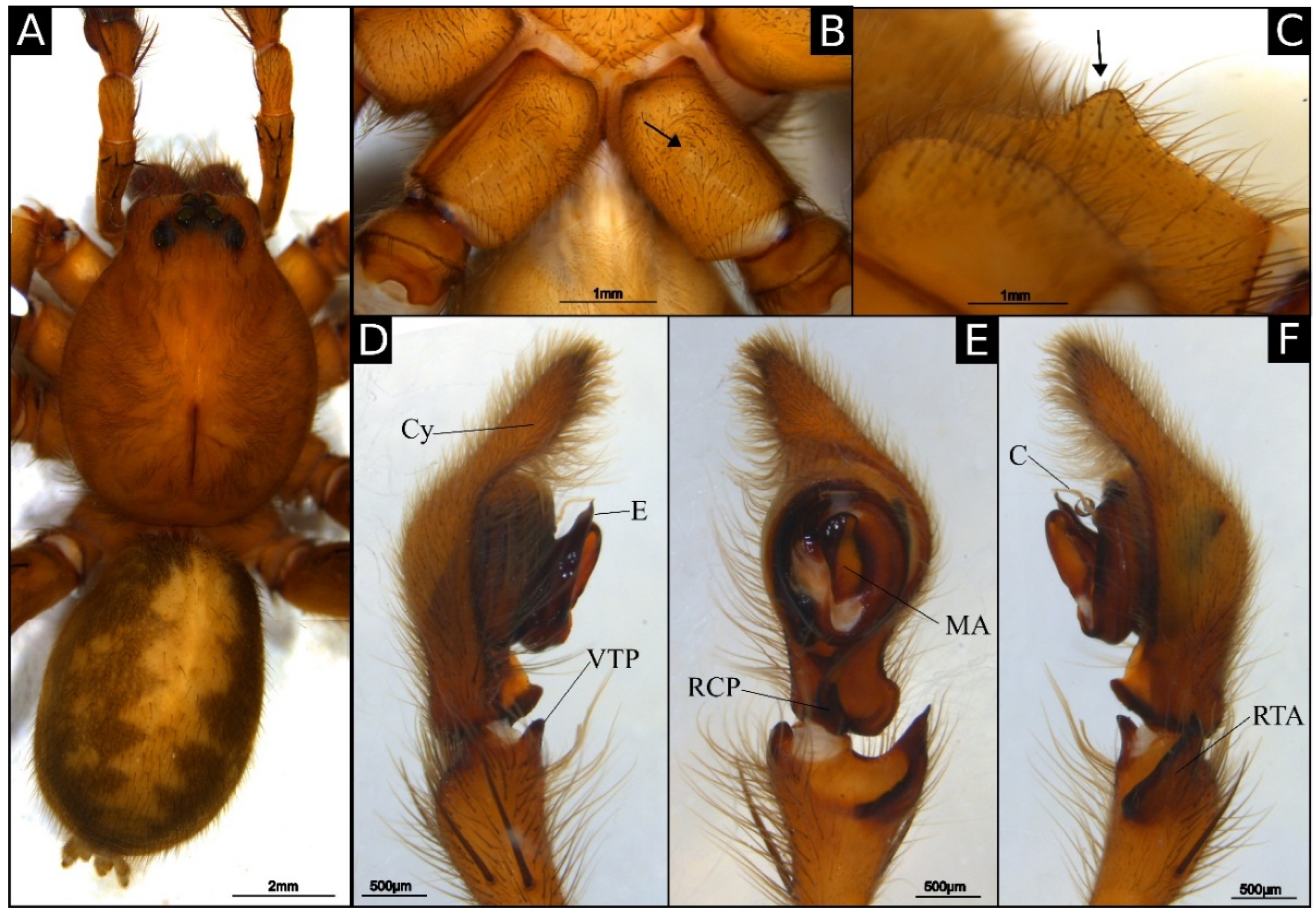
Description of male holotype (IBSP 175354): Coloration: orange carapace, black eye borders and reddish-brown thoracic groove (
Figure 1A). Reddish brown chelicerae. Endites orange, distally yellow. Labium and sternum orange. Legs orange with darker metatarsus and tarsi. Abdomen with grayish yellow dorsal band, gray laterally and ventrally gray with cream central area. Total length 16.8. Carapace 9 long and 7 wide. Clypeus 0.20 high. Eye diameters: AME 0.20, ALE 0.15, PME 0.25, PLE 0.25. Chelicerae: cheliceral glands next to promarginal teeth, oval, with large number of very small pores (
Figure 3B,C); promargin with 3 teeth, the median almost twice as high as the laterals; retromargin with 4 similar-sized teeth (
Figure 3A). Leg measurements: I: femur 9.5/patella 4.2/tibia 11.0/metatarsus 9.8/tarsus 3.8/total 38.3; II: 8.9/4.0/8.3/7.8/3.0/32.0; III: 7.0/3.4/7.0/7.2/2.6/27.2; IV: 10.3/3.7/9.7/11.8/3.0/38.5. Leg formula: 4123. Leg spination: tibia I-II v2-2-2-2-2, r1-1-0, p1-1-0, III-IV v2-2-2, r1-1-0, p1-1-0; metatarsus I v2-2-0, r1-1-0, p1-1-0, II v2-2-0, r1-1-1, p1-1-1, III v2-2-0, r1-1-2, p1-1-2, IV v2-2-2, r1-1-2, p1-1-2. Palp (
Figure 1D–F,
Figure 3D–F,
Figure 4A,B and
Figure 5A,B): tibia as long as cymbium; retrolateral tibial apophysis (RTA) strongly sclerotized, with wide base and acute tip, excavated anteriorly; ventral tibial process (VTP) short and round; cymbium drop-shaped ventrally, with large retrolateral cymbial process (RCP) and anterior projection; subtegulum visible next to the embolus; tegulum suboval, with spermatic duct visible medially; embolus wide with rectangular base and rounded tip, slightly curved retrolaterally; median apophysis cup-shaped, excavated laterally; conductor cylindrical, as long as the embolus; conductos hyaline, covering the embolus tip.
Female paratype (IBSP 175353): Coloration as in male, except labium and endites red-brownish (
Figure 2A). Abdomen yellow ventrally. Total length 21.5. Carapace 10.8 long and 8.2 wide. Clypeus 0.35 high. Eye diameters: AME 0.20, ALE 0.15, PME 0.25, PLE 0.25. Chelicerae with cheliceral glands and teeth as in male (
Figure 4C,D). Pedipalp claw with six teeth (
Figure 4E). Leg measurements: I: femur 10.0/patella 4.8/tibia 10.2/metatarsus 8.5/tarsus 3.5/total 37.0; II: 9.8/4.3/8.8/7.5/3.1/33.5; III: 7.8/3.7/7.2/7.4/2.8/28.9; IV: 10.8/4.0/9.6/12.5/3.5/40.4. Leg formula: 4123. Leg spination as in male, except: tibia I-II, III-IV metatarsus I v2-2-2, r0, p0, II v2-2-2, r1-0-0, p1-0-0, III v2-2-2, p1-1-2, IV v2-2-1-2. Epigynum (
Figure 2B,
Figure 4F and
Figure 5B): median sector (MS) sulcated; copulatory opening located anteriorly, not visible ventrally; lateral projections (LP) small, short, extending until the posterior margin sublateral of the epigyne; posterior lateral projection (PLP) relatively short. Vulva (
Figure 2C and
Figure 5C): straight copulatory ducts; short; spermathecae large and oval; fertilization ducts short, originating at the basal area of the spermathecae.
Variation: Males (n = 07): total length 11.2–17.1; carapace length 5.8–9.5; femur I length 5.2–10.1. Females (n = 10): total length 11.5–21.5; carapace length 5.8–10.8; femur I length 6.3–10.2.
Other material examined: BRAZIL. Pará: Parauapebas, Serra dos Carajás, Cave N1_0004 (GEM-1200) (6°02′23″ S 50°16′12″ W), 16/VII−06/VIII/2014, 1 ♀ (IBSP 208163); 1 ♀ (IBSP 208164); Cave N1_0015 (GEM-1211) (6°02′02″ S 50°16′16″ W), 11/VI−02/VII/2014, 1 ♀ (IBSP 208159); Cave N1_0025 (GEM-1221) (6°01′49″ S 50°16′20″ W), 04/IX−06/X/2014, 1 ♀ (IBSP 208169); Cave N1_0035 (GEM-1231) (6°01′49″ S 50°16′29″ W), 04/IX−06/X/2014, 1 ♀ (MPEG 37258, ex IBSP 208170); Cave N1_0088 (GEM-1286) (6°01′02″ S 50°17′04″ W), 1 ♀ (IBSP 208172); Cave N1_0098 (GEM-1296) (6°01′08″ S 50°17′05″ W), 1 ♀ (IBSP 208173); Cave N1_0174 (GEM-1374) (6°01′28″ S 50°17′53″ W), 16/VII−06/VIII/2014, 1 ♂ (IBSP 208585); all collected by Equipe Carste et al.; Flona de Carajás, Cave N1_0075 (GEM-1273), 28/XI-03/X/2007, R. Andrade et al., 1 ♀ (IBSP 115496); Cave N3_0023 (GEM-1911) (6°02′35″ S 50°13′10″ W), 02-23/VIII/2013, 1 ♀ (IBSP 178289); Cave N3_0031 (GEM-1920) (6°02′37″ S 50°13′09″ W), 26/IX−17/X/2012, 1 ♀ (IBSP 178284); Cave N3_0036 (GEM-1925) (6°02′46″ S 50°13′13″ W), 05−17/III/2013, 1 ♂ (IBSP 178286); Cave N3_0043 (GEM-1983) (6°02′12″ S 50°13′04″ W), 1 ♀ (IBSP 178285); Cave N3_0047 (GEM-2011) (6°02′27″ S 50°13′40″ W), 03−17/IV/2013, 1 ♀ (IBSP 178287); Cave N3_0052 (GEM-2016) (6°02′25″ S 50°13′42″ W), 02−23/VIII/2013, 1 ♀ (IBSP 178290); all collected by Equipe Carste; Cave N4WS_0050(GEM-1168)/0051 (6°04′43″ S 50°11′34″ W), 01−09/VI/2011, Cave N4WS_0064 (GEM-1843) (6°04′52″ S 50°11′43″ W), 10−19/V/2011, 1 ♀ (IBSP 174014); all collected by F. P. Franco and C. A. R. Souza et al.; Cave N4E_0033 (GEM-989) (6°02′25″ S 50°09′36″ W), 15−22/IX/2009, 1 ♀ (IBSP 176093); Cave N4E_0044 (GEM-1113) (6°01′55″ S 50°09′50″ W), 24-30/VII/2009, 1 ♀ (IBSP 176095); Cave N4E_0067 (GEM-1533) (6°01′55″ S 50°09′02″ W), 1 ♀ (IBSP 176091); Cave N4E_0083 (GEM-1552) (6°01′59″ S 50°09′22″ W), 1 ♀ (IBSP 173998); Cave N4E_0092 (GEM-1586) (6°02′22″ S 50°09′31″ W), 1 ♀ (IBSP 176092); 1 ♀ (IBSP 177768); Cave N5S_0011 (GEM-1036) (6°06′18″ S 50°07′47″ W), 14−23/X/2009, 1 ♀ (IBSP 176097); Cave N5S_0011 (GEM-1036) (6°06′18″ S 50°07′47″ W), 1 ♀ (IBSP 176098); all collected by R. Andrade and I. Cizauskas et al.; Canaã dos Carajás, Cave S11B (9297508 567507), 22/VI/2018, 1 ♀ (IBSP 264736); Cave S11B_0012B (929691 566641), 06/VI/2018, 1 ♀ (IBSP 264731); Cave S11B_0083 (9297921 567344), 09/VI/2018, 1 ♂ (IBSP 264730); Cave S11B_0090 (9298175 567177), 07/VI/2018, 1 ♀ (IBSP 264728); Cave S11B_0101 (9298331 566682), 14/VI/2018, 1 ♀ (IBSP 264729); 1 ♀ (IBSP 264735); Cave S11B_0109 (9296727 565924), 13/VI/2018, 1 ♀ (IBSP 264733); S11B_0109 (9296727 565924), 16/XI/2018, 1 ♀ (IBSP 264732); Cave S11B_0109B (9296727 565924), 13/VI/2018, 1 ♀ (IBSP 264734); Cave S11B_0193 (9299065 564802), 23/VI/2018, 1 ♀ (IBSP 264727); all collected by Ativo Ambiental; FLONA de Carajás, Cave S11A_0007 (6°21′06″ S 50°26′36″ W), 23/VIII−02/IX/2007, 1 ♀ (IBSP 174408); Cave S11D_0079 (725) (6°23′33″ S 50°18′56″ W), 01−14/VII/2010, 1 ♀ (IBSP 175355); Cave S11D_0091 (737) (6°23′43″ S 50°19′19″ W), 13−30/I/2010, 1 ♀ (IBSP 175384); all collected by R. Andrade and I. Cizauskas et al. coll.; Serra dos Carajás, Cave SB_0114 (GEM-1527) (6°21′10″ S 49°58′37″ W), 20−26/VI/2013, 1 ♀ (IBSP 195240); Cave SB_0125 (GEM-1593) (6°21′15″ S 49°59′10″ W), 20−26/VI/2013, 1 ♀ (IBSP 195241); 1 ♀ (IBSP 195666); Cave SB_0126 (GEM-1594) (6°21′15″ S 49°59′10″ W), 1 ♀ (IBSP 195242); Cave SB_0201 (6°20′24″ S 49°57′58″ W), 10−20/IX/2013, 1 ♀ (IBSP 195243); Cave SB_0214 (6°18′12″ S 49°58′32″ W), 08−22/V/2013, 2♂ (IBSP 195664); Cave SB_0226 (6°21′30″ S 49°59′34″ W), 1 ♀ (MPEG 37259; ex IBSP 195239); Cave SB_0237 (6°20′34″ S 49°54′15″ W), 12−22/X/2013, 1 ♀ (IBSP 195245); all collected by C.A.R. Souza et. al.; Cave S11D_0037 (680) (6°24′46″ S 50°21′31″ W), 19−22/II/2010, R. Andrade and I. Cizauskas et al. coll., 1 ♂ (IBSP 175385; Photos); Cave S11D_105 (6°23′50″ S 50°22′01″ W), 30/VII−02/IX/2011, R. Andrade and I. Cizauskas et al. coll., 1 ♀ (IBSP 175872, Photos); Curionópolis, Cave CRIS-029 (6°27′31″ S 49°42′32″ W), 1 ♀ (IBSP 174628); Cave CRIS-035 (6°27′32″ S 49°42′16″ W), 1 ♀ (IBSP 174640), all collected in 29/VII−06/VIII/2008 by R. Andrade et al.; Serra dos Carajás, Cave SL_0060 (05°58′46″ S 49°37′22″ W), 10/VI/2010, R. Zampaulo coll., 1 ♀ (IBSP 190021).
Distribution: Known only from Serra de Carajás, in the municipalities of Parauapebas, Canaã dos Carajás, and Curionópolis, in Southeastern Pará state, Brazil.
Natural history: Fifty-nine specimens (
n = 59) were collected, eight males and fifty-one females, distributed in 51 caves located in different ferruginous outcrops in the Carajás region. Both were observed in illuminated areas as well as in twilight regions of the cavities. The female of
Parabatinga danielae sp.
n. selects the subterranean environment and fixes the egg sac to the cavity wall (
Figure 6A,B), as observed in other cave-dwelling Ctenid species, such as
Ctenus fasciatus Mello-Leitão, 1943 [
8] (Cizauskas et al., 2022 [
9]). The morphological characteristics observed in the evaluated specimens suggest that these spiders are able to complete their life cycle in hypogean habitats, since, no specimens have been sampled outside the cave environment.