Herzog Vindicated: Integrative Taxonomy Reveals That Trichostomum brachydontium (Pottiaceae, Bryophyta) Comprises Several Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. DNA Sequencing
2.3. Data Analysis
2.4. Morphological Study
3. Results
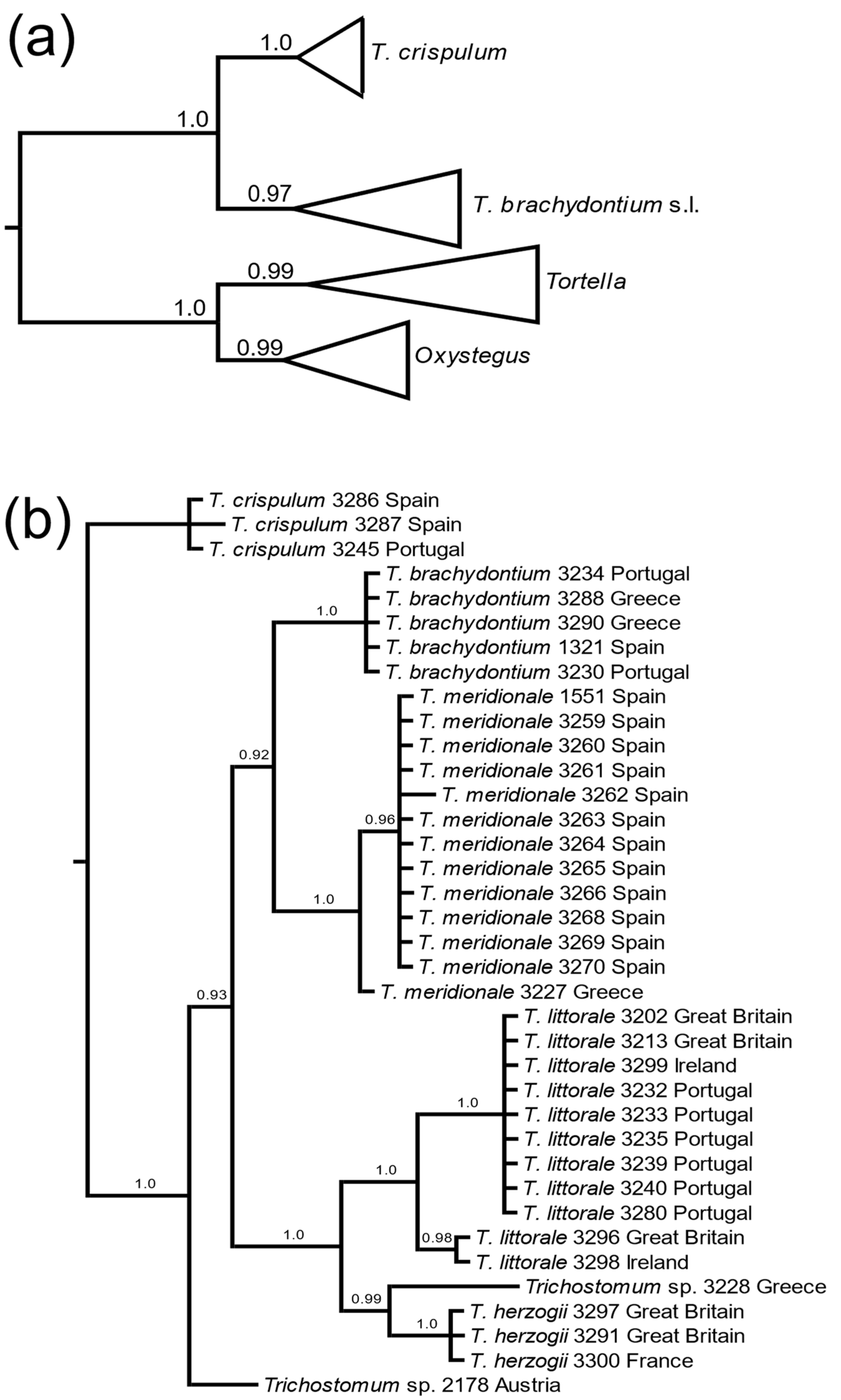
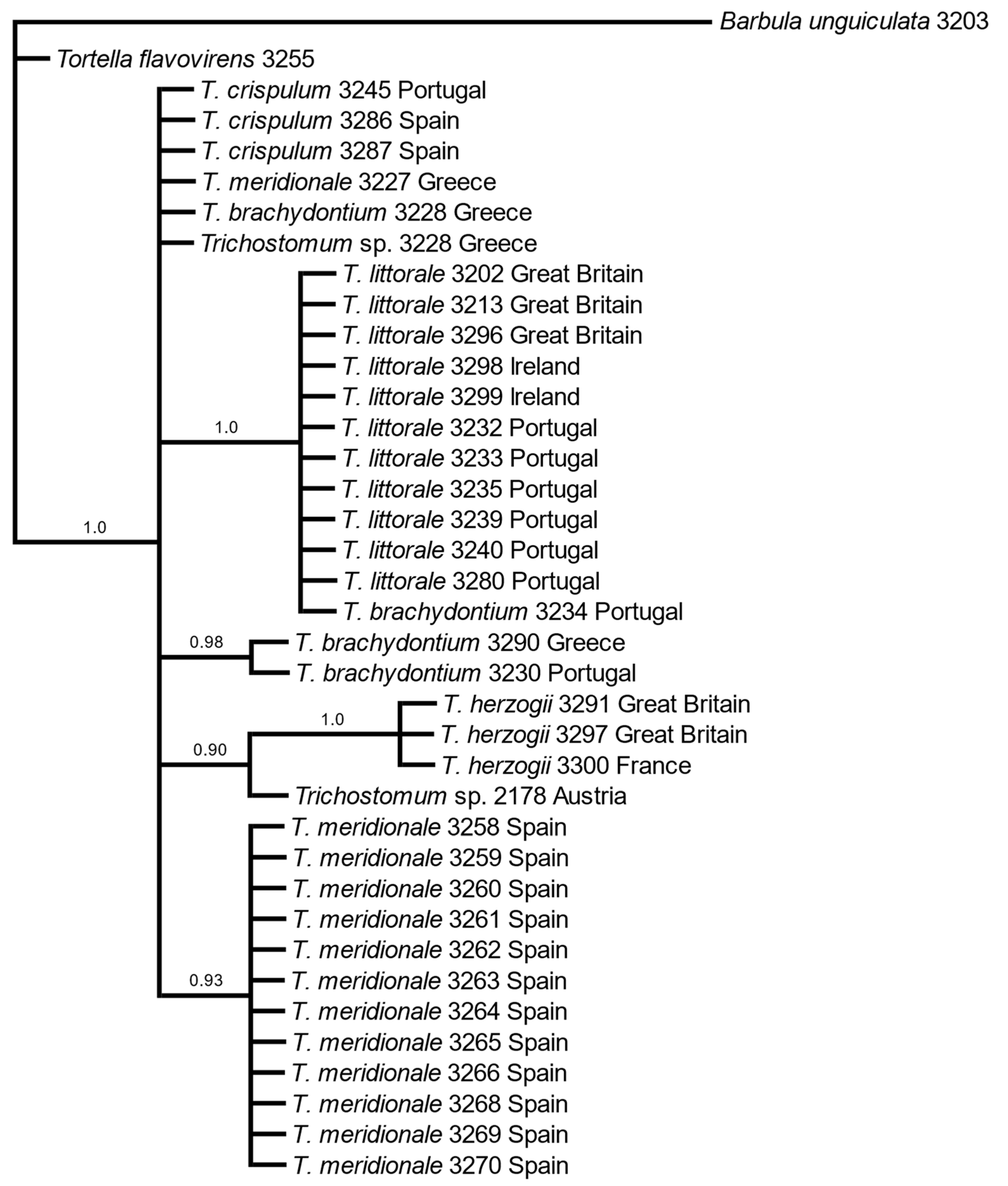
3.1. Molecular Data
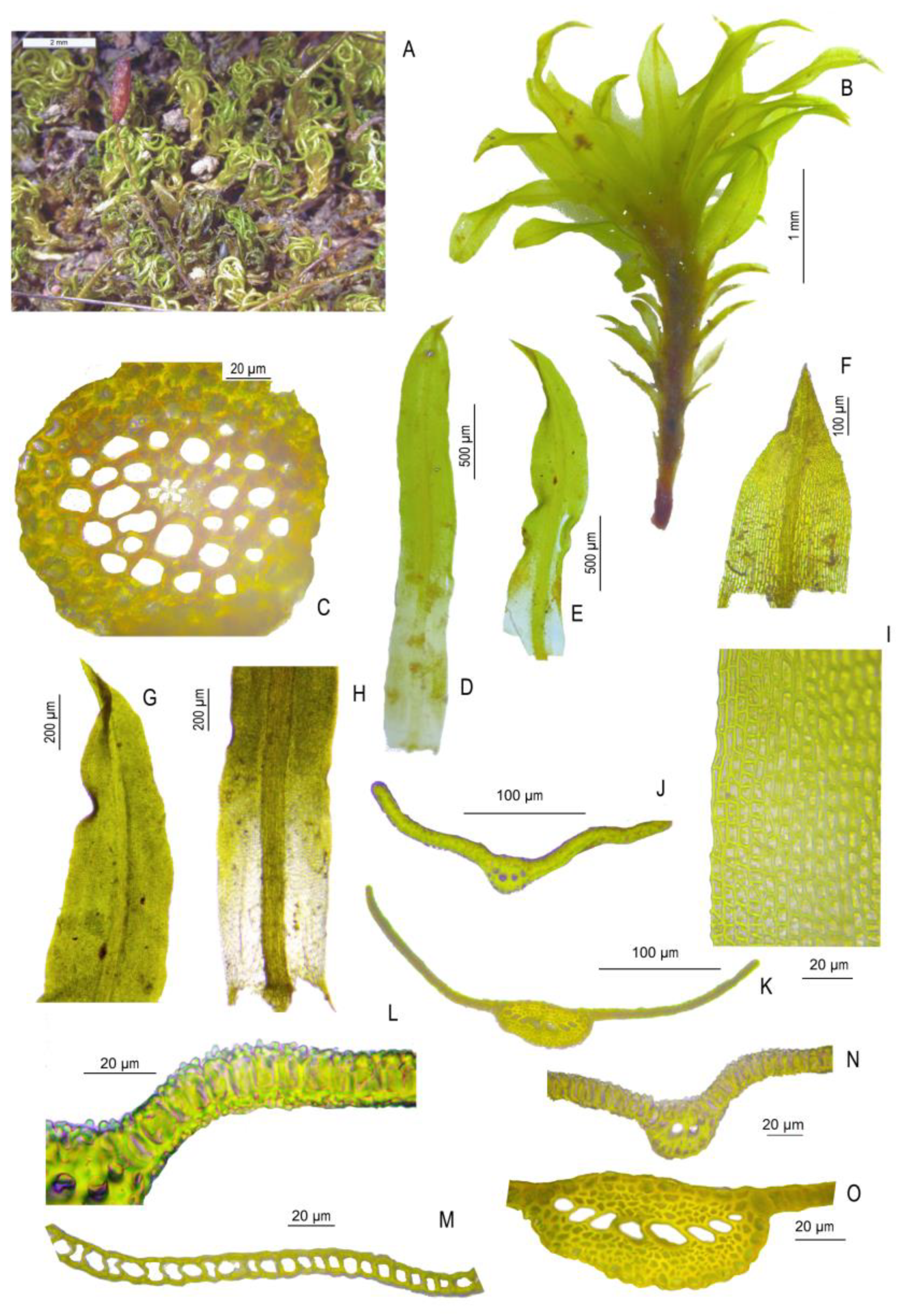
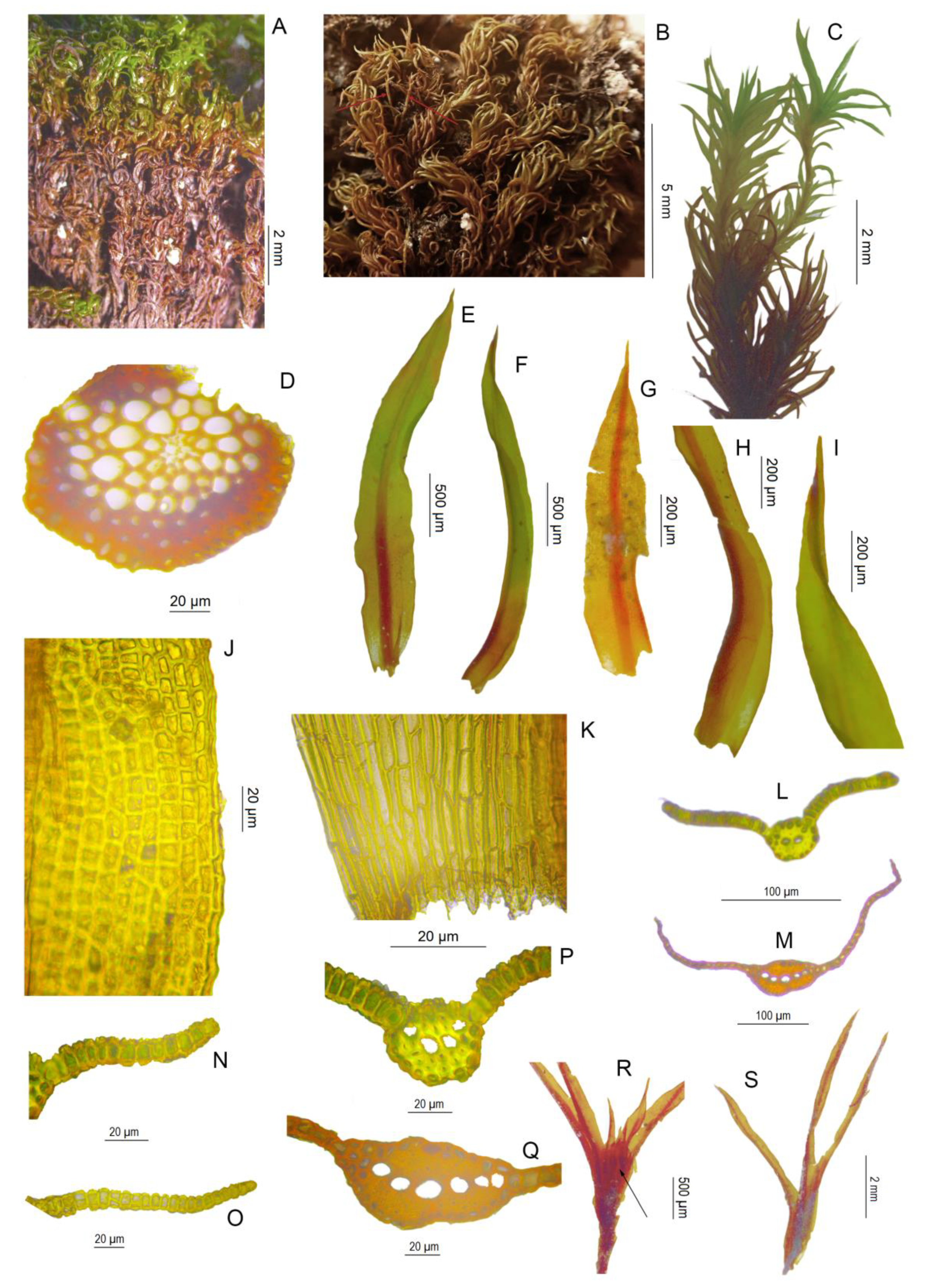
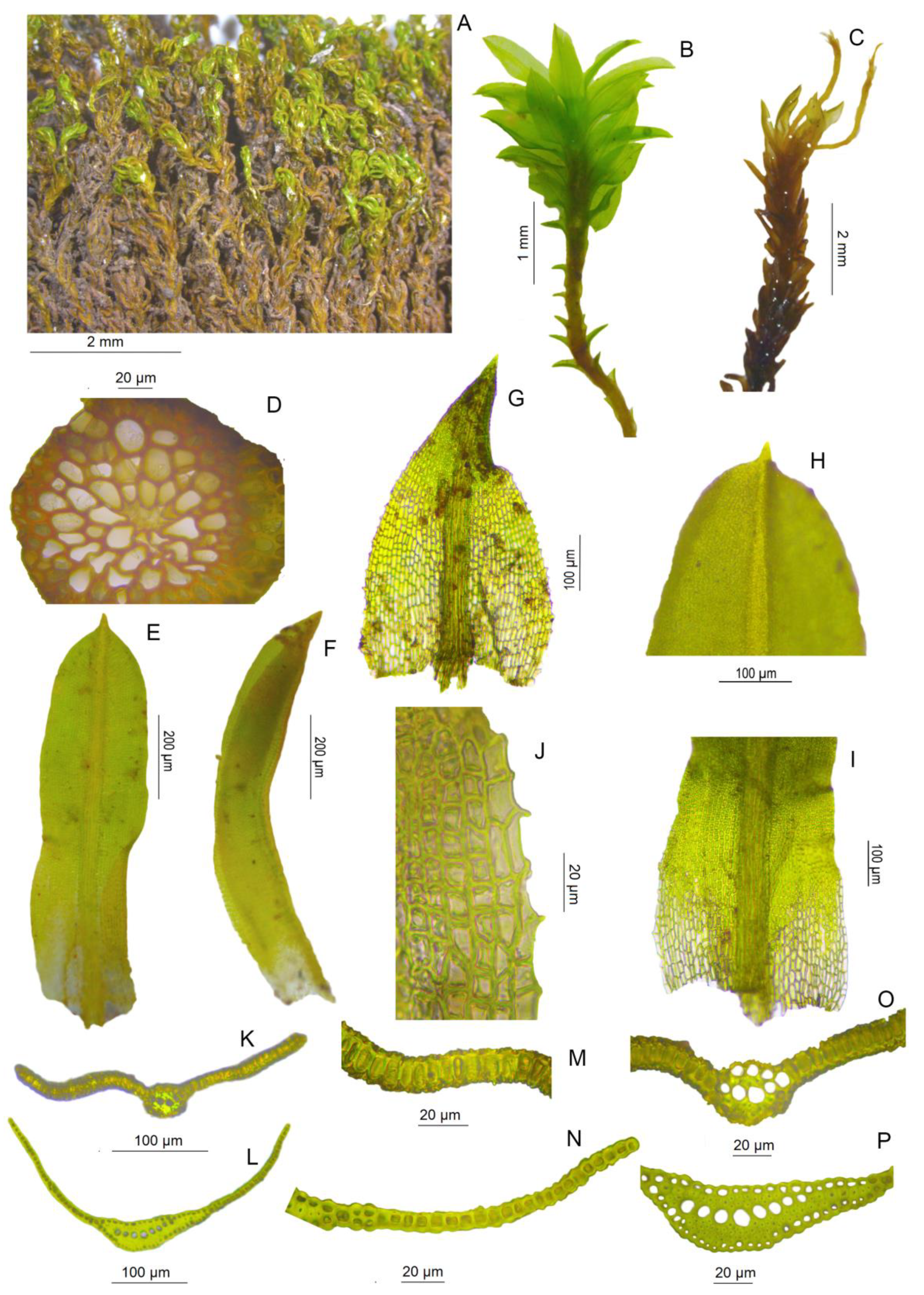
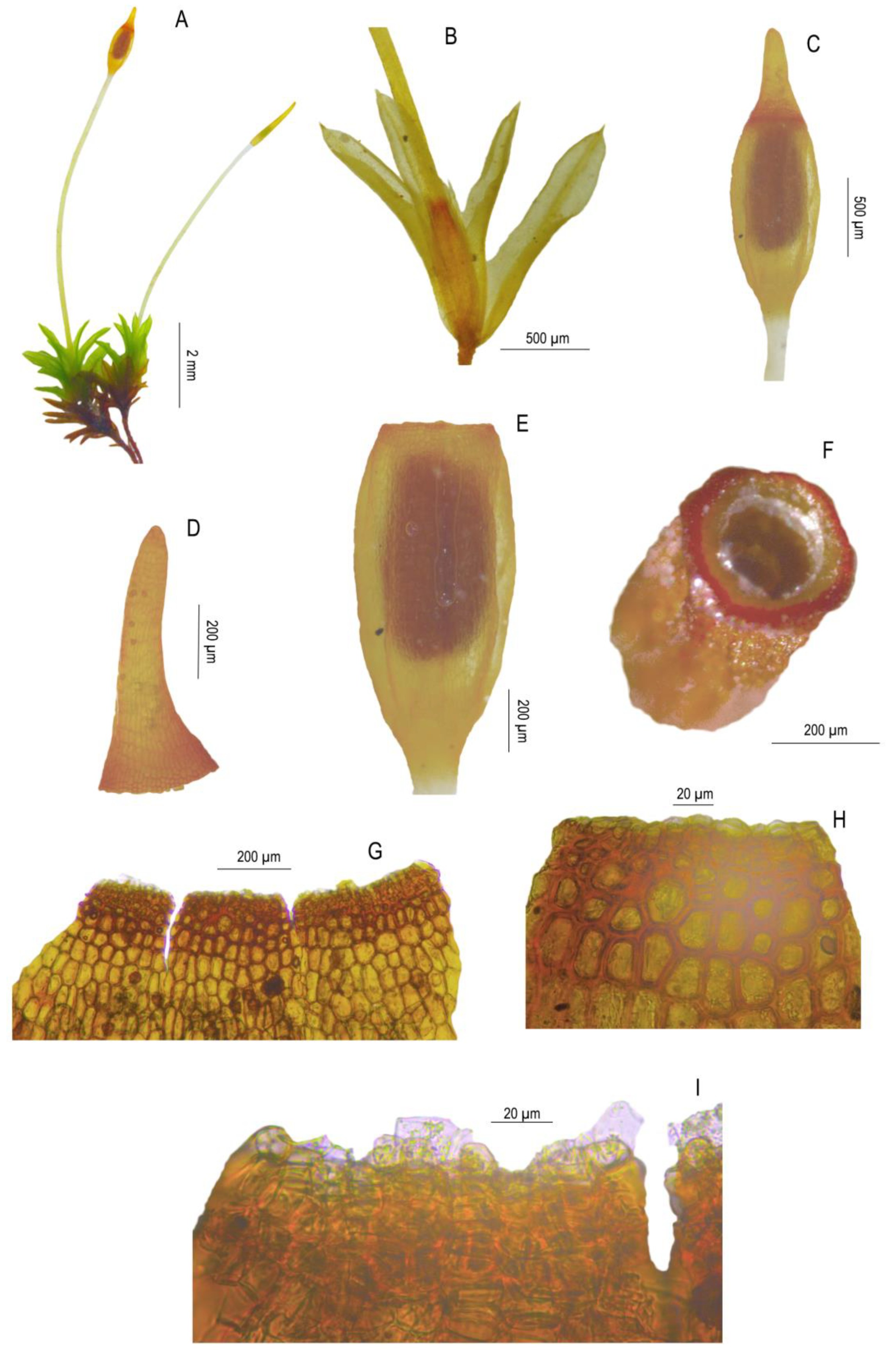
3.2. Morphological Study
4. Discussion
5. Taxonomic Treatment
5.1. The List of Taxa
5.2. Key to Species
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Code (Haplotype) | Locality (Voucher) | GenBank Accession Numbers ITS/rbcL |
| Trichostomum brachydontium s.s. | ||
| 1321 | SPAIN, Andalusia, Cádiz, Los Barrios, Sierra de Ojén, Canuto del Cebrillo, 28.12.1999, J. Guerra & R.M. Ros s.n., MUB 12650 | AY796252 [22]/- |
| 3230 | PORTUGAL, Algarve, Serra de Monchique, Vale de Cova da Serra, 17.05.2020, R.D. Porley 01, Herb. Porley, duplicate MUB 60458. | OM234698/OM260133 |
| 3234 | PORTUGAL, Algarve, Serra de Monchique, Barranco Parral North, 06.04.2014, R.D. Porley 05, Herb. Porley, duplicate MUB 60459 | OM234695/OM260134 |
| 3288 | GREECE, Cyclades, Andros, Frousei, near Palestou, ca. 240 m, 19.03.2016, T.L. Blockeel 45/085, Herb. Blockeel, duplicate MUB 60460 | OM234696/OM260097 |
| 3290 | GREECE, Cyclades, Andros, above Arnim near Vourkoti, ca. 670 m, 19.03.2016, T.L. Blockeel 45/129, Herb. Blockeel, duplicate MUB 60461. | OM234697/OM260099 |
| - | ITALY, Sardinia, prope Cagliari, Feb. 1827, Müller, JE 04008873 (Lectotype of T. brachydontium). | - |
| - | ITALY, Sardinia, prope Cagliari, Feb. 1827, Müller, JE 04008874 (Syntype of T. brachydontium). | - |
| - | ITALY, Toscana, Firenze, Giardino di Boboli, 29.03.1988, C. Cortini s.n., CAME | - |
| - | ITALY, Toscana, Alpi Apuane, lungo il torrente Renara fra Guadine e Casania (Massa), 20.11.1988, C. Cortini & M. Aleffi s.n., CAME | - |
| - | ITALY, Toscana, Alpi Apuane, Desiate (Riomagno), 19.11.1988, C. Cortini & M. Aleffi s.n., CAME | - |
| - | ITALY, Sardinia, Chia, Domus de Maria, 07.04.2006, A. Cogoni s.n., CAG | - |
| - | ITALY, Sardinia, Rio Santa Lucia, Assemini, 01.10.2020, A. Cogoni s.n., CAG | - |
| - | ITALY, Sardinia, Is Pauceris, Assemini, 25.05.2021, A. Cogoni s.n., CAG (var. cylindricum) | - |
| - | MOROCCO, Bab Taza, ascensión al Jbel Bouhalla, El Maounzil, 35°04′45″ N, 5°10′05″ W, 1100 m, 17.03.1997, M.J. Cano, M.T. Gallego & R.M. Ros s.n. MUB 10961 | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Ribeira de Seixoso, 07.01.2010, R.D. Porley s.n., Herb. Porley, duplicate MUB 60462 | - |
| - | PORTUGAL, Algarve, Carrapateira, Costa Vicentina PN, 27.21.2007, R.D. Porley 14, Herb. Porley, duplicate MUB 60463 | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Ribeira do Seixoso, 07.01.2010, R.D. Porley 31, Herb. Porley, duplicate MUB 60464 | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Ribeira do Seixoso, 07.01.2010, R.D. Porley 32, Herb. Porley, duplicate MUB 60465 | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Ribeira do Seixoso, 05.02.2010, R.D. Porley 33, Herb. Porley, duplicate MUB 60466 | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Ribeira do Seixoso, 07.01.2010, R.D. Porley 34, Herb. Porley, duplicate MUB 60467 | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Ribeira do Seixoso, 05.02.2010, R.D. Porley 35, Herb. Porley, duplicate MUB 60468 | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Ribeira do Seixoso, 07.01.2010, R.D. Porley 36, Herb. Porley, duplicate MUB 60469 | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Ribeira de Boina, S. of Brejão, 22.12.2016, R.D. Porley 37, Herb. Porley, duplicate MUB 60470 | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Ribeira do Seixoso, 18.01.2010, R.D. Porley 45, Herb. Porley, duplicate MUB 60471 | - |
| - | PORTUGAL, Algarve, Monte do Galo, near Cerca dos Pomares, 12.11.2021, R.D. Porley s.n., Herb. Porley | - |
| - | PORTUGAL, Madeira, S. Vicente, Folhadal, entre o 1° e 2° Tunel a partir da Encumeada, 29.02.1982, M. Pita 5, LISU1492332 | - |
| - | PORTUGAL, Madeira, Ribeiro Frio, 12.05.1979, C. Sérgio 2365, LISU162400 | - |
| - | PORTUGAL, Madeira, Faldas do Pico Jorge, Levada para a Ribeiro Bonito,12.04.1988, C. Sérgio & M. Nóbrega 6132, LISU162405 | - |
| - | PORTUGAL, Madeira, Fanal de Baixo, Riberia Funda descendo para a Ribeira, 28.06.1988, C. Sérgio & M. Nóbrega 6132, LISU162406 | - |
| - | SPAIN, Balearic Islands, Mallorca, cruce de Carretera Sacalobra y carretera a Pollensa, cerca del chiringuito, 39°49′05″ N, 2°49′05″ E, 580 m, 16.04.1999, M.J. Cano, M.T. Gallego & M.C Sánchez-Moya, MUB 11128 | - |
| - | SPAIN, Canary Islands, Tenerife, Anaga, Junto a Puente, 29.10.2003. R.M. Ros, A. Losada & J.M. González-Mancebo, MUB 16625 | - |
| - | GREAT BRITAIN, England, N. Somerset, Walton-in-Gordano, 31.01.1989, R.D. Porley 1392, Herb. Porley | - |
| - | GREAT BRITAIN, Wales, Montgomeryshire, Guilsfield, 02.07.1978, M.J. Wigginton s.n., Herb. Porley | - |
| Trichostomum herzogii | ||
| 3291 | GREAT BRITAIN, England, Derbyshire, Water-cum-Jolly Dale, near Cressbrook, 30.04.2021, T.L. Blockeel 50/064, Herb. Blockeel, duplicate MUB 60473 | OM234712/OM260100 |
| 3297 | GREAT BRITAIN, England, Miller’s Dale, T.L. Blockeel 50/123, DNA 3297, Herb. Blockeel, duplicate MUB 60474 | OM234711/OM260102 |
| 3300 | FRANCE, Ain, Lélex, Pont du Rouffes, 01.07.2020, V. Hugonnot s.n., Herb. Hugonnot, duplicates Herb. Porley, MUB 60457 | OM234726/OM260122 |
| - | FRANCE, prope Aix en Provence, 30.06.1874, Philibert, HA.H3400241 (sub Hymenostomum unguiculatum) | - |
| - | GERMANY, auf Kalkfelsen des Hohensteins bei Warburg (collector unknown s.n., s.d.) Lectotype, JE04008876 (Lectotype of Trichostomum cuspidatum) | - |
| - | GERMANY, am Massenkalkfels des Hohenstein bei Warstein (s.d.) H. Müller, JE04008877 (Isotype of Trichostomum cuspidatum) | - |
| - | GERMANY, Warstein, H. Müller (s.d.) JE04008878 (Isotype of Trichostomum cuspidatum) | - |
| - | GERMANY, in Klüften der Massenkalkfelsen im Lürmekethal [Lörmecketal] u. am Hohen Stein bei Warstein im Sauerland u. bei Höxter, H. Müller au Wenck, JE04008875 | - |
| - | GREAT BRITAIN, Wales, Breconshire, Craig Cerrig Gleisand NNR, 950 m, R.D. Porley 1470, Herb. Porley | - |
| - | GREAT BRITAIN, England, Shropshire, Alberbury, R.D. Porley 1391, Herb. Porley | - |
| - | ITALY, Toscana, Afuane, Can. del Rio, 680 m, 27.07.1990. M. Aleffi, CAME | - |
| Trichostomum littorale | ||
| 3202 | GREAT BRITAIN, Cornwall, Kynance Cove, Lizard, 13.02.2011, J.A. Norton 130211, Herb. D. Callaghan, duplicate MUB 60475 | OM 234703/OM260112 |
| 3213 | GREAT BRITAIN, Shetland Islands, Eshaness, 31.08.2014, S.V.O’ Leary s.n., Herb. D. Callaghan 310814, duplicate MUB 60476 | OM234704/OM260094 |
| 3232 | PORTUGAL, Algarve, Serra de Monchique, Barbelote amphitheatre, 527 m, 27.10.2016, R.D. Porley 03, Herb. R. Porley, duplicate MUB 60477 | OM234705/OM260107 |
| 3233 | PORTUGAL, Algarve, Serra de Monchique, Picota, 737 n, 02.03.2017, R.D. Porley 04, Herb. Porley, duplicate MUB 60478 | OM234706/OM260108 |
| 3235 | PORTUGAL, Algarve, Serra de Monchique, Barranco do Lajeado, N of Caldas, 227 m, 20.05.2016, R.D. Porley 06, Herb. Porley, duplicate MUB 60479 | OM234707/OM260109 |
| 3239 | PORTUGAL, Algarve, Serra de Monchique, Chilrão, 480 m, 30.07.2015, R. Porley 10, Herb. Porley, duplicate MUB 60480 | OM234708/OM260110 |
| 3240 | PORTUGAL, Algarve, Serra de Monchique, Fóia, Penedo do Buraco, 740 m, 06.02.2015, R.D. Porley 11, Herb. Porley, duplicate MUB 60481 | OM234709/OM260111 |
| 3280 | PORTUGAL, Algarve, Serra de Monchique, Ribeira da Cerca, W. of Marmelete, 19.08.2015, R.D. Porley 41, Herb. Porley, duplicate MUB 60482 | OM234699/OM260095 |
| 3296 | GREAT BRITAIN, Wales, Upper part of Cwm yr Allt-Lwyd, 17.09.2021, T.L. Blockeel 50/152, Herb. Blockeel, duplicates Herb. Porley, MUB 60483 | OM234700/OM260101 |
| 3298 | IRELAND, County Kerry, Torc Cascade, ca. 140 m, N.G. Hodgetts 11187, Herb. N.G. Hodgetts, duplicates Herb. Porley, MUB 60484 | OM234701/OM260103 |
| 3299 | IRELAND, County Kerry, Torc, Muckross Lake, N.G. Hodgetts 11181, Herb. Hodgetts, duplicates Herb. Porley, MUB 60485 | OM234702/OM260104 |
| - | IRELAND, NY 01449062 (Lectotype) | - |
| - | GREAT BRITAIN, Whitsand Bay Cornwall, F. Brent, 0 3.02.1868, NY 01449063 (Syntype) | - |
| - | GREAT BRITAIN, a little East of Fairlight Down near Hastings, Sussex, July 1847, W.M., NY 01449064 (Syntype) | - |
| - | GREAT BRITAIN, Scotland, Orkney, Egilsay, 6 m, 12.07.2006, R.D. Porley 2917, Herb. Porley | - |
| - | GREAT BRITAIN, England, North Devon, Bideford Bay, Saunton, 8 m, 05.08.2003, R.D. Porley 2644, Herb. Porley | - |
| - | ITALY, Prov. La Spezia, Bachschlucht ca. 1 km vor Pignone an der Str. nach La Spezia im Castanea-Wald, 25.03.1965, R. Düll s.n., CAME. | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Barbelote amphitheatre, 600 m, 02.04.2015, R.D. Porley 02, Herb. Porley, duplicate MUB 60486 | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Ribeira de Seixoso, 125 m, 05.02.2010, R.D. Porley 07, Herb. Porley, duplicate MUB 60487 | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Ribeira de Seixoso, 125 m, 05.02.2010, R.D. Porley 09, Herb. Porley, duplicate MUB 60488 | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Fóia, north side, 815 m, 03.12.2015, R.D. Porley 12, Herb. Porley, duplicate MUB 60489 | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Fóia, Penado do Buraco, 05.02.2015, R.D. Porley 24, Herb. Porley | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Chilrão, 500 m, 28.02.2014, R.D. Porley 29, Herb. Porley | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Ribeira do Seixoso, 125 m, 05.02.2010, R.D. Porley 30, Herb. Porley | - |
| - | PORTUGAL, Algarve, Ribeira da Cerca, Cerca dos Pomares, 50 m, 26.06.2013, R.D. Porley 39, Herb. Porley | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Ribeira da Cerca, W. of Marmelete, 180 m, 19.08.2020, R.D. Porley 40, Herb. Porley | - |
| - | PORTUGAL, Algarve, Serra de Monchique, Ribeira da Cerca, W. of Marmelete, 163 m, 05.08.2020, R.D. Porley 43, Herb. Porley | - |
| - | SPAIN, Andalusia, Cádiz, Medina-Sidonia, Sierra Blanquilla, arroyo del Alisoso, 16.04.2000, J. Guerra s.n., MUB 11257 | - |
| - | GREAT BRITAIN, Scotland, Outer Hebrides, Clash na Bearnaich, Hirta, St. Kilda, 100 m, 09.07.2013, R.D. Porley s.n., Herb. Porley | - |
| Trichostomum meridionale | ||
| 1551 | SPAIN, Murcia Region, Bullas, Salto Lucero, 650 m, 20.11.2002, R.M. Ros s.n., MUB 14240 (Paratype) | AY796255/- |
| 3227 | GREECE, Eastern Aegean Islands, Rhodes, ca 1.2 km SE of Moni Artamiti, ca, 240 m, 08.03.2018, T.L. Blockeel 47/027, Herb. Blockeel, duplicate MUB 60472 (Paratype) | OM234718/OM260105 |
| 3259 | SPAIN, Murcia Region, Sierra Minera de Cartagena-La Unión, Rambla del Gorguel, 131 m, 04.03.2020, R.M. Ros, O. Werner & P. Egea Benavente s.n., MUB 60287, duplicate Herb. Porley (Paratype) | OM234713/OM260119 |
| 3260 | SPAIN, Murcia Region, Cartagena, Atamaría, camino que sube al Monte de las Cenizas, en la carretera RM-514, 151 m, 04.03.2020, DNA 3260, R.M. Ros, O. Werner & P. Egea Benavente s.n., MUB 60288, duplicate Herb. Porley (Paratype) | OM234714/OM260120 |
| 3261 | SPAIN, Murcia Region, Cartagena, Atamaría, camino que sube al Monte de las Cenizas, en la carretera RM-514, 151 m, 04.03.2020, DNA 3261, R.M. Ros, O. Werner & P. Egea Benavente s.n., MUB 60289, duplicate Herb. Porley (Paratype) | OM234715/OM260123 |
| 3262 | SPAIN, Murcia Region, Cartagena, Calarreona, 0 m, 04.03.2020, DNA 3262, R.M. Ros, O. Werner & P. Egea Benavente s.n., MUB 60290, duplicate Herb. Porley (Paratype) | OM234716/OM260124 |
| 3263 | SPAIN, Murcia Region, Cartagena, Calarreona, 0 m, 04.03.2020, DNA 3263, R.M. Ros, O. Werner & P. Egea Benavente s.n., MUB 60291, duplicate Herb. Porley (Paratype) | OM234717/OM260125 |
| 3264 | SPAIN, Murcia Region, Las Torres de Cotillas, Urbanización Los Romeros, calle I, parcela en la zona más alta, 144 m, 07.03.2020, R.M. Ros s.n., MUB 60293 (Paratype) | OM234719/OM260126 |
| 3265 | SPAIN, Murcia Region, Las Torres de Cotillas, Urbanización Los Romeros, calle I, parcela en la zona más alta, 144 m, 02.05.2020, R.M. Ros s.n., MUB 60296, duplicate Herb. Porley (Paratype) | OM234720/OM260127 |
| 3266 | SPAIN, Murcia Region, Murcia, Rambla del Puerto de La Cadena, 260 m, 26.03.2019, R.M. Ros & M. Farag s.n., MUB 60292, duplicate Herb. Porley (Paratype) | OM234721/OM260128 |
| 3268 | SPAIN, Murcia Region, Murcia, El Valle Perdido, proximidades a Santuario de la Fuensanta, 143 m, 14.03.2020, R.M. Ros & O. Werner s.n., MUB 60295, duplicate Herb. Porley (Holotype) | OM234722/OM260130 |
| 3269 | SPAIN, Murcia Region, Murcia, El Valle Perdido, proximidades a Santuario de la Fuensanta, 143 n, 14.03.2020, R.M. Ros & O. Werner s.n., MUB 60294, duplicate Herb. Porley (Paratype) | OM234723/OM260131 |
| 3270 | SPAIN, Murcia Region, Cartagena, Punta de la Azohía, 50 m, 06.03.2009, R.M. Ros s.n., MUB 29467, duplicate Herb. Porley (Paratype) | OM234724/OM260132 |
| - | ITALY, Sardinia, S. Elia, Cagliari, pianoro per Cala Fighera, 17.05.2001, A. Cogoni s.n., CAG | - |
| - | ITALY, Sardinia, Casa Laura, Giara di Gesturi, Genoni, 08.03.2002, A. Cogoni s.n., CAG (sample with intermediate characters with T. littorale) | - |
| - | SPAIN, Alicante, Cabo de la Nao, (Jávea), M.J. Cano s.n, MUB 5565 | - |
| - | SPAIN, Almería, camino a Río de Aguas desde El Cerrón (Sorbas), 18.03.1988, J.J. Martínez-Sánchez, R.M. Ros & J. Guerra s.n., MUB 6101 | - |
| Trichostomum sp. | ||
| 2178 | AUSTRIA, Styria, Hochschwab massif, Pfarrerlacke W of Tragöss, ca 930 m, 06.10.2006, H. Köckinger 12338, Herb. Köckinger, duplicates Herb. Porley, MUB 60490 | OM234727/OM260121 |
| 3228 | GREECE, Kithira Island, ca 1.5 km east of Limnionas, 140 m, 27.03.2019, T.L. Blockeel 48/098, Herb. Blockeel, duplicates Herb. Porley, MUB 60491 | OM234710/OM260106 |
| Trichostomum crispulum | ||
| 3245 | PORTUGAL, Algarve, Senhora da Rocha, Lagoa, 17.03.2013, R.D. Porley 16, Herb. Porley, duplicate MUB 60492 | OM234693/OM260091 |
| 3286 | SPAIN, Galicia, Orense, Monasterio de San Estevo de Ribas de Sil, 20.08.2021, R.M. Ros & A. Werner Ros s.n., MUB 60493 | OM234691/OM260092 |
| 3287 | SPAIN, Andalusia, Jaén, Sierras del Sur de Jaén, carretera hacia Monumento Natural Quejigo del Carbón o del Amo, 04.05.2021, R.M. Ros & O. Werner s.n., MUB 60494 | OM234692/OM260093 |
| Outgroup specimens | ||
| Barbula unguiculata 3203 | GREAT BRITAIN, Durslton Head, Dorset, A. Norton 81213, Herb. Callaghan, duplicate MUB 60495 | -/OM260089 |
| Oxystegus sp. | MALAWI, Zomba plateau, peak road below Malumbe Peak, D. Long 12608, BM | AY854390 [22,25]/- |
| Oxystegus tenuirostris | AUSTRIA, Styria, Stubalpe, Granitzgraben SE of Weisskirchen, H. Köckinger 14245a, GZU, MUB 31054 | HM049797 [25]/- |
| Tortella alpicola | SPAIN, Sierra Nevada, Collado de La Mosca, S. Rams s.n., MUB 21926 | HM049809 [25]/- |
| Tortella flavovirens | GREECE, Pelopónnisos, M.J. Cano et al. s.n., MUB 11940 | AY796262 [22]/- |
| Tortella flavovirens 3255 | PORTUGAL, Algarve, Serra de Monchique, Gralhos, Via Alagarviana, 545 m, 26.01.2018, R.D. Porley 26, Herb. Porley | -/OM260117 |
| Tortella humilis | SPAIN, Albacete, R.M. Ros & O. Werner s.n., MUB 17767 | AY796260 [22]/- |
| Tortella squarrosa | CZECH REPUBLIC, Tmaň-Kotýz, J. Košnar 1266, CBFS 562 | JX679950 [55]/- |
| Tortella tortuosa | MOROCCO, Rif, M.J. Cano & R.M. Ros, s.n., MUB 10397 | AY796266 [22]/- |
References
- Blockeel, T.L. Trichostomum brachydontium. In Atlas of British & Irish Bryophytes; Blockeel, T.L., Bosanquet, S.D.S., Hill, M.O., Preston, C.D., Eds.; Pisces Publications: Newbury, UK, 2014; p. 141. ISBN 978-1-874357-62-9. [Google Scholar]
- Zander, R.H. Trichostomum. In Flora of North America North of Mexico; Flora of North America Editorial Committee, Ed.; Oxford University Press: New York, NY, USA, 2007; Volume 27, pp. 488–494. [Google Scholar]
- Thouvenot, L.; Bardat, J. Contribution to the bryophyte flora of New Caledonia. I. New taxa and amendments. Cryptogam. Bryol. 2013, 34, 37–47. [Google Scholar] [CrossRef]
- Guerra, J. Trichostomum Bruch. In Flora Briofítica Ibérica; Guerra, J., Cano, M.J., Ros, R.M., Eds.; Universidad de Murcia y Sociedad Española de Briología: Murcia, Spain, 2006; Volume 3, pp. 76–83. [Google Scholar]
- Müller, F.A. Erstes Verzeichnis sardinischer Laubmoose, wie auch derjenigen welche von meinen Freunde Herrn Fleischer bei Smyrna aufgefunden worden sind, nebst Beschreibungen und Abbildungen einiger neuen Arten. Flora 1829, 12, 385–399. [Google Scholar]
- Herzog, T. Studien über den Formenkreis des Trichostomum mutabile Br. Abh. Kais. Leopold.-Carol. Dtsch. Akad. Nat. Nova Acta 1907, 73, 453–481. [Google Scholar]
- Rungby, S. A contribution to the bryophytic flora of Spain and Morocco, especially the area between Gandía and Alcoy. Bot. Not. 1962, 115, 61–64. [Google Scholar]
- Koppe, F. Bryologische Beobachtungen auf der Insel Mallorca. Bot. Not. 1965, 118, 25–48. [Google Scholar]
- Renauld, F.; Cardot, J. Mousses des Canaries récoltées par M. Albert Tullgren et coup d’oeil sur la flore bryologique des îles atlantiques. Bull. L’herbier Boissier Sér. 2 1902, 2, 433–453. [Google Scholar]
- Sérgio, C. Acerca da identidade de Hyophila contorta (Kunze) Jaeg. Pottiaceae da Ilha da Madeira. Port. Acta Biol. Série B Sist. Ecol. Biogeogr. E Paleontol. 1985, 14, 168–172. [Google Scholar]
- Sérgio, C.; Sim-Sim, M.; Carvalho, M. Annotated catalogue of Madeiran bryophytes. Bol. do Mus. Munic. do Funchal (Hist. Nat.) Supl. 2006, 10, 5–163. [Google Scholar]
- Hill, M.O.; Bell, N.; Bruggeman-Nannenga, M.A.; Brugués, M.; Cano, M.J.; Enroth, J.; Flatberg, K.; Frahm, J.-P.; Gallego, M.; Garilleti, R.; et al. An annotated checklist of the mosses of Europe and Macaronesia. J. Bryol. 2006, 28, 198–267. [Google Scholar] [CrossRef]
- Ros, R.M.; Mazimpaka, V.; Abou-salama, U.; Aleffi, M.; Blockeel, T.L.; Brugués, M.; Cros, R.M.; Dia, M.G.; Dirkse, G.M.; Draper, I.; et al. Mosses of the Mediterranean, an annotated checklist. Cryptogam. Bryol. 2013, 34, 99–283. [Google Scholar] [CrossRef]
- Hodgetts, N.G.; Söderström, L.; Blockeel, T.L.; Caspari, S.; Ignatov, M.S.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An annotated checklist of bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- Braithwaite, R. The British Moss-Flora; L. Reeve & Co.: London, UK, 1887; Volume 1, pp. 1–315. [Google Scholar]
- Dixon, H.N.; Jameson, H.G. The Student’s Handbook of British Mosses, 1st ed.; V.T. Sumfield: Eastbourne, UK, 1896; pp. 1–582. [Google Scholar]
- Dixon, H.N. The Student’s Handbook of British Mosses, reprint 3rd ed.; Sumfield & Day: Eastbourne, UK, 1954; p. XLVI + 1-582. [Google Scholar]
- Smith, A.J.E. The Moss Flora of Britain and Ireland; Cambridge University Press: Cambridge, UK, 1978; p. VIII + 1-706. [Google Scholar]
- Smith, A.J.E. The Moss Flora of Britain and Ireland, 2nd ed.; Cambridge University Press: Cambridge, UK, 2004; p. XII + 1-1012. [Google Scholar]
- Renner, M.A.M. Opportunities and challenges presented by cryptic bryophyte species. Telopea 2020, 23, 41–60. [Google Scholar] [CrossRef]
- Werner, O.; Ros, R.M.; Cano, M.J.; Guerra, J. Molecular phylogeny of Pottiaceae (Musci) based on chloroplast rps4 sequence data. Plant Syst. Evol. 2004, 243, 147–164. [Google Scholar] [CrossRef]
- Werner, O.; Ros, R.M.; Grundmann, M. 2005. Molecular phylogeny of Trichostomoideae (Pottiaceae, Bryophyta) based on nr ITS sequence data. Taxon 2005, 54, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Werner, O.; Köckinger, H.; Magdy, M.; Ros, R.M. On the systematic position of Tortella arctica and Trichostomum arcticum (Bryophyta, Pottiaceae). Nova Hedwig. 2014, 98, 273–293. [Google Scholar] [CrossRef]
- Ros, R.M.; Werner, O. The circumscription of the genus Pottiopsis (Pottiaceae, Bryophyta) based on morphology and molecular sequence data. Nova Hedwig. Beih. 2007, 131, 65–79. [Google Scholar]
- Köckinger, H.; Werner, O.; Ros, R.M. A new taxonomic approach to the genus Oxystegus (Pottiaceae, Bryophyta) in Europe based on molecular data. Nova Hedwig. 2010, 138, 31–49. [Google Scholar]
- Köckinger, H.; Lüth, M.; Werner, O.; Ros, R.M. Tortella mediterranea (Pottiaceae), a new species from southern Europe, its molecular affinities, and taxonomic notes on T. nitida. Bryologist 2018, 121, 560–570. [Google Scholar] [CrossRef]
- Werner, O.; Ros, R.M.; Guerra, J. Direct amplification and NaOH extraction: Two rapid and simple methods for preparing bryophyte DNA for polymerase chain reaction (PCR). J. Bryol. 2002, 24, 127–131. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols a Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Kress, W.J.; Erickson, D.L. A two-locus global DNA barcode for land plants: The coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS ONE 2007, 2, e508. [Google Scholar] [CrossRef] [Green Version]
- Kress, W.J.; Erickson, D.L.; Jones, F.A.; Swenson, N.G.; Perez, R.; Sanjur, O.; Bermingham, E. Plant DNA barcodes and a community phylogeny of a tropical forest dynamics plot in Panama. Proc. Natl. Acad. Sci. USA 2009, 106, 18621–18626. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Donath, A.; Stadler, P.F. Split-inducing indels in phylogenomic analysis. Algorithms Mol. Biol. 2018, 13, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-Coffee. A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huelsenbeck, J.P.; Ronquist, F. MrBayes: Bayesian inference of phylogeny. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huelsenbeck, J.P.; Larget, B.; Alfaro, M.E. Bayesian phylogenetic model selection using reversible jump Markov chain Monte Carlo. Mol. Biol. Evol. 2004, 21, 1123–1133. [Google Scholar] [CrossRef]
- Stöver, B.C.; Müller, K.F. TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinformatics 2010, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Maddison, W.P. Gene trees in species trees. Syst. Biol. 1997, 46, 523–536. [Google Scholar] [CrossRef]
- Pampilo, P.; Nei, M. Relationships between gene trees and species trees. Mol. Biol. Evol. 1988, 5, 568–583. [Google Scholar]
- Zander, R.H. Evolutionary analysis of five bryophyte families using virtual fossils. An. Jardín Botánico Madr. 2009, 66, 263–277. [Google Scholar] [CrossRef]
- Mönkemeyer, W. Die Laubmoose Europas, Andreales-Bryales; Akademische Verlagsgesellschaft: Leipzig, Germany, 1927; pp. 1–960. [Google Scholar]
- Eckel, P.M. Re-evaluation of Tortella (Musci, Pottiaceae) in conterminous U.S.A. and Canada with a treatment of the European species Tortella nitida. Bull. Buffalo Soc. Nat. Sci. 1998, 36, 117–191. [Google Scholar]
- Husnot, P.T. Muscologia Gallica; F. Savy, Libraire: Paris, France, 1884–1890; pp. 1–458. [Google Scholar]
- Schimper, W.P. Synopsis Muscorum Europaeorum, Praemissa Introductione de Elementis Bryologicis Tractante, 2nd ed.; Schweizerbart: Stuttgart, Germany, 1876; p. CXXX 1-866. [Google Scholar]
- Wörz, A. The “Botanische Reiseverein” A 19th-century joint stock company for the collecting of herbarium specimens. Huntia 2007, 13, 121–141. [Google Scholar]
- Stafleu, F.A.; Cowan, R.S. Taxonomic Literature, 2nd ed.; Bohn, Scheltema & Holkema Utrecht/Dr. W. Junk b.v.: The Hague, The Netherlands, 1981; Volume 3, pp. 1–980. [Google Scholar]
- Hodgetts, N.; Lockhart, N. Checklist and Country Status of European Bryophytes–Update 2020; Irish Wildlife Manuals, 123; National Parks and Wildlife Service: Dublin, Ireland, 2020; pp. 1–214. [Google Scholar]
- Mitten, W. New or rare British mosses. J. Bot. Br. Foreign 1868, 6, 97–99. [Google Scholar]
- Herzog, T. Geographie der Moose; Gustav Fischer: Jena, Germany, 1926; p. XI + 1-439. [Google Scholar]
- Frey, W.; Frahm, J.-P.; Fischer, E.; Lobin, W. The Liverworts, Mosses and Ferns of Europe, English Edition; Blockeel, T.L., Ed.; Harley Books: Colchester, UK, 2006; p. XV + 1-512. [Google Scholar]
- Cortes Latorre, C. Aportaciones a la Briología española (dos musgos nuevos para la Flora española). An. Inst. Botánico A. J. Cavanilles 1956, 13, 135–147. [Google Scholar]
- Esteve Chueca, F.; Cortes Latorre, C. El estrato liquénico-muscinal de la durilignosa de la Sierra de Cartagena. An. Inst. Botánico A. J. Cavanilles 1956, 13, 121–128. [Google Scholar]
- Kučera, J.; Košnar, J.; Werner, O. Partial generic revision of Barbula (Musci: Pottiaceae): Re-establishment of Hydrogonium and Streblotrichum, and the new genus Gymnobarbula. Taxon 2013, 62, 21–39. [Google Scholar] [CrossRef]


| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1 T. crispulum | 0.67 | ||||||
| 2 T. brachydontium | 12.3 | 0.0 | |||||
| 3 T. littorale | 17.0 | 13.6 | 1.3 | ||||
| 4 T. herzogii | 16.3 | 11.0 | 8.6 | 0.0 | |||
| 5 T. meridionale | 11.3 | 7.0 | 13.6 | 12.0 | 0.3 | ||
| 6 Trichostomum sp. 2178 Austria | 10.3 | 8.0 | 11.6 | 11.0 | 7.0 | - | |
| 7 Trichostomum sp. 3228 Greece | 17.3 | 12.0 | 11.6 | 7.0 | 8.2 | 14.0 | - |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1 T. crispulum | 0.00 | ||||||
| 2 T. brachydontium | 0.17 | 1.67 | |||||
| 3 T. littorale | 2.00 | 1.17 | 0.00 | ||||
| 4 T. herzogii | 3.00 | 3.17 | 5.00 | 0.0 | |||
| 5 T. meridionale | 0.85 | 1.01 | 2.85 | 3.85 | 0.15 | ||
| 6 Trichostomum sp. 2178 Austria | 1.0 | 1.17 | 3.00 | 2.00 | 1.85 | - | |
| 7 Trichostomum sp. 3228 Greece | 0.00 | 0.17 | 2.00 | 3.00 | 0.85 | 1.00 | - |
| Traits | T. brachydontium | T. herzogii | T. littorale | T. meridionale |
|---|---|---|---|---|
| Plants size | (0.7-)1.0–2.0 cm | (1.0-)2.0–3.0(-5.0) cm | (0.5-)1.5–3.0 cm | 0.3–0.5(-1.0) cm |
| Color of plants | bright green above, brown below | yellowish green above, reddish below | olive-green above, brown below | bright green above, yellow to brown below |
| Life-form | tuft | tuft | tuft/cushion | dense and short turf |
| Stem tomentum | present | present | absent | absent |
| Stem ramification | distinctly interrupted | distinctly interrupted or not | distinctly interrupted | distinctly interrupted or not |
| Stem apex | sometimes distinctly comose | not distinctly comose | distinctly comose | distinctly comose |
| Flagelliform shoots | not seen | occasional | frequent | not seen |
| Leaves when dry | crispate | incurved or crispate | incurved or slightly crispate | incurved |
| Leaves when moist | patent to spreading, apical leaves with the tips recurved | erect to erect-patent, apical leaves spreading | erect to erect-patent, comal leaves spreading or with the tips recurved | erect to erect-patent, comal leaves spreading |
| Median and upper leaf shape | narrowly lingulate to narrowly lanceolate or ensiform | linear-lanceolate | lingulate, oblong-lingulate sometimes slightly lanceolate or spatulate | lingulate to spatulate, occasionally panduriform or lanceolate |
| Median and upper leaves size | (1.35-)1.80–3.00(-3.80) × 0.30–0.50(-0.60) mm | (1.22-)2.00–3.45(-3.61) × (0.21-)0.35–0.50(-0.57) mm | (0.60-)1.00–1.90(-2.30) × 0.20–0.40(-0.50) mm | 0.90–1.90(-2.30) × (0.20-)0.30–0.50(-0.60) mm |
| Length/width ratio | 5.0–8.0:1 | 5.0–7.6(−8.9):1 | 3.0–4.6(−6.6):1 | 3.4–4.0:1 |
| Lamina | flat to slightly canaliculate, twisted, undulate, unistratose, fragile | strongly canaliculate, not twisted, undulate or not, rarely bistratose in small patches below, not fragile | canaliculate, rarely flat, not twisted, slightly undulate, rarely bistratose in small patches below, not fragile | flat to canaliculate, not twisted, slightly undulate, unistratose, not fragile |
| Basal lamina color | yellowish or hyaline, contrasting with upper lamina | reddish, concolorous with upper lamina | yellowish or hyaline, contrasting with upper lamina | yellowish or hyaline, contrasting with upper lamina |
| Leaf apex | short-acuminate or more rarely acute | long-acuminate to subulate | obtuse, mucronate | obtuse, mucronate |
| Basal margin teeth | absent or weakly developed | absent or weakly developed | usually prominent | absent or weakly developed |
| Costa | gradually narrowing from base to apex | gradually narrowing from base to apex | gradually narrowing from base to apex | gradually narrowing from mid-leaf to apex |
| Length of excurrent costa | (50-)90–150 µm | 70–150 µm | 20–85 µm | 25–60 µm |
| Basal lamina costa cross-section | plane-convex | biconvex | plane-convex | plane-convex |
| Mid-basal and paracostal basal cells | thin-walled | usually thick-walled, obscurely nodulose | sometimes thick-walled | thin-walled |
| Sporophyte | often present, seta (5-)10–20 mm long, urn 1.2–2.2 mm long, peristome up to 250 µm or rudimentary | not seen | not seen | often present, seta 5–10 mm long, urn 1.0–1.5 mm long, peristome absent or rudimentary up to 55 µm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ros, R.M.; Werner, O.; Porley, R.D. Herzog Vindicated: Integrative Taxonomy Reveals That Trichostomum brachydontium (Pottiaceae, Bryophyta) Comprises Several Species. Taxonomy 2022, 2, 57-88. https://doi.org/10.3390/taxonomy2010005
Ros RM, Werner O, Porley RD. Herzog Vindicated: Integrative Taxonomy Reveals That Trichostomum brachydontium (Pottiaceae, Bryophyta) Comprises Several Species. Taxonomy. 2022; 2(1):57-88. https://doi.org/10.3390/taxonomy2010005
Chicago/Turabian StyleRos, Rosa M., Olaf Werner, and Ron D. Porley. 2022. "Herzog Vindicated: Integrative Taxonomy Reveals That Trichostomum brachydontium (Pottiaceae, Bryophyta) Comprises Several Species" Taxonomy 2, no. 1: 57-88. https://doi.org/10.3390/taxonomy2010005
APA StyleRos, R. M., Werner, O., & Porley, R. D. (2022). Herzog Vindicated: Integrative Taxonomy Reveals That Trichostomum brachydontium (Pottiaceae, Bryophyta) Comprises Several Species. Taxonomy, 2(1), 57-88. https://doi.org/10.3390/taxonomy2010005








