Description of a New Scaled Species of Ptychostomella (Gastrotricha: Macrodasyida) from the Brazilian Coast and a Cladistics Analysis of the Genus
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Phylogenetic Analysis
2.2.1. Ingroup Taxa
2.2.2. Outgroup Taxa
2.2.3. Character Coding and Analysis
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tetranchyroderma esarabdophorum Tongiorgi & Balsamo, 1984 | 2 | 0 | 1 | 1 | 0 | 1 | 1 | 3 | 1 | 0 | 0 | [11] |
| Tetranchyroderma quadritentaculatum Todaro, Balsamo & Tongiorgi, 1992 | 2 | 0 | 1 | 1 | 0 | 1 | 1 | 3 | 1 | 0 | 0 | [12] |
| Ptychostomella bergensis Clausen, 1996 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | [13] |
| Ptychostomella brachycephala (Levi, 1954) | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | [14] |
| Ptychostomella helana Roszczak, 1939 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | - | 0 | 0 | 0 | [15] |
| Ptychostomella jejuensis Lee, Hwang & Chang, 2009 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | [16] |
| Ptychostomella higginsi Clausen, 2004 | 2 | 1 | 0 | 1 | 1 | 0 | 1 | 3 | 0 | 0 | 0 | [14] |
| Ptychostomella lamelliphora Todaro, 2013 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 1 | [3] |
| Ptychostomella lepidota Clausen, 2000 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | [17] |
| Ptychostomella mediterranea Remane, 1927 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | - | 0 | 0 | 0 | [18] |
| Ptychostomella ommatophora Remane, 1927 | 2 | 0 | 0 | ? | ? | 0 | 0 | - | 0 | 0 | 0 | [18] |
| Ptychostomella orientalis Lee & Chang, 2003 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | [19] |
| Ptychostomella papillata Lee & Chang, 2003 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | [19] |
| Ptychostomella pectinata Remane, 1926 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | - | 0 | 0 | 0 | [20] |
| Ptychostomella tyrrhenica Hummon, Todaro & Tongiorgi, 1993 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | [21] |
| Ptychostomella sebastiana sp. nov. | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | [present study] |
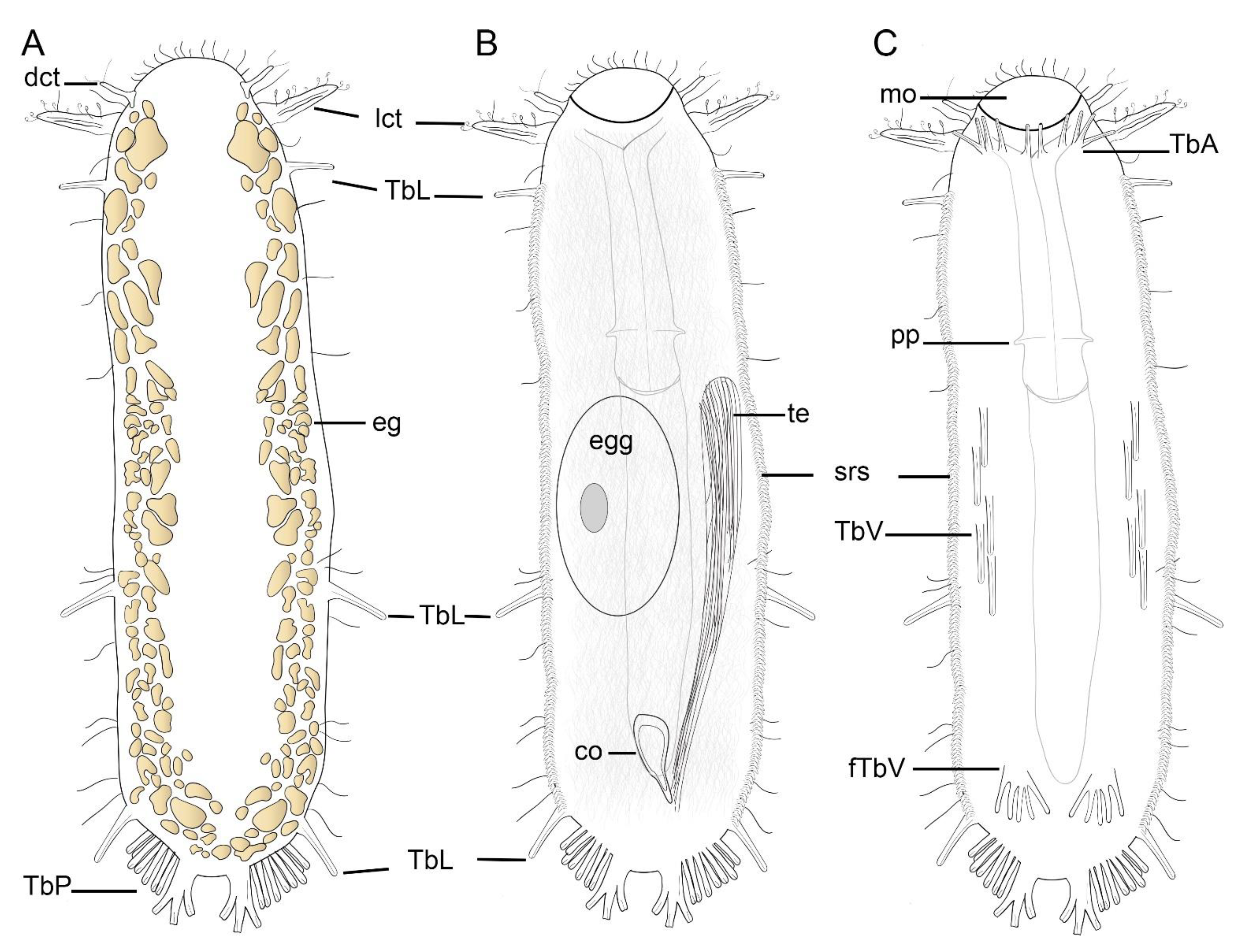
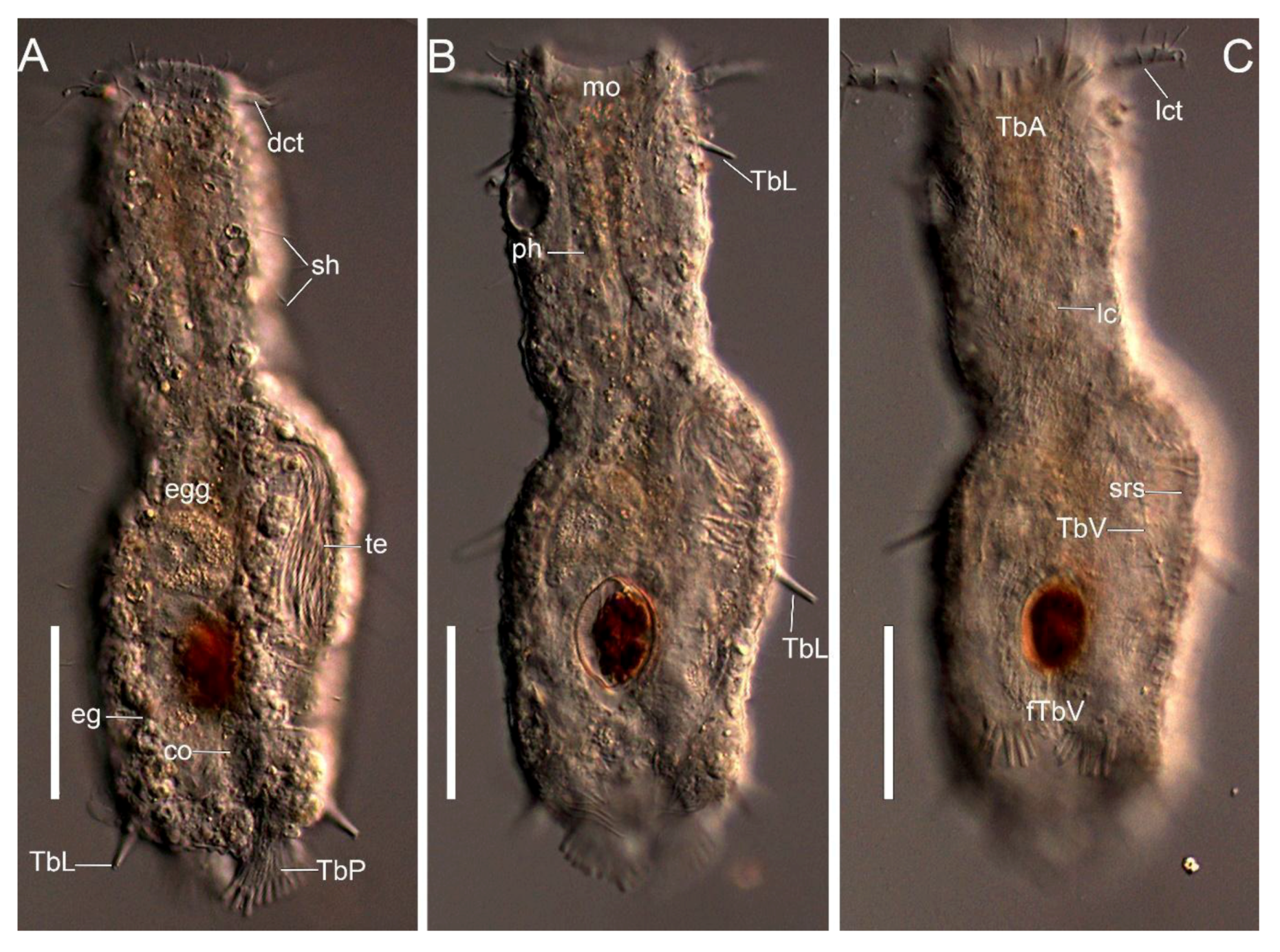
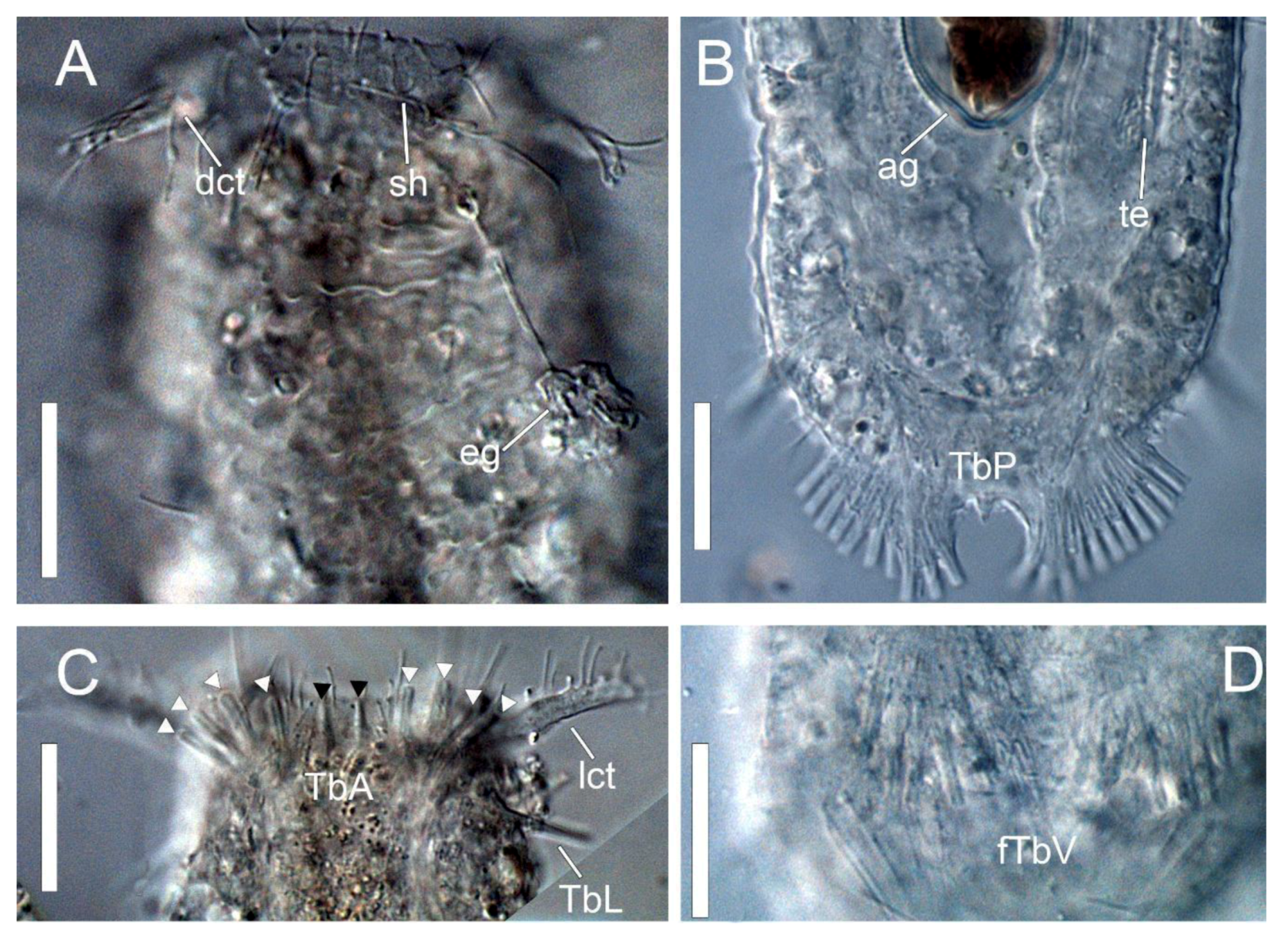
2.3. Morphological Description of the Characters
3. Results
3.1. Taxonomic Account
- Order Macrodasyida Remane, 1925 [Rao & Clausen, 1970]
- Family Taumastodermatidae Remane, 1927
- Subfamily Taumastodermatinae Remane, 1927
- Genus Ptychostomella Remane, 1926
- Ptychostomella sebastiana sp. nov.
Taxonomic Remarks
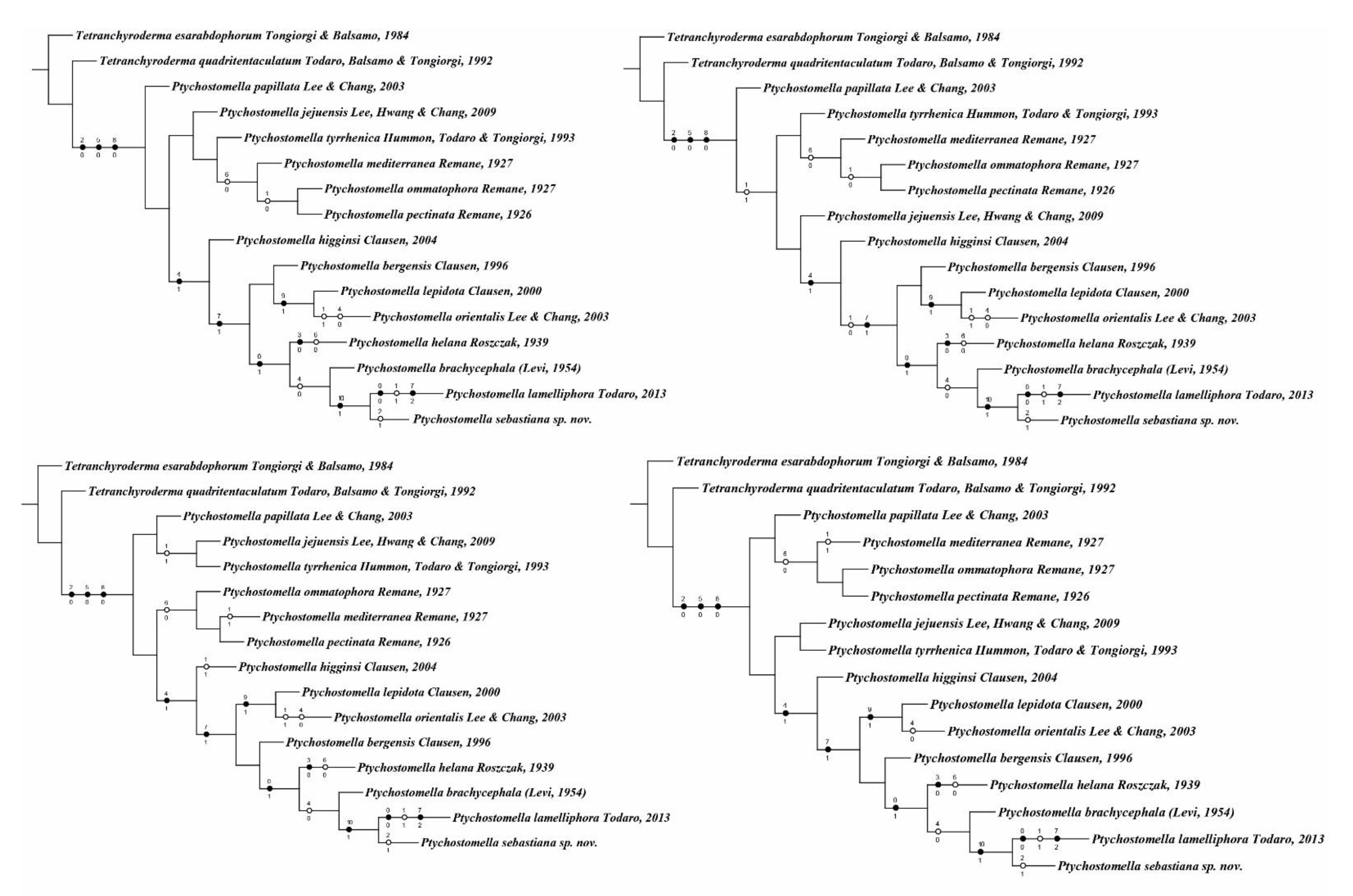
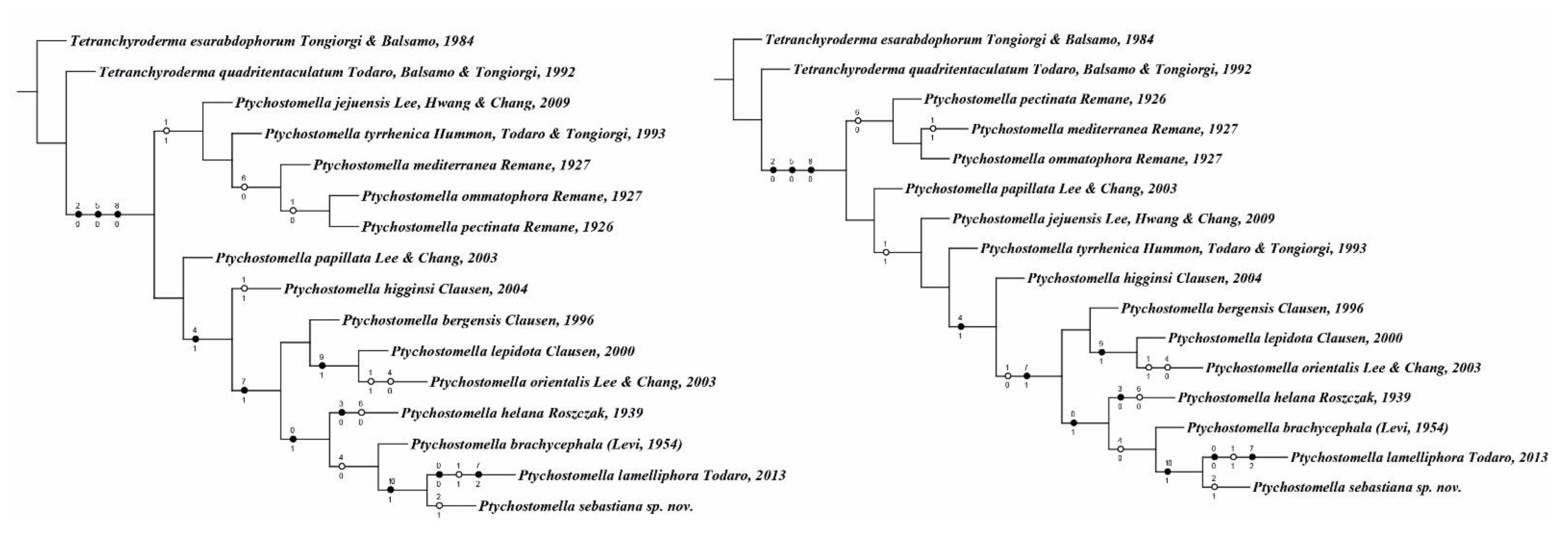
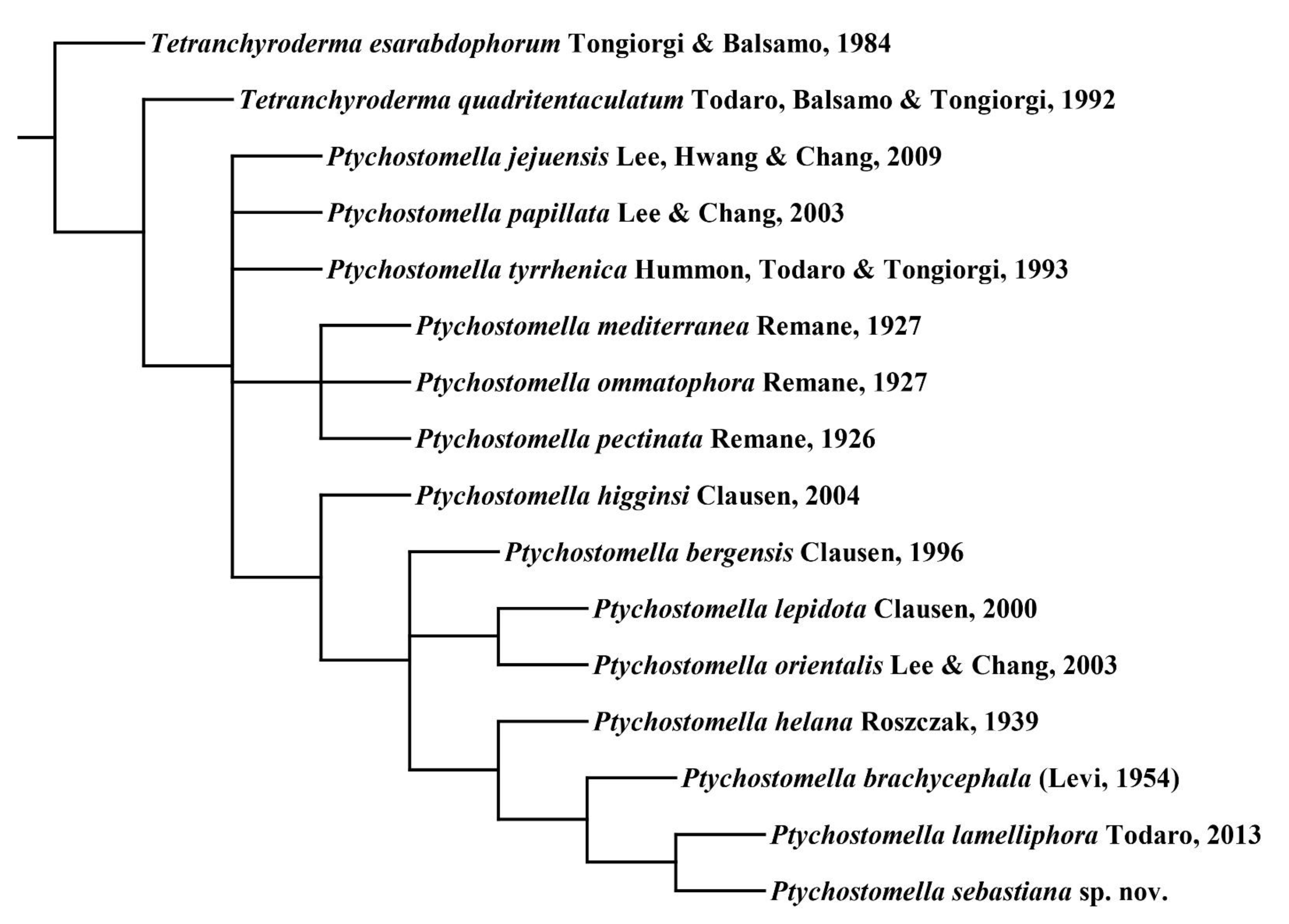
3.2. Phylogenetic Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Todaro, M.A.; Kånneby, T.; Dal Zotto, M.; Jondelius, U. Phylogeny of Thaumastodermatidae (Gastrotricha: Macrodasyida) inferred from nuclear and mitochondrial sequence data. PLoS ONE 2011, 6, e0017892. [Google Scholar] [CrossRef]
- Todaro, M.A. Marine and Freshwater Gastrotricha. Available online: http://www.gastrotricha.unimore.it (accessed on 30 September 2021).
- Todaro, M. A new non-naked species of Ptychostomella (Gastrotricha) from Brazil. Zookeys 2013, 289, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, E.E. Gastrotricha. In Introduction to the Study of Meiofauna; Higgins, R.P., Thiel, H., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1988; pp. 302–321. [Google Scholar]
- Pfannkuche, O.; Thiel, H. Sampling processing. In Introduction to the Study Meiofauna; Higgins, R.P., Thiel, H., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1988; Volume 1, pp. 134–145. [Google Scholar]
- Hummon, W.D.; Balsamo, M.; Todaro, M.A. Italian marine Gastrotricha: I. Six new and one redescribed species of Chaetonotida. Boll. Zool. 1992, 59, 499–516. [Google Scholar] [CrossRef]
- Bosco, I.; Lourenço, A.P.; Guidi, L.; Balsamo, M.; Hochberg, R.; Garraffoni, A.R.S. Integrative description of a new species of Acanthodasys Remane, 1927 (Gastrotricha, Macrodasyida, Thaumastodermatidae) based on four distinct morphological techniques and molecular data. Zool. Anz. 2020, 286, 31–42. [Google Scholar] [CrossRef]
- Forey, P.L.; Kitching, I.J. Experiments in coding multistate characters. In Homology and Systematics: Coding Characters for Phylogenetic Analysis; Scotland, R., Pennington, T., Eds.; Taylor & Francis: London, UK, 2000; pp. 54–80. [Google Scholar]
- Pleijel, F. On character coding for phylogeny reconstruction. Cladistics 1995, 11, 309–315. [Google Scholar] [CrossRef]
- Hawkins, J.A.; Hughes, C.E.; Scotland, R.W. Primary Homology Assessment, Characters and Character States. Cladistics 1997, 13, 275–283. [Google Scholar] [CrossRef]
- Tongiorgi, P.; Balsamo, M. A new Tetranchyroderma species (Gastrotricha, Macrodasyoidea) from the Adriatic coast. Boll. Zool. 1984, 51, 335–338. [Google Scholar] [CrossRef]
- Balsamo, M.; Todaro, M.A.; Tongiorgi, P. Marine gastrotrichs from the Tuscan achipelago (Tyrrhenian Sea): II. Chaetonotida, with description of three new species. Boll. Zool. 1992, 59, 487–498. [Google Scholar] [CrossRef]
- Clausen, C. Three new species of Gastrotricha Macrodasyida from the Bergenarea, Western Norway. Sarsia 1996, 81, 119–219. [Google Scholar] [CrossRef]
- Clausen, C. Gastrotricha from the Faroe Bank. Sarsia 2004, 89, 423–458. [Google Scholar] [CrossRef]
- Roszczak, R. Die Psammitgastrotricha des polnischen Ostseestrandes. Zool. Pol. 1939, 4, 1–24. [Google Scholar]
- Lee, J.M.; WookHwang, U.; Chang, C.Y. A new gastrotrich species of the genus Ptychostomella (macrodasyida, thaumastodermatidae) from south korea. Anim. Cells Syst. 2009, 13, 25–30. [Google Scholar] [CrossRef]
- Clausen, C.; Båmstedt, U. Gastrotricha Macrodasyida from the Tromsø region, northern Norway. Sarsia 2000, 85, 357–384. [Google Scholar] [CrossRef]
- Remane, A. Neue Gastrotricha Macrodasyoidea. Zool. Jahrb. Abt. Syst. Ökologie Geogr. Tiere 1927, 54, 230–242. [Google Scholar]
- Lee, J.M.; Chang, C.Y. Two New Marine Gastrotrichs of the Genus Ptychostomella (Macrodasyida, Thaumastodermatidae) from South Korea. Zool. Sci. 2003, 20, 481–489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Remane, A. Morphologie und verwandtschaftsbeziehungen der aberranten gastrotrichen I. Z. Morphol. Ökologie Tiere 1926, 5, 625–754. [Google Scholar] [CrossRef]
- Hummon, W.D.; Tod Aro, M.A.; Tongiorgi, P. Italian marine gastrotricha: II. one new genus and ten new species of macrodasyida. Bolletino Zool. 1993, 60, 109–127. [Google Scholar] [CrossRef]
- Goloboff, P.A.; Farris, J.S.; Nixon, K.C. TNT: Tree Analysis Using New Technology. Syst. Biol. 2003, 54, 176–178. [Google Scholar]
- Goloboff, P.A.; Farris, J.S.; Nixon, K.C. TNT, a free program for phylogenetic analysis. Cladistics 2008, 24, 774–786. [Google Scholar] [CrossRef]
- Marcos Mirande, J. Weighted parsimony phylogeny of the family Characidae (Teleostei: Characiformes). Cladistics 2009, 25, 574–613. [Google Scholar] [CrossRef]
- Nixon, K.C. WinClada Version. 1.00.08. Published by the Author. Ithaca, N.Y. 2002. Available online: http://www.cladistics.com/ (accessed on 16 February 2007).
- Kieneke, A. A new species of Thaumastoderma (Gastrotricha: Macrodasyida) from the Antarctic deep sea with a phylogenetic analysis of the whole genus. J. Mar. Biol. Assoc. UK 2010, 90, 575–584. [Google Scholar] [CrossRef]
- Garraffoni, A.R.S.; Araújo, T.Q. Phylogeny of Pseudostomella Swedmark, 1956 (Gastrotricha: Macrodasyida) based on morphological data and first insights on the historical biogeography of Thaumastodermatidae. Proc. Biol. Soc. Washingt. 2017, 130, 222–238. [Google Scholar] [CrossRef]
- Fonseca, G.; Fontaneto, D.; Di Domenico, M. Addressing biodiversity shortfalls in meiofauna. J. Exp. Mar. Biol. Ecol. 2018, 502, 26–38. [Google Scholar] [CrossRef]

| 0. Cephalic sensorial organs–Lateral tentacle type |
|
• 0 Short • 1 Long • 2 absent |
| 1. Cephalic sensorial organs–Knob-like organ |
|
• 0 absent • 1 present |
| 2. Cephalic sensorial organs–Dorsolateral tentacle |
|
• 0 absent • 1 present |
| 3. Anterior adhesive tubes–Inserted along the mouth margin |
|
• 0 absent • 1 present |
| 4. Anterior adhesive tubes–Inserted in a ventrolateral column |
|
• 0 absent • 1 present |
| 5. Dorsal adhesive tubes |
|
• 0 absent • 1 present |
| 6. Ventral adhesives at U85 |
|
• 0 absent • 1 present |
| 7. Ventral adhesives at U85–Type |
|
• 0 Paired • 1 Foot-like • 2 Bulked with many adhesive tube • 3 Single |
| 8. Dorsal ornamentation–Ancres |
|
• 0 absent • 1 present |
| 9. Dorsal ornamentation–Scale-like cuticular elevation |
|
• 0 absent • 1 present |
| 10. Lateral ornamentation–Plates |
|
• 0 absent • 1 present |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, T.Q.; Garraffoni, A.R.S. Description of a New Scaled Species of Ptychostomella (Gastrotricha: Macrodasyida) from the Brazilian Coast and a Cladistics Analysis of the Genus. Taxonomy 2021, 1, 278-289. https://doi.org/10.3390/taxonomy1040022
Araújo TQ, Garraffoni ARS. Description of a New Scaled Species of Ptychostomella (Gastrotricha: Macrodasyida) from the Brazilian Coast and a Cladistics Analysis of the Genus. Taxonomy. 2021; 1(4):278-289. https://doi.org/10.3390/taxonomy1040022
Chicago/Turabian StyleAraújo, Thiago Q., and André R. S. Garraffoni. 2021. "Description of a New Scaled Species of Ptychostomella (Gastrotricha: Macrodasyida) from the Brazilian Coast and a Cladistics Analysis of the Genus" Taxonomy 1, no. 4: 278-289. https://doi.org/10.3390/taxonomy1040022
APA StyleAraújo, T. Q., & Garraffoni, A. R. S. (2021). Description of a New Scaled Species of Ptychostomella (Gastrotricha: Macrodasyida) from the Brazilian Coast and a Cladistics Analysis of the Genus. Taxonomy, 1(4), 278-289. https://doi.org/10.3390/taxonomy1040022





