Cytogeography of the East Asian Tulips (Amana, Liliaceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Observations and Sampling
2.2. Chromosome Observation and Geographical Distribution Analysis
3. Results
3.1. Chromosome Number and Ploidy Levels
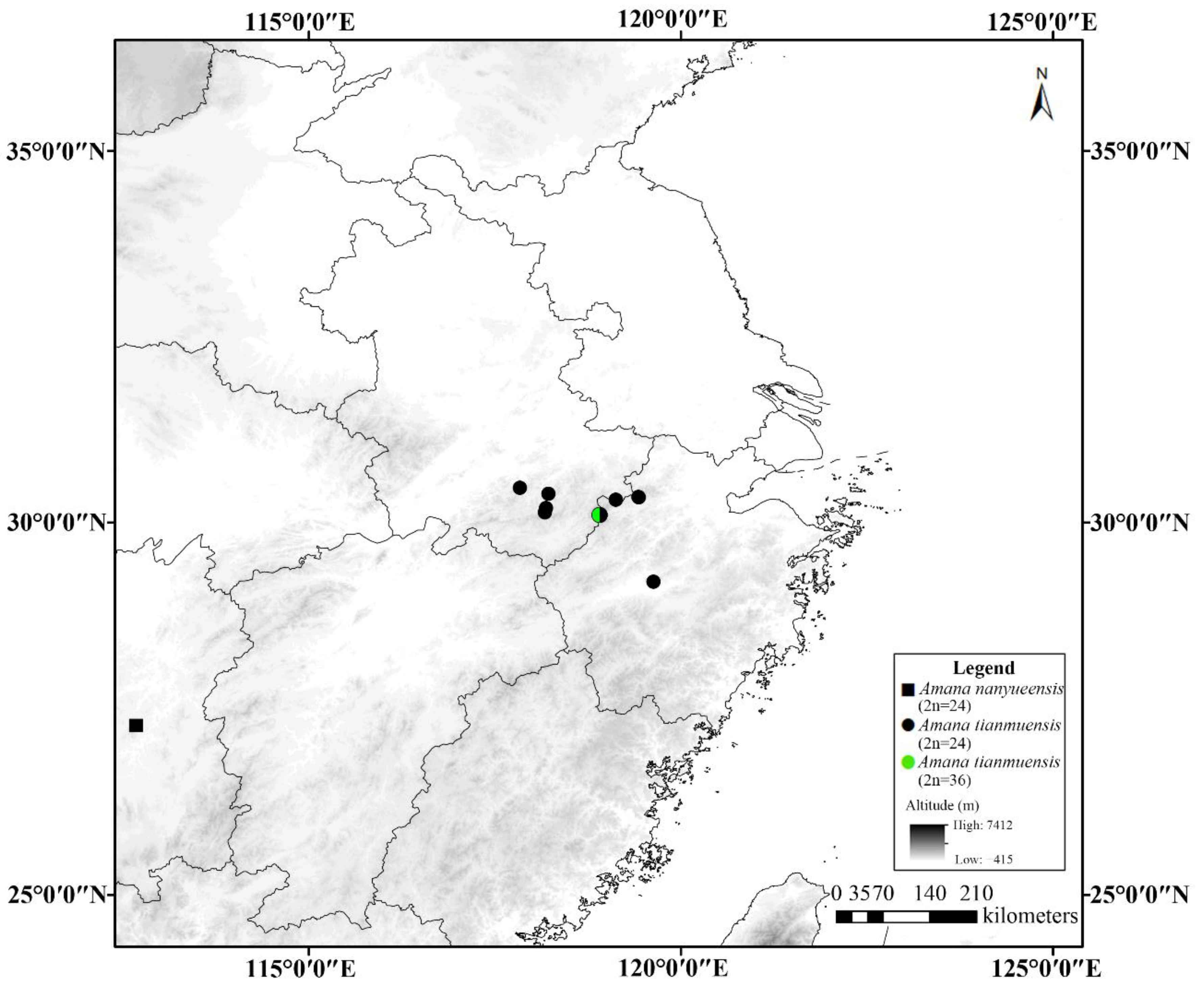
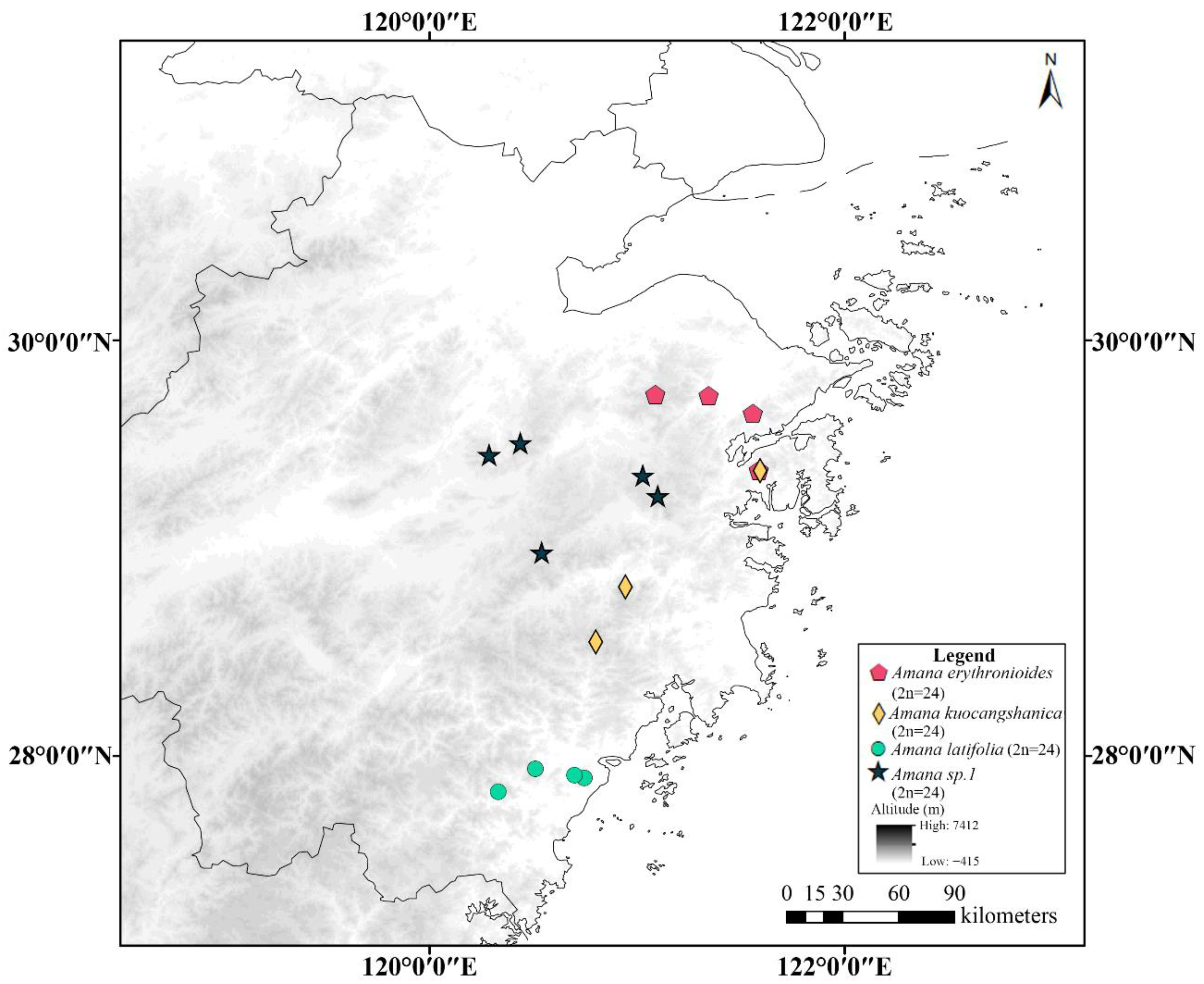
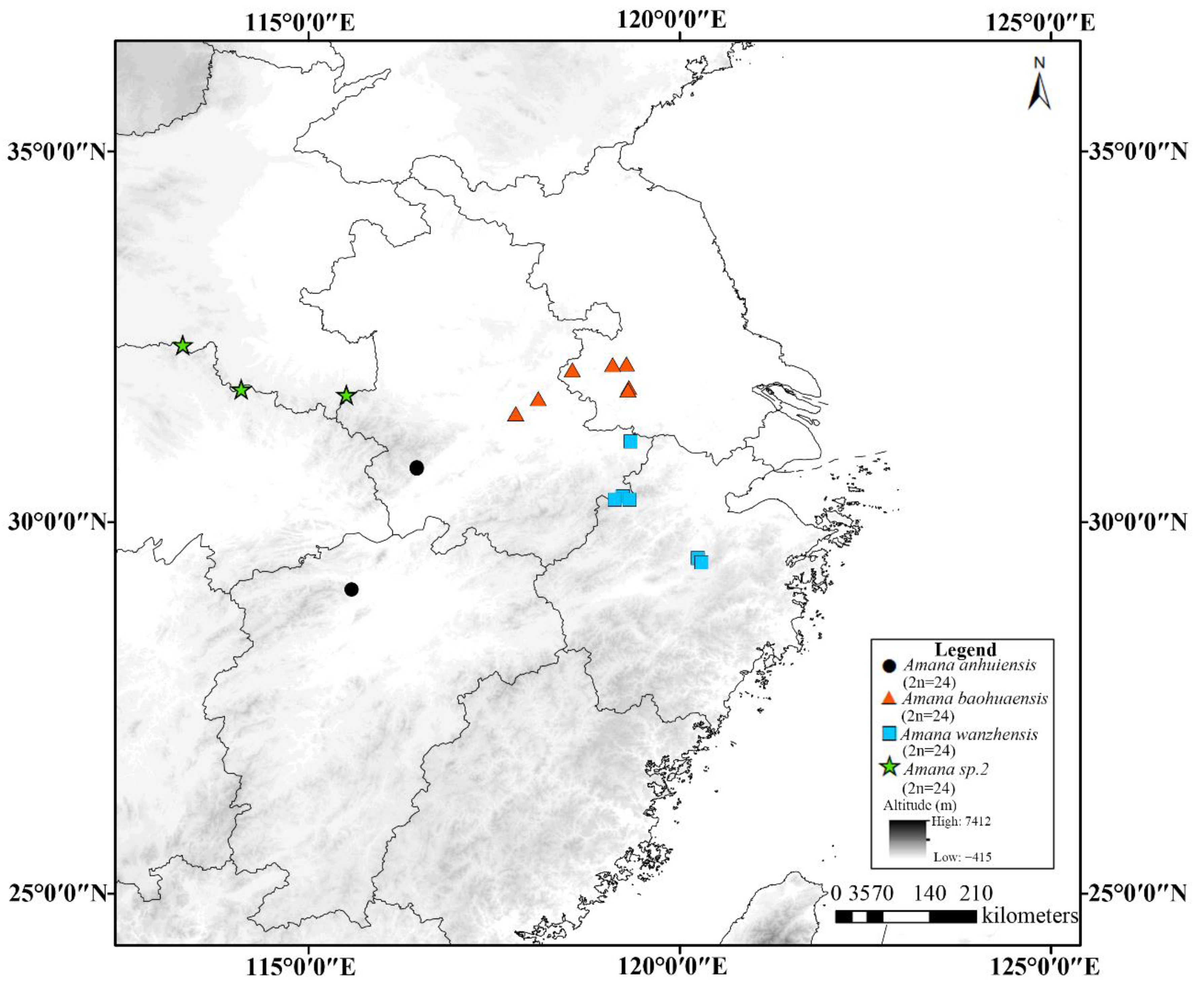
3.2. Geographical Distribution Pattern of Cytotypes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- APG IV. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar]
- Lu, R.S.; Yang, T.; Chen, Y.; Wang, S.Y.; Cai, M.Q.; Cameron, K.M.; Li, P.; Fu, C.X. Comparative plastome genomics and phylogenetic analyses of Liliaceae. Bot. J. Linn. Soc. 2021, 196, 279–293. [Google Scholar] [CrossRef]
- Peruzzi, L. A new infrafamilial taxonomic setting for Liliaceae, with a key to genera and tribes. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2016, 150, 1341–1347. [Google Scholar] [CrossRef]
- Honda, M. Amana a new genus of Liliaceae. Bull. Biogeograpical Soc. Jpn. 1935, 6, 19–21. [Google Scholar]
- Tan, D.Y.; Zhang, Z.; Li, X.R.; Hong, D.Y. Restoration of the genus Amana Honda (Liliaceae) based on a cladistic analysis of morphological characters. Acta Phytotaxon. Sin. 2005, 43, 262–270. [Google Scholar]
- Li, P.; Lu, R.S.; Xu, W.Q.; Ohi-Toma, T.; Cai, M.Q.; Qiu, Y.X.; Cameron, K.M.; Fu, C.X. Comparative Genomics and Phylogenomics of East Asian Tulips (Amana, Liliaceae). Front. Plant Sci. 2017, 8, 451. [Google Scholar] [CrossRef] [PubMed]
- Struik, G.J. Growth patterns of some native annual and perennial herbs in southern Wisconsin. Ecology 1965, 46, 401–420. [Google Scholar] [CrossRef]
- Mckenna, M.F.; Houle, G. Why are annual plants rarely spring ephemerals? New Phytol. 2000, 148, 295–302. [Google Scholar] [CrossRef]
- Ohwi, J.; Kitagawa, M. New Flora of Japan; Shibundo Co. Ltd.: Tokyo, Japan, 1992. [Google Scholar]
- Chen, X.Q.; Mordak, H.V. Tulipa Linnaeus. In Flora of China; Wu, Z.-Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2000; Volume 24, pp. 123–126. [Google Scholar]
- Shen, X.S. A new species of Tulipa (Liliaceae) from China. Acta Bot. Yunnanica 2001, 23, 39–40. [Google Scholar]
- Tan, D.Y.; Li, X.R.; Hong, D.Y. Amana kuocangshanica (Liliaceae) a new species from south–east China. Bot. J. Linn. Soc. 2007, 154, 435–442. [Google Scholar] [CrossRef]
- Han, B.X.; Zhang, K.; Huang, L.Q. Amana wanzhensis (Liliaceae) a new species from Anhui China. Phytotaxa 2014, 177, 118–124. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Xing, Q.; Lu, G.Y.; Lu, X.; Zhao, Q.; Song, X.W.; Han, B.X. Amana baohuaensis (Liliaceae) a new species from East China. Phytotaxa 2019, 427, 43–50. [Google Scholar] [CrossRef]
- Wang, M.Z.; Fan, X.K.; Zhang, Y.H.; Wu, J.; Mao, L.M.; Zhang, S.L.; Cai, M.Q.; Li, M.H.; Zhu, Z.S.C.; Zhao, M.S.; et al. Phylogenomics and integrative taxonomy reveal two new species of Amana (Liliaceae). Plant Divers. 2022; in press. [Google Scholar]
- Yuan, W.B. Genetic Diversity Research of Aconitum carmichaeli Debx; Chinese Academy of Agricultural Sciences: Beijing, China, 2013; pp. 22–24. [Google Scholar]
- Peruzzi, L.; Leitch, I.J.; Caparelli, K.F. Chromosome diversity and evolution in Liliaceae. Ann. Bot. 2009, 103, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.N.; Jin, X.F.; Gao, Z. Karyotype analysis on three species of Liliaceae. J. Zhejiang For. Sci. Technol. 2002, 22, 22–25. [Google Scholar]
- Deng, A.H.; Li, K.; Chen, Y.; Liu, C.Y.; Guo, Q.S.; Zhu, Z.B.; Miao, Y.Y. Karyotype analysis of different populations of Tulipa edulis. Chin. Herb. Med. 2016, 39, 493–498. [Google Scholar]
- Minami, M.; Sakala, M.; Wrightsell, J. Using ArcMap; ESRI: Redlands, CA, USA, 2000. [Google Scholar]
- Sato, D. Karyotype alteration and phylogeny in Liliaceae and allied families. Jpn. J. Bot. 1943, 12, 57–161. [Google Scholar]
- Noguchi, J.; Kowano, S. Brief notes on the chromosomes of Japanese plants (3). Jpn. J. Bot. 1974, 49, 75–86. [Google Scholar]
- De Bodt, S.; Maere, S.; Van de Peer, Y. Genome duplication and the origin of angiosperms. Trends Eco. Evol 2005, 20, 591–597. [Google Scholar] [CrossRef]
- Soltis, D.E.; Albert, V.A.; Leebens-Mack, J.; Bell, C.D.; Paterson, A.H.; Zheng, C.F.; Sankoff, D.; de Pamphilis, C.W.; Wall, P.K.; Soltis, P.S. Polyploidy and angiosperm diversification. Am. J. Bot. 2009, 96, 336–348. [Google Scholar] [CrossRef]
- Tank, D.C.; Eastman, J.M.; Pennell, M.W.; Soltis, P.S.; Soltis, D.E.; Hinchliff, C.E.; Brown, J.W.; Sessa, E.B.; Harmon, L.J. Nested radiations and the pulse of angiosperm diversification: Increased diversification rates often follow whole genome duplications. New Phytol. 2015, 207, 454–467. [Google Scholar] [CrossRef]
- Soltis, P.S.; Soltis, D.E. Ancient WGD events as drivers of key innovations in angiosperms. Curr. Opin. Plant Biol. 2016, 30, 159–165. [Google Scholar] [CrossRef]
- Landis, J.B.; Soltis, D.E.; Li, Z.; Marx, H.E.; Barker, M.S.; Tank, D.C.; Soltis, P.S. Impact of whole-genome duplication events on diversification rates in angiosperms. Am. J. Bot. 2018, 105, 348–363. [Google Scholar] [CrossRef]
- Carta, A.; Bedini, G.; Peruzzi, L. A deep dive into the ancestral chromosome number and genome size of flowering plants. New Phytol. 2020, 228, 1097–1106. [Google Scholar] [CrossRef]
- Vidal-Russell, R.; Tadey, M.; Urfusová, R.; Urfus, T.; Souto, C.P. Evolutionary importance of the relationship between cytogeography and climate: New insights on creosote bushes from North and South America. Plant Divers. 2021. [Google Scholar] [CrossRef]
- Carta, A.; Peruzzi, L. Testing the large genome constraint hypothesis: Plant traits, habitat and climate seasonality in Liliaceae. New Phytol. 2016, 2, 709–716. [Google Scholar] [CrossRef]
- Levin, D.A. Polyploidy and novelty in flowering plants. Am. Nat. 1983, 122, 1–25. [Google Scholar] [CrossRef]
- Udall, J.A.; Wendel, J.F. Polyploidy and crop improvement. Crop. Sci. 2006, 46, S3–S14. [Google Scholar] [CrossRef]
- McArthur, E.D.; Sanderson, S.C. Cytogeography and chromosome evolution of subgenus Tridentatae of Artemisia (Asteraceae). Am. J. Bot. 1999, 86, 1754–1775. [Google Scholar] [CrossRef]
- Brochmann, C.; Brysting, A.K.; Alsos, I.G.; Borgen, L.; Grundt, H.H.; Scheen, A.C.; Elven, R. Polyploidy in arctic plants. Biol. J. Linn. Soc. 2004, 82, 521–536. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Gavrilenko, T.; Stephenson, S.; Bamberg, J.; Salas, A.; Spooner, D.M. Geographical and environmental range expansion through polyploidy in wild potatoes (Solanum section Petota). Glob. Ecol. Biogeogr. 2007, 16, 485–495. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.F.; Zhong, C.H.; Huang, H.W. Morphological and cytotype variation of wild kiwifruit (Actinidia chinensis complex) along an altitudinal and longitudinal gradient in central-west China. Bot. J. Linn. Soc. 2010, 164, 72–83. [Google Scholar] [CrossRef]
- Patrick, J.M. Cytogeography and genome size variation in the Claytonia perfoliata (Portulacaceae) polyploid complex. Ann. Bot. 2012, 110, 1195–1203. [Google Scholar]
- Silveira, R.M.; Machado, R.M.; Forni-Martins, E.R.; Verola, C.F.; Costa, I.R. Environmental variations drive polyploid evolution in neotropical Eugenia species (Myrtaceae). Genet. Mol. Res. 2016, 15, gmr15048842. [Google Scholar] [CrossRef]
- Mousavizadeh, S.J.; Gil, J.; Moreno, R.; Mashayekhi, K. Asparagus ploidy distribution related to climates adaptation in Iran. Environ Dev. Sustain. 2021. [Google Scholar] [CrossRef]
- Peruzzi, L.; Góralski, G.; Joachimiak, A.J.; Bedini, G. Does actually mean chromosome number increase with latitude in vascular plants? An answer from the comparison of Italian, Slovak and Polish floras. Comp. Cytogenet. 2012, 6, 371–377. [Google Scholar] [CrossRef][Green Version]
- Li, J. Ecological Significance of Polyploidy in the Invasion of Solidago Canadensis in China. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2011. [Google Scholar]
- Thompson, K.A.; Husband, B.C.; Maherali, H. Climatic niche differences between diploid and tetraploid cytotypes of Chamerion angustifolium (Onagraceae). Am. J. Bot. 2014, 101, 1868–1875. [Google Scholar] [CrossRef]
- McAllister, C.; Blaine, R.; Kron, P.; Bennett, B.; Garrett, H.; Kidson, J.; Matzenbacher, B.; Glotzbach, A.; Miller, A.J. Environmental correlates of cytotype distribution in Andropogon gerardii (Poaceae). Am. J. Bot. 2015, 102, 92–102. [Google Scholar] [CrossRef]
- Molgo, I.E.; Soltis, D.E.; Soltis, P.S. Cytogeography of Callisia section Cuthbertia (Commelinaceae). Comp. Cytogenet. 2017, 11, 553–577. [Google Scholar] [CrossRef]
- Mráz, P.; Šingliarová, B.; Urfus, T.; Krahulec, F. Cytogeography of Pilosella officinarum (Compositae): Altitudinal and longitudinal differences in ploidy level distribution in the Czech Republic and Slovakia and the general pattern in Europe. Ann. Bot. 2008, 101, 59–71. [Google Scholar] [CrossRef]
- Elías, G.; Sartor, M.; Solís-Neffa, V.G. Patterns of cytotype variation of Turnera sidoides subsp. pinnatifida (Turneraceae) in mountain ranges of central Argentina. J. Plant Res. 2011, 124, 25–34. [Google Scholar] [CrossRef]
- Husband, B.C.; Sabara, H.A. Reproductive isolation between autotetraploids and their diploid progenitors in fi reweed, Chamerion angustifolium (Onagraceae). New Phytol. 2003, 161, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Schönswetter, P.; Lachmayer, M.; Lettner, C.; Prehsler, D.; Rechnitzer, S.; Reich, D.S.; Sonnleitner, M.; Wagner, I.; Hülber, K.; Schneeweiss, G.M.; et al. Sympatric diploid and hexaploid cytotypes of Senecio carniolicus (Asteraceae) in the Eastern Alps are separated along an altitudinal gradient. J. Plant Res. 2007, 120, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Pockman, W.T.; Sperry, J.S. Freezing-induced xylem cavitation and the northern limit of Larrea tridentata. Oecologia 1997, 109, 19–27. [Google Scholar] [CrossRef]
- Sunmonu, N.; Kudo, G. Warm temperature conditions restrict the sexual reproduction and vegetative growth of the spring ephemeral Gagea lutea (Liliaceae). Plant Ecol. 2015, 216, 1419–1431. [Google Scholar] [CrossRef]
- Liu, K.B. Quaternary history of the temperate forests of China. Quat. Sci. Rev. 1988, 7, 1–20. [Google Scholar] [CrossRef]
- Ying, T.S. Species diversity and distribution pattern of seed plants in China. Biodivers. Sci. 2001, 9, 393–398, (In Chinese with an English Abstract). [Google Scholar]
- Iijima, A.; Tada, R. Evolution of tertiary sedimentary basins of Japan in reference to opening of the Japan Sea. J. Fac. Sci. Univ. Tokyo Sect. II 1990, 22, 121–171. [Google Scholar]
- Maruyama, S.; Isozaki, Y.; Kimura, G.; Terabayashi, M. Paleogeographic maps of the Japanese Islands: Plate tectonics synthesis from 750 Ma to the present. Isl. Arc 1997, 6, 121–142. [Google Scholar] [CrossRef]
- Otofuji, Y.I.; Matsuda, T.; Nohda, S. Opening mode of the Japan Sea inferred from the paleomagnetism of the Japan Arc. Nature 1985, 317, 603–604. [Google Scholar] [CrossRef]
- Otofuji, Y.I. Large tectonic movement of the Japan Arc in late Cenozoic times inferred from paleomagnetism: Review and synthesis. Isl. Arc 1996, 5, 229–249. [Google Scholar] [CrossRef]
- Kitamura, A.; Takano, O.; Takada, H.; Omote, H. Late Pliocene-early Pleistocene paleoceanographic evolution of the Sea of Japan. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 172, 81–98. [Google Scholar] [CrossRef]
- Kitamura, A.; Kimoto, K. History of the inflow of the warm Tsushima Current into the Sea of Japan between 3.5 and 0.8 Ma. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 236, 355–366. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Suzuki, H.; Shinohara, A.; Harada, M.; Wakana, S.; Sakaizumi, M.; Han, S.H.; Lin, L.K.; Kryukov, A.P. Molecular phylogeny of East Asian moles inferred from the sequence variation of the mitochondrial cytochrome b gene. Genes Genet. Syst. 2000, 75, 17–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shinohara, A.; Suzuki, H.; Tsuchiya, K.; Zhang, Y.P.; Luo, J.; Jiang, X.L.; Wang, Y.X.; Campbell, K.L. Evolution and biogeography of talpid moles from continental East Asia and the Japanese islands inferred from mitochondrial and nuclear gene sequences. Zool. Sci. 2004, 21, 1177–1185. [Google Scholar] [CrossRef]
- Kawamura, K.; Parrenin, F.; Lisiecki, L.; Uemura, R.; Vimeux, F.; Severinghaus, J.P.; Hutterli, M.A.; Nakazawa, T.; Aoki, S.; Jouzel, J.; et al. Northern Hemisphere forcing of climatic cycles in Antarctica over the past 360,000 years. Nature 2007, 448, 912–916. [Google Scholar] [CrossRef]


| Pop. | Species | Voucher Number | Chromosome Numbers | Latitude | Longitude | Elevation | Locality |
|---|---|---|---|---|---|---|---|
| 1 | Amana edulis | / | 24 | 40.1161 | 124.3584 | / | China, Liaoning Province, Dandong City, Zhenxin District (Deng, 2016) |
| 2 | A. edulis | / | 24 | 39.0989 | 121.7955 | / | China, Liaoning Province, Dalian City, Mt. Dahei (Deng, 2016) |
| 3 | A. edulis | LP161230 | 24 | 37.4546 | 121.5833 | 45 | China, Shandong Province, Yantai City, Yangma Island |
| 4 | A. edulis | LP161195 | 24 | 36.6298 | 117.0413 | 283 | China, Shandong Province, Jinan City, Mt. Fohui |
| 5 | A. edulis | LP161194 | 24 | 36.2551 | 117.0746 | 749 | China, Shandong Province, Taian City, Mt. Tai |
| 6 | A. edulis | / | 48 | 36.2200 | 127.2400 | / | South Korea, Daejeon |
| 7 | A. edulis | / | 48 | 35.9167 | 140.4333 | / | Japan, Tokyo, Koishikawa Botanical Garden (Sato, 1943) |
| 8 | A. edulis | / | 48 | 35.7783 | 139.4968 | 56 | Japan, Tokyo, Akitsu. HigashiMurayama (Noguchi and Kowano, 1974) |
| 9 | A. edulis | LP161180 | 24 | 35.6164 | 116.9902 | 73 | China, Shandong Province, Qufu County, Cemetery of Confucius |
| 10A | A. edulis | LP161174 | 24 | 35.6017 | 117.1556 | 335 | China, Shandong Province, Sishui County, Mt. Ge |
| 10B | A. edulis | LP161175 | 24 | 35.6013 | 117.1583 | 240 | China, Shandong Province, Sishui County, Mt. Ge |
| 11A | A. edulis | LP161176 | 24 | 35.5972 | 117.1950 | 226 | China, Shandong Province, Sishui County, Mt. Matou |
| 11B | A. edulis | LP161177 | 24 | 35.5969 | 117.1975 | 302 | China, Shandong Province, Sishui County, Mt. Matou |
| 11C | A. edulis | LP161178 | 24 | 35.5946 | 117.2050 | 330 | China, Shandong Province, Sishui County, Mt. Matou |
| 11D | A. edulis | LP161179 | 24 | 35.5930 | 117.2016 | 349 | China, Shandong Province, Sishui County, Mt. Matou |
| 12 | A. edulis | / | 24 | 35.3000 | 113.9000 | / | China, Henan Province, Xinxiang City (Deng, 2016) |
| 13 | A. edulis | LP161123 | 24 | 35.1313 | 117.4130 | 198 | China, Shandong Province, Zaozhuang City, Yunfengshan Village |
| 14 | A. edulis | LP173025 | 24 | 34.9805 | 111.4382 | 910 | China, Shanxi Province, Xia County, Wulongmiao |
| 15 | A. edulis | LP173072 | 48 | 34.7155 | 119.4267 | 183 | China, Jiangsu Province, Lianyungang City, Mt. Yuntai |
| 16A | A. edulis | LP207907 | 24 | 34.2433 | 117.1736 | 104 | China, Jiangsu Province, Xuzhou City, Mt. Yunlong |
| 16B | A. edulis | LP173071 | 24 | 34.2433 | 117.1737 | 104 | China, Jiangsu Province, Xuzhou City, Mt. Yunlong |
| 17 | A. edulis | LP173073 | 24 | 33.5989 | 119.0568 | 7 | China, Jiangsu Province, Huai’an City, Mt. Bochi |
| 18 | A. edulis | LP173057 | 48 | 33.1717 | 113.0545 | 295 | China, Henan Province, Wugang County, Dengtaijia |
| 19A | A. edulis | LP173055 | 24 | 33.0600 | 112.0579 | 354 | China, Henan Province, Nanyang City, Mt. Du |
| 19B | A. edulis | LP207916 | 24 | 33.0600 | 112.0579 | 354 | China, Henan Province, Nanyang City, Mt. Du |
| 20 | A. edulis | LP196198 | 48 | 32.4635 | 118.9308 | 15 | China, Jiangsu Province, Nanjing City, Mt. Guizi |
| 21 | A. edulis | WMZ1490 | 48 | 32.3954 | 113.2989 | 249 | China, Henan Province, Tongbai County, Tayuan Temple |
| 22 | A. edulis | WMZ1493 | 48 | 32.3067 | 113.4549 | 161 | China, Henan Province, Tongbai County, Huangjialaozhuang |
| 23 | A. edulis | WMZ1422 | 48 | 32.2956 | 118.2864 | 87 | China, Anhui Province, Chuzhou City, Mt. Langya |
| 24 | A. edulis | WMZ1416 | 48 | 32.1251 | 119.0896 | 129 | China, Jiangsu Province, Jurong County, Mt. Baohua |
| 25 | A. edulis | WMZ1418 | 48 | 32.1008 | 118.5875 | 102 | China, Jiangsu Province, Nanjing City, Mt. Lao |
| 26 | A. edulis | WMZ1420 | 48 | 32.0625 | 118.5523 | 87 | China, Jiangsu Province, Nanjing City, Shiziling |
| 27 | A. edulis | WMZ1408 | 24 | 31.8154 | 119.3090 | 269 | China, Jiangsu Province, Jurong County, Mt. Mao |
| 28 | A. edulis | WMZ1488 | 24 | 31.7980 | 114.0873 | 394 | China, Hubei Province, Guangshui County, Heilongtan |
| 29 | A. edulis | WMZ1482 | 24 | 31.7153 | 115.5012 | 379 | China, Henan Province, Shangnan County, Liluocheng Village |
| 30 | A. edulis | WMZ1424 | 24 | 31.6710 | 118.0846 | 41 | China, Anhui Province, Hanshan County, Mt. Baochan |
| 31 | A. edulis | WMZ1486 | 24 | 31.5727 | 114.6192 | 660 | China, Hubei Province, Hong’an County, Mt. Tiantai |
| 32 | A. edulis | WMZ1485 | 48 | 31.5719 | 114.6154 | 590 | China, Hubei Province, Hong’an County, Mt. Tiantai |
| 33 | A. edulis | WMZ1426 | 24 | 31.4650 | 117.7859 | 132 | China, Anhui Province, Wuwei County, Loujialong |
| 34A | A. edulis | LP207905 | 48 | 31.2615 | 119.7530 | 82 | China, Jiangsu Province, Yixing County, Haoshan Village |
| 34B | A. edulis | WMZ1404 | 48 | 31.2614 | 119.7528 | 88 | China, Jiangsu Province, Yixing County, Haoshan Village |
| 35 | A. edulis | LP172908 | 24 | 31.0643 | 119.2697 | 322 | China, Jiangsu Province, Liyang County, Mt. Wawu |
| 36 | A. edulis | WMZ1477 | 24 | 30.4191 | 117.2035 | 90 | China, Anhui Province, Chizhou City, Yaogang |
| 37 | A. edulis | LJK54 | 48 | 30.3866 | 118.2291 | 473 | China, Anhui Province, Huangshan County, Qiaoshan Village |
| 38 | A. edulis | WMZ1471 | 48 | 30.3846 | 118.2460 | 614 | China, Anhui Province, Huangshan City, Qiaoshan Village |
| 39 | A. edulis | WMZ1494 | 48 | 30.1979 | 115.1092 | 158 | China, Hubei Province, Huangshi City, Feiyun Cave |
| 40 | A. edulis | WMZ1496 | 48 | 29.9485 | 114.7491 | 85 | China, Hubei Province, Daye County, Caomen Village |
| 41A | A. edulis | LP150069 | 48 | 29.7446 | 121.0833 | 892 | China, Zhejiang Province, Yuyao County, Mt. Siming |
| 41B | A. edulis | WMZ1432 | 48 | 29.7426 | 121.0842 | 880 | China, Zhejiang Province, Yuyao County, Mt. Siming |
| 41C | A. edulis | WMZ1430 | 48 | 29.7398 | 121.0869 | 861 | China, Zhejiang Province, Yuyao County, Mt. Siming |
| 42A | A. edulis | WMZ1434 | 48 | 29.6488 | 121.5570 | 571 | China, Zhejiang Province, Ningbo City, Mt. Jin’e |
| 42B | A. edulis | LP184953 | 48 | 29.6458 | 121.5575 | 564 | China, Zhejiang Province, Ningbo City, Mt. Jin’e |
| 42C | A. edulis | WMZ1436 | 48 | 29.6386 | 121.5596 | 458 | China, Zhejiang Province, Ningbo City, Mt. Jin’e |
| 43A | A. edulis | WMZ1456 | 48 | 29.5147 | 120.2411 | 564 | China, Zhejiang Province, Zhuji County, Fenglinxia Village |
| 43B | A. edulis | LP184930 | 48 | 29.5138 | 120.2438 | 447 | China, Zhejiang Province, Zhuji County, Fenglinxia Village |
| 44 | A. edulis | WMZ1459 | 48 | 29.4565 | 120.2906 | 234 | China, Zhejiang Province, Zhuji County, Banqiu Village |
| 45 | A. edulis | LP184939 | 48 | 29.3811 | 121.6190 | 263 | China, Zhejiang Province, Ninghai County, Mt. Cha |
| 46A | A. edulis | LP184936 | 48 | 29.3773 | 121.5875 | 628 | China, Zhejiang Province, Ninghai County, Mt. Cha |
| 46B | A. edulis | WMZ1440 | 48 | 29.3772 | 121.5875 | 671 | China, Zhejiang Province, Ninghai County, Mt. Cha |
| 47 | A. edulis | WMZ1438 | 48 | 29.3428 | 121.7588 | 268 | China, Zhejiang Province, Xiangshan County, Dalingyan |
| 48 | A. edulis | WMZ1453 | 24 | 29.2085 | 119.6260 | 558 | China, Zhejiang Province, Jinhua City, Shuanglong Scenic Area |
| 49 | A. edulis | LP184860 | 48 | 29.0454 | 120.2871 | 331 | China, Zhejiang Province, Zhuji County, Banqiu Village |
| 50 | A. edulis | LP161162 | 48 | 28.8559 | 121.1082 | 561 | China, Zhejiang Province, Linhai County, Mt. Chenghuang |
| 51A | A. nanyueensis | WMZ1463 | 24 | 27.2881 | 112.6932 | 1067 | China, Hunan Province, Hengyang City, Mt. Heng |
| 51B | A. nanyueensis | LP196219 | 24 | 27.2881 | 112.6932 | 1055 | China, Hunan Province, Hengyang City, Mt. Heng |
| 52 | A. nanyueensis | WMZ1464 | 24 | 27.2767 | 112.6746 | 1063 | China, Hunan Province, Hengyang City, Mt. Heng |
| 53 | A. tianmuensis | WMZ1473 | 24 | 30.4728 | 117.8345 | 736 | China, Anhui Province, Qingyang County, Mt. Jiuhua |
| 54A | A. tianmuensis | WMZ1470 | 24 | 30.3884 | 118.2182 | 629 | China, Anhui Province, Huangshan City, Yuxiang |
| 54B | A. tianmuensis | LJK51 | 24 | 30.3878 | 118.2169 | 692 | China, Anhui Province, Huangshan City, Yuxiang |
| 54C | A. tianmuensis | LP173012 | 24 | 30.3878 | 118.2169 | 692 | China, Anhui Province, Huangshan City, Yuxiang |
| 55A | A. tianmuensis | WMZ1504 | 24 | 30.3497 | 119.4262 | 1437 | China, Zhejiang Province, Hangzhou County, Mt. Tianmu |
| 55B | A. tianmuensis | LJK42 | 24 | 30.3425 | 119.4332 | 1104 | China, Zhejiang Province, Hangzhou County, Mt. Tianmu |
| 55C | A. tianmuensis | WMZ1465 | 24 | 30.3423 | 119.4333 | 1108 | China, Zhejiang Province, Hangzhou County, Mt. Tianmu |
| 56A | A. tianmuensis | LJK45 | 24 | 30.3085 | 119.1215 | 1120 | China, Zhejiang Province, Hangzhou City, Zhexitianchi |
| 56B | A. tianmuensis | WMZ1505 | 24 | 30.3082 | 119.1214 | 1118 | China, Zhejiang Province, Hangzhou City, Zhexitianchi |
| 57 | A. tianmuensis | WMZ1472 | 24 | 30.2008 | 118.1846 | 629 | China, Anhui Province, Huangshan City, Yuanlinchang |
| 58 | A. tianmuensis | WMZ1506 | 24 | 30.1426 | 118.1704 | 1620 | China, Anhui Province, Huangshan City, Mt. Huang |
| 59A | A. tianmuensis | LJK48 | 24 | 30.1097 | 118.9013 | 902 | China, Zhejiang Province, Hangzhou City, Qingliangfeng Botanical Garden |
| 59B | A. tianmuensis | WMZ1502 | 36 | 30.1097 | 118.9014 | 872 | China, Zhejiang Province, Hangzhou City, Qingliangfeng Botanical Garden |
| 60A | A. tianmuensis | LJK19 | 24 | 29.2085 | 119.6263 | 577 | China, Zhejiang Province, Jinhua City, Shuanglong Scenic Area |
| 60B | A. tianmuensis | LP207913 | 24 | 29.2085 | 119.6263 | 577 | China, Zhejiang Province, Jinhua City, Shuanglong Scenic Area |
| 60C | A. tianmuensis | LP173009 | 24 | 29.2085 | 119.6263 | 577 | China, Zhejiang Province, Jinhua City, Shuanglong Scenic Area |
| 61 | A. erythronioides | WMZ1429 | 24 | 29.7398 | 121.0869 | 845 | China, Zhejiang Province, Yuyao County, Mt. Siming |
| 62A | A. erythronioides | LP184944 | 24 | 29.7339 | 121.3429 | 169 | China, Zhejiang Province, Ningbo City, Tianhu Scenic Area |
| 62B | A. erythronioides | LJK24 | 24 | 29.7286 | 121.3461 | 148 | China, Zhejiang Province, Ningbo City, Tianhu Scenic Area |
| 62C | A. erythronioides | WMZ1433 | 24 | 29.7283 | 121.3456 | 118 | China, Zhejiang Province, Ningbo City, Tianhu Scenic Area |
| 63 | A. erythronioides | WMZ1435 | 24 | 29.6489 | 121.5570 | 573 | China, Zhejiang Province, Ningbo City, Mt. Jin’e |
| 64A | A. erythronioides | LP184934 | 24 | 29.3735 | 121.5855 | 492 | China, Zhejiang Province, Ninghai County, Mt. Cha |
| 64B | A. erythronioides | WMZ1439 | 24 | 29.3735 | 121.5854 | 509 | China, Zhejiang Province, Ninghai County, Mt. Cha |
| 65 | A. kuocangshanica | WMZ1441 | 24 | 29.3752 | 121.5909 | 460 | China, Zhejiang Province, Ninghai County, Mt. Cha |
| 66 | A. kuocangshanica | WMZ1443 | 24 | 28.8150 | 120.9432 | 868 | China, Zhejiang Province, Linhai County, Mt. Kuocang |
| 67 | A. kuocangshanica | WMZ1448 | 24 | 28.5512 | 120.7998 | 865 | China, Zhejiang Province, Yongjia County, Mt. Sihai |
| 68A | A. latifolia | WMZ1445 | 24 | 27.9379 | 120.5080 | 401 | China, Zhejiang Province, Wenzhou City, Jinbao Village |
| 68B | A. latifolia | LP173010 | 24 | 27.9423 | 120.5061 | 400 | China, Zhejiang Province, Wenzhou City, Jinbao Village |
| 69 | A. latifolia | WMZ1444 | 24 | 27.9079 | 120.6970 | 324 | China, Zhejiang Province, Wenzhou City, Mt. Daluo |
| 70 | A. latifolia | LP172995 | 24 | 27.8950 | 120.7445 | 694 | China, Zhejiang Province, Wenzhou City, Mt. Daluo |
| 71 | A. latifolia | WMZ1446 | 24 | 27.8273 | 120.3295 | 336 | China, Zhejiang Province, Rui’an County, Huayan National Forest Park |
| 72A | Amana sp.1 | LJK10 | 24 | 29.5070 | 120.4370 | 438 | China, Zhejiang Province, Zhuji County, Mt. Dongbai |
| 72B | Amana sp.1 | WMZ1461 | 24 | 29.5068 | 120.4370 | 430 | China, Zhejiang Province, Zhuji County, Mt. Dongbai |
| 73A | Amana sp.1 | LP184959 | 24 | 29.4501 | 120.2861 | 432 | China, Zhejiang Province, Zhuji County, Banqiu Village |
| 73B | Amana sp.1 | WMZ1458 | 24 | 29.4500 | 120.2860 | 413 | China, Zhejiang Province, Zhuji County, Banqiu Village |
| 74A | Amana sp.1 | LP150073 | 24 | 29.3517 | 121.0251 | 588 | China, Zhejiang Province, Xinchang County, Malikeng Village |
| 74B | Amana sp.1 | WMZ1451 | 24 | 29.3514 | 121.0259 | 629 | China, Zhejiang Province, Xinchang County, Malikeng Village |
| 74C | Amana sp.1 | LP161171 | 24 | 29.3513 | 121.0255 | 636 | China, Zhejiang Province, Xinchang County, Malikeng Village |
| 75A | Amana sp.1 | WMZ1450 | 24 | 29.2525 | 121.0977 | 990 | China, Zhejiang Province, Tiantai County, Huading National Forest Park |
| 75B | Amana sp.1 | LP161169 | 24 | 29.2523 | 121.0959 | 962 | China, Zhejiang Province, Tiantai County, Huading National Forest Park |
| 76 | Amana sp.1 | WMZ1449 | 24 | 28.9799 | 120.5393 | 747 | China, Zhejiang Province, Pan’an County, Mt. Dapan |
| 77 | A. wanzhensis | WMZ1427 | 24 | 31.0871 | 119.3349 | 307 | China, Anhui Province, Guangde County, Xiaojiawan |
| 78A | A. wanzhensis | LJK44 | 24 | 30.3487 | 119.2296 | 713 | China, Anhui Province, Ningguo County, Longmentou Village |
| 78B | A. wanzhensis | WMZ1466 | 24 | 30.3480 | 119.2298 | 720 | China, Anhui Province, Ningguo County, Longmentou Village |
| 79 | A. wanzhensis | LJK46 | 24 | 30.3065 | 119.3163 | 1120 | China, Zhejiang Province, Hangzhou City, Zhexitianchi |
| 80 | A. wanzhensis | WMZ1469 | 24 | 30.3064 | 119.1196 | 1126 | China, Zhejiang Province, Hangzhou City, Zhexitianchi |
| 81 | A. wanzhensis | WMZ1455 | 24 | 29.5148 | 120.2410 | 572 | China, Zhejiang Province, Zhuji County, Fenglinxia Village |
| 82 | A. wanzhensis | WMZ1460 | 24 | 29.4565 | 120.2906 | 244 | China, Zhejiang Province, Zhuji County, Banqiu Village |
| 83 | A. baohuaensis | WMZ1413 | 24 | 32.1385 | 119.2762 | 259 | China, Jiangsu Province, Zhenjiang City, Mt. Chaofeng |
| 84 | A. baohuaensis | WMZ1415 | 24 | 32.1251 | 119.0896 | 216 | China, Jiangsu Province, Jurong County, Mt. Baohua |
| 85 | A. baohuaensis | WMZ1419 | 24 | 32.0559 | 118.5476 | 346 | China, Jiangsu Province, Nanjing City, Shiziling |
| 86A | A. baohuaensis | WMZ1407 | 24 | 31.8160 | 119.3090 | 333 | China, Jiangsu Province, Jurong County, Mt. Mao |
| 86B | A. baohuaensis | LP172906 | 24 | 31.8156 | 119.3090 | 235 | China, Jiangsu Province, Jurong County, Mt. Mao |
| 87 | A. baohuaensis | WMZ1409 | 24 | 31.7883 | 119.2961 | 107 | China, Jiangsu Province, Jurong County, Mt. Mao |
| 88 | A. baohuaensis | WMZ1423 | 24 | 31.6714 | 118.0888 | 74 | China, Anhui Province, Hanshan County, Mt. Baochan |
| 89A | A. anhuiensis | LJK62 | 24 | 30.7412 | 116.4531 | 1183 | China, Anhui Province, Qianshan County, Mt. Tianzhu |
| 89B | A. anhuiensis | CMQ2015075 | 24 | 30.7410 | 116.4526 | 1207 | China, Anhui Province, Qianshan County, Mt. Tianzhu |
| 90 | A. anhuiensis | WMZ1480 | 24 | 30.7235 | 116.4537 | 708 | China, Anhui Province, Qianshan County, Mt. Tianzhu |
| 91A | A. anhuiensis | WMZ1499 | 24 | 29.0968 | 115.5768 | 711 | China, Jiangxi Province, Yongxiu County, Mt. Yunju |
| 91B | A. anhuiensis | LP173014 | 24 | 29.0963 | 115.5767 | 703 | China, Jiangxi Province, Yongxiu County, Mt. Yunju |
| 92 | Amana sp.2 | WMZ1489 | 24 | 32.3954 | 113.2989 | 248 | China, Henan Province, Tongbai County, Tayuan Temple |
| 93 | Amana sp.2 | WMZ1487 | 24 | 31.7973 | 114.0872 | 529 | China, Hubei Province, Guangshui County, Heilongtan |
| 94 | Amana sp.2 | WMZ1483 | 24 | 31.7212 | 115.5038 | 496 | China, Henan Province, Shangnan County, Liluocheng Village |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Wang, M.; Zhu, Z.; Cai, M.; Lee, J.; Li, P. Cytogeography of the East Asian Tulips (Amana, Liliaceae). Taxonomy 2022, 2, 145-159. https://doi.org/10.3390/taxonomy2010012
Wu J, Wang M, Zhu Z, Cai M, Lee J, Li P. Cytogeography of the East Asian Tulips (Amana, Liliaceae). Taxonomy. 2022; 2(1):145-159. https://doi.org/10.3390/taxonomy2010012
Chicago/Turabian StyleWu, Jing, Meizhen Wang, Zhangshichang Zhu, Minqi Cai, Joongku Lee, and Pan Li. 2022. "Cytogeography of the East Asian Tulips (Amana, Liliaceae)" Taxonomy 2, no. 1: 145-159. https://doi.org/10.3390/taxonomy2010012
APA StyleWu, J., Wang, M., Zhu, Z., Cai, M., Lee, J., & Li, P. (2022). Cytogeography of the East Asian Tulips (Amana, Liliaceae). Taxonomy, 2(1), 145-159. https://doi.org/10.3390/taxonomy2010012






