Insights into the Electrocatalytic Activity of Mixed-Valence Mn3+/Mn4+ and Fe2+/Fe3+ Transition Metal Oxide Materials
Abstract
1. Introduction
2. Experimental Method
2.1. Materials and Reagents
2.2. Electrode Manufacturing Method
2.3. Electrochemical Study
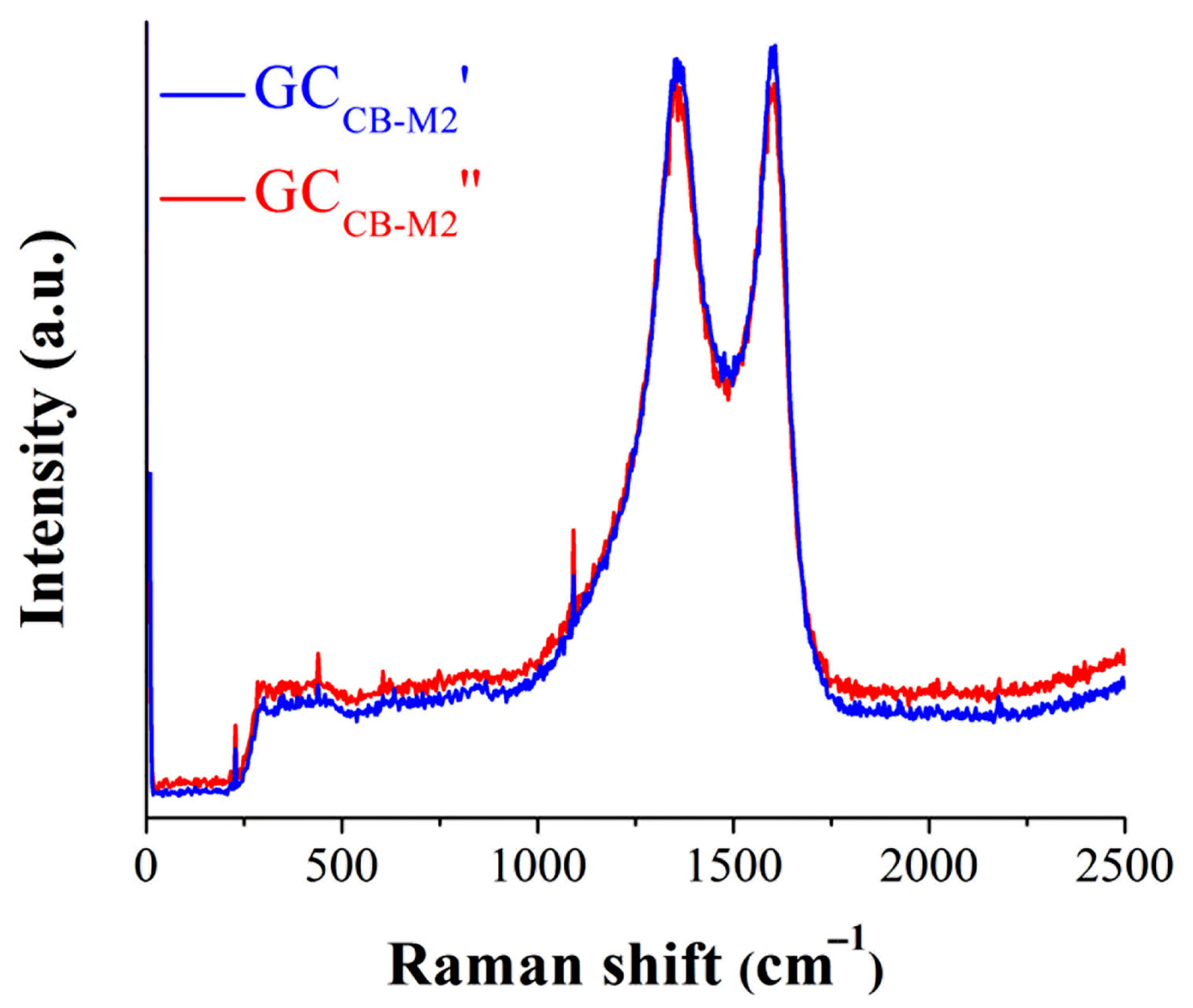
2.4. Physical–Chemical Characterizations
3. Results and Discussion
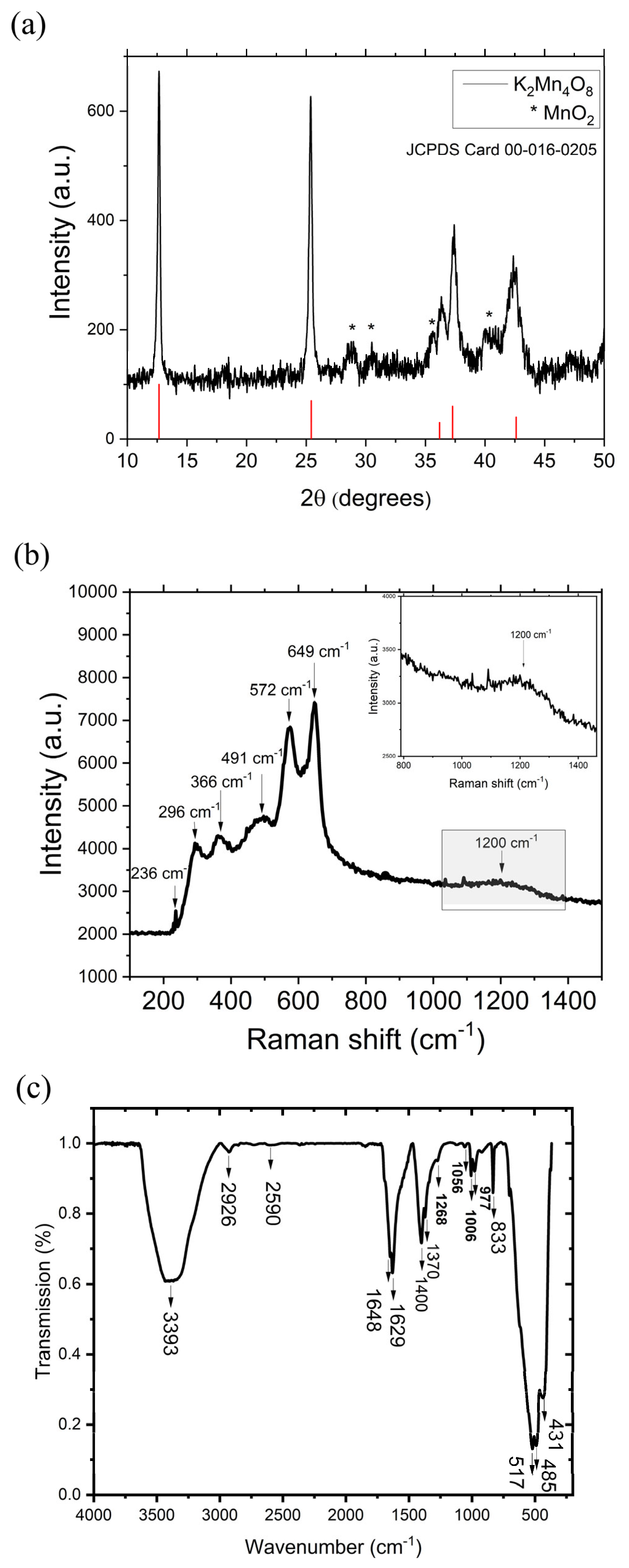
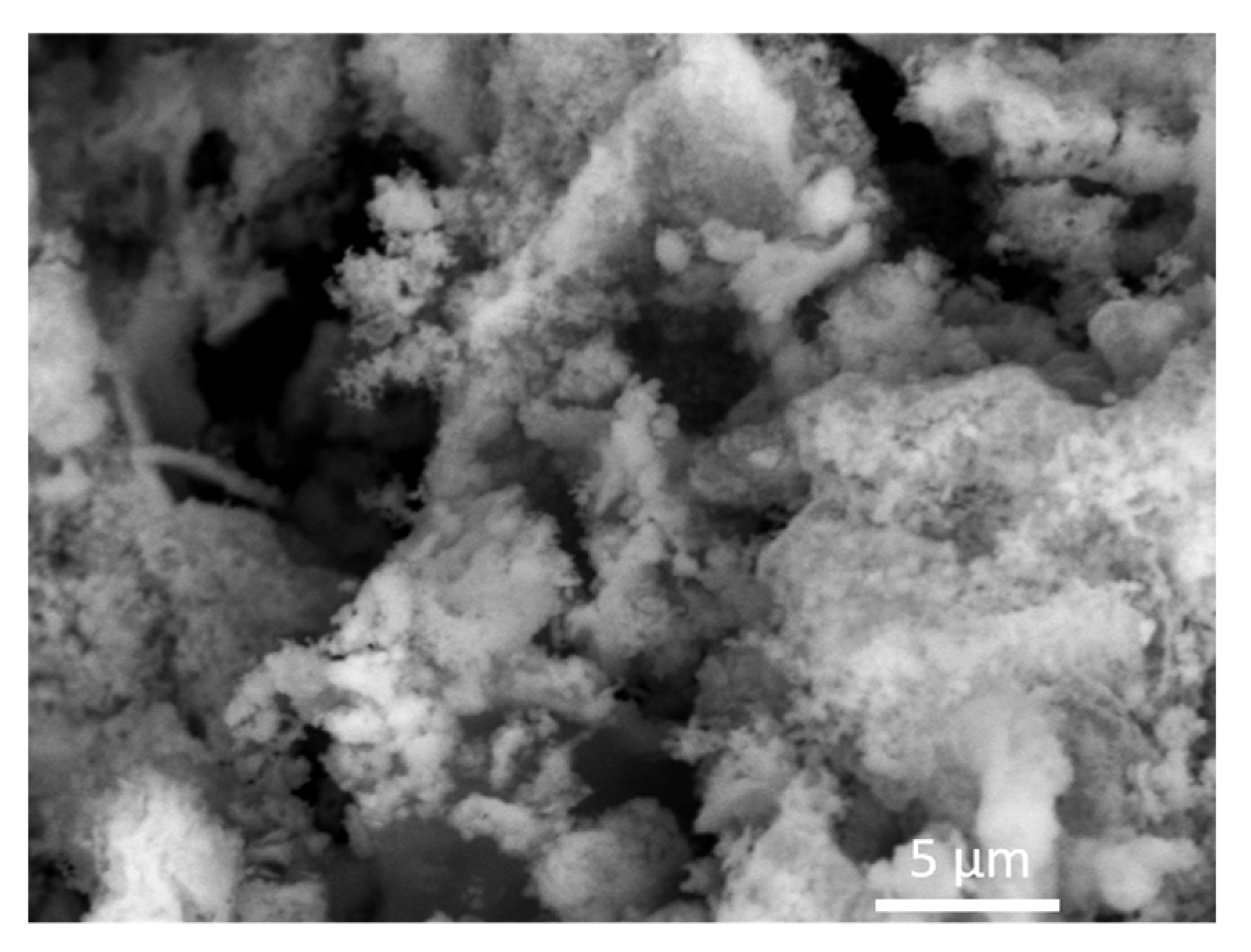
3.1. Structural and Morphological Analysis of Birnessite-Type K2Mn4O8 Materials
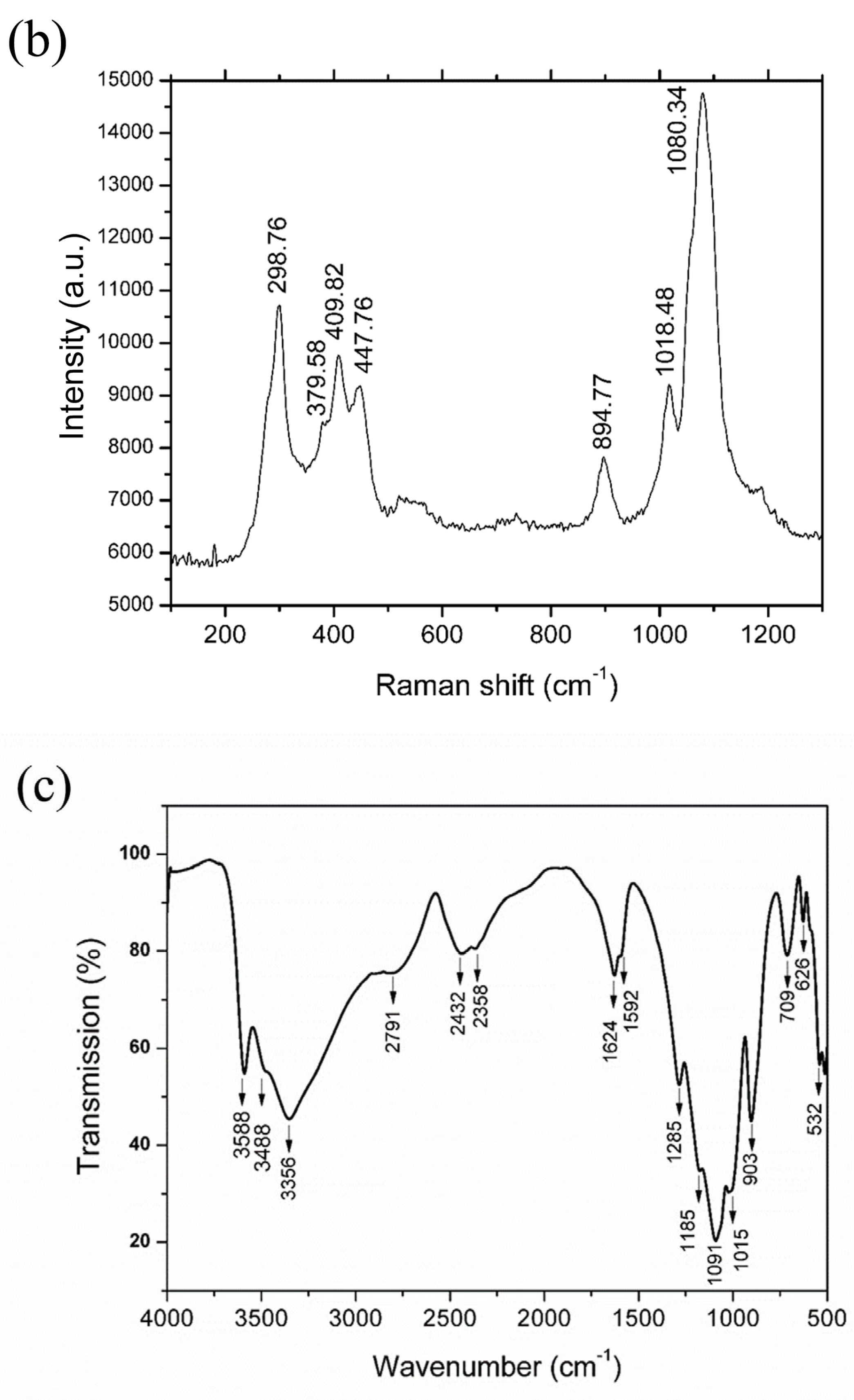
3.2. Structural and Morphological Analysis of Mixed-Valence Iron Phosphate Fe3(PO3OH)4(H2O)4 Materials
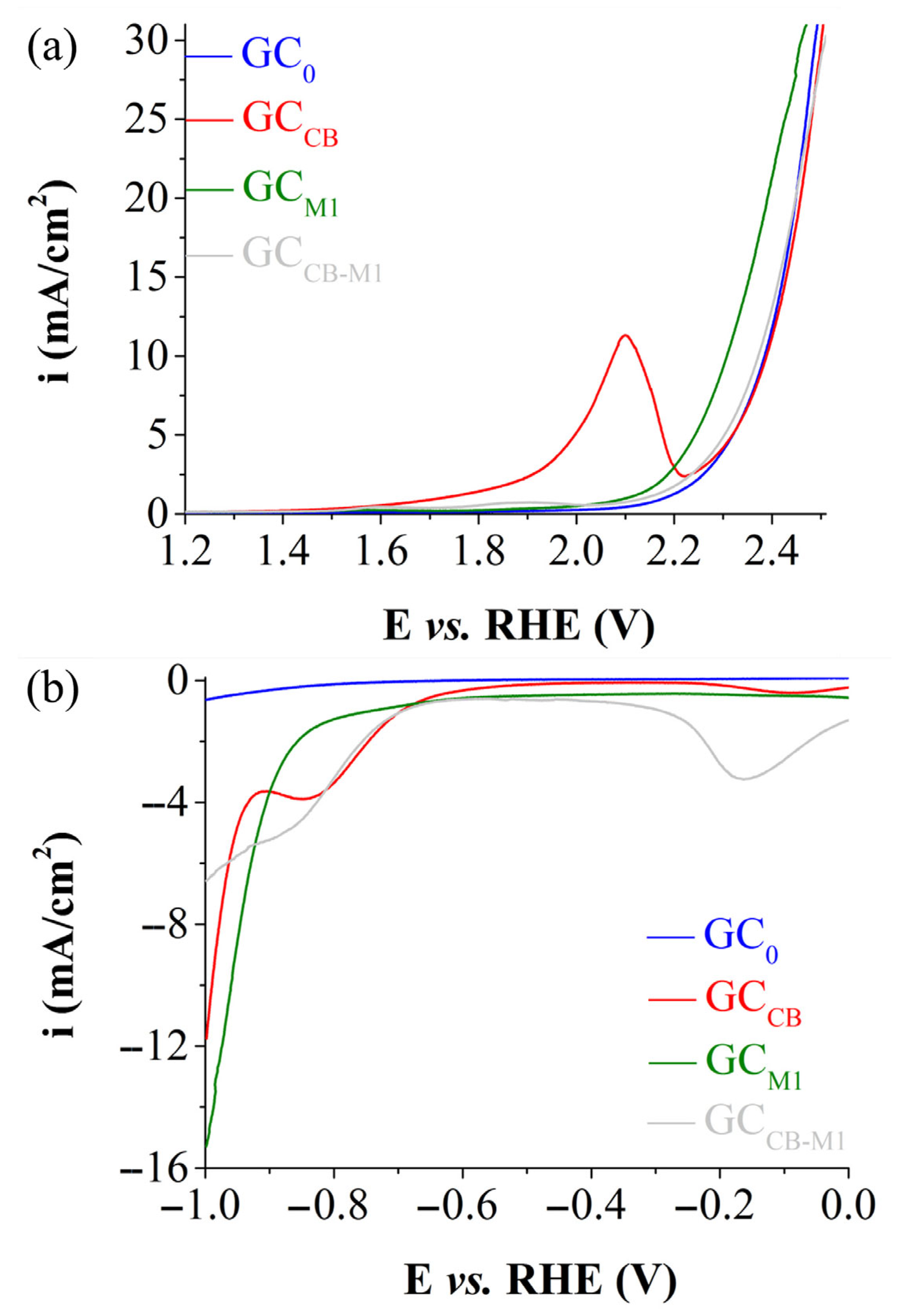
3.3. Electrochemical Study of K2Mn4O8-Based Electrodes
3.4. Electrochemical Study of Fe3(PO3OH)4(H2O)4-Based Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A global assessment: Can renewable energy replace fossil fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Chowdhury, C.R.; Yadav, D.; Verma, R.; Dutta, S.; Jaiswal, K.S.; Sangmesh, B.; Karuppasamy, K.S.K. Renewable and sustainable clean energy development and impact on social, economic, and environmental health. Energy Nexus 2022, 7, 100118. [Google Scholar] [CrossRef]
- de Lange, D.E. Climate action now: Energy industry restructuring to accelerate the renewable energy transition. J. Clean. Prod. 2024, 443, 141018. [Google Scholar] [CrossRef]
- Reda, B.; Elzamar, A.A.; AlFazzani, S.; Ezzat, S.M. Green hydrogen as a source of renewable energy: A step towards sustainability, an overview. Environ. Dev. Sustain. 2024, 1–21. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Z.-S. Scalable production of high-performance electrocatalysts for electrochemical water splitting at large current densities. eScience 2024, 5, 100334. [Google Scholar] [CrossRef]
- Raveendran, A.; Chandran, M.; Dhanusuraman, R. A comprehensive review on the electrochemical parameters and recent material development of electrochemical water splitting electrocatalysts. RSC Adv. 2023, 13, 3843–3876. [Google Scholar] [CrossRef] [PubMed]
- Araujo, H.; Sljukic, B.; Gago, S.; Santos, D.M.F. The current state of transition metal-based electrocatalysts (oxides, alloys, POMs, and MOFs) for oxygen reduction, oxygen evolution, and hydrogen evolution reactions. Front. Energy Res. 2024, 12, 1373522. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liu, J.; Wu, H.; Wu, K.; Lyu, C.; Wu, J.; Lau, W.-M.; Wu, Q.; Zheng, J. The role of manganese-based catalyst in electrocatalytic water splitting: Recent research and progress. Mater. Today Phys. 2023, 36, 101169. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, Y.; Liu, Y.; Xu, Z.; Li, H. Recent Advances in Manganese-Based Materials for Electrolytic Water Splitting. Int. J. Mol. Sci. 2023, 24, 6861. [Google Scholar] [CrossRef]
- Dutta, B.; Clarke, R.; Raman, S.; Shaffer, T.D.; Achola, L.; Nandi, P.; Suib, S.L. Lithium promoted mesoporous manganese oxide catalyzed oxidation of allyl ethers. Nat. Commun. 2019, 10, 655. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Shi, J.; Tan, D.; Liu, L.; Zhang, F.; Lu, C.; Su, Z.; Tan, X.; Cheng, X.; et al. Manganese acting as a high-performance heterogeneous electrocatalyst in carbon dioxide reduction. Nat. Commun. 2019, 10, 2980. [Google Scholar] [CrossRef]
- Lin, B.; Zhu, X.; Fang, L.; Liu, X.; Li, S.; Zhai, T.; Xue, L.; Guo, Q.; Xu, J.; Xia, H. Birnessite Nanosheet Arrays with High K Content as a High-Capacity and Ultrastable Cathode for K-Ion Batteries. Adv. Mater. 2019, 31, 1900060. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Pan, Z.; Feng, F.; Gu, Y.; Du, J.; Zhao, Y. Design of CeMnCu ternary mixed oxides as soot combustion catalysts based on optimized Ce/Mn and Mn/Cu ratios in binary mixed oxides. Appl. Catal. B Environ. 2020, 268, 118422. [Google Scholar] [CrossRef]
- Yu, D.; Ren, Y.; Yu, X.; Fan, X.; Wang, L.; Wang, R.; Zhao, Z.; Cheng, K.; Chen, Y.; Sojka, Z.; et al. Facile synthesis of birnessite-type K2Mn4O8 and cryptomelane-type K2−xMn8O16 catalysts and their excellent catalytic performance for soot combustion with high resistance to H2O and SO2. Appl. Catal. B Environ. 2021, 285, 119779. [Google Scholar] [CrossRef]
- Weng, J.; Duan, J.; Sun, C.; Liu, P.; Li, A.; Zhou, P.; Zhou, J. Construction of hierarchical K0.7Mn0.7Mg0.3O2 microparticles as high capacity & long cycle life cathode materials for low-cost potassium-ion batteries. Chem. Eng. J. 2020, 392, 123649. [Google Scholar] [CrossRef]
- Guo, R.; Lai, X.; Huang, J.; Du, X.; Yan, Y.; Sun, Y.; Zou, G.; Xiong, J. Phosphate-based electrocatalysts for water splitting: Recent progress. ChemElectroChem 2018, 5, 3822–3834. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.; Kim, S.H.; Park, J.; Kim, S.; Kwon, S.-H.; Bae, J.-S.; Park, Y.S.; Kim, Y. Cobalt–iron–phosphate hydrogen evolution reaction electrocatalyst for solar-driven alkaline seawater electrolyzer. Nanomaterials 2021, 11, 2989. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, P.; Maiyalagan, T. Phosphorus-doped cobalt sulphide nanocubes as an electrocatalyst for the hydrogen evolution reaction in an alkaline medium. New J. Chem. 2025, 49, 3997–4006. [Google Scholar] [CrossRef]
- Poienar, M.; Taranu, B.-O.; Svera, P.; Sfirloaga, P.; Vlazan, P. Disclosing the thermal behaviour, electrochemical and optical properties of synthetic Fe3(PO4)2(OH)2 materials. J. Therm. Anal. Calorim. 2022, 147, 11839–11855. [Google Scholar] [CrossRef]
- Poienar, M.; Gutmann, M.J.; Pascut, G.L.; Petrícek, V.; Stenning, G.; Vlazan, P.; Sfirloaga, P.; Paulmann, C.; Tolkiehn, M.; Manuel, P.; et al. Phase Transitions and Physical Properties of the Mixed Valence Iron Phosphate Fe3(PO3OH)4(H2O)4. Materials 2022, 15, 8059. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Rus, F.S.; Fagadar-Cosma, E. A3B Zn(II)-porphyrin-coated carbon electrodes obtained using different procedures and tested for water electrolysis. Coatings 2024, 14, 1048. [Google Scholar] [CrossRef]
- Sfirloaga, P.; Bognár, S.; Taranu, B.-O.; Vlazan, P.; Poienar, M.; Merkulov, D.Š. Co- and Sn-doped YMnO3 perovskites for electrocatalytic water-splitting and photocatalytic pollutant degradation. Coatings 2025, 15, 475. [Google Scholar] [CrossRef]
- Chang, J.; Lv, Q.; Li, G.; Ge, J.; Liu, C.; Xing, W. Core-shell structured Ni12P5/Ni3(PO4)2 hollow spheres as difunctional and efficient electrocatalysts for overall water electrolysis. Appl. Catal. B Environ. 2017, 204, 486–496. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Fagadar-Cosma, E. The pH influence on the water-splitting electrocatalytic activity of graphite electrodes modified with symmetrically substituted metalloporphyrins. Nanomaterials 2022, 12, 3788. [Google Scholar] [CrossRef]
- Ciriaco, M.L.F.; Silva-Pereira, M.I.; Nunes, M.R.; Costa, F.M. Electrochemical behaviour of BaSn0.9Sb0.1O3 coated titanium electrodes. Port. Electrochim. Acta 1999, 17, 149–156. [Google Scholar] [CrossRef]
- Taranu, B.-O. Different electrode modification protocols for evaluating the water-splitting properties of a P(V)-metalloporphyrin. J. Serbian Chem. Soc. 2025, 90, 447–459. [Google Scholar] [CrossRef]
- Hao, S.-M.; Qu, J.; Yang, J.; Gui, C.-X.; Wang, Q.-Q.; Li, Q.-J.; Li, X.; Yu, Z.-Z. K2Mn4O8/Reduced Graphene Oxide Nanocomposites for Excellent Lithium Storage and Adsorption of Lead Ions. Chem. Eur. J. 2016, 22, 3397–3404. [Google Scholar] [CrossRef]
- Yin, H.; Liu, Y.; Koopal, L.K.; Feng, X.; Chu, S.; Zhu, M.; Liu, F. High Co-doping promotes the transition of birnessite layer symmetry from orthogonal to hexagonal. Chem. Geol. 2015, 410, 12–20. [Google Scholar] [CrossRef]
- Liu, C.; Luo, S.; Huang, H.; Zhai, Y.; Wang, Z. Layered potassium-deficient P2- and P3-type cathode materials KxMnO2 for K-ion batteries. Chem. Eng. J. 2019, 356, 53–59. [Google Scholar] [CrossRef]
- Hironaka, Y.; Kubota, K.; Komaba, S. P2- and P3-KxCoO2 as an electrochemical potassium intercalation host. Chem. Commun. 2017, 53, 3693–3696. [Google Scholar] [CrossRef]
- Kim, H.; Seo, D.-H.; Kim, J.C.; Bo, S.-H.; Liu, L.; Shi, T.; Cede, G. Investigation of Potassium Storage in Layered P3-Type K0.5MnO2 Cathode. Adv. Mater. 2017, 29, 1702480. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.; Massot, M.; Baddour-Hadjean, R.; Franger, S.; Bach, S.; Pereira-Ramos, J.-P. Raman spectra of birnessite manganese dioxides. Solid State Ionics 2003, 159, 345. [Google Scholar] [CrossRef]
- Julien, C.; Massot, M. Spectroscopic studies of the local structure in positive electrodes for lithium batteries. Phys. Chem. Chem. Phys. 2002, 4, 4226. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, F.; Huang, X.; Li, B.; Guan, G.; Zhang, W.; Zou, R.; Lu, X.; Hu, J. Hydrophilic K2Mn4O8 nanoflowers as a sensitive photothermal theragnosis synergistic platform for the ablation of cancer. New J. Chem. 2018, 42, 3714–3721. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, D.; Zhuo, R.; Li, S.; Wu, Z.; Wang, J.; Ren, P.; Yan, P.; Geng, Z. Design, hydrothermal synthesis and electrochemical properties of porous birnessite-type manganese dioxide nanosheets on graphene as a hybrid material for supercapacitors. J. Power Sources 2013, 242, 78–85. [Google Scholar] [CrossRef]
- Ogata, A.; Komaba, S.; Baddour-Hadjean, R.; Pereira-Ramos, J.-P.; Kumagai, N. Doping effects on structure and electrode performance of K-birnessite-type manganese dioxides for rechargeable lithium battery. Electrochim. Acta 2008, 53, 3084–3093. [Google Scholar] [CrossRef]
- Ren, G.; Sun, M.; Sun, Y.; Li, Y.; Wang, C.; Lu, A.; Ding, H. A cost-effective birnessite–silicon solar cell hybrid system with enhanced performance for dye decolorization. RSC Adv. 2017, 7, 47975. [Google Scholar] [CrossRef]
- Gailloot, A.-C.; Flot, D.; Drits, V.A.; Manceau, A.; Burghammer, M.; Lanson, B. Structure of Synthetic K-rich Birnessite Obtained by High-Temperature Decomposition of KMnO4. I. Two-Layer Polytype from 800 °C Experiment. Chem. Mater. 2003, 15, 4666–4678. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, F.; Cao, S.; Yin, K.; Chang, X.; Fan, R.; Fan, C.; Dong, L.; Yin, Y.; Chen, X. Hierarchical K2Mn4O8 nanoflowers: A novel photothermal conversion material for efficient solar vapor generation. Sol. Energy Mater. Sol. Cells 2019, 200, 110043. [Google Scholar] [CrossRef]
- Martinez-Alonso, S.; Rustad, J.R.; Goetz, A.F.H. Ab initio quantum mechanical modeling of infrared vibrational frequencies of the OH group in dioctahedral phyllosilicates. Part II: Main physical factors governing the OH vibrations. Am. Mineral. 2002, 87, 1224. [Google Scholar] [CrossRef]
- Vencato, I.; Mascarenhas, Y.P.; Mattievich, E. The crystal structure of FeFe2(PO3OH)4(H2O)4: A new synthetic compound of mineralogic interest. Am. Mineral. 1986, 71, 222. [Google Scholar]
- Frost, R.L.; Xi, Y.; Lopez, A.; Scholz, R.; de Carvalho Lana, C.; de Souza, B.F. Vibrational spectroscopic characterization of the phosphate mineral barbosalite Fe2+Fe23+(PO4)2(OH)2—Implications for the molecular structure. J. Mol. Struct. 2013, 1051, 292–298. [Google Scholar] [CrossRef]
- Battle, P.D.; Cheetham, A.K.; Gleitzer, C.; Harrison, W.T.; Long, G.J.; Longworth, G. A novel magnetic phase transition in anhydrous iron(III) phosphate, FePO4. J. Phys. C Solid State Phys. 1982, 15, L919–L924. [Google Scholar] [CrossRef]
- Povarennykh, A.S. The use of infrared spectra for the determination of minerals. Am. Miner. 1978, 63, 956–959. [Google Scholar]
- Chukanov, N.V.; Chervonnyi, A.D. Infrared Spectroscopy of Minerals and Related Compounds; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Hou, W.; Zhou, C.; Li, Q.; Zhou, H.; Liu, Z.; Yang, L.; Wu, C.; Zhao, H.; Dong, S. Preparation and overall water-splitting performance study of amorphous nickel-copper-phosphide. J. Alloys Compd. 2024, 1002, 175314. [Google Scholar] [CrossRef]
- Aralekallu, S.; Lokesh, K.S.; Singh, V. Advanced bifunctional catalysts for energy production by electrolysis of earth-abundant water. Fuel 2024, 357 Pt A, 129753. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Fagadar-Cosma, E.; Sfirloaga, P.; Poienar, M. Free-base porphyrin aggregates combined with nickel phosphite for enhanced alkaline hydrogen evolution. Energies 2023, 16, 1212. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Vlazan, P.; Svera, P.; Poienar, M.; Sfirloaga, P. New functional hybrid materials based on clay minerals for enhanced electrocatalytic activity. J. Alloys Compd. 2022, 892, 162239. [Google Scholar] [CrossRef]
- Poienar, M.; Svera, P.; Taranu, B.-O.; Ianasi, C.; Sfirloaga, P.; Buse, G.; Veber, P.; Vlazan, P. Electrochemical investigation of the OER activity for nickel phosphite-based compositions and its morphology-dependent fluorescence properties. Crystals 2022, 12, 1803. [Google Scholar] [CrossRef]
- Wang, J.; Zang, W.; Liu, X.; Sun, J.; Xi, S.; Liu, W.; Kou, Z.; Shen, L.; Wang, J. Switch Volmer-Heyrovsky to Volmer-Tafel pathway for efficient acidic electrocatalytic hydrogen evolution by correlating Pt single atoms with clusters. Small 2024, 20, 2309427. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Hu, B.-C.; Wu, P.; Liang, H.-W.; Yu, Z.-L.; Lin, Y.; Zheng, Y.-R.; Li, Z.; Yu, S.-H. Mo2C nanoparticles embedded within bacterial cellulose-derived 3D N-doped carbon nanofiber networks for efficient hydrogen evolution. NPG Asia Mater. 2016, 8, e288. [Google Scholar] [CrossRef]
- Cong, Z.; Shuaiyang, W.; Junfeng, R.; Wanliang, M. Amorphous catalysts for electrochemical water splitting. China Pet. Process. Petrochem. Technol. 2022, 24, 1–13. [Google Scholar]
- Pardanaud, C.; Cartry, G.; Lajaunie, L.; Arenal, R.; Buijnsters, J.G. Investigating the Possible Origin of Raman Bands in Defective sp2/sp3 Carbons below 900 cm−1: Phonon Density of States or Double Resonance Mechanism at Play? C—J. Carbon Res. 2019, 5, 79. [Google Scholar] [CrossRef]
- Wei, C.; Rao, R.R.; Peng, J.; Huang, B.; Stephens, I.E.L.; Risch, M.; Xu, Z.J.; Shao-Horn, Y. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv. Mater. 2019, 31, 1806296. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Mamgain, H.; Jacob, C. Influence of hydrogen on chemical vapour synthesis of different carbon nanostructures using propane as precursor and nickel as catalyst. Bull. Mater. Sci. 2014, 37, 1197–1204. [Google Scholar] [CrossRef]
- Lai, F.; Shang, H.; Jiao, Y.; Chen, X.; Zhang, T.; Liu, X. Recent progress and perspective on electrocatalysis in neutral media: Mechanisms, materials, and advanced characterizations. Mater. Interdiscip. Mater. 2024, 3, 492–529. [Google Scholar] [CrossRef]
- Luo, Z.; Cong, C.; Zhang, J.; Xiong, Q.; Yu, T. The origin of sub-bands in the Raman D-band of graphene. Carbon 2012, 50, 4252–4258. [Google Scholar] [CrossRef]
- Dychalska, A.; Popielarski, P.; Franków, W.; Fabisiak, K.; Paprocki, K.; Szybowicz, M. Study of CVD diamond layers with amorphous carbon admixture by Raman scattering spectroscopy. Mater. Sci.-Pol. 2015, 33, 799–805. [Google Scholar] [CrossRef]
- Shaheen, S.; Ali, S.A.; Mir, U.F.; Sadiq, I.; Ahmad, T. Recent Advances in Transition Metal Phosphide Nanocatalysts for H2 Evolution and CO2 Reduction. Catalysts 2023, 13, 1046. [Google Scholar] [CrossRef]
- Luo, Q.; Zhao, Y.; Sun, L.; Wang, C.; Xin, H.; Song, J.; Li, D.; Ma, F. Interface oxygen vacancy enhanced alkaline hydrogen evolution activity of cobalt-iron phosphide/CeO2 hollow nanorods. Chem. Eng. J. 2022, 437, 135376. [Google Scholar] [CrossRef]
- Liu, L.; Chen, L.; Liang, J.; Liu, L.; Han, H. A Novel Ratiometric Probe Based on Nitrogen-Doped Carbon Dots and Rhodamine B Isothiocyanate for Detection of Fe3+ in Aqueous Solution. J. Anal. Methods Chem. 2016, 2016, 4939582. [Google Scholar] [CrossRef]
- Burba, C.M.; Palmer, J.M.; Holinsworth, B.S. Laser-induced phase changes in olivine FePO4: A warning on characterizing LiFePO4-based cathodes with Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 225–228. [Google Scholar] [CrossRef]
- Suryanto, B.H.R.; Wang, Y.; Hocking, R.K.; Adamson, W.; Zhao, C. Overall electrochemical splitting of water at the heterogeneous interface of nickel and iron oxide. Nat. Commun. 2019, 10, 5599. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Chakraborty, P.; Kumar, B.; Mandal, S.; Dey, S.K. Fe-Based Materials for Electrocatalytic Water Splitting: A Mini Review. ChemCatChem 2024, 16, e202400622. [Google Scholar] [CrossRef]
- Hui, T.; Zheng, T.; Cheng, X.; Li, T.; Zhang, R.; Meng, X.; Liu, H.; Liu, Z.; Xu, C. A review of plasma treatment on nano-microstructure of electrochemical water splitting catalysts. Chin. J. Struct. Chem. 2025, 44, 100520. [Google Scholar] [CrossRef]
- Liu, S.; Wei, Y.; Wang, M.; Shen, Y. The future of alkaline water splitting from the perspective of electrocatalysts-seizing today’s opportunities. Coord. Chem. Rev. 2025, 522, 216190. [Google Scholar] [CrossRef]
- Tran, D.T.; Tran, P.K.L.; Malhotra, D.; Nguyen, T.H.; Nguyen, T.T.A.; Duong, N.T.A.; Kim, N.H.; Lee, J.H. Current status of developed electrocatalysts for water splitting technologies: From experimental to industrial perspective. Nano Converg. 2025, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, D.; Feng, X.; Zhi, C.; Jia, Y.; Zhang, J.; Zhang, Y.; Chen, Y.; Shi, L.; Shi, J.-W. Design and modification of layered double hydroxides-based compounds in electrocatalytic water splitting: A review. Small 2025, 21, 2412576. [Google Scholar] [CrossRef]
- Sadeghi, E.; Chamani, S.; Sadat Peighambardoust, N.; Aydemir, U. Unveiling the Potential of Metal Diborides for Electrocatalytic Water Splitting: A Comprehensive Review. Energy Environ. Mater. 2025, 8, e12873. [Google Scholar] [CrossRef]
- Jia, H.; Yao, Y.; Gao, Y.; Lu, D.; Du, P. Pyrolyzed cobalt porphyrin-based conjugated mesoporous polymers as bifunctional catalysts for hydrogen production and oxygen evolution in water. Chem. Commun. 2016, 52, 13483–13486. [Google Scholar] [CrossRef]
- Cui, S.; Qian, M.; Liu, X.; Sun, Z.; Du, P. A copper porphyrin-based conjugated mesoporous polymer-derived bifunctional electrocatalyst for hydrogen and oxygen evolution. ChemSusChem 2016, 9, 2365. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Du, A.; Gao, G.; Chen, J.; Yan, X.; Brown, C.L.; Yao, X. Defect graphene as a trifunctional catalyst for electrochemical reactions. Adv. Mater. 2016, 28, 9532–9538. [Google Scholar] [CrossRef]
- Qu, K.; Zheng, Y.; Jiao, Y.; Zhang, X.; Dai, S.; Qiao, S.Z. Polydopamine-inspired, dual heteroatom-doped carbon nanotubes for highly efficient overall water splitting. Adv. Energy Mater. 2017, 7, 1602068. [Google Scholar] [CrossRef]
- Shit, S.; Chhetri, S.; Jang, W.; Murmu, N.C.; Koo, H.; Samanta, P.; Kuila, T. Cobalt sulfide/nickel sulfide heterostructure directly grown on nickel foam: An efficient and durable electrocatalyst for overall water splitting application. ACS Appl. Mater. Inter. 2018, 10, 27712–27722. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Sun, H.; Wang, H.; Rong, F.; He, L.; Lou, Y.; Zhang, S.; Zhang, Z.; Du, M. CoOx/CoNy nanoparticles encapsulated carbon-nitride nanosheets as an efficiently trifunctional electrocatalyst for overall water splitting and Zn-air battery. Appl. Catal. B Environ. 2020, 279, 119407. [Google Scholar] [CrossRef]
- Wang, A.; Cheng, L.; Zhao, W.; Shen, X.; Zhu, W. Electrochemical hydrogen and oxygen evolution reactions from a cobalt-porphyrin-based covalent organic polymer. J. Colloid Interface Sci. 2020, 579, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Zeng, L.; He, L.; Sun, S.; Tong, Y.; Zhang, J. Imine gels based on ferrocene and porphyrin and their electrocatalytic property. Chem. Asian J. 2020, 15, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, D.; Li, J.; Shi, Q.; Zhao, J.; Hu, Y.; Zeng, F.; Wang, N. Covalent metalloporphyrin polymer coated on carbon nanotubes as bifunctional electrocatalysts for water splitting. Inorg. Chem. 2022, 61, 10198–10204. [Google Scholar] [CrossRef] [PubMed]
- Ocuane, N.; Ge, Y.; Sandoval-Pauker, C.; Villagrán, D. Bifunctional porphyrin-based metal–organic polymers for electrochemical water splitting. Dalton Trans. 2024, 53, 2306–2317. [Google Scholar] [CrossRef]
- Kousar, N.; Giddaerappa; Sannegowda, L.K. Hybrid cobalt phthalocyanine polymer as a potential electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 50, 37–47. [Google Scholar] [CrossRef]


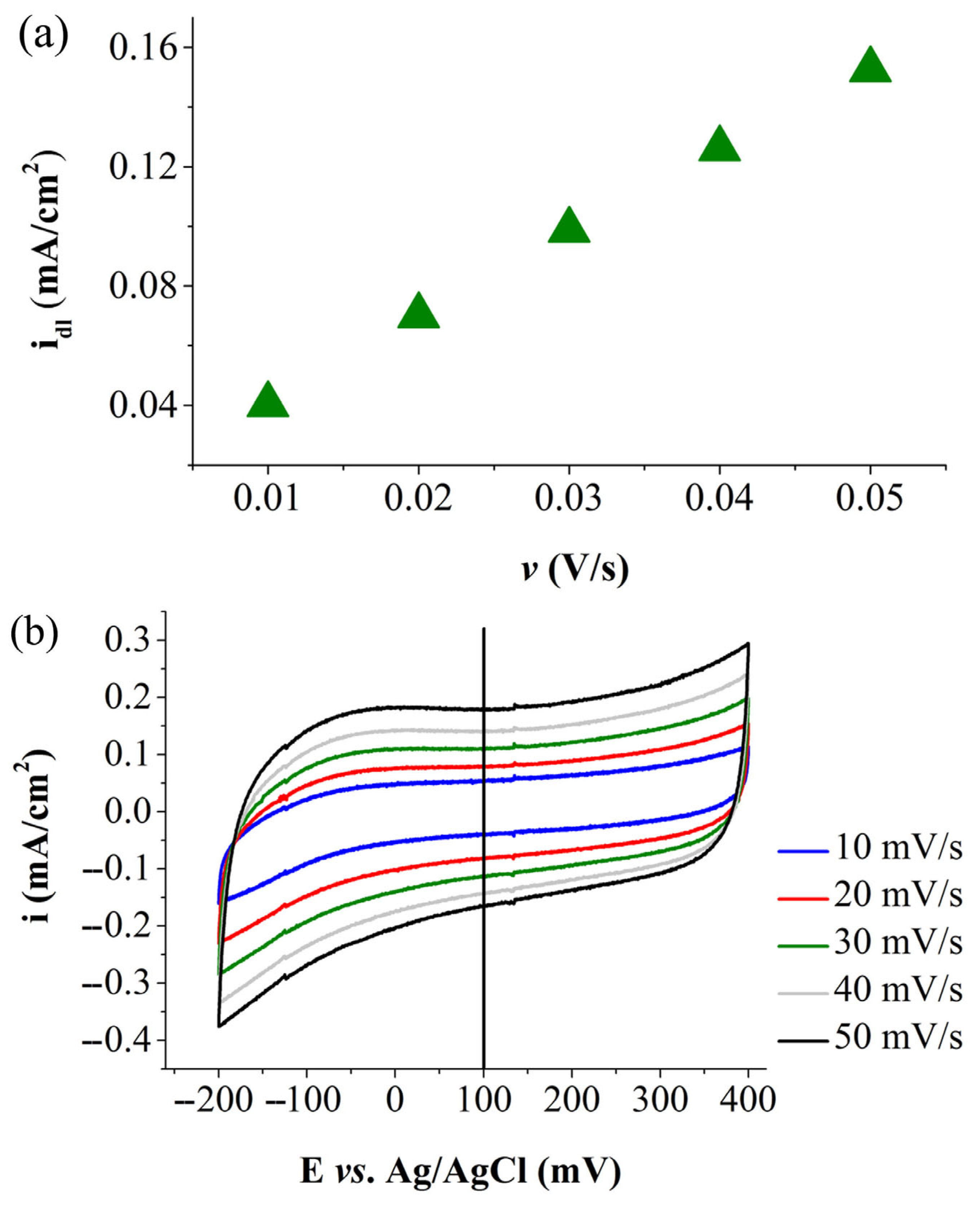

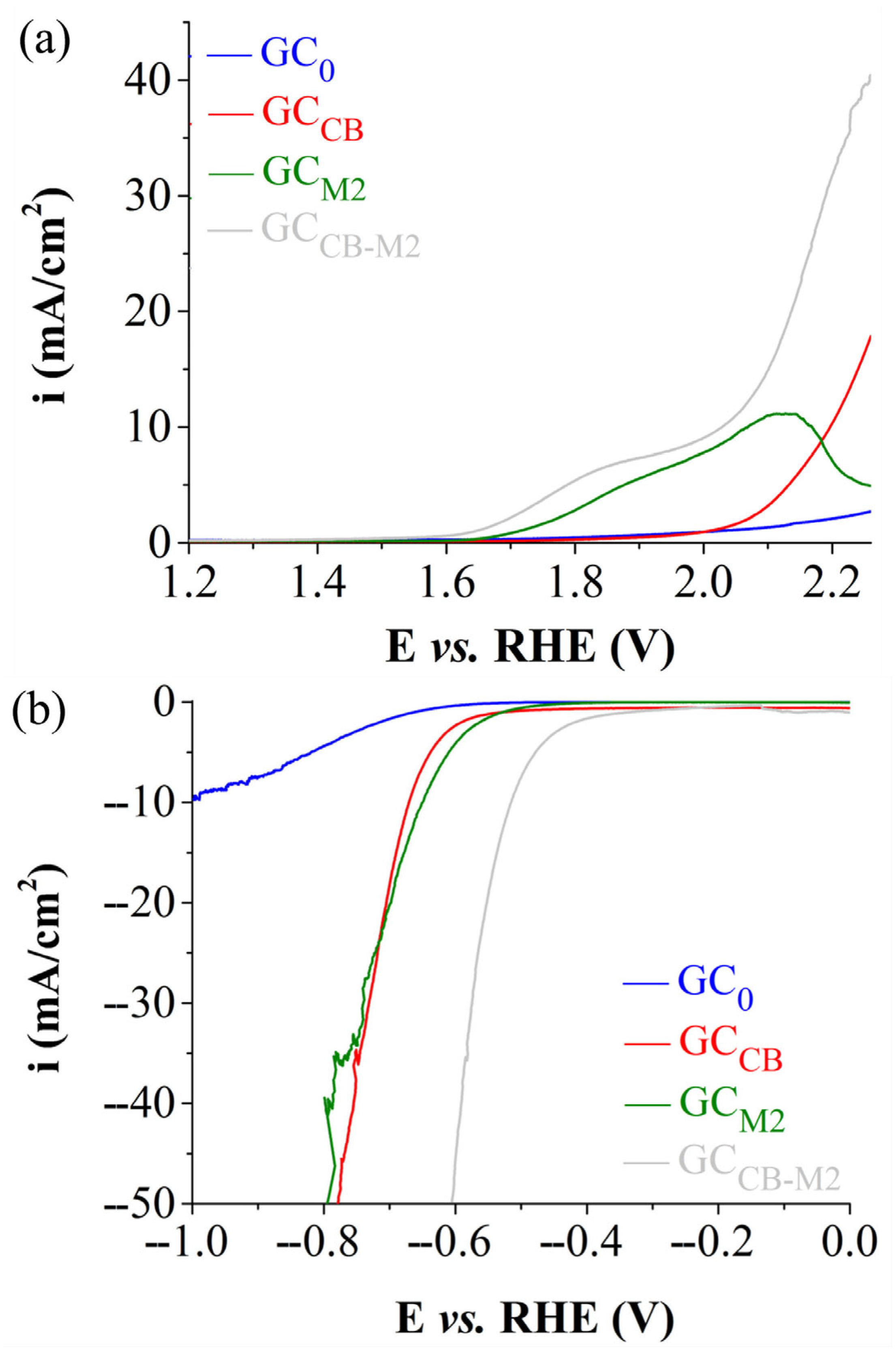
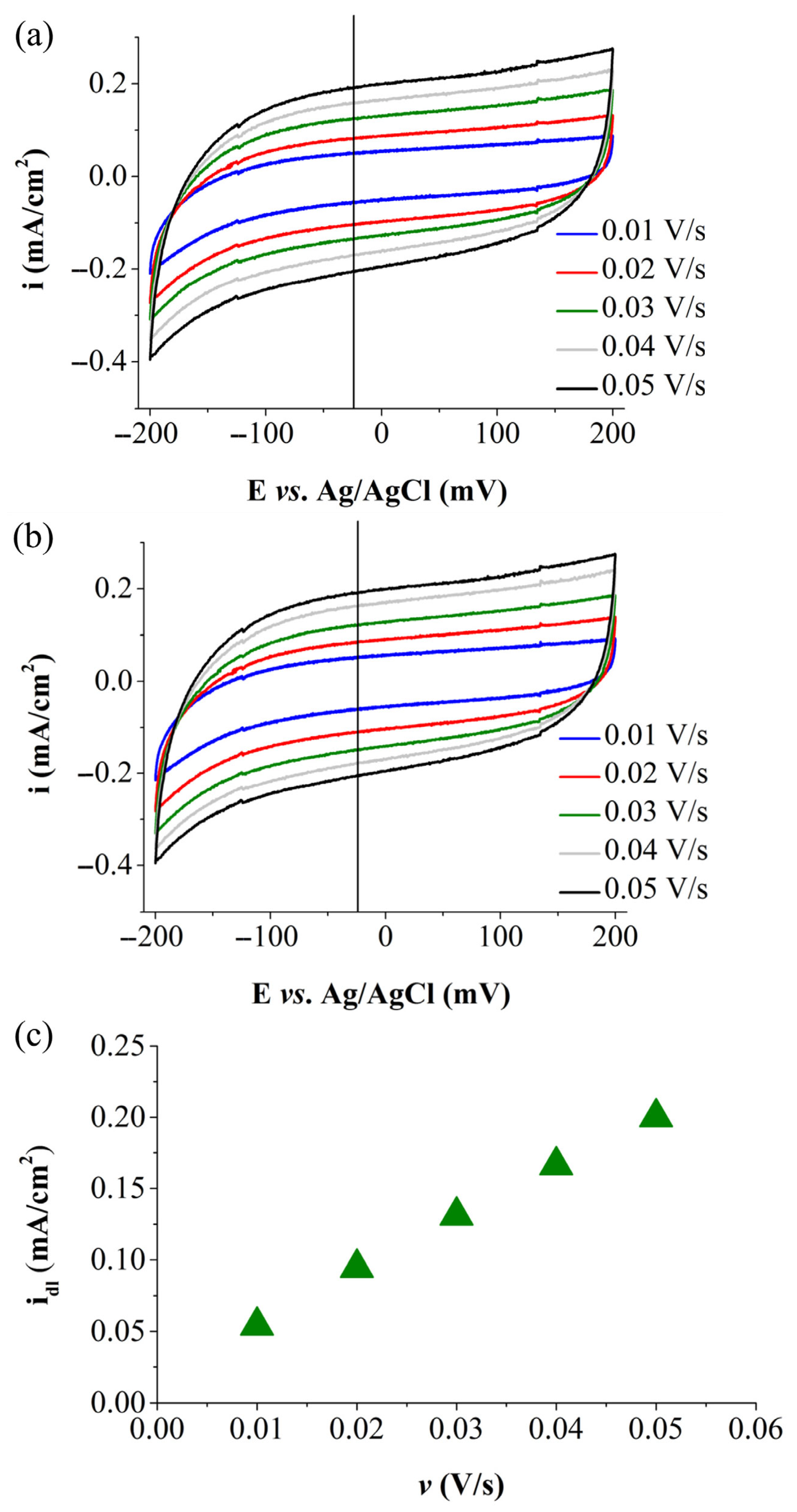
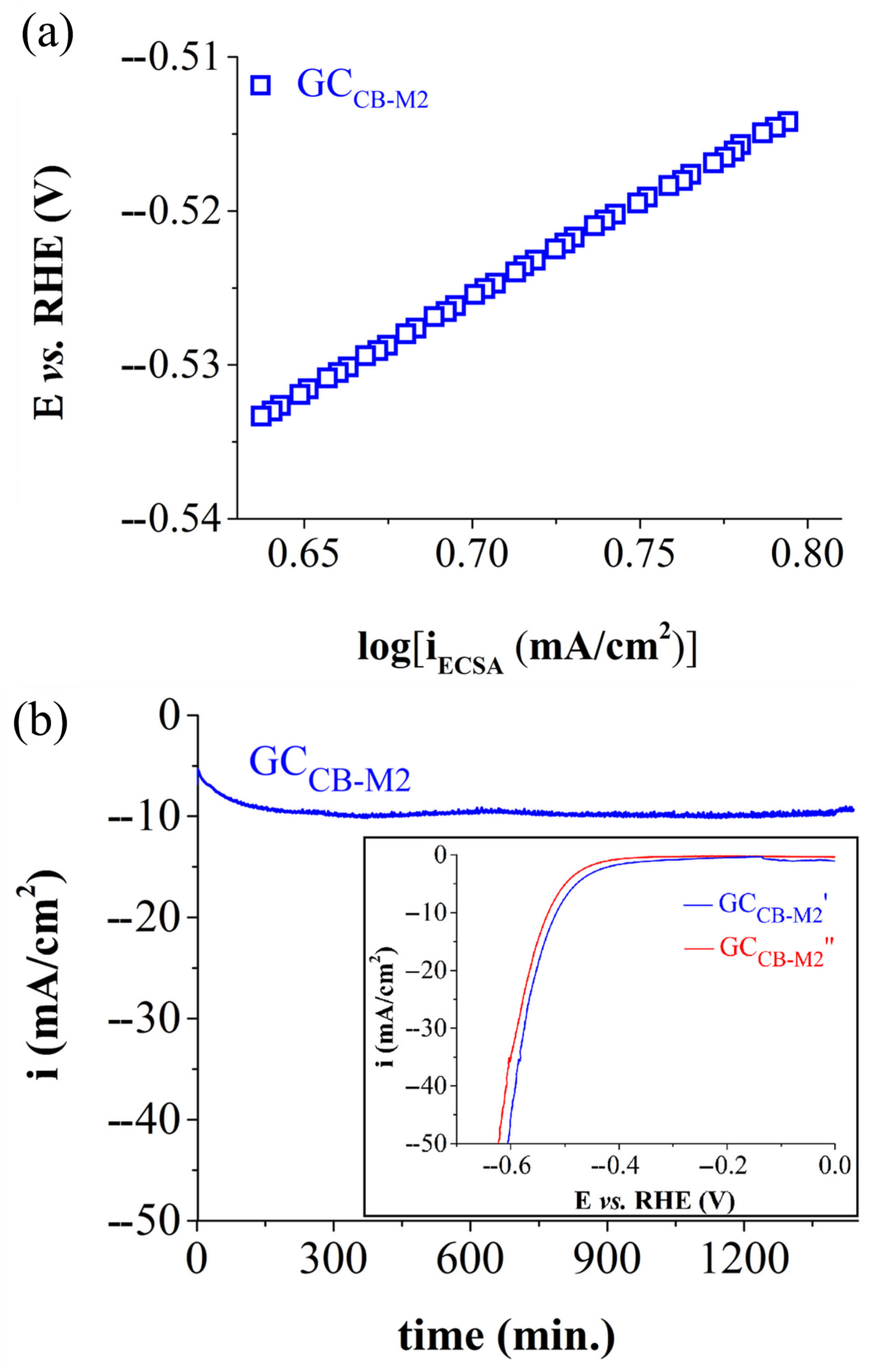
| Electrode Labels | K2Mn4O8 (mg) | Fe3(PO3OH)4(H2O)4 (mg) | Carbon Black (mg) | Nafion Solution (µL) | Ethanol (µL) |
|---|---|---|---|---|---|
| GC0 | - | - | - | - | - |
| GCCB | - | - | 5 | 50 | 450 |
| GCM1 | 5 | - | - | 50 | 450 |
| GCCB-M1 | 5 | - | 5 | 100 | 900 |
| GCM2 | - | 5 | - | 50 | 450 |
| GCCB-M2 | - | 5 | 5 | 100 | 900 |
| Electrode | Cdl (mF/cm2) | R2 | Rf | ECSA (cm2) |
|---|---|---|---|---|
| GCM1 | 2.805 | 0.9992 | 46.75 | 13.09 |
| Electrode | Cdl (mF/cm2) | R2 | Rf | ECSA (cm2) |
|---|---|---|---|---|
| GCCB-M2 | 3.637 | 0.9984 | 60.617 | 16.973 |
| Electrode Labels | GCM1 | GCCB-M2 |
|---|---|---|
| Parameters | ||
| ηOER (V) at i = 10 mA/cm2 | 1.07 | 0.80 |
| ηHER (V) at i = −10 mA/cm2 | 0.957 | 0.515 |
| Cdl (mF/cm2) | 2.805 (R2 = 0.9992) | 3.637 (R2 = 0.9984) |
| Rf | 46.75 | 60.617 |
| ECSA (cm2) | 13.09 | 16.973 |
| OER Tafel slope (V) | 0.180 (R2 = 0.9998) | - |
| HER Tafel slope (V) | 0.142 (R2 = 0.9993) | 0.122 (R2 = 0.9998) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taranu, B.-O.; Svera, P.; Buse, G.; Poienar, M. Insights into the Electrocatalytic Activity of Mixed-Valence Mn3+/Mn4+ and Fe2+/Fe3+ Transition Metal Oxide Materials. Solids 2025, 6, 48. https://doi.org/10.3390/solids6030048
Taranu B-O, Svera P, Buse G, Poienar M. Insights into the Electrocatalytic Activity of Mixed-Valence Mn3+/Mn4+ and Fe2+/Fe3+ Transition Metal Oxide Materials. Solids. 2025; 6(3):48. https://doi.org/10.3390/solids6030048
Chicago/Turabian StyleTaranu, Bogdan-Ovidiu, Paula Svera, Gabriel Buse, and Maria Poienar. 2025. "Insights into the Electrocatalytic Activity of Mixed-Valence Mn3+/Mn4+ and Fe2+/Fe3+ Transition Metal Oxide Materials" Solids 6, no. 3: 48. https://doi.org/10.3390/solids6030048
APA StyleTaranu, B.-O., Svera, P., Buse, G., & Poienar, M. (2025). Insights into the Electrocatalytic Activity of Mixed-Valence Mn3+/Mn4+ and Fe2+/Fe3+ Transition Metal Oxide Materials. Solids, 6(3), 48. https://doi.org/10.3390/solids6030048






