DFT Study of Oxygen Ion Migration in Mg-Doped Cubic Zirconia
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Investigation of the Electronic Properties of Magnesium-Stabilized Cubic Zirconia
3.2. Investigation of Ionic Conductivity
3.2.1. Ionic Conductivity via Oxygen Vacancy-Mediated Oxygen Ion Migration
3.2.2. Ionic Conductivity of ZrO2 Stabilized with MgO Aligned Along the Migration Path
3.2.3. Ionic Conductivity of ZrO2 Stabilized with MgO with Spatially Distributed Mg Ions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DFT | Density functional theory |
| DOS | A function that describes the number of electronic states at each energy level that are available to be occupied in a system. |
| PDOS | A decomposition of the total DOS into contributions from specific atoms or orbitals helps to understand which atoms or orbitals contribute to particular energy levels. |
| B3LYP | A hybrid Density Functional Theory (DFT) functional that combines Becke’s three-parameter exchange functional with the Lee-Yang-Parr correlation functional. |
References
- Danilov, V.P.; Borisova, E.S.; Shukshin, V.E.; Runina, K.I.; Strekalov, P.V.; Mayakova, M.N.; Petrova, O.B. Structure and optical properties of ZrO2–Sc2O3 solid solution system obtained by the coprecipitation method. Glass Ceram. 2025, 81, 428–437. [Google Scholar] [CrossRef]
- Sathya, A.; Anburaj, D.B.; Porkalai, V.; Muthuvel, A.; Al-Zaqri, N. Hydrothermal synthesis of ZrO2 nanoparticles: Study on structural, optical, morphology properties, and photocatalyst activity. Phys. Solid State 2025, 67, 196–206. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, J.; Chen, X.; Wang, Z.; Zhao, Y. Oxygen defect-rich CeO2−x@ZrO2 Mott–Schottky electrocatalyst with tunable interfacial charge redistribution for accelerated sulfur redox kinetics in lithium–sulfur batteries. Chem. Eng. J. 2025, 512, 162371. [Google Scholar] [CrossRef]
- Jin, H.-B.; Zhang, Z.-H.; Ma, P.; Li, H.-B. Synergically enhancing lithium-ion storage performance of silicon anode by designing shelled structure with reduced graphene oxide and ZrO2. Rare Met. 2025, 44, 2393–2404. [Google Scholar] [CrossRef]
- Jammee, R.; Kolganov, A.; Groves, M.C.; Pidko, E.A.; Sydora, O.L.; Conley, M.P. C–H bond activation by sulfated zirconium oxide is mediated by a sulfur-centered Lewis superacid. Angew. Chem. Int. Ed. 2024, 63, e202421699. [Google Scholar] [CrossRef]
- Florez, J.; Diaz-Uribe, C.E.; Vallejo, W.; Duran, F.; Puello, E.; Salazar, J.; Zarate, X.; Schott, E. Study of methylene blue removal and photocatalytic degradation on zirconia thin films modified with Mn-Anderson polyoxometalates. Dalton Trans. 2025, 54, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Lin, L.; Cen, Z.; Ke, Y.; Xu, Q.; Zhu, J.; Mei, X.; Xia, Z.; Zheng, X.; Wang, Y.; et al. Production of branched alkanes by upcycling of waste polyethylene over controlled acid sites of SO4/ZrO2–Al2O3 catalyst. Angew. Chem. Int. Ed. 2025, 64, e202417923. [Google Scholar] [CrossRef]
- Huang, Q.A.; Cao, Y.; Satou, K.; Murayama, H.; Yoshizawa, A.; Yamamoto, E.; Nakayama, A.; Ishida, T.; Kitagawa, Y.; Ishimaru, Y.; et al. Active and stable Au/ZrO2 catalysts for isomerization of allylic esters: A practical application of heterogeneous gold catalysis. Appl. Catal. B Environ. Energy 2025, 373, 125351. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, T.; Wang, H.; Zhang, Z.; Yin, H.; Wang, S.; Waterhouse, G.I.N.; Wang, X.; Ma, C.; Li, P. Synergistic Adsorption-Catalysis Regulation Effect for Carbon Dioxide Over Hierarchically-Structured Cu/Zro2 Nts; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Luo, J.; Yao, C.; Zhou, Y.; Qian, Y.; Wang, L.; Lu, C.; Li, X. Facile Oxygen Functionalization Strategy for Mediating the Activity–Stability Trade-Off for Metal Encapsulated Catalysts. Surf. Interfaces 2025, 72, 107209. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Dong, M.; Wang, J.; Fan, W. Effect of metal-support interaction on catalytic performance of Pd/ZrOx in CO2 hydrogenation to formate. Chem. Res. Chin. Univ. 2025, 41, 529–538. [Google Scholar]
- Lee, S.H.; Lee, T.Y.; Lim, H.H.; Cho, J.W.; An, C.; Chae, S.C. Symmetry engineering in antiferroelectric ZrO2 thin films via split-up behavior. ACS Appl. Electron. Mater. 2025, 7, 2146–2152. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Song, X.-F.; Li, C.; Zhu, H.-F. The influence of Ce element doping on the mechanical properties of ZrO2 ceramic from first-principles calculations. Solid State Ion. 2025, 424, 116865. [Google Scholar] [CrossRef]
- Yuan, J.; Dai, J.-Q.; Zhao, M.-W.; Zhong, Y.-Y.; Deng, D.-W. First-principles study on thermodynamic stability and electronic structures of the ferroelectric binary HfO2 and ZrO2 (001) polar surfaces. Surf. Interfaces 2025, 42, 105523. [Google Scholar] [CrossRef]
- Kadyrzhanov, K.K.; Kozlovskiy, A.A.; Konuhova, M.; Popov, A.I.; Shlimas, D.D.; Borgekov, D.B. Determination of gamma radiation shielding efficiency by radiation-resistant composite ZrO2–Al2O3–TiO2–WO3–Nb2O5 ceramics. Opt. Mater. 2024, 154, 115752. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Konuhova, M.; Shlimas, D.I.; Borgekov, D.B.; Zdorovets, M.V.; Shakirziyanov, R.I.; Garanin, Y.A.; Volodina, N.O.; Popov, A.I. Study of the effect of nanostructured grains on the radiation resistance of zirconium dioxide ceramics during gas swelling under high-dose irradiation with helium ions. ES Mater. Manuf. 2024, 24, 1165. [Google Scholar] [CrossRef]
- Kenzhina, I.E.; Kozlovskiy, A.L.; Begentayev, M.; Blynskiy, P.; Tolenova, A.; Popov, A.I. Study of phase transformations in ZrO2 ceramics stabilized by Y2O3 and their role in changing strength characteristics and heat resistance. Sustainability 2025, 17, 4284. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Konuhova, M.; Borgekov, D.B. Study of irradiation temperature effect on radiation-induced polymorphic transformation mechanisms in ZrO2 ceramics. Opt. Mater. 2024, 156, 115994. [Google Scholar] [CrossRef]
- Imanova, G. Modeling defect formation in nano-ZrO2 under He and H+ irradiation. Mod. Phys. Lett. B 2024, 38, 2450206. [Google Scholar] [CrossRef]
- Costantini, J.M.; Gutierrez, G.; Lelong, G.; Guillaumet, M.; Rahman, M.M.; Yasuda, K. Raman spectroscopy study of damage in swift heavy ion-irradiated ceramics. J. Raman Spectrosc. 2022, 53, 1614–1624. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Shen, T.; Chai, J.; Niu, L.; Li, S.; Jin, P.; Zheng, H.; Wang, Z. Irradiation response of Al2O3–ZrO2 ceramic composite under He ion irradiation. J. Eur. Ceram. Soc. 2021, 41, 2883–2891. [Google Scholar] [CrossRef]
- Dauletbekova, A.; Zvonarev, S.; Nikiforov, S.; Akilbekov, A.; Shtang, T.; Karavannova, N.; Akylbekova, A.; Ishchenko, A.; Akhmetova-Abdik, G.; Baymukhanov, Z.; et al. Luminescence properties of ZrO2:Ti ceramics irradiated with electrons and high-energy Xe ions. Materials 2024, 17, 1307. [Google Scholar] [CrossRef]
- Bandarenka, H.; Burko, A.; Laputsko, D.; Dronina, L.; Kovalchuk, N.; Podelinska, A.; Shapel, U.; Popov, A.I.; Bocharov, D. Ultraviolet exposure improves SERS activity of graphene-coated Ag/ZrO2 substrates. Crystals 2023, 13, 1570. [Google Scholar] [CrossRef]
- Nikiforov, S.V.; Kortov, V.S.; Kiryakov, A.N.; Konev, S.F.; Men’shenina, A.A. Increasing the luminescence yield of zirconia. Tech. Phys. Lett. 2017, 43, 1074–1076. [Google Scholar] [CrossRef]
- Swami, S.K.; Khan, J.I.; Dutta, V.; Lee, J.; Laquai, F.; Chaturvedi, N. Spray-deposited aluminum-doped zinc oxide as an efficient electron transport layer for inverted organic solar cells. ACS Appl. Energy Mater. 2023, 6, 2906–2913. [Google Scholar] [CrossRef]
- Kate, R.S.; Deokate, R.J. Effect of cobalt doping on electrochemical properties of sprayed nickel oxide thin films. Mater. Sci. Energy Technol. 2020, 3, 830–839. [Google Scholar] [CrossRef]
- Ricca, C.; Ringuedé, A.; Cassir, M.; Adamo, C.; Labat, F. A comprehensive DFT investigation of bulk and low-index surfaces of ZrO2 polymorphs. J. Comput. Chem. 2015, 36, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Benali, A.; Luo, Y.; Crabb, E.; Lopez-Bezanilla, A.; Ratcliff, L.E.; Jokisaari, A.M.; Heinonen, O. Zirconia and hafnia polymorphs: Ground-state structural properties from diffusion Monte Carlo. Phys. Rev. Mater. 2018, 2, 075001. [Google Scholar] [CrossRef]
- Rajesh, G.; Akilandeswari, S.; Govindarajan, D.; Thirumalai, K. Enhancement of photocatalytic activity of ZrO2 nanoparticles by doping with Mg for UV light photocatalytic degradation of methyl violet and methyl blue dyes. J. Mater. Sci. Mater. Electron. 2020, 31, 4058–4072. [Google Scholar] [CrossRef]
- Marfin, A.Y.; Nikiforov, S.V.; Ananchenko, D.V.; Zyryanov, S.S.; Yakovlev, G.A.; Denisov, E.I. Thermoluminescence of monoclinic ZrO2 after electron irradiation. AIP Conf. Proc. 2022, 2466, 030012. [Google Scholar]
- Sredojević, D.; Lazić, V.; Pirković, A.; Periša, J.; Murafa, N.; Spremo-Potparević, B.; Živković, L.; Topalović, D.; Zarubica, A.; Krivokuća, M.J.; et al. Toxicity of silver nanoparticles supported by surface-modified zirconium dioxide with dihydroquercetin. Nanomaterials 2022, 12, 3195. [Google Scholar] [CrossRef]
- Osinkin, D.A.; Antonova, E.P.; Lesnichyova, A.S.; Tropin, E.S.; Chernov, M.E.; Chernov, E.I.; Farlenkov, A.S.; Khodimchuk, A.V.; Eremin, V.A.; Kovrova, A.I.; et al. Application of promising electrode materials in contact with a thin-layer ZrO2-based supporting electrolyte for solid oxide fuel cells. Energies 2020, 13, 1190. [Google Scholar] [CrossRef]
- Qi, S.; Porotnikova, N.M.; Ananyev, M.V.; Kuzmin, A.V.; Eremin, V.A.; Pankratov, A.A.; Molchanova, N.G.; Reznitskikh, O.G.; Farlenkov, A.S.; Vovkotrub, E.G.; et al. High-temperature glassy-ceramic sealants SiO2–Al2O3–BaO–MgO and SiO2–Al2O3–ZrO2–CaO–Na2O for solid oxide electrochemical devices. Trans. Nonferrous Met. Soc. China 2016, 26, 2916–2924. [Google Scholar] [CrossRef]
- Farlenkov, A.S.; Ananyev, M.V.; Eremin, V.A.; Porotnikova, N.M.; Kurumchin, E.K. Particle coarsening influence on oxygen reduction in LSM–YSZ composite materials. Fuel Cells 2015, 15, 131–139. [Google Scholar]
- Nikiforov, S.; Dauletbekova, A.; Gerasimov, M.; Kasatkina, Y.; Denisova, O.; Lisitsyn, V.; Golkovski, M.; Akylbekova, A.; Bazarbek, A.-D.; Akilbekov, A.; et al. Thermoluminescent and dosimetric properties of zirconium dioxide ceramics irradiated with high doses of pulsed electron beam. Crystals 2023, 13, 1585. [Google Scholar] [CrossRef]
- Ananchenko, D.V.; Nikiforov, S.V.; Sobyanin, K.V.; Konev, S.F.; Dauletbekova, A.K.; Akhmetova-Abdik, G.; Akilbekov, A.T.; Popov, A.I. Paramagnetic defects and thermoluminescence in irradiated nanostructured monoclinic zirconium dioxide. Materials 2022, 15, 8624. [Google Scholar] [CrossRef]
- Borik, M.A.; Bredikhin, S.I.; Bublik, V.T.; Kulebyakin, A.V.; Kuritsyna, I.E.; Lomonova, E.E.; Milovich, P.O.; Myzina, V.A.; Osiko, V.V.; Ryabochkina, P.A.; et al. Structure and conductivity of yttria- and scandia-doped zirconia crystals grown by skull melting. J. Am. Ceram. Soc. 2017, 100, 5536–5547. [Google Scholar] [CrossRef]
- Gayathri, P.; Balasubramani, V.; Balraju, P.; Sayed, M.A.; Shkir, M. Ultra-high photosensitivity response in MIS SBDs enabled by Zn-integrated ZrO2@Zn interfacial layers for photovoltaic devices. Phys. B Condens. Matter 2025, 714, 417506. [Google Scholar]
- Aboraia, A.M.; Sharaf, I.M.; Alradaddi, S.; Trabelsi, A.B.G.; Alkallas, F.H. Advanced supercapacitors benefit from the electrode material Gd-Enhanced Cubic-ZrO2. Phys. B Condens. Matter 2025, 714, 417519. [Google Scholar]
- Cheng, Z.; Ren, H.; Wang, Y.; Ta, S.; Zhang, P.; Yang, Y.; Xu, S.; Goodman, B.A.; Deng, W. Effects of Yb2O3 as stabilizer and sensitizer on the luminescence properties of cubic ZrO2 single crystals. Cryst. Growth Des. 2022, 22, 5481–5488. [Google Scholar]
- Thammachart, M.; Meeyoo, V.; Risksomboon, T.; Osuwan, S. Catalytic activity of CeO2–ZrO2 mixed oxide catalysts prepared via sol–gel technique: CO oxidation. Catal. Today 2001, 68, 53–61. [Google Scholar] [CrossRef]
- Li, P.; Chen, I.-W.; Penner-Hahn, J.E. Effect of dopants on zirconia stabilization—An X-ray absorption study: II, tetravalent dopants. J. Am. Ceram. Soc. 1994, 77, 1281–1288. [Google Scholar] [CrossRef]
- Winczewski, J.P.; Zeiler, S.; Gabel, S.; Maestre, D.; Merle, B.; Gardeniers, J.; Arce, A.S. Additive manufacturing of 3D yttria-stabilized zirconia microarchitectures. Mater. Des. 2024, 238, 112701. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, J.; Chi, Q.; Li, W.; Yu, Y.; Fei, W. Recent advances in design and applications of heteroatom-doped carbon with single-atom active sites. Chem. Rev. 2020, 120, 1710–1766. [Google Scholar] [CrossRef]
- Haering, C.; Roosen, A.; Schichl, H.; Schnöller, M. Mechanical properties of tape-cast electrolyte substrates for solid oxide fuel cells. Solid State Ion. 2005, 176, 261–268. [Google Scholar] [CrossRef]
- King, A.; Singh, R.; Anand, R.; Behera, S.K.; Nayak, B.B. Phase and luminescence behaviour of Ce-doped zirconia nanopowders for latent fingerprint visualisation. Optik 2021, 242, 167087. [Google Scholar] [CrossRef]
- Xie, Y.; Ma, Z.; Liu, L.; Su, Y.; Zhao, H.; Liu, Y.; Zhang, Z.; Duan, H.; Li, J.; Xie, E. Enhanced photocatalytic activity in ZrO2–TiO2 nanocomposite films deposited by magnetron sputtering. Appl. Phys. Lett. 2010, 97, 141916. [Google Scholar] [CrossRef]
- Furasova, A.D.; Ivanovski, V.; Yakovlev, A.V.; Milichko, V.A.; Vinogradov, V.V.; Vinogradov, A.V. Inkjet fabrication of highly efficient luminescent Eu-doped ZrO2 nanostructures. Nanoscale 2017, 9, 13069–13078. [Google Scholar] [CrossRef] [PubMed]
- Winczewski, J.; Herrera, M.; Cabriel, C.; Izeddin, I.; Gabel, S.; Merle, B.; Arce, A.S.; Gardeniers, H. Additive manufacturing of 3D luminescent ZrO2:Eu3+ architectures. Adv. Opt. Mater. 2022, 10, 2102758. [Google Scholar] [CrossRef]
- Kurakhmedov, A.E.; Morzabayev, A.K.; Tleubay, I.; Berguzinov, A.; Kozlovskiy, A.L. Study of the mechanisms of polymorphic transformations in zirconium dioxide upon doping with magnesium oxide, as well as establishing the relationship between structural changes and strength properties. Ceramics 2023, 6, 1164–1178. [Google Scholar] [CrossRef]
- Malyi, O.I.; Wu, P.; Kulish, V.V.; Bai, K.; Chen, Z. Formation and migration of oxygen and zirconium vacancies in cubic zirconia and zirconium oxysulfide. Solid State Ion. 2012, 212, 117–122. [Google Scholar] [CrossRef]
- Kurakhmedov, A.E.; Alin, M.; Temir, A.M.; Ivanov, I.A.; Bikhert, Y.V.; Ungarbayev, Y.O.; Zdorovets, M.V.; Kozlovskiy, A.L. Study of the effect of doping ZrO2 ceramics with MgO to increase the resistance to polymorphic transformations under the action of irradiation. Nanomaterials 2021, 11, 3172. [Google Scholar] [CrossRef]
- Perevalov, T.V.; Islamov, D.R. Oxygen polyvacancies as conductive filament in zirconia: First principle simulation. ECS Trans. 2017, 80, 357–362. [Google Scholar] [CrossRef]
- Xue, Q.; Huang, X.; Wang, L.; Zhang, H.; Zhang, J. Computational and experimental investigations of defect interaction and ionic conductivity in doped zirconia. Phys. Rev. Appl. 2018, 10, 014032. [Google Scholar] [CrossRef]
- Dhingra, A.; Thakur, O.P.; Pandey, R. Structure–property relationship and electronic structure calculation of cubic YSZ solid electrolyte for electrochemical applications. J. Electron. Mater. 2025, 54, 1949–1960. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Erba, A.; Desmarais, J.K.; Casassa, S.; Civalleri, B.; Dona, L.; Bush, I.J.; Searle, B.; Maschio, L.; Edith-Dazr, L.; Cossard, A.; et al. CRYSTAL23: A program for computational solid-state physics and chemistry. J. Chem. Theory Comput. 2022, 19, 6891–6932. [Google Scholar] [CrossRef]
- Muhammad, I.D.; Awang, M. Modelling the interatomic potential of cubic zirconia. Appl. Mech. Mater. 2013, 446–447, 151–157. [Google Scholar] [CrossRef]
- French, R.H.; Glass, S.J.; Ohuchi, F.S.; Xu, Y.-N.; Ching, W.Y. Experimental and theoretical determination of the electronic structure and optical properties of three phases of ZrO2. Phys. Rev. B 1994, 49, 5133–5142. [Google Scholar] [CrossRef] [PubMed]
- Kröger, F.A. The Chemistry of Imperfect Crystals, 2nd ed.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1974; Volume 2. [Google Scholar]
- Li, W.; Sunarso, J.; Yang, Y.; Chen, Y.; Ge, C.; Wang, W.; Guo, Y.; Ran, R.; Zhou, W. Strategies for improving oxygen ionic conducting in perovskite oxides and their practical applications. Energy Rev. 2024, 3, 100085. [Google Scholar] [CrossRef]
- Rauf, S.; Hanif, M.B.; Mushtaq, N.; Tayyab, Z.; Ali, N.; Shah, M.Y.; Javed, Q.; Rasool, K.; Khan, M.A.; Xu, W. Modulating the energy band structure of the Mg-doped Sr0.5Pr0.5Fe0.2Mg0.2Ti0.6O3−δ electrolyte with boosted ionic conductivity and electrochemical performance for solid oxide fuel cells. ACS Appl. Mater. Interfaces 2022, 14, 43067–43084. [Google Scholar] [CrossRef] [PubMed]
- Kulish, V.V.; Malyi, O.I.; Persson, C.; Wu, P. Evaluation of phosphorene as anode material for Na-ion batteries: A first-principles study. Phys. Chem. Chem. Phys. 2015, 17, 13921–13928. [Google Scholar] [CrossRef]
- Hinuma, Y.; Graciani, J.; Stacchiola, D.; Senanayake, S.D.; Rodríguez, J.A.; Ganduglia-Pirovano, M.V. Density functional theory calculations of oxygen vacancy formation and subsequent molecular adsorption on zirconia surfaces. J. Phys. Chem. C 2018, 122, 1687–1696. [Google Scholar] [CrossRef]
- Han, Z.-K.; Liu, W.; Gao, Y. Advancing the understanding of oxygen vacancies in ceria: Insights into their formation, behavior, and catalytic roles. JACS Au 2025, 5, 1549–1569. [Google Scholar] [CrossRef]
- Emery, A.A.; Wolverton, C. High-throughput DFT calculations of formation energy, stability, and oxygen vacancy formation energy of ABO3 perovskites. Sci. Data 2017, 4, 160124. [Google Scholar] [CrossRef]
- Kaptagay, G.A.; Satanova, B.M.; Abuova, A.U.; Konuhova, M.; Zakiyeva, Z.Y.; Tolegen, U.Z.; Koilyk, N.O.; Abuova, F.U. Effect of rhodium doping for photocatalytic activity of barium titanate. Opt. Mater. X 2025, 25, 100382. [Google Scholar] [CrossRef]
- Inerbaev, T.M.; Abuova, A.U.; Zakiyeva, Z.Y.; Abuova, F.U.; Mastrikov, Y.A.; Sokolov, M.; Gryaznov, D.; Kotomin, E.A. Effect of Rh doping on optical absorption and oxygen evolution reaction activity on BaTiO3 (001) surfaces. Molecules 2024, 29, 2707. [Google Scholar] [CrossRef]
- Abuova, A.U.; Mastrikov, Y.A.; Kotomin, E.A.; Kawazoe, Y.; Inerbaev, T.M.; Akilbekov, A.T. First-principles modeling of Ag adsorption on the LaMnO3 (001) surfaces. Solid State Ion. 2015, 273, 46–50. [Google Scholar] [CrossRef]
- Abuova, F.U.; Kotomin, E.A.; Lisitsyn, V.M.; Akilbekov, A.T.; Piskunov, S. Ab initio modeling of radiation damage in MgF2 crystals. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2014, 326, 314–317. [Google Scholar] [CrossRef]
- Dauletbekova, A.; Abuova, F.; Akilbekov, A.; Kotomin, E.; Piskunov, S. First-principles modeling of the H color centers in MgF2 crystals. Phys. Status Solidi C 2013, 10, 160–164. [Google Scholar] [CrossRef]
- Zhukovskii, Y.F.; Platonenko, A.; Piskunov, S.; Kotomin, E.A. Ab initio simulations on migration paths of interstitial oxygen in corundum. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2016, 374, 29–34. [Google Scholar] [CrossRef]
- Kaewmeechai, C.; Strand, J.; Shluger, A.L. Structure and migration mechanisms of oxygen interstitial defects in β-Ga2O3. Phys. Status Solidi B 2025, 263, 2400652. [Google Scholar] [CrossRef]
- Usseinov, A.B.; Akilbekov, A.T.; Kotomin, E.A.; Karipbayev, Z.T. First-principles calculations of CO2 adsorption on the (10-10) ZnO surface. AIP Conf. Proc. 2019, 2174, 020181. [Google Scholar] [CrossRef]
- Kilo, M.; Argirusis, C.; Borchardt, G.; Jackson, R.A. Oxygen diffusion in yttria-stabilised zirconia—Experimental results and molecular dynamics calculations. Phys. Chem. Chem. Phys. 2003, 5, 2219–2224. [Google Scholar] [CrossRef]
- Momenzadeh, L.; Belova, I.V.; Murch, G.E. Analysis of thermotransport and thermal and ionic conductivity in doped lanthanum gallate (LSGM) using molecular dynamics. Solid State Ion. 2022, 377, 115881. [Google Scholar] [CrossRef]
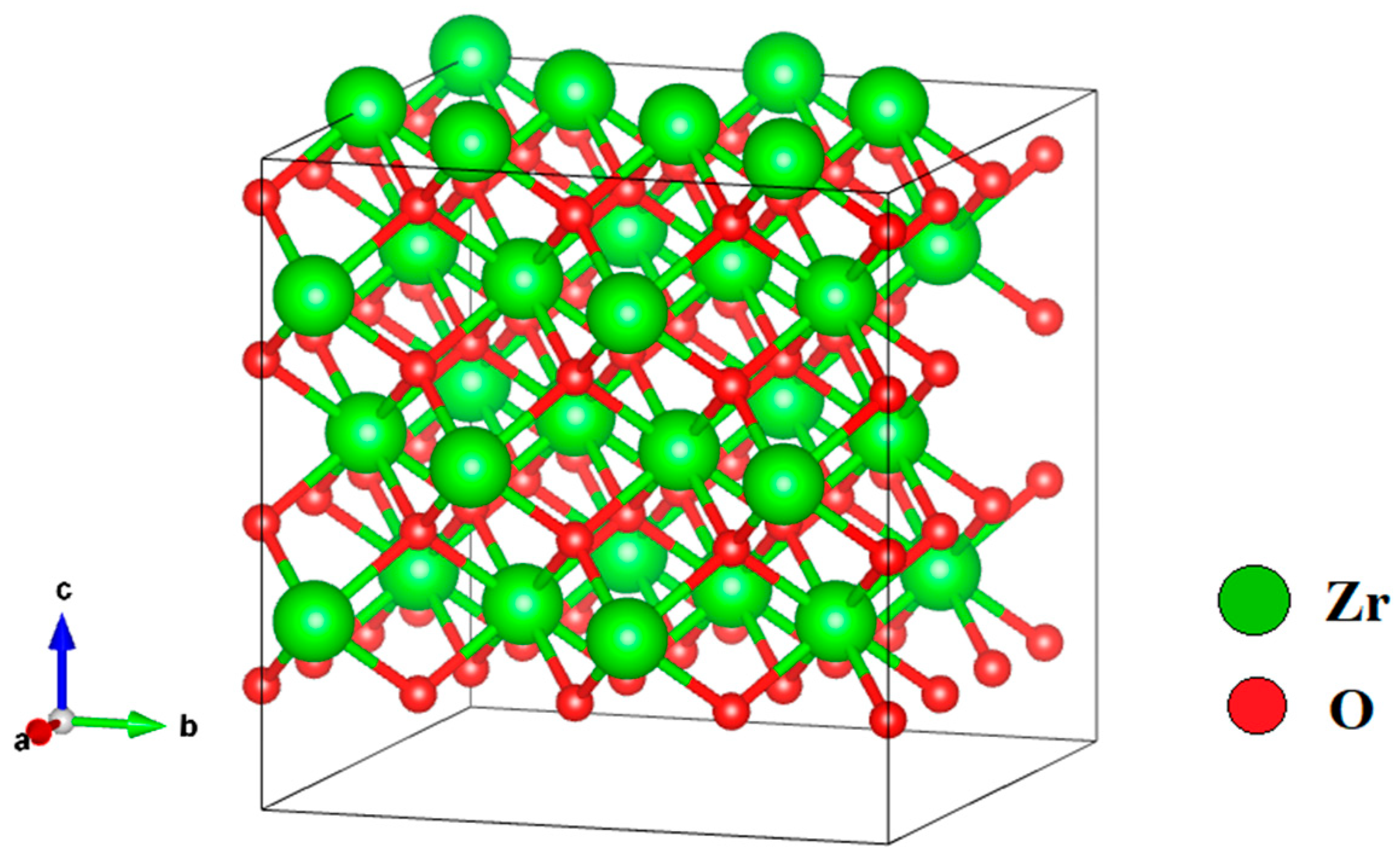
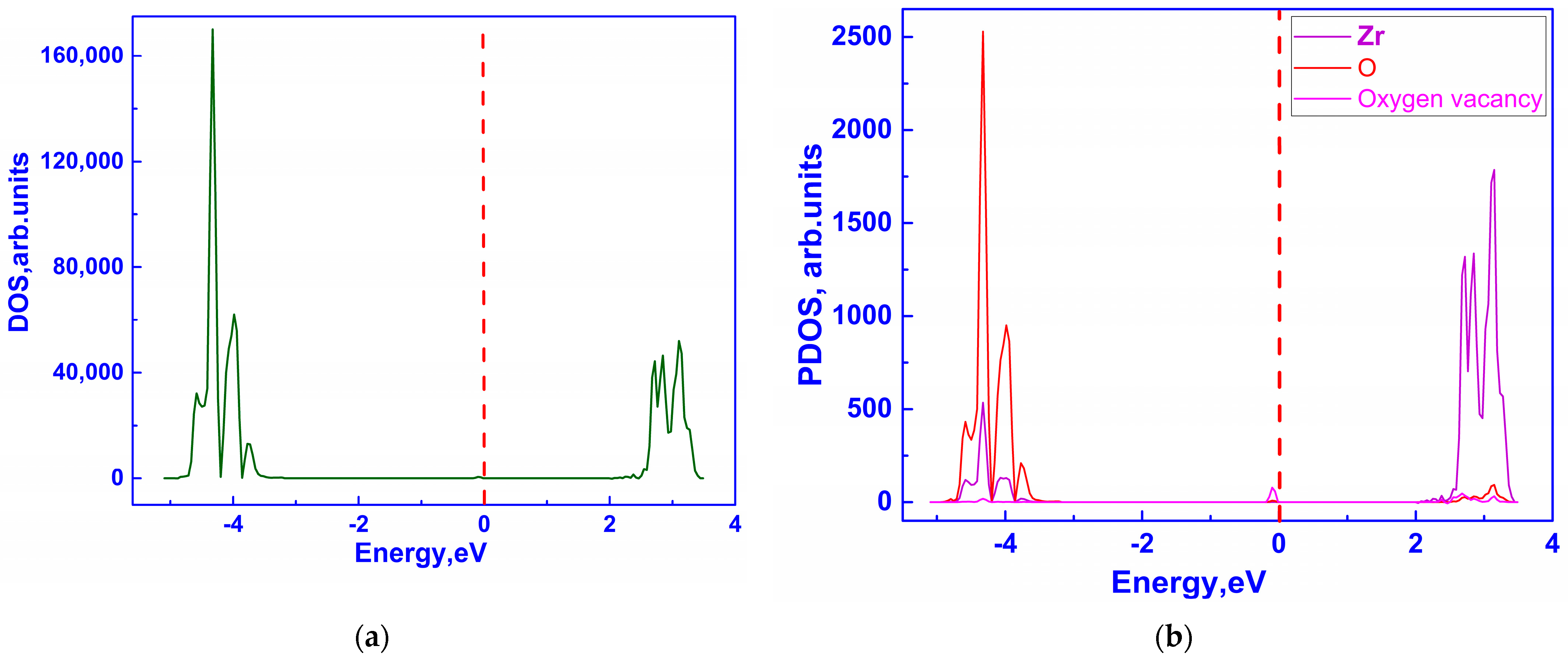
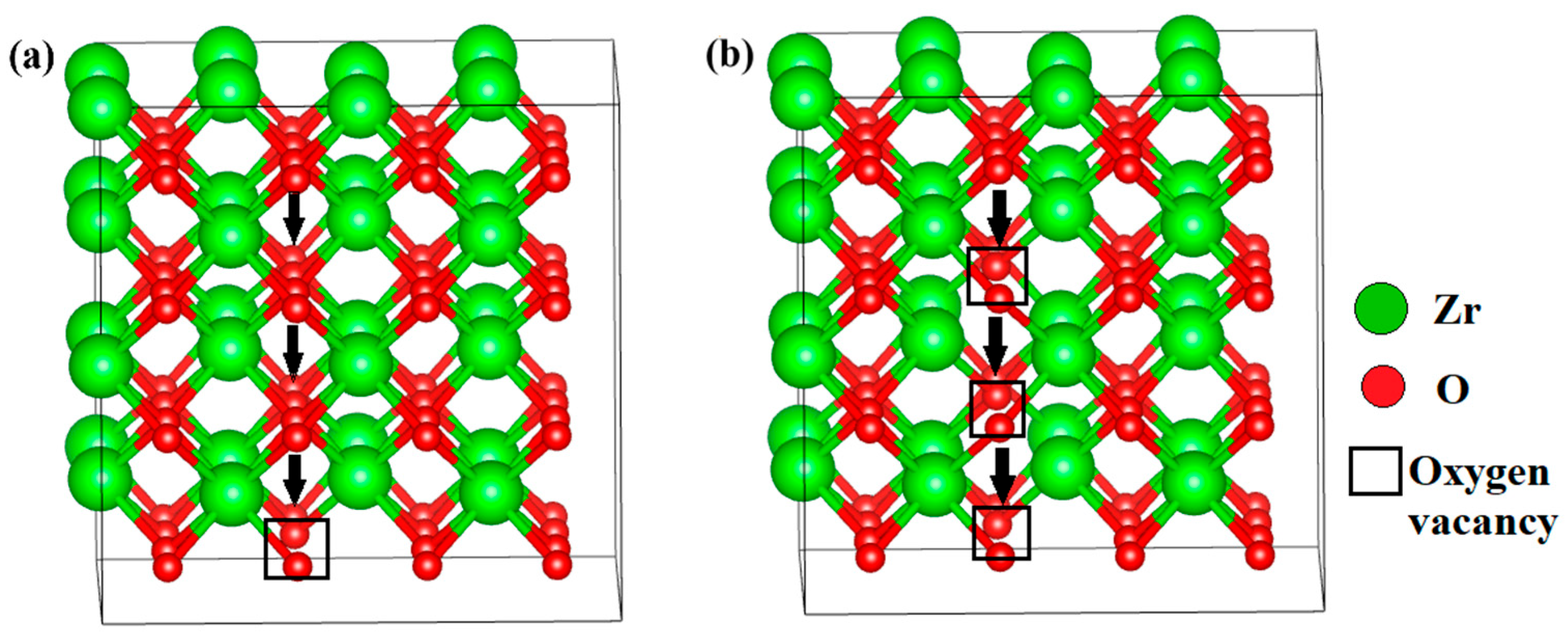
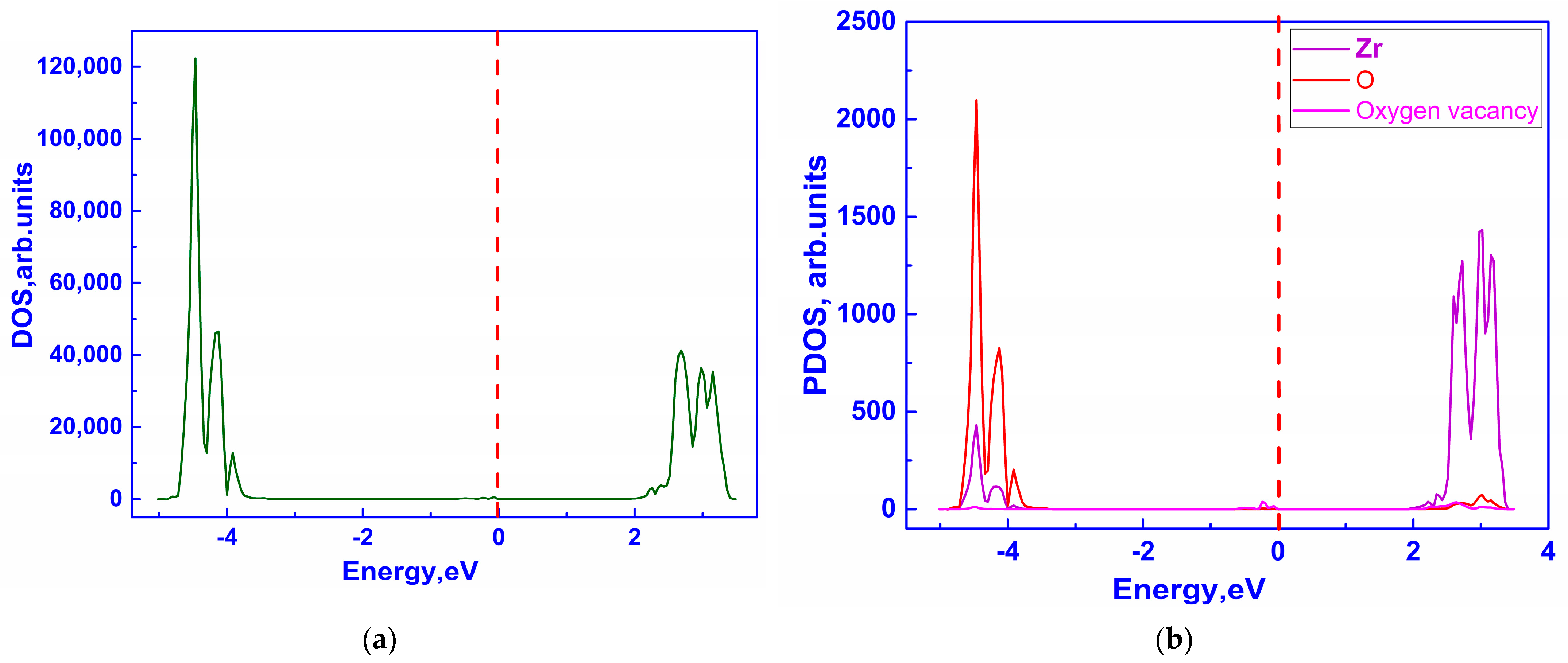
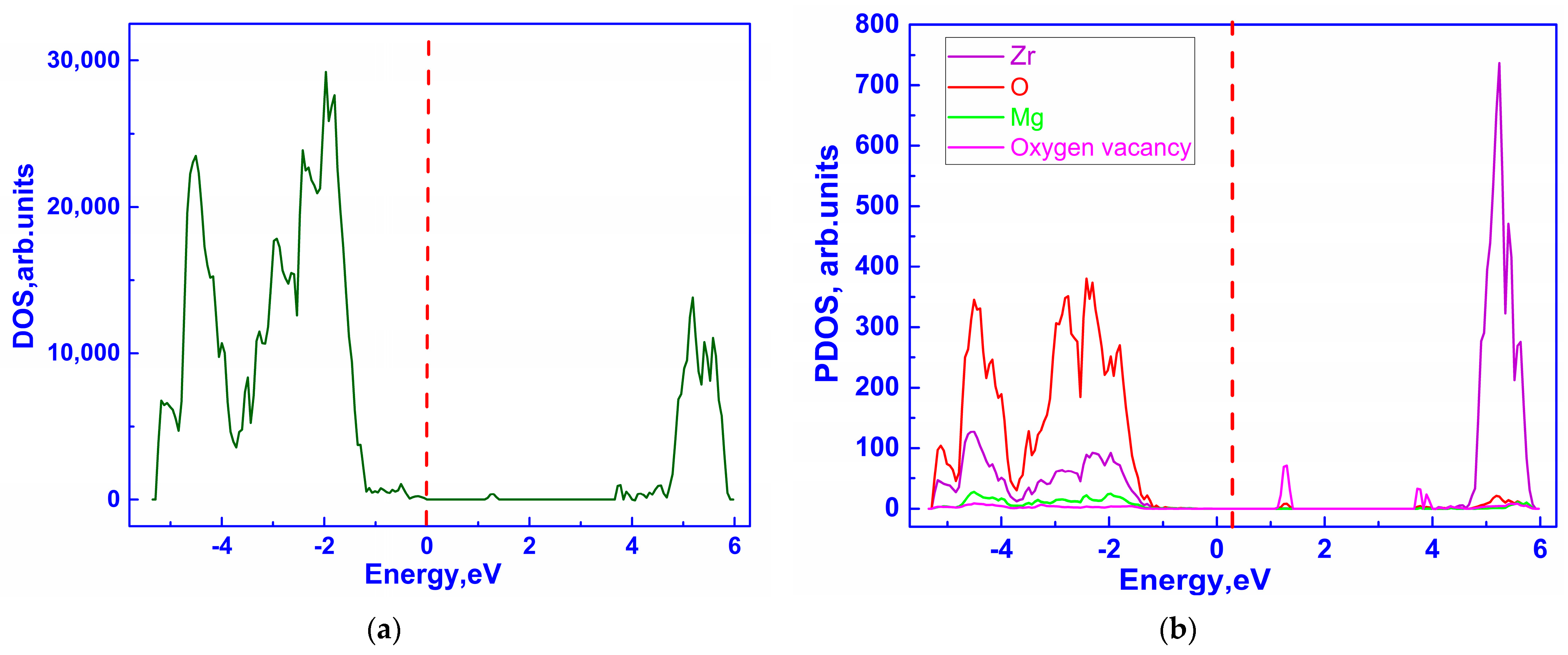


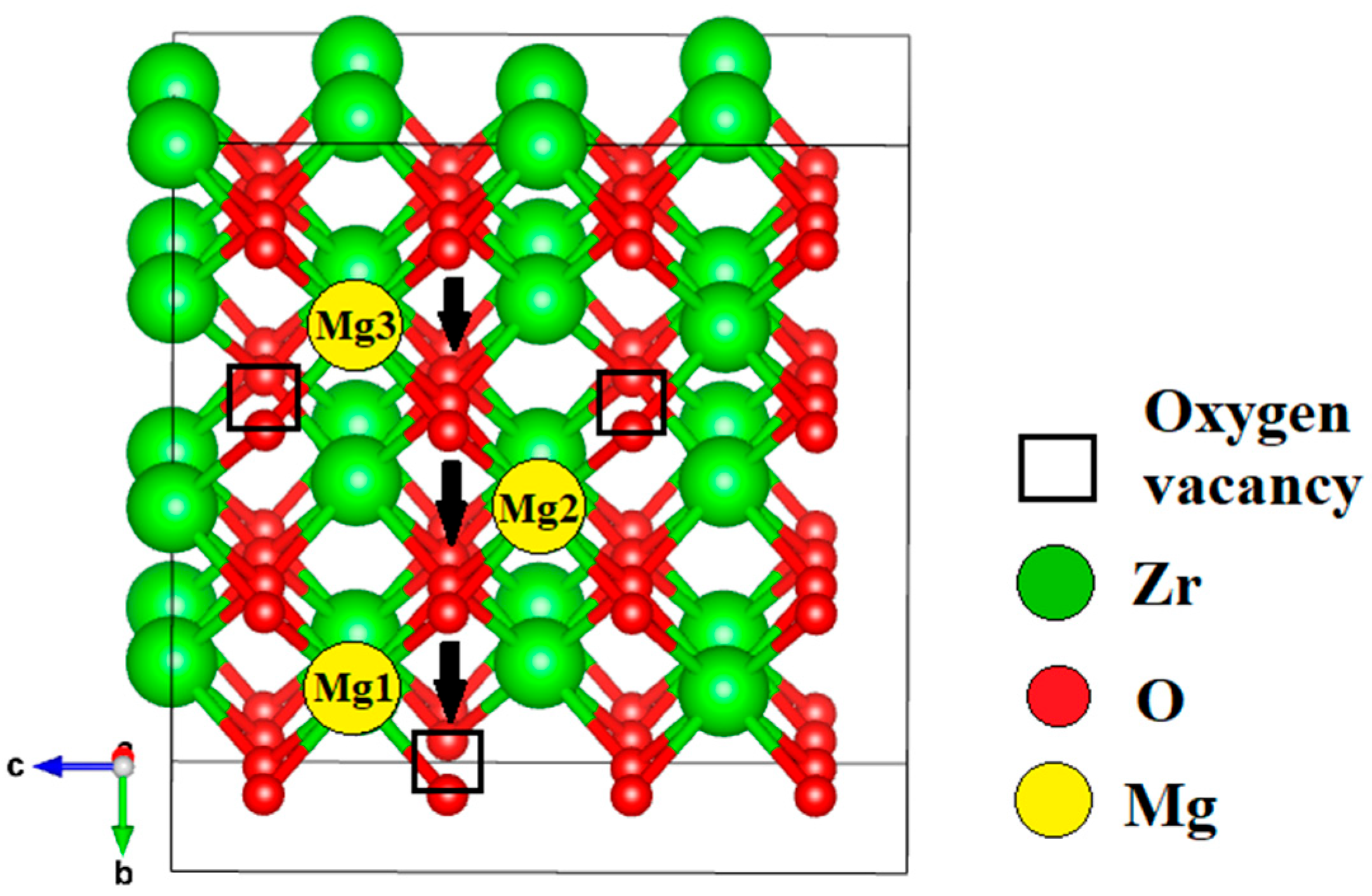
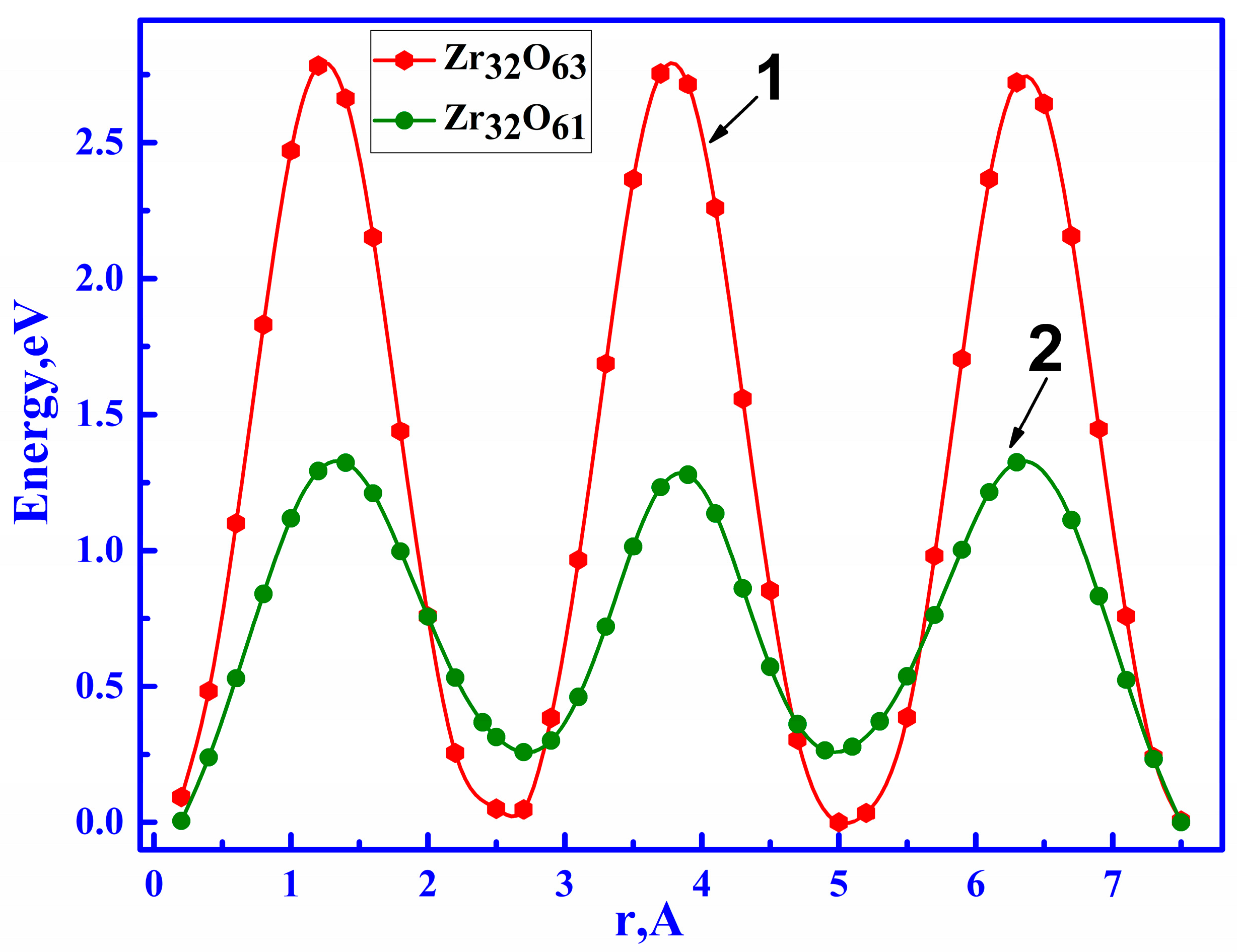
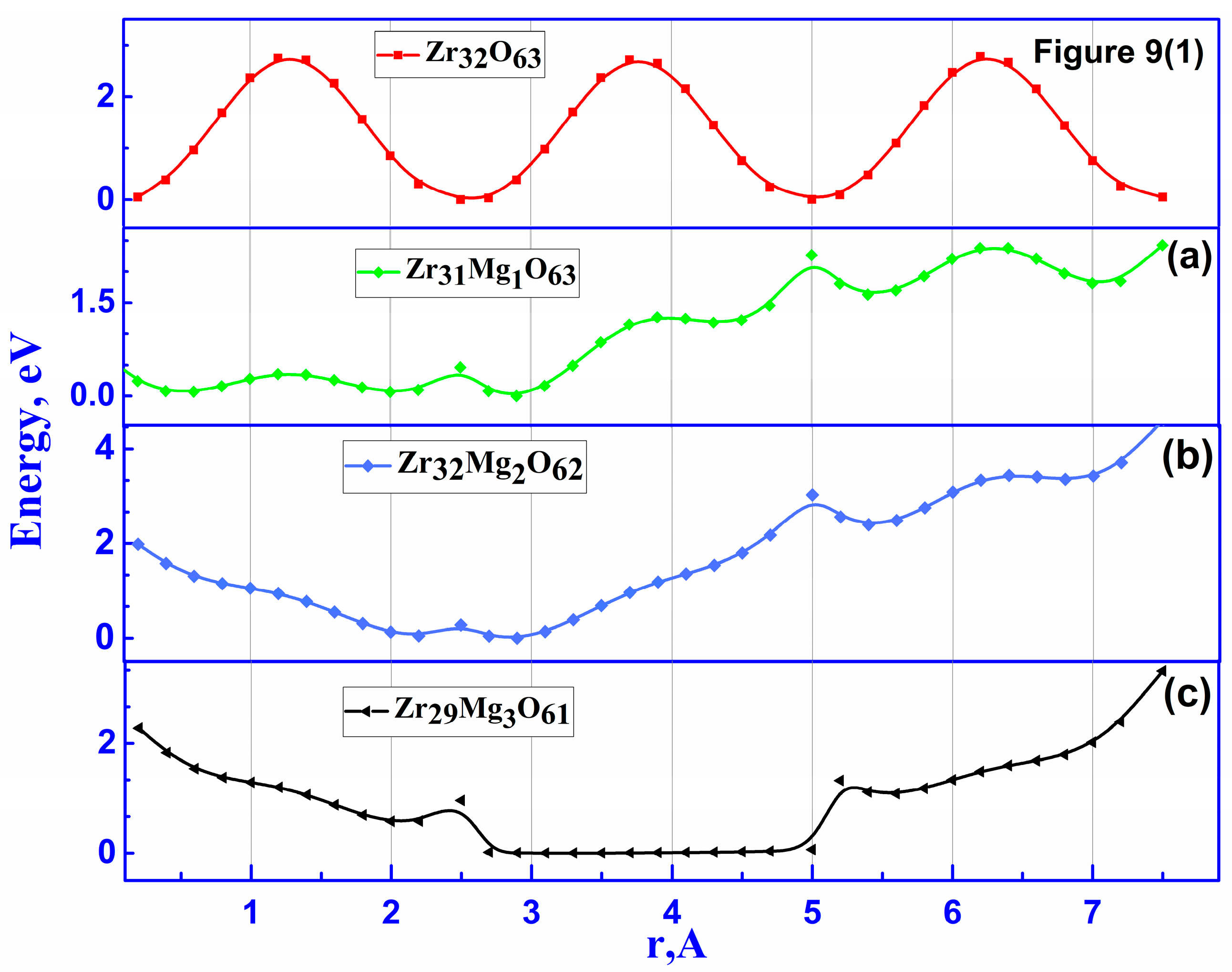
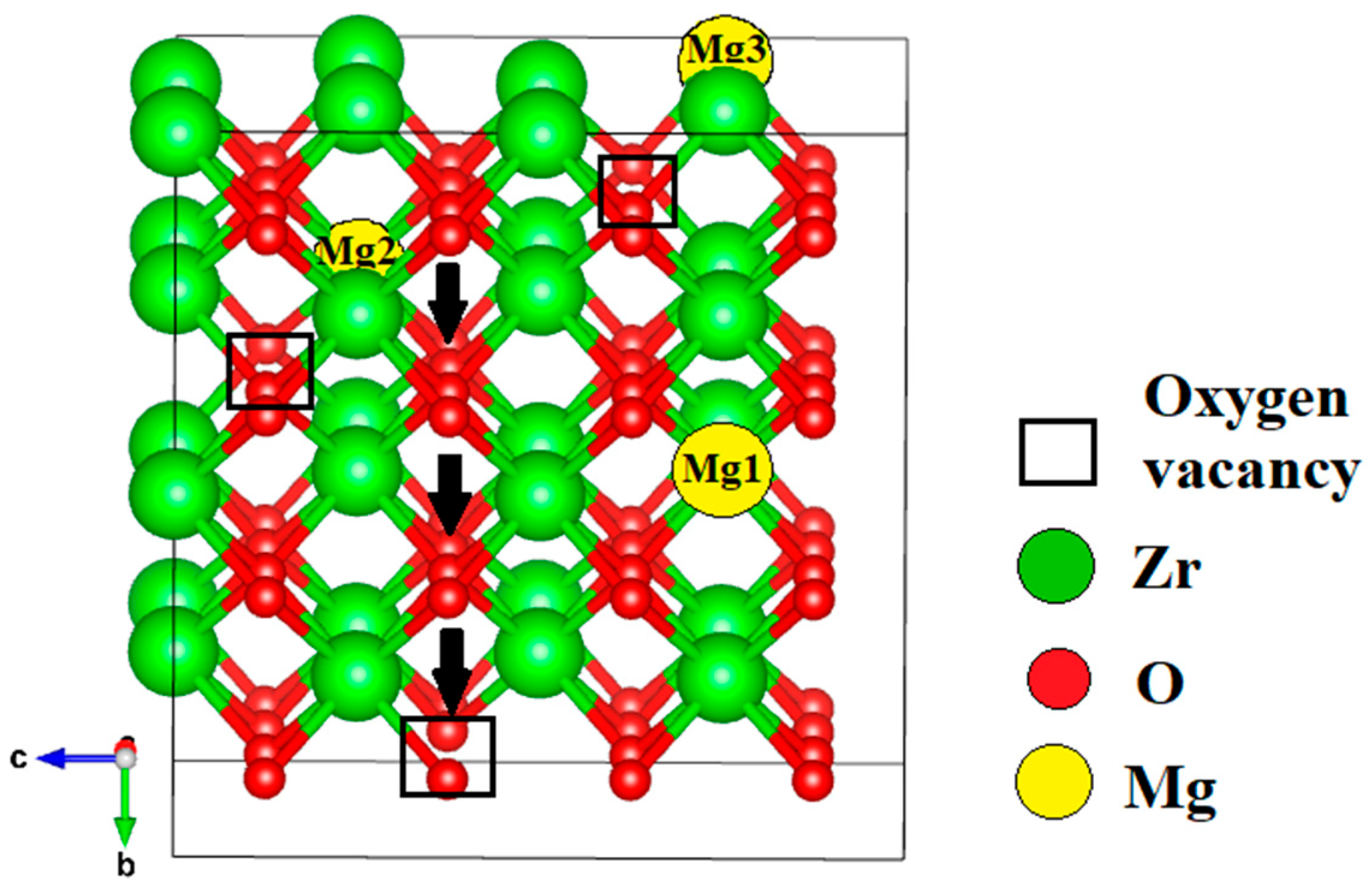
| Configuration | Local Effect | Average Symmetry | Space Group | Lattice Change |
|---|---|---|---|---|
| Zr32O64 (ideal) | Perfect cubic lattice | Cubic | Fm-3m | a = b = c |
| Zr32O63 (1 VO) | Zr shifts toward vacancy; distortion | Locally tetragonal | Local tetragonal/rhombohedral | Local anisotropy |
| Zr32O61 (3 VO) | Vacancy chain ⟨001⟩; elongation | Tetragonal | P42/nmc | c/a > 1 |
| Mg near VO (1–3) | Mg–VO dipoles; stronger distortions; high Mg restores cubic | Tetragonal → Cubic-like | P42/nmc → Fm-3m | c/a > 1 → ≈ 1 |
| Mg outside VO (1–3) | Weak background effect; lattice stable | Mostly cubic | Fm-3m | a ≈ b ≈ c |
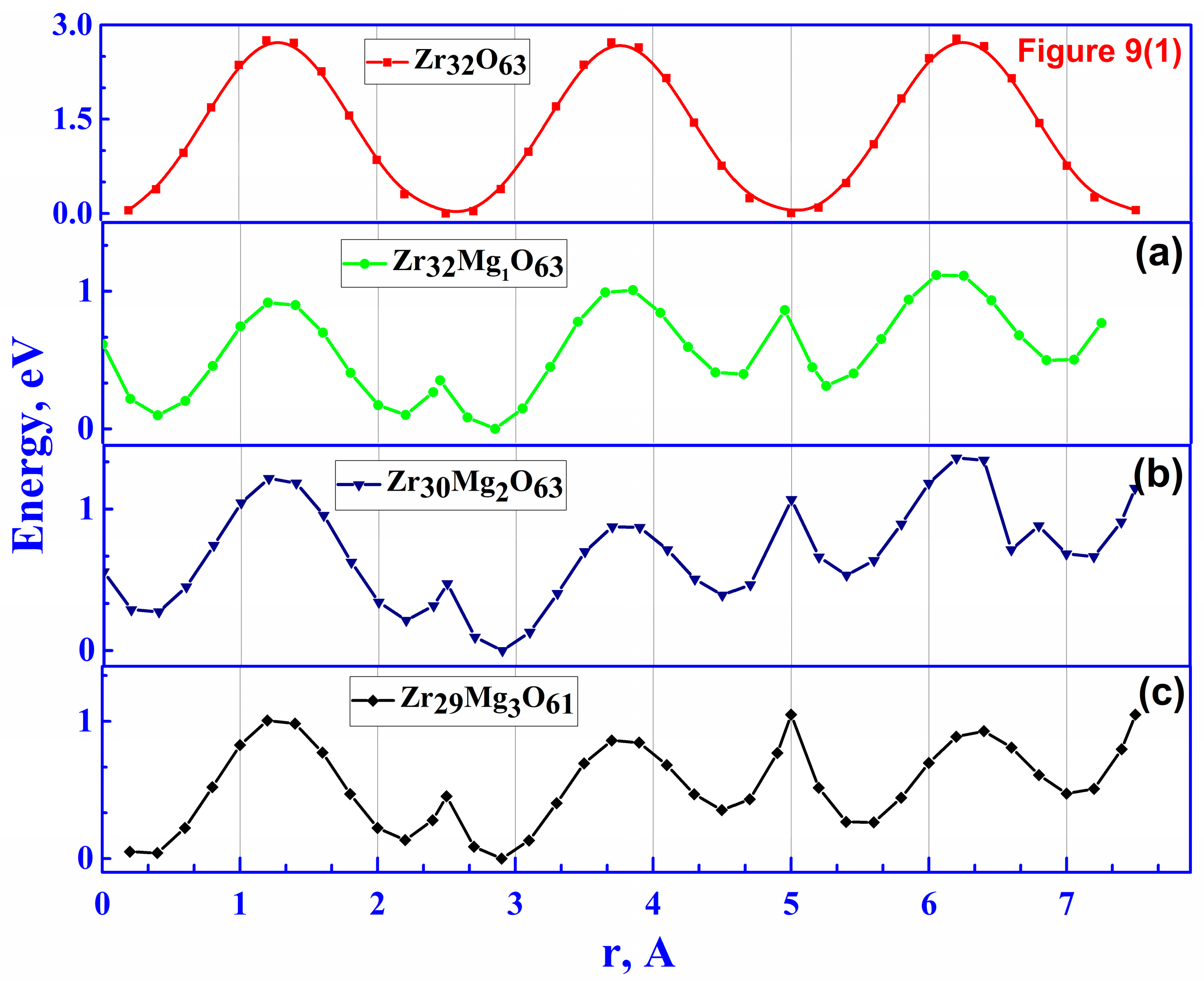
| Doping Configuration | Number of Mg | Min. Barrier, eV | Max. Barrier, eV |
|---|---|---|---|
| Zr31Mg1O63 | 1 | 0.4 | 2.4 |
| Zr30Mg2O62 | 2 | 0.5 | 3.1 |
| Zr29Mg3O61 | 3 | 0.8 | 1.32 |
| Doping Configuration | Number of Mg | Min. Barrier, eV | Max. Barrier, eV |
|---|---|---|---|
| Zr31Mg1O63 | 1 | 0.4 | 1.12 |
| Zr30Mg2O62 | 2 | 0.5 | 1.34 |
| Zr29Mg3O61 | 3 | 0.5 | 1.05 |
| Configuration | Mg Position | Total Energy (eV) | ΔE = Econfig − EZr32O64 (eV) |
|---|---|---|---|
| Zr32O64 | −171,752.37 | 0.00 | |
| Zr32O63 | −169,742.54 | +2009.83 | |
| Zr32O61 | −165,599.06 | +6153.31 | |
| Zr31Mg1O63 | along pathway | −173,916.22 | −2163.85 |
| Zr30Mg2O62 | −176,037.62 | −4285.25 | |
| Zr29Mg3O61 | −178,159.57 | −6407.20 | |
| Zr31Mg1O63 | outside pathway | −173,914.32 | −2161.95 |
| Zr30Mg2O62 | −176,034.90 | −4282.53 | |
| Zr29Mg3O61 | −178,158.75 | −6406.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salikhodzha, Z.M.; Bairbayeva, G.B.; Popov, A.I.; Kassymkhanova, R.N.; Zhangylyssov, K.B.; Popova, E.; Konuhova, M. DFT Study of Oxygen Ion Migration in Mg-Doped Cubic Zirconia. Solids 2025, 6, 55. https://doi.org/10.3390/solids6040055
Salikhodzha ZM, Bairbayeva GB, Popov AI, Kassymkhanova RN, Zhangylyssov KB, Popova E, Konuhova M. DFT Study of Oxygen Ion Migration in Mg-Doped Cubic Zirconia. Solids. 2025; 6(4):55. https://doi.org/10.3390/solids6040055
Chicago/Turabian StyleSalikhodzha, Zhussupbek M., Guldari B. Bairbayeva, Anatoli I. Popov, Raigul N. Kassymkhanova, Keleshek B. Zhangylyssov, Elena Popova, and Marina Konuhova. 2025. "DFT Study of Oxygen Ion Migration in Mg-Doped Cubic Zirconia" Solids 6, no. 4: 55. https://doi.org/10.3390/solids6040055
APA StyleSalikhodzha, Z. M., Bairbayeva, G. B., Popov, A. I., Kassymkhanova, R. N., Zhangylyssov, K. B., Popova, E., & Konuhova, M. (2025). DFT Study of Oxygen Ion Migration in Mg-Doped Cubic Zirconia. Solids, 6(4), 55. https://doi.org/10.3390/solids6040055








