MXene-Based Composites for Energy Harvesting and Energy Storage Devices
Abstract
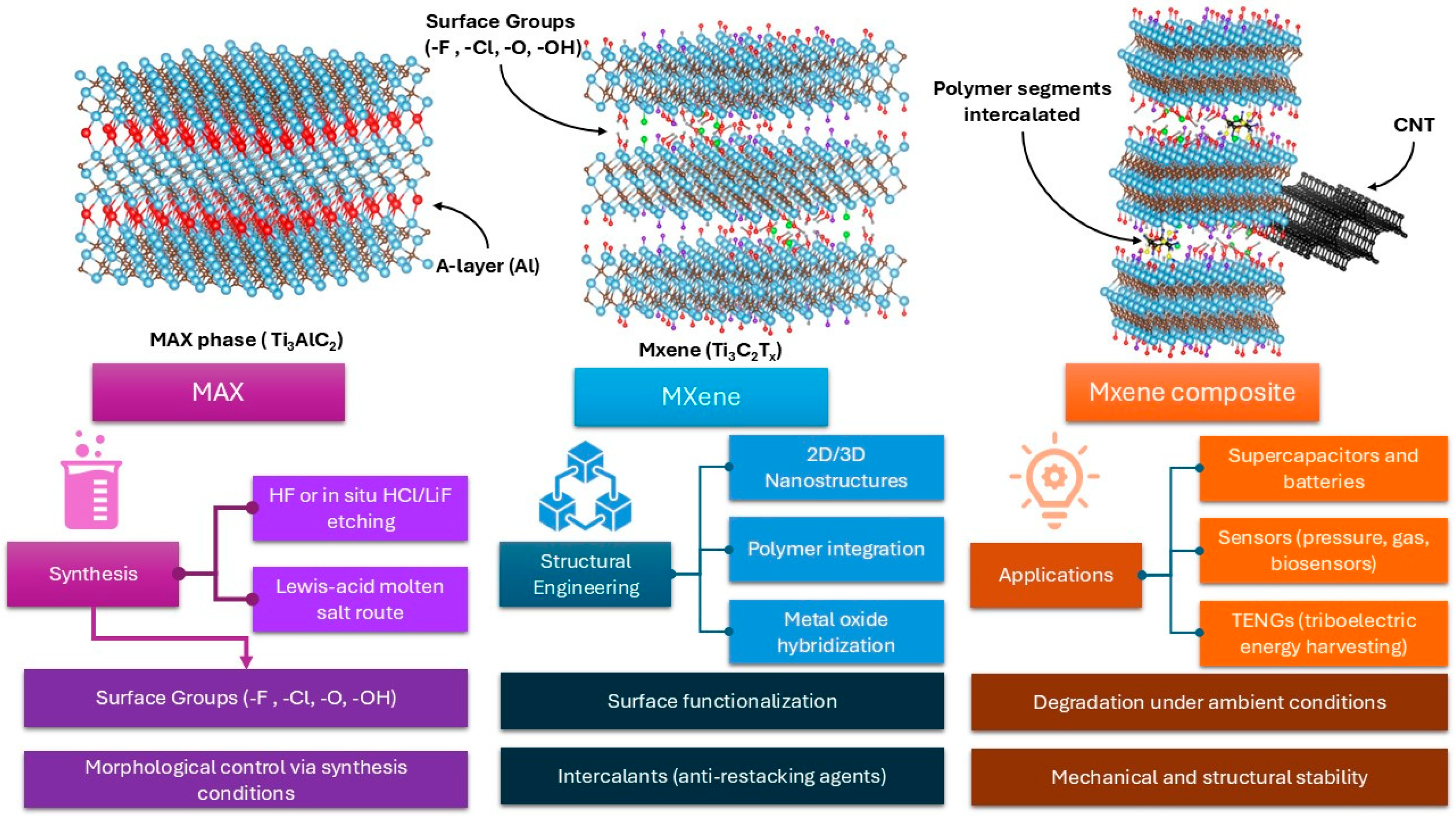
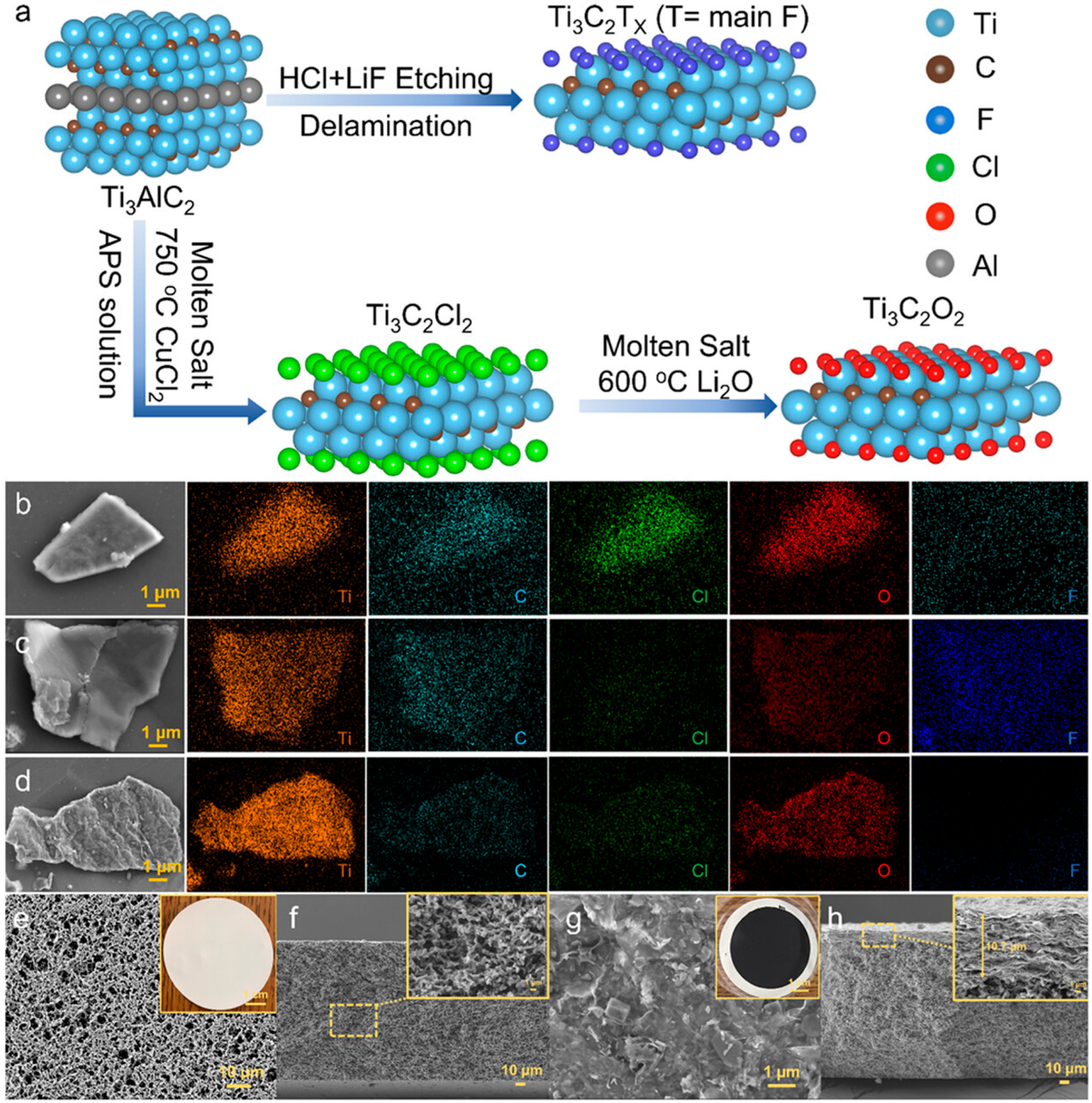
1. Introduction
2. Strategies for Improvement in the Electrochemical Performance of MXene
2.1. MXene Composites for Supercapacitors and Batteries
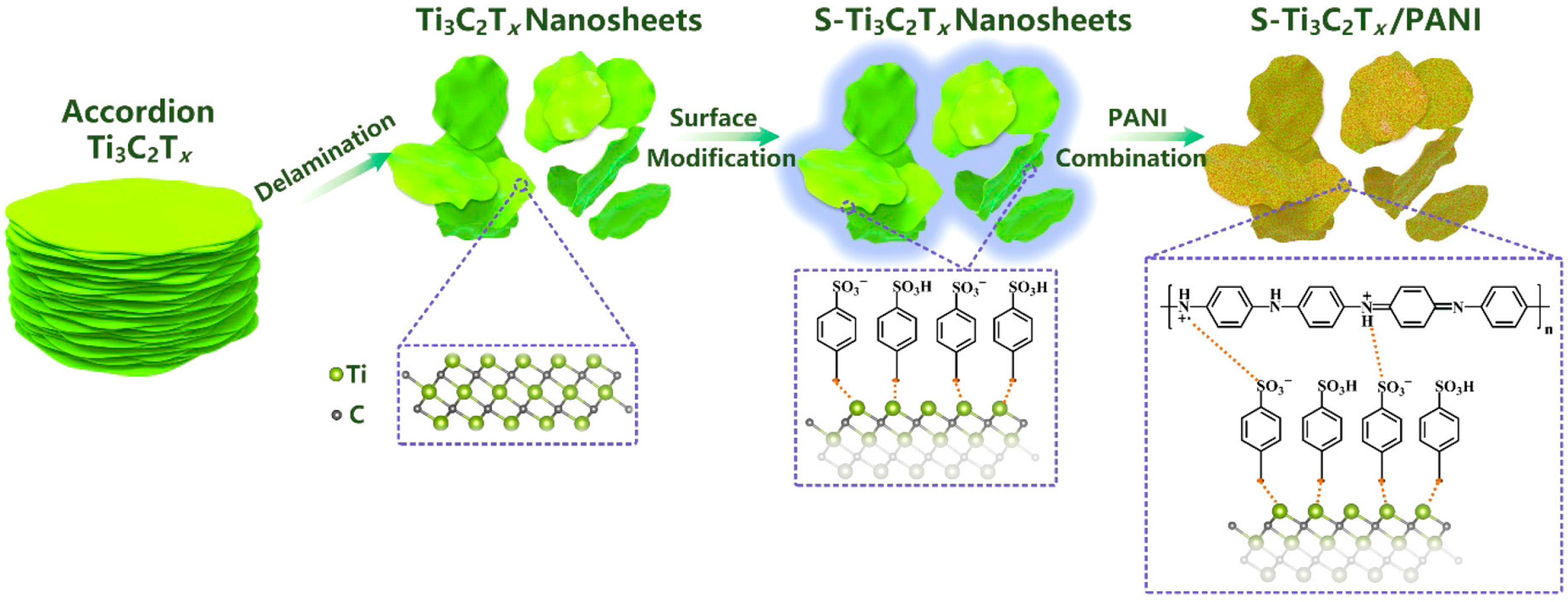
2.1.1. MXene + Conducting Polymers Composites
2.1.2. MXene + Carbon Derivative Nanomaterials
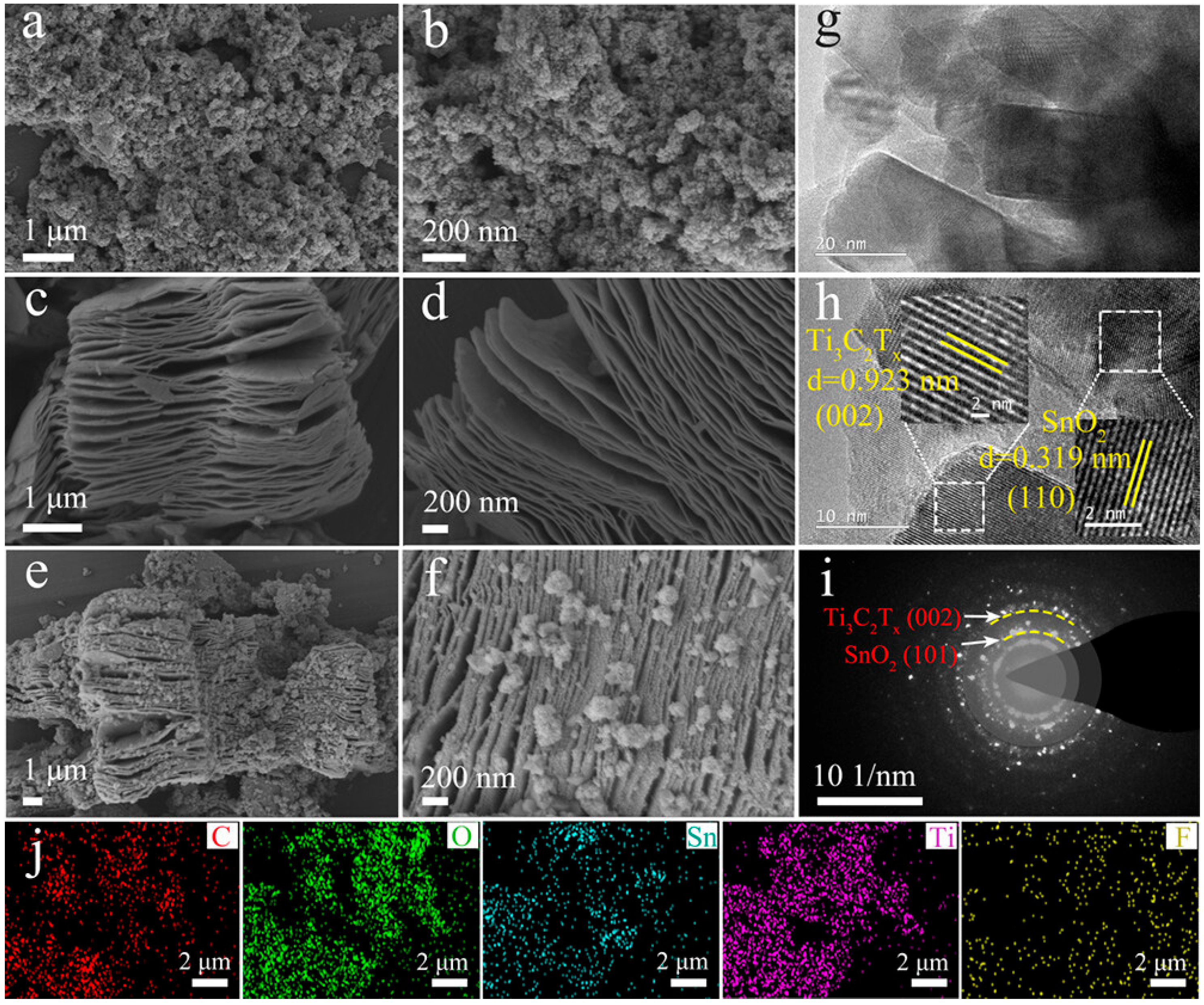
2.1.3. MXene + Metal Oxides and Ceramics Composites
3. Hybrid Applications of MXene-Based Composites
3.1. MXenes in Hybrid Energy Storage Devices
3.1.1. MXene-Based Hybrid Supercapacitors
3.1.2. MXenes as Electrodes in Batteries
3.2. MXenes in Triboelectric Nanogenerators (TENGs)
3.2.1. Integration of MXene in TENGs for Self-Powered Devices
3.2.2. Applications in Wearable Sensors and Sustainable Energy Systems
3.3. MXenes in Sensors and Flexible Electronics
3.3.1. Development of Hybrid Sensors for Biomedical, Gas, and Pressure Detection
3.3.2. Emerging Applications in Wearable and Flexible Electronics
4. Challenges and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | activated carbon |
| AI | artificial intelligence |
| Bi | bismuth |
| CDP | cyclodextrin polymer |
| CNC | cellulose nanocrystals |
| CNT | carbon nanotube |
| Co9S8 | cobalt sulfide |
| COF | covalent organic framework |
| CoO | cobalt(II) oxide |
| CuO | copper(II) oxide |
| CV | cyclic voltammetry |
| DEC | diethyl carbonate |
| DEGDME | diethylene glycol dimethyl ether |
| DMC | dimethyl carbonate |
| DMSO | dimethyl sulfoxide |
| DOL/DME | 1,3-dioxolane/dimethoxyethane |
| EC | ethylene carbonate |
| EDLC | electrical double-layer capacitance |
| EDX | energy-dispersive X-ray spectroscopy |
| EMI | electromagnetic interference |
| EMIMTFSI | 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide |
| FeVO4 | iron vanadate |
| FVO | FeVO4 (iron vanadate) |
| g-C3N4 | graphitic Carbon Nitride |
| GCD | galvanostatic charge–discharge |
| GeOx | germanium oxide |
| H2SO4 | sulfuric acid |
| HRTEM | high-resolution transmission electron microscopy |
| K4[Fe(CN)6] | potassium ferrocyanide |
| KOH | potassium hydroxide |
| LiFePO4 | lithium iron phosphate |
| LiPF6 | lithium hexafluorophosphate |
| LiTFSI | lithium bis(trifluoromethanesulfonyl)imide |
| ML | machine learning |
| MnO2 | manganese dioxide |
| Mo2TiC2TX | double transition metal MXene composed of molybdenum and titanium atoms |
| Mo4VC4Tx | molybdenum vanadium carbide MXene |
| MOF | metal-organic framework |
| MoS2 | molybdenum disulfide |
| MWCNT | multi-walled carbon nanotube |
| NaClO4 | sodium perchlorate |
| NaPF6 | sodium hexafluorophosphate |
| Nb2C | niobium carbide |
| NH2 | amine group |
| NH3 | ammonia |
| NH4Cl | ammonium chloride |
| NiCoP | nickel cobalt phosphide |
| Ni-MOF | nickel-based metal-organic framework |
| NPO | nickel phosphate |
| NVP | sodium vanadium phosphate |
| p(PFDMA) | poly(perfluorodecyl methacrylate) |
| PAAm | polyacrylamide |
| PANI | polyaniline |
| Pav | average power density |
| PEDOT:PSS | poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) |
| PLA | polylactic acid |
| p-MC | 3D interconnected porous MXene/carbon dot film |
| PVA | polyvinyl alcohol |
| PVP | Polyvinylpyrrolidone |
| rGO | reduced graphene oxide |
| S- Ti3C2Tx | sulfonation of Ti3C2Tx |
| SA | sodium alginate |
| SDS | sodium dodecyl sulfate |
| SEM | scanning electron microscopy |
| SPE | solid polymer electro |
| SSA | specific surface area |
| TEM | transmission electron microscopy |
| Ti2C | titanium carbide |
| Ti3C2 | titanium carbide |
| Ti3C2Tx | titanium carbide MXene |
| Ti3CN | nitrogen-containing titanium carbide |
| TiO2 | titanium dioxide |
| TiVC | titanium-vanadium solid solution MXene |
| TNO | titanium–niobium oxide |
| UN-Ti3C2Tx | urea-assisted nitrogen-doped Ti3C2Tx |
| VS2 | vanadium disulfide |
| V2CTx | vanadium carbide MXene with surface terminations |
| V4C3TX | vanadium carbide MXene with four vanadium and three carbon layers, functionalized with surface terminations |
| ZIF | zeolitic imidazolate framework |
| Zn | zinc |
| ZnS | zinc sulfide |
| ZnSO4 | zinc sulfate |
| βCD | beta-cyclodextrin |
References
- Aghmadi, A.; Mohammed, O.A. Energy Storage Systems: Technologies and High-Power Applications. Batteries 2024, 10, 141. [Google Scholar] [CrossRef]
- Krichen, M.; Basheer, Y.; Qaisar, S.M.; Waqar, A. A Survey on Energy Storage: Techniques and Challenges. Energies 2023, 16, 2271. [Google Scholar] [CrossRef]
- Ryu, H.; Yoon, H.J.; Kim, S.W. Hybrid Energy Harvesters: Toward Sustainable Energy Harvesting. Adv. Mater. 2019, 31, 1802898. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, R.; Singh, D. Energy harvesting in wireless sensor networks: A taxonomic survey. Int. J. Energy Res. 2021, 45, 118–140. [Google Scholar] [CrossRef]
- Yan, W.; Ma, L.; Xu, J.; Guo, Y.; Hu, H.; Zhi, C.; Ho, D. Battery-Sensor Hybrid: A New Gas Sensing Paradigm with Complete Energy Self-Sufficiency. ACS Appl. Mater. Interfaces 2021, 13, 46507–46517. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Meng, X. Overview of transition metal-based composite materials for supercapacitor electrodes. Nanoscale Adv. 2020, 2, 5516–5528. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Z.; Liu, J.; Li, C.; Mao, F.; Wu, H.; Zhang, Q. Enhancing the Performance of a Battery-Supercapacitor Hybrid Energy Device through Narrowing the Capacitance Difference between Two Electrodes via the Utilization of 2D MOF-Nanosheet-Derived Ni@Nitrogen-Doped-Carbon Core-Shell Rings as Both Negative and P. ACS Appl. Mater. Interfaces 2020, 12, 47482–47489. [Google Scholar] [CrossRef]
- Khuje, S.; Alshatnawi, F.; Alhendi, M.; Yu, J.; Sheng, A.; Huang, Y.; Zhuang, C.G.; Armstrong, J.; Zhou, C.; Poliks, M.; et al. High-Temperature Oxidation-Resistant Printed Copper Conductors. Adv. Electron. Mater. 2023, 9, 2200979. [Google Scholar] [CrossRef]
- Liang, Z.; Nafis, M.S.; Rodriguez, D.; Ban, C. Surface Science and Engineering for Electrochemical Materials. Acc. Chem. Res. 2024, 57, 3102–3112. [Google Scholar] [CrossRef]
- Mateen, A.; Khan, A.J.; Zhou, Z.; Mujear, A.; Farid, G.; Yan, W.; Li, H.; Li, J.; Bao, Z. Silicon Nanowires via Metal-Assisted Chemical Etching for Energy Storage Applications. ChemSusChem 2024, 18, e202400777. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Wasiucionek, M.; Garbarczyk, J.E. Towards higher electric conductivity and wider phase stability range via nanostructured glass-ceramics processing. Nanomaterials 2021, 11, 1321. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.; Bououdina, M.; Usman, M.; Khan, A.; Luo, W.; Wang, C. Designing State-of-the-Art Gas Sensors: From Fundamentals to Applications. Chem. Rec. 2024, 24, e202300350. [Google Scholar] [CrossRef] [PubMed]
- Nunes Simonetti, E.A.; Cardoso de Oliveira, T.; Enrico do Carmo Machado, Á.; Coutinho Silva, A.A.; Silva dos Santos, A.; de Simone Cividanes, L. TiO2 as a gas sensor: The novel carbon structures and noble metals as new elements for enhancing sensitivity—A review. Ceram. Int. 2021, 47, 17844–17876. [Google Scholar] [CrossRef]
- Tovar-Lopez, F.J. Recent Progress in Micro- and Nanotechnology-Enabled Sensors for Biomedical and Environmental Challenges. Sensors 2023, 23, 5406. [Google Scholar] [CrossRef]
- Costa, R.S.; Pires, A.L.; Pereira, A.M.; Pereira, C.R. Multifunctional energy harvesting and storage textile technology based on thermionic effect. J. Power Sources 2023, 587, 233712. [Google Scholar] [CrossRef]
- Huo, D.; Yang, X.; Pan, Y.; Su, J. Gravity-Switch-Triggered Triboelectric Nanogenerator for Multi-Directional Wave Energy Harvesting. Adv. Mater. Technol. 2025, 10, 2401796. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Shukla, P.S.; Varma, G.D.; Venkataraman, D.; Bag, M. Photorechargeable Hybrid Halide Perovskite Supercapacitors. ACS Appl. Mater. Interfaces 2022, 14, 35592–35599. [Google Scholar] [CrossRef]
- Teixeira, J.S.; Costa, R.S.; Guedes, A.; Pereira, A.M.; Pereira, C.R. Fabrication of CNT-N@Manganese Oxide Hybrid Nanomaterials through a Versatile One-Pot Eco-Friendly Route toward Engineered Textile Supercapacitors. ACS Appl. Eng. Mater. 2024, 2, 1170–1189. [Google Scholar] [CrossRef]
- Cheng, T.; Yang, X.; Yang, S.; Li, L.; Liu, Z.-T.; Qu, J.; Meng, C.-F.; Li, X.-C.; Zhang, Y.-Z.; Lai, W.-Y. Flexible Transparent Bifunctional Capacitive Sensors with Superior Areal Capacitance and Sensing Capability based on PEDOT:PSS/MXene/Ag Grid Hybrid Electrodes. Adv. Funct. Mater. 2023, 33, 2210997. [Google Scholar] [CrossRef]
- Li, Y.; Shao, H.; Lin, Z.; Lu, J.; Liu, L.; Duployer, B.; Persson, P.O.Å.; Eklund, P.; Hultman, L.; Li, M.; et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 2020, 19, 894–899. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, L.; Zhou, J.; Sun, Z. Microscopic origin of MXenes derived from layered MAX phases. RSC Adv. 2015, 5, 25403–25408. [Google Scholar] [CrossRef]
- Fu, Z.H.; Zhang, Q.F.; Legut, D.; Si, C.; Germann, T.C.; Lookman, T.; Du, S.Y.; Francisco, J.S.; Zhang, R.F. Stabilization and strengthening effects of functional groups in two-dimensional titanium carbide. Phys. Rev. B 2016, 94, 104103. [Google Scholar] [CrossRef]
- Nahirniak, S.; Ray, A.; Saruhan, B. Challenges and Future Prospects of the MXene-Based Materials for Energy Storage Applications. Batteries 2023, 9, 126. [Google Scholar] [CrossRef]
- Luo, X.; Zhu, L.; Wang, Y.C.; Li, J.; Nie, J.; Wang, Z.L. A Flexible Multifunctional Triboelectric Nanogenerator Based on MXene/PVA Hydrogel. Adv. Funct. Mater. 2021, 31, 2104928. [Google Scholar] [CrossRef]
- Wicklein, B.; Valurouthu, G.; Yoon, H.; Yoo, H.; Ponnan, S.; Mahato, M.; Kim, J.; Ali, S.S.; Park, J.Y.; Gogotsi, Y.; et al. Influence of MXene Composition on Triboelectricity of MXene-Alginate Nanocomposites. ACS Appl. Mater. Interfaces 2024, acsami.4c03298. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Patel, A.; Shankar, S.; Driscoll, N.; Zhou, C.; Rex, T.S.; Vitale, F.; Gallagher, M.J. An Open-Source Mouse Chronic EEG Array System with High-Density MXene-Based Skull Surface Electrodes. eNeuro 2024, 11, ENEURO.0512-22.2023. [Google Scholar] [CrossRef]
- Zhang, H.; Lan, D.; Wu, B.; Chen, X.; Li, X.; Li, Z.; Dai, F. Electrospun Piezoelectric Scaffold with External Mechanical Stimulation for Promoting Regeneration of Peripheral Nerve Injury. Biomacromolecules 2023, 24, 3268–3282. [Google Scholar] [CrossRef]
- Liang, C.; Wang, H.; Lin, Z.; Zhang, C.; Liu, G.; Hu, Y. Augmented wound healing potential of photosensitive GelMA hydrogel incorporating antimicrobial peptides and MXene nanoparticles. Front. Bioeng. Biotechnol. 2023, 11, 1310349. [Google Scholar] [CrossRef]
- Donthula, K.; Malothu, U.R.; Araga, R.; Vooradi, R.; Patnaikuni, V.S.; Reddy, M.V.; Kakunuri, M. Flexible polyaniline/MXene/CNF composite nanofibrous mats as high-performance supercapacitor electrodes. Polym. Compos. 2023, 44, 7571–7584. [Google Scholar] [CrossRef]
- Zhang, S.; Tu, T.; Li, T.; Cai, Y.; Wang, Z.; Zhou, Y.; Wang, D.; Fang, L.; Ye, X.; Liang, B. 3D MXene/PEDOT:PSS Composite Aerogel with a Controllable Patterning Property for Highly Sensitive Wearable Physical Monitoring and Robotic Tactile Sensing. ACS Appl. Mater. Interfaces 2022, 14, 23877–23887. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Sui, C.; Zhao, C.; Zhao, Y.; Cheng, G.; Li, J.; Wen, L.; Hao, W.; Sang, Y.; Zhou, Y.; et al. Supertough MXene/Sodium Alginate Composite Fiber Felts Integrated with Outstanding Electromagnetic Interference Shielding and Heating Properties. Nano Lett. 2024, 24, 8098–8106. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Zahra, S.A.; Khan, S.A.; Akinwande, D.; Minár, J.; Rizwan, S. Experimental and computational analysis of mno2@v2c-mxene for enhanced energy storage. Nanomaterials 2021, 11, 1707. [Google Scholar] [CrossRef] [PubMed]
- Myint, W.; Lolupiman, K.; Yang, C.; Woottapanit, P.; Limphirat, W.; Kidkhunthod, P.; Muzakir, M.; Karnan, M.; Zhang, X.; Qin, J. Exploring the Electrochemical Superiority of V2O5/TiO2@Ti3C2-MXene Hybrid Nanostructures for Enhanced Lithium-Ion Battery Performance. ACS Appl. Mater. Interfaces 2024, 16, 53764–53774. [Google Scholar] [CrossRef]
- Ruan, T.; Wang, B.; Lv, Q.; Jiang, Y.; Wang, D. Interface coupling in FeOOH/MXene heterojunction for highly reversible lithium-ion storage. Mater. Today Energy 2021, 19, 100584. [Google Scholar] [CrossRef]
- Sun, Z.; Peng, J.; Yang, S.; Jin, R.; Liu, C.; Huang, Q. Synthesis of Low-Crystalline MnO2/MXene Composites for Capacitive Deionization with Efficient Desalination Capacity. Metals 2023, 13, 1047. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, J.; Luo, X.; Miao, Y.; Zhang, Y.; Xu, W.; Yang, L.; Liang, Y.; Weng, W.; Zhu, M. Fabrication of a fibrous MnO2@MXene/CNT electrode for high-performance flexible supercapacitor. Ceram. Int. 2020, 46, 11874–11881. [Google Scholar] [CrossRef]
- Pathak, M.; Mane, P.; Chakraborty, B.; Rout, C.S. Facile in-situ grown spinel MnCo2O4/MWCNT and MnCo2O4/Ti3C2 MXene composites for high-performance asymmetric supercapacitor with theoretical insight. J. Energy Storage 2023, 66, 107475. [Google Scholar] [CrossRef]
- Zhou, Z.; Panatdasirisuk, W.; Mathis, T.S.; Anasori, B.; Lu, C.; Zhang, X.; Liao, Z.; Gogotsi, Y.; Yang, S. Layer-by-layer assembly of MXene and carbon nanotubes on electrospun polymer films for flexible energy storage. Nanoscale 2018, 10, 6005–6013. [Google Scholar] [CrossRef]
- Habib, T.; Zhao, X.; Shah, S.A.; Chen, Y.; Sun, W.; An, H.; Lutkenhaus, J.L.; Radovic, M.; Green, M.J. Oxidation stability of Ti3C2Tx MXene nanosheets in solvents and composite films. npj 2D Mater. Appl. 2019, 3, 8. [Google Scholar] [CrossRef]
- Sunderiya, S.; Suragtkhuu, S.; Purevdorj, S.; Ochirkhuyag, T.; Bat-Erdene, M.; Myagmarsereejid, P.; Slattery, A.D.; Bati, A.S.R.; Shapter, J.G.; Odkhuu, D.; et al. Understanding the oxidation chemistry of Ti3C2T (MXene) sheets and their catalytic performances. J. Energy Chem. 2024, 88, 437–445. [Google Scholar] [CrossRef]
- Wu, T.; Kent, P.R.C.; Gogotsi, Y.; Jiang, D. How Water Attacks MXene. Chem. Mater. 2022, 34, 4975–4982. [Google Scholar] [CrossRef]
- Huang, S.; Mochalin, V.N. Hydrolysis of 2D Transition-Metal Carbides (MXenes) in Colloidal Solutions. Inorg. Chem. 2019, 58, 1958–1966. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, N.; Hegh, D.; Usman, K.A.S.; Guan, G.; Qin, S.; Jurewicz, I.; Yang, W.; Razal, J.M. Freezing Titanium Carbide Aqueous Dispersions for Ultra-long-term Storage. ACS Appl. Mater. Interfaces 2020, 12, 34032–34040. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Zhao, X.; Zhang, P.; Wei, Y.; Qiao, N.; Yang, B.; Soomro, R.A.; Zhang, R.; Xu, B. Effect of Sodium Dodecyl Sulfate on Stability of MXene Aqueous Dispersion. Adv. Sci. 2023, 10, 2300273. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, N.; Zhang, Z.; Wang, G.; Cheng, K. Free-standing 3D porous energy hydrogels enabled by ion-induced gelation strategy for High-performance supercapacitors. Appl. Surf. Sci. 2022, 604, 154636. [Google Scholar] [CrossRef]
- Akir, S.; Lontio Fomekong, R.; Chacko, L.; Děkanovský, L.; Mazánek, V.; Sturala, J.; Koňáková, D.; Sofer, Z. Nanoengineering bismuth-modified vanadium carbide MXene for enhanced electrochemical performance in neutral electrolyte: A pathway toward high-performance supercapacitors. J. Energy Storage 2024, 85, 110962. [Google Scholar] [CrossRef]
- Jin, Y.; Geng, J.; Wang, Y.; Zhao, Z.; Chen, Z.; Guo, Z.; Pei, L.; Ren, F.; Sun, Z.; Ren, P.-G. Flexible all-solid-state asymmetric supercapacitor based on Ti3C2Tx MXene/graphene/carbon nanotubes. Surfaces and Interfaces 2024, 53, 104999. [Google Scholar] [CrossRef]
- Xiong, K.; Wang, P.; Yang, G.; Liu, Z.; Zhang, H.; Jin, S.; Xu, X. Functional Group Effects on the Photoelectronic Properties of MXene (Sc2CT2, T = O, F, OH) and Their Possible Photocatalytic Activities. Sci. Rep. 2017, 7, 15095. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, C.; Quan, X.; Li, L.; Wang, Y.; Zhou, J. Lysozyme Adsorption on Different Functionalized MXenes: A Multiscale Simulation Study. Langmuir 2021, 37, 5932–5942. [Google Scholar] [CrossRef]
- Song, H.; Jiang, D.E. First principles insights into stability of defected MXenes in water. Nanoscale 2023, 15, 16010–16015. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Sha, X.; Liang, J.; Yang, G.; Hu, X.; Wen, Y.; Liu, M.; Zhou, N.; Zhang, X.; Wei, Y. Construction of ionic liquid functionalized MXene with extremely high adsorption capacity towards iodine via the combination of mussel-inspired chemistry and Michael addition reaction. J. Colloid Interface Sci. 2021, 601, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jin, Y.; Hu, M.; Ren, W.; Xie, Y.; Wang, Y. BC6N as a promising sulfur host material for lithium-sulfur batteries. Appl. Surf. Sci. 2022, 577, 151843. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Z.; Zhou, H.; Sharma, A.; Deng, J.; Ke, C. Physical adsorption and oxidation of ultra-thin MoS2 crystals: Insights into surface engineering for 2D electronics and beyond. Nanotechnology 2023, 34, 405701. [Google Scholar] [CrossRef]
- Lee, T.W.; Chen, C.C.; Chen, C. Chemical Stability and Transformation of Molybdenum Disulfide Nanosheets in Environmental Media. Environ. Sci. Technol. 2019, 53, 6282–6291. [Google Scholar] [CrossRef]
- Zhang, W.; Matsuda, K.; Miyauchi, Y. Photostability of Monolayer Transition-Metal Dichalcogenides in Ambient Air and Acidic/Basic Aqueous Solutions. ACS Omega 2019, 4, 10322–10327. [Google Scholar] [CrossRef]
- Zhao, X.; Dall’Agnese, C.; Chu, X.; Zhao, S.; Chen, G.; Gogotsi, Y.; Gao, Y.; Dall’Agnese, Y. Electrochemical Behavior of Ti3C2Tx MXene in Environmentally Friendly Methanesulfonic Acid Electrolyte. ChemSusChem 2019, 12, 4480–4486. [Google Scholar] [CrossRef]
- Mehdi, S.M.Z.; Ghulam Abbas, H.; Ali, M.; Rizvi, S.B.H.; Choi, S.R.; Goak, J.C.; Seo, Y.; Kumar, S.; Lee, N. Enhanced Electrochemical Performance and Theoretical Insights of Ni-Intercalated Ti3C2Tx MX ene. ENERGY Environ. Mater. 2025. [Google Scholar] [CrossRef]
- Huang, P.; Jia, H.; Wang, T.; Xu, Y.; Zhang, L.; Wei, X.; Jia, H.; Wen, S.; Lv, K.; Liu, D. Effects of Modification Degrees on the Colloidal Stability of Amphiphilic Janus Graphene Oxide in Aqueous Solution with and without Electrolytes. Langmuir 2021, 37, 10061–10070. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.; Wang, W.; Fortner, J. Organic functionalized graphene oxide behavior in water. Nanomaterials 2020, 10, 1228. [Google Scholar] [CrossRef]
- Iakunkov, A.; Nordenström, A.; Boulanger, N.; Hennig, C.; Baburin, I.; Talyzin, A.V. Temperature-dependent swelling transitions in MXene Ti3C2Tx. Nanoscale 2022, 14, 10940–10949. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Fu, Q.; Wu, W.; Gao, H.; Zhang, X.; Wang, B. Understanding the different diffusion mechanisms of hydrated protons and potassium ions in titanium carbide mxene. ACS Appl. Mater. Interfaces 2019, 11, 7087–7095. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, W.; Guo, B.; Cui, M.; Ma, H.; Zhang, Z.; Zhang, R. Facile fabrication of ultrathin graphene film with ultrahigh electrical conductivity and superb electromagnetic interference shielding effectiveness. J. Mater. Chem. C 2021, 9, 214–222. [Google Scholar] [CrossRef]
- Guo, T.; Zhou, D.; Deng, S.; Jafarpour, M.; Avaro, J.; Neels, A.; Heier, J.; Zhang, C. Rational Design of Ti3C2Tx MXene Inks for Conductive, Transparent Films. ACS Nano 2023, 17, 3737–3749. [Google Scholar] [CrossRef]
- Ranjan, B.; Kaur, D. Achieving enhanced pseudocapacitance in MoS2 nanowires rationally sputtered over NiMnIn shape memory alloy for flexible Na-ion supercapacitor. J. Energy Storage 2023, 71, 108122. [Google Scholar] [CrossRef]
- Lee, J.J.; Chae, S.-H.; Lee, J.J.; Lee, M.S.; Yoon, W.; Kwac, L.K.; Kim, H.G.; Shin, H.K. Waste-Wood-Isolated Cellulose-Based Activated Carbon Paper Electrodes with Graphene Nanoplatelets for Flexible Supercapacitors. Molecules 2023, 28, 7822. [Google Scholar] [CrossRef]
- Wu, W.; Wang, C.; Zhao, C.; Wei, D.; Zhu, J.; Xu, Y. Facile strategy of hollow polyaniline nanotubes supported on Ti3C2-MXene nanosheets for High-performance symmetric supercapacitors. J. Colloid Interface Sci. 2020, 580, 601–613. [Google Scholar] [CrossRef]
- Park, S.; Park, B.; Jeon, S.-P.; Kang, Y.; Kim, J.; Park, S.K.; Kim, Y.-H. Effects of Ti Doping on the Electrical Properties and Gate-Bias Stability of Amorphous Zinc–Tin–Oxide Thin-Film Transistors. ACS Appl. Electron. Mater. 2023, 5, 3416–3425. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, H.; Liu, W.; Wang, S.; Wang, X.; Wang, M.; Ma, W.; Sun, G.; Liu, J. Design of Ti3C2Tx/SnO2-Selective Ethanolamine Sensor. ACS Appl. Nano Mater. 2024, 7, 4324–4335. [Google Scholar] [CrossRef]
- Shi, M.; Wang, R.; Li, L.; Chen, N.; Xiao, P.; Yan, C.; Yan, X. Redox-Active Polymer Integrated with MXene for Ultra-Stable and Fast Aqueous Proton Storage. Adv. Funct. Mater. 2023, 33. [Google Scholar] [CrossRef]
- Li, J.; Song, J.; Luo, L.; Zhang, H.; Feng, J.; Zhao, X.; Guo, X.; Dong, H.; Chen, S.; Liu, H.; et al. Synergy of MXene with Se Infiltrated Porous N-Doped Carbon Nanofibers as Janus Electrodes for High-Performance Sodium/Lithium–Selenium Batteries. Adv. Energy Mater. 2022, 12, 2200894. [Google Scholar] [CrossRef]
- Lu, C.; Li, A.; Li, G.; Yan, Y.; Zhang, M.; Yang, Q.; Zhou, W.; Guo, L. S-Decorated Porous Ti3C2 MXene Combined with In Situ Forming Cu2Se as Effective Shuttling Interrupter in Na–Se Batteries. Adv. Mater. 2021, 33. [Google Scholar] [CrossRef]
- Liu, N.; Li, Q.; Wan, H.; Chang, L.; Wang, H.; Fang, J.; Ding, T.; Wen, Q.; Zhou, L.; Xiao, X. High-temperature stability in air of Ti3C2Tx MXene-based composite with extracted bentonite. Nat. Commun. 2022, 13, 5551. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, T.; Meng, Z.; Wang, C.; Dong, W.; Liu, J.; Yang, S.; Hou, X.; Cheng, X.; Liu, W.; et al. Self-intercepting interference of hydrogen-bond induced flexible hybrid film to facilitate lithium extraction. Chem. Eng. J. 2023, 458, 141403. [Google Scholar] [CrossRef]
- Hakim, M.W.; Fatima, S.; Tahir, R.; Iqbal, M.Z.; Li, H.; Rizwan, S. Ni-intercalated Mo2TiC2Tx free-standing MXene for excellent gravimetric capacitance prepared via electrostatic self-assembly. J. Energy Storage 2023, 61, 106662. [Google Scholar] [CrossRef]
- Tran, M.H.; Schäfer, T.; Shahraei, A.; Dürrschnabel, M.; Molina-Luna, L.; Kramm, U.I.; Birkel, C.S. Adding a New Member to the MXene Family: Synthesis, Structure, and Electrocatalytic Activity for the Hydrogen Evolution Reaction of V4C3Tx. ACS Appl. Energy Mater. 2018, 1, 3908–3914. [Google Scholar] [CrossRef]
- Bin, X.; Sheng, M.; Luo, Y.; Que, W. Self-Assembling Delaminated V4C3Tx MXene into Highly Stable Pseudocapacitive Flexible Film Electrode for Supercapacitors. Adv. Mater. Interfaces 2022, 9, 2200231. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, S.; Huang, Y.; Tong, P.; Zhao, B.; Zhu, X.; Sun, Y. Synthesis and lithium ion storage performance of two-dimensional V4C3 MXene. Chem. Eng. J. 2019, 373, 203–212. [Google Scholar] [CrossRef]
- Lin, Z.; Barbara, D.; Taberna, P.-L.; Van Aken, K.L.; Anasori, B.; Gogotsi, Y.; Simon, P. Capacitance of Ti3C2Tx MXene in ionic liquid electrolyte. J. Power Sources 2016, 326, 575–579. [Google Scholar] [CrossRef]
- Shao, H.; Lin, Z.; Xu, K.; Taberna, P.L.; Simon, P. Electrochemical study of pseudocapacitive behavior of Ti3C2Tx MXene material in aqueous electrolytes. Energy Storage Mater. 2019, 18, 456–461. [Google Scholar] [CrossRef]
- Ghotia, S.; Kumar, A.; Sudarsan, V.; Dwivedi, N.; Singh, S.; Kumar, P. Multilayered Ti3C2Tx MXenes: A prominent materials for hydrogen storage. Int. J. Hydrogen Energy 2024, 52, 100–107. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef]
- Chen, C.; Xie, X.; Anasori, B.; Sarycheva, A.; Makaryan, T.; Zhao, M.; Urbankowski, P.; Miao, L.; Jiang, J.; Gogotsi, Y. MoS2-on-MXene Heterostructures as Highly Reversible Anode Materials for Lithium-Ion Batteries. Angew. Chemie-Int. Ed. 2018, 57, 1846–1850. [Google Scholar] [CrossRef]
- Deysher, G.; Shuck, C.E.; Hantanasirisakul, K.; Frey, N.C.; Foucher, A.C.; Maleski, K.; Sarycheva, A.; Shenoy, V.B.; Stach, E.A.; Anasori, B.; et al. Synthesis of Mo4VAlC4 MAX Phase and Two-Dimensional Mo4VC4 MXene with Five Atomic Layers of Transition Metals. ACS Nano 2020, 14, 204–217. [Google Scholar] [CrossRef]
- Hussain, I.; Rehman, F.; Saraf, M.; Zhang, T.; Wang, R.; Das, T.; Luo, Z.; Gogotsi, Y.; Zhang, K. Electrochemical Properties of Mo4VC4Tx MXene in Aqueous Electrolytes. ACS Appl. Mater. Interfaces 2024, 16, 38053–38060. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, D.; Wu, Q.; Wang, H.; Wang, L.; Liu, B.; Zhou, A.; He, J. MXene: A new family of promising hydrogen storage medium. J. Phys. Chem. A 2013, 117, 14253–14260. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Hu, R.; Liu, B.; Zhang, Y.; Zhu, K.; Yan, J.; Ye, K.; Cheng, K.; Wang, G.; Cao, D. MXene-derived TiO2/reduced graphene oxide composite with an enhanced capacitive capacity for Li-ion and K-ion batteries. J. Mater. Chem. A 2019, 7, 5363–5372. [Google Scholar] [CrossRef]
- Wang, G.; Yang, Z.; Nie, X.; Wang, M.; Liu, X. A Flexible Supercapacitor Based on Niobium Carbide MXene and Sodium Anthraquinone-2-Sulfonate Composite Electrode. Micromachines 2023, 14, 1515. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tian, M.; Wang, H.; Wei, C.; Sun, Z.; Rummeli, M.H.; Strasser, P.; Sun, J.; Yang, R. Mildly Oxidized MXene (Ti3C2, Nb2C, and V2C) Electrocatalyst via a Generic Strategy Enables Longevous Li-O2 Battery under a High Rate. ACS Nano 2021, 15, 19640–19650. [Google Scholar] [CrossRef] [PubMed]
- Yazdanparast, S.; Soltanmohammad, S.; Fash-White, A.; Tucker, G.J.; Brennecka, G.L. Synthesis and Surface Chemistry of 2D TiVC Solid-Solution MXenes. ACS Appl. Mater. Interfaces 2020, 12, 20129–20137. [Google Scholar] [CrossRef]
- Baig, M.M.; Gul, I.H.; Baig, S.M.; Shahzad, F. 2D MXenes: Synthesis, properties, and electrochemical energy storage for supercapacitors—A review. J. Electroanal. Chem. 2022, 904, 115920. [Google Scholar] [CrossRef]
- Shankar, S.; Murphy, B.B.; Driscoll, N.; Shekhirev, M.; Valurouthu, G.; Shevchuk, K.; Anayee, M.; Cimino, F.; Gogotsi, Y.; Vitale, F. Effect of the deposition process on the stability of Ti3C2Tx MXene films for bioelectronics. 2D Mater. 2023, 10, 44001. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Ke, T.; Rajavel, K.; Yang, K.; Lin, D. Dispersibility and Photochemical Stability of Delaminated MXene Flakes in Water. Small 2020, 16, 2002433. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Oh, M.S.; Cho, A.; Ryu, J.; Kim, Y.J.; Kang, H.; Cho, S.Y.; Im, S.G.; Kim, S.J.; Jung, H.T. Simple Approach to Enhance Long-Term Environmental Stability of MXene Using Initiated Chemical Vapor Deposition Surface Coating. ACS Nano 2023, 17, 10898–10905. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y.; Wang, Y.; Liu, Z.; Dong, K.; Zhang, S. Effect of Interlayer Spaces and Interfacial Structures on High-Performance MXene/Ionic Liquid Supercapacitors: A Molecular Dynamics Simulation. Langmuir 2024, 40, 2220–2229. [Google Scholar] [CrossRef]
- Benchakar, M.; Loupias, L.; Garnero, C.; Bilyk, T.; Morais, C.; Canaff, C.; Guignard, N.; Morisset, S.; Pazniak, H.; Hurand, S.; et al. One MAX phase, different MXenes: A guideline to understand the crucial role of etching conditions on Ti3C2Tx surface chemistry. Appl. Surf. Sci. 2020, 530, 147209. [Google Scholar] [CrossRef]
- Gurzęda, B.; Boulanger, N.; Nordenström, A.; Dejoie, C.; Talyzin, A.V. Pristine MXene: In Situ XRD Study of MAX Phase Etching with HCl+LiF Solution. Adv. Sci. 2024, 11, 2408448. [Google Scholar] [CrossRef]
- Carey, M.S.; Barsoum, M.W. Scalable and sustainable production of Ti3C2Tz MXene and fluorine recovery from wastewater through cryolite precipitation. RSC Adv. 2022, 12, 30846–30850. [Google Scholar] [CrossRef]
- Ma, Q.; Gao, J.; Moussa, B.; Young, J.; Zhao, M.; Zhang, W. Electrosorption, Desorption, and Oxidation of Perfluoroalkyl Carboxylic Acids (PFCAs) via MXene-Based Electrocatalytic Membranes. ACS Appl. Mater. Interfaces 2023, 15, 29149–29159. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Li, M.; Zhang, Q.; Shi, G.; Li, Y.; Hou, C.; Wang, H. MXene-Coated Air-Permeable Pressure-Sensing Fabric for Smart Wear. ACS Appl. Mater. Interfaces 2020, 12, 46446–46454. [Google Scholar] [CrossRef]
- Wang, T.; Wang, C.; Xu, X.; Li, Z.; Li, D. One-step electrodeposition synthesized aunps/mxene/ERGO for selectivity nitrite sensing. Nanomaterials 2021, 11, 1892. [Google Scholar] [CrossRef]
- Jiang, X.; Jia, H.; Chen, X.; Li, J.; Chen, Y.; Jia, J.; Zhao, G.; Yu, L.; Zhu, G.; Zhu, Y. Broadening the Voltage Window of 3D-Printed MXene Micro-Supercapacitors with a Hybridized Electrolyte. Molecules 2024, 29, 1393. [Google Scholar] [CrossRef]
- Liao, Y.; Bin, X.; Xu, J.; He, X.; Que, W. Nitrogen-Doped MXene Electrodes for High-Voltage Window Supercapacitors in Organic Electrolytes. Chemistry 2025, 7, 13. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, X.; Xie, Y.; Wang, Z.; Huang, J.; Qu, Y.; Mu, D.; Zhang, X.; Yang, W.; Zhang, H. Additive Engineering Enables Ionic-Liquid Electrolyte-Based Supercapacitors To Deliver Simultaneously High Energy and Power Density. ACS Sustain. Chem. Eng. 2023, 11, 5685–5695. [Google Scholar] [CrossRef]
- Wang, X.; Mathis, T.S.; Li, K.; Lin, Z.; Vlcek, L.; Torita, T.; Osti, N.C.; Hatter, C.; Urbankowski, P.; Sarycheva, A.; et al. Influences from solvents on charge storage in titanium carbide MXenes. Nat. Energy 2019, 4, 241–248. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.; Wang, Z.; Qiu, J. Development of quasi-solid-state anode-free high-energy lithium sulfide-based batteries. Nat. Commun. 2022, 13, 4415. [Google Scholar] [CrossRef] [PubMed]
- Karmur, R.S.; Gogoi, D.; Biswas, A.; Prathibha, C.; Das, M.R.; Ghosh, N.N. Nanocomposite having hierarchical architecture of MXene-WO3 nanorod@rGOsponge and porous carbon for cathode and anode materials for high-performance flexible all-solid-state asymmetric supercapacitor device. Appl. Surf. Sci. 2023, 623, 157042. [Google Scholar] [CrossRef]
- Likitaporn, C.; Okhawilai, M.; Kasemsiri, P.; Qin, J.; Potiyaraj, P.; Uyama, H. High electrolyte uptake of MXene integrated membrane separators for Zn-ion batteries. Sci. Rep. 2022, 12, 19915. [Google Scholar] [CrossRef]
- Ionescu, D.; Kovaci, M. Prediction of the Specific Energy of Supercapacitors with Polymeric Materials Using Advanced Molecular Dynamics Simulations. Polymers 2024, 16, 3404. [Google Scholar] [CrossRef]
- Liu, S.; Yin, T.; Li, Z.; Wang, J. Potentiometric/time resolved chronopotentiometric sensing for an all-solid-state ion-selective electrode based on MXene/MWCNTs as solid contact. Sensors Actuators B Chem. 2024, 405, 135269. [Google Scholar] [CrossRef]
- Jia, Z.; Li, Z.; Ma, S.; Zhang, W.; Chen, Y.; Luo, Y.; Jia, D.; Zhong, B.; Razal, J.M.; Wang, X.; et al. Constructing conductive titanium carbide nanosheet (MXene) network on polyurethane/polyacrylonitrile fibre framework for flexible strain sensor. J. Colloid Interface Sci. 2021, 584, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, S.; Qiu, R.; Yang, X.; Zhu, F. MXene/sPS nanocomposites: Rheological, electrical conductivity, polymorphism, mechanical, thermal, and flammability properties. RSC Adv. 2024, 14, 25793–25801. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kang, D.; Nguyen, V.H.; Nasir, N.; Hong, H.; Kim, M.; Nguyen, D.C.; Lee, Y.J.; Lee, N.; Seo, Y. Application of Titanium-Carbide MXene-Based Transparent Conducting Electrodes in Flexible Smart Windows. ACS Appl. Mater. Interfaces 2021, 13, 40976–40985. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.T.; Chuang, E.; Lin, S.C. Advancing Lithium Battery Performance through Porous Conductive Polyaniline-Modified Graphene Composites Additive. Nanomaterials 2024, 14, 509. [Google Scholar] [CrossRef]
- Khan, S.; Alkhedhe, M.; Raza, R.; Ahmad, M.A.; Majid, A.; Tag El Din, E.M. Electrochemical Investigation of PANI:PPy/AC and PANI:PEDOT/AC Composites as Electrode Materials in Supercapacitors. Polymers 2022, 14, 1976. [Google Scholar] [CrossRef]
- Cirillo, C.; Iuliano, M.; Scarpa, D.; Iovane, P.; Borriello, C.; Portofino, S.; Galvagno, S.; Sarno, M. Silver-Doped Reduced Graphene Oxide/PANI-DBSA-PLA Composite 3D-Printed Supercapacitors. Nanomaterials 2024, 14, 1681. [Google Scholar] [CrossRef]
- He, T.; Li, X.; Sun, B.; Lin, L.; Guo, F.; Diao, G.; Piao, Y.; Zhang, W. Preparation of cyclodextrin polymer-functionalized polyaniline/MXene composites for high-performance supercapacitor. RSC Adv. 2024, 14, 13685–13693. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, J.; Wang, Y.; Zheng, R.; Liu, Q.; Shang, X.; Shao, J.; Wan, Z.; Luo, J.; Jia, C. Approach to Significantly Enhancing the Electrochromic Performance of PANi by In Situ Electrodeposition of the PANi@MXene Composite Film. ACS Appl. Mater. Interfaces 2023, 15, 58940–58954. [Google Scholar] [CrossRef]
- Guan, X.; Feng, W.; Wang, X.; Venkatesh, R.; Ouyang, J. Significant Enhancement in the Seebeck Coefficient and Power Factor of p-Type Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) through the Incorporation of n-Type MXene. ACS Appl. Mater. Interfaces 2020, 12, 13013–13020. [Google Scholar] [CrossRef]
- Habibpour, S.; Zarshenas, K.; Zhang, M.; Hamidinejad, M.; Ma, L.; Park, C.B.; Yu, A. Greatly Enhanced Electromagnetic Interference Shielding Effectiveness and Mechanical Properties of Polyaniline-Grafted Ti3C2Tx MXene-PVDF Composites. ACS Appl. Mater. Interfaces 2022, 14, 21521–21534. [Google Scholar] [CrossRef]
- Alex, M.; Khan, K.R.B.; Al-Othman, A.; Al-Sayah, M.H.; Al Nashash, H. MXene-Based Flexible Electrodes for Electrophysiological Monitoring. Sensors 2024, 24, 3260. [Google Scholar] [CrossRef]
- Kim, J.; Ryu, J.H.; Jang, M.; Park, S.; Kim, M.; Lee, K.H.; Choi, S.; Yoon, Y.; Jung, H.K.; Lee, S.S.; et al. One-Dimensional π-d Conjugated Coordination Polymer Intercalated MXene Compound for High-Performance Supercapacitor Electrode. Small Methods 2023, 7, 2201539. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, Z.; Zhang, W.; Jiang, Y.; Peng, J.; Wu, D.; Chen, B.; Wei, W.; Chen, X.; Liu, Z.; et al. Sulfonic-Group-Grafted Ti3C2Tx MXene: A Silver Bullet to Settle the Instability of Polyaniline toward High-Performance Zn-Ion Batteries. ACS Nano 2021, 15, 9065–9075. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, Q.; He, J.; Li, S.; Ming, R.; Wei, Y.; Xu, Y.; Liu, J.; Zhang, L. A synergistic effect of MXene/MWCNT enables self-healable and low percolation elastomer sensor: A combined experiment and all-atom molecular dynamics simulation. Compos. Sci. Technol. 2023, 242, 110155. [Google Scholar] [CrossRef]
- Guo, T.; Fu, M.; Zhou, D.; Pang, L.; Su, J.; Lin, H.; Yao, X.; Sombra, A.S.B. Flexible Ti3C2Tx/Graphene Films with Large-Sized Flakes for Supercapacitors. Small Struct. 2021, 2, 2100015. [Google Scholar] [CrossRef]
- Gao, M.; Xie, Y.; Yang, W.; Lu, L. Fabrication of novel electrochemical sensor based on MXene/MWCNTs composite for sensitive detection of synephrine. Int. J. Electrochem. Sci. 2020, 15, 4619–4630. [Google Scholar] [CrossRef]
- Shi, X.; Guo, F.; Hou, K.; Guan, G.; Lu, L.; Zhang, Y.; Xu, J.; Shang, Y. Highly Flexible All-Solid-State Supercapacitors Based on MXene/CNT Composites. Energy Fuels 2023, 37, 9704–9712. [Google Scholar] [CrossRef]
- Chen, J.; Feng, M.; Lian, X.; Zheng, F.; Xu, C.; Wang, K.; Niu, H. Rational design of MXene/MWCNT/TOCNF film for flexible supercapacitor. Ceram. Int. 2024, 50, 29895–29903. [Google Scholar] [CrossRef]
- Xu, S.; Wei, G.; Li, J.; Han, W.; Gogotsi, Y. Flexible MXene-graphene electrodes with high volumetric capacitance for integrated co-cathode energy conversion/storage devices. J. Mater. Chem. A 2017, 5, 17442–17451. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Dhanjai; Sinha, A.; Li, Y.; Shen, Y.; Huang, Y. An electrochemical sensor for ifosfamide, acetaminophen, domperidone, and sumatriptan based on self-assembled MXene/MWCNT/chitosan nanocomposite thin film. Microchim. Acta 2020, 187, 402. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.; Xie, J.; Li, J.; Huang, H.; Liang, X.; Huang, D.; Lan, Z.; Liu, H.; Li, G.; et al. Potassium Ion-Assisted Self-Assembled MXene-K-CNT Composite as High-Quality Sulfur-Loaded Hosts for Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2024, 16, 39771–39783. [Google Scholar] [CrossRef]
- Chen, K.; Yan, X.; Li, J.; Jiao, T.; Cai, C.; Zou, G.; Wang, R.; Wang, M.; Zhang, L.; Peng, Q. Preparation of self-assembled composite films constructed by chemically-modified mxene and dyes with surface-enhanced raman scattering characterization. Nanomaterials 2019, 9, 284. [Google Scholar] [CrossRef]
- Pan, Z.; Ji, X. Facile synthesis of nitrogen and oxygen co-doped C@Ti3C2 MXene for high performance symmetric supercapacitors. J. Power Sources 2019, 439, 227068. [Google Scholar] [CrossRef]
- Afolabi, M.A.; Xiao, D.; Chen, Y. The Impact of Surface Chemistry and Synthesis Conditions on the Adsorption of Antibiotics onto MXene Membranes. Molecules 2024, 29, 148. [Google Scholar] [CrossRef]
- Khaledialidusti, R.; Mahdavi, E.; Barnoush, A. Stabilization of 2D graphene, functionalized graphene, and Ti2CO2 (MXene) in super-critical CO2: A molecular dynamics study. Phys. Chem. Chem. Phys. 2019, 21, 12968–12976. [Google Scholar] [CrossRef]
- Ali, A.; Hantanasirisakul, K.; Abdala, A.; Urbankowski, P.; Zhao, M.Q.; Anasori, B.; Gogotsi, Y.; Aïssa, B.; Mahmoud, K.A. Effect of Synthesis on Performance of MXene/Iron Oxide Anode Material for Lithium-Ion Batteries. Langmuir 2018, 34, 11325–11334. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, T.; Rozmysłowska-Wojciechowska, A.; Matyszczak, G.; Wrzecionek, M.; Olszyna, A.; Peter, A.; Mihaly-Cozmuta, A.; Nicula, C.; Mihaly-Cozmuta, L.; Podsiadło, S.; et al. Ti2C MXene Modified with Ceramic Oxide and Noble Metal Nanoparticles: Synthesis, Morphostructural Properties, and High Photocatalytic Activity. Inorg. Chem. 2019, 58, 7602–7614. [Google Scholar] [CrossRef] [PubMed]
- Rozmysłowska-Wojciechowska, A.; Karwowska, E.; Poźniak, S.; Wojciechowski, T.; Chlubny, L.; Olszyna, A.; Ziemkowska, W.; Jastrzębska, A.M. Influence of modification of Ti3C2 MXene with ceramic oxide and noble metal nanoparticles on its antimicrobial properties and ecotoxicity towards selected algae and higher plants. RSC Adv. 2019, 9, 4092–4105. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Han, L.; Li, H.; Wang, J.; Lu, T.; Pan, L. In-situ fabrication of few-layered MoS2 wrapped on TiO2-decorated MXene as anode material for durable lithium-ion storage. J. Colloid Interface Sci. 2021, 604, 30–38. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, L.; Zhang, B.; Wu, H. 2D-Ti3C2 as hard, conductive substrates to enhance the electrochemical performance of MnO2 for supercapacitor applications. Ceram. Int. 2018, 44, 17539–17543. [Google Scholar] [CrossRef]
- Tang, H.; Jiang, M.; Ren, E.; Zhang, Y.; Lai, X.; Cui, C.; Jiang, S.; Zhou, M.; Qin, Q.; Guo, R. Integrate electrical conductivity and Li+ ion mobility into hierarchical heterostructure Ti3C2@CoO/ZnO composites toward high-performance lithium ion storage. Energy 2020, 212, 118696. [Google Scholar] [CrossRef]
- Seredych, M.; Shuck, C.E.; Pinto, D.; Alhabeb, M.; Precetti, E.; Deysher, G.; Anasori, B.; Kurra, N.; Gogotsi, Y. High-Temperature Behavior and Surface Chemistry of Carbide MXenes Studied by Thermal Analysis. Chem. Mater. 2019, 31, 3324–3332. [Google Scholar] [CrossRef]
- Guo, J.; Legum, B.; Anasori, B.; Wang, K.; Lelyukh, P.; Gogotsi, Y.; Randall, C.A. Cold Sintered Ceramic Nanocomposites of 2D MXene and Zinc Oxide. Adv. Mater. 2018, 30, 1801846. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Jiang, X.; Li, Y.; Lu, X.; Luan, S.; Wang, Y.; Li, Y.; Gao, F. Metal−organic frameworks-derived MnO2/Mn3O4 microcuboids with hierarchically ordered nanosheets and Ti3C2 MXene/Au NPs composites for electrochemical pesticide detection. J. Hazard. Mater. 2019, 373, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Myndrul, V.; Coy, E.; Babayevska, N.; Zahorodna, V.; Balitskyi, V.; Baginskiy, I.; Gogotsi, O.; Bechelany, M.; Giardi, M.T.; Iatsunskyi, I. MXene nanoflakes decorating ZnO tetrapods for enhanced performance of skin-attachable stretchable enzymatic electrochemical glucose sensor. Biosens. Bioelectron. 2022, 207, 114141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Javed, M.S.; Ren, H.; Batool, S.; Ahmad, A.; Tao, R.; Albaqami, M.D.; Khan, S.; Wang, X.; Han, W. Optimization of Ti3C2Tx performance through synergistic enhancement of GeOx/Ti3C2Tx heterostructures for ammonium-ion storage. Chem. Eng. J. 2025, 504, 158582. [Google Scholar] [CrossRef]
- Rajeeve, A.D.; Yamuna, R.; Vinoba, M.; Bhagiyalakshmi, M. β-Cyclodextrin-Stabilized CuO/MXene Nanocomposite as an Electrode Material for High-Performance Supercapacitors. Langmuir 2023, 39, 17688–17699. [Google Scholar] [CrossRef]
- Shao, H.; Xu, K.; Wu, Y.C.; Iadecola, A.; Liu, L.; Ma, H.; Qu, L.; Raymundo-Piñero, E.; Zhu, J.; Lin, Z.; et al. Unraveling the charge storage mechanism of Ti3C2T xmxene electrode in acidic electrolyte. ACS Energy Lett. 2020, 5, 2873–2880. [Google Scholar] [CrossRef]
- Sharma, A.; Mane, P.; Chakraborty, B.; Rout, C.S. 1T-VS2/MXene Hybrid as a Superior Electrode Material for Asymmetric Supercapacitors: Experimental and Theoretical Investigations. ACS Appl. Energy Mater. 2021, 4, 14198–14209. [Google Scholar] [CrossRef]
- Siddu, P.; Sree Raj, K.A.; Radhakrishnan, S.; Mun Jeong, S.; Sekhar Rout, C. 3D Ternary Hybrid of VSe2/e-MXene/CNT with a Promising Energy Storage Performance for High Performance Asymmetric Supercapacitor. Batter. Supercaps 2024, 8, e202400466. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Yang, G.; Zhang, L.; Zhang, K.; Lucia, L.A.; Meng, L.; Wang, X.; Chen, J. Multifunctional hydrogel enabled by tannin derived nanoparticles system for the application of strain sensing and human machine interfaces. Colloids Surfaces A Physicochem. Eng. Asp. 2025, 711, 136367. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, Y.; Feng, L.; Meng, J.; Yang, Z.; Wu, H.; Li, P.; Zhu, Z.; Zhao, B.; Wei, Q. A flexible multifunctional triboelectric nanogenerator based on bio-inspired nanocellulose/tannic acid@MXene-composited hydrogel for human healthcare. Int. J. Biol. Macromol. 2025, 306, 141261. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, L.; Wu, T.; Song, H.; Wan, Y.; Zhang, M. High-Performance Conductive Hydrogel Prepared by an Electrohydrodynamic Printing Method for Strain Sensors and Self-Powered Triboelectric Nanogenerator. ACS Appl. Nano Mater. 2025, 8, 589–601. [Google Scholar] [CrossRef]
- Salami, N. First-principles realistic prediction of gas adsorption on two-dimensional Vanadium Carbide (MXene). Appl. Surf. Sci. 2022, 581, 152105. [Google Scholar] [CrossRef]
- Mondal, A.; Faraz, M.; Dahiya, M.; Khare, N. Double-Layer Electronegative Structure-Based Triboelectric Nanogenerator for Enhanced Performance Using Combined Effect of Enhanced Charge Generation and Improved Charge Trapping. ACS Appl. Mater. Interfaces 2024, 16, 50659–50670. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Xu, H.; Fan, J.; Pang, D.; Zheng, Y.; Chen, G.; Du, F.; Gogotsi, Y.; Dall’Agnese, Y.; Gao, Y. Synergy of ferric vanadate and MXene for high performance Li- and Na-ion batteries. Chem. Eng. J. 2022, 436, 135012. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Hou, Z.; Mei, H.; Feng, Y.; Xu, B.; Sun, D. Self-assembly of MOF on MXene nanosheets and in-situ conversion into superior nickel phosphates/MXene battery-type electrode. Chem. Eng. J. 2021, 425, 130602. [Google Scholar] [CrossRef]
- Akıllı, A.; Taymaz, B.H.; Eskizeybek, V.; Kamış, H. Enhanced performance of MXene-based supercapacitor via new activated carbon-nafion composite cathode. J. Phys. Chem. Solids 2025, 199, 112523. [Google Scholar] [CrossRef]
- Shivade, D.S.; Kurade, A.N.; Bhosale, R.K.; Kundale, S.S.; Shelake, A.R.; Patil, A.D.; Waifalkar, P.P.; Kamat, R.K.; Teli, A.M.; Dongale, T.D. Folic acid-assisted in situ solvothermal synthesis of Ni-MOF/MXene composite for high-performance supercapacitors. J. Energy Storage 2024, 100, 113754. [Google Scholar] [CrossRef]
- Baasanjav, E.; Raj, K.A.S.; Hakkeem, H.; Rout, C.S.; Jeong, S.M. High-performance asymmetric supercapacitors based on 2D MXene/NiCoP hybrid and ZIF derived porous nanocarbon. J. Mater. Sci. Technol. 2025, 228, 42–53. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, N.; Zhou, B.; Zhou, X.; Pu, B.; Bai, J.; Tang, Q.; Liu, Y.; Yang, W. NH3-Induced In Situ Etching Strategy Derived 3D-Interconnected Porous MXene/Carbon Dots Films for High Performance Flexible Supercapacitors. Nano-Micro Lett. 2023, 15, 231. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Wu, B.; Wei, H.; Guo, H.; El-Bahy, Z.M.; Liu, B.; He, M.; Melhi, S.; Shi, X.; et al. Interfacial-engineered robust and high performance flexible polylactic acid/polyaniline/MXene electrodes for high-perfarmance supercapacitors. J. Mater. Sci. Technol. 2024, 203, 201–210. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Gao, Y.; Li, L.; Bao, L.; Li, X. Physicochemical double protection enables stable MXene for high-rate performance hybrid supercapacitors. J. Mater. Sci. Technol. 2025, 211, 89–97. [Google Scholar] [CrossRef]
- Li, J.; Qiang, X.; Jia, B.; Wang, L.; Wu, X. Etching and surface self-assembly of Ni-MOF/MXene hybrids for excellent flexible Pseudocapacitance. Appl. Surf. Sci. 2025, 695, 162867. [Google Scholar] [CrossRef]
- Xu, C.; Tong, L.; Zhang, W.; Zhao, X.; Yang, L.; Yin, S. One-step synthesis of g-C3N4/TiVCTx MXene electrodes for lithium-ion batteries and supercapacitors. Chem. Eng. J. 2024, 497, 154449. [Google Scholar] [CrossRef]
- Yu, L.; He, X.; Tang, L.; Wang, X.; Cai, W.; He, Y.; Sun, Y.; Zhu, G.; Rosen, J.; Jin, H. Understanding mechanisms of fast sodium storage kinetics for MXene/MoS2@C in ether electrolytes. J. Power Sources 2025, 641, 236852. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, Y.; Yue, M.; Cao, Y.; Wang, G.; Wang, Y. Encapsulating FeSe2 nanorods in Na+ pre-intercalated 3D porous Ti3C2Tx MXene nanostructures for advanced sodium storage performance. J. Power Sources 2025, 640, 236679. [Google Scholar] [CrossRef]
- Pathak, D.D.; Neem, M.; Kumar, G.; Modak, B.; Neogi, S.; Mandal, B.P. Diamondoid covalent organic framework-MXene composite for cathode host in lithium sulfur battery. J. Energy Storage 2025, 117, 116176. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.; Xiao, J. Flower-like Ti3C2Tx-TiO2 modified with ZnS nanoparticles as adsorption-catalytic cathodic material for lithium–sulfur batteries. J. Energy Storage 2025, 118, 116241. [Google Scholar] [CrossRef]
- Wei, J.; Cao, H.; Tian, Y.; Zhang, X. MXene-encapsulated titanium-niobium oxide microspheres for fast and stable lithium storage. Electrochim. Acta 2023, 464, 142957. [Google Scholar] [CrossRef]
- Luo, A.; CUI, Y.; Wang, J.; Ju, Z.; Zhuang, Q.; Shi, Y. SnS2 anchored on MXene etched by hydrofluoric acid for sodium-ion battery anode material. Solid State Ionics 2023, 399, 116271. [Google Scholar] [CrossRef]
- Niu, Y.; Feng, W.; Lei, Z.; Hu, W.; Zheng, X.; Su, W.; Zhang, L. MXene surface-attached Ni2P on lithium-sulfur battery catalytic effect. J. Electroanal. Chem. 2023, 946, 117743. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Nan, Y.; Zhou, H.; Xu, H. Enhanced of ZIF-8 and MXene decorated triboelectric nanogenerator for droplet energy harvesting. Chem. Eng. J. 2025, 506, 160137. [Google Scholar] [CrossRef]
- Wei, R.; Sun, S. A MXene-based double-network conductive hydrogel triboelectric nanogenerator for intelligent sports monitoring. Energy Reports 2025, 13, 3773–3781. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, L.; Wang, D.; Yang, Y.; Mi, Q.; Zhang, J. Multifunctional latex/ polytetrafluoroethylene-based triboelectric nanogenerator for self-powered organ-like mxene/metal-organic framework-derived cuo nanohybrid ammonia sensor. ACS Nano 2021, 15, 2911–2919. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Luo, J.; Xu, W.; Zhang, C.; Yuan, H.; Zhang, Y.; Pang, Y. High-performance triboelectric nanogenerator based on NH2-MXene/TiO2@sodium alginate composite film for self-powered cathodic protection. Chem. Eng. J. 2025, 506, 159837. [Google Scholar] [CrossRef]
- Anwer, S.; Umair Khan, M.; Alazzam, A.; Mohammad, B.; Abu Nada, E. 2D Ti3C2Tx-MXene and its superstructures as dual-function electrodes for triboelectric nanogenerators for self-powered electronics. Chem. Eng. J. 2024, 502, 157985. [Google Scholar] [CrossRef]
- Jalali, A.; Gupta, T.; Pakharenko, V.; Rejeb, Z.B.; Kheradmandkeysomi, M.; Sain, M.; Park, C.B. Lightweight 3D-printed chitosan/MXene aerogels for advanced electromagnetic shielding, energy harvesting, and thermal management. Carbohydr. Polym. 2025, 352, 123252. [Google Scholar] [CrossRef]
- Sarikurt, S.; Çakir, D.; Keçeli, M.; Sevik, C. The influence of surface functionalization on thermal transport and thermoelectric properties of MXene monolayers. Nanoscale 2018, 10, 8859–8868. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zschiesche, H.; Antonietti, M.; Daffos, B.; Tarakina, N.V.; Gibilaro, M.; Chamelot, P.; Massot, L.; Duployer, B.; Taberna, P.L.; et al. Tuning the Surface Chemistry of MXene to Improve Energy Storage: Example of Nitrification by Salt Melt. Adv. Energy Mater. 2023, 13, 2202709. [Google Scholar] [CrossRef]
- Yu, K.; Ren, J.; Liao, W.; Hu, B.; Bai, C.; Li, Z.; Zhang, X.; Chhattal, M.; Li, N.; Qiang, L. Maintaining the 2D Structure of MXene via Self-Assembled Monolayers for Efficient Lubrication in High Humidity. Small 2024, 20, 2402143. [Google Scholar] [CrossRef]
- Wang, L.; Gao, S.; Li, W.; Zhu, A.; Li, H.; Zhao, C.; Zhang, H.; Wang, W.-H.; Wang, W. Machine learning assisted screening of MXenes pseudocapacitive materials. J. Power Sources 2023, 564, 232834. [Google Scholar] [CrossRef]
- Li, X.; Qiu, J.; Cui, H.; Chen, X.; Yu, J.; Zheng, K. Machine Learning Accelerated Discovery of Functional MXenes with Giant Piezoelectric Coefficients. ACS Appl. Mater. Interfaces 2024, 16, 12731–12743. [Google Scholar] [CrossRef]
- Shrestha, S.; Barvenik, K.J.; Chen, T.; Yang, H.; Li, Y.; Kesavan, M.M.; Little, J.M.; Whitley, H.C.; Teng, Z.; Luo, Y.; et al. Machine intelligence accelerated design of conductive MXene aerogels with programmable properties. Nat. Commun. 2024, 15, 4685. [Google Scholar] [CrossRef]

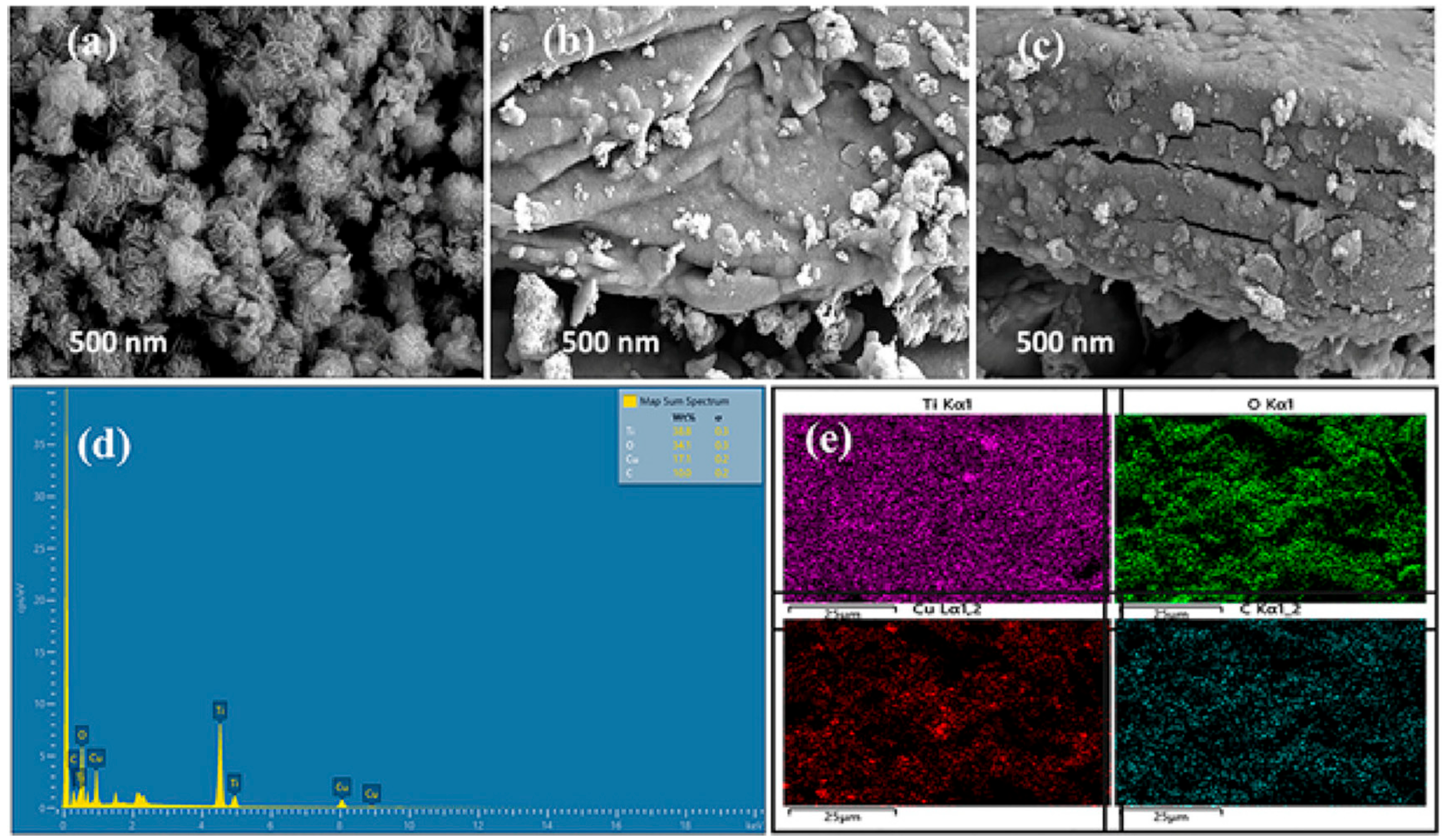
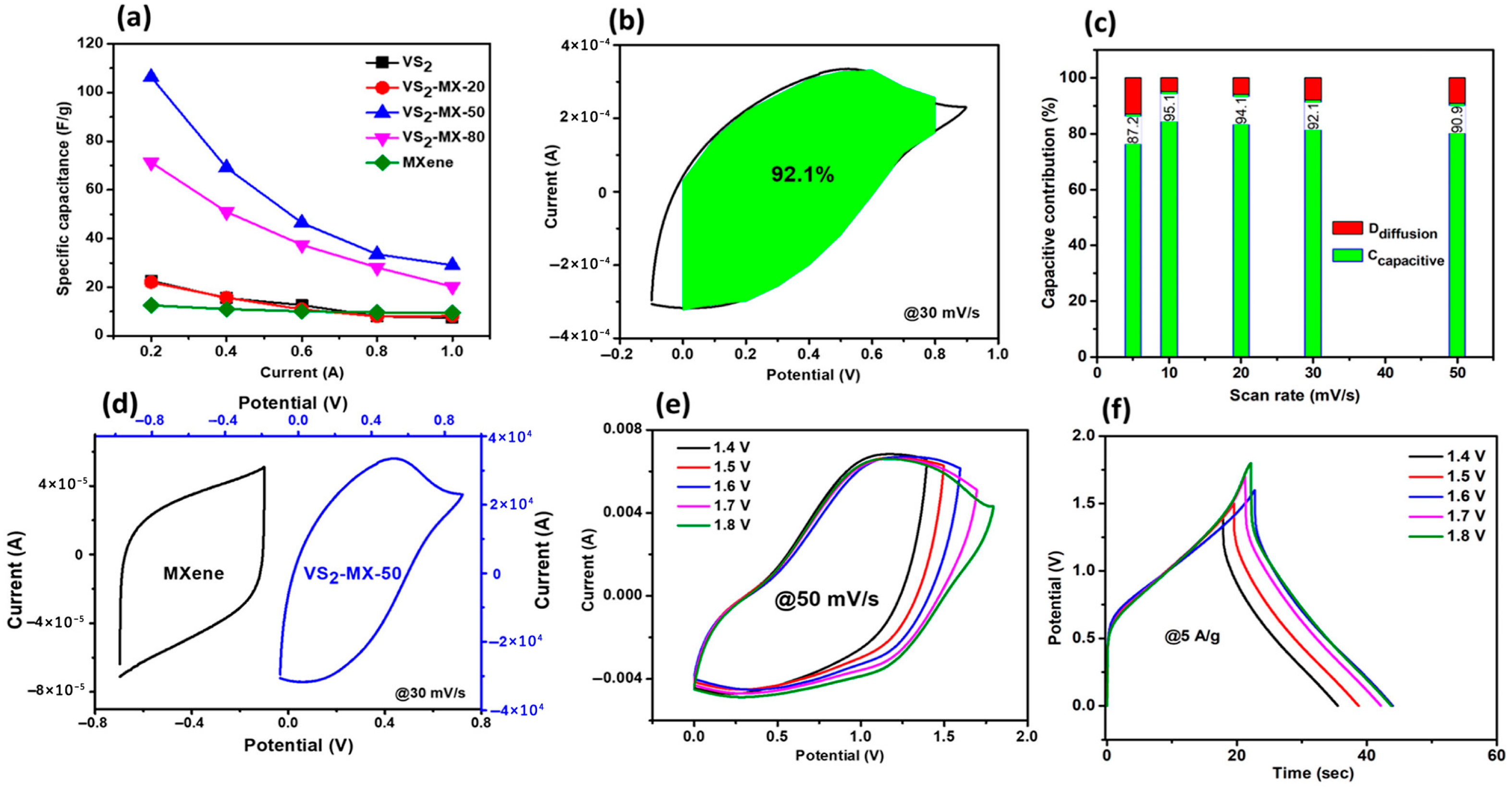
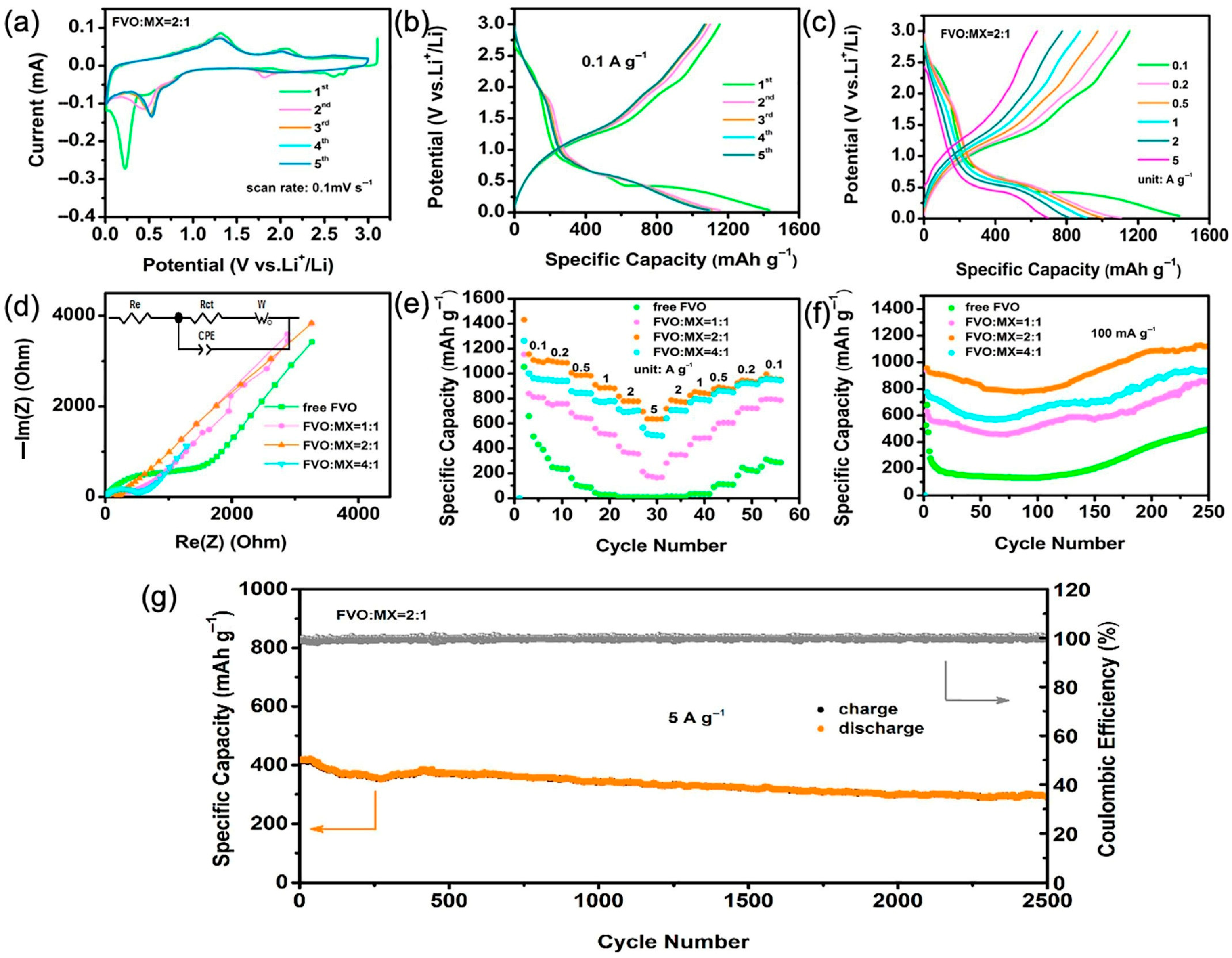
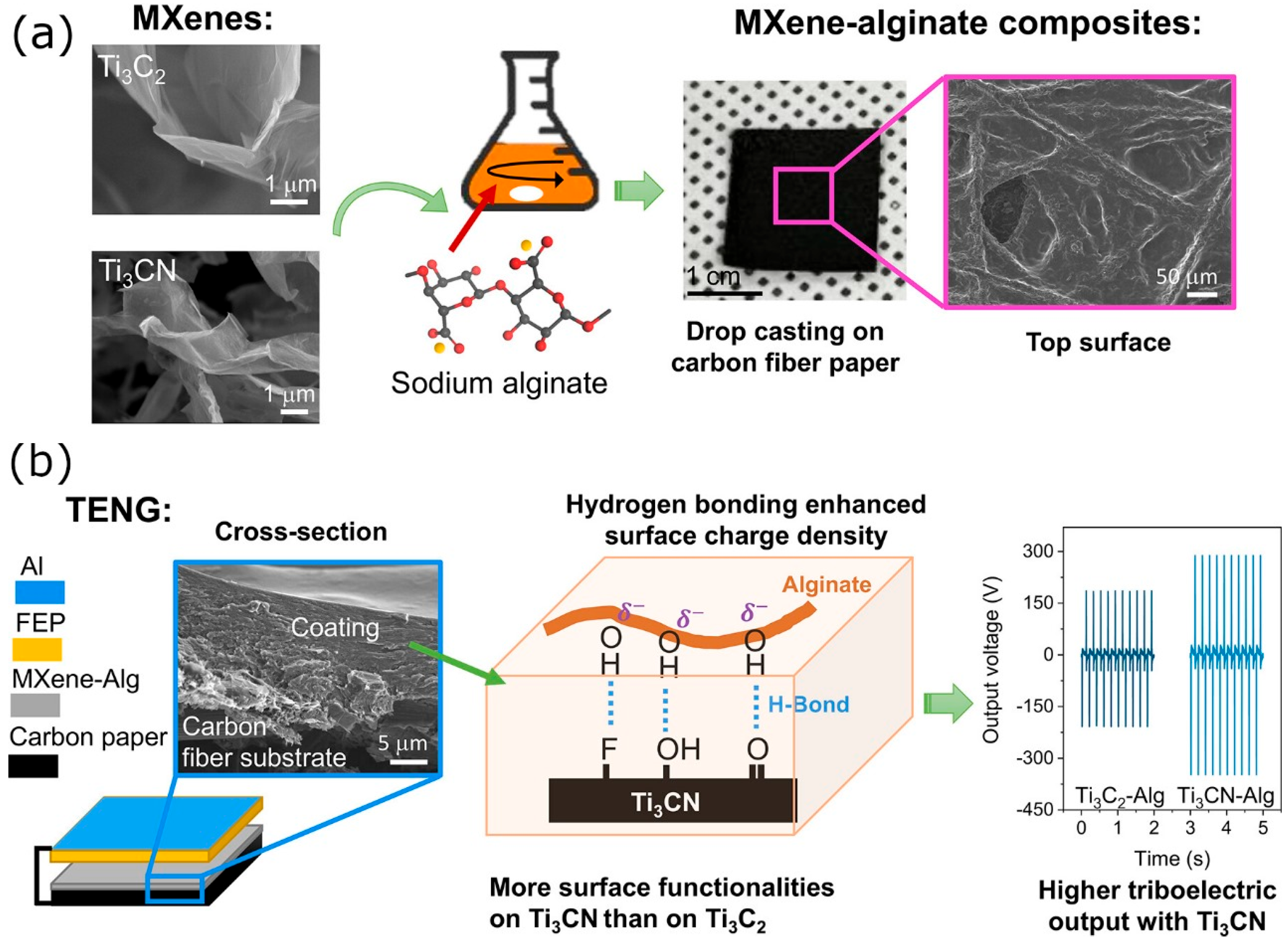

| Structure | Device | Electrolyte | Specific Capacitance | Energy Density Wh·kg−1 | Power Density W·kg−1 | Voltage Window | Capacitance Retention | Ref. |
|---|---|---|---|---|---|---|---|---|
| GeOx@Ti3C2Tx//AC | Hybrid Supercapacitor | 1 M (NH4)2SO4 (aqueous) | 141 F·g−1 at 1 A·g−1 | 51.4 | 800.6 | 1.6 V | 92.6% at 20 A·g−1 after 10,000 cycles | [147] |
| MXene-NPO//PPD-rGO | Hybrid Supercapacitor | KOH/PVA gel | 187 F·g−1 at 1 A·g−1 | 72.6 | 932 | 1.6 V | 94% after 10,000 cycles | [159] |
| MXene//AC-Nafion | Asymmetric Supercapacitor | 3 M H2SO4 | 555 F·g−1 at 1 A·g−1 | 81.2 | 1023 | 2 V | 93% after 5000 cycles at 1 A·g−1 | [160] |
| N-doped Ti3C2Tx (UN-Ti3C2Tx) | Symmetric Supercapacitor | 1 M EMIMTFSI/LiTFSI in ACN | 147 F·g−1 at 0.5 A·g−1 | 29.4 | 600 | 2.4 V | - | [104] |
| VSe2/e-MXene/CNT | Asymmetric Supercapacitor | 0.5 M K2SO4 (aqueous) | 101 F·g−1 at 1.6 A·g−1 | 35.91 | 1280 | 1.6 V | ~99% after 5000 cycles | [151] |
| Ni-MOF/MXene Composite | Hybrid Supercapacitor | 1 M KOH (aqueous) | 716.19 F·g−1 at 1 A·g−1 | 23.28 | 2,841 | 1.5 V | 74.22% after 2000 cycles | [161] |
| MXene-WO3 nanorods–rGOsp//Porous carbon | Asymmetric Supercapacitor | PVA gel + 2 M KOH + 0.1 M K4[Fe(CN)6] | 123.4 F·g−1 at 2 A·g−1 | 34 | 1450 | 1.45 V | 86% after 3000 cycles at 5 A·g−1 | [108] |
| 2D MXene/NiCoP + ZIF-derived porous carbon | Asymmetric Supercapacitor | 2 M KOH (aqueous) | 1754.0 F·g−1 at 3 mA·cm−2 | 54.3 | 565.6 | 1.6 V | 93.8% after 10,000 cycles 20 mA·cm−2 | [162] |
| 3D-interconnected porous MXene/carbon dot film (p-MC) | Flexible All-Solid-State Supercapacitor | PVA/H2SO4 gel | 688.9 F·g−1 at 2 A·g−1 | 20 | 600 | 1.2 V | 90% after 10,000 cycles 8 A·g−1 | [163] |
| PLA/PANI/MXene (PPM) Composite | Symmetric Supercapacitor | 1 M PVA/H2SO4 gel | 193.7 F·g−1 at 0.25 A·g−1 | 9.3 | 291.3 | 0.6 V | 80.3% 5000 cycles at 2 A·g−1 | [164] |
| CDP-MX/PA (PANI/MXene + cyclodextrin polymer) | High-Performance Supercapacitor | 1 M H2SO4 | 523.8 F·g−1 at 1 A·g−1 | 27.7 | 700 | 1.4 V | 86.5% 5000 cycles at 5 A·g−1 | [118] |
| 2D MXene-PVP-Co9S8//MoS2 | Hybrid Supercapacitor | 6 M KOH | 277 F·g−1 at 1 A·g−1 | 111 | 845 | 1.7 V | 84% 10,000 cycles at 10 A·g−1 | [165] |
| MXene-NPO//AC | Asymmetric Supercapacitor | PVA/KOH gel | 332 F·g−1 at 1 A·g−1 | 118 | 799.7 | 1.6 V | 99.7% to 95.1% at 5 A·g−1 for 1000 cycles | [166] |
| Structure | Device | Electrolyte | Current Rate | Initial Capacity | Cycles | Final Capacity | Coulombic Efficiency | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ti3C2Tx//FeVO4 (2:1) | Li-ion Battery | LiPF6 in EC/DMC | 5 A·g−1 | 418 mAh·g−1 | 2500 | ~70% | 99.4% | [158] |
| Ti3C2Tx//FeVO4 | Na-ion Battery | NaClO4 in EC/DMC | 5 A·g−1 | 129 mAh·g−1 | 5000 | ~81% | 99.52% | [158] |
| g-C3N4/TiVCTx-30//LiFePO4 | Li-ion Battery | LiPF6 in EC/DEC | 1 A·g−1 | 558.3 mAh·g−1 | 1000 | 76.17% | 97.9% after 50 cycles | [167] |
| S-Ti3C2Tx/PANI//Zn | Zn-ion Battery | 2 M ZnSO4 | 15 A·g−1 | 160 mAh·g−1 | 5000 | ~64% | ~100% | [124] |
| MXene/MoS2@C//NVP | Na-ion Battery | NaPF6 in DEGDME | 1 A·g−1 | 230 mAh·g−1 | 100 | 82.2% | 97.8% at 0.1 A·g−1 | [168] |
| FeSe2 nanorods-MXene | Na-ion Battery | NaCF3SO3 in diglyme | 10 A·g−1 | 368 mAh·g−1 | 3000 | ~87.5% | ~100% | [169] |
| 3D COF/MXene/S | Li–S Battery | LiTFSI in DOL/DME + 1% LiNO3 | 0.5 A·g−1 | - | 300 | 73% 379 mAh·g−1 | 95% | [170] |
| Ti3C2Tx-TiO2/ZnS | Li–S Battery | Li2S6 in DOL/DME | 0.5 C | 1002.9 mAh·g−1 | 500 | ~56% | - | [171] |
| TNO@MXene | Li-ion Battery | LiPF6 in EC/DEC | 20 C | 200.3 mAh·g−1 | 1400 | ~66% | 94.4%. | [172] |
| MXene@SnS2 | Na-ion Battery | NaPF6 in DME | 1 | 288.1 mAh·g−1 | 100 | 86.1% | 82.9% | [173] |
| MXene@Ni2P-1/S | Li–S Battery | Li2S6 em DOL/DME | 1 C | ~690 mAh·g−1 | 300 | 72.8% | 99.8% | [174] |
| Structure/Composition | Open-Circuit Voltage (V) | Short-Circuit Current (μA) | Power Output | Ref. |
|---|---|---|---|---|
| SA/MXene/PAAm Hydrogel TENG | 491.98 | 75.41 | 2.54 mW with 3 MΩ. | [176] |
| TA@CNC/MXene Hydrogel (N-TENG) | 68.04 | 1.02 | 69.97 mW·m−2 | [152] |
| Ti3CN–Alginate TENG | 670 | 15 | 0.28 W·m−2 | [27] |
| MXene/CuO composite | 810 | 34 | 10.84 W·m−2 | [177] |
| PAM/CNF/MXene Hydrogel | 67.5 (under 100% strain) | 0.13 | [154] | |
| Chitosan aerogel + 2 wt% MXene | 110 | 1.9 | [180] | |
| BW-TENG (Al/B-MX/Kapton/PVA) | 120 | 25 | 5.66 W·m−2 | [179] |
| NMTS-TENG (NH2-MXene/TiO2) | 357.6 | 55.1 | 3.9 mW | [178] |
| ZIF-8/MXene@PDMS | 162 | 6.1 | [175] |
| Sensing Material | Sensor Type | Target Analyte | Mechanism | Performance | Application | Ref. |
|---|---|---|---|---|---|---|
| MXene/MWCNT/ITT Elastomer | Strain Sensor (Self-healing) | Mechanical deformation | Piezoresistive (dynamic bonding) | Self-healing efficiency up to 100%@80 °C; recovery of 74.3% mechanical and 94.8% electrical properties; GF = 1.0; low threshold (3.5 wt%) for conduction | Wearable strain sensors, e-skin, robotics | [125] |
| Ti3C2Tx/PDMS/Glycerol Composite | Biomedical (ECG, EMG) | Biopotentials (skin) | Electrochemical | Bulk impedance: 280–111 Ω; conductivity: 0.462–1.533 mS/cm; charge storage: 0.665–1.99 mC/cm2; elongation: 139–144%; | Wearable skin biosignal sensing (ECG, EMG), long-term electrode applications | [122] |
| AChE-Chit/MXene/Au NPs/MnO2/Mn3O4/GCE | Electrochemical Sensor | Pesticides (methamidophos) | Enzymatic inhibition electrochemical | LOD: 1.34 × 10−13 M; range: 10−12–10−6 M; recovery 95.2–101.3% | Electrochemical pesticide detection | [145] |
| Ti3C2Tx/CuO (MOF-derived) | Gas Sensor (Ammonia) | Ammonia (NH3) | Triboelectric | Response ~24.8@100 ppm; TENG: 810 V, 34 μA, 10.84 W/m2; | Wearable flexible format Self-powered ammonia detection | [177] |
| Ti3C2Tx + p(PFDMA) | Stability-Enhanced Gas | VOC gases | Surface modification (polymer coating) | SNR retained under 50 °C/100% RH for 3 weeks; SNR 8.3× higher than uncoated MXene; | Enhanced environmental stability and sensor durability | [95] |
| MXene/SA Double-Network Hydrogel | TENG (Triboelectric Generator) | Mechanical motion (foot/joint) | Triboelectric | VOC: 491.98 V; ISC: 75.41 μA; QSC: 83.93 nC; power: 2.54 mW@3 MΩ; +84% output vs. conventional | Sports monitoring, motion sensing, self-powered wearable electronics | [176] |
| PAM/CNF/MXene Hydrogel | Strain Sensor + TENG | Strain (1–550%) | Triboelectric + piezoresistive | Strain: 1–550%; GF: 6.73; response time: 100 ms; recovery: 110 ms; Voc: 67.5 V @100% strain; 550% elongation, 0.31 MPa | Motion sensing, writing recognition, energy harvesting, info transmission | [154] |
| 3D-Printed Chitosan/MXene Aerogel | TENG + EMI Shielding | Mechanical motion + EM signals | Triboelectric + electromagnetic attenuation | TENG output: 22 V (0 wt%), 110 V (2 wt%), current: 1.9 µA; EMI shielding: up to 28 dB (81% absorption); | Energy harvesting, EMI shielding, thermal insulation | [180] |
| PEDOT:PSS/MXene/Ag Grid | FTS (Flexible Transparent Supercapacitor) | Human activity | Capacitive + sensing (Electrochemical) | Transparency: 60–71%; areal capacitance: 3.7–12 mF/cm2; optical transmittance ≈ 89% | Energy storage + sensing + transparency for smart wearable devices | [19] |
| Tannin/CNC/MXene (TMCN) Hydrogel | Strain Sensor + TENG | Motion, biosignals, handwriting | Ionic conduction + triboelectric | Strain: up to 3718%; toughness: 12.14 MJ/m3; gauge factor: 14.5; Voc: 68.04 V; Isc: 1.02 μA; Qsc: 22.66 nC; Power: 69.97 mW/m2; | Motion sensing, self-powered messaging, healthcare (Morse code) | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotius, J.A.A.; Oliveira, H.P.d. MXene-Based Composites for Energy Harvesting and Energy Storage Devices. Solids 2025, 6, 41. https://doi.org/10.3390/solids6030041
Fotius JAA, Oliveira HPd. MXene-Based Composites for Energy Harvesting and Energy Storage Devices. Solids. 2025; 6(3):41. https://doi.org/10.3390/solids6030041
Chicago/Turabian StyleFotius, Jorge Alexandre Alencar, and Helinando Pequeno de Oliveira. 2025. "MXene-Based Composites for Energy Harvesting and Energy Storage Devices" Solids 6, no. 3: 41. https://doi.org/10.3390/solids6030041
APA StyleFotius, J. A. A., & Oliveira, H. P. d. (2025). MXene-Based Composites for Energy Harvesting and Energy Storage Devices. Solids, 6(3), 41. https://doi.org/10.3390/solids6030041







