Abstract
Thrixopelma nadineae sp. nov. is described from Loja, Ecuador, based on the male, which possesses distinctive palpal bulb, metatarsal and tibial apophysis morphology. The new species represents the second species of Thrixopelma to be recorded from Ecuador.
1. Introduction
The theraphosid spiders of Ecuador are poorly known; presently, 20 valid species are recognized by the World Spider Catalog [1]: Amazonius elenae (Schmidt, 1994), Avicularia hirschii Bullmer, Thierer-Lutz & Schmidt, 2006, Avicularia juruensis Mello-Leitão, 1923, Avicularia lynnae Fukushima & Bertani, 2017, Avicularia purpurea Kirk, 1990, Avicularia rufa Schiapelli & Gerschman, 1945, Cyclosternum gaujoni Simon, 1889, Cyclosternum janthinum (Simon, 1889), Cyclosternum schmardae Ausserer, 1871, Cymbiapophysa velox (Pocock, 1903), Megaphobema velvetosoma Schmidt, 1995, Neischnocolus yupanquii (Pérez-Miles, Gabriel & Gallon, 2008), Pamphobeteus augusti (Simon, 1889), Pamphobeteus petersi Schmidt, 2002, Pamphobeteus ultramarinus Schmidt, 1995, Pamphobeteus vespertinus (Simon, 1889), Psalmopoeus ecclesiasticus Pocock, 1903, Reversopelma petersi Schmidt, 2001, Tapinauchenius cupreus Schmidt & Bauer, 1996, Thrixopelma longicolli (Schmidt, 2003), and one further species may possibly be distributed in Ecuador: Cymbiapophysa yimana Gabriel & Sherwood, 2020. Many Ecuadorian theraphosid taxa need modern redescription (pers. obs.).
The genus Thrixopelma Schmidt, 1994, is currently only represented by T. longicolli in Ecuador and was recently revised by Sherwood et al. [2]. Whilst working through undetermined mygalmorph material loaned to DS from the Zoologisches Museum, Universität Hamburg, we discovered a new species of the genus. A second male was subsequently located by DS whilst working through undetermined mygalomorph material at the Natural History Museum, London.
In this work, we describe a new species of Thrixopelma from Loja, Ecuador, based on the male, which possesses a unique combination of palpal bulb and tibial apophysis morphology in conjunction with a strongly curved metatarsus I.
2. Materials and Methods
The specimens were examined under a binocular microscope. Photographs of the palpal bulb and tibial apophysis of the holotype were made using a Leica M125C auto-montage. Description style follows Sherwood et al. [3]. Abbreviations for museum collections follow Evenhuis [4]. Abbreviations, Institutes: BMNH = Natural History Museum, London, United Kingdom; ZMH = Zoologisches Museum, Universität Hamburg, Germany. Structures: ALE = anterior lateral eyes, AME = anterior median eyes, PLE = posterior lateral eyes, PME = posterior median eyes; PB = prolateral branch (of tibial apophysis), RB = retrolateral branch (of tibial apophysis). Other: coll. = collector; det. = determined by. Leg spine terminology follows Petrunkevitch [5] with the modifications proposed by Bertani [6]: d = dorsal, v = ventral, r = retrolateral, p = prolateral. Palpal bulb terminology follows Bertani [7] and Gabriel [8]: A = apical keel, PI = prolateral inferior keel, PS = prolateral superior keel, RI = retrolateral inferior keel, RS = retrolateral superior keel, SA = subapical keel, TH = tegular heel; with the additions proposed by Gabriel & Sherwood [9]: ER = embolic ridge, PR = prolateral ridge, PAR = prolateral apical ridge, PC = prolateral crease. Leg formulae start with the longest leg to the shortest in order of decreasing size, e.g., 4, 1, 2, 3. Urticating setae terminology follows Cooke, Roth & Miller [10]. All measurements are in mm. Podomeres were measured from the femur to tarsus and do not include coxa or trochanter measurements. Percentages for metatarsal scopulae show their comparative extent of length to that of the total length of the ventral aspect of the metatarsus. The map was made using SimpleMappr (Shorthouse [11]). In accordance with Article 8 of the International Code of Zoological Nomenclature, this work was registered in ZooBank (urn:lsid:zoobank.org:pub:995014E5-91F0-42A2-9A1A-1F9760617BD9) prior to publication. A map of the type locality is given in the Supplementary Information.
3. Results
Thrixopelma nadineae sp. nov.
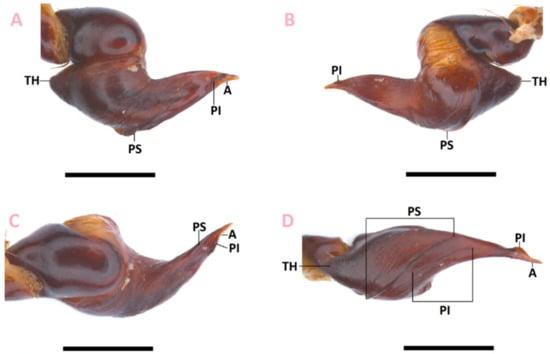
Figure 1.
Thrixopelma nadineae sp. nov. holotype male (ZMH-0000888), palpal bulb (left hand side), (A) prolateral view, (B) retrolateral view, (C) dorsal view, (D) ventral view. Scale bars = 1 mm.

Figure 2.
Thrixopelma nadineae sp. nov. holotype male (ZMH-0000888), tibial apophysis (left hand side), (A) prolateral view, (B) ventral view, (C) retrolateral view, (D) retro-ventral view. Scale bars = 1 mm.
Type material: Holotype ♂ (ZMH-0000888), Loja, Ecuador, E. W. Witt leg., ded.16.I.1898; paratype ♂ (BMNH), Ecuador: Loja, Zamora Huico, in house at 2000 m, 1997, coll. P. Lewis & B. Klitgaard, Mygalomorphae det. P. Hillyard, NHM London.
Diagnosis: Thrixopelma nadineae sp. nov. can be distinguished from all known male congeners by the absence of a palpal tibial apophysis (present in all known congeners), the presence of a basal crest on the PS (unknown in all known congeners), the absence of a basal crest on the PI (present in all known congeners), the position of the medial crest of the PI (comparatively more anterior than in all other known congeners), the developed PS (PS weakly developed in all known congeners) (Figure 1), the strongly curved metatarsus I (slightly curved in all known congeners), the elongate and finger-like RB (not so in all known congeners) (Figure 2) and its diminutive size. T. nadineae sp. nov. is further distinguished from the male of T. longicolli by the developed PI (PI well-developed in T. longicolli) (see Figure 1).
Etymology: The epithet is a matronym honouring Nadine Dupérré (Collections Manager, Zoologisches Museum, Universität Hamburg), our friend and colleague, in recognition of her numerous contributions to arachnology, especially on the taxonomy of spiders from Ecuador.
Description of holotype male (ZMH-0000888): Total length including chelicerae: 24.2. Carapace: length 11.4, width 4.1. Caput: slightly raised. Ocular tubercle: slightly raised, length 0.9, width 1.3. Eyes: AME > ALE, ALE > PLE, PLE > PME, anterior eye row procurved, posterior row slightly recurved. Clypeus: narrow; clypeal fringe: medium. Fovea: deep, recurved. Chelicera: length 2.5, width 1.8. Abdomen: length 10.3, width 7.1. Maxilla with 130–150 cuspules covering approximately 60% of the proximal edge. Labium: length 1.2, width 1.1, with 35–40 cuspules mostly separated by 0.5–1.0 times the width of a cuspule. Labio-sternal mounds: separate. Sternum: length 4.8, width 3.8, with three pairs of sigilla. Tarsi I–IV divided by line of setae. Metatarsal scopulae: I 86%; II 42%; III 30%; IV 24%. Lengths of legs and palpal segments: see Table 1, legs 4,1,2,3. Spination: femur I d 0–2–2, II d 0–2–0, III d 2–2–2, IV d 0–0–2, palp d 0–0–3, patella I p 0–0–1, II p 0–1–0, III p 0–0–1, r 0–0–1, IV p 0–0–2, palp d 0–0–3, tibia I d 2–2–0, v 3–2–2, II d 1–1–0, v 3–2–3, III d 2–2–0, v 2–2–2, IV d 2–2–0, v 4–3–2, palp p 1–0–0, r 0–2–1, metatarsus I d 0–1–0, v 2–0–1 (apical), II d 0–2–0, v 1–1–2 (apical), III d 2–2–2, v 3–4–5 (3 apical), IV d 2–2–2, v 2–3–5 (3 apical). Femur IV with fine but firm spine-like setae aligned on dorsal face on lateral borders. Tibia I with paired tibial apophysis, RB almost twice as long as PB, RB finger-like in shape in apical third (Figure 2). Femur III: incrassate. Palpal tibia: slightly incrassate, retrolateral apophysis absent. Metatarsus I: strongly curved (see Figure 2). Posterior lateral spinnerets with three segments, basal 1.4, median 0.9, digitiform apical 1.1. Lateral median spinnerets with one segment. Palpal bulb with TH; embolus angular and wide, with slight tapering at the very apex; PS elongate, disjunct, originating ventrally at base of the bulb and running prolaterally towards the apex of the embolus, with developed basal crest, PI developed, elongate, disjunct, originating ventrally at the base of the bulb and running prolaterally towards the apex of the embolus, with a developed medial crest and lacking a basal crest (Figure 1; Table 2). Urticating setae: Type III present dorsally. Colour: alcohol preserved brown.

Table 1.
Thrixopelma nadineae sp. nov. holotype male (ZMH-0000888), podomere lengths.

Table 2.
Bulb keel morphology of Thrixopelma nadineae sp. nov. and other species of Thrixopelma Schmidt, 1994, sensu Sherwood et al. [2] where males are properly known. Format follows Bertani [7] and Gabriel [8] with modifications based on the new palpal bulb features detailed by Gabriel & Sherwood [9]. Homologous keels present: weakly developed (+), developed (++), well-developed (+++), or absent (–).
Female: Unknown.
Distribution: Known only from the type locality, Loja, Ecuador (Figure S1).
Remarks: The right-hand side palpal bulb of the holotype has some deformity and was thus not considered in more detail in the analysis. The left-hand and right-hand side palpal bulbs of the paratype male in BMNH were consulted to ensure that the characters found in the left-hand side palpal bulb of the holotype were uniform for the species and this was indeed the case.
4. Discussion
Thrixopelma nadineae sp. nov. is the second species of Thrixopelma known from Ecuador. We suspect more undescribed taxa exist in the country, given the high levels of endemism known for other spider groups (e.g., Dupérré & Tapia [12]; Dupérré et al. [13]) and its ecoregional structure. The discovery of T. nadineae sp. nov. is interesting as this species has many divergent traits in comparison to its recently redescribed male congeners T. lagunas and T. longicolli. Most notably, in this species the PS is developed and has a basal crest. Conversely, a basal crest has only been found on the PI in T. lagunas and T. longicolli (Sherwood et al. [2]) and the same is true for several other (presently undescribed) species (pers. obs.). The total body length of T. nadineae sp. nov. is also 10.6 millimetres less that of the next smallest known male congener (i.e., the holotype male of T. lagunas (SMF 66757-84), see Sherwood et al. [2]). These findings demonstrate that much is still to be discovered about South American theraphosines. It also demonstrates that interesting discoveries are still to be made when working through unsorted historical museum material, adding further weight to broader discussions about the value of natural history museums (e.g., Allmon [14]; Bradley et al. [15]; Mares [16]; Nudds & Pettitt [17]; Suarez & Tsutsui [18]).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/taxonomy2030020/s1, Figure S1. Map showing the type locality of Thrixopelma nadineae sp. nov. (pink star).
Author Contributions
Conceptualization, D.S. and R.G; investigation, D.S. and R.G.; methodology, D.S. and R.G.; validation, D.S. and R.G., visualization, D.S. and R.G.; writing—original draft preparation, D.S.; writing—review and editing, D.S. and R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We wish to thank Nadine Dupérré (Zoologisches Museum, Universität Hamburg) for loan of this specimen, amongst other interesting material, and Darren Mann, James Hogan, and Zoë Simmons (Oxford University Museum of Natural History) for allowing use of the auto-montage at OUMNH (sponsored by the A. McCrae bequest).
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Spider Catalog. The World Spider Catalog, Version 23.0; Naturhistorisches Museum Bern: Bern, Switzerland, 2022. Available online: http://wsc.nmbe.ch (accessed on 23 April 2022).
- Sherwood, D.; Gabriel, R.; Kaderka, R.; Lucas, S.M.; Brescovit, A.D. Stabilizing a chaotic taxonomy: Redescription and redefinition of the genera Lasiodorides Schmidt & Bischoff, 1997 and Thrixopelma Schmidt, 1994 (Araneae: Theraphosidae). Arachnology 2021, 18, 893–917. [Google Scholar]
- Sherwood, D.; Fabiano-da-Silva, W.; Gabriel, R.; Lucas, S.M. Redescription of Nesipelma insulare Schmidt & Kovařík, 1996 with a revised generic diagnosis for Nesipelma Schmidt & Kovařík, 1996 and a transfer from Cyrtopholis Simon, 1892 (Araneae: Theraphosidae). Arachnology 2020, 18, 462–467. [Google Scholar]
- Evenhuis, N.L. The Insect and Spider Collections of the World; Bishop Museum: Honolulu, HI, USA, 1997; Available online: http://hbs.bishopmuseum.org/codens (accessed on 23 April 2022).
- Petrunkevitch, A. Arachnida from Panama. Trans. Connect. Acad. Arts Sci. 1925, 27, 51–248. [Google Scholar]
- Bertani, R. Revision, cladistic analysis, and zoogeography of Vitalius, Nhandu, and Proshapalopus; with notes on other theraphosine genera (Araneae, Theraphosidae). Arq. Zool. 2001, 36, 265–356. [Google Scholar]
- Bertani, R. Male palpal bulbs and homologous features in Theraphosinae (Araneae, Theraphosidae). J. Arachnol. 2000, 28, 29–42. [Google Scholar] [CrossRef]
- Gabriel, R. Revised taxonomic placement of the species in the Central American genera Davus O. Pickard-Cambridge, 1892, Metriopelma Becker, 1878, and Schizopelma F. O. Pickard-Cambridge, 1897, with comments on species in related genera (Araneae: Theraphosidae). Arachnology 2016, 17, 61–92. [Google Scholar] [CrossRef]
- Gabriel, R.; Sherwood, D. Revised taxonomic placement of Pseudhapalopus Strand, 1907, with notes on some related taxa (Araneae: Theraphosidae). Arachnology 2020, 18, 301–316. [Google Scholar] [CrossRef]
- Cooke, J.A.L.; Roth, V.D.; Miller, F.H. The urticating hairs of theraphosid spiders. Am. Mus. Novit. 1972, 2498, 1–43. [Google Scholar]
- Shorthouse, D.P. 2010: SimpleMappr, an online tool to produce publication-quality point maps. Available online: https://www.simplemappr.net (accessed on 23 April 2022).
- Dupérré, N.; Tapia, E. Megadiverse Ecuador: A review of Mysmenopsis (Araneae, Mysmenidae) of Ecuador, with the description of twenty-one new kleptoparasitic spider species. Zootaxa 2020, 4761, 1–81. [Google Scholar] [CrossRef] [PubMed]
- Dupérré, N.; Tapia, E.; Quandt, D.; Crespo-Pérez, V.; Harms, D. From the lowlands to the highlands of Ecuador, a study of the genus Masteria (Araneae, Mygalomorphae, Dipluridae) with description of seven new species. Zootaxa 2021, 5005, 538–568. [Google Scholar] [CrossRef] [PubMed]
- Allmon, W.D. The value of natural history collections. Curator 2001, 37, 83–89. [Google Scholar] [CrossRef]
- Bradley, R.D.; Bradley, L.C.; Garner, H.J.; Baker, R.J. Assessing the value of natural history collections and addressing issues regarding long-term growth and care. BioScience 2014, 64, 1150–1158. [Google Scholar] [CrossRef]
- Mares, M.A. Natural science collections: America’s irreplaceable resource. BioScience 2009, 59, 544–545. [Google Scholar] [CrossRef][Green Version]
- Nudds, J.R.; Pettitt, C.W. The Value and Valuation of Natural Science Collections, Proceedings of the International Conference, Manchester, UK, 1995; The Geological Society: London, UK, 1997; 276p. [Google Scholar]
- Suarez, A.V.; Tsutsui, N.D. The value of museum collections for research and society. BioScience 2004, 54, 66–74. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).