Green-Synthesized Zinc Oxide Nanoparticles with Enhanced Release Behavior for Sustainable Agricultural Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnO Nanoparticles
2.2. Physicochemical Characterizations of ZnO Nanoparticles
2.3. Release Behavior Study of Synthesized Green ZnO Nanoparticles
3. Results
3.1. Physiochemical Characterization of the Green-Synthesized ZnO NPs
3.2. Optical Properties Measurement Using UV–Visible Absorption Spectroscopy and Energy Gap Calculations
3.3. Controlled Release of Zn2+ Ions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pudhuvai, B.; Koul, B.; Das, R.; Shah, M.P. Nano-Fertilizers (NFs) for Resurgence in Nutrient Use Efficiency (NUE): A Sustainable Agricultural Strategy. Curr. Pollut. Rep. 2024, 11, 1–29. [Google Scholar] [CrossRef]
- Saurabh, K.; Prakash, V.; Dubey, A.K.; Ghosh, S.; Kumari, A.; Sundaram, P.K.; Jeet, P.; Sarkar, B.; Upadhyaya, A.; Das, A.; et al. Enhancing sustainability in agriculture with nanofertilizers. Discov. Appl. Sci. 2024, 6, 1–23. [Google Scholar] [CrossRef]
- Arora, P.K.; Tripathi, S.; Omar, R.A.; Chauhan, P.; Sinhal, V.K.; Singh, A.; Srivastava, A.; Garg, S.K.; Singh, V.P. Next-generation fertilizers: The impact of bionanofertilizers on sustainable agriculture. Microb. Cell Factories 2024, 23, 1–8. [Google Scholar] [CrossRef]
- Chinnasamy, G.; Chandrasekharan, S.; Bhatnagar, S. Biosynthesis of Silver Nanoparticles from Melia azedarach: Enhancement of Antibacterial, Wound Healing, Antidiabetic and Antioxidant Activities. Int. J. Nanomed. 2019, 14, 9823–9836. [Google Scholar] [CrossRef]
- Ganguly, S.; Sengupta, J. Green synthesis of metal oxide nanoparticles. In Handbook of Green and Sustainable Nanotechnology; Shanker, U., Manviri, R., Chaudhury, M.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 303–327. [Google Scholar] [CrossRef]
- Dinga, E.; Mthiyane, D.M.N.; Marume, U.; Botha, T.L.; Horn, S.; Pieters, R.; Wepener, V.; Ekennia, A.; Onwudiwe, D.C. Biosynthesis of ZnO nanoparticles using Melia azedarach seed extract: Evaluation of the cytotoxic and antimicrobial potency. OpenNano. 2022, 8, 100068. [Google Scholar] [CrossRef]
- Maciel, M.V.; Morais, S.M.; Bevilaqua, C.M.L.; Camurça-Vasconcelos, A.L.F.; Costa, C.T.C.; Castro, C.M.S. Ovicidal and larvicidal activity of Melia azedarach extracts on Haemonchuscontortus. Vet. Parasitol. 2006, 140, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Naturae 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Haq, S.I.; Nisar, M.; Zahoor, M.; Ikram, M.; Islam, N.U.; Ullah, R.; Alotaibi, A. Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties. Green Process. Synth. 2023, 12, 20230029. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Eixenberger, J.E.; Anders, C.B.; Hermann, R.J.; Brown, R.J.; Reddy, K.M.; Punnoose, A.; Wingett, D.G. Rapid dissolution of ZnO nanoparticles induced by biological buffers significantly impacts cytotoxicity. Chem. Res. Toxicol. 2017, 30, 1641–1651. [Google Scholar] [CrossRef]
- Alhasaniah, A.H.; Aldabaan, N.A.; Reddy, D.; Alzahrani, H.A.; Shaikh, I.A.; Mahnashi, M.H.; Mannasaheb, B.A.; Muddapur, U.M.; Khan, A.A.; Bahafi, A.; et al. Harnessing Asystasia gangetica Flower Extract and Green Synthesized Zinc Oxide Nanoparticles for Combating Multidrug–Resistant Pathogens. ChemistrySelect 2025, 10, e00965. [Google Scholar] [CrossRef]
- Mahajan, M.; Kumar, S.; Gaur, J.; Kaushal, S.; Somvanshi, A.; Kaur, H.; Singh, G.; Hatshan, M.R.; Kumar, S.; Lotey, G.S. Role of cellulose, phenolic compounds, and water-soluble proteins in ZnO nanoparticle synthesis using Mangifera indica leaf extract for photocatalytic and antioxidant investigations. Colloids Surf. A Physicochem. Eng. Asp. 2025, 720, 137066. [Google Scholar] [CrossRef]
- SelvaKumar, M.; Jeyamurugan, R.; Jose, P.A.; Ponvel, K.M.; Sundarajan, M. Green Synthesis, Characterization, and Biological Evaluation of CuO–ZnO Nanoparticles Using Cyphostemma Setosum Leaf Extract. ChemistrySelect 2025, 10, e06062. [Google Scholar] [CrossRef]
- Aspoukeh, P.K.; Barzinjy, A.A.; Hamad, S.M. Synthesis, properties and uses of ZnO nanorods: A mini review. Int. Nano Lett. 2022, 12, 153–168. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today: Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, S.; Zhong, L.; Zhao, X.; Wang, L. Effects of zinc oxide nanoparticles on growth, development, and flavonoid synthesis in Ginkgo biloba. Int. J. Mol. Sci. 2023, 24, 15775. [Google Scholar] [CrossRef]
- Khan, S.H.; Suriyaprabha, R.; Pathak, B.; Fulekar, M.H. Development of zinc oxide nanoparticle by sonochemical method and study of their physical and optical properties. In AIP Conference Proceedings. International Conference on Mathematics, Engineering and Computer Science, Kuala Lumpur, Malaysia, 26 February 2016; Publishing LLC: Melville, NY, USA, 2016; Volume 1724. [Google Scholar] [CrossRef]
- Chen, H.; Song, Y.; Wang, Y.; Wang, H.; Ding, Z.; Fan, K. Zno nanoparticles: Improving photosynthesis, shoot development, and phyllosphere microbiome composition in tea plants. J. Nanobiotechnol. 2024, 22, 1–28. [Google Scholar] [CrossRef]
- Gupta, A.; Bharati, R.; Kubes, J.; Popelkova, D.; Praus, L.; Yang, X.; Severova, L.; Skalicky, M.; Brestic, M. Zinc oxide nanoparticles application alleviates salinity stress by modulating plant growth, biochemical attributes and nutrient homeostasis in Phaseolus vulgaris L. Front. Plant Sci. 2024, 15, 1432258. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Ayesh, A.S.; Abdel-Rahem, R.A. Optical and electrical properties of polycarbonate/MnCl2 composite films. J. Plast. Film Sheeting 2008, 24, 109–124. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Abd El-Mageed, T.A.; Babalghith, A.O.; Selim, S.; Mohamed, A.M. Green synthesis and characterization of ZnO nanoparticles using Pelargonium odoratissimum (L.) aqueous leaf extract and their antioxidant, antibacterial and anti-inflammatory activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef]
- Hoseinpour, V.; Souri, M.; Ghaemi, N.; Shakeri, A. Optimization of green synthesis of ZnO nanoparticles by Dittrichia graveolens (L.) aqueous extract. Health Biotechnol. Biopharma. 2017, 1, 39–49. [Google Scholar]
- Duong, T.B.N.; Pham, P.-Q.; Tran, A.T.; Bui, D.T.; Pham, A.T.T.; Nguyen, T.C.T.; Nguyen, L.H.T.; Ung, T.D.T.; Hoang, N.V.; Pham, N.K. Correlation between organic residuals of green synthesized nanoparticles and resistive switching behavior. RSC Adv. 2024, 14, 36340–36350. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Musa, I.; Qamhieh, N. Study of optical energy gap and quantum confinment effects in Zinc Oxide nanoparticles and nanorods. Dig. J. Nanomater. Biostruct. 2019, 14, 119–125. [Google Scholar]
- Dhandapani, K.V.; Anbumani, D.; Gandhi, A.D.; Annamalai, P.; Muthuvenkatachalam, B.S.; Kavitha, P.; Ranganathan, B. Green route for the synthesis of zinc oxide nanoparticles from Melia azedarach leaf extract and evaluation of their antioxidant and antibacterial activities. Biocatal. Agric. Biotechnol. 2020, 24, 101517. [Google Scholar] [CrossRef]
- Ekanayake, S.A.; Godakumbura, P.I. Synthesis of a Dual-Functional Nanofertilizer by Embedding ZnO and CuO Nanoparticles on an Alginate-Based Hydrogel. ACS Omega 2021, 6, 26262–26272. [Google Scholar] [CrossRef]
- Yuvaraj, M.; Subramanian, K.S.; Cyriac, J. Efficiency of zinc oxide nanoparticles as controlled release nanofertilizer for rice (Oryza sativa L). J. Plant Nutr. 2023, 46, 4477–4493. [Google Scholar] [CrossRef]
- Rodrigues, S.; Avellan, A.; Bland, G.D.; Miranda, M.C.R.; Larue, C.; Wagner, M.; Moreno-Bayona, D.A.; Castillo-Michel, H.; Lowry, G.V.; Rodrigues, S.M. Effect of a Zinc Phosphate Shell on the Uptake and Translocation of Foliarly Applied ZnO Nanoparticles in Pepper Plants (Capsicum annuum). Environ. Sci. Technol. 2024, 58, 3213–3223. [Google Scholar] [CrossRef]
- Deger, A.G.; Çevik, S.; Kahraman, O.; Turunc, E.; Yakin, A.; Binzet, R. Effects of green and chemically synthesized ZnO nanoparticles on Capsicum annuum under drought stress. Acta Physiol. Plant. 2025, 47, 1–17. [Google Scholar] [CrossRef]
- Aalami, Z.; Hoseinzadeh, M.; Manesh, P.H.; Aalami, A.H.; Es’HAghi, Z.; Darroudi, M.; Sahebkar, A.; Hosseini, H.A. Synthesis, characterization, and photocatalytic activities of green sol-gel ZnO nanoparticles using Abelmoschus esculentus and Salvia officinalis: A comparative study versus co-precipitation-synthesized nanoparticles. Heliyon 2024, 10, e24212. [Google Scholar] [CrossRef]
- Irede, E.L.; Awoyemi, R.F.; Owolabi, B.; Aworinde, O.R.; Kajola, R.O.; Hazeez, A.; Raji, A.A.; Ganiyu, L.O.; Onukwuli, C.O.; Onivefu, A.P.; et al. Cutting-edge developments in zinc oxide nanoparticles: Synthesis and applications for enhanced antimicrobial and UV protection in healthcare solutions. RSC Adv. 2024, 14, 20992–21034. [Google Scholar] [CrossRef] [PubMed]
- David, C.A.; Galceran, J.; Rey-Castro, C.; Puy, J.; Companys, E.; Salvador, J.; Monné, J.; Wallace, R.; Vakourov, A. Dissolution Kinetics and Solubility of ZnO Nanoparticles Followed by AGNES. J. Phys. Chem. C 2012, 116, 11758–11767. [Google Scholar] [CrossRef]
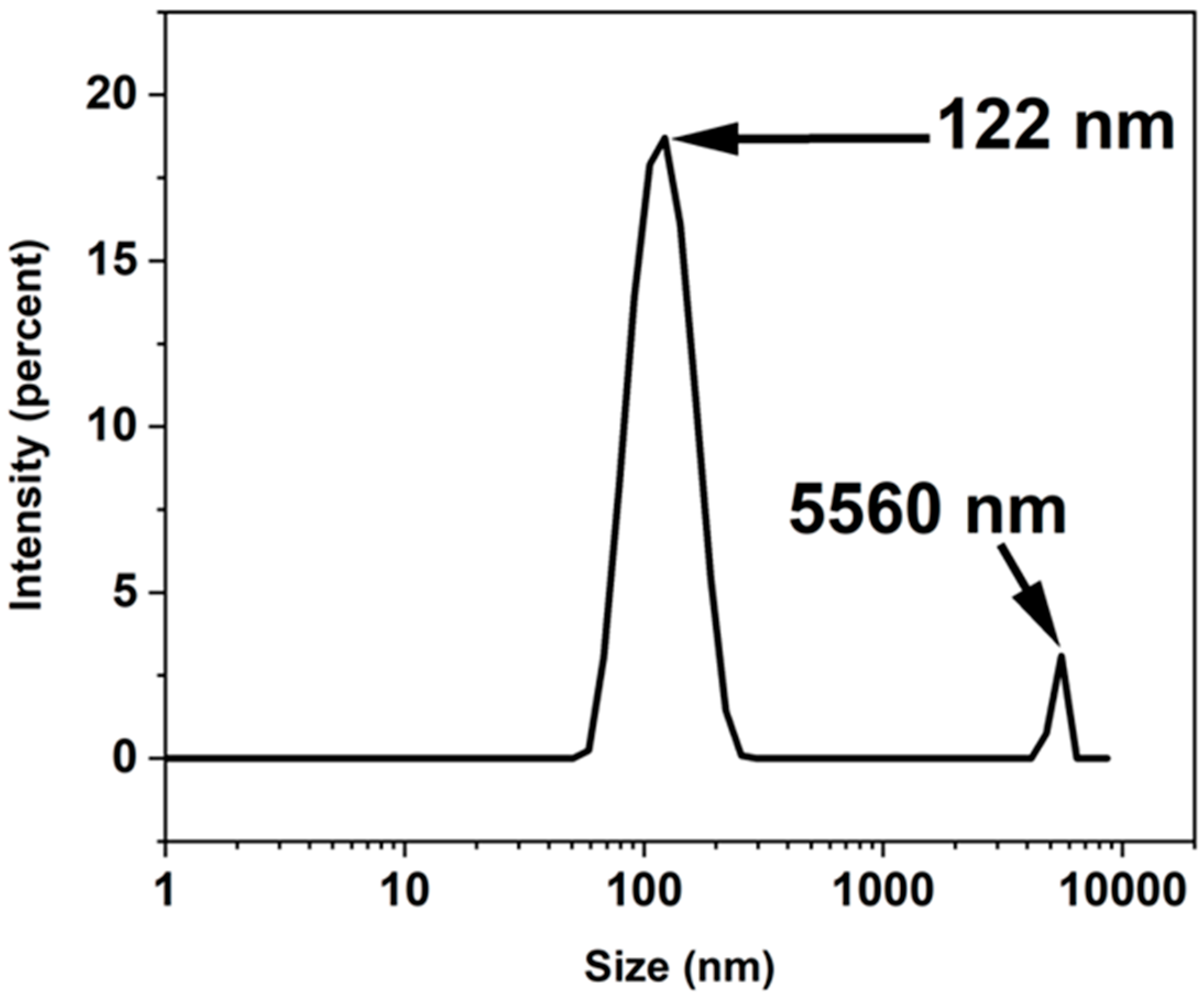
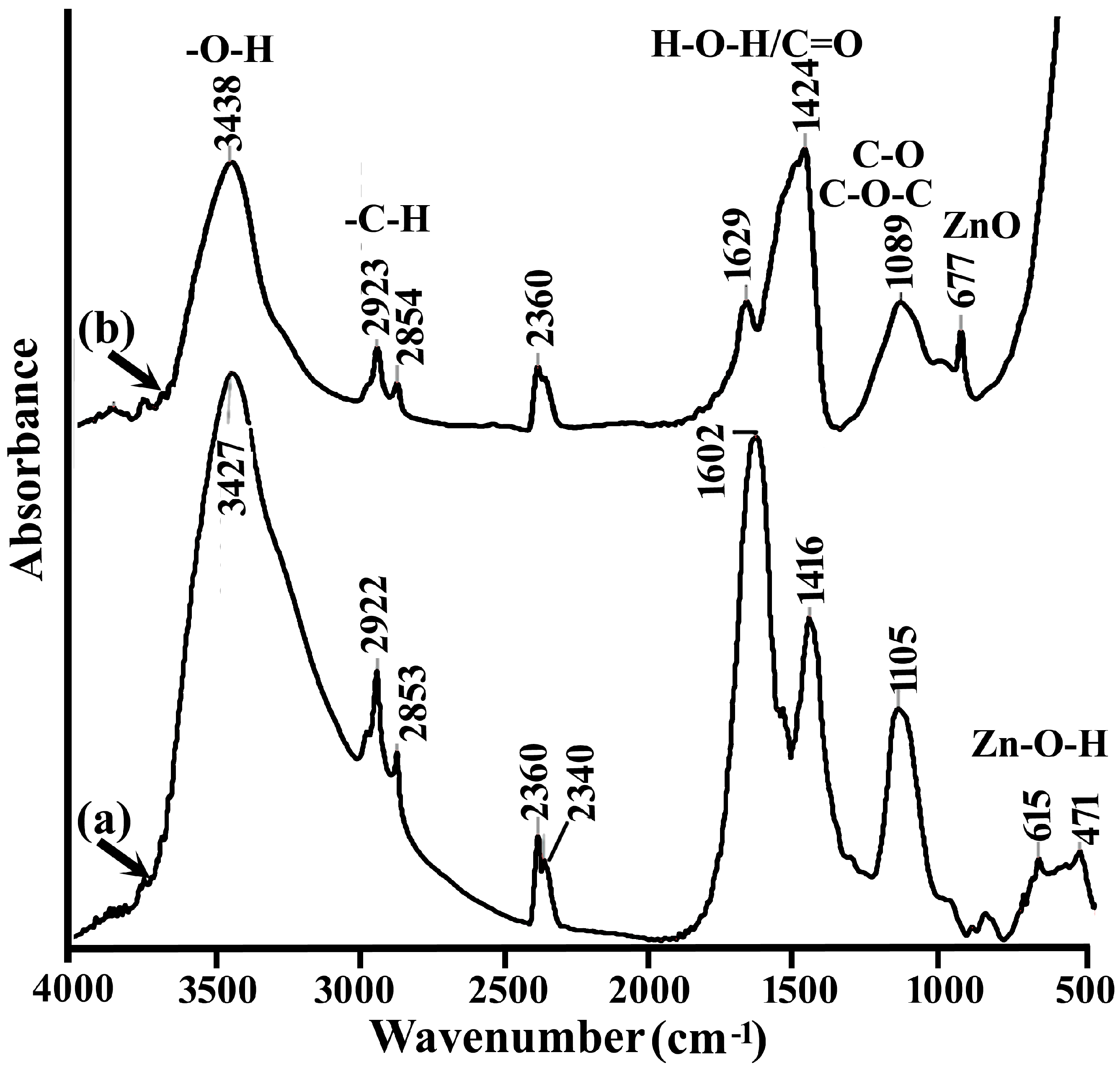
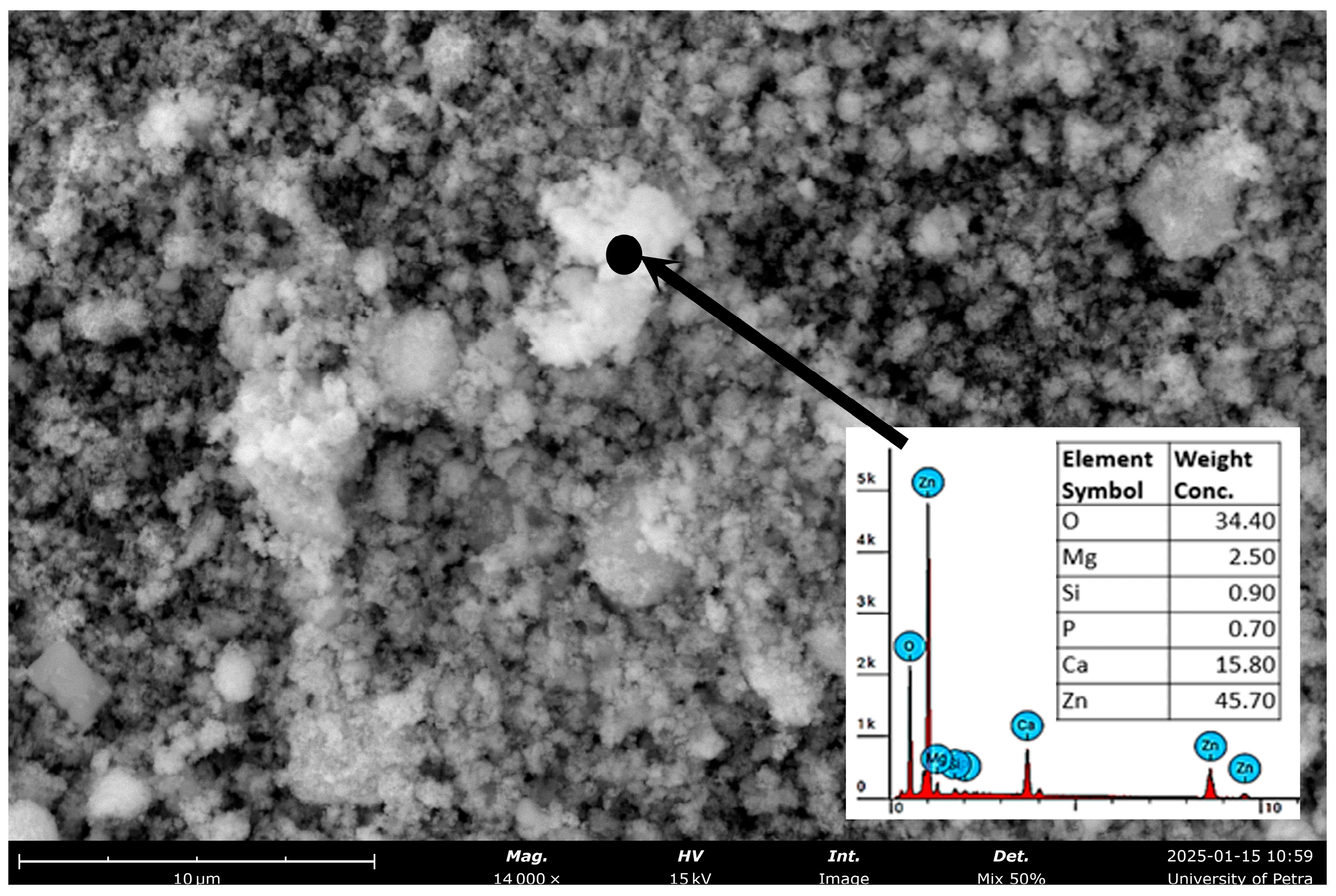
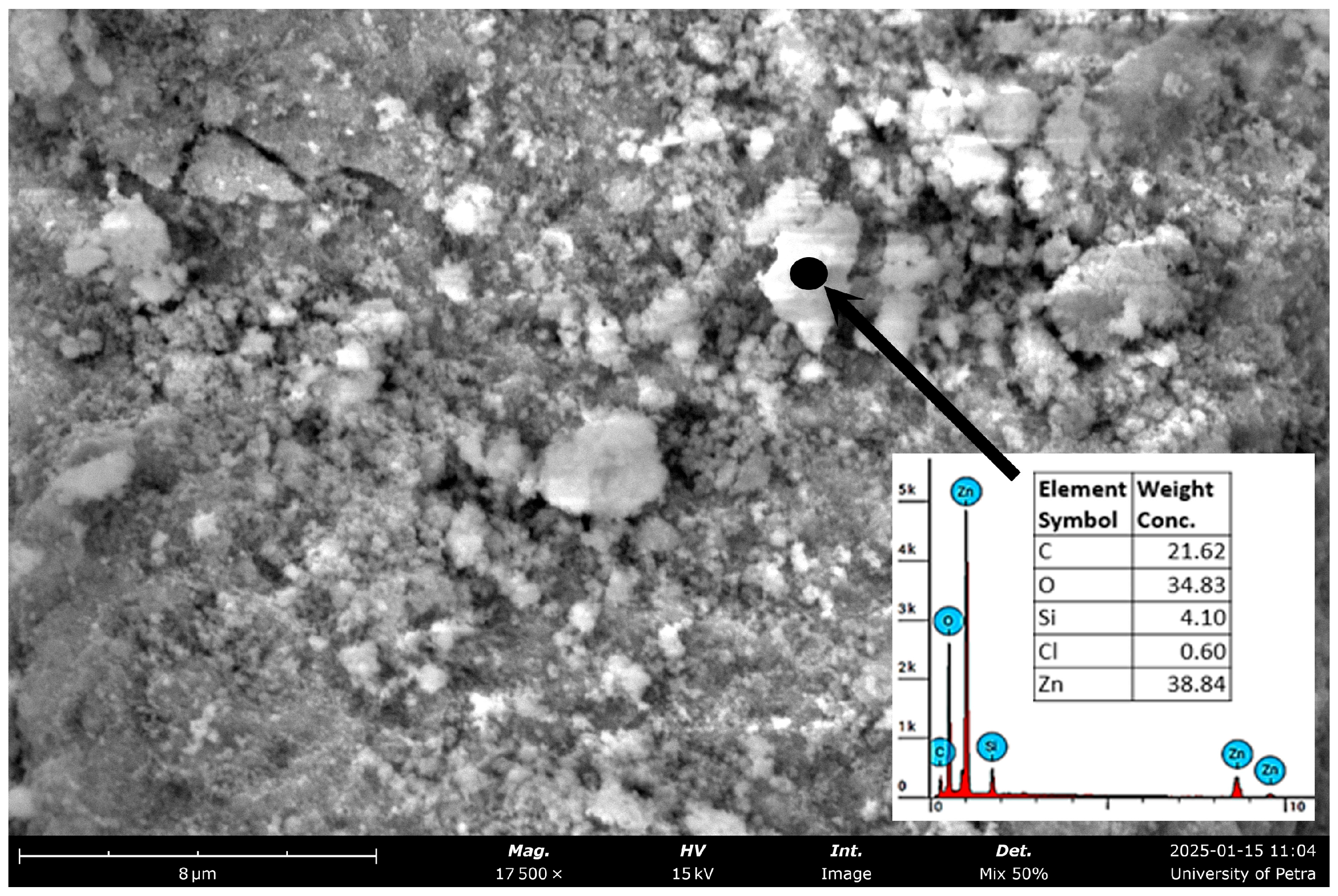
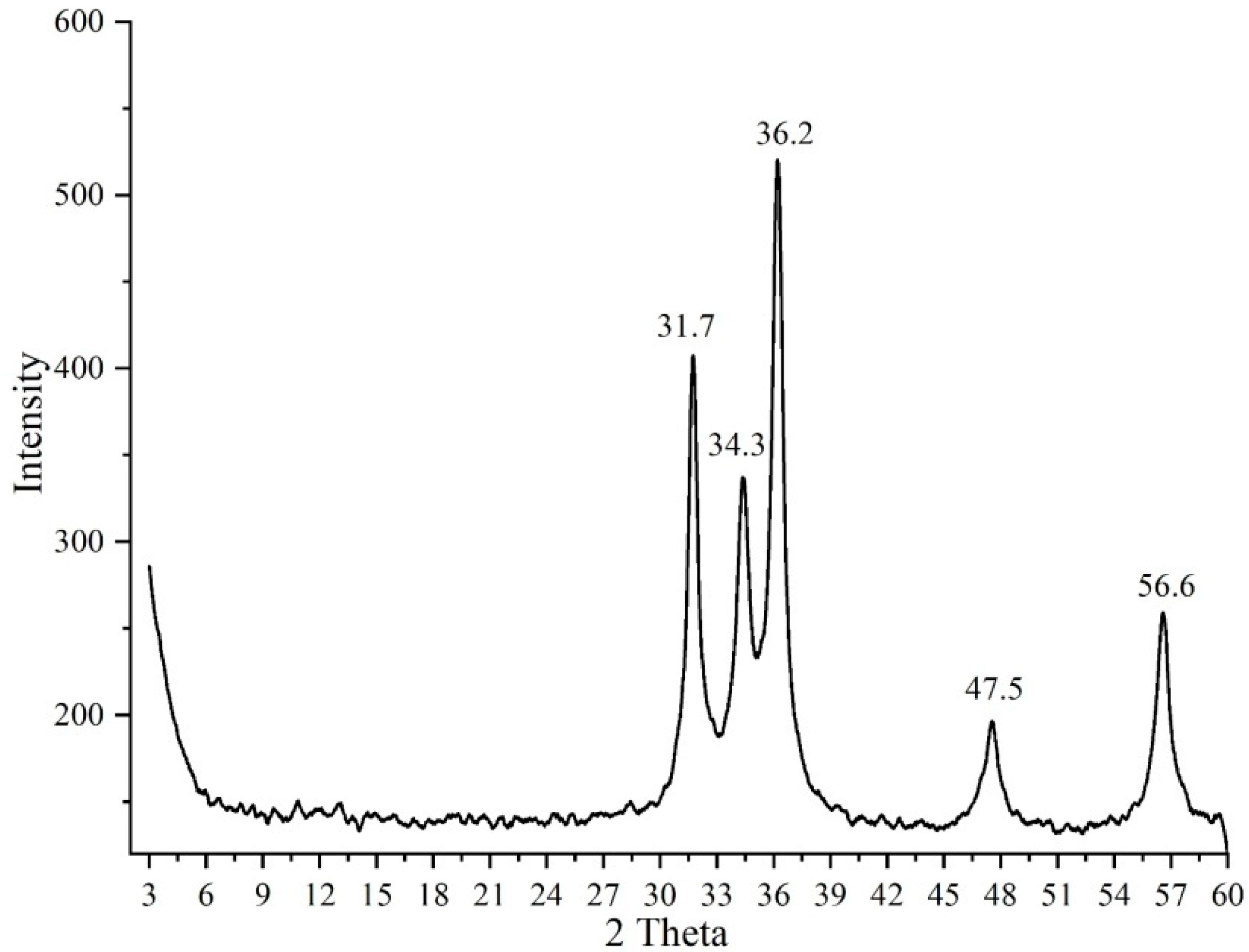
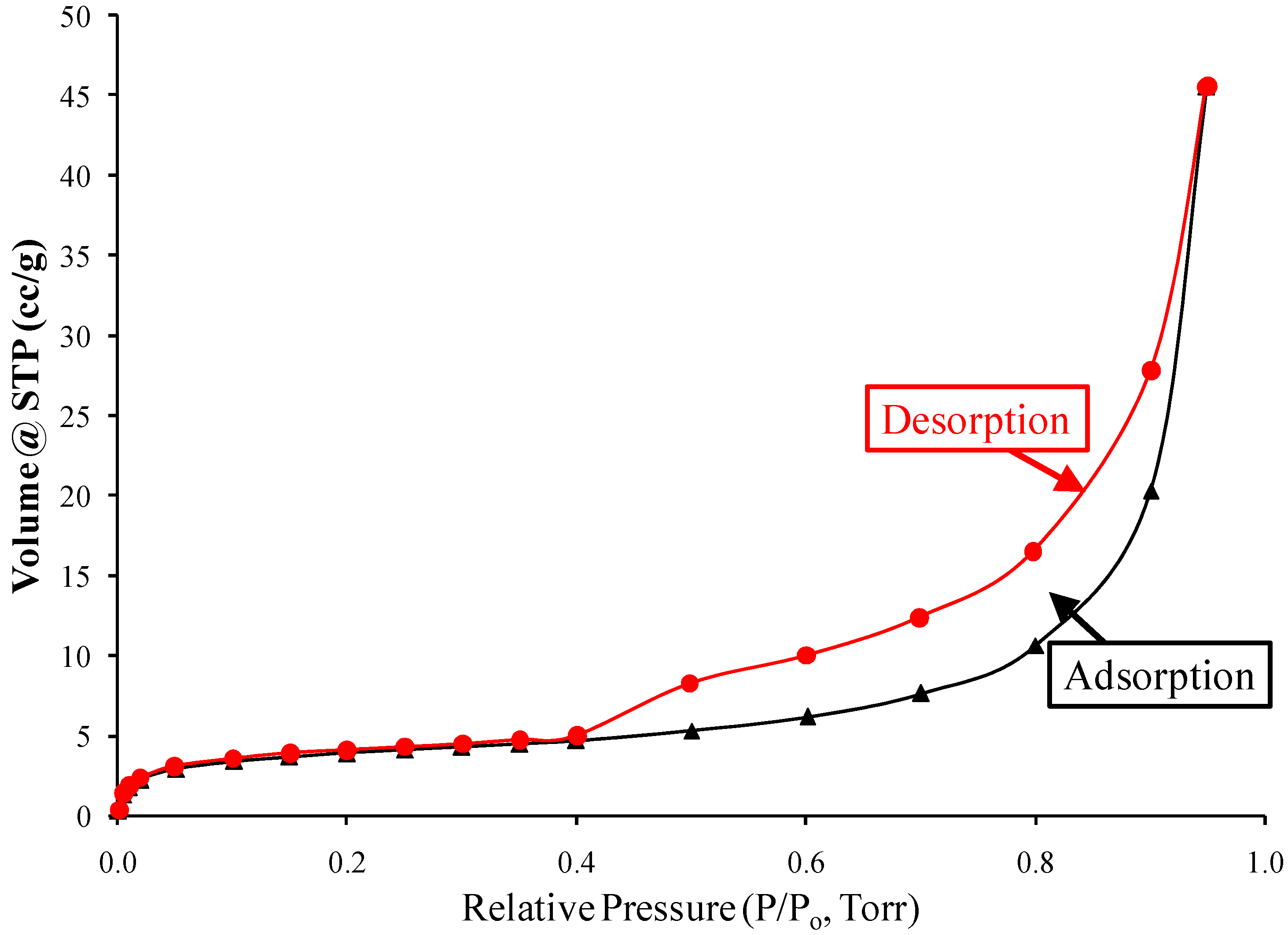

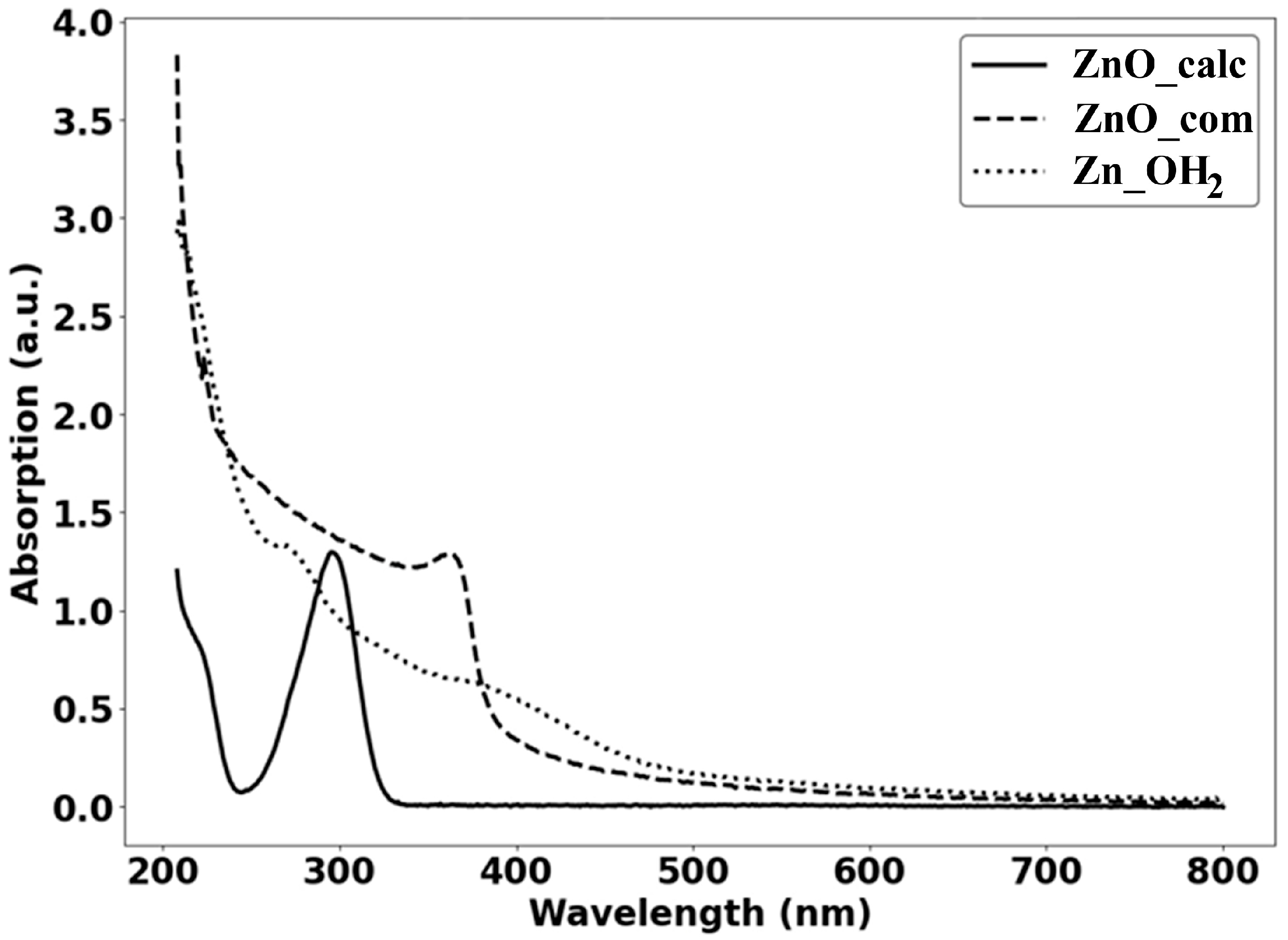
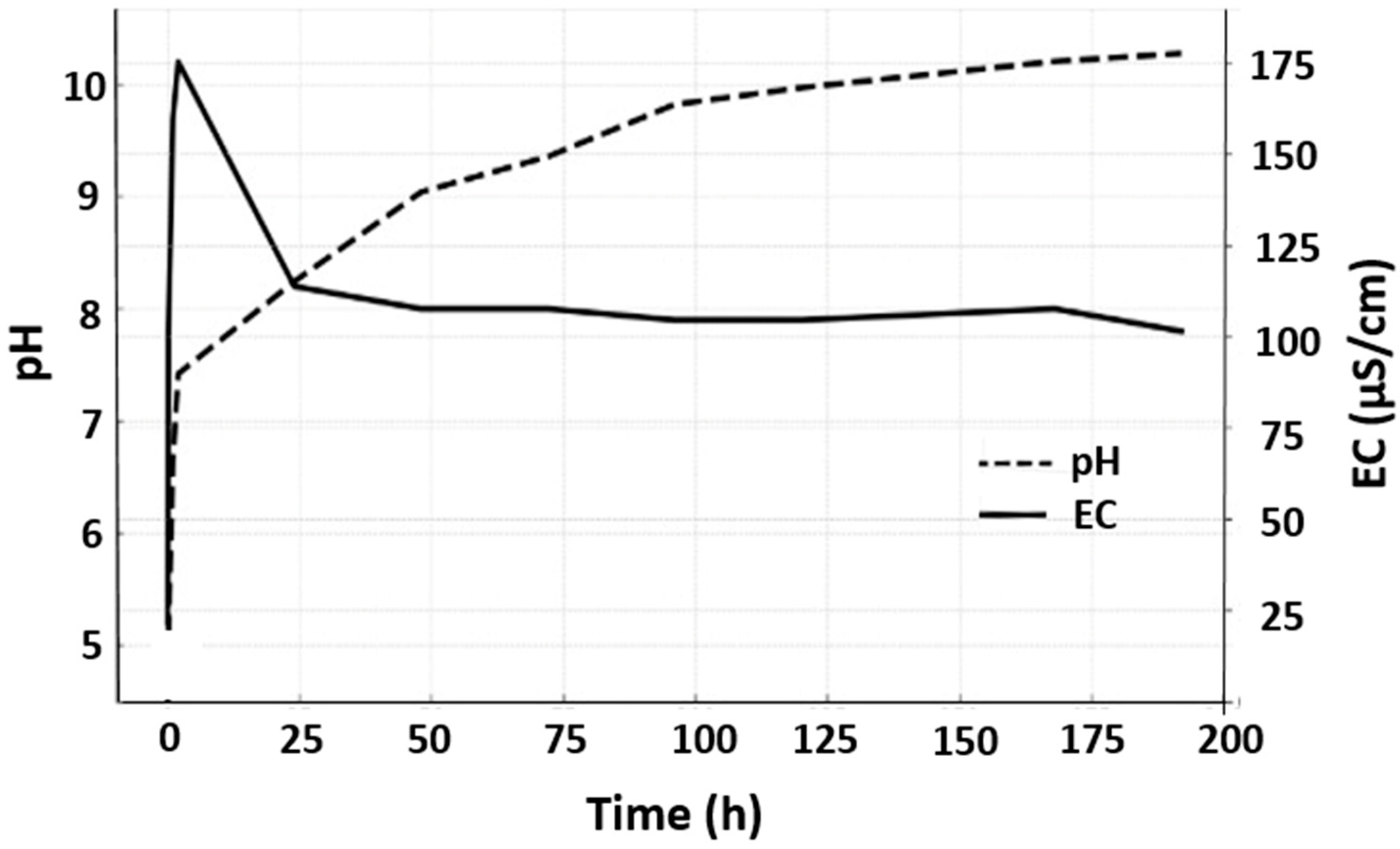
| Miller Index | Peak Position (2θ Deg) | FWHM | Crystallite Size D (nm) |
|---|---|---|---|
| (100) | 31.7 | 0.4456 | 18.5 |
| (002) | 34.4 | 0.8405 | 9.9 |
| (101) | 36.2 | 0.5012 | 16.7 |
| (102) | 47.5 | 0.8909 | 9.7 |
| (110) | 56.6 | 0.9489 | 9.5 |
| Synthesis Method | pH Behavior over Time | EC Behavior over Time | Zn2+ Release Characteristics | Ref. |
|---|---|---|---|---|
| Sol–gel | Gradually increases with time as dissolution proceeds | EC steadily increases over longer period | Moderate-to-high release rate is attributed to the porous structure, which increases surface reactivity and facilitates ion exchange. | [35] |
| Hydrothermal | Stable pH profile; minimal variation | Low and stable EC values | Exhibits the lowest Zn2+ release rate owing to its dense crystal structure and minimal surface defects that restrict dissolution. | [36] |
| Precipitation | Decreases slightly with time as Zn2+ hydrolyzes | Rapid EC rise initially, stabilizing after a few hours | Moderate release rate resulting from particle aggregation and the presence of residual ions that promote initial Zn2+ release. | [37] |
| Present Green synthesis study | Rapid early increase (peak ≈ 10 at ~few h) then stabilizes to mildly alkaline ~8–8.5 over long term | EC: sharp early rise, then gradual increase and plateau (~170 µS/cm by 200 h). EC correlates with ionic release. | Controlled and sustained Zn2+ release is attributed to phytochemical capping agents that decrease solubility and enable gradual dissolution. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Sharif, R.; Ayesh, A.S.; Esaifan, M.; Mazahrih, N.; Bani Hani, N.; Al Rjoub, B.; Rayya, E.; Abu Salem, M. Green-Synthesized Zinc Oxide Nanoparticles with Enhanced Release Behavior for Sustainable Agricultural Applications. Solids 2025, 6, 59. https://doi.org/10.3390/solids6040059
Al Sharif R, Ayesh AS, Esaifan M, Mazahrih N, Bani Hani N, Al Rjoub B, Rayya E, Abu Salem M. Green-Synthesized Zinc Oxide Nanoparticles with Enhanced Release Behavior for Sustainable Agricultural Applications. Solids. 2025; 6(4):59. https://doi.org/10.3390/solids6040059
Chicago/Turabian StyleAl Sharif, Riyad, Ayman S. Ayesh, Muayad Esaifan, Naem Mazahrih, Nabeel Bani Hani, Bayan Al Rjoub, Eva Rayya, and Majd Abu Salem. 2025. "Green-Synthesized Zinc Oxide Nanoparticles with Enhanced Release Behavior for Sustainable Agricultural Applications" Solids 6, no. 4: 59. https://doi.org/10.3390/solids6040059
APA StyleAl Sharif, R., Ayesh, A. S., Esaifan, M., Mazahrih, N., Bani Hani, N., Al Rjoub, B., Rayya, E., & Abu Salem, M. (2025). Green-Synthesized Zinc Oxide Nanoparticles with Enhanced Release Behavior for Sustainable Agricultural Applications. Solids, 6(4), 59. https://doi.org/10.3390/solids6040059










