Electric Field-Enhanced SPR Sensors with AuNPs and CQDs for Rapid and Low-Detection-Limit Detection of Co2+
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Preparation of Prismatic SPR Co2+ Sensing Chip
2.3. An Applied Planar Electric Field on Prismatic SPR Sensor Chip
2.4. Characterization
2.5. Performance of Prismatic SPR Co2+ Sensing Chip
3. Results
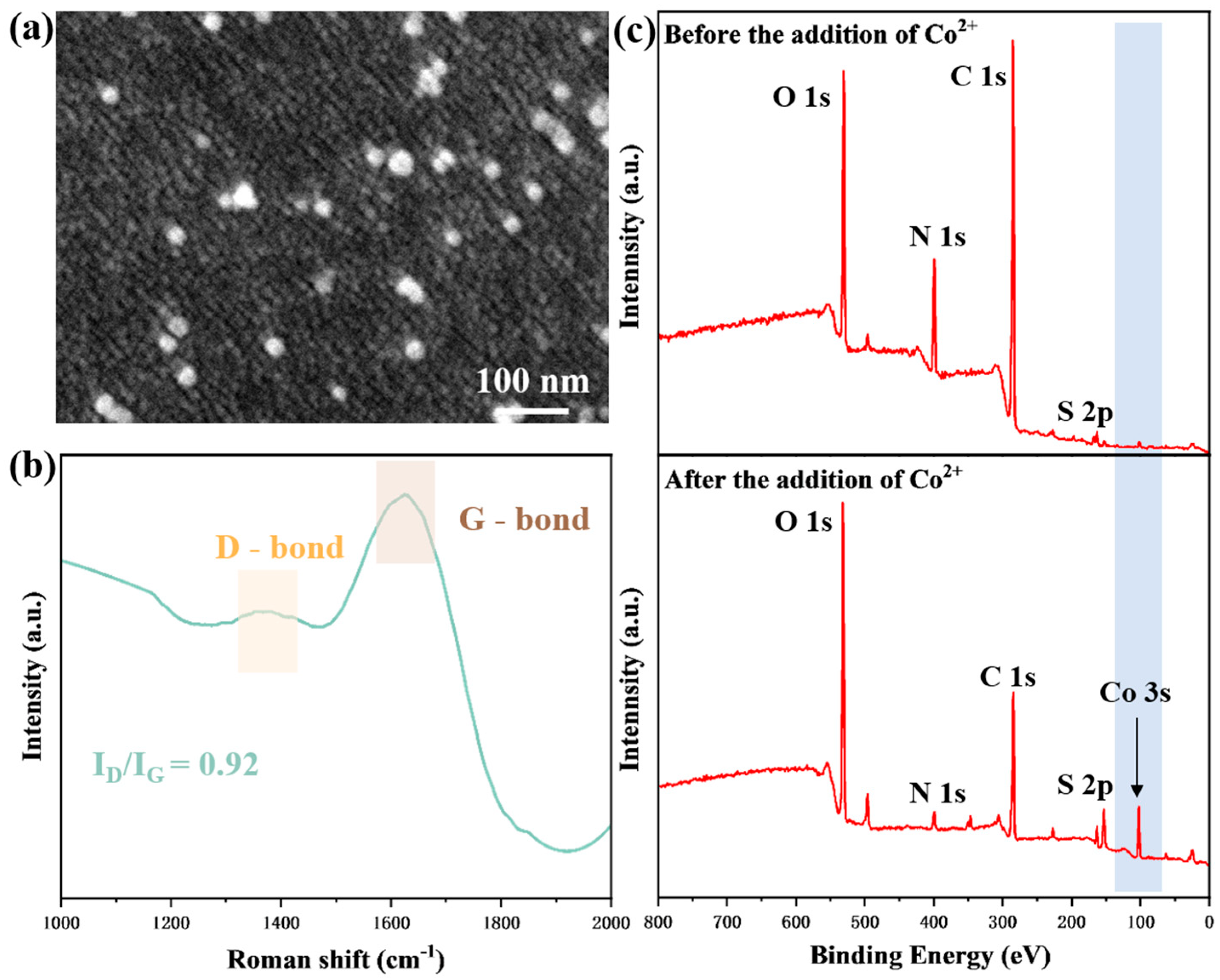
3.1. Characterization of Prismatic SPR Co2+ Sensing Chip
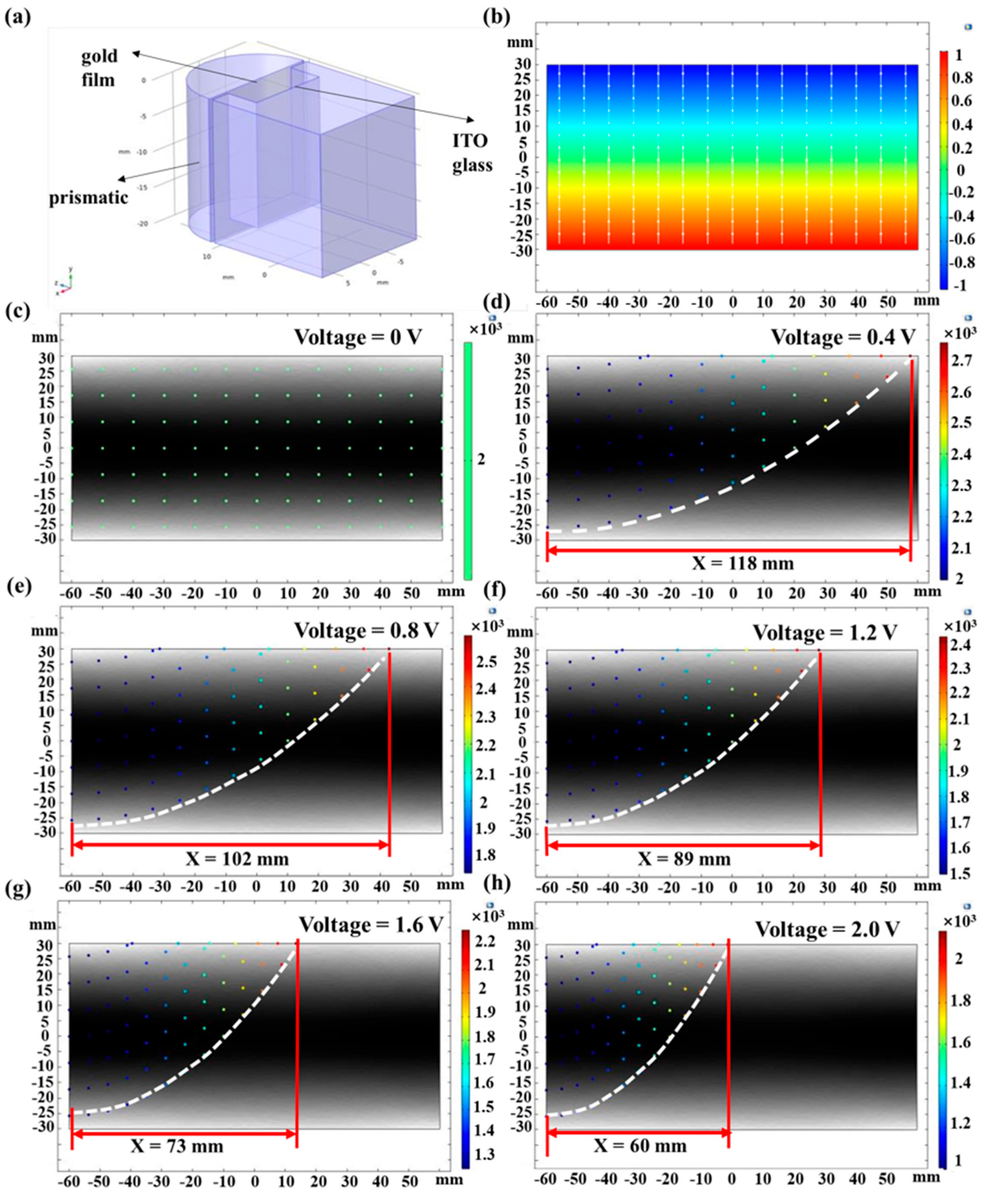
3.2. Simulation of Prismatic SPR Sensing Chip with Planar Electric Field Applied
3.3. Response Time and Resonance Angle of Prismatic SPR Chip Under Different Voltages
3.4. Response Time and Resonance Angle of Prismatic SPR Chip Under Different Electrolyte Concentrations
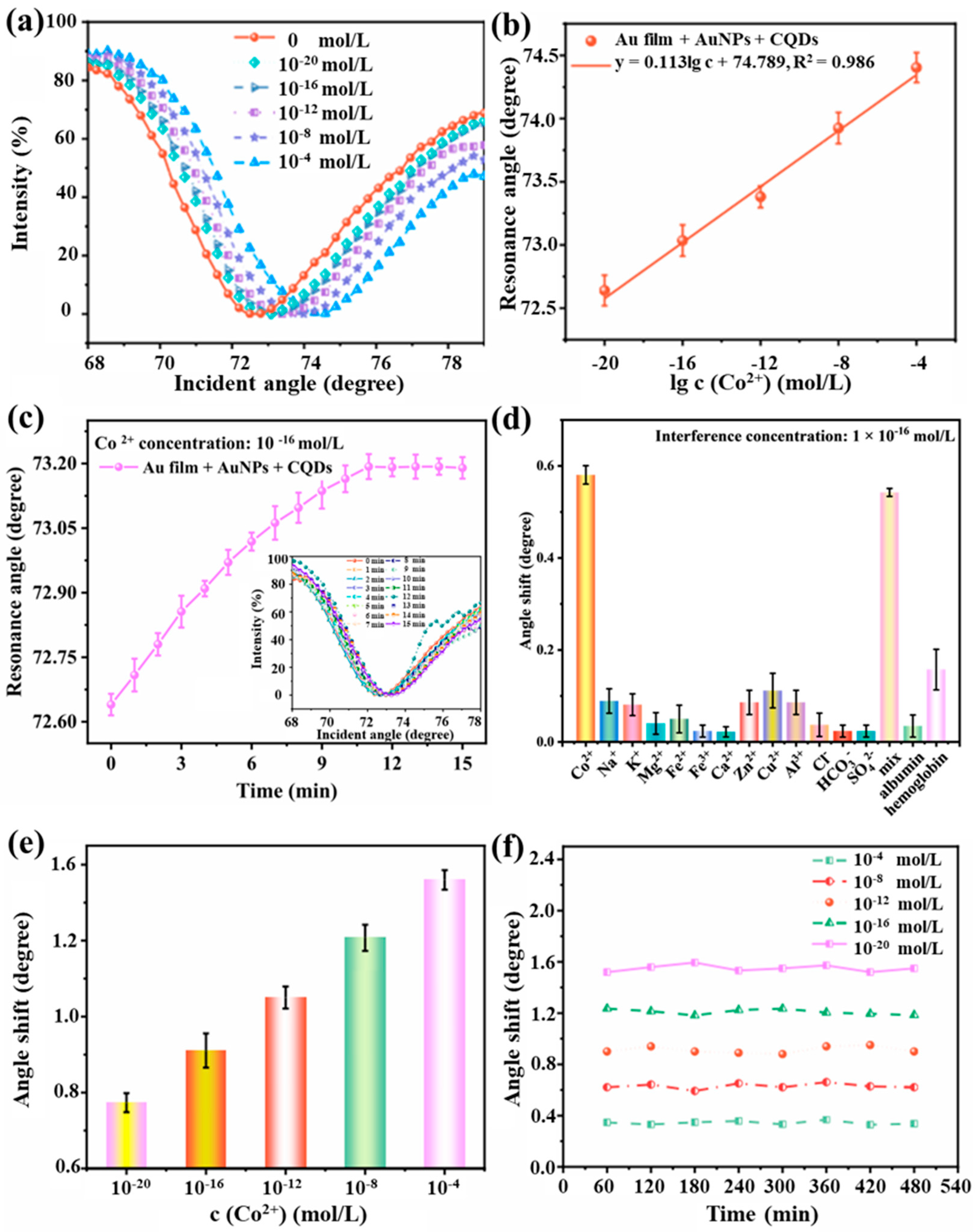
3.5. Detection of Co2+
3.6. Selectivity, Repeatability, and Stability of the Prismatic SPR Sensing Chips
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelbasir, S.M.; El-Shewaikh, A.M.; El-Sheikh, S.M.; Ali, O.I. Novel modified chitosan nanocomposites for Co(II) ions removal from industrial wastewater. J. Water Process Eng. 2021, 41, 102008. [Google Scholar] [CrossRef]
- Mohammadi, A.; Tamang, S.; Rethinasabapathy, M.; Ranjith, K.S.; Safarkhani, M.; Kwak, C.H.; Roh, C.; Huh, Y.S.; Han, Y.-K. Eco-friendly synthesis of rod-like hydroxyapatite on spherical carbon: A dual-function composite for selective cobalt removal and enhanced oxygen evolution reaction. J. Hazard. Mater. 2025, 487, 137164. [Google Scholar] [CrossRef]
- He, Z.; Zhu, J.; Li, X.; Weng, G.-J.; Li, J.-J.; Zhao, J.-W. Surface etching-dependent geometry tailoring and multi-spectral information of Au@AuAg yolk-shell nanostructure with asymmetrical pyramidal core: The application in Co2+ determination. J. Colloid Interface Sci. 2022, 625, 340–353. [Google Scholar] [CrossRef]
- Shi, J.; Lu, C.; Yan, D.; Ma, L. High selectivity sensing of cobalt in HepG2 cells based on necklace model microenvironment-modulated carbon dot-improved chemiluminescence in Fenton-like system. Biosens. Bioelectron. 2013, 45, 58–64. [Google Scholar] [CrossRef]
- Vashisht, D.; Kaur, K.; Jukaria, R.; Vashisht, A.; Sharma, S.; Mehta, S.K. Colorimetric chemosensor based on coumarin skeleton for selective naked eye detection of cobalt (II) ion in near aqueous medium. Sens. Actuators B 2019, 280, 219–226. [Google Scholar] [CrossRef]
- Hussain, M.M.; Asiri, A.M.; Arshad, M.N.; Rahman, M.M. Development of selective Co2+ ionic sensor based on various derivatives of benzenesulfonohydrazide (BSH) compound: An electrochemical approach. Chem. Eng. J. 2018, 339, 133–143. [Google Scholar] [CrossRef]
- Kravets, V.G.; Strudwick, A.; Grigorenko, A.N. Ultrathin Gold for Robust Multi-Element Surface Plasmon Resonance Biosensing. Adv. Mater. Interfaces 2025, 12, 2400925. [Google Scholar] [CrossRef]
- Park, J.-H.; Byun, J.-Y.; Yim, S.-Y.; Kim, M.-G. A Localized Surface Plasmon Resonance (LSPR)-based, simple, receptor-free and regeneratable Hg2+ detection system. J. Hazard. Mater. 2016, 307, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Wang, R.; Jiang, M.; Li, E.; Li, Y.; Yan, X.; Wang, T.; Ren, Z. Polydopamine functionalized graphene oxide for high sensitivity micro-tapered long period fiber grating sensor and its application in detection Co2+ ions. Opt. Fiber Technol. 2022, 68, 102807. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.; Ma, R.; He, M.; Shi, H.; Yi, Q. High-throughput biomolecular interaction analysis probing by an array fluorescent biosensor platform. Sens. Actuators B 2018, 259, 888–893. [Google Scholar] [CrossRef]
- Kravets, V.G.; Wu, F.; Yu, T.; Grigorenko, A.N. Metal-Dielectric-Graphene Hybrid Heterostructures with Enhanced Surface Plasmon Resonance Sensitivity Based on Amplitude and Phase Measurements. Plasmonics 2022, 17, 973–987. [Google Scholar] [CrossRef]
- Gao, C.; Lu, Z.; Liu, Y.; Zhang, Q.; Chi, M.; Cheng, Q.; Yin, Y. Highly Stable Silver Nanoplates for Surface Plasmon Resonance Biosensing. Angew. Chem. Int. Ed. 2012, 51, 5629–5633. [Google Scholar] [CrossRef]
- Stebunov, Y.V.; Yakubovsky, D.I.; Fedyanin, D.Y.; Arsenin, A.V.; Volkov, V.S. Superior Sensitivity of Copper-Based Plasmonic Biosensors. Langmuir 2018, 34, 4681–4687. [Google Scholar] [CrossRef]
- Jia, Y.; Peng, Y.; Bai, J.; Zhang, X.; Cui, Y.; Ning, B.; Cui, J.; Gao, Z. Magnetic nanoparticle enhanced surface plasmon resonance sensor for estradiol analysis. Sens. Actuators B 2018, 254, 629–635. [Google Scholar] [CrossRef]
- Lee, E.G.; Park, K.M.; Jeong, J.Y.; Lee, S.H.; Baek, J.E.; Lee, H.W.; Jung, J.K.; Chung, B.H. Carbon nanotube-assisted enhancement of surface plasmon resonance signal. Anal. Biochem. 2011, 408, 206–211. [Google Scholar] [CrossRef]
- Aubé, A.; Charbonneau, D.M.; Pelletier, J.N.; Masson, J.-F. Response Monitoring of Acute Lymphoblastic Leukemia Patients Undergoing l-Asparaginase Therapy: Successes and Challenges Associated with Clinical Sample Analysis in Plasmonic Sensing. ACS Sens. 2016, 1, 1358–1365. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, Y.; Wei, J. Recent advances in surface-enhanced raman spectroscopy (SERS): Finite-difference time-domain (FDTD) method for SERS and sensing applications. Trends Anal. Chem. 2016, 75, 162–173. [Google Scholar] [CrossRef]
- Jain, S.; Choudhary, K.; Kumar, S. Photonic crystal fiber-based SPR sensor for broad range of refractive index sensing applications. Opt. Fiber Technol. 2022, 73, 103030. [Google Scholar] [CrossRef]
- Yao, H.; Zhong, S. High-mode spoof SPP of periodic metal grooves for ultra-sensitive terahertz sensing. Opt. Express 2014, 22, 25149–25160. [Google Scholar] [CrossRef] [PubMed]
- Saad, D.R.; Alismaeel, Z.T.; Abbar, A.H. Cobalt Removal from Simulated Wastewaters Using a Novel Flow-by Fixed Bed Bio-electrochemical Reactor. Chem. Eng. Process. 2020, 156, 108097. [Google Scholar] [CrossRef]
- Mehta, V.N.; Desai, M.L.; Basu, H.; Kumar Singhal, R.; Kailasa, S.K. Recent developments on fluorescent hybrid nanomaterials for metal ions sensing and bioimaging applications: A review. J. Mol. Liq. 2021, 333, 115950. [Google Scholar] [CrossRef]
- Devi, P.; Saini, S.; Kim, K.-H. The advanced role of carbon quantum dots in nanomedical applications. Biosens. Bioelectron. 2019, 141, 111158. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Li, L.; Wang, D.; Chen, M.; Zeng, Z.; Xiong, W.; Wu, X.; Guo, C. Carbon dots: Synthesis, properties and biomedical applications. J. Mater. Chem. B 2021, 9, 6553–6575. [Google Scholar] [CrossRef]
- Gupta, A.; Chaudhary, A.; Mehta, P.; Dwivedi, C.; Khan, S.; Verma, N.C.; Nandi, C.K. Nitrogen-doped, thiol-functionalized carbon dots for ultrasensitive Hg(ii) detection. Chem. Commun. 2015, 51, 10750–10753. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Shan, X.; Chai, L.; Chen, J.; Feng, H. Simultaneous Detection of Multiple DNA Targets by Integrating Dual-Color Graphene Quantum Dot Nanoprobes and Carbon Nanotubes. Chem. Eur. J. 2014, 20, 16065–16069. [Google Scholar] [CrossRef]
- Mowery, J.L.; Chen, J. Recent Biomedical Applications of Carbon Quantum Dots in Cancer Treatment. J. Phys. Chem. C. 2024, 128, 16291–16301. [Google Scholar] [CrossRef]
- Chen, W.; Chen, T.; Fang, M.; Zhu, W.; Li, C. Continuous Detection of Cobalt Ions and Pyrophosphates by NAC-CdTe Quantum Dots Fluorescence Probes. J. Fluoresc. 2025, 35, 2261–2272. [Google Scholar] [CrossRef]
- Kong, D.; Yan, F.; Han, Z.; Xu, J.; Guo, X.; Chen, L. Cobalt(ii) ions detection using carbon dots as an sensitive and selective fluorescent probe. RSC Adv. 2016, 6, 67481–67487. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Liu, Q.; Chen, M.; Chen, X. Facile synthesis of B,N-doped CQDs as versatile fluorescence probes for sensitive detection of cobalt ions in environmental water and biological samples. Microchem. J. 2021, 163, 105888. [Google Scholar] [CrossRef]
- Hou, J.; Xia, B.; Niu, K.; Wang, J.; Li, J.; Wang, N. Ultrasensitive optical fiber SPR sensor enhanced by Au-NPs-film modified with functionalized CQDs for label-free detecting cobalt (II) ion. Anal. Chim. Acta 2024, 1320, 343030. [Google Scholar] [CrossRef]
- Salihoglu, O.; Balci, S.; Kocabas, C. Plasmon-polaritons on graphene-metal surface and their use in biosensors. Appl. Phys. Lett. 2012, 100, 213110. [Google Scholar] [CrossRef]
- Kretschmann, E.; Raether, H. Notizen: Radiative Decay of Non Radiative Surface Plasmons Excited by Light. Naturforsch. A 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Saikia, M.; Das, T.; Dihingia, N.; Fan, X.; Silva, L.F.O.; Saikia, B.K. Formation of carbon quantum dots and graphene nanosheets from different abundant carbonaceous materials. Diamond Relat. Mater. 2020, 106, 107813. [Google Scholar] [CrossRef]
- Zhan, J.; Peng, R.; Wei, S.; Chen, J.; Peng, X.; Xiao, B. Ethanol-Precipitation-Assisted Highly Efficient Synthesis of Nitrogen-Doped Carbon Quantum Dots from Chitosan. ACS Omega 2019, 4, 22574–22580. [Google Scholar] [CrossRef]
- Yang, Q.; Duan, J.; Yang, W.; Li, X.; Mo, J.; Yang, P.; Tang, Q. Nitrogen-doped carbon quantum dots from biomass via simple one-pot method and exploration of their application. Appl. Surf. Sci. 2018, 434, 1079–1085. [Google Scholar] [CrossRef]
- Qu, S.; Wang, X.; Lu, Q.; Liu, X.; Wang, L. A Biocompatible Fluorescent Ink Based on Water-Soluble Luminescent Carbon Nanodots. Angew. Chem. Int. Ed. 2012, 51, 12215–12218. [Google Scholar] [CrossRef]
- Wahyuni, S.; Riswan, M.; Adrianto, N.; Dharmawan, M.Y.; Tumbelaka, R.M.; Cuana, R.; Istiqomah, N.I.; Jiananda, A.; Garcia, S.; Suharyadi, E. Localized surface plasmon resonance properties dependence of green-synthesized Fe3O4/Ag composite nanoparticles on Ag concentration and an electric field for biosensor application. Photonics Nanostruct. Fundam. Appl. 2023, 57, 101191. [Google Scholar] [CrossRef]
- Zhou, K.; Huang, J.; Xiang, D.; Deng, A.; Du, J.; Liu, H. Decoupled water electrolysis: Flexible strategy for pure hydrogen production with small voltage inputs. J. Energy Chem. 2024, 94, 340–356. [Google Scholar] [CrossRef]
- Qiu, J.; Yao, J.; Feng, Z.; Huang, B.; Luo, Z.; Wang, L. Enhancing Water Electrolysis Performance by Bubble Behavior Management. Small Methods 2025, 9, 2402105. [Google Scholar] [CrossRef]
- Yan, Y.; Wu, M.; Zhou, L.; Chen, W.; Han, L.; Gao, G.; Cui, Y.; Sun, Z.; Cabot, A. Enhancing Electrocatalytic Activity Through Targeted Local Electrolyte Micro-Environment. Adv. Funct. Mater. 2025, 35, 2419328. [Google Scholar] [CrossRef]
- You, K.E.; Uddin, N.; Kim, T.H.; Fan, Q.H.; Yoon, H.J. Highly sensitive detection of biological substances using microfluidic enhanced Fabry-Perot etalon-based optical biosensors. Sens. Actuators B 2018, 277, 62–68. [Google Scholar] [CrossRef]
- You, B.; Yue, Y.; Sun, M.; Li, J.; Jia, D. Design of a Real-Time Salinity Detection System for Water Injection Wells Based on Fuzzy Control. Sensors 2021, 21, 3086. [Google Scholar] [CrossRef]
- Chantaramethakul, J.; Choophun, N.; Chokradjaroen, C.; Watthanaphanit, A.; Saito, N.; Panomsuwan, G. Morphological Evolution of Gold Nanoparticles Synthesized via Solution Plasma Sputtering: Effect of Sodium Chloride Concentration and Storage Time. J. Phys. Chem. C 2023, 127, 3184–3193. [Google Scholar] [CrossRef]
- Brown, M.A.; Goel, A.; Abbas, Z. Effect of Electrolyte Concentration on the Stern Layer Thickness at a Charged Interface. Angew. Chem. Int. Ed. 2016, 55, 3790–3794. [Google Scholar] [CrossRef]
- Mubeen, S.; Zhang, S.; Kim, N.; Lee, S.; Krämer, S.; Xu, H.; Moskovits, M. Plasmonic Properties of Gold Nanoparticles Separated from a Gold Mirror by an Ultrathin Oxide. Nano Lett. 2012, 12, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X.-Z.; Song, H.; Zhao, W.-M.; Jing, J.-Y. A dual channel self-compensation optical fiber biosensor based on coupling of surface plasmon polariton. Opt. Laser Technol. 2020, 124, 106002. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, C.; Xia, B.; Wang, N.; Chen, X.; Jing, X.; Chen, M.; Xu, X. An enhanced SPR optical fiber biosensor using Ti3C2Tx MXene/AuNPs for label-free and sensitive detection of human IgG. Nanoscale 2024, 16, 18477–18487. [Google Scholar] [CrossRef]
- Wang, N.; Ga, L.; Ai, J. Synthesis of fluorescent BSA templated silver nanomaterials and it’s application of detection of Zn2+ and Co2+. Mater. Res. Express 2018, 5, 105703. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Wang, Y.; Xu, J.; Xiong, Z.; Zhao, X.; Xia, Y. Nitrogen and sulfur Co-doped carbon dots as selective and visual sensors for monitoring cobalt ions. Opt. Mater. 2021, 112, 110787. [Google Scholar] [CrossRef]
- Karami, C.; Taher, M.A. Colorimetric Sensor of Cobalt Ions in Aqueous Solution Using Gold Nanoparticles Modified with Glycyrrhizic Acid. Plasmonics 2018, 13, 1315–1323. [Google Scholar] [CrossRef]
- Liao, S.; Zhu, F.; Zhao, X.; Yang, H.; Chen, X. A reusable P, N-doped carbon quantum dot fluorescent sensor for cobalt ion. Sens. Actuators B Chem. 2018, 260, 156–164. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Lou, T.; Pan, D.; Chen, L.; Qu, C.; Chen, Z. Label-free colorimetric sensing of cobalt(ii) based on inducing aggregation of thiosulfate stabilized gold nanoparticles in the presence of ethylenediamine. Analyst 2012, 137, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Mazur, F.; Liu, L.; Li, H.; Huang, J.; Chandrawati, R. Core-satellite gold nanoparticle biosensors for monitoring cobalt ions in biological samples. Sens. Actuators B 2018, 268, 182–187. [Google Scholar] [CrossRef]


| Biosensor | LOD (mol/L) | Time | Concentration Range (mol/L) | Ref. |
|---|---|---|---|---|
| Nitrogen and sulfur Co-doped carbon dots as selective and visual sensors | 2.6 × 10−8 | / | 1−5 × 10−5 | [49] |
| A reusable P, N-doped carbon quantum dot fluorescent sensor | 5.3 × 10−8 | / | 0–6 ×10−5 | [51] |
| Synthesis of fluorescent BSA templated silver AuNPs | 1.327 × 10−6 | / | 6 × 10−4–10−3 | [48] |
| Label-free colorimetric sensing based on inducing aggregation of thiosulfate-stabilized AuNPs | 4 × 10−8 | 20 min | 1 × 10−7–7 × 10−7 | [52] |
| AuNPs Modified with Glycyrrhizic Acid | 4 × 10−10 | 10 min | 1.6 × 10−5–5 × 10−2 | [50] |
| Core-satellite AuNPs biosensors | 10−8 | 15 min | 10−7–10−5 | [53] |
| Carbon dots as a sensitive and selective fluorescent probe | 4.5 × 10−7 | 30 min | 0–2 × 10−4 | [28] |
| PDA-GO | 10−7 | / | 10−7–10−2 | [9] |
| Prismatic SPR Sensor Chip with an applied planar electric field | 4.96 × 10−20 | 11 min | 10−20–10−4 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, X.; Chen, M.; Ma, X.; Xu, X.; Wang, N.; Niu, K.; Chen, X.; Wang, Y.; Zhu, J.; Hou, J.; et al. Electric Field-Enhanced SPR Sensors with AuNPs and CQDs for Rapid and Low-Detection-Limit Detection of Co2+. Solids 2025, 6, 49. https://doi.org/10.3390/solids6030049
Jing X, Chen M, Ma X, Xu X, Wang N, Niu K, Chen X, Wang Y, Zhu J, Hou J, et al. Electric Field-Enhanced SPR Sensors with AuNPs and CQDs for Rapid and Low-Detection-Limit Detection of Co2+. Solids. 2025; 6(3):49. https://doi.org/10.3390/solids6030049
Chicago/Turabian StyleJing, Xinyue, Minxuan Chen, Xingye Ma, Xinrui Xu, Ning Wang, Kunpeng Niu, Xiaohan Chen, Yihao Wang, Jiayi Zhu, Jianguo Hou, and et al. 2025. "Electric Field-Enhanced SPR Sensors with AuNPs and CQDs for Rapid and Low-Detection-Limit Detection of Co2+" Solids 6, no. 3: 49. https://doi.org/10.3390/solids6030049
APA StyleJing, X., Chen, M., Ma, X., Xu, X., Wang, N., Niu, K., Chen, X., Wang, Y., Zhu, J., Hou, J., & Wang, Z. (2025). Electric Field-Enhanced SPR Sensors with AuNPs and CQDs for Rapid and Low-Detection-Limit Detection of Co2+. Solids, 6(3), 49. https://doi.org/10.3390/solids6030049








