Catalytic Direct Decomposition of NOx Using Non-Noble Metal Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
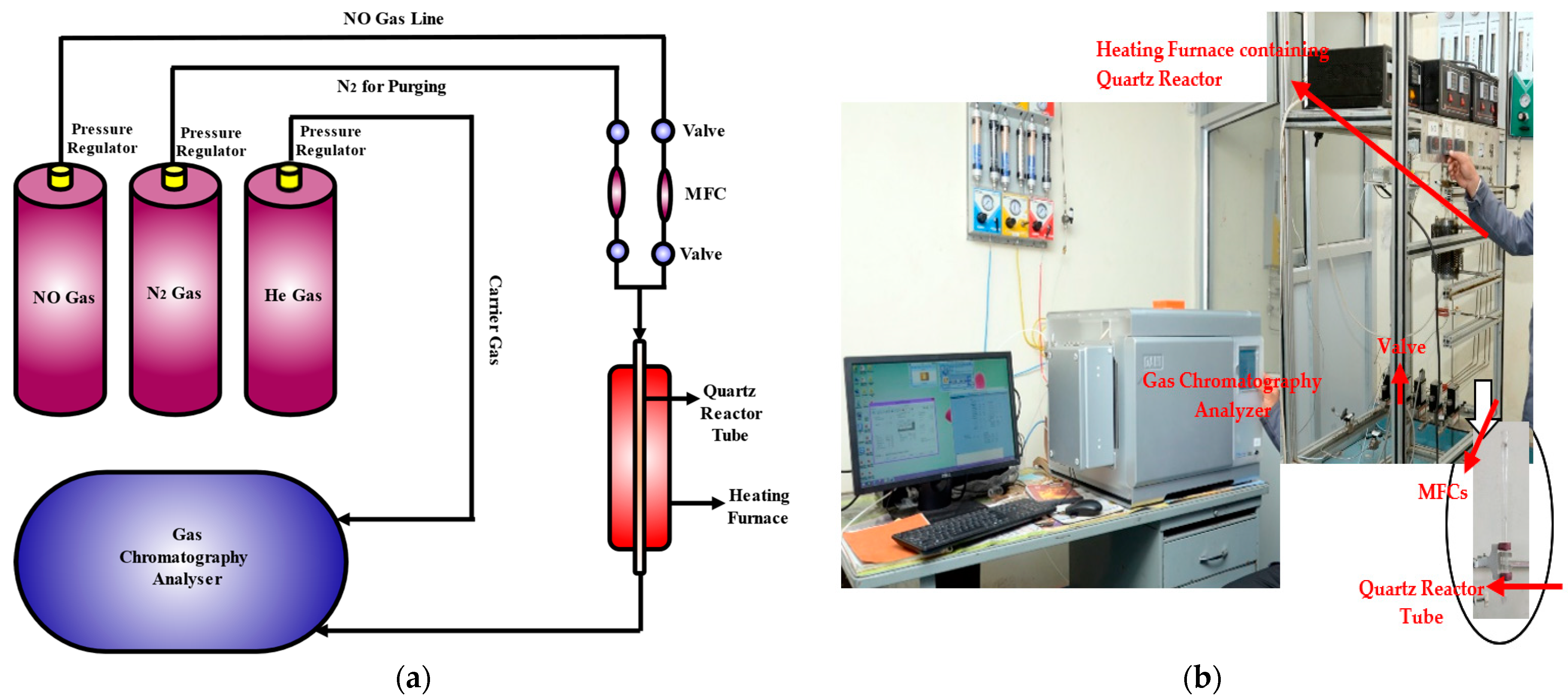
2.3. Experimental Setup
2.4. Characterization Methods
3. Result and Discussion
3.1. Material Characteristics
3.1.1. Surface Area
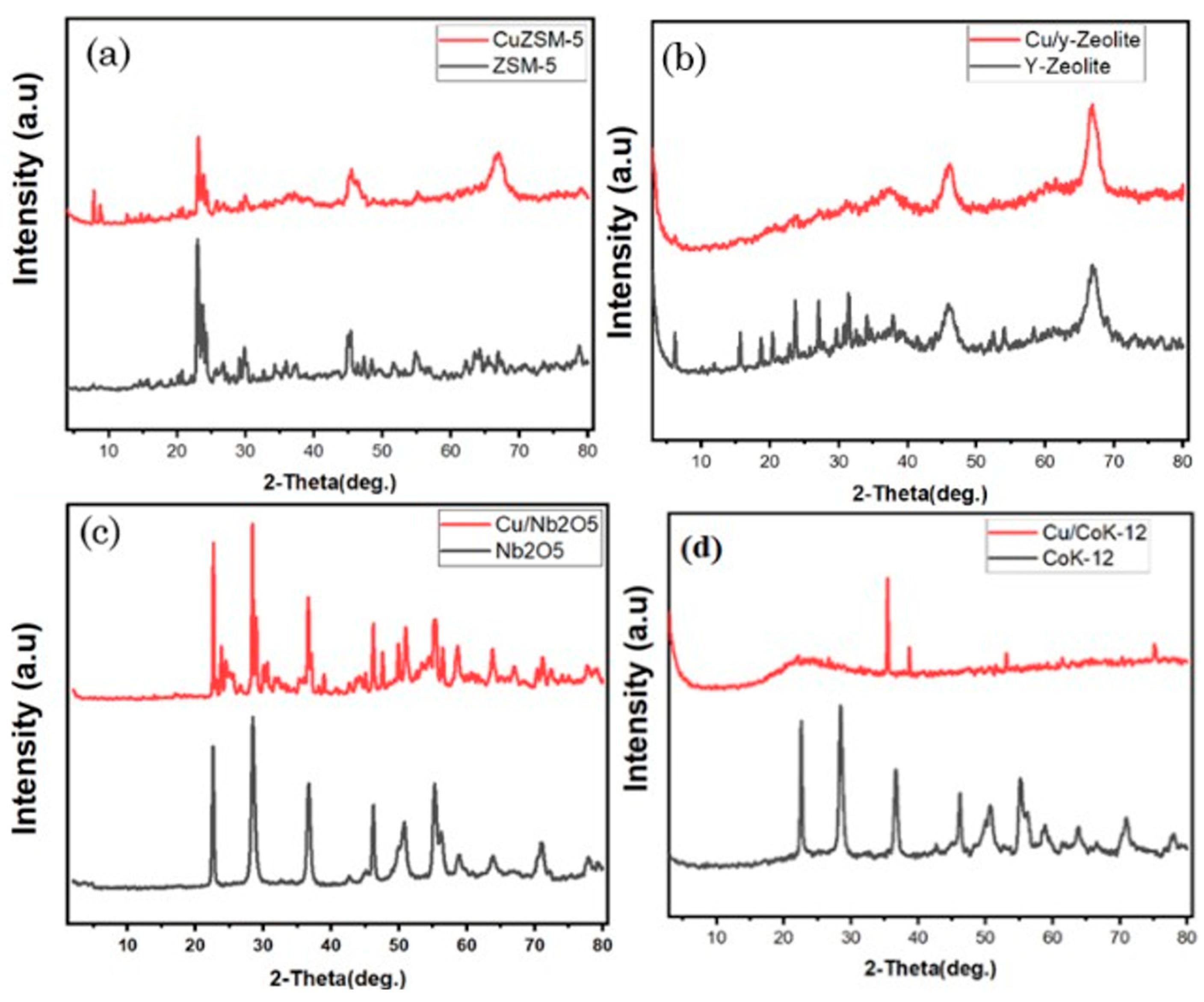
3.1.2. X-ray Diffraction
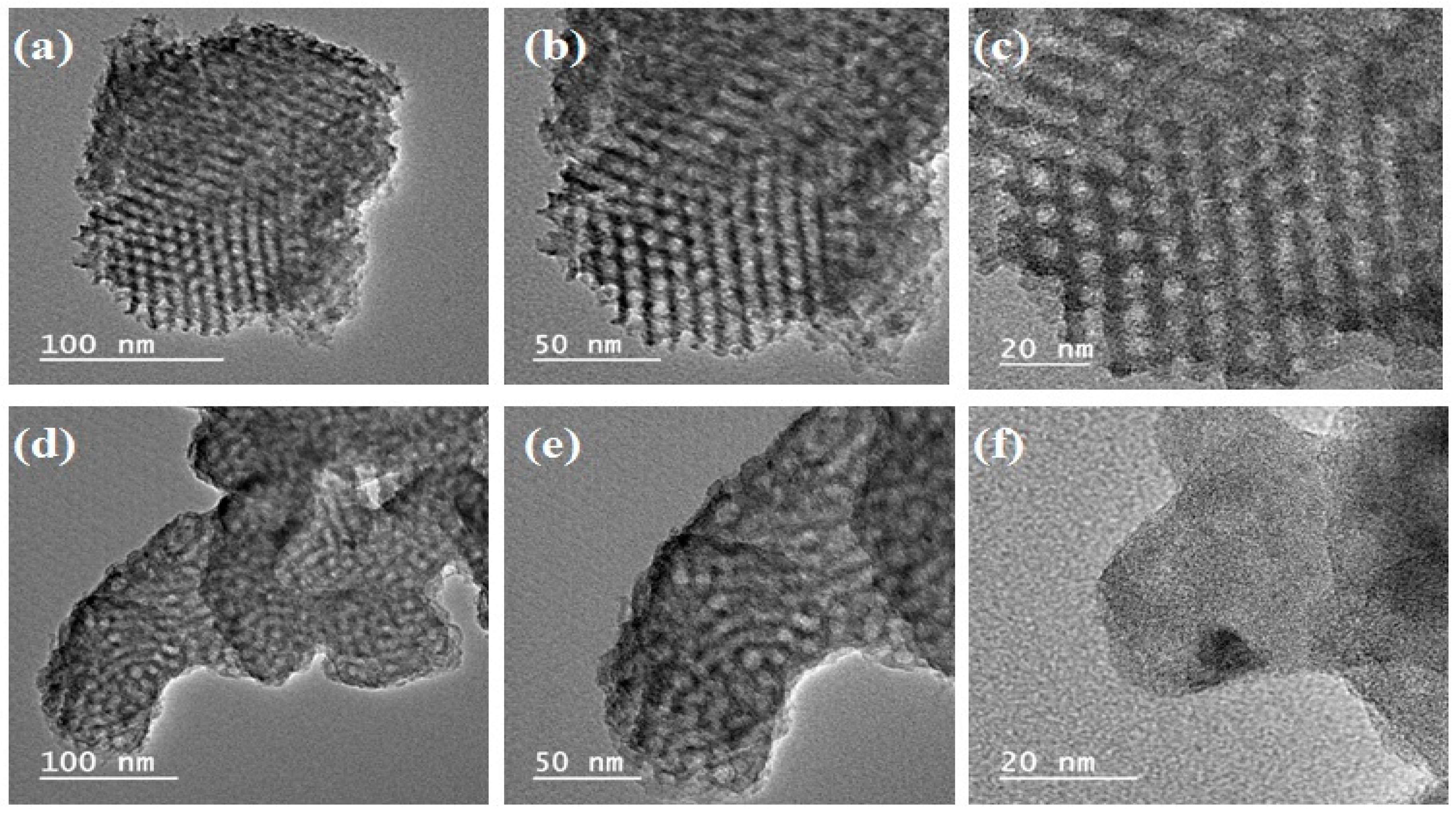
3.1.3. Transmission Electron Microscopy (TEM)
3.1.4. Scanning Electron Microscopy (SEM)
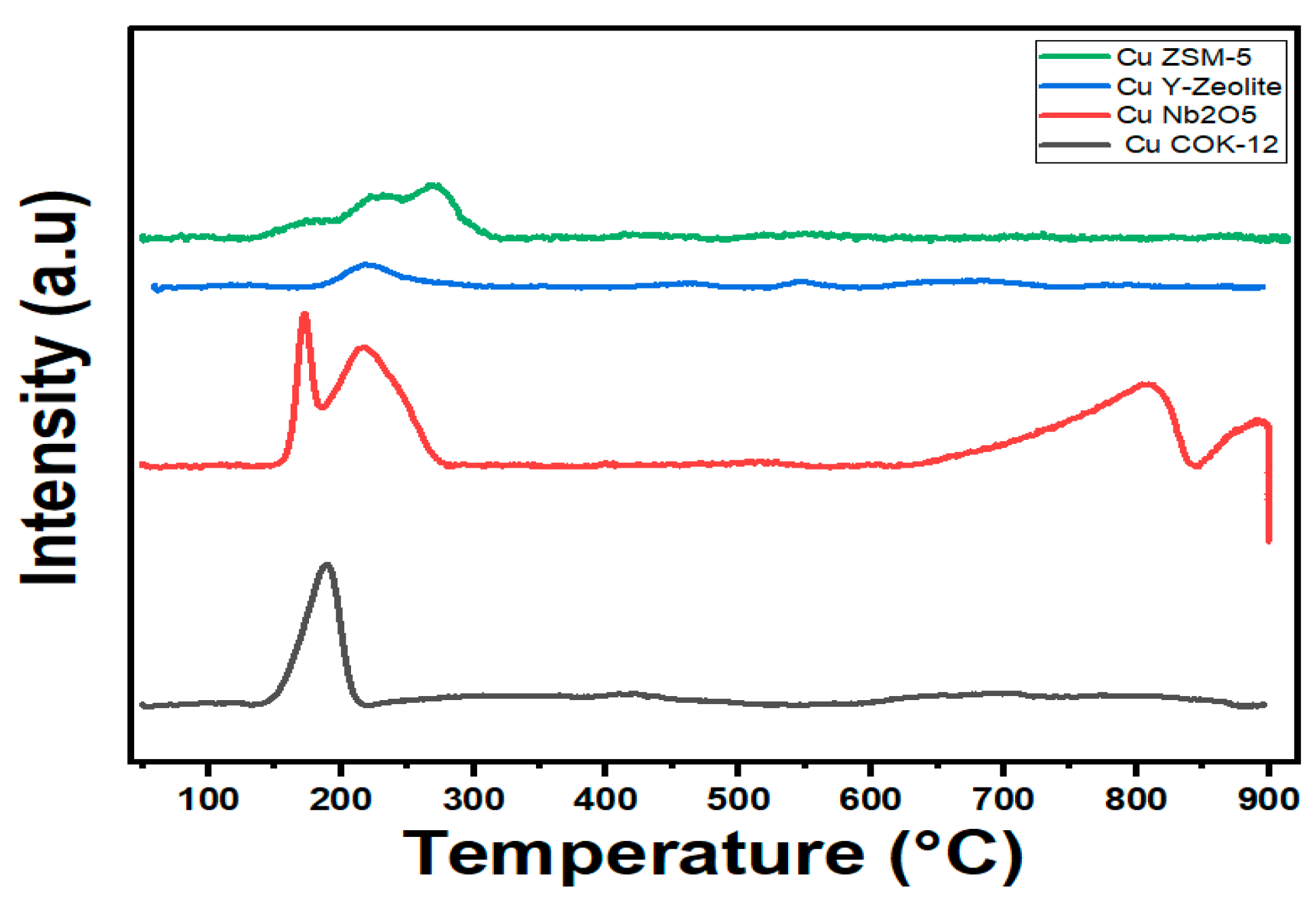
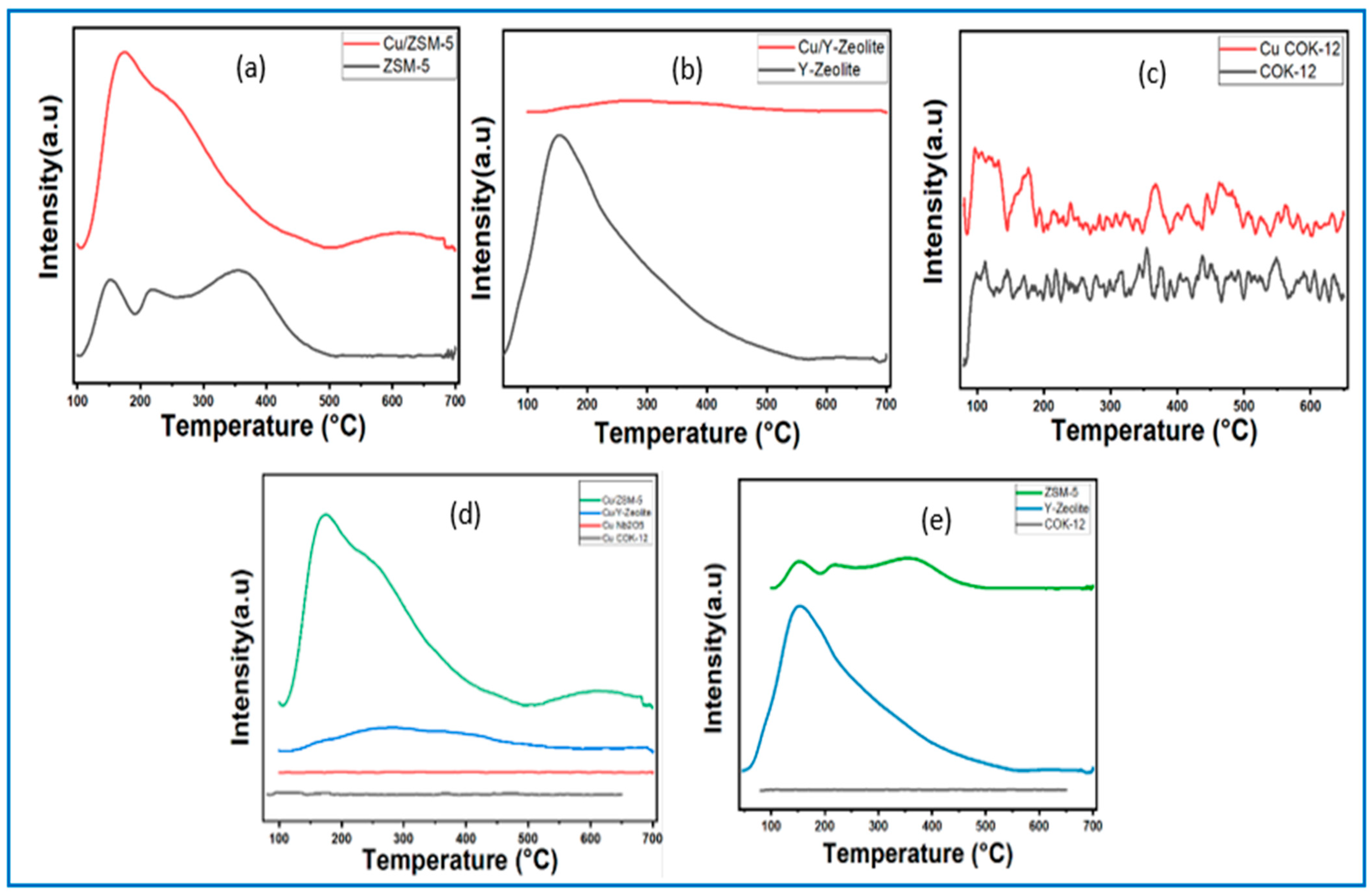
3.1.5. Temperature Programmed Reduction (TPR)
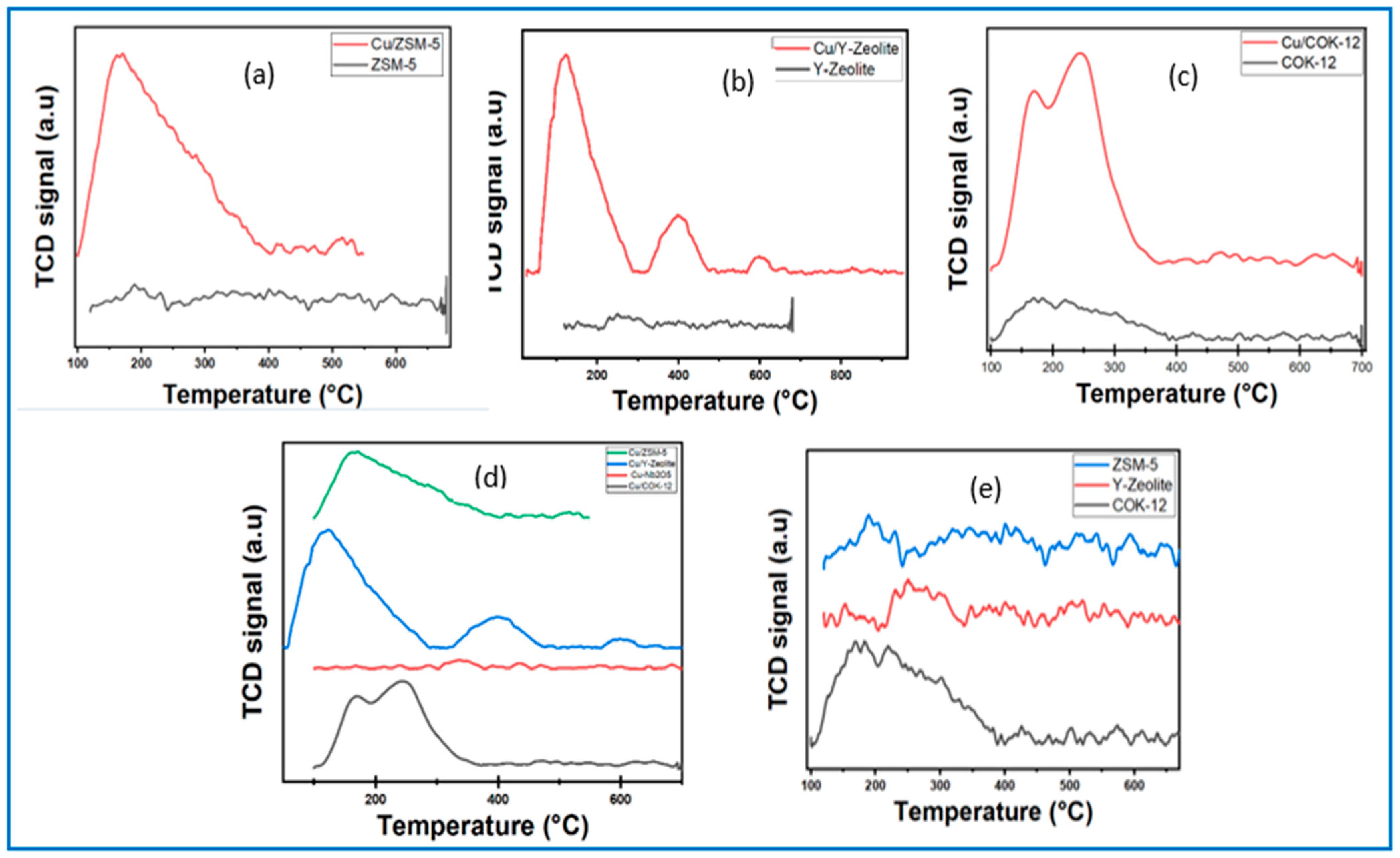
3.1.6. Temperature Programmed Desorption (TPD)
CO2-Temperature Programmed Desorption (TPD)
NH3-Temperature Programmed Desorption (TPD)
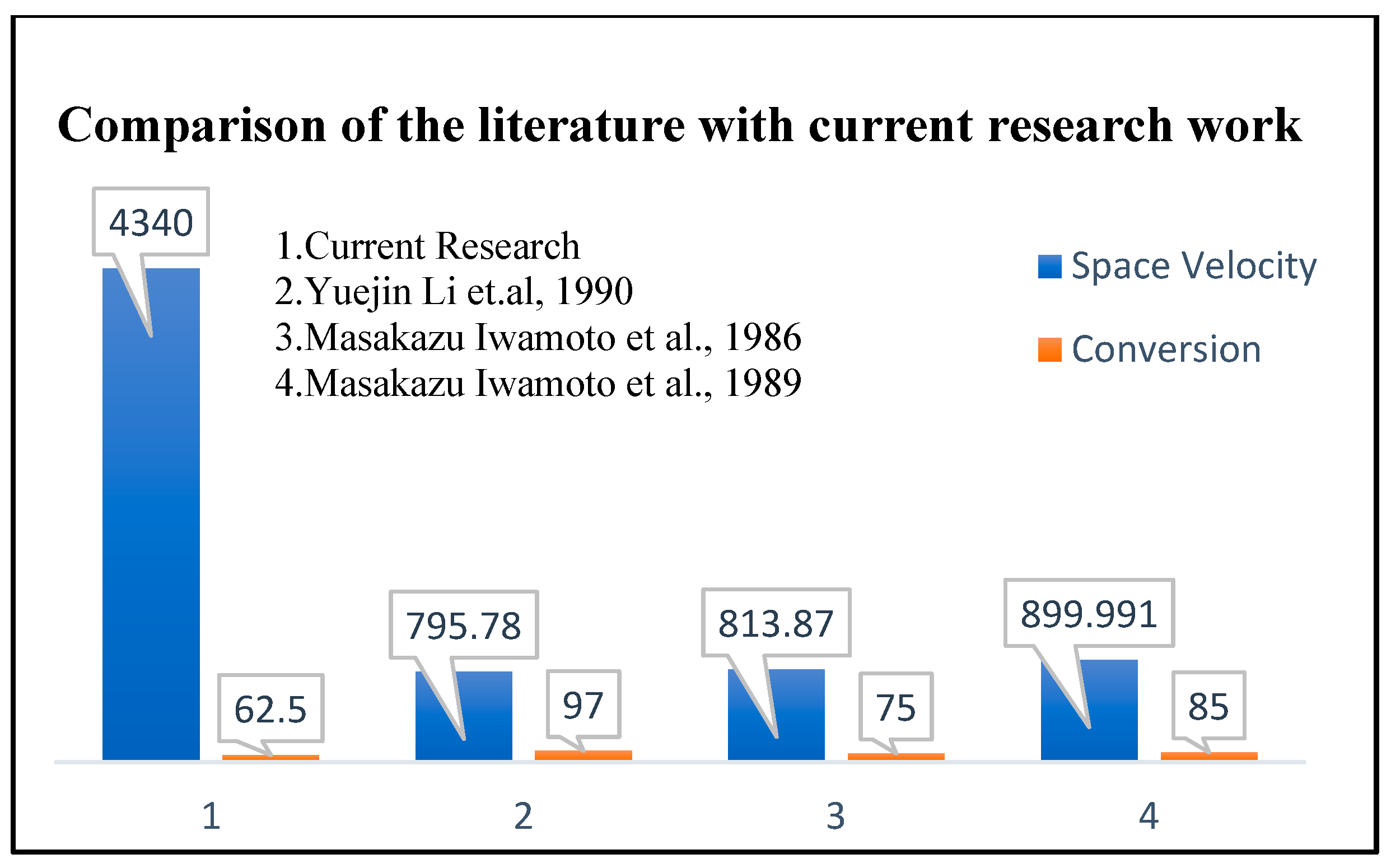
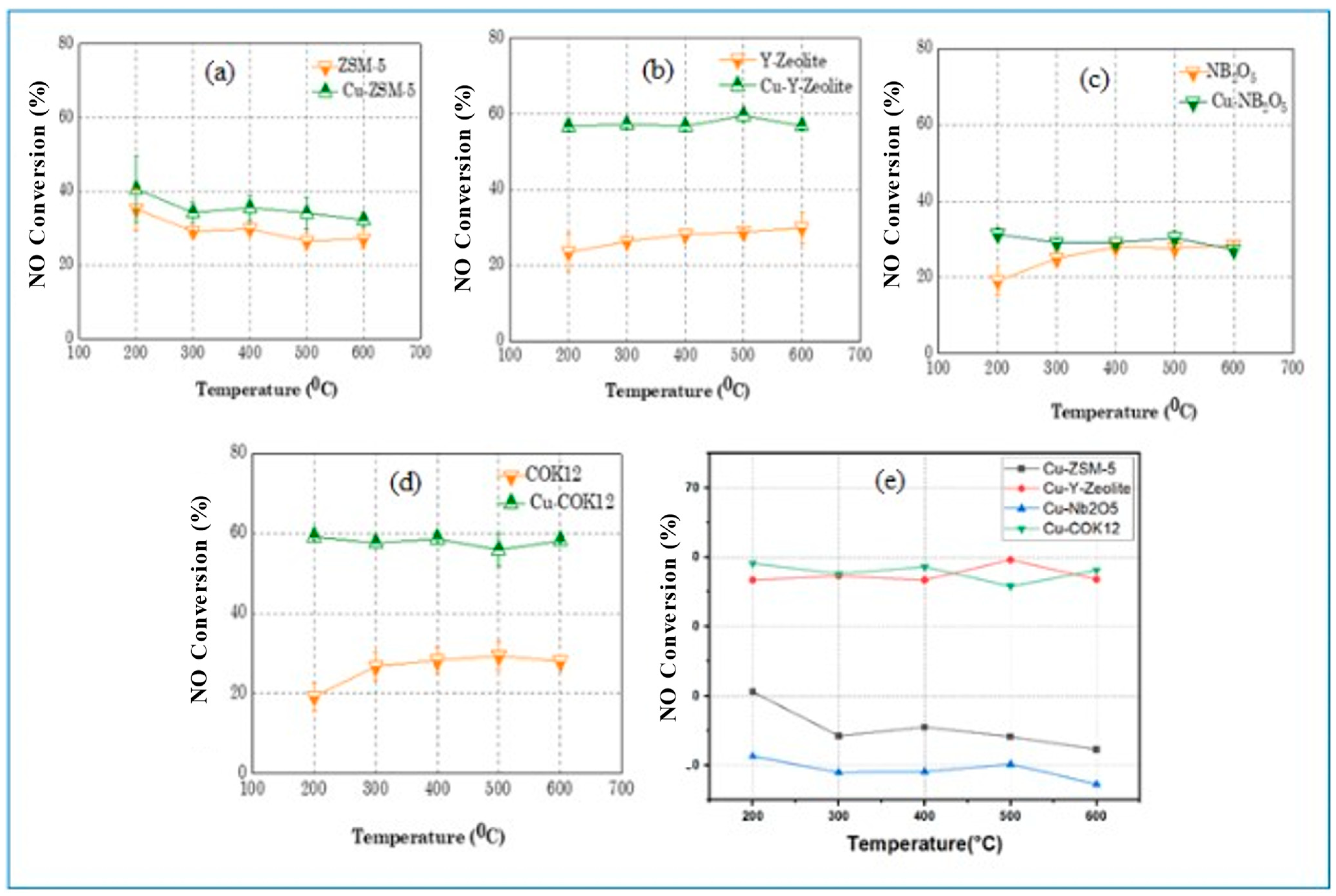
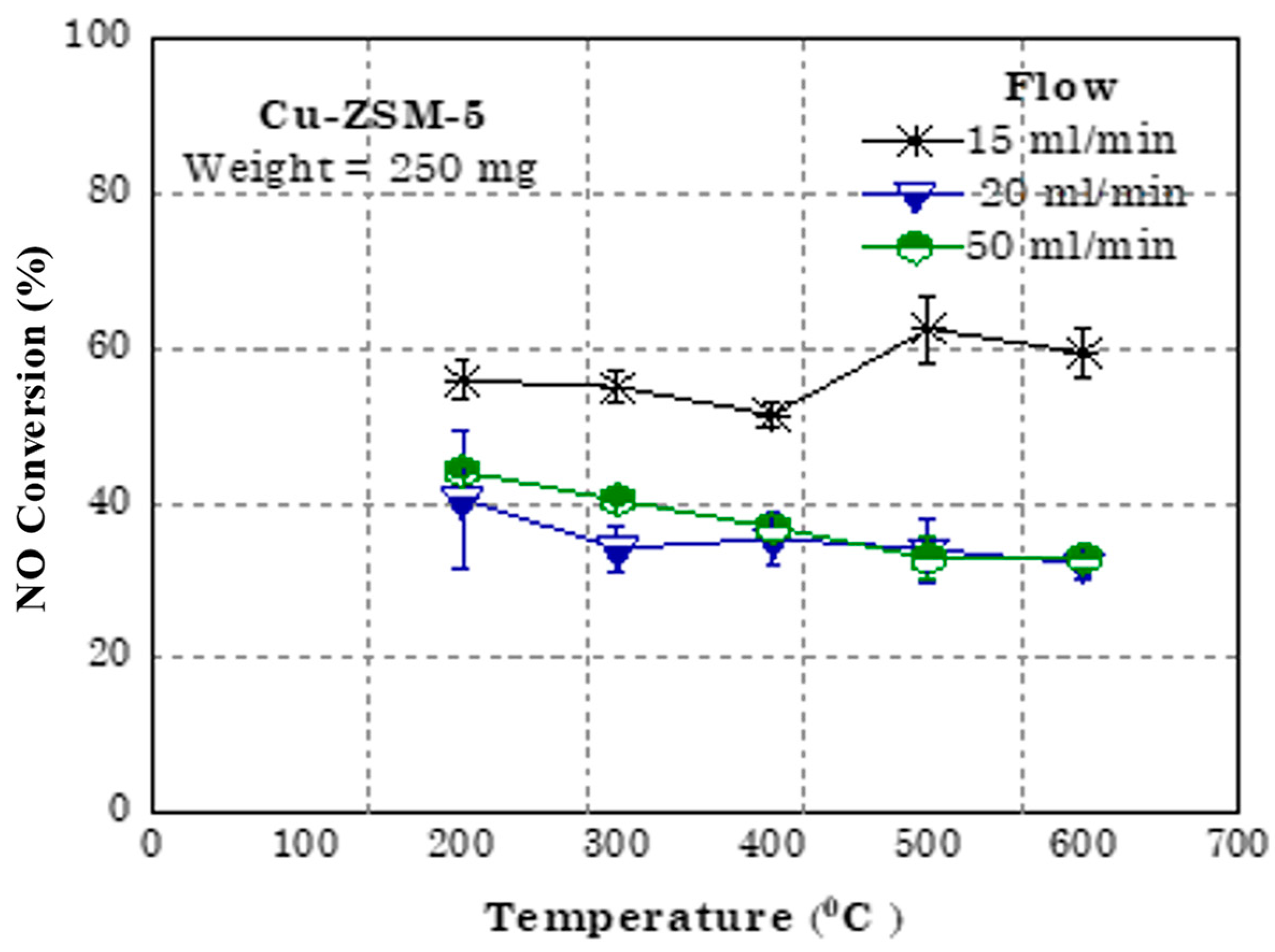
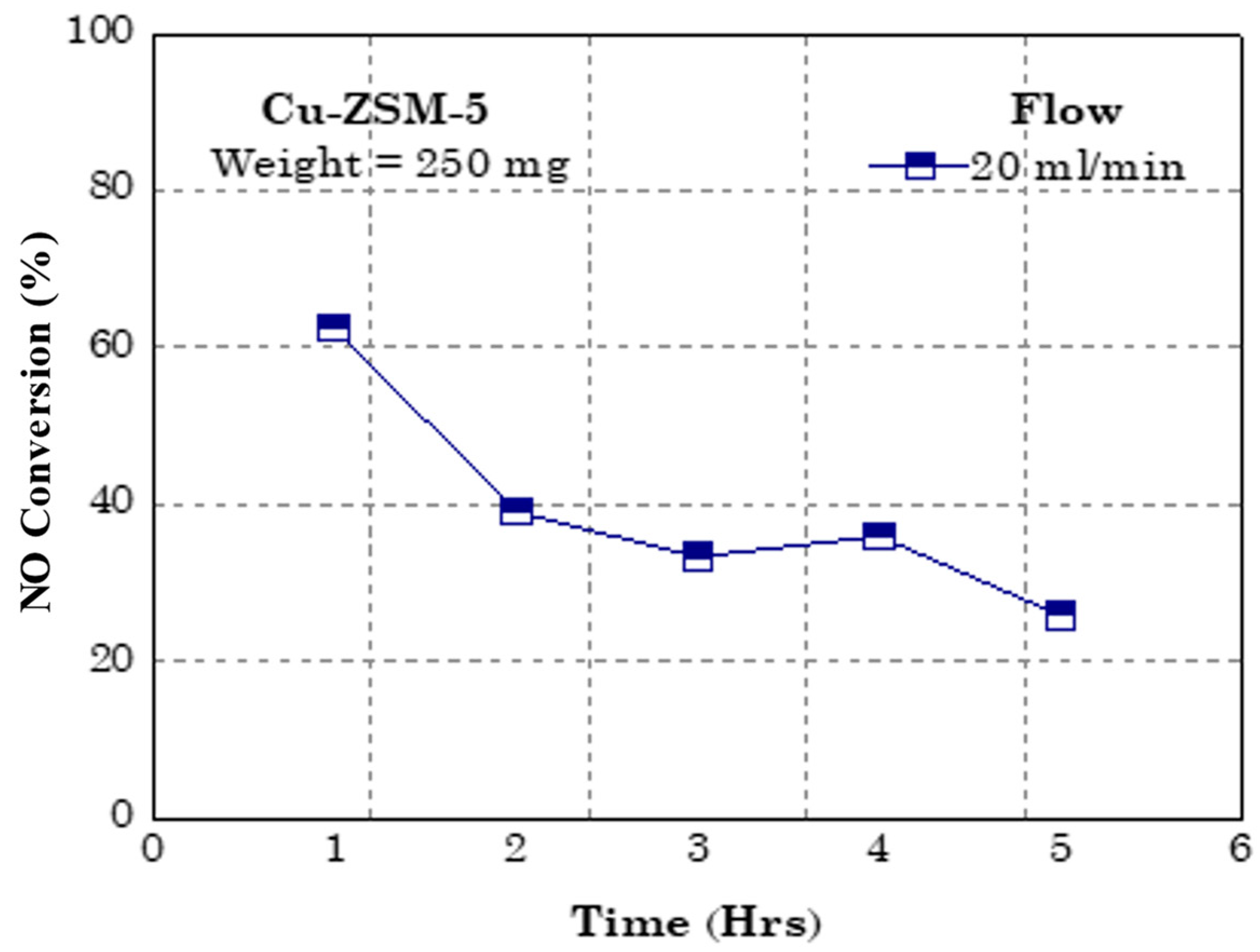
4. Application towards NO Conversion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ravina, M.; Caramitti, G.; Panepinto, D.; Zanetti, M. Air quality and photochemical reactions: Analysis of NOx and NO2 concentrations in the urban area of Turin, Italy. Air Qual. Atmos. Health 2022, 15, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Alli, A.S.; Clark, S.; Hughes, A.; Ezzati, M.; Beddows, A.; Vallarino, J.; Nimo, J.; Bedford-Moses, J.; Baah, S.; et al. Nitrogen oxides (NO and NO2) pollution in the Accra metropolis: Spatiotemporal patterns and the role of meteorology. Sci. Total Environ. 2021, 803, 149931. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.J.; Derwent, R. Air Pollution and Climate Change: The Basics; Routledge: London, UK, 2022. [Google Scholar]
- Mohite, V.T. Pollution and Pollution Control. In Emerging Trends in Environmental Biotechnology; CRC Press: Boca Raton, FL, USA, 2022; pp. 11–21. [Google Scholar]
- Bharti, S.; Chauhan, B.V.; Garg, A.; Vedrtnam, A.; Shukla, M. Potential of E-Fuels for Decarbonization of Transport Sector. In Greener and Scalable E-fuels for Decarbonization of Transport; Springer: Berlin/Heidelberg, Germany, 2022; pp. 9–32. [Google Scholar]
- Chauhan, B.V.S.; Sayyed, I.; Vedrantam, A.; Garg, A.; Bharti, S.; Shukla, M. State of the Art in Low-Temperature Combustion Technologies: HCCI, PCCI, and RCCI. In Advanced Combustion for Sustainable Transport; Springer: Berlin/Heidelberg, Germany, 2022; pp. 95–139. [Google Scholar]
- Garg, A.; Chauhan, B.V.; Vedrantam, A.; Jain, S.; Bharti, S. Potential and Challenges of Using Biodiesel in a Compression Ignition Engine. In Potential and Challenges of Low Carbon Fuels for Sustainable Transport; Springer: Berlin/Heidelberg, Germany, 2022; pp. 289–317. [Google Scholar]
- Cellek, M.S. The decreasing effect of ammonia enrichment on the combustion emission of hydrogen, methane, and propane fuels. Int. J. Hydrog. Energy 2022, 47, 19916–19934. [Google Scholar] [CrossRef]
- Bender, W.R. Lean pre-mixed combustion. In The Gas Turbine Handbook; NETL: Washington, DC, USA, 2006; pp. 217–227. [Google Scholar]
- Kim, J.-H.; Kim, J.-H.; Kim, H.-S.; Kim, H.-J.; Kang, S.-H.; Ryu, J.-H.; Shim, S.-S. Reduction of NOx Emission from the Cement Industry in South Korea: A Review. Atmosphere 2022, 13, 121. [Google Scholar] [CrossRef]
- Patel, B.; Barot, M.R.; Tala, J. Emission Control Technology for Stationary Internal Combustion Engines; Manufacturers of Emission Controls Association: Arlington, VA, USA, 2015. [Google Scholar]
- Masui, T.; Uejima, S.; Tsujimoto, S.; Nagai, R.; Imanaka, N. Direct NO decomposition over C-type cubic Y2O3–Pr6O11–Eu2O3 solid solutions. Catal. Today 2015, 242, 338–342. [Google Scholar] [CrossRef]
- Tofan, C.; Klvana, D.; Kirchnerova, J. Direct decomposition of nitric oxide over perovskite-type catalysts: Part I. Activity when no oxygen is added to the feed. Appl. Catal. A Gen. 2002, 223, 275–286. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Yuan, F.; Zhang, G.; Fu, H. Direct NO decomposition over La2−xBaxNiO4 catalysts containing BaCO3 phase. Appl. Catal. B Environ. 2008, 82, 255–263. [Google Scholar] [CrossRef]
- Sadek, R.; Chalupka, K.A.; Mierczynski, P.; Rynkowski, J.; Gurgul, J.; Dzwigaj, S. Cobalt Based Catalysts Supported on Two Kinds of Beta Zeolite for Application in Fischer-Tropsch Synthesis. Catalysts 2019, 9, 497. [Google Scholar] [CrossRef]
- Xie, P.; Ji, W.; Li, Y.; Zhang, C. NO direct decomposition: Progress, challenges and opportunities. Catal. Sci. Technol. 2020, 11, 374–391. [Google Scholar] [CrossRef]
- Green, E.T.; Hinshelwood, C.N. CCXXIV—The catalytic decomposition of nitric oxide at the surface of platinum. J. Chem. Soc. 1926, 129, 1709–1713. [Google Scholar] [CrossRef]
- Jellinek, H.H.G.; Chaudhuri, A.; Takada, K. Inhibition of Hydrocyanic Acid Evolution from Polyurethanes during Oxidative Degradation. Polym. J. 1978, 10, 253–268. [Google Scholar] [CrossRef][Green Version]
- Shelef, M.; Otto, K.; Gandhi, H. The heterogeneous decomposition of nitric oxide on supported catalysts. Atmospheric Environ. (1967) 1969, 3, 107–122. [Google Scholar] [CrossRef]
- Tsujimoto, S.; Nishimura, C.; Masui, T.; Imanaka, N. Direct decomposition of nitrogen monoxide on (Ho, Zr, Pr)2O3+δ Catalysts. Catal. Commun. 2014, 43, 84–87. [Google Scholar] [CrossRef]
- Tsujimoto, S.; Wang, X.; Masui, T.; Imanaka, N. Direct Decomposition of NO into N2 and O2 on C-type Cubic Y2O3–ZrO2 and Y2O3–ZrO2–BaO. Bull. Chem. Soc. Jpn. 2011, 84, 807–811. [Google Scholar] [CrossRef]
- Imanaka, N.; Masui, T.; Masaki, H. Direct Decomposition of Nitric Oxide over C-Type Cubic (Gd1–x–yYxBay)2O3–y Solid Solutions. Adv. Mater. 2007, 19, 3660–3663. [Google Scholar] [CrossRef]
- Doi, Y.; Haneda, M.; Ozawa, M. Direct decomposition of NO on Ba catalysts supported on rare earth oxides. J. Mol. Catal. A: Chem. 2013, 383–384, 70–76. [Google Scholar] [CrossRef]
- Tsujimoto, S.; Nishimura, C.; Masui, T.; Imanaka, N. Coexisting Gas-resistant C-type Cubic Yb2O3–Tb4O7 Catalysts for Direct NO Decomposition. Chem. Lett. 2011, 40, 708–710. [Google Scholar] [CrossRef]
- Masaki, H.; Masui, T.; Imanaka, N. Direct decomposition of nitric oxide into nitrogen and oxygen over C-type cubic Y2O3–ZrO2 solid solutions. J. Alloy. Compd. 2008, 451, 406–409. [Google Scholar] [CrossRef]
- Tsujimoto, S.; Mima, K.; Masui, T.; Imanaka, N. Direct decomposition of NO on C-type cubic rare earth oxides based on Y2O3. Chem. Lett. 2010, 39, 456–457. [Google Scholar] [CrossRef]
- Imanaka, N.; Masui, T. Advances in direct NOx decomposition catalysts. Appl. Catal. A Gen. 2012, 431, 1–8. [Google Scholar] [CrossRef]
- Vannice, M.; Walters, A.B.; Zhang, X. The Kinetics of NOxDecomposition and NO Reduction by CH4overLa2O3and Sr/La2O3. J. Catal. 1996, 159, 119–126. [Google Scholar] [CrossRef]
- Xie, S.; Mestl, G.; Rosynek, M.P.; Lunsford, J.H. Decomposition of nitric oxide over barium oxide supported on magnesium oxide. 1. Catalytic results and in situ Raman spectroscopic evidence for a barium—Nitro intermediate. J. Am. Chem. Soc. 1997, 119, 10186–10191. [Google Scholar] [CrossRef]
- Xie, S.; Rosynek, M.P.; Lunsford, J.H. Catalytic reactions of NO over 0–7 mol% Ba/MgO catalysts: I. The direct decomposition of NO. J. Catal. 1999, 188, 24–31. [Google Scholar] [CrossRef]
- Matsumoto, S.; Yokota, K.; Kimura, M.; Sekizawa, K.; Kasahara, S. Research on new DeNO [sub x] catalysts for automotive engines. Catal. Today 1994, 22, 127–146. [Google Scholar] [CrossRef]
- Komvokis, V.G.; Iliopoulou, E.F.; Vasalos, I.A.; Triantafyllidis, K.S.; Marshall, C.L. Development of optimized Cu–ZSM5 deNOx catalytic materials both for HC-SCR applications and as FCC catalytic additives. Appl. Catal. A Gen. 2007, 325, 345–352. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Sachtler, W.M. Activity and durability of Fe/ZSM5 catalysts for lean burn NOx reduction in the presence of water vapor. Catal. Today 1998, 42, 73–83. [Google Scholar] [CrossRef]
- Curtin, T.; Grange, P.; Delmon, B. The direct decomposition of nitrogen monoxide. Catal. Today 1997, 35, 121–127. [Google Scholar] [CrossRef]
- Jia, L.; Yu, Y.; Li, Z.-P.; Qin, S.-N.; Guo, J.-R.; Zhang, Y.-Q.; Wang, J.-C.; Zhang, J.-C.; Fan, B.-G.; Jin, Y. Study on the Hg0 removal characteristics and synergistic mechanism of iron-based modified biochar doped with multiple metals. Bioresour. Technol. 2021, 332, 125086. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S. Fixed bed reactor dynamics and control–A review. In Dynamics and Control of Chemical Reactors and Distillation Columns; Elsevier: Amsterdam, The Netherlands, 1988; pp. 11–24. [Google Scholar]
- Stegehake, C.; Riese, J.; Grünewald, M. Modeling and Validating Fixed-Bed Reactors: A State-of-the-Art Review. ChemBioEng Rev. 2019, 6, 28–44. [Google Scholar] [CrossRef]
- Abdi, M.R.; Shakur, H.R.; Saraee, K.R.E.; Sadeghi, M. Effective removal of uranium ions from drinking water using CuO/X zeolite based nanocomposites: Effects of nano concentration and cation exchange. J. Radioanal. Nucl. Chem. Artic. 2014, 300, 1217–1225. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, J.; Bhaskar, T. High surface area biochar from Sargassum tenerrimum as potential catalyst support for selective phenol hydrogenation. Environ. Res. 2020, 186, 109533. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, L.; Yu, W.; Xie, H. Intriguingly high thermal conductivity increment for CuO nanowires contained nanofluids with low viscosity. Sci. Rep. 2018, 8, 5282. [Google Scholar] [CrossRef] [PubMed]
- Kannapu, H.P.R.; Suh, Y.-W.; Narani, A.; Vaddeboina, V.; Burri, D.R.; Seetha, R.R.K. One-pot synthesis of ethylbenzene/1-phenylethanol and γ-butyrolactone from simultaneous acetophenone hydrogenation and 1, 4-butanediol dehydrogenation over copper based catalysts: Effects of the support. RSC Adv. 2017, 7, 35346–35356. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, J.; Wang, L.; Xie, W.; Chen, P.; Hou, Z.; Zheng, X. Biodiesel derived glycerol hydrogenolysis to 1, 2-propanediol on Cu/MgO catalysts. Bioresour. Technol. 2010, 101, 7088–7092. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jindal, M.; Rawat, S.; Kumar, J.; Sripadi, P.; Yang, B.; Thallada, B. Upgradation of sugarcane bagasse lignin: Fractionation to cyclic alcohols production. Catal. Today 2022, 408, 182–193. [Google Scholar] [CrossRef]
- Pochamoni, R.; Narani, A.; Gurram, V.R.; Gudimella, M.D.; Potharaju, P.S.; Burri, D.R.; Rao, K.S. Molybdenum oxide supported on COK12: A novel catalyst for oxidative dehydrogenation of ethylbenzene using CO2. Indian J. Chem. 2014, 53, 493–498. [Google Scholar]
- Lisi, L.; Pirone, R.; Russo, G.; Stanzione, V. Cu-ZSM5 based monolith reactors for NO decomposition. Chem. Eng. J. 2009, 154, 341–347. [Google Scholar] [CrossRef]
- Da Costa, P.; Modén, B.; Meitzner, G.D.; Lee, D.K.; Iglesia, E. Spectroscopic and chemical characterization of active and inactive Cu species in NO decomposition catalysts based on Cu-ZSM5. Phys. Chem. Chem. Phys. 2002, 4, 4590–4601. [Google Scholar] [CrossRef]
- Modén, B.; Da Costa, P.; Fonfé, B.; Lee, D.K.; Iglesia, E. Kinetics and mechanism of steady-state catalytic NO decomposition reactions on Cu–ZSM5. J. Catal. 2002, 209, 75–86. [Google Scholar] [CrossRef]
- Moden, B.; da Costa, P.; Lee, D.K.; Iglesia, E. Catalytic NO Decomposition on Cu-ZSM5: Kinetically Relevant Elementary Steps and Speciation and Role of Cu Structures. In Proceedings of the 18th North American Catalysis Society Meeting, Cancun, Mexico, 1–6 June 2003. [Google Scholar]
- Ganemi, B.; Björnbom, E.; Paul, J. Conversion and in situ FTIR studies of direct NO decomposition over Cu-ZSM5. Appl. Catal. B: Environ. 1998, 17, 293–311. [Google Scholar] [CrossRef]
- Palella, B.I.; Lisi, L.; Pirone, R.; Russo, G.; Notaro, M. Enhancement of hydrothermal stability of Cu-ZSM5 catalyst for NO decomposition. Kinet. Catal. 2006, 47, 728–736. [Google Scholar] [CrossRef]
- Pirone, R.; Ciambelli, P.; Palella, B.; Russo, G. Simultaneous NO and N2O decomposition on Cu-ZSM5. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2000; pp. 911–916. [Google Scholar]
- Schay, Z.; Guczi, L.; Kiricsi, I. Transient kinetic study on NO decomposition over Cu-ZSM-5 catalysts. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1998; pp. 347–355. [Google Scholar]
- Li, Y.; Hall, W.K. Stoichiometric catalytic decomposition of nitric oxide over copper-exchanged zeolite (CuZSM-5) catalysts. J. Phys. Chem. 1990, 94, 6145–6148. [Google Scholar] [CrossRef]
- Iwamoto, M.; Furukawa, H.; Mine, Y.; Uemura, F.; Mikuriya, S.-I.; Kagawa, S. Copper (II) ion-exchanged ZSM-5 zeolites as highly active catalysts for direct and continuous decomposition of nitrogen monoxide. J. Chem. Soc. Chem. Commun. 1986, 16, 1272–1273. [Google Scholar] [CrossRef]
- Iwamoto, M.; Yahiro, H.; Mine, Y.; Kagawa, S. Excessively copper ion-exchanged ZSM-5 zeolites as highly active catalysts for direct decomposition of nitrogen monoxide. Chem. Lett. 1989, 18, 213–216. [Google Scholar] [CrossRef]
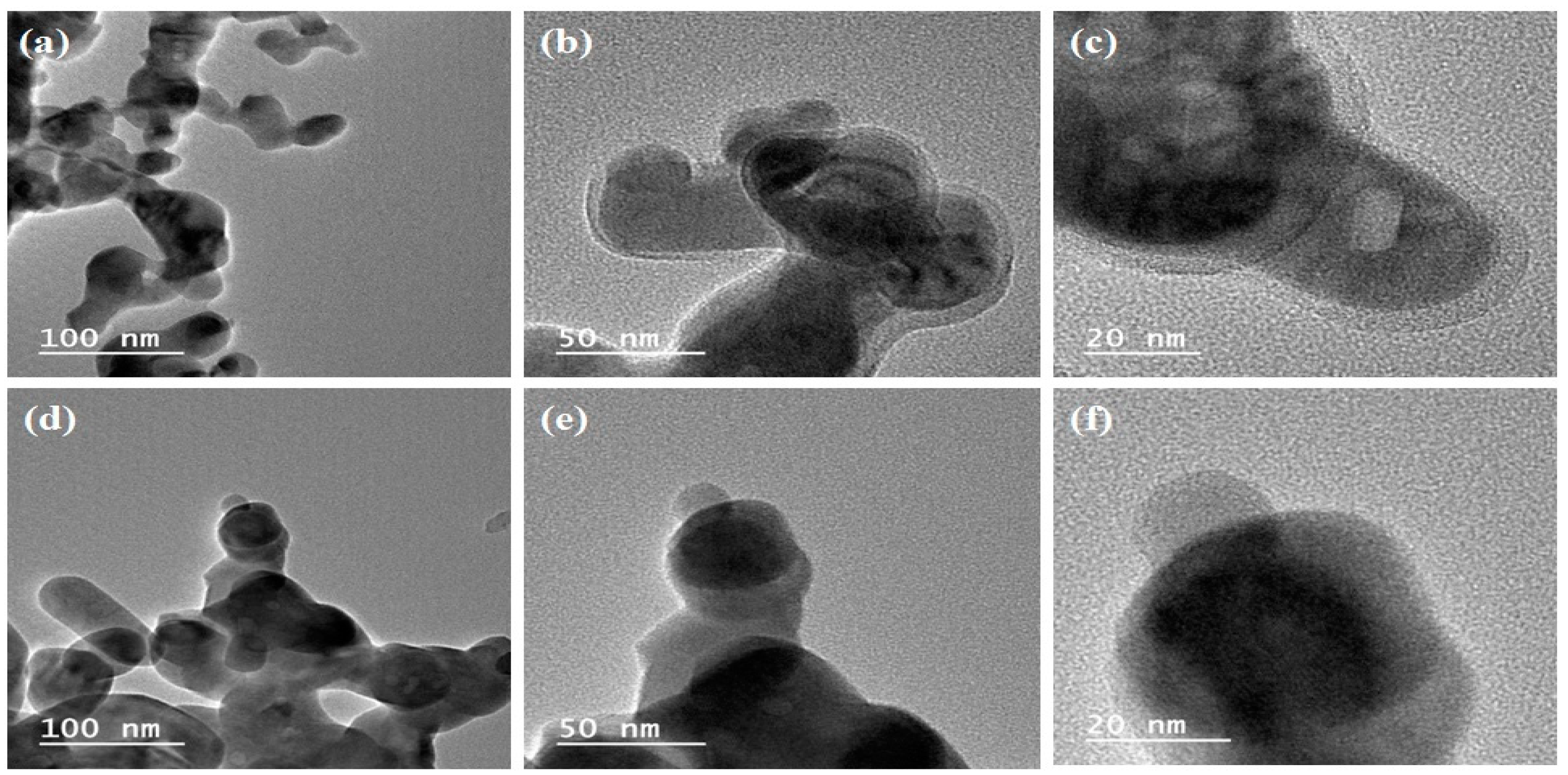
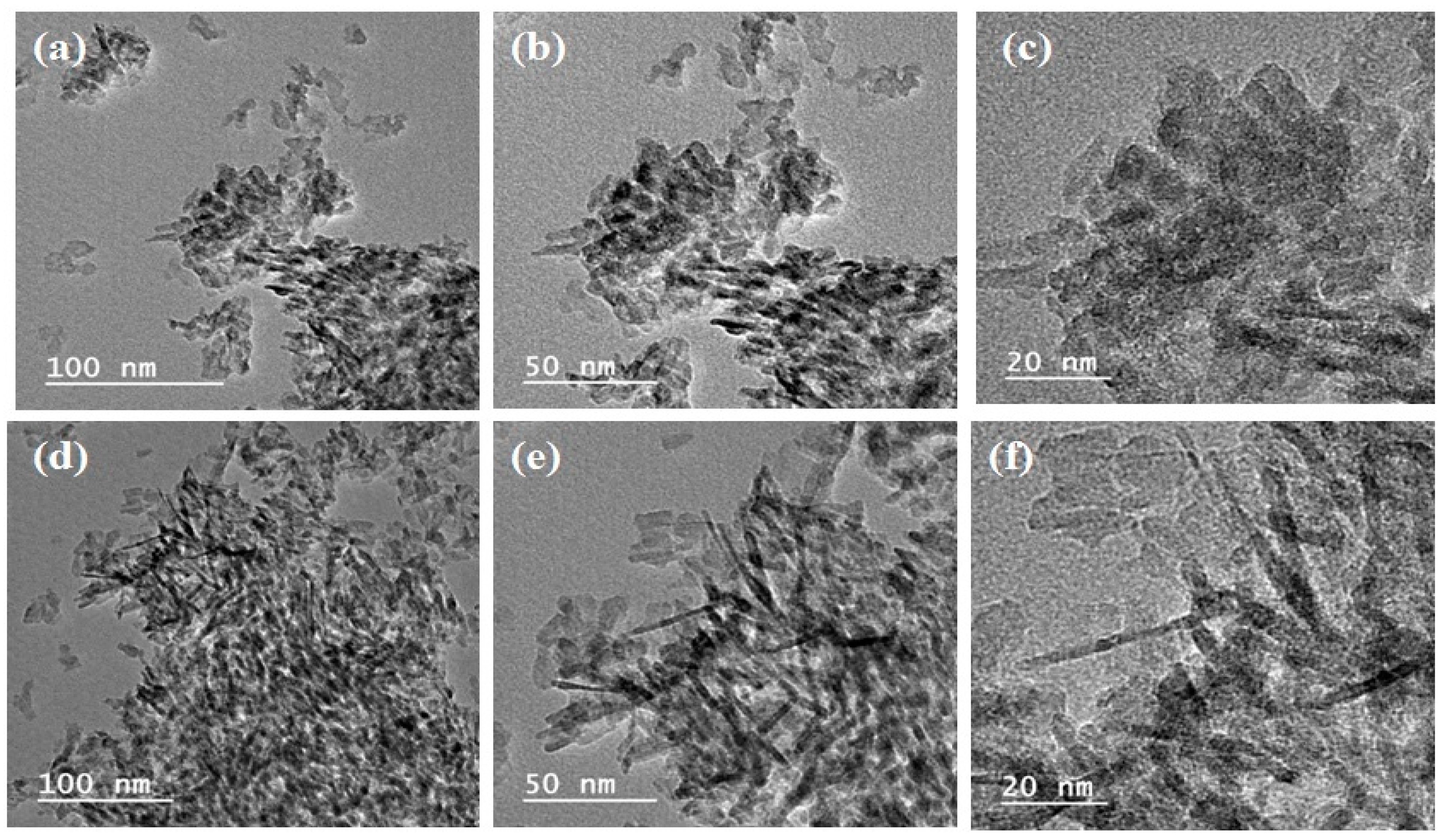
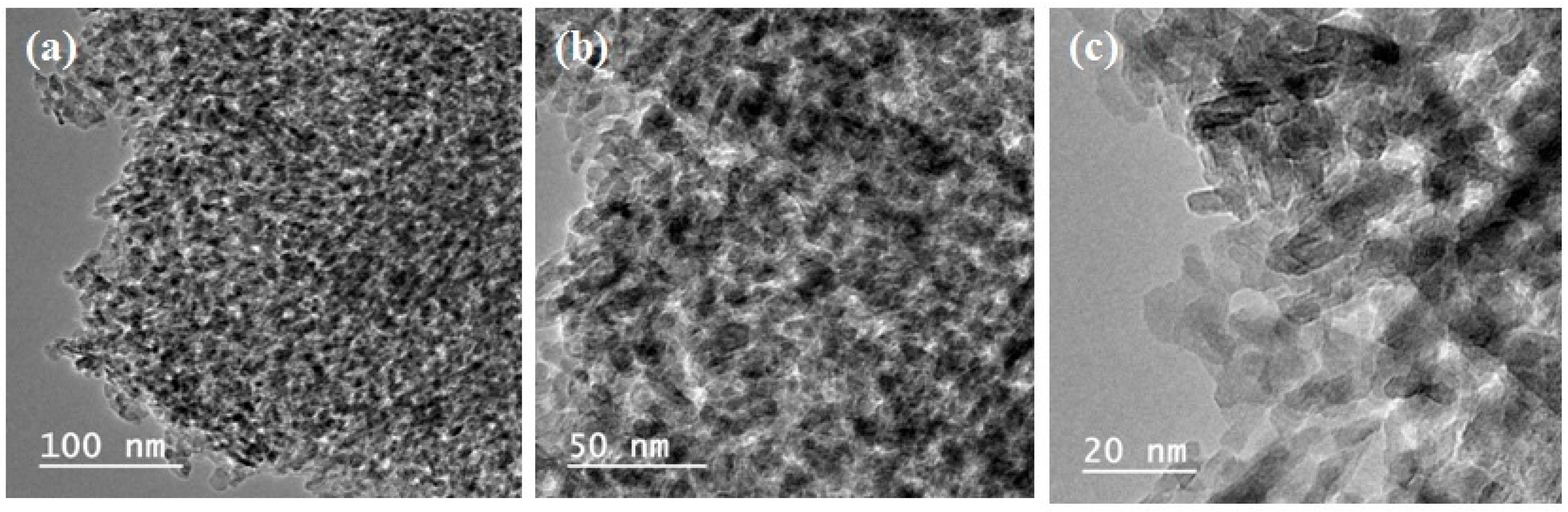


| Catalyst | Surface Area, m2/g | Pore Volume, cm3/g | Pore Size, nm |
|---|---|---|---|
| COK12 | 270 | 0.79 | 11.80 |
| 3% Cu-COK12 | 323 | 0.72 | 8.90 |
| Nb2O5 | 15.4 | 0.06 | 16.00 |
| 3% Cu-Nb2O5 | 6.5 | 0.08 | 53.6 |
| Y-Zeolite | 325 | 0.47 | 7.45 |
| 3% Cu-Y-Zeolite | 230 | 0.50 | 8.70 |
| ZSM5 | 293 | 0.20 | 5.90 |
| 3% Cu-ZSM5 | 228 | 0.37 | 6.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shukla, M.K.; Chauhan, B.V.S.; Verma, S.; Dhar, A. Catalytic Direct Decomposition of NOx Using Non-Noble Metal Catalysts. Solids 2022, 3, 665-683. https://doi.org/10.3390/solids3040041
Shukla MK, Chauhan BVS, Verma S, Dhar A. Catalytic Direct Decomposition of NOx Using Non-Noble Metal Catalysts. Solids. 2022; 3(4):665-683. https://doi.org/10.3390/solids3040041
Chicago/Turabian StyleShukla, M. K., Balendra V. S. Chauhan, Sneha Verma, and Atul Dhar. 2022. "Catalytic Direct Decomposition of NOx Using Non-Noble Metal Catalysts" Solids 3, no. 4: 665-683. https://doi.org/10.3390/solids3040041
APA StyleShukla, M. K., Chauhan, B. V. S., Verma, S., & Dhar, A. (2022). Catalytic Direct Decomposition of NOx Using Non-Noble Metal Catalysts. Solids, 3(4), 665-683. https://doi.org/10.3390/solids3040041