Recent Progress on Synthesis and Electrochemical Performance of Iron Fluoride Conversion Cathodes for Li-Ion Batteries
Abstract
1. Introduction
2. Cathode Materials for Lithium-Ion Batteries
2.1. Intercalation-Type Cathode Materials
2.2. Conversion-Type Cathodes
3. Conversion Mechanisms and Crystal Structure Engineering
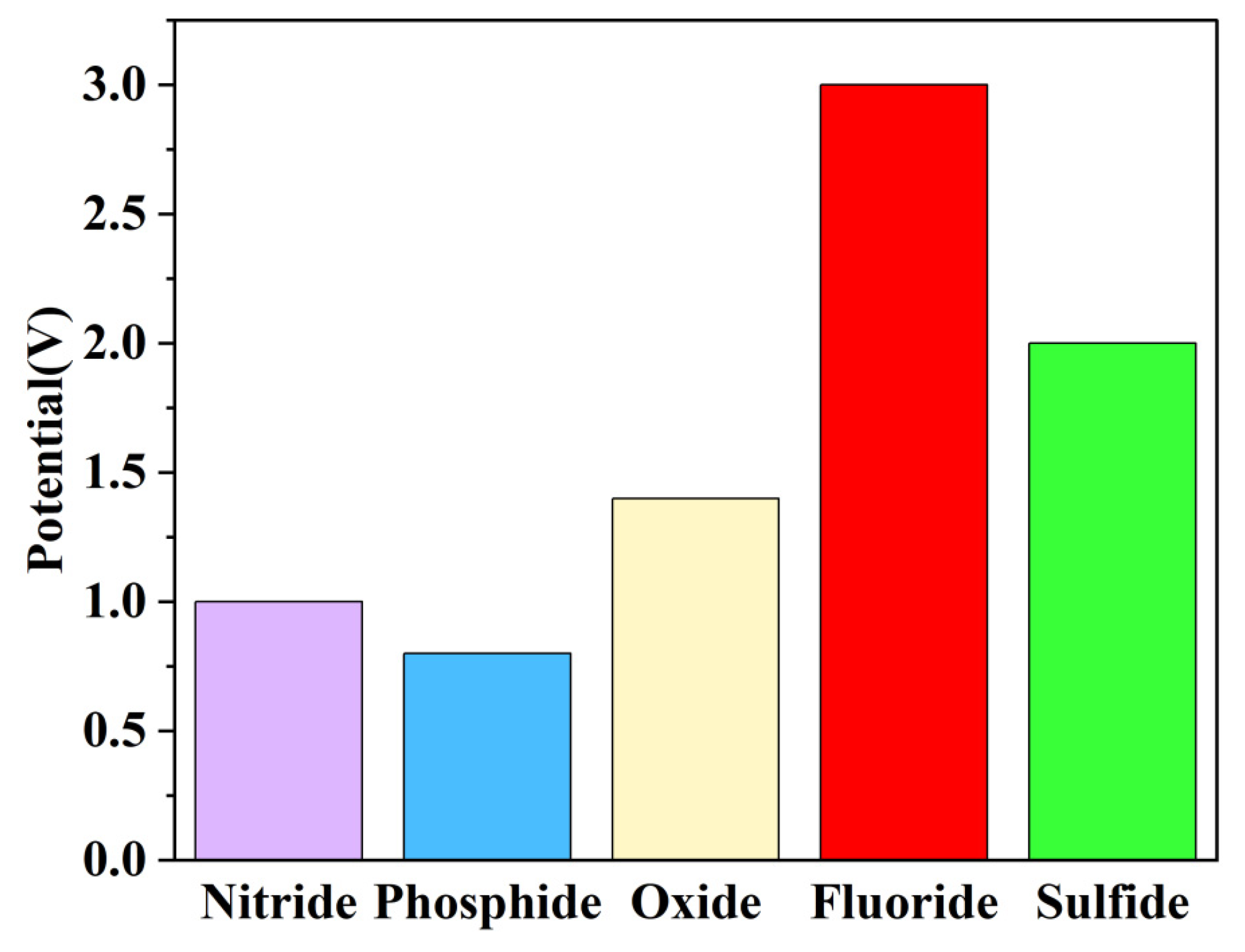
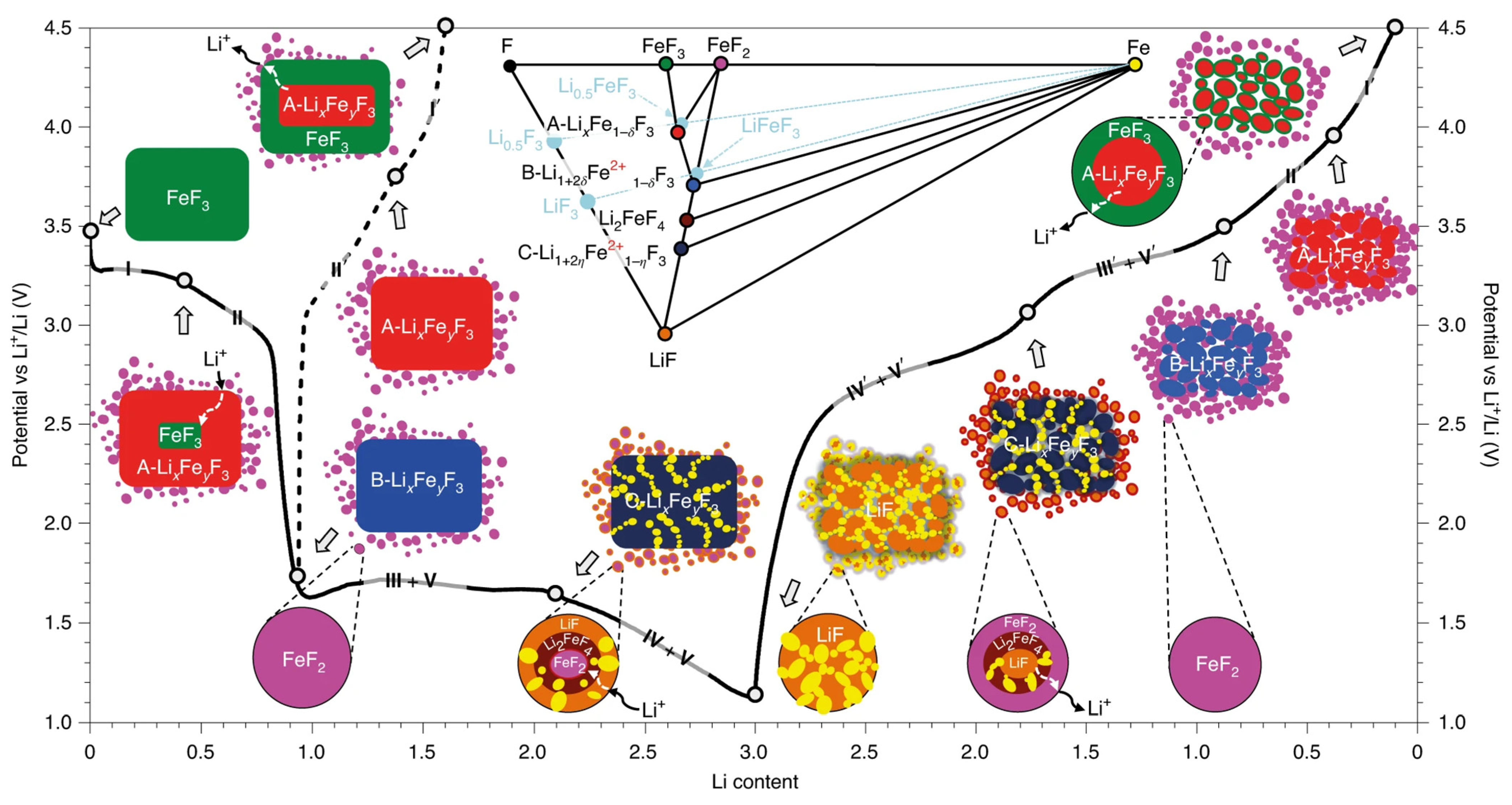
3.1. Conversion Mechanism of Iron Fluoride
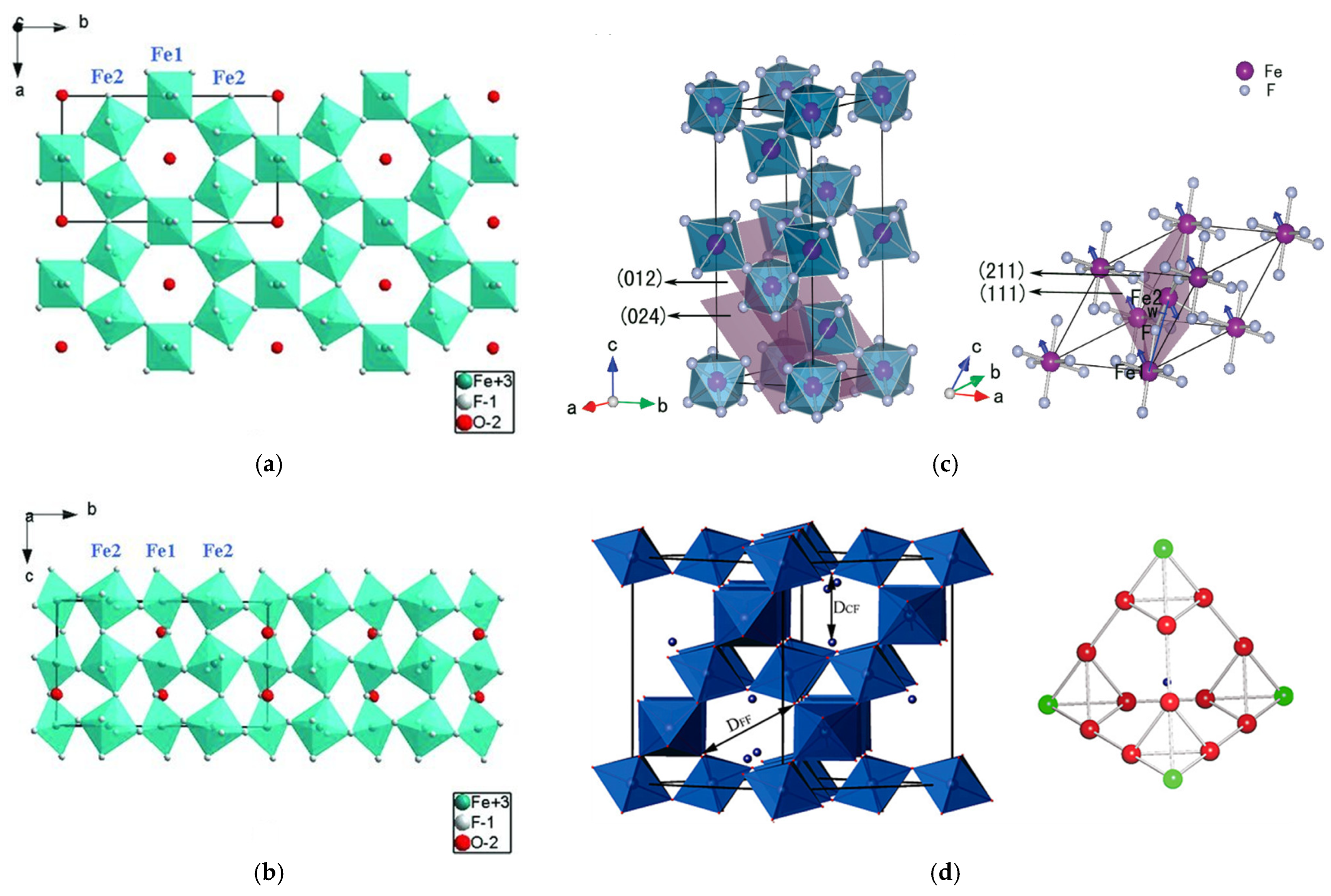
3.2. Iron Fluorides with Different Crystal Structures
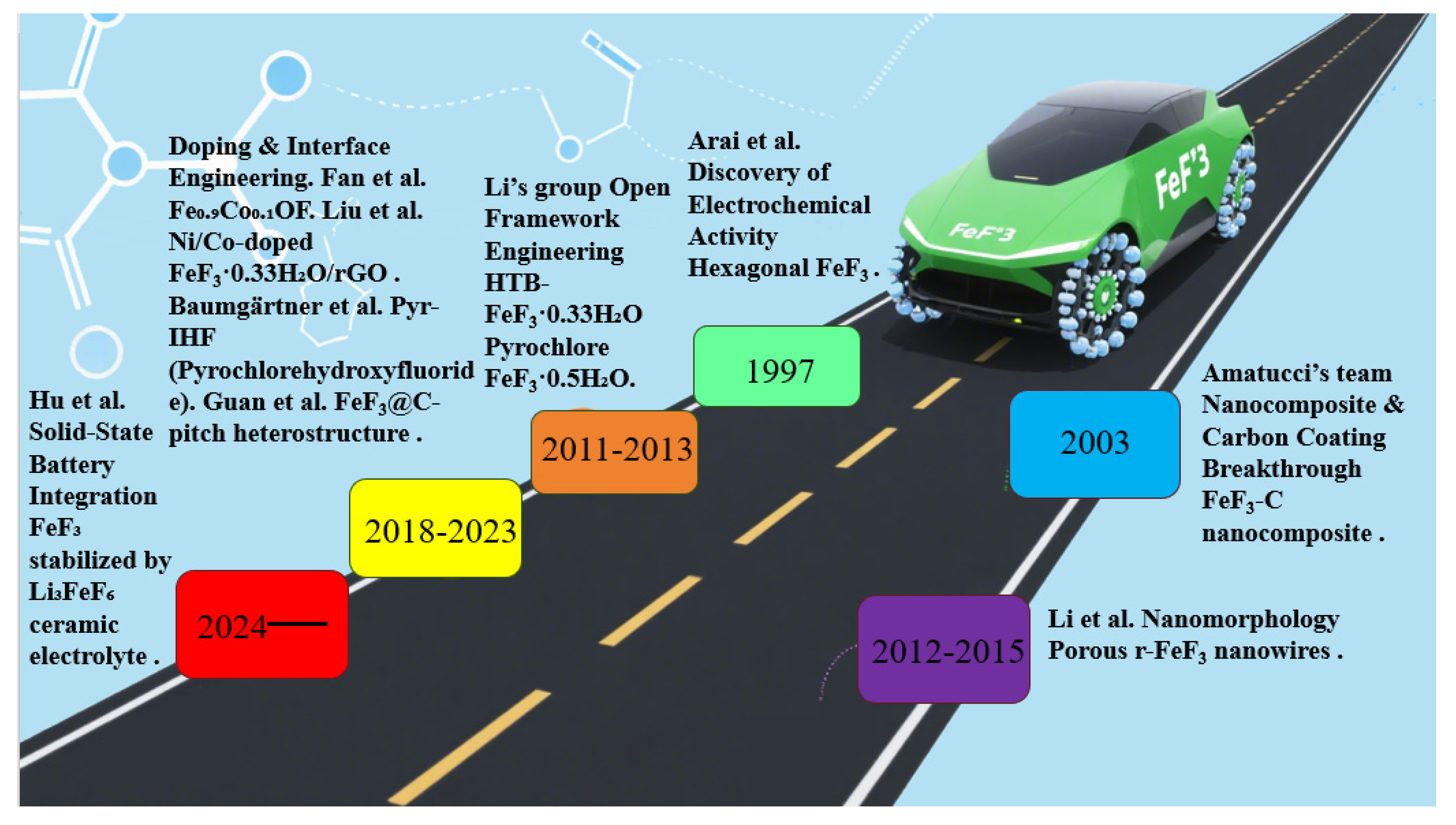
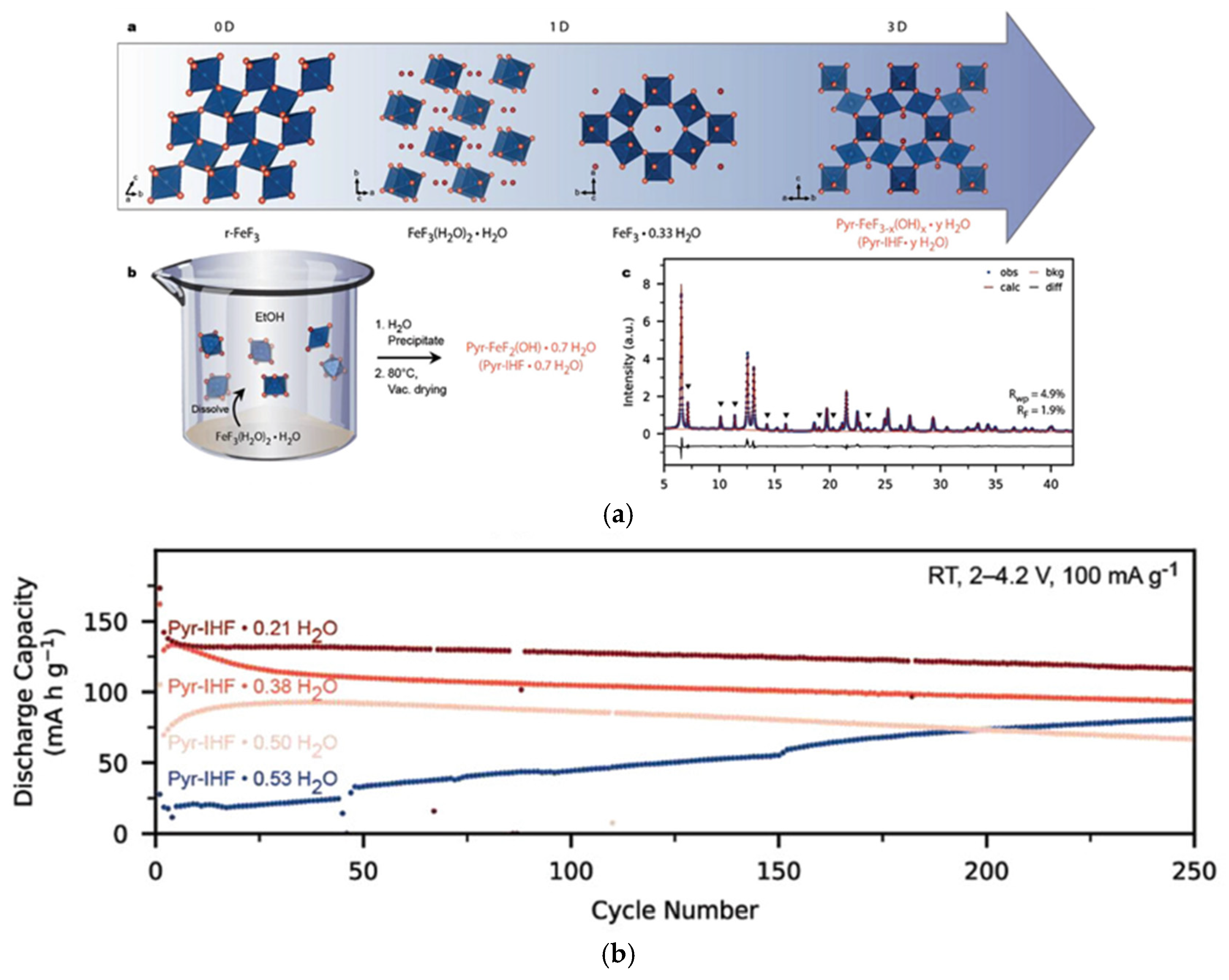
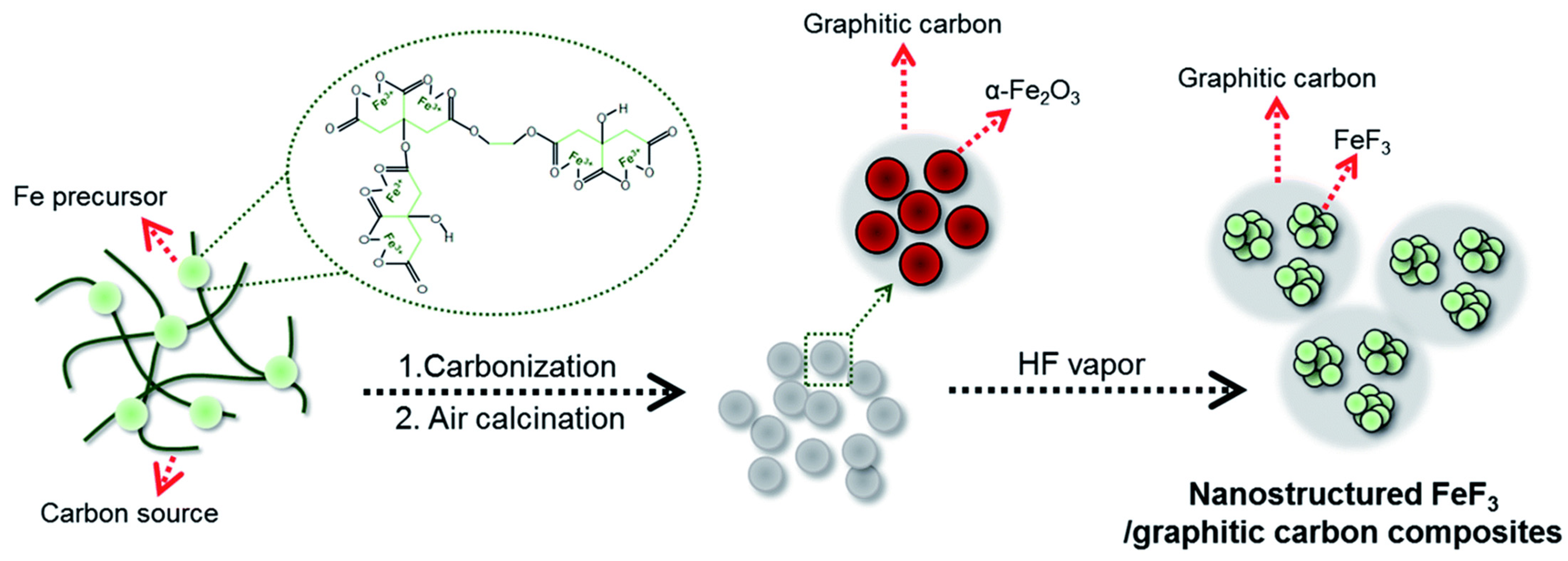
3.3. Synthesis–Structure–Performance Correlations
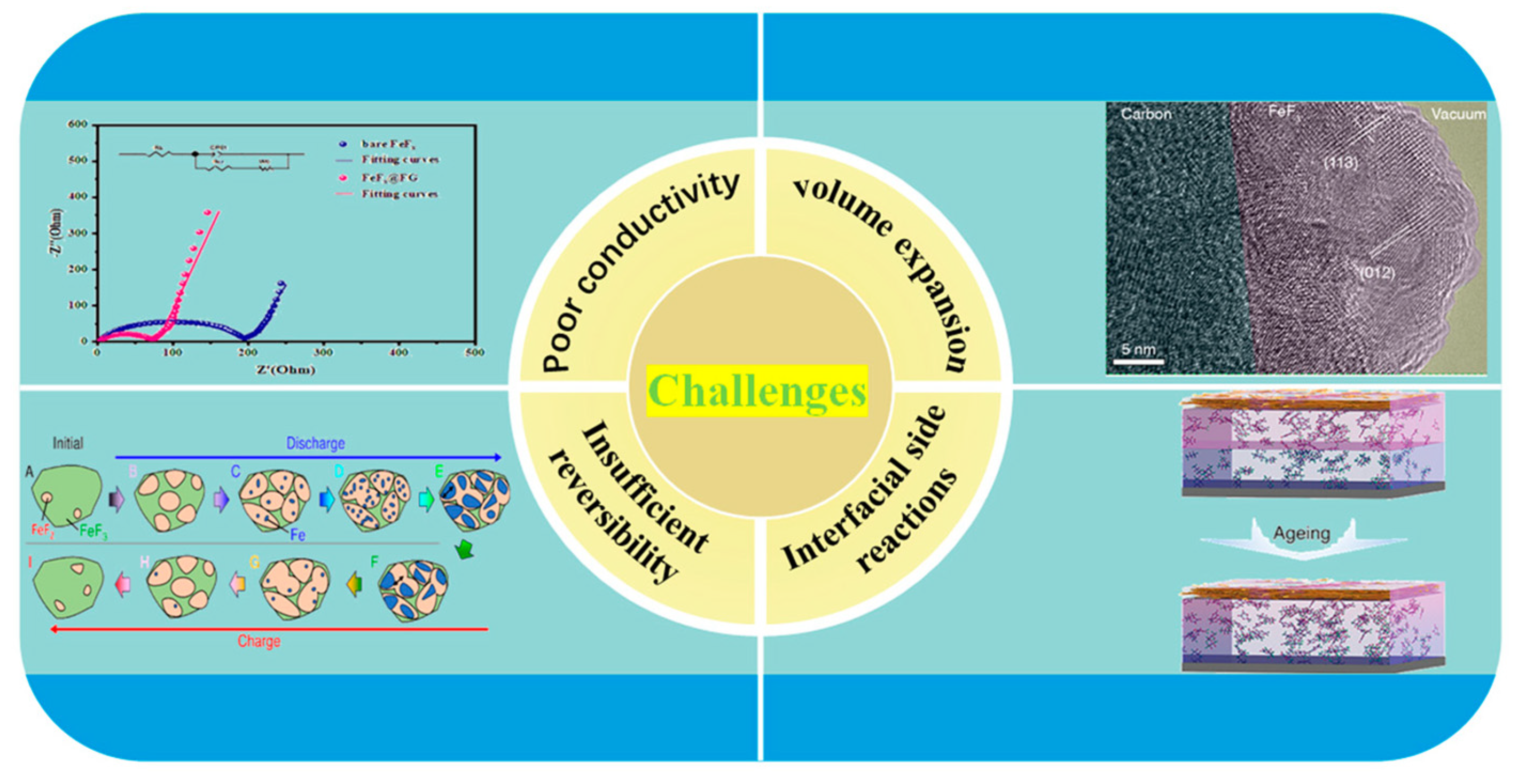
4. Key Challenges and Issues of Iron Fluoride Cathode Materials
4.1. Poor Intrinsic Conductivity
4.2. Severe Volume Effects During Cycling
4.3. Poor Reversibility of Conversion Reactions
4.4. Complex Interfacial Side Reactions
5. Modification of Iron Fluoride Cathode Materials
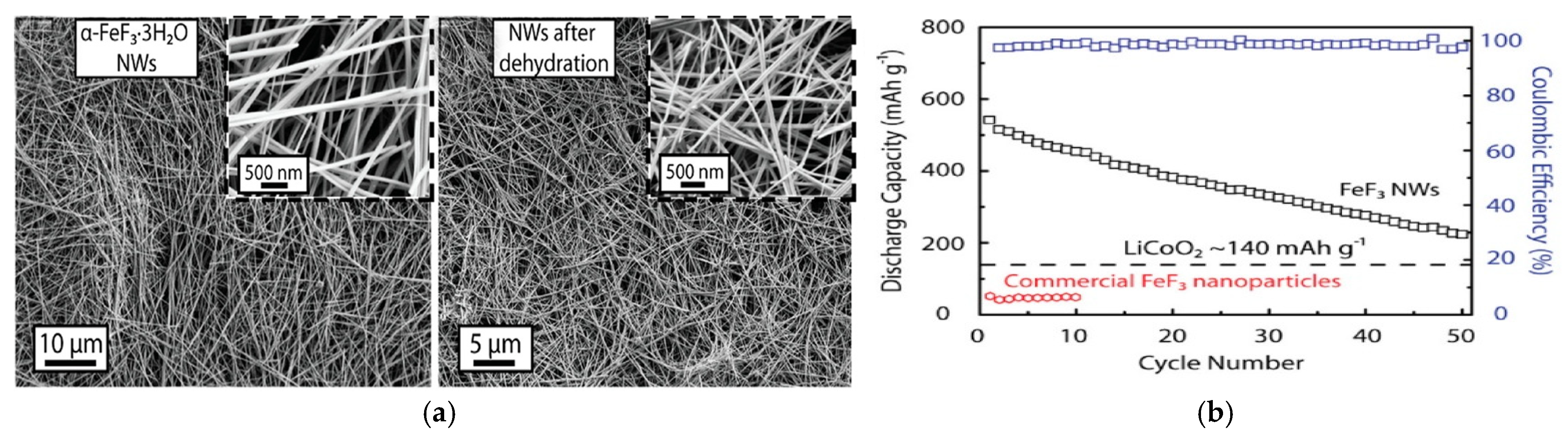
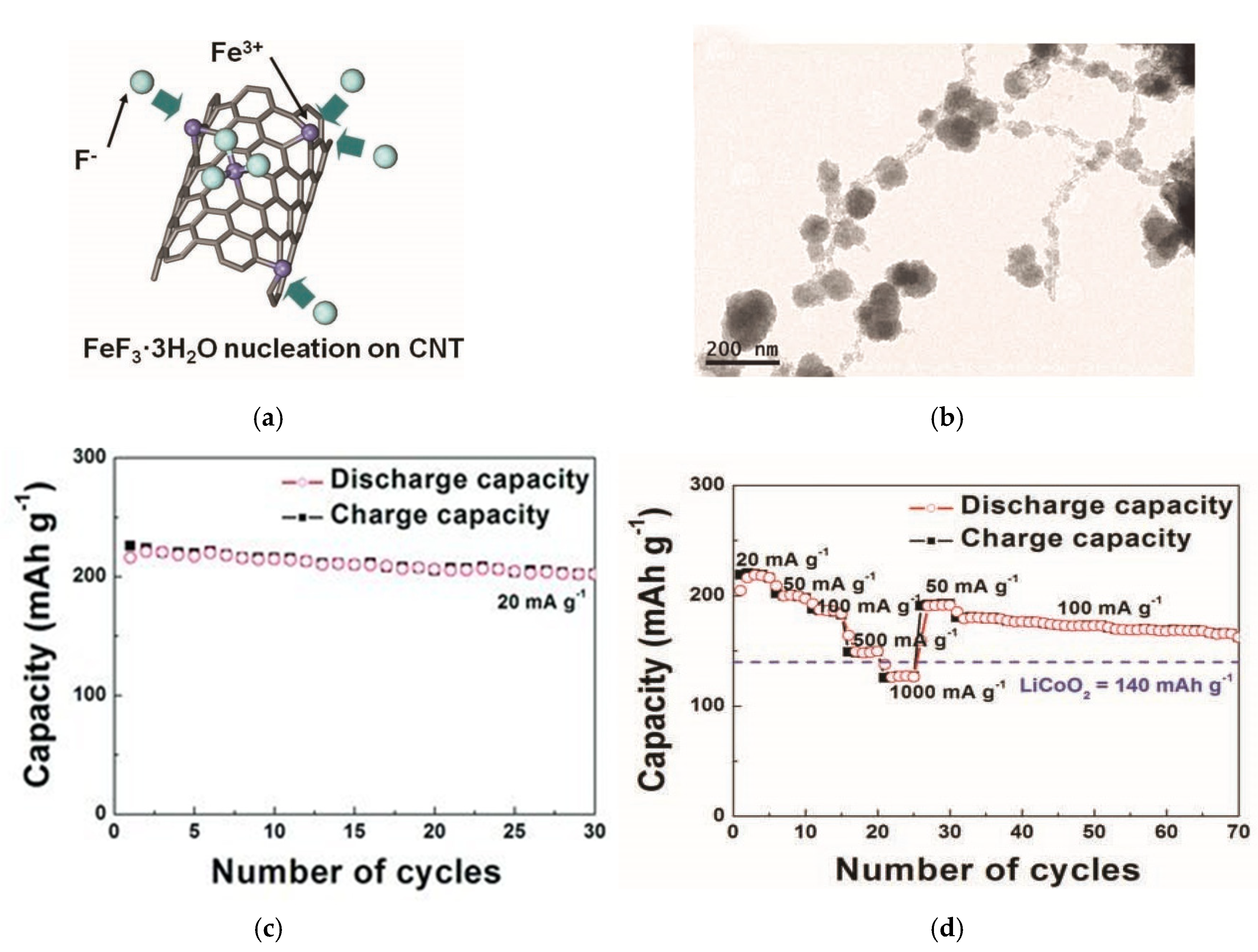
5.1. Nanoengineering
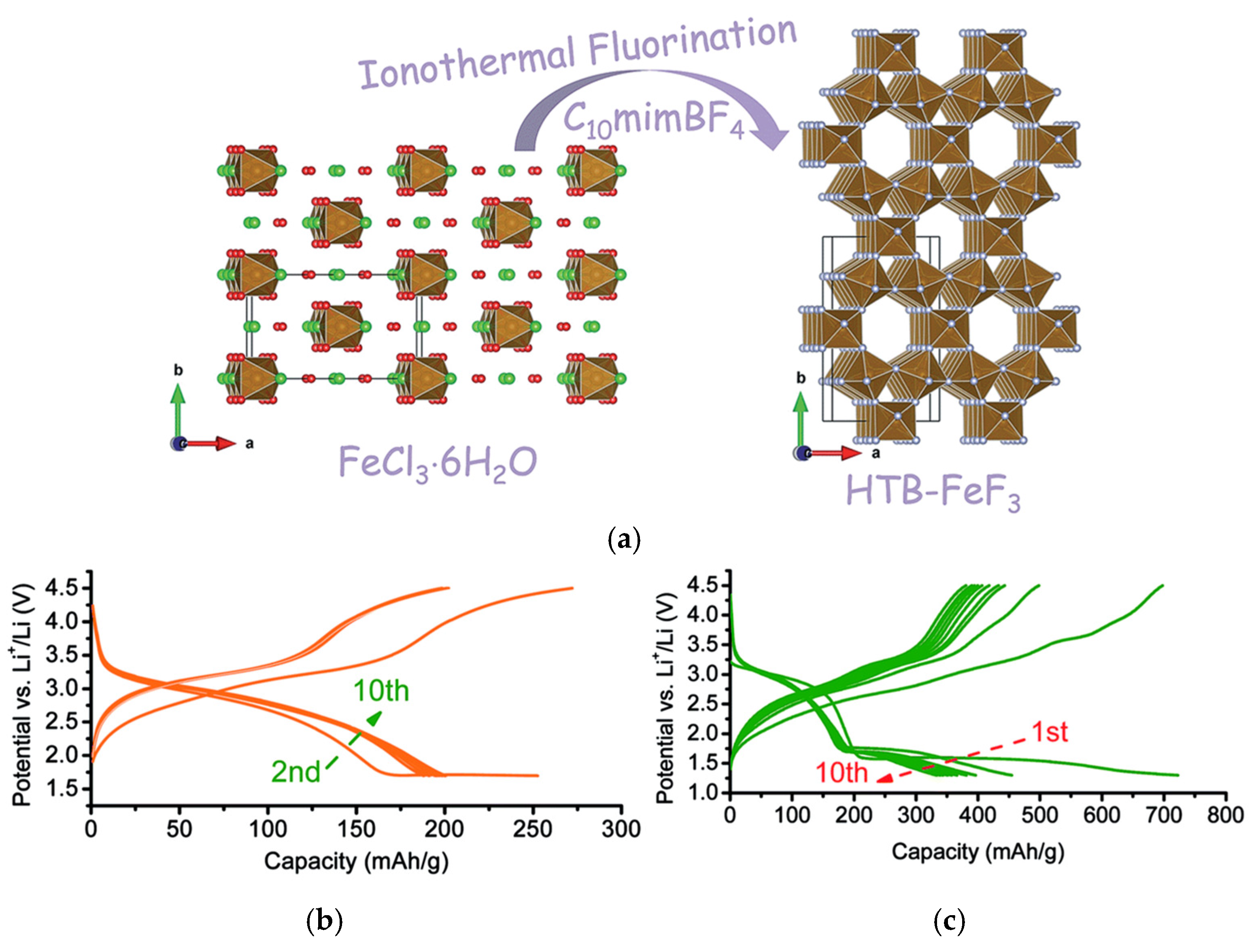
5.2. Open-Framework Strategy
5.3. Anionic/Cationic Doping
5.4. Surface/Interface Modification
5.5. Comparative Performance of Modified Iron Fluoride Cathodes
6. Summary and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, T.; Liu, Y.; Chen, G.H.; Liu, H.J.; Han, Y.Y.; Zhai, S.H.; Zhang, L.Q.; Pan, Y.Y.; Li, Q.H.; Li, Q. Pseudocapacitance-Enhanced Storage Kinetics of 3D Anhydrous Iron (III) Fluoride as a Cathode for Li/Na-Ion Batteries. Nanomaterials 2022, 12, 4041. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y. Flexible Tellurium-Based Electrode for High-Performance Lithium-Tellurium Battery. Nanomaterials 2021, 11, 2903. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Robert, R.; Chernova, N.A.; Pereira, N.; Omenya, F.; Badway, F.; Hua, X.; Ruotolo, M.; Zhang, R.G.; Wu, L.J.; et al. Conversion Reaction Mechanisms in Lithium Ion Batteries: Study of the Binary Metal Fluoride Electrodes. J. Am. Chem. Soc. 2011, 133, 18828–18836. [Google Scholar] [CrossRef]
- Su, Y.; Liu, S.X.; Zhu, D.D.; Luo, Y.; Zhang, X.D.; Yan, J.T.; Chen, J.Z.; Geng, L.; Guo, B.Y.; Li, H.; et al. Cryo-TEM studies of binder free high performance FeF2 cathode based full cells enabled by surface engineering. Energy Storage Mater. 2023, 59, 102779. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, D.H.; Gwon, H.; Kim, J.; Kang, K. Fabrication of FeF Nanoflowers on CNT Branches and Their Application to High Power Lithium Rechargeable Batteries. Adv. Mater. 2010, 22, 5260–5264. [Google Scholar] [CrossRef]
- Baumgärtner, J.F.; Wörle, M.; Guntlin, C.P.; Krumeich, F.; Siegrist, S.; Vogt, V.; Stoian, D.C.; Chernyshov, D.; van Beek, W.; Kravchyk, K.V.; et al. Pyrochlore-Type Iron Hydroxy Fluorides as Low-Cost Lithium-Ion Cathode Materials for Stationary Energy Storage. Adv. Mater. 2023, 35, e2304158. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, B.B.; Wu, T.J.; Li, H.; Liu, X.L.; Wang, G.; Chen, M.F.; Yang, Z.H.; Bai, Y.S.; Wang, X.Y. rGO-Encapsulated Co/Ni dual-doped FeF3 0.33H2O nanoparticles enabling a high-rate and long-life iron (III) fluoride-lithium battery. Chem. Eng. J. 2023, 451, 138774. [Google Scholar] [CrossRef]
- Kim, T.; Jae, W.J.; Kim, H.; Park, M.; Han, J.M.; Kim, J. A cathode material for lithium-ion batteries based on graphitized carbon-wrapped FeF nanoparticles prepared by facile polymerization. J. Mater. Chem. A 2016, 4, 14857–14864. [Google Scholar] [CrossRef]
- Arai, H.; Okada, S.; Sakurai, Y.; Yamaki, J. Cathode performance and voltage estimation of metal trihalides. J. Power Sources 1997, 68, 716–719. [Google Scholar] [CrossRef]
- Badway, F.; Cosandey, F.; Pereira, N.; Amatucci, G.G. Carbon metal fluoride nanocomposites-High-capacity reversible metal fluoride conversion materials as rechargeable positive electrodes for Li batteries. J. Electrochem. Soc. 2003, 150, A1318–A1327. [Google Scholar] [CrossRef]
- Li, C.L.; Gu, L.; Tong, J.W.; Tsukimoto, S.; Maier, J. A Mesoporous Iron-Based Fluoride Cathode of Tunnel Structure for Rechargeable Lithium Batteries. Adv. Funct. Mater. 2011, 21, 1391–1397. [Google Scholar] [CrossRef]
- Li, C.L.; Yin, C.L.; Gu, L.; Dinnebier, R.E.; Mu, X.K.; van Aken, P.A.; Maier, J. An FeF3 0.5H2O Polytype: A Microporous Framework Compound with Intersecting Tunnels for Li and Na Batteries. J. Am. Chem. Soc. 2013, 135, 11425–11428. [Google Scholar] [CrossRef]
- Li, L.S.; Meng, F.; Jin, S. High-Capacity Lithium-Ion Battery Conversion Cathodes Based on Iron Fluoride Nanowires and Insights into the Conversion Mechanism. Nano. Lett. 2012, 12, 6030–6037. [Google Scholar] [CrossRef]
- Fan, X.L.; Hu, E.Y.; Ji, X.; Zhu, Y.Z.; Han, F.D.; Hwang, S.Y.; Liu, J.; Bak, S.M.; Ma, Z.H.; Gao, T.; et al. High energy-density and reversibility of iron fluoride cathode enabled via an intercalation-extrusion reaction. Nat. Commun. 2018, 9, 2324. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, X.D.; Gao, Z.R.; Su, Y.; Liu, S.X.; Wu, F.X.; Ren, X.L.; He, X.; Song, B.H.; Lyu, P.; et al. Boosting the Cycle Performance of Iron Trifluoride Based Solid State Batteries at Elevated Temperatures by Engineering the Cathode Solid Electrolyte Interface. Small 2024, 20, e2307116. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.X.; Zhao, L.; Zhou, Y.; Qiu, X.M.; Wu, J.; Wu, G.; Bao, N.Z. Hetero-Packing Nanostructures of Iron (III) Fluoride Nanocomposite Cathode for High-Rate and Long-Life Rechargeable Lithium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2301680. [Google Scholar] [CrossRef]
- Hasan, K.; Tom, N.; Yuce, M.R. Navigating Battery Choices in IoT: An Extensive Survey of Technologies and Their Applications. Batteries 2023, 9, 580. [Google Scholar] [CrossRef]
- Comanescu, C. Ensuring Safety and Reliability: An Overview of Lithium-Ion Battery Service Assessment. Batteries 2025, 11, 6. [Google Scholar] [CrossRef]
- Kaur, G.; Gates, B.D. Review-Surface Coatings for Cathodes in Lithium Ion Batteries: From Crystal Structures to Electrochemical Performance. J. Electrochem. Soc. 2022, 169, 043504. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.M.; Eshetu, G.G.; Laruelle, S.; Grugeon, S.; Zaghib, K.; Julien, C.; Mauger, A.; Guyomard, D.; Rojo, T.; et al. From Solid-Solution Electrodes and the Rocking-Chair Concept to Today’s Batteries. Angew. Chem. Int. Edit. 2020, 59, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Kang, Y.Q.; Zhao, Y.; Wang, L.; Liu, J.L.; Li, Y.X.; Liang, Z.; He, X.M.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Ma, D.L.; Zhang, R.L.; Hu, X.; Chen, Y.; Xiao, C.F.; He, F.; Zhang, S.; Chen, J.B.; Hu, G.Z. Insights into the electrochemical performance of metal fluoride cathodes for lithium batteries. Energy Mater 2022, 2, 200027. [Google Scholar] [CrossRef]
- Lee, D.; Kim, H.; Jeong, S.M. Unveiling the Future of Li-Ion Batteries: Real-Time Insights into the Synthesis of Advanced Layered Cathode Materials. ACS Energy Lett. 2024, 9, 4255–4264. [Google Scholar] [CrossRef]
- Palacín, M.R. Recent advances in rechargeable battery materials: A chemist’s perspective. Chem. Soc. Rev. 2009, 38, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, N.; Suntharam, N.M.; Bashir, S.; Vengadaesvaran, B.; Abd Rahim, N.; Gunasekaran, Y.; Ramesh, S.; Ramesh, K.; Uttran, A. Advancements in cathode materials for lithium-ion batteries: An overview of future prospects. Ionics 2025, 31, 1153–1180. [Google Scholar] [CrossRef]
- Yi, T.F.; Zhu, Y.R.; Zhu, X.D.; Shu, J.; Yue, C.B.; Zhou, A.N. A review of recent developments in the surface modification of LiMnO as cathode material of power lithium-ion battery. Ionics 2009, 15, 779–784. [Google Scholar] [CrossRef]
- Gong, Z.L.; Yang, Y. Recent advances in the research of polyanion-type cathode materials for Li-ion batteries. Energy Environ. Sci. 2011, 4, 3223–3242. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, B.; Huang, X.J. A 3.9 V polyanion-type cathode material for Li-ion batteries. Prog. Nat. Sci. Mater. Int. 2011, 21, 211–215. [Google Scholar] [CrossRef][Green Version]
- Xu, B.; Qian, D.N.; Wang, Z.Y.; Meng, Y.S.L. Recent progress in cathode materials research for advanced lithium ion batteries. Mat. Sci. Eng. R 2012, 73, 51–65. [Google Scholar] [CrossRef]
- Yang, B.; Yin, B.; Chen, H.; Zheng, Y.F. Corrosion resistance of insulating refractories for the synthesis of lithium-ion battery LiCoO cathode materials. Int. J. Appl. Ceram. Technol. 2025, 22, e14948. [Google Scholar] [CrossRef]
- Islam, M.S.; Fisher, C.A.J. Lithium and sodium battery cathode materials: Computational insights into voltage, diffusion and nanostructural properties. Chem. Soc. Rev. 2014, 43, 185–204. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.T.; Son, D.Y.; Ono, L.K.; Qi, Y.B. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Nasajpour-Esfahani, N.; Garmestani, H.; Bagheritabar, M.; Jasim, D.J.; Toghraie, D.; Dadkhah, S.; Firoozeh, H. Comprehensive review of lithium-ion battery materials and development challenges. Renew. Sustain. Energy Rev. 2024, 203, 114783. [Google Scholar] [CrossRef]
- Wang, L.L.; Ma, J.; Wang, C.; Yu, X.R.; Liu, R.; Jiang, F.; Sun, X.W.; Du, A.B.; Zhou, X.H.; Cui, G.L. A Novel Bifunctional Self-Stabilized Strategy Enabling 4.6 V LiCoO2 with Excellent Long-Term Cyclability and High-Rate Capability. Adv. Sci. 2019, 6, 1900355. [Google Scholar] [CrossRef] [PubMed]
- Song, L.B.; Du, J.L.; Xiao, Z.L.; Jiang, P.; Cao, Z.; Zhu, H.L. Research Progress on the Surface of High-Nickel Nickel-Cobalt-Manganese Ternary Cathode Materials: A Mini Review. Front. Chem. 2020, 8, 00761. [Google Scholar] [CrossRef]
- Chen, S.Y.; Mi, C.H.; Su, L.H.; Gao, B.; Fu, Q.B.; Zhang, X.G. Improved performances of mechanical-activated LiMnO/MWNTs cathode for aqueous rechargeable lithium batteries. J. Appl. Electrochem. 2009, 39, 1943–1948. [Google Scholar] [CrossRef]
- Aurbach, D.; Levi, M.D.; Gamulski, K.; Markovsky, B.; Salitra, G.; Levi, E.; Heider, U.; Heider, L.; Oesten, R. Capacity fading of LiMnO spinel electrodes studied by XRD and electroanalytical techniques. J. Power Sources 1999, 81, 472–479. [Google Scholar] [CrossRef]
- Sun, X.D.; Zhang, L. Outstanding Li-storage performance of LiFePO@MWCNTs cathode material with 3D network structure for lithium-ion batteries. J. Phys. Chem. Solids 2018, 116, 216–221. [Google Scholar] [CrossRef]
- Li, C.Z.; Yuan, H.Y.; Yang, Z.J. Enhanced electrochemical performance of boron-doped graphene-decorated LiFePO@C cathode material for lithium-ion batteries. Solid State Ion. 2020, 352, 115366. [Google Scholar] [CrossRef]
- Guilmard, M.; Croguennec, L.; Delmas, C. Thermal Stability of Lithium Nickel Oxide Derivatives. Part II: LixNi0.70Co0.15Al0.15O2 and LixNi0.90Mn0.10O2 (x = 0.50 and 0.30). Comparison with LixNi1.02O2 and LixNi0.89Al0.16O2. Chem. Mater. 2003, 15, 4484–4493. [Google Scholar] [CrossRef]
- Ju, J.W.; Lee, E.J.; Yoon, C.S.; Myung, S.T.; Sun, Y.K. Optimization of Layered Cathode Material with Full Concentration Gradient for Lithium-Ion Batteries. J. Phys. Chem. C 2014, 118, 175–182. [Google Scholar] [CrossRef]
- Kim, S.W.; Pyun, S.I. Thermodynamic and kinetic approaches to lithium intercalation into a LiMnO electrode using Monte Carlo simulation. Electrochim. Acta. 2001, 46, 987–997. [Google Scholar] [CrossRef]
- Wang, X.Q.; Nakamura, H.; Yoshio, M. Capacity fading mechanism for oxygen defect spinel as a 4 V cathode material in Li-ion batteries. J. Power Sources 2002, 110, 19–26. [Google Scholar] [CrossRef]
- Yamada, A.; Chung, S.C.; Hinokuma, K. Optimized LiFePO for lithium battery cathodes. J. Electrochem. Soc. 2001, 148, A224–A229. [Google Scholar] [CrossRef]
- Bai, T.S.; Li, D.P.; Xiao, S.Y.; Ji, F.J.; Zhang, S.; Wang, C.; Lu, J.Y.; Gao, Q.; Ci, L.J. Recent progress on single-atom catalysts for lithium-air battery applications. Energy Environ. Sci. 2023, 16, 1431–1465. [Google Scholar] [CrossRef]
- Hu, B.; Xu, J.; Fan, Z.J.; Xu, C.; Han, S.C.; Zhang, J.X.; Ma, L.B.; Ding, B.; Zhuang, Z.C.; Kang, Q.; et al. Covalent Organic Framework Based Lithium-Sulfur Batteries: Materials, Interfaces, and Solid-State Electrolytes. Adv. Energy Mater. 2023, 13, 2203540. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Kang, K. Conversion-Based Cathode Materials for Rechargeable Sodium Batteries. Adv. Energy Mater. 2018, 8, 1702646. [Google Scholar] [CrossRef]
- Skurtveit, A.; Brennhagen, A.; Park, H.; Cavallo, C.; Koposov, A.Y. Benefits and Development Challenges for Conversion-Alloying Anode Materials in Na-Ion Batteries. Front. Energy Res. 2022, 10, 897755. [Google Scholar] [CrossRef]
- Yu, S.H.; Feng, X.R.; Zhang, N.; Seok, J.; Abruña, H.D. Understanding Conversion-Type Electrodes for Lithium Rechargeable Batteries. Acc. Chem. Res. 2018, 51, 273–281. [Google Scholar] [CrossRef]
- Amatucci, G.G.; Pereira, N. Fluoride based electrode materials for advanced energy storage devices. J. Fluorine. Chem. 2007, 128, 243–262. [Google Scholar] [CrossRef]
- Wu, F.X.; Yushin, G. Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ. Sci. 2017, 10, 435–459. [Google Scholar] [CrossRef]
- Wu, F.X.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Li, H.; Balaya, P.; Maier, J. Li-storage via heterogeneous reaction in selected binary metal fluorides and oxides. J. Electrochem. Soc. 2004, 151, A1878–A1885. [Google Scholar] [CrossRef]
- Thieu, D.T.; Hammad, M.; Bhatia, H.; Diemant, T.; Chakravadhanula, V.S.K.; Behm, R.J.; Kübel, C.; Fichtner, M. CuF as Reversible Cathode for Fluoride Ion Batteries. Adv. Funct. Mater. 2017, 27, 1701051. [Google Scholar] [CrossRef]
- Fang, S.F.; Pei, J.H.; Guo, L.P.; Qin, Y.M. Porous MnF2 with micro-nanostructures coated by a nitrogen doped carbon shell as an anode for enhanced lithium storage. Appl. Surf. Sci. 2024, 652, 159323. [Google Scholar] [CrossRef]
- Oh, J.; Lim, E.; Chun, J.; Jo, C. Nickel fluoride (NiF)/porous carbon nanocomposite synthesized via ammonium fluoride (NHF) treatment for lithium-ion battery cathode applications. J. Power Sources 2022, 521, 230935. [Google Scholar] [CrossRef]
- Guan, Q.; Cheng, J.L.; Li, X.D.; Ni, W.; Wang, B. Porous CoF Spheres Synthesized by a One-Pot Solvothermal Method as High Capacity Cathode Materials for Lithium-Ion Batteries. Chin. J. Chem. 2017, 35, 48–54. [Google Scholar] [CrossRef]
- Wilson, K.L.; Halajko, A.; Badway, F.; Harrop, B.; Amatucci, G.G. Iron Metal Based Positive Electrodes for Metal Fluoride High Energy Lithium Batteries. J. Electrochem. Soc. 2025, 172, 010531. [Google Scholar] [CrossRef]
- Sun, L.D.; Li, Y.; Feng, W. Metal Fluoride Cathode Materials for Lithium Rechargeable Batteries: Focus on Iron Fluorides. Small Methods 2023, 7, e2201152. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, M.; Zhang, Y.Q.; Zhou, Y.B.; Li, R.L.; Yue, W.B. FeF3 0.33H2O nanoparticles decorated Ti3C2 MXene as high-performance bifunctional sulfur host for lithium-sulfur batteries. J. Energy Storage 2024, 99, 113401. [Google Scholar] [CrossRef]
- Hua, X.; Eggeman, A.S.; Castillo-Martínez, E.; Robert, R.; Geddes, H.S.; Lu, Z.H.; Pickard, C.J.; Meng, W.; Wiaderek, K.M.; Pereira, N.; et al. Revisiting metal fluorides as lithium-ion battery cathodes. Nat. Mater. 2021, 20, 841–850. [Google Scholar] [CrossRef]
- Li, R.F.; Wu, S.Q.; Yang, Y.; Zhu, Z.Z. Structural and Electronic Properties of Li-Ion Battery Cathode Material FeF. J. Phys. Chem. C 2010, 114, 16813–16817. [Google Scholar] [CrossRef]
- Li, H.; Richter, G.; Maier, J. Reversible formation and decomposition of LiF clusters using transition metal fluorides as precursors and their application in rechargeable Li batteries. Adv. Mater. 2003, 15, 736–739. [Google Scholar] [CrossRef]
- Doe, R.E.; Persson, K.A.; Meng, Y.S.; Ceder, G. First-Principles Investigation of the Li-Fe-F Phase Diagram and Equilibrium and Nonequilibrium Conversion Reactions of Iron Fluorides with Lithium. Chem. Mater. 2008, 20, 5274–5283. [Google Scholar] [CrossRef]
- Yamakawa, N.; Jiang, M.; Key, B.; Grey, C.P. Identifying the Local Structures Formed during Lithiation of the Conversion Material, Iron Fluoride, in a Li Ion Battery: A Solid-State NMR, X-ray Diffraction, and Pair Distribution Function Analysis Study. J. Am. Chem. Soc. 2009, 131, 10525–10536. [Google Scholar] [CrossRef]
- Li, C.L.; Gu, L.; Tong, J.W.; Maier, J. Carbon Nanotube Wiring of Electrodes for High-Rate Lithium Batteries Using an Imidazolium-Based Ionic Liquid Precursor as Dispersant and Binder: A Case Study on Iron Fluoride Nanoparticles. ACS Nano 2011, 5, 2930–2938. [Google Scholar] [CrossRef]
- Lian, J.L.; Wu, Y.; Guo, Y.C.A.; Zhao, Z.Y.; Zhang, Q.H.; Hou, Y.; Chen, L.X.; Lu, B.; Pan, X.H.; Ye, Z.Z.; et al. Design of hierarchical and mesoporous FeF/rGO hybrids as cathodes for superior lithium-ion batteries. Chin. Chem. Lett. 2022, 33, 3931–3935. [Google Scholar] [CrossRef]
- Shi, Y.S.; Xu, X.Z.; Li, J.; Li, J.Y.; Yin, P.P.; Jiang, Q.T.; Wang, J.J.; Li, W.B.; Xu, K.H.; Zhang, K.; et al. Graphitized Carbon-Coated Iron Fluoride Nanocavities for Enhanced Kinetics of Multielectron Cathode Conversion Reactions. ACS Appl. Mater. Interfaces 2023, 15, 41504–41515. [Google Scholar] [CrossRef]
- Qu, J.Y.; Ren, Z.J.; Yan, L.; Zhu, Y.C.; Hu, J.; Tang, Y.X.; Chen, Z. Mixed crystal FeF x submicron spheres loaded on fluorinated graphene as cathode materials for Lithium-Ion batteries. J. Electroanal. Chem. 2024, 960, 118195. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takabayashi, Y.; Kibino, K.; Kimura, K.; Inoishi, A.; Yano, A.; Yoshii, K.; Sakaebe, H.; Shikano, M.; Nakatani, T.; et al. Changes in Structure and Valence of Iron-Based Positive Electrodes in All-Solid-State Fluoride Batteries. J. Phys. Chem. C 2025, 129, 11831–11837. [Google Scholar] [CrossRef]
- Choi, C.; Yoon, H.; Kang, S.; Kim, D.I.; Hong, J.; Shin, M.; Yoo, D.J.; Kim, M. Achieving High Stability and Capacity in Micron-Sized Conversion-Type Iron Fluoride Li-Metal Batteries. Adv. Sci. 2024, 11, e2410114. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.Y.; Wei, S.Y.; Wang, X.; Liu, M.; Hu, H. Iron fluoride microspheres by titanium dioxide surface modification as high capacity cathode of Li-ion batteries. J. Alloy Compd. 2017, 719, 331–340. [Google Scholar] [CrossRef]
- Fotouhi, A.; Auger, D.J.; O’Neill, L.; Cleaver, T.; Walus, S. Lithium-Sulfur Battery Technology Readiness and Applications-A Review. Energies 2017, 10, 1937. [Google Scholar] [CrossRef]
- Fan, X.L.; Luo, C.; Lamb, J.L.; Zhu, Y.J.; Xu, K.; Wang, C.S. PEDOT Encapsulated FeOF Nanorod Cathodes for High Energy Lithium-Ion Batteries. Nano. Lett. 2015, 15, 7650–7656. [Google Scholar] [CrossRef]
- Zhou, H.; Ruther, R.E.; Adcock, J.; Zhou, W.; Dai, S.; Nanda, J. Controlled Formation of Mixed Nanoscale Domains of High Capacity FeO-FeF Conversion Compounds by Direct Fluorination. Acs. Nano. 2015, 9, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Xu, H.M.; Ru, H.; Shi, Y.L.; Zhuang, Q.C.; Cui, Y.L.; Ju, Z.C.; Cui, Y.H. Nanosized FeF3 0.33H2O as Cathode Material for High-Performance Li-Ion Batteries. J. Electrochem. Soc. 2021, 168, 030501. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Cao, Y.L.; Ai, X.P.; Yang, H.X. Reversible Three-Electron Redox Behaviors of FeF Nanocrystals as High-Capacity Cathode-Active Materials for Li-Ion Batteries. J. Phys. Chem. C 2010, 114, 3190–3195. [Google Scholar] [CrossRef]
- Li, J.; Fu, L.C.; Xu, Z.W.; Zhu, J.J.; Yang, W.L.; Li, D.Y.; Zhou, L.P. Electrochemical properties of carbon-wrapped FeF nanocomposite as cathode material for lithium ion battery. Electrochim. Acta. 2018, 281, 88–98. [Google Scholar] [CrossRef]
- Lu, L.; Li, S.; Li, J.; Lan, L.F.; Lu, Y.; Xu, S.J.; Huang, S.; Pan, C.Y.; Zhao, F.H. High-Performance Cathode Material of FeF·0.33HO Modified with Carbon Nanotubes and Graphene for Lithium-Ion Batteries. Nanoscale Res. Lett. 2019, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wu, X.Y.; Luo, S.F.; Gao, M.T.; Cai, D.M.; Xie, Y.; Zhu, L.C.; Yuan, Z.Z. Novel synthesis of FeF3 0.33H2O@hollow acetylene black nanosphere and its long-life electrochemical properties as a cathode for lithium-ion batteries. Carbon 2024, 226, 119188. [Google Scholar] [CrossRef]
- Yun, B.; Maulana, A.Y.; Lee, D.; Song, J.; Futalan, C.M.; Moon, D.; Kim, J. The Effect of Ni Doping on FeOF Cathode Material for High-Performance Sodium-Ion Batteries. Small 2024, 20, e2308011. [Google Scholar] [CrossRef]
- Liu, Y.D.; Yu, Y.K.; Yang, F.; Zhu, G.Q.; Yu, K.; Kou, R.H.; Sun, C.J.; Liu, Y.Z.; Xu, J.Y.; Liu, C.; et al. Reversible Iron Oxyfluoride (FeOF)-Graphene Composites as Sustainable Cathodes for High Energy Density Lithium Batteries. Small 2023, 19, e2206947. [Google Scholar] [CrossRef]
- Huang, Q.; Turcheniuk, K.; Ren, X.L.; Magasinski, A.; Gordon, D.; Bensalah, N.; Yushin, G. Insights into the Effects of Electrolyte Composition on the Performance and Stability of FeF Conversion-Type Cathodes. Adv. Energy Mater. 2019, 9, e1803323. [Google Scholar] [CrossRef]
- Xiao, A.B.W.; Lee, H.J.; Capone, I.; Robertson, A.; Wi, T.U.; Fawdon, J.; Wheeler, S.; Lee, H.W.; Grobert, N.; Pasta, M. Understanding the conversion mechanism and performance of monodisperse FeF nanocrystal cathodes. Nat. Mater. 2020, 19, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Lei, M.; Yao, Z.G.; Zheng, Y.J.; Hu, J.L.; Lai, C.A.Z.; Li, C.L. Construction of solid-liquid fluorine transport channel to enable highly reversible conversion cathodes. Sci. Adv. 2021, 7, eabj1491. [Google Scholar] [CrossRef]
- Huang, Q.; Turcheniuk, K.; Ren, X.L.; Magasinski, A.; Song, A.Y.; Xiao, Y.R.; Kim, D.; Yushin, G. Cycle stability of conversion-type iron fluoride lithium battery cathode at elevated temperatures in polymer electrolyte composites. Nat. Mater. 2019, 18, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.D.; Wang, Y.; Kong, L.C.; Chen, S.S.; Peng, C.; Zheng, J.H.; Li, Y.; Feng, W. Designing mesostructured iron (II) fluorides with a stable in situ polymer electrolyte interface for high-energy-density lithium-ion batteries. Escience 2024, 4, 100188. [Google Scholar] [CrossRef]
- Zheng, Z.Z.; Shi, J.; Xiao, X.J.; Li, X.; Chen, J.K.; Zhu, C.F. Synthesis by Electrolysis of Iron-Based Fluoride as Cathode Materials for Lithium Ion Batteries. Electron. Mater. Lett. 2024, 20, 306–316. [Google Scholar] [CrossRef]
- He, D.F.; Zhang, Y.L.; Cao, D.; Sun, M.F.; Xia, J.W.; Yang, Y.; Ding, Y.J.; Chen, H.Q. A flexible free-standing FeF/reduced graphene oxide film as cathode for advanced lithium-ion battery. J. Alloys Compd. 2022, 909, 164702. [Google Scholar] [CrossRef]
- Fu, W.B.; Zhao, E.B.; Sun, Z.F.; Ren, X.L.; Magasinski, A.; Yushin, G. Iron Fluoride-Carbon Nanocomposite Nanofibers as Free-Standing Cathodes for High-Energy Lithium Batteries. Adv. Funct. Mater. 2018, 28, 1801711. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.Y.; Wei, S.Y.; Hu, H.; Zhang, R.; Liu, L. Cr-doped Fe2F5·H2O with open framework structure as a high performance cathode material of sodium-ion batteries. Electrochim. Acta. 2018, 269, 479–489. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Q.; Chen, B.B.; Lei, H.L.; Liu, L.; Wu, C.; Wang, X.Y.; Yang, Z.H. Band-Gap Engineering of FeF3 0.33H2O Nanosphere via Ni Doping as a High-Performance Lithium-Ion Battery Cathode. ACS Sustain. Chem. Eng. 2020, 8, 15651–15660. [Google Scholar] [CrossRef]
- Park, J.; Yang, Y.; Park, H.; Sundar, A.; Lee, S.; Kinnibrugh, T.L.; Son, S.B.; Lee, E.; Zapol, P.; Klie, R.F.; et al. Entropy-Stabilized Multication Fluorides as a Conversion-Type Cathode for Li-Ion Batteries–Impact of Element Selection. ACS Appl. Mater. Interfaces 2024, 16, 57151–57161. [Google Scholar] [CrossRef]
- Ali, G.; Lee, J.H.; Chang, W.; Cho, B.W.; Jung, H.G.; Nam, K.W.; Chung, K.Y. Lithium intercalation mechanism into FeF3·0.5H2O as a highly stable composite cathode material. Sci. Rep. 2017, 7, 42237. [Google Scholar] [CrossRef]
- Shi, Y.L.; Shen, M.F.; Xu, S.D.; Zhuang, Q.C.; Jiang, L.; Qiang, Y.H. Electrochemical impedance spectroscopy investigation of the FeF/C cathode for lithium-ion batteries. Solid State Ion. 2012, 222, 23–30. [Google Scholar] [CrossRef]
- Song, H.W.; Cui, H.; Wang, C.X. Extremely high-rate capacity and stable cycling of a highly ordered nanostructured carbon-FeF2 battery cathode. J. Mater. Chem. A 2015, 3, 22377–22384. [Google Scholar] [CrossRef]
- Chu, Q.X.; Xing, Z.C.; Ren, X.B.; Asiri, A.M.; Al-Youbi, A.O.; Alamry, K.A.; Sun, X.P. Reduced graphene oxide decorated with FeF nanoparticles: Facile synthesis and application as a high capacity cathode material for rechargeable lithium batteries. Electrochim. Acta 2013, 111, 80–85. [Google Scholar] [CrossRef]
- Lee, J.; Kang, B. Superior electrochemical performance of N-doped nanocrystalline FeF/C with a single-step solid-state process. Chem. Commun. 2016, 52, 12100–12103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.S.; Zhang, D.; Zhang, X.T.; Song, H.H.; Chen, X.H. Carbon-Nanotube-Encapsulated FeF Nanorods for High-Performance Lithium-Ion Cathode Materials. Acs Appl. Mater. Inter. 2014, 6, 21223–21229. [Google Scholar] [CrossRef]
- Wu, W.; Wang, X.Y.; Wang, X.; Yang, S.Y.; Liu, X.M.; Chen, Q.Q. Effects of MoS doping on the electrochemical performance of FeF cathode materials for lithium-ion batteries. Mater. Lett. 2009, 63, 1788–1790. [Google Scholar] [CrossRef]
- Chen, G.H.; Zhou, X.Z.; Bai, Y.; Yuan, Y.F.; Li, Y.; Chen, M.Z.; Ma, L.; Tan, G.Q.; Hu, J.P.; Wang, Z.H.; et al. Enhanced lithium storage capability of FeF3·0.33H2O single crystal with active insertion site exposed. Nano Energy 2019, 56, 884–892. [Google Scholar] [CrossRef]
- Fan, X.L.; Zhu, Y.J.; Luo, C.; Gao, T.; Suo, L.M.; Liou, S.C.; Xu, K.; Wang, C.S. lithiated FeF/C nanocomposite as high energy conversion-reaction cathode for lithium-ion batteries. J. Power Sources 2016, 307, 435–442. [Google Scholar] [CrossRef]

| Name | Lithium Cobalt Oxide (LCO) | Nickel Cobalt Manganese (NCM) | Lithium Manganese Oxide (LMO) | Lithium Iron Phosphate (LFP) |
|---|---|---|---|---|
| Chemical Formula | LiCoO2 | LiNixCoyMn1−x−yO2 | LiMn2O4 | LiFePO4 |
| Crystal Structure [31,32] | 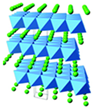 | 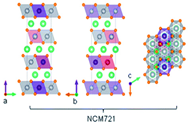 | 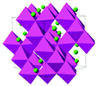 | 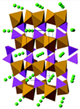 |
| Structure | Layered | Layered | Spinel | Olivine |
| Theoretical Capacity (mAh·g−1) | 274 | 273–285 | 148 | 170 |
| Practical Capacity (mAh·g−1) | 135–150 | 155–220 | 100–120 | 130–140 |
| Li+ Diffusion Coefficient (cm2/s) | 10−12–10−11 | 10−11–10−10 | 10−14–10−12 | 10−16–10−14 |
| Conductivity (S/cm) | 10−3 | 10−5 | 10−5 | 10−9 |
| Advantages [33] | High voltage plateau, simple synthesis, good cycling stability [34] | Stable high discharge voltage, high energy density and capacity [35] | Good rate capability, low cost, environmentally friendly [36,37] | Low cost, excellent cycling and thermal stability [38], eco-friendly [39] |
| Disadvantages | Low Li+ utilization, environmental pollution [40], poor safety | Li/Ni cation mixing at high voltage leads to poor cycling, low electronic conductivity [41] | Low capacity, poor cycling stability [42,43] | Poor low-temperature performance, low conductivity, slow Li+ diffusion [39,44] |
| MFn | Gf (kJ·mol−1) | Electromotive Force/V | Theoretical Capacity (mAh·g−1) |
|---|---|---|---|
| LiF | −589 | — | 0 |
| TiF3 | −1361 | 1.396 | 767 |
| VF3 | −1227 | 1.863 | 745 |
| MnF2 | −807 | 1.919 | 577 |
| MnF3 | −1000 | 2.647 | 719 |
| FeF2 | −663 | 2.664 | 571 |
| FeF3 | −972 | 2.742 | 712 |
| BiF3 | −902 | 3.124 | 302 |
| CoF2 | −627 | 2.854 | 553 |
| CoF3 | −719 | 3.617 | 694 |
| NiF2 | −604 | 2.964 | 554 |
| CuF2 | −492 | 3.553 | 528 |
| ZnF2 | −714 | 2.404 | 518 |
| SnF2 | −601 | 2.984 | 342 |
| AgF | −187 | 4.156 | 211 |
| PbF2 | −617 | 2.903 | 218 |
| CaF2 | −1173 | 0.0259 | 686 |
| BaF2 | −1158 | 0.104 | 306 |
| Property | ReO3-FeF3 | Pyrochlore-FeF3·0.5H2O | HTB-FeF3·0.33H2O |
|---|---|---|---|
| Structure | Corner-sharing [FeF6] octahedra forming 3D cubic channels, Small pore size (~2.5 Å) | 3D open framework with H2O in interstitial sites, Larger channels (~4.5 Å) | 1D hexagonal tunnels with H2O in the center, Expanded interlayer spacing (~7.2 Å) |
| Lattice water content | None | 0.5 H2O | 0.33 H2O |
| Reaction mechanism | Dominant intercalation (~3.0 V) | Intercalation + partial conversion (~3.0 V and ~2.0 V) | Intercalation + reversible conversion (~3.0 V and ~2.0 V) |
| Theoretical capacity (mAh·g–1) | ~237 (intercalation) | ~300 (mixed) | ~600 (mixed) |
| Advantages | High working voltage (~3.0 V), Low polarization | High structural stability, Enhanced Li+ diffusion due to H2O | High capacity, Good rate capability (~50% retention at 10C) |
| Disadvantages | Metastable (transforms to hexagonal phase), Low capacity | Structural collapse upon dehydration, Moderate capacity | Sensitive to H2O content, Capacity fading over long cycles |
| Modification strategies | Doping (Ti4+, Co2+)for stabilization, Nanostructuring | Carbon coating for conductivity, Interface engineering | Graphene composition, Precise H2O control |
| Synthesis Method | Typical Product | Capacity (mAh·g−1) |
|---|---|---|
| Solid-State (Ball-milling/Mechanochemistry) | Anhydrous ReO3-FeF3 [62] | 237 |
| FeF3/C composites [10] | 450–712 | |
| Liquid-Phase (Hydrothermal/Solvothermal) | HTB-FeF3·0.33H2O [11] | 600 |
| Pyrochlore-FeF3·0.5H2O [6] | 300 | |
| Gas-Phase Fluorination (F2/HF treatment) | High-purity FeF3 [14] | 200–400 |
| FeOXFY oxyfluorides [14] | 500–600 |
| Samples | Voltage Range /V | Current Density /mAg–1 | Capacity/mAh g−1 | Cycle Number |
|---|---|---|---|---|
| FeF2.2(OH)0.8·0.33H2O | 1−4.5 | 20 | 170 [88] | 50 |
| FE-FeF2 | 1–4.5 | 50 | 177 [93] | 30 |
| FeF3·0.33H2O/C + G | 1.8–4.5 | 47.4 | 193.1 [79] | 50 |
| Cr-doped Fe2F5·H2O | 1.0–4.0 | 20 | 171 [91] | 100 |
| FeF3·0.5H2O | 1.7–4.5 | 11 | 145 [94] | 100 |
| FeF3·0.5H2O | 1.7–4.5 | 24 | 135 [12] | 300 |
| FeF3/C/RGO | 1.0–4.0 | 100 | 220 [89] | 200 |
| FeF3/C | 1.5–4.5 | 7.12 | 250 [95] | 100 |
| FeF2-CMK-3 | 1.5–4.5 | 500 | 529 [96] | 100 |
| FeF3/rGO | 1.7–4.5 | 1000 | 146 [97] | 50 |
| N-doped FeF3/C | 2.0–4.5 | 5000 | 95 [98] | 250 |
| FeF3-carbon nanofiber | 1.0–4.0 | 100 | 550 [90] | 400 |
| FeF2@CNT nanorods | 1.3–4.3 | 50 | 263 [99] | 50 |
| FeF3/MoS2 | 2.0–4.5 | 23.7 | 169.6 [100] | 30 |
| Ni-doped FeF3·0.33H2O | 1.5–4.5 | 200 | 264 [92] | 100 |
| FeF3·0.33H2O | 2.0–4.5 | 200 | 167 [101] | 100 |
| Lithiated FeF3/C | 1.0–4.5 | 25 | 400 [102] | 30 |
| Material Type | Commercial Viability | Manufacturing Feasibility | Environmental Concerns |
|---|---|---|---|
| Iron Fluoride (FeF3) | Low cost Highest capacity (712 mAh·g−1 theoretical) | HF fluorination (high-risk) High energy input | Cobalt/nickel-free Hydrometallurgical recycling (HF handling) Medium carbon emissions |
| Lithium Cobalt Oxide (LCO) | High cost Medium capacity (274 mAh·g−1) | Solid-state reaction (mature) Medium energy input | Cobalt mining pollution Pyrometallurgical recycling (high energy) High carbon emissions |
| NCM811 | Medium-high cost High capacity (220 mAh·g−1) | Co-precipitation (complex) High energy input | Nickel/cobalt ecological impact Pyrometallurgical challenges Very high carbon emissions |
| Lithium Iron Phosphate (LFP) | Low cost Low capacity (170 mAh·g−1) | Solid-phase synthesis (simple) Low energy input | Heavy-metal-free Easy acid-leach recycling Low carbon emissions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Yang, Z.; Zheng, Y.; Chen, Z. Recent Progress on Synthesis and Electrochemical Performance of Iron Fluoride Conversion Cathodes for Li-Ion Batteries. Solids 2025, 6, 47. https://doi.org/10.3390/solids6030047
Tian J, Yang Z, Zheng Y, Chen Z. Recent Progress on Synthesis and Electrochemical Performance of Iron Fluoride Conversion Cathodes for Li-Ion Batteries. Solids. 2025; 6(3):47. https://doi.org/10.3390/solids6030047
Chicago/Turabian StyleTian, Jiabin, Ziyi Yang, Yayun Zheng, and Zhengfei Chen. 2025. "Recent Progress on Synthesis and Electrochemical Performance of Iron Fluoride Conversion Cathodes for Li-Ion Batteries" Solids 6, no. 3: 47. https://doi.org/10.3390/solids6030047
APA StyleTian, J., Yang, Z., Zheng, Y., & Chen, Z. (2025). Recent Progress on Synthesis and Electrochemical Performance of Iron Fluoride Conversion Cathodes for Li-Ion Batteries. Solids, 6(3), 47. https://doi.org/10.3390/solids6030047






