Intrinsic Defect-Related Photoluminescence in Single-Crystalline Tin Dioxide
Abstract
1. Introduction
2. Experimental Details
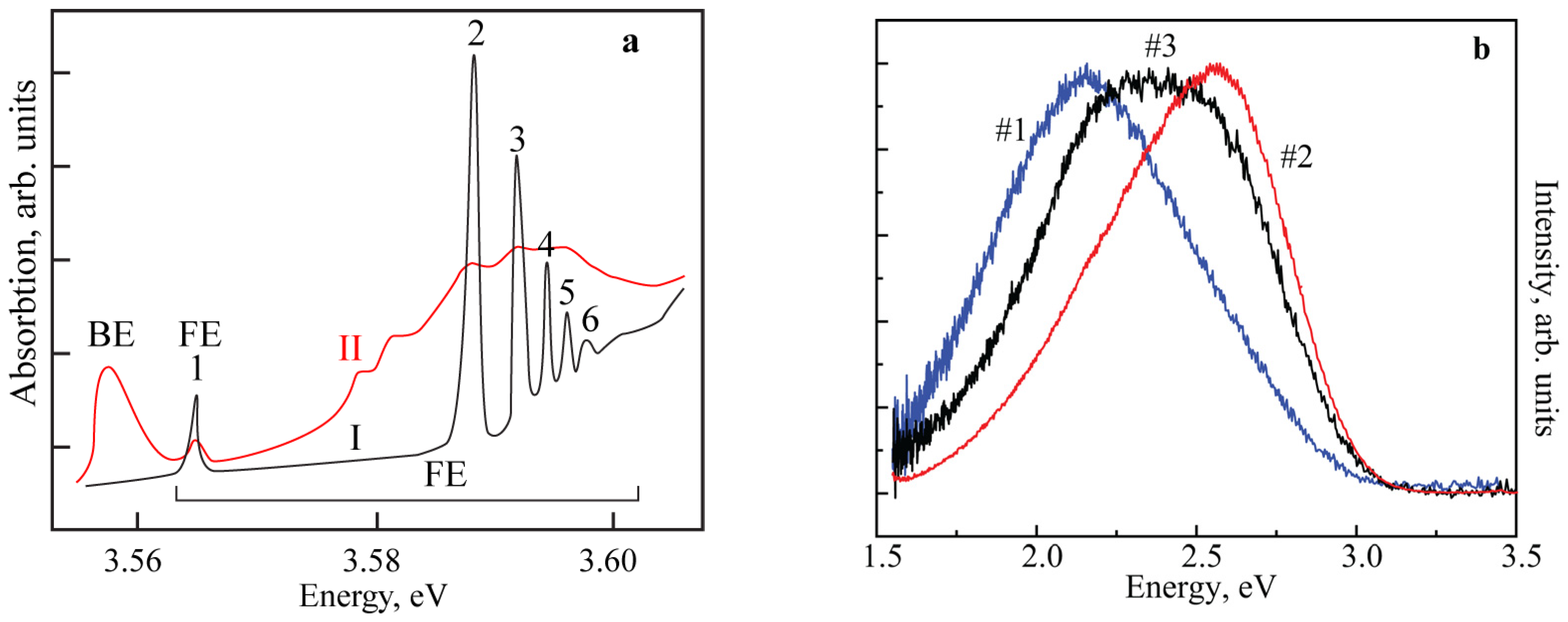
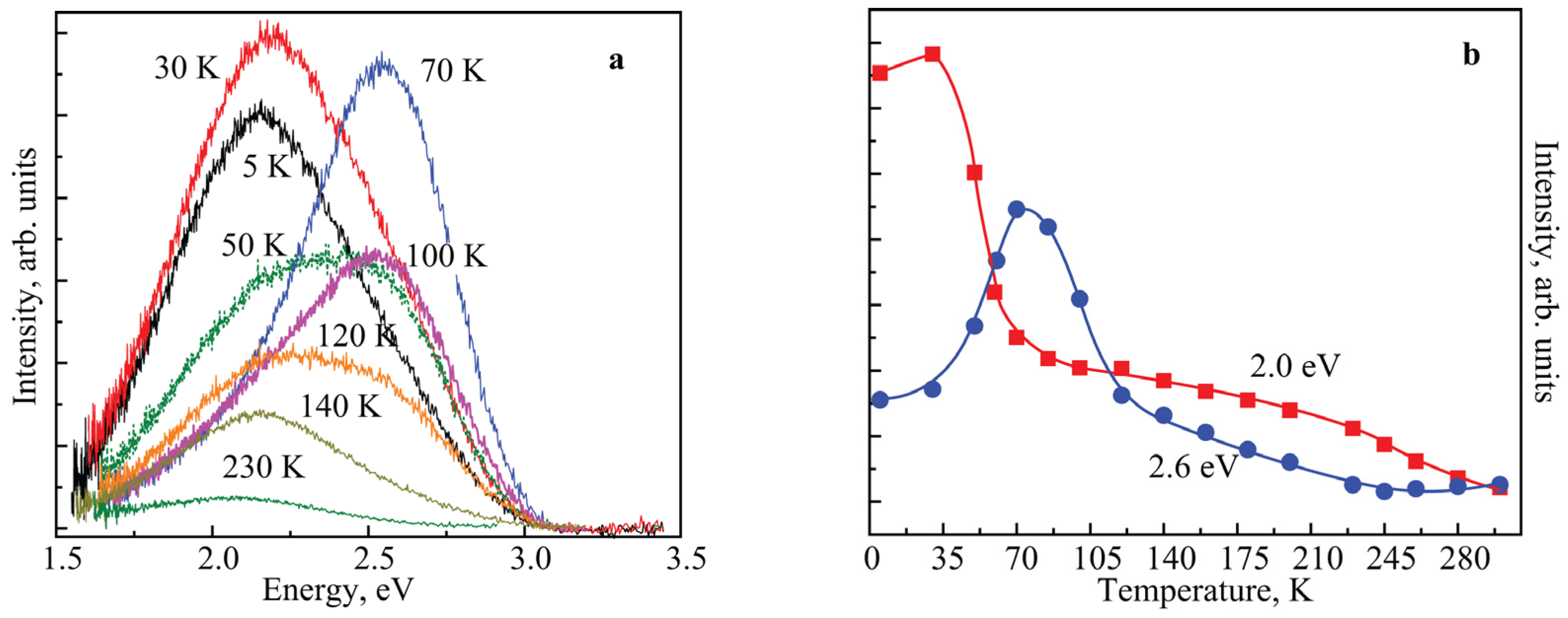
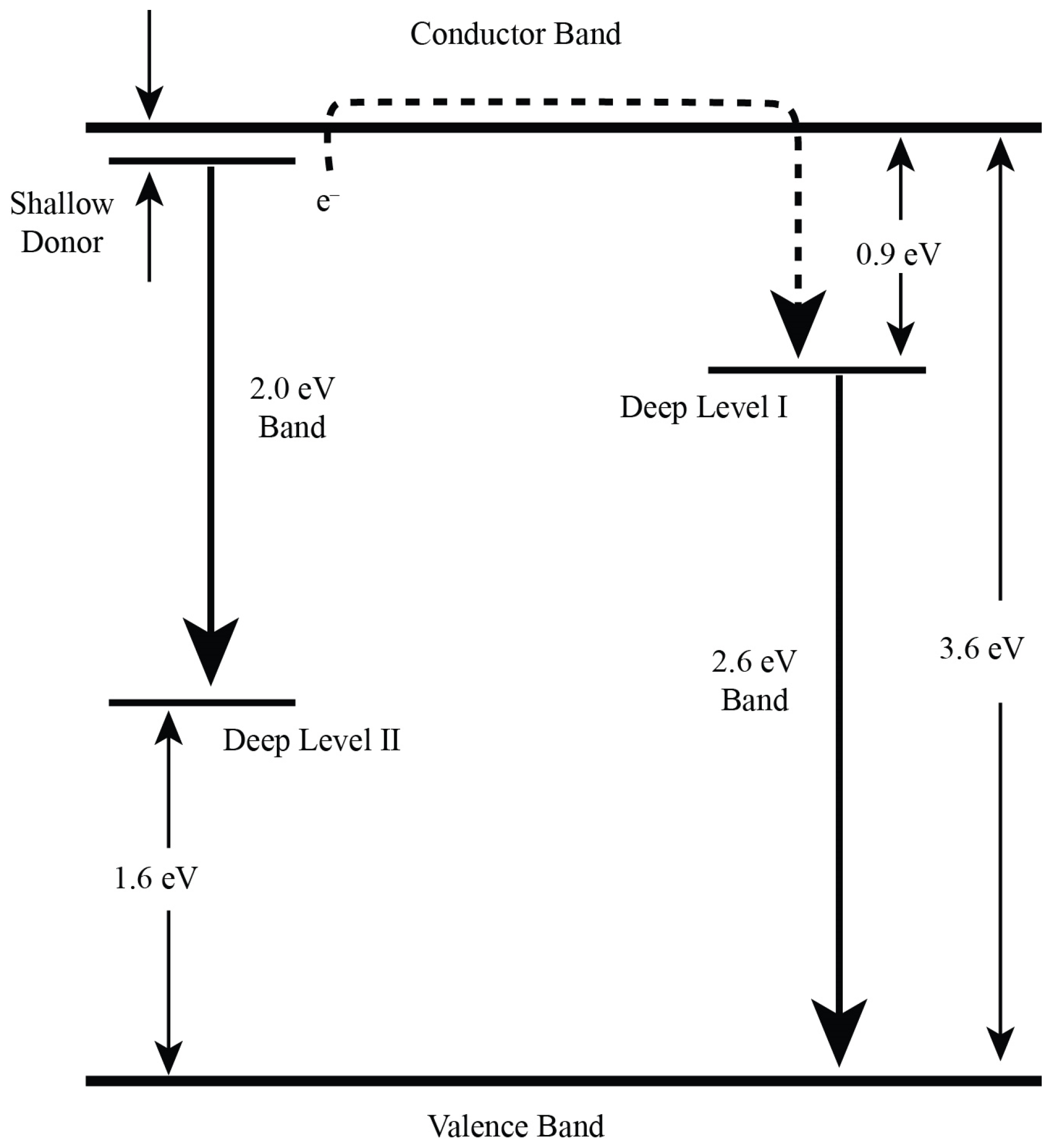
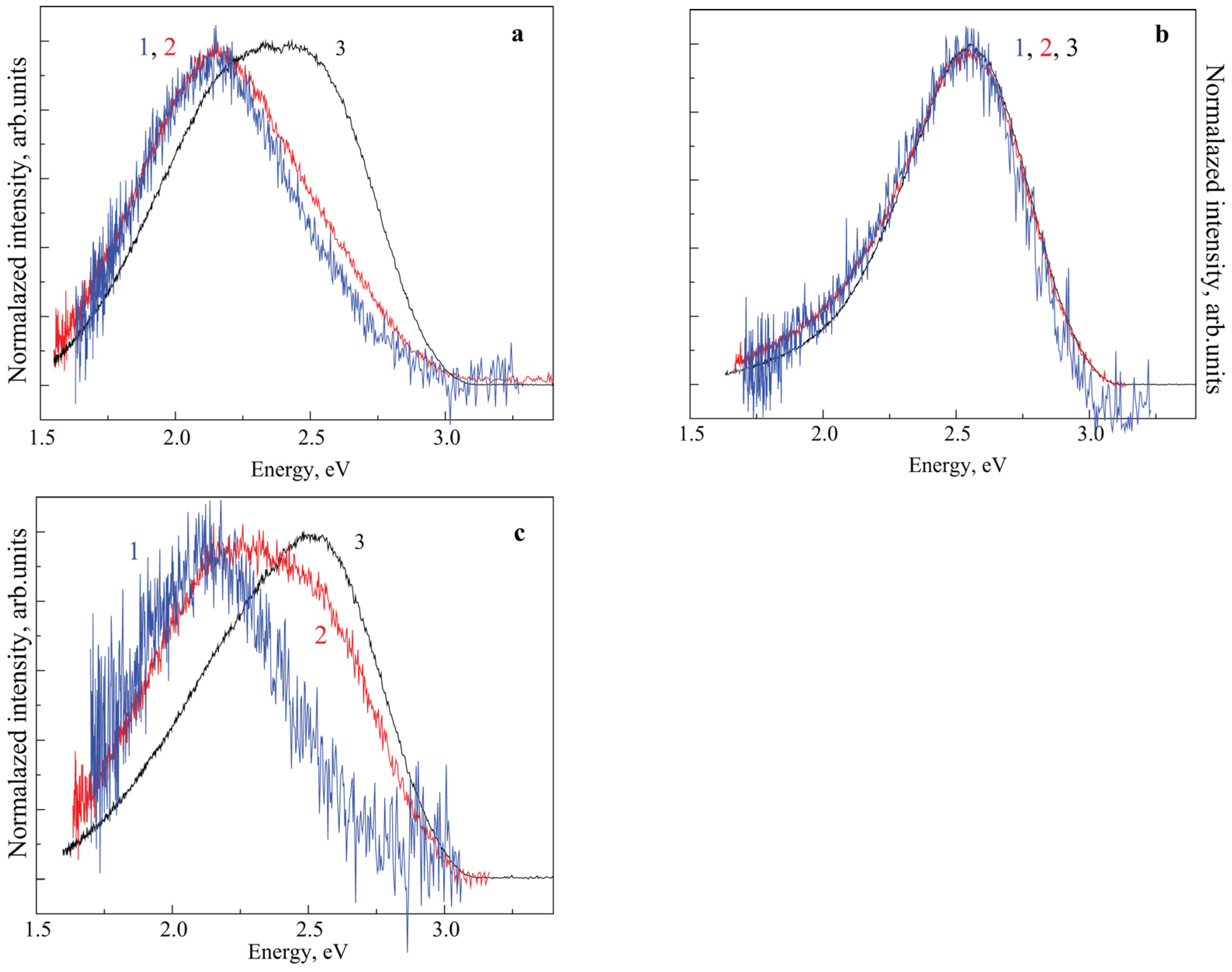
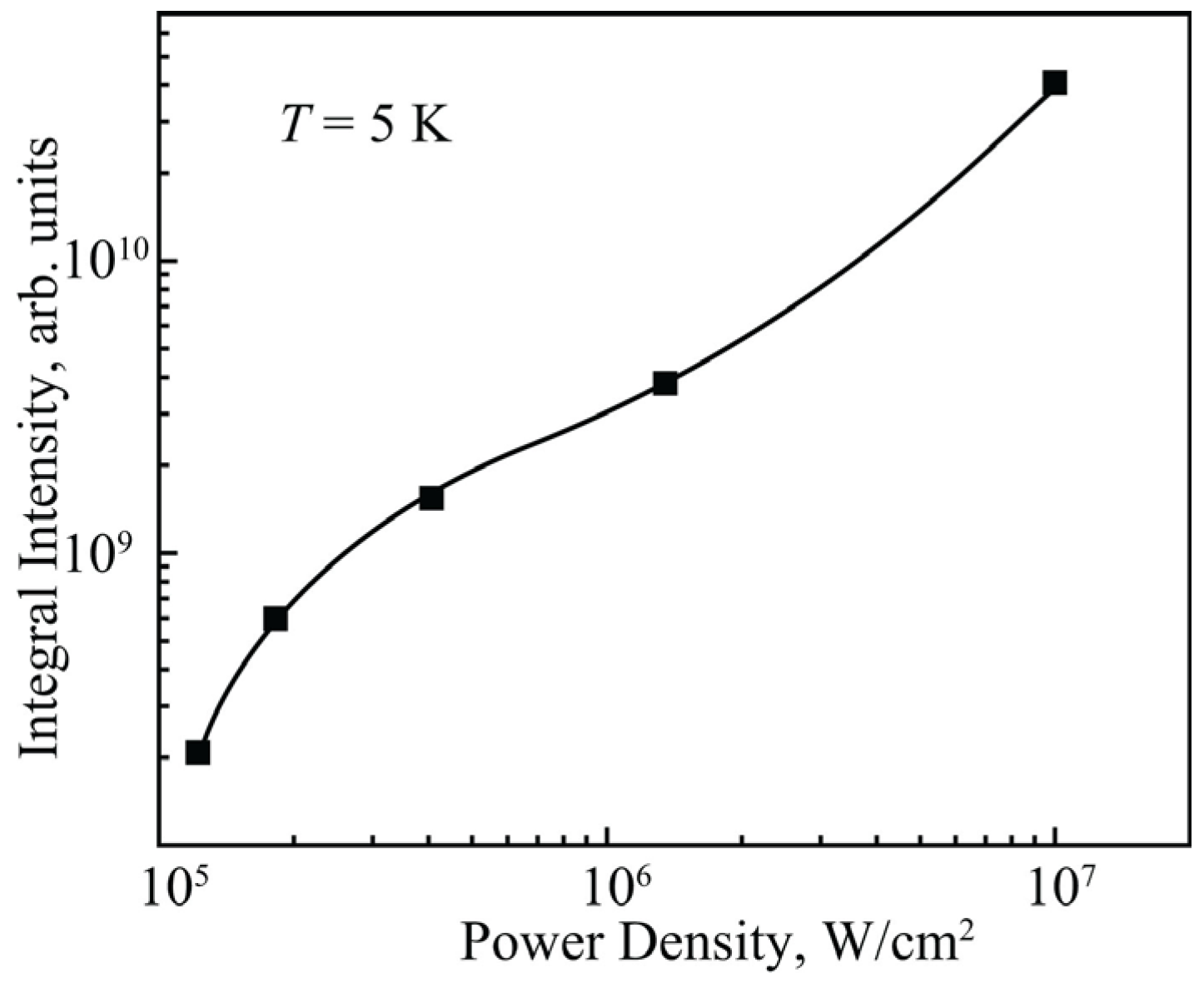
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fleischer, K.; Arca, E.; Smith, C.; Shvets, I.V. Aluminium doped Zn1−xMgxO—A transparent conducting oxide with tunable optical and electrical properties. Appl. Phys. Lett. 2012, 101, 121918. [Google Scholar] [CrossRef]
- Akagawa, M.; Fujiwara, H. Optical characterization of textured SnO2:F layers using spectroscopic ellipsometry. J. Appl. Phys. 2012, 112, 083507. [Google Scholar] [CrossRef]
- Liu, Y.S.; Hsieh, C.I.; Wu, Y.J.; Wei, Y.S.; Lee, P.M.; Liu, C.Y. Transparent p-type AlN:SnO2 and p-AlN:SnO2/n-SnO2:In2O3 p-n junction fabrication. Appl. Phys. Lett. 2012, 101, 122107. [Google Scholar] [CrossRef]
- Shen, W. Properties of SnO2 based gas-sensing thin films prepared by ink-jet printing. Sens. Actuators B Chem. 2012, 166–167, 110. [Google Scholar] [CrossRef]
- Choi, E.; Lee, D.; Shin, H.J.; Kim, N.; Valladares, L.D.L.S.; Seo, J. Role of oxygen vacancy sites on the temperature-dependent photoluminescence of SnO2 nanowires. J. Phys. Chem. C 2021, 125, 14974. [Google Scholar] [CrossRef]
- Costa, I.M.; Teodoro, M.D.; Zaghete, M.A.; Chiquito, A.J. Influence of the metastable state (V++) on the electronic properties of SnO2 nanowires under the influence of light. J. Appl. Phys. 2020, 128, 115702. [Google Scholar] [CrossRef]
- Zhou, J.X.; Zhang, M.S.; Hong, J.M.; Yin, Z. Raman spectroscopic and photoluminescence study of single-crystalline SnO2 nanowires. Solid State Commun. 2006, 138, 242. [Google Scholar] [CrossRef]
- Luo, S.; Chu, P.K. Origin of low-temperature photoluminescence from SnO2 nanowires fabricated by thermal evaporation and annealed in different ambients. Appl. Phys. Lett. 2006, 88, 183112. [Google Scholar] [CrossRef]
- Pramata, A.D.; Suematsu, K.; Quitain, A.T.; Sasaki, M.; Kida, T. Synthesis of Highly Luminescent SnO2 Nanocrystals: Analysis of their Defect-Related Photoluminescence Using Polyoxometalates as Quenchers. Adv. Funct. Mater. 2017, 27, 1704620. [Google Scholar] [CrossRef]
- Lee, E.J.; Ribeiro, C.; Giraldi, T.R.; Longo, E.; Leite, E.R.; Varela, J.A. Photoluminescence in quantum-confined SnO2 nanocrystals: Evidence of free exciton decay. Appl. Phys. Lett. 2004, 84, 1745. [Google Scholar] [CrossRef]
- Kazem, H.A.; Salman, H.I. The synthesis and characterization of SnO2 nanostructure using hydrothermal method. AIP Conf. Proc. 2025, 3395, 050005. [Google Scholar]
- Park, S.Y.; Zhu, K. Advances in SnO2 for efficient and stable n–i–p perovskite solar cells. Adv. Mater. 2022, 34, 2110438. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Zhang, Y.; Zhang, H.; Han, W.; Zhou, T.; Sun, Y.; Pang, S.; Wang, S.J. Enhanced ethanol sensing properties of SnO2/CdSnO3 heterostructure via a facile carbon incorporation strategy. Alloys Compd. 2025, 1047, 185061. [Google Scholar] [CrossRef]
- Pham, V.T.; Le, T.H.; Chu, M.H.; Hoang, B.T.; Vu, T.T.; Tran, T.Q.H.; Nguyen, X.S.; Tran, N.K. Effects of annealing temperature on the structure, morphology, and photocatalytic properties of SnO2/rGO nanocomposites. Nanotechnology 2021, 32, 015201. [Google Scholar] [CrossRef]
- Aguekian, V.F.; Stepanov, Y.A.; Akai, I.; Karasawa, T.; Komatsu, T. Exciton luminescence in tin dioxide single crystals. J. Phys. Soc. Jpn. 1993, 62, 4516. [Google Scholar] [CrossRef]
- Agekian, V.F.; Serov, A.Y.; Filosofov, N.G. Light emission from tin-dioxide crystals. Semiconductors 2014, 48, 442. [Google Scholar] [CrossRef]
- Gastev, C.V.; Kuzmina, I.P.; Lazarevskaya, O.A.; Sokolov, N.S.; Yakovlev, N.L. Luminescence and optical detection of EPR-triplet states in CuO2 crystals. Sov. Phys. Solid State 1983, 25, 2338. [Google Scholar]
- Kiliç, Ç.; Zunger, A. On the possibility of p-type SnO2. Phys. Rev. Lett. 2002, 88, 095501. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, D.O.; Watson, G.W. On the possibility of p-type SnO2. J. Mater. Chem. 2012, 22, 25236. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agekyan, V.F.; Filosofov, N.G.; Serov, A.Y.; Shtrom, I.V. Intrinsic Defect-Related Photoluminescence in Single-Crystalline Tin Dioxide. Solids 2025, 6, 68. https://doi.org/10.3390/solids6040068
Agekyan VF, Filosofov NG, Serov AY, Shtrom IV. Intrinsic Defect-Related Photoluminescence in Single-Crystalline Tin Dioxide. Solids. 2025; 6(4):68. https://doi.org/10.3390/solids6040068
Chicago/Turabian StyleAgekyan, Vadim F., Nikolai G. Filosofov, Alexey Yu. Serov, and Igor V. Shtrom. 2025. "Intrinsic Defect-Related Photoluminescence in Single-Crystalline Tin Dioxide" Solids 6, no. 4: 68. https://doi.org/10.3390/solids6040068
APA StyleAgekyan, V. F., Filosofov, N. G., Serov, A. Y., & Shtrom, I. V. (2025). Intrinsic Defect-Related Photoluminescence in Single-Crystalline Tin Dioxide. Solids, 6(4), 68. https://doi.org/10.3390/solids6040068





