Doped Tin Dioxide (d-SnO2) and Its Nanostructures: Review of the Theoretical Aspects, Photocatalytic and Biomedical Applications
Abstract
1. Introduction
2. SnO2 Structural and Theoretical Aspects
2.1. Computational Tools and Density Functional Theory
2.2. SnO2 Theoretical Models
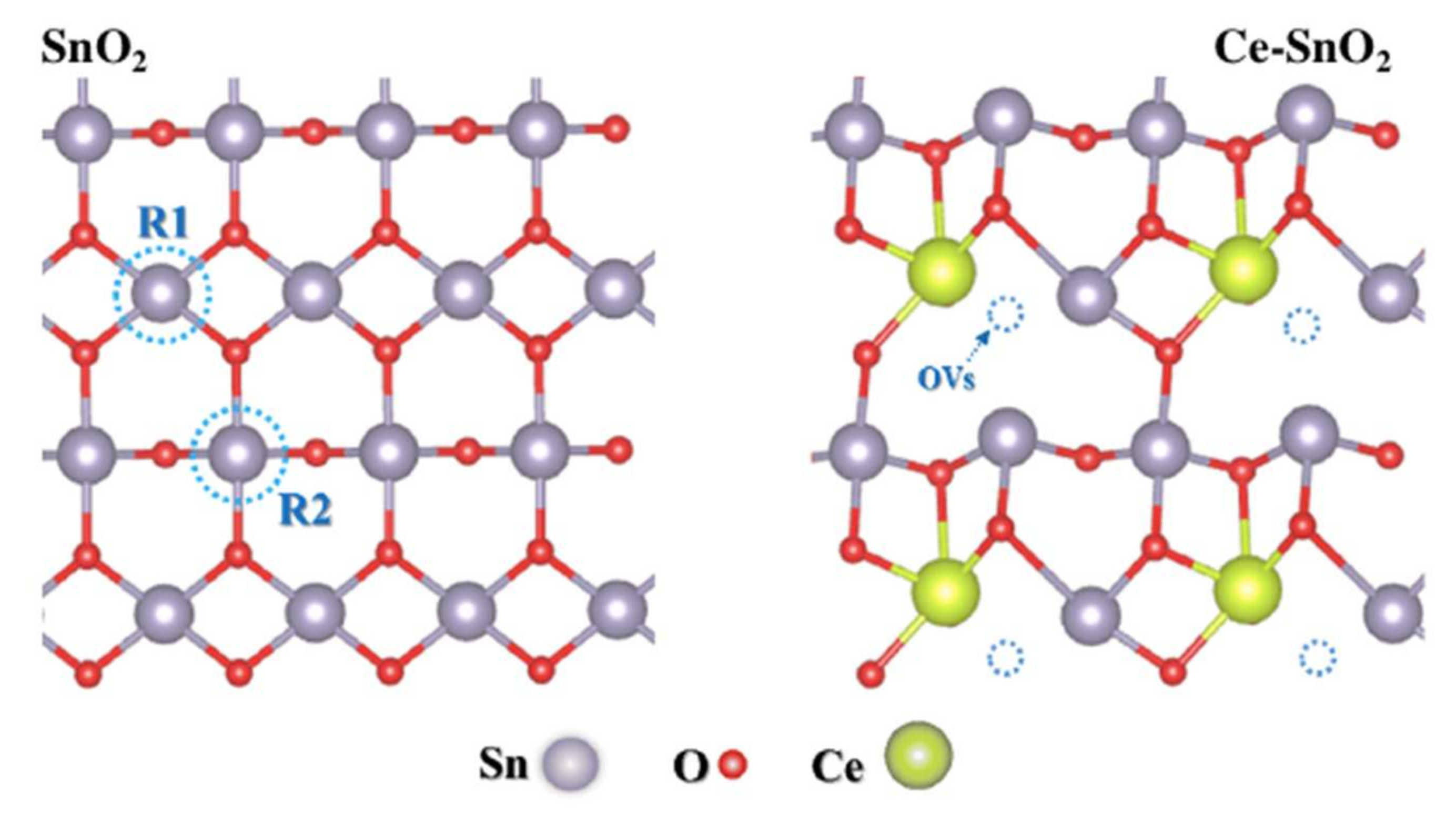
2.3. Doped SnO2 (d-SnO2)
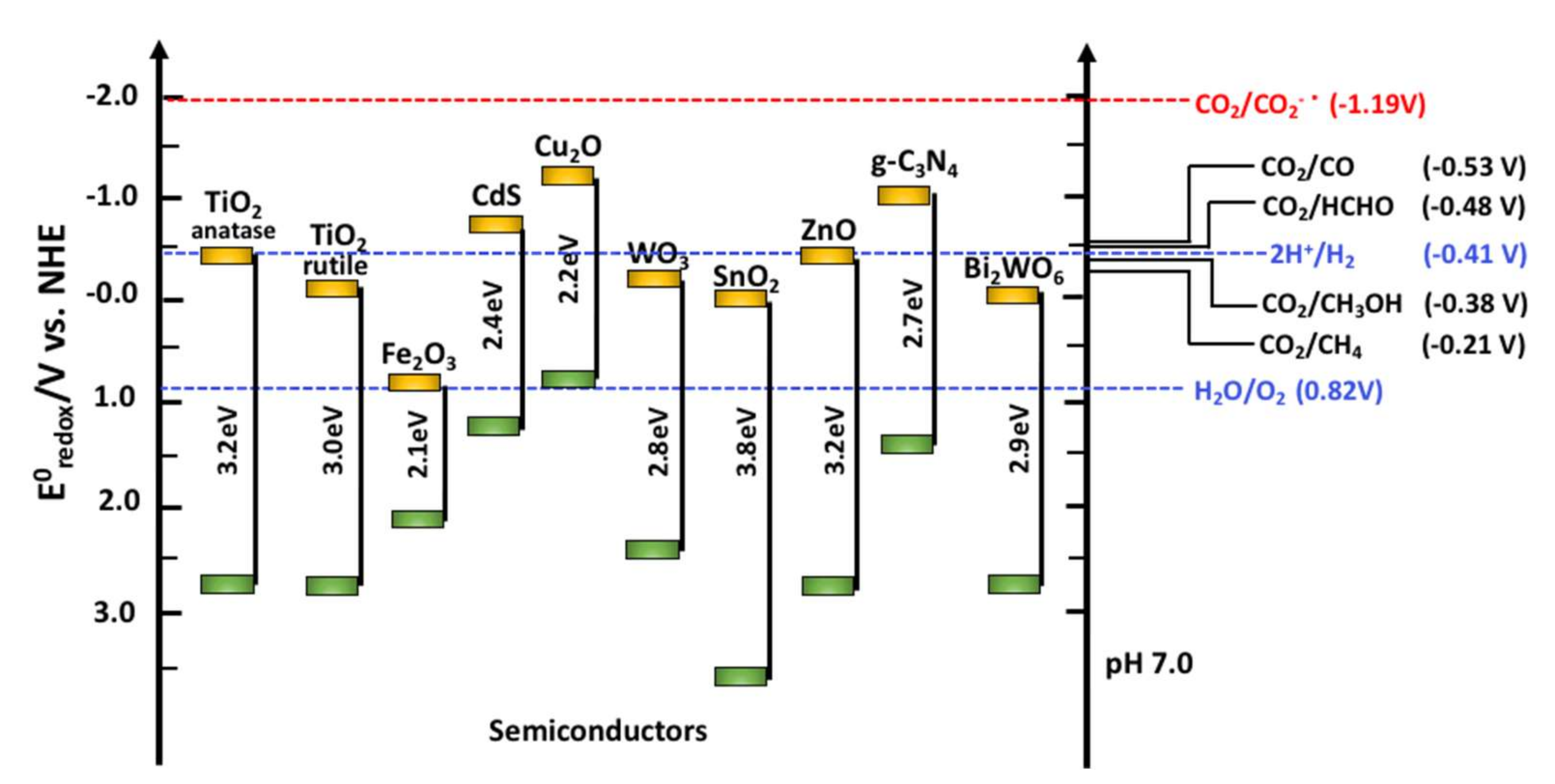
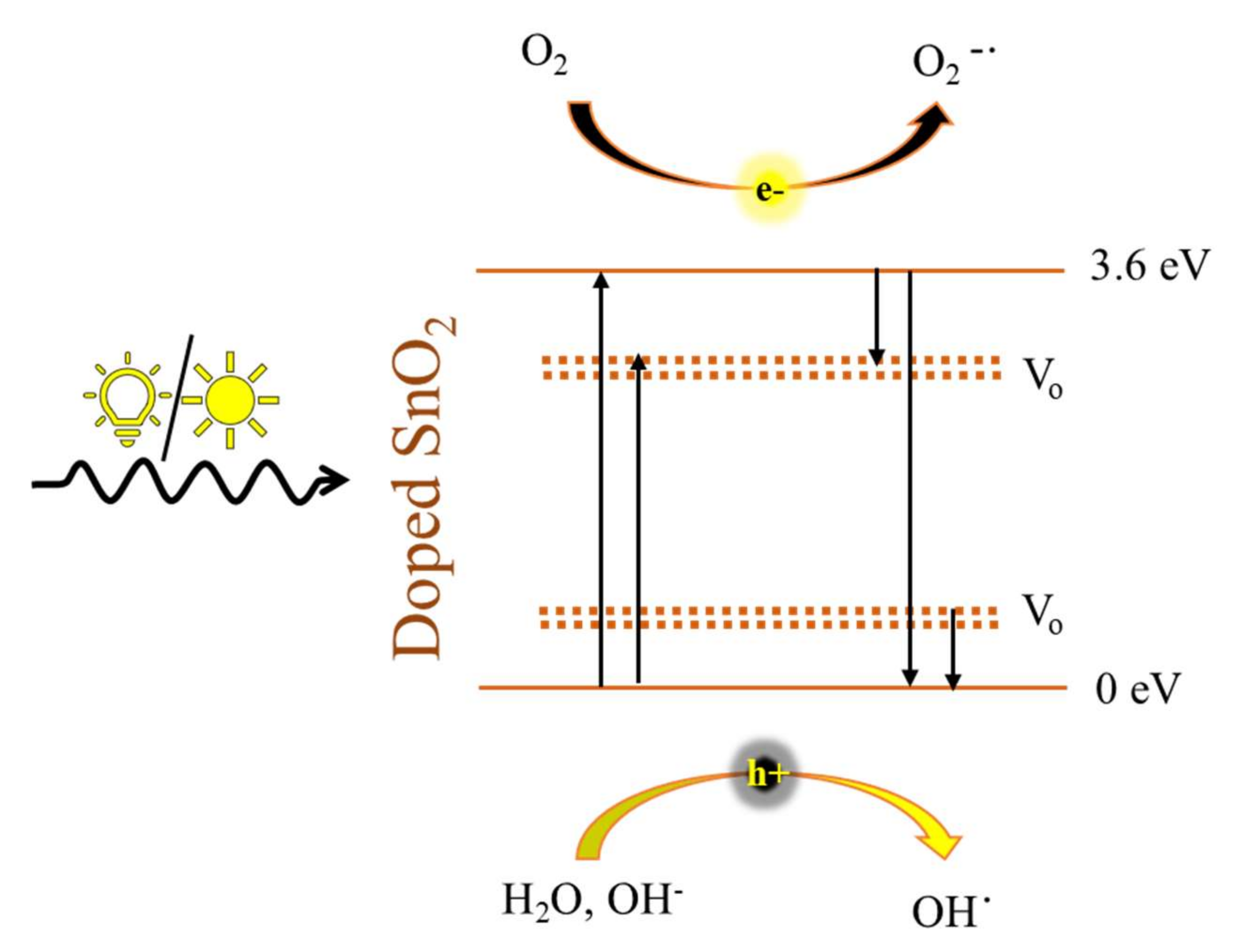
3. SnO2 and d-SnO2 Photocatalysis
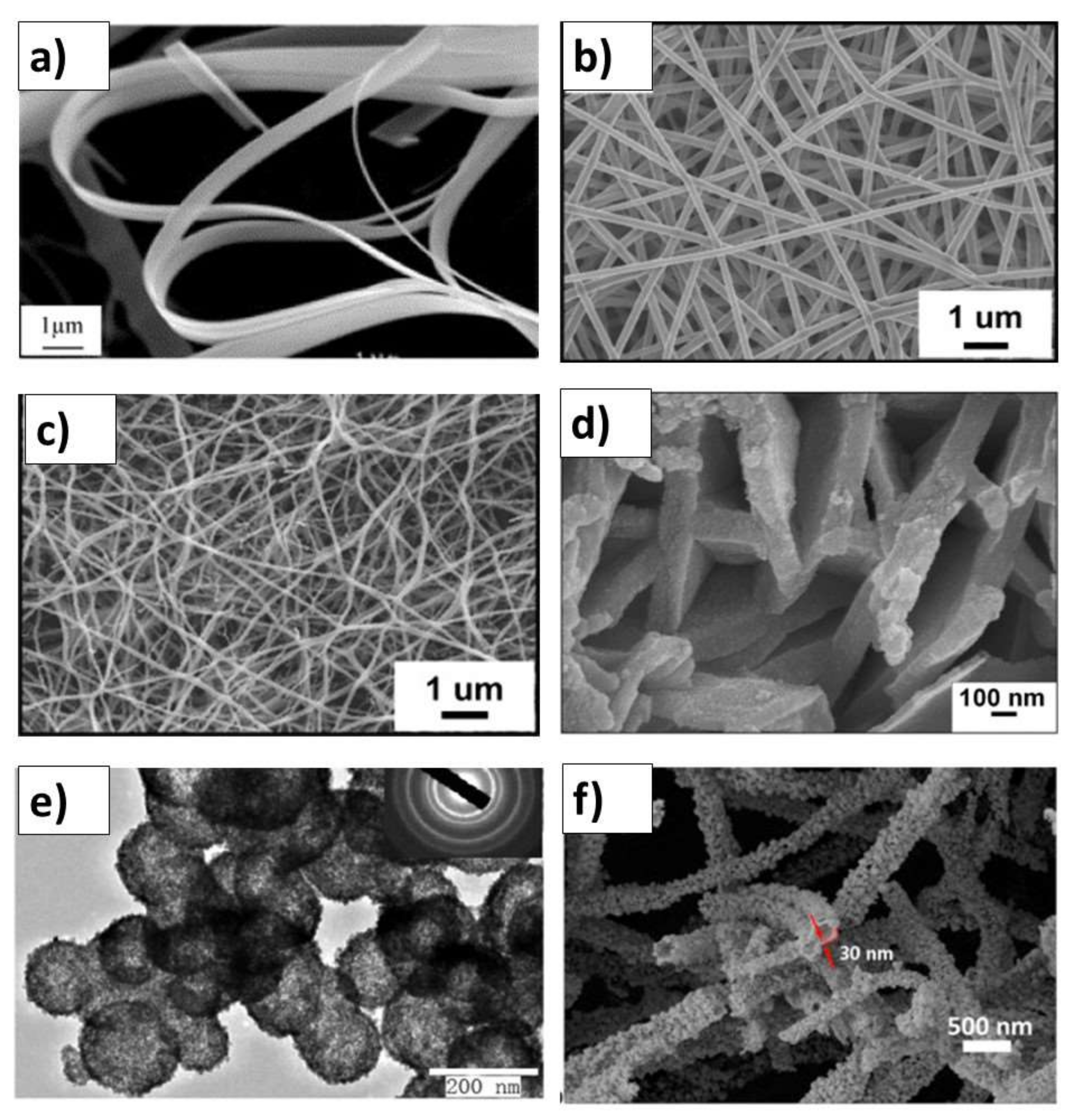
SnO2 and d-SnO2 Morphological Control and Its Impact on the Photocatalysis
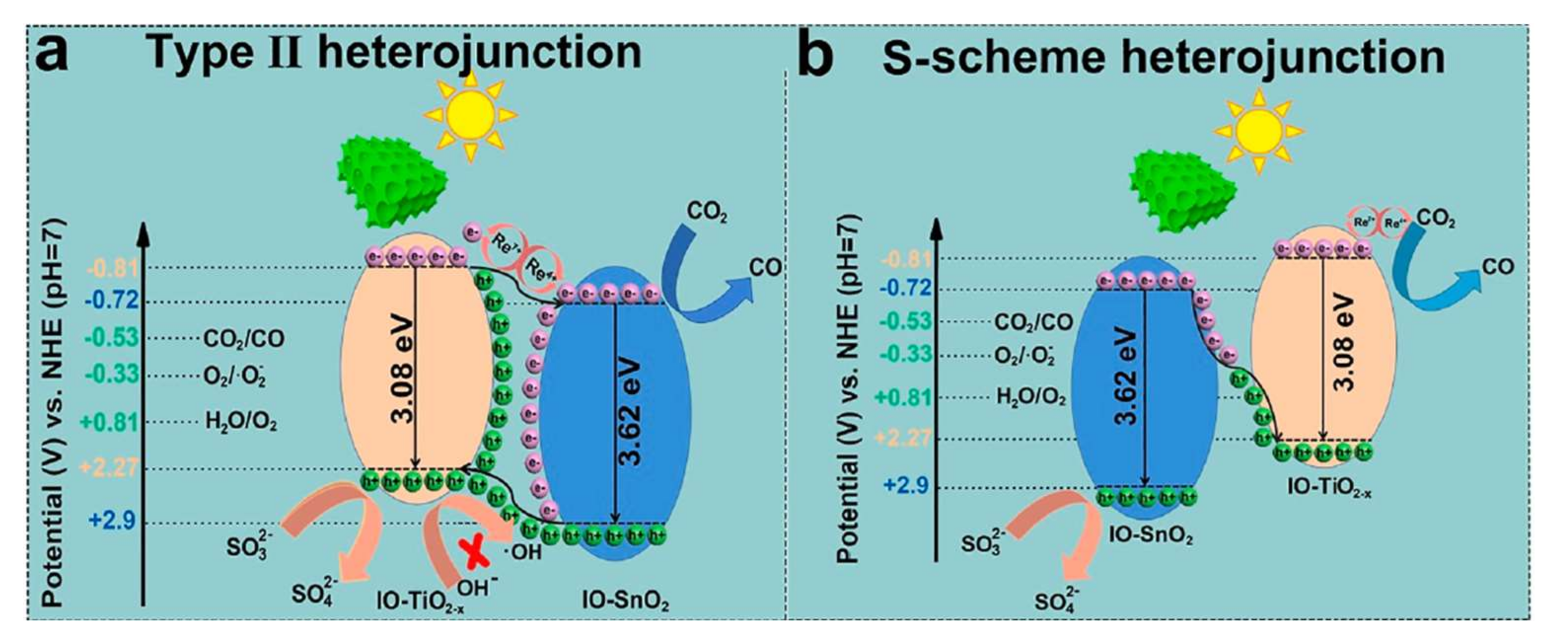
4. SnO2 and d-SnO2 Applied to CO2 Reduction
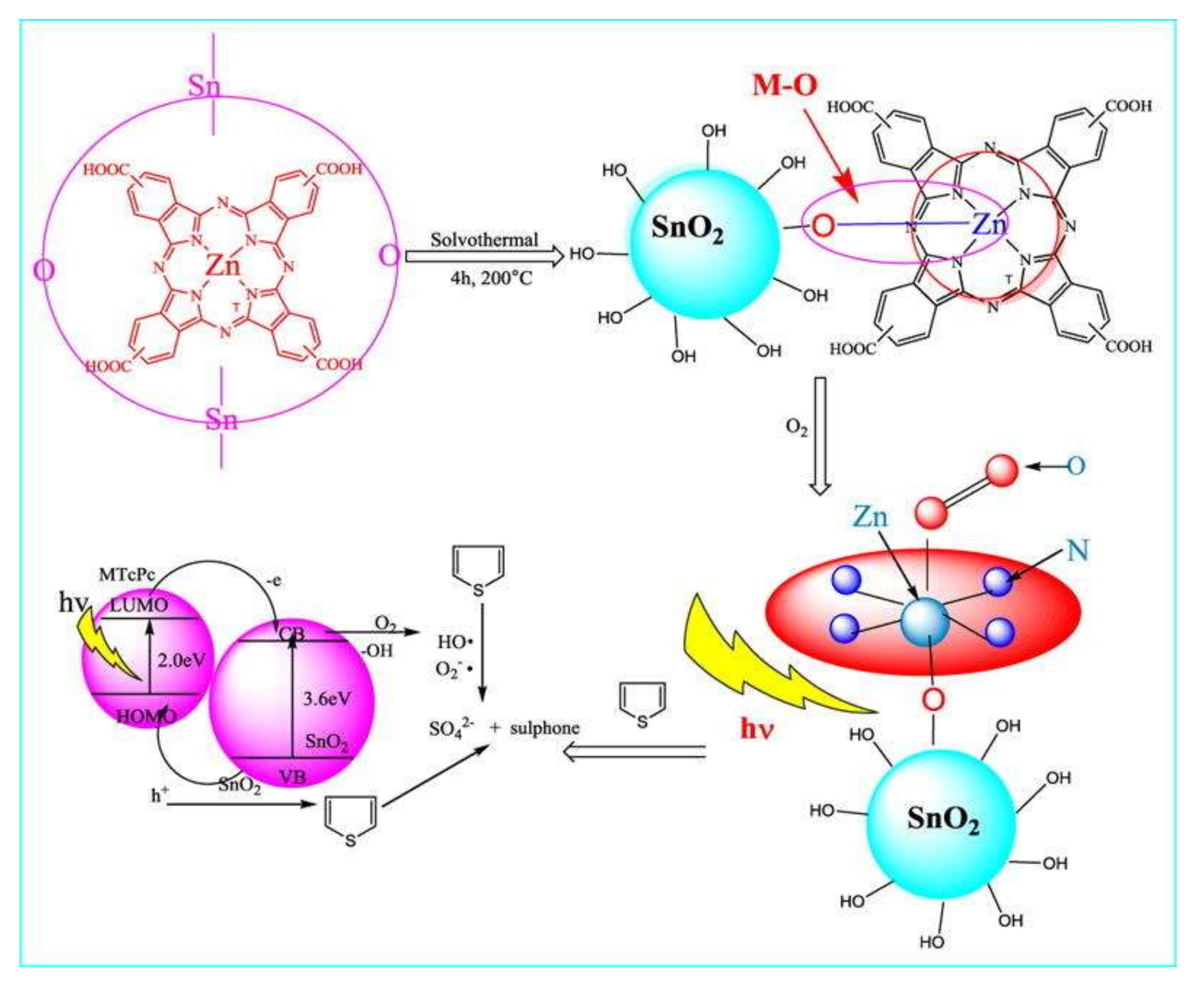
5. SnO2 and Doped-SnO2 Nanostructures Applied to Fuels Desulfurization
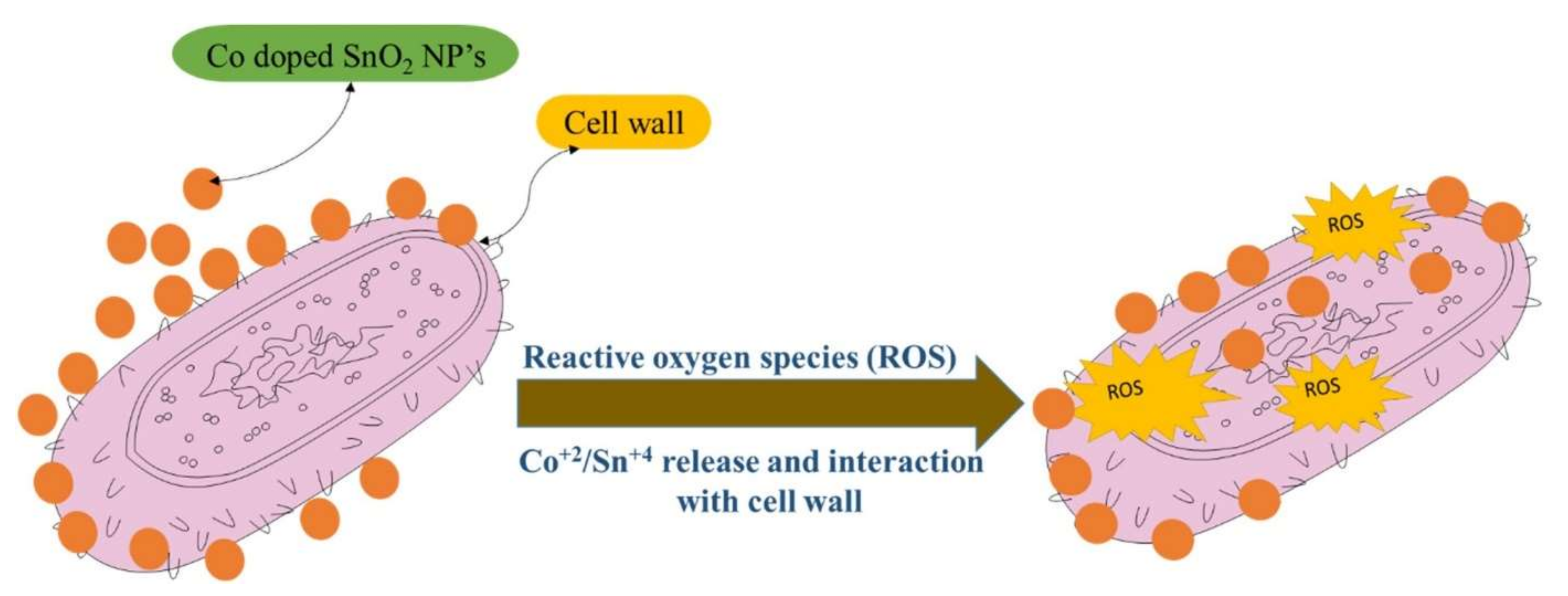
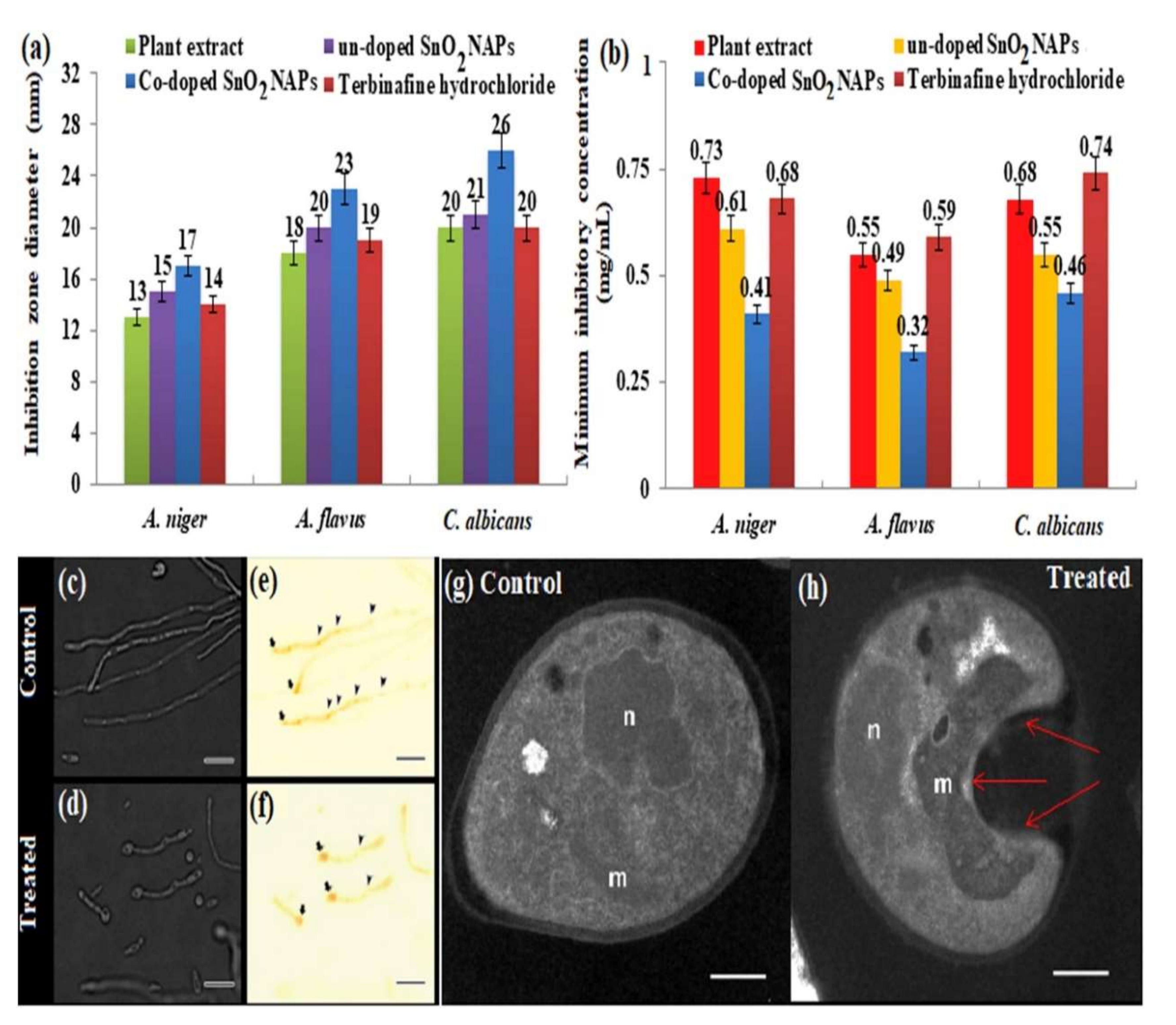
6. Biological Applications
7. Further Applications and Future Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baranowska-Korczyc, A.; Mackiewicz, E.; Ranoszek-Soliwoda, K.; Grobelny, J.; Celichowski, G. Core/shell ag/SnO2 nanowires for visible light photocatalysis. Catalysts 2022, 12, 30. [Google Scholar] [CrossRef]
- Dohčević-Mitrovic, Z.D.; Araújo, V.D.; Radović, M.; Aškrabić, S.; Costa, G.R.; Bernardi, M.I.B.; Djokić, D.M.; Stojadinović, B.; Nikolić, M.G. Influence of oxygen vacancy defects and cobalt doping on optical, electronic and photocatalytic properties of ultrafine SnO2-δ nanocrystals. Process. Appl. Ceram. 2020, 14, 102–112. [Google Scholar] [CrossRef]
- Manjula, N.; Selvan, G.; Balu, A.R. Photocatalytic Performance of SnO2:Mo Nanopowders Against the Degradation of Methyl Orange and Rhodamine B Dyes Under Visible Light Irradiation. J. Electron. Mater. 2019, 48, 401–408. [Google Scholar] [CrossRef]
- Chu, L.; Duo, F.; Zhang, M.; Wu, Z.; Sun, Y.; Wang, C.; Dong, S.; Sun, J. Doping induced enhanced photocatalytic performance of SnO2:Bi3+ quantum dots toward organic pollutants. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 589, 124416. [Google Scholar] [CrossRef]
- Khatoon, Z.; Fouad, H.; Alothman, O.Y.; Hashem, M.; Ansari, Z.A.; Ansari, S.A. Doped SnO2 nanomaterials for e-nose based electrochemical sensing of biomarkers of lung cancer. ACS Omega 2020, 5, 27645–27654. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Li, J.; Yang, F.; Cao, K.; Bao, Q.; Sun, Y.; Yuan, J. Antimony-Doped Tin Oxide Nanocrystals for Enhanced Photothermal Theragnosis Therapy of Cancers. Front. Bioeng. Biotechnol. 2020, 8, 673. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, U.; Jagtap, S. Electrochemical studies on doped SnO2 nanocomposite for selective detection of lung cancer biomarkers. AIP Adv. 2021, 11, 125327. [Google Scholar] [CrossRef]
- Kwak, C.H.; Kim, T.H.; Jeong, S.Y.; Yoon, J.W.; Kim, J.S.; Lee, J.H. Humidity-Independent Oxide Semiconductor Chemiresistors Using Terbium-Doped SnO2 Yolk-Shell Spheres for Real-Time Breath Analysis. ACS Appl. Mater. Interfaces 2018, 10, 18886–18894. [Google Scholar] [CrossRef]
- Shin, J.; Choi, S.J.; Lee, I.; Youn, D.Y.; Park, C.O.; Lee, J.H.; Tuller, H.L.; Kim, I.D. Thin-wall assembled SnO2 fibers functionalized by catalytic Pt nanoparticles and their superior exhaled-breath-sensing properties for the diagnosis of diabetes. Adv. Funct. Mater. 2013, 23, 2357–2367. [Google Scholar] [CrossRef]
- Shariati, M. The field effect transistor DNA biosensor based on ITO nanowires in label-free hepatitis B virus detecting compatible with CMOS technology. Biosens. Bioelectron. 2018, 105, 58–64. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Nguyen, H.H.; Mai, A.T. On-chip selective growth of SnO2 nanowires for DNA sensor development. Sens. Actuators A Phys. 2020, 312, 112171. [Google Scholar] [CrossRef]
- Kandasamy, M.; Seetharaman, A.; Sivasubramanian, D.; Nithya, A.; Jothivenkatachalam, K.; Maheswari, N.; Gopalan, M.; Dillibabu, S.; Eftekhari, A. Ni-Doped SnO2 Nanoparticles for Sensing and Photocatalysis. ACS Appl. Nano Mater. 2018, 1, 5823–5836. [Google Scholar] [CrossRef]
- Babu, B.; Neelakanta Reddy, I.; Yoo, K.; Kim, D.; Shim, J. Bandgap tuning and XPS study of SnO2 quantum dots. Mater. Lett. 2018, 221, 211–215. [Google Scholar] [CrossRef]
- Carolin, L.R.; Samuel, S.S.A. Hierarchical nanostructures of In–SnO2 with enhanced photo catalytic activity for the degradation of RR 120 dye. J. Mater. Sci. Mater. Electron. 2020, 31, 12796–12806. [Google Scholar] [CrossRef]
- Jain, S.K.; Fazil, M.; Naaz, F.; Pandit, N.A.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Silver-doped SnO2nanostructures for photocatalytic water splitting and catalytic nitrophenol reduction. New J. Chem. 2022, 46, 2846–2857. [Google Scholar] [CrossRef]
- Babu, B.; Kadam, A.N.; Ravikumar, R.V.S.S.N.; Byon, C. Enhanced visible light photocatalytic activity of Cu-doped SnO2 quantum dots by solution combustion synthesis. J. Alloys Compd. 2017, 703, 330–336. [Google Scholar] [CrossRef]
- Ragupathy, S.; Manikandan, V.; Devanesan, S.; Ahmed, M.; Ramamoorthy, M.; Priyadharsan, A. Enhanced sun light driven photocatalytic activity of Co doped SnO2 loaded corn cob activated carbon for methylene blue dye degradation. Chemosphere 2022, 295, 133848. [Google Scholar] [CrossRef]
- Kumar, S.; Bhawna; Yadav, S.K.; Gupta, A.; Kumar, R.; Ahmed, J.; Chaudhary, M.; Suhas; Kumar, V. B-doped SnO2 nanoparticles: A new insight into the photocatalytic hydrogen generation by water splitting and degradation of dyes. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- Chandran, D.; Nair, L.S.; Balachandran, S.; Rajendra Babu, K.; Deepa, M. Structural, optical, photocatalytic, and antimicrobial activities of cobalt-doped tin oxide nanoparticles. J. Sol-Gel Sci. Technol. 2015, 76, 582–591. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, L. Synthesis and characterization of antimony-doped tin oxide (ATO) nanoparticles by a new hydrothermal method. Mater. Chem. Phys. 2004, 87, 10–13. [Google Scholar] [CrossRef]
- Wang, J.; Pang, Z.; Jin, F.; Ge, M. Precipitation method to prepare conductive F-doped SnO2-coated rutile TiO2 whisker and its application in coating. J. Mater. Sci. Mater. Electron. 2021, 32, 20583–20597. [Google Scholar] [CrossRef]
- Pan, S.; Li, G. Recent Progress in p-Type Doping and Optical Properties of SnO2 Nanostructures for Optoelectronic Device Applications. Recent Pat. Nanotechnol. 2011, 5, 138–161. [Google Scholar] [CrossRef] [PubMed]
- Chaparadza, A.; Rananavare, S.B. Towards p-type conductivity in SnO2 nanocrystals through Li doping. Nanotechnology 2010, 21, 035708. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.S.; Li, G.H.; Wang, L.B.; Shen, Y.D.; Wang, Y.; Mei, T.; Hu, X. Atomic nitrogen doping and p-type conduction in SnO2. Appl. Phys. Lett. 2009, 95, 10–13. [Google Scholar] [CrossRef]
- Callister, W.D.J.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; ISBN 9780470448649. [Google Scholar]
- White, M.A. Properties of Materials; Oxford University Press: Oxford, UK, 1999; 334p. [Google Scholar]
- Wang, L.; Wang, Y.; Su, D.; Zhao, Y. Enhancement of visible light photocatalytic activity over bistructural SnO2 nanobelts. Superlattices Microstruct. 2018, 114, 416–420. [Google Scholar] [CrossRef]
- Umar, A.; Ammar, H.Y.; Kumar, R.; Almas, T.; Ibrahim, A.A.; AlAssiri, M.S.; Abaker, M.; Baskoutas, S. Efficient H2 gas sensor based on 2D SnO2 disks: Experimental and theoretical studies. Int. J. Hydrogen Energy 2020, 45, 26388–26401. [Google Scholar] [CrossRef]
- Zhang, F.; Teng, X.; Shi, W.; Song, Y.; Zhang, J.; Wang, X.; Li, H.; Li, Q.; Li, S.; Hu, H. SnO2 nanoflower arrays on an amorphous buffer layer as binder-free electrodes for flexible lithium-ion batteries. Appl. Surf. Sci. 2020, 527, 146910. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Dong, L.; Wang, J. Fabricating pod-like SnO2 hierarchical micro-nanostructures for enhanced acetone gas detection. Mater. Sci. Semicond. Process. 2021, 121, 105451. [Google Scholar] [CrossRef]
- Geraldo, V.; Briois, V.; Scalvi, L.V.A.; Santilli, C.V. EXAFS investigation on Sb incorporation effects to electrical transport in SnO2 thin films deposited by sol-gel. J. Eur. Ceram. Soc. 2007, 27, 4265–4268. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Vermeire, F.H.; Green, W.H. Transfer learning for solvation free energies: From quantum chemistry to experiments. Chem. Eng. J. 2021, 418, 129307. [Google Scholar] [CrossRef]
- Filippatos, P.-P.; Kelaidis, N.; Vasilopoulou, M.; Davazoglou, D.; Chroneos, A. Impact of boron and indium doping on the structural, electronic and optical properties of SnO2. Sci. Rep. 2021, 11, 13031. [Google Scholar] [CrossRef] [PubMed]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Bloch, F. Über die Quantenmechanik der Elektronen in Kristallgittern. Zeitschrift für Phys. 1929, 52, 555–600. [Google Scholar] [CrossRef]
- Kratzer, P.; Neugebauer, J. The Basics of Electronic Structure Theory for Periodic Systems. Front. Chem. 2019, 7, 106. [Google Scholar] [CrossRef]
- Fritsch, D.; Morgan, B.J.; Walsh, A. Self-Consistent Hybrid Functional Calculations: Implications for Structural, Electronic, and Optical Properties of Oxide Semiconductors. Nanoscale Res. Lett. 2017, 12, 19. [Google Scholar] [CrossRef]
- Gilani, R.; Rehman, S.U.; Butt, F.K.; Ul Haq, B.; Aleem, F. Elucidating the First-Principles Calculations of SnO2 Within DFT Framework and Beyond: A Library for Optimization of Various Pseudopotentials. Silicon 2018, 10, 2317–2328. [Google Scholar] [CrossRef]
- Gracia, L.; Beltrán, A.; Andrés, J. Characterization of the High-Pressure Structures and Phase Transformations in SnO2. A Density Functional Theory Study. J. Phys. Chem. B 2007, 111, 6479–6485. [Google Scholar] [CrossRef]
- Borges, P.D.; Scolfaro, L.M.R.; Leite Alves, H.W.; da Silva, E.F. DFT study of the electronic, vibrational, and optical properties of SnO2. Theor. Chem. Acc. 2010, 126, 39–44. [Google Scholar] [CrossRef]
- Beltrán, A.; Andrés, J.; Longo, E.; Leite, E.R. Thermodynamic argument about SnO2 nanoribbon growth. Appl. Phys. Lett. 2003, 83, 635–637. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, Y.; Yi, D.; Sun, S.; Yu, Z. Environment-dependent surface structures and stabilities of SnO2 from the first principles. J. Appl. Phys. 2012, 111, 63504. [Google Scholar] [CrossRef]
- Oviedo, J.; Gillan, M.J. Energetics and structure of stoichiometric SnO2 surfaces studied by first-principles calculations. Surf. Sci. 2000, 463, 93–101. [Google Scholar] [CrossRef]
- Laranjeira, J.A.S.; Fabris, G.S.L.; Ferrer, M.M.; Albuquerque, A.R.; Sambrano, J.R. Morphological Transformation Network of Nanoparticles via DFT Simulations. Cryst. Growth Des. 2020, 20, 4600–4611. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Shi, C.; Li, L.; Qin, H.; Hu, J. Sensing mechanism of SnO2(1 1 0) surface to H2: Density functional theory calculations. Sens. Actuators B Chem. 2015, 220, 279–287. [Google Scholar] [CrossRef]
- Maleki, F.; Pacchioni, G. A DFT study of formic acid decomposition on the stoichiometric SnO2 surface as a function of iso-valent doping. Surf. Sci. 2022, 718, 122009. [Google Scholar] [CrossRef]
- García, E.V.; Carbajal-Franco, G.; López-Galán, O.A. DFT transition state study of the catalyzed oxidation of methane on SnO2 surfaces. Catal. Today 2021, 392–393, 41–48. [Google Scholar] [CrossRef]
- Ganose, A.M.; Scanlon, D.O. Band gap and work function tailoring of SnO2 for improved transparent conducting ability in photovoltaics. J. Mater. Chem. C 2016, 4, 1467–1475. [Google Scholar] [CrossRef]
- Awa, K.; Akashi, R.; Akita, A.; Naya, S.; Kobayashi, H.; Tada, H. Highly Efficient and Selective Oxidation of Ethanol to Acetaldehyde by a Hybrid Photocatalyst Consisting of SnO2 Nanorod and Rutile TiO2 with Heteroepitaxial Junction. ChemPhysChem 2019, 20, 2155–2161. [Google Scholar] [CrossRef]
- Beloufa, N.; Cherchab, Y.; Louhibi-Fasla, S.; Daoud, S.; Rekab-Djabri, H.; Chahed, A. Theoretical investigation of structural, electronic and optical properties of Sc-doped SnO2. Comput. Condens. Matter 2022, 30, e00642. [Google Scholar] [CrossRef]
- Medina Chanduvi, H.H.; Mudarra Navarro, A.M.; Bilovol, V.; Errico, L.A.; Gil Rebaza, A.V. Structural, Electronic, Magnetic, and Hyperfine Properties of V-doped SnO2 (Sn1–xVxO2, x: 0, 0.042, 0.084, and 0.125). A DFT-Based Study. J. Phys. Chem. C 2021, 125, 11702–11713. [Google Scholar] [CrossRef]
- Albanese, E.; Di Valentin, C.; Pacchioni, G.; Sauvage, F.; Livraghi, S.; Giamello, E. Nature of Paramagnetic Species in Nitrogen-Doped SnO2: A Combined Electron Paramagnetic Resonance and Density Functional Theory Study. J. Phys. Chem. C 2015, 119, 26895–26903. [Google Scholar] [CrossRef]
- Nath, P.; Chakraborti, A.; Sanyal, D. Ab initio calculation of magnetic properties of p-block element doped ZnO. RSC Adv. 2014, 4, 45598–45602. [Google Scholar] [CrossRef]
- Luitel, H.; Roy, S.; Sanyal, D. Ab-initio calculation of the magnetic properties of P and As doped SnO2. Comput. Condens. Matter 2018, 14, 36–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wei, Z.; Liu, Q.; Wang, C.; Ma, J. Electrochemical CO2 reduction over nitrogen-doped SnO2 crystal surfaces. J. Energy Chem. 2019, 33, 22–30. [Google Scholar] [CrossRef]
- Li, W.; Ding, C.; Li, J.; Ren, Q.; Bai, G.; Xu, J. Sensing mechanism of Sb, S doped SnO2 (1 1 0) surface for CO. Appl. Surf. Sci. 2020, 502, 144140. [Google Scholar] [CrossRef]
- Náfrádi, M.; Alapi, T.; Bencsik, G.; Janáky, C. Impact of reaction parameters and water matrices on the removal of organic pollutants by TiO2 /led and ZnO/led heterogeneous photocatalysis using 365 and 398 nm radiation. Nanomaterials 2022, 12, 5. [Google Scholar] [CrossRef]
- De Almeida, J.C.; Correâ, M.T.; Koga, R.H.; Del Duque, D.M.S.; Lopes, O.F.; Da Silva, G.T.S.T.; Ribeiro, C.; De Mendoncą, V.R. Crystallization time in ZnO: The role of surface OH groups in its photoactivity. New J. Chem. 2020, 44, 18216–18224. [Google Scholar] [CrossRef]
- de Mendonça, V.R.; Avansi, W.; Arenal, R.; Ribeiro, C. A building blocks strategy for preparing photocatalytically active anatase TiO2/rutile SnO2 heterostructures by hydrothermal annealing. J. Colloid Interface Sci. 2017, 505, 454–459. [Google Scholar] [CrossRef]
- Silva, G.; Lopes, O.; Dias, E.; Torres, J.; Nogueira, A.; Faustino, L.; Prado, F.; Patrocínio, A.; Ribeiro, C. ReduÇÃo de CO2 em hidrocarbonetos e oxigenados: Fundamentos, estrategias e desafios. Quim. Nova 2021, 44, 963–981. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, L.; Zhang, W. Preparation of Cl doped SnO powder with excellent photocatalytic property by mechanical alloying. Ceram. Int. 2019, 45, 8908–8913. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Luque, R. Heterogeneous photocatalysis. ChemEngineering 2021, 5, 26. [Google Scholar] [CrossRef]
- Mubeen, S.; Singh, N.; Lee, J.; Stucky, G.D.; Moskovits, M.; McFarland, E.W. Synthesis of chemicals using solar energy with stable photoelectrochemically active heterostructures. Nano Lett. 2013, 13, 2110–2115. [Google Scholar] [CrossRef] [PubMed]
- Ali Baig, A.B.; Rathinam, V.; Ramya, V. Facile fabrication of Zn-doped SnO2 nanoparticles for enhanced photocatalytic dye degradation performance under visible light exposure. Adv. Compos. Hybrid Mater. 2021, 4, 114–126. [Google Scholar] [CrossRef]
- Castro, S.; Albo, J.; Irabien, A. Photoelectrochemical Reactors for CO2 Utilization. ACS Sustain. Chem. Eng. 2018, 6, 15877–15894. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Han, Y.; Wu, X.; Ma, Y.; Gong, L.; Qu, F.; Fan, H. Porous SnO2 nanowire bundles for photocatalyst and Li ion battery applications. CrystEngComm 2011, 13, 3506–3510. [Google Scholar] [CrossRef]
- Vadivel, S.; Rajarajan, G. Effect of W doping on structural, optical and photocatalytic activity of SnO2 nanostructure thin films. J. Mater. Sci. Mater. Electron. 2015, 26, 7127–7133. [Google Scholar] [CrossRef]
- Parthibavarman, M.; Sathishkumar, S.; Jayashree, M.; BoopathiRaja, R. Microwave Assisted Synthesis of Pure and Ag Doped SnO2 Quantum Dots as Novel Platform for High Photocatalytic Activity Performance. J. Clust. Sci. 2019, 30, 351–363. [Google Scholar] [CrossRef]
- Sery, A.A.; Mohamed, W.A.A.; Hammad, F.F.; Khalil, M.M.H.; Farag, H.K. Synthesis of pure and doped SnO2 and NiO nanoparticles and evaluation of their photocatalytic activity. Mater. Chem. Phys. 2022, 275, 125190. [Google Scholar] [CrossRef]
- Ahmed, A.; Naseem Siddique, M.; Alam, U.; Ali, T.; Tripathi, P. Improved photocatalytic activity of Sr doped SnO2 nanoparticles: A role of oxygen vacancy. Appl. Surf. Sci. 2019, 463, 976–985. [Google Scholar] [CrossRef]
- Wrighton, M.S.; Morse, D.L.; Ellis, A.B.; Ginley, D.S.; Abrahamson, H.B. Photoassisted Electrolysis of Water by Ultraviolet Irradiation of an Antimony Doped Stannic Oxide Electrode. J. Am. Chem. Soc. 1976, 98, 44–48. [Google Scholar] [CrossRef]
- Asaithambi, S.; Sakthivel, P.; Karuppaiah, M.; Murugan, R.; Yuvakkumar, R.; Ravi, G. Preparation of SnO2 Nanoparticles with Addition of Co Ions for Photocatalytic Activity of Brilliant Green Dye Degradation. J. Electron. Mater. 2019, 48, 2183–2194. [Google Scholar] [CrossRef]
- Cao, Y.; Huang, L.; Bai, Y.; Jermsittiparsert, K.; Hosseinzadeh, R.; Rasoulnezhad, H.; Hosseinzadeh, G. Synergic effect of oxygen vacancy defect and shape on the photocatalytic performance of nanostructured TiO2 coating. Polyhedron 2020, 175, 114214. [Google Scholar] [CrossRef]
- Matović, B.; Luković, J.; Stojadinović, B.; Aškrabić, S.; Zarubica, A.; Babić, B.; Dohčević-Mitrović, Z. Influence of Mg doping on structural, optical and photocatalytic performances of ceria nanopowders. Process. Appl. Ceram. 2017, 11, 304–310. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.; Duan, L.; Shen, H.; Liu, R. Controlling oxygen vacancies and enhanced visible light photocatalysis of CeO2/ZnO nanocomposites. J. Photochem. Photobiol. A Chem. 2020, 392, 112156. [Google Scholar] [CrossRef]
- Liu, J.; Ji, X.; Shi, J.; Wang, L.; Jian, P.; Yan, X.; Wang, D. Experimental and theoretical investigation of the tuning of electronic structure in SnO2 via Co doping for enhanced styrene epoxidation catalysis. Catal. Sci. Technol. 2022, 12, 1499–1511. [Google Scholar] [CrossRef]
- Xu, X.; Ding, X.; Yang, X.; Wang, P.; Li, S.; Lu, Z.; Chen, H. Oxygen vacancy boosted photocatalytic decomposition of ciprofloxacin over Bi2MoO6: Oxygen vacancy engineering, biotoxicity evaluation and mechanism study. J. Hazard. Mater. 2019, 364, 691–699. [Google Scholar] [CrossRef]
- Karmakar, K.; Sarkar, A.; Mandal, K.; Khan, G.G. Investigating the Role of Oxygen Vacancies and Lattice Strain Defects on the Enhanced Photoelectrochemical Property of Alkali Metal (Li, Na, and K) Doped ZnO Nanorod Photoanodes. ChemElectroChem 2018, 5, 1147–1152. [Google Scholar] [CrossRef]
- Putri, N.A.; Fauzia, V.; Iwan, S.; Roza, L.; Umar, A.A.; Budi, S. Mn-doping-induced photocatalytic activity enhancement of ZnO nanorods prepared on glass substrates. Appl. Surf. Sci. 2018, 439, 285–297. [Google Scholar] [CrossRef]
- Ali Baig, A.B.; Rathinam, V.; Palaninathan, J. Facile synthesis of Ce-doped SnO2 nanoparticles with enhanced performance for photocatalytic degradation of organic dye. J. Iran. Chem. Soc. 2021, 18, 13–27. [Google Scholar] [CrossRef]
- Song, X.; Qin, G.; Cheng, G.; Jiang, W.; Chen, X.; Dai, W.; Fu, X. Oxygen defect-induced NO− intermediates promoting NO deep oxidation over Ce doped SnO2 under visible light. Appl. Catal. B Environ. 2021, 284, 119761. [Google Scholar] [CrossRef]
- Nascimento, E.P.; Firmino, H.C.T.; Neves, G.A.; Menezes, R.R. A review of recent developments in tin dioxide nanostructured materials for gas sensors. Ceram. Int. 2022, 48, 7405–7440. [Google Scholar] [CrossRef]
- Chen, N.; Liu, B.; Zhang, P.; Wang, C.; Du, Y.; Chang, W.; Hong, W. Enhanced photocatalytic performance of Ce-doped SnO2 hollow spheres by a one-pot hydrothermal method. Inorg. Chem. Commun. 2021, 132, 108848. [Google Scholar] [CrossRef]
- Li, Y.; Gao, S.; Zhang, B.; Mao, H.; Tang, X. Electrospun Ag-Doped SnO2 Hollow Nanofibers with High Antibacterial Activity. Electron. Mater. Lett. 2020, 16, 195–206. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Xue, Y.; Zhou, M.; Cao, R.; Chen, P.; Zeng, X. Hierarchical SnO2 hollow nanotubes as anodes for high performance lithium-ion battery. J. Mater. Sci. Mater. Electron. 2021, 32, 22944–22952. [Google Scholar] [CrossRef]
- Sun, S.H.; Meng, G.W.; Zhang, G.X.; Gao, T.; Geng, B.Y.; Zhang, L.D.; Zuo, J. Raman scattering study of rutile SnO2 nanobelts synthesized by thermal evaporation of Sn powders. Chem. Phys. Lett. 2003, 376, 103–107. [Google Scholar] [CrossRef]
- Park, J.Y.; Asokan, K.; Choi, S.W.; Kim, S.S. Growth kinetics of nanograins in SnO2 fibers and size dependent sensing properties. Sens. Actuators B Chem. 2011, 152, 254–260. [Google Scholar] [CrossRef]
- Wang, A.R.; Xiao, H. Controllable preparation of SnO2 nanoplates and nanoparticles via hydrothermal oxidation of SnS2 nanoplates. Mater. Lett. 2009, 63, 1221–1223. [Google Scholar] [CrossRef]
- Ma, X.; Song, H.; Guan, C. Interfacial oxidation-dehydration induced formation of porous SnO2 hollow nanospheres and their gas sensing properties. Sens. Actuators B Chem. 2013, 177, 196–204. [Google Scholar] [CrossRef]
- Man, J.; Liu, K.; Du, Y.; Sun, J. Self-assemble SnO2 porous nanotubes as high-performance anodes for lithium-ion batteries. Mater. Chem. Phys. 2020, 256, 123669. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Ahmaruzzaman, M. A novel and green process for the production of tin oxide quantum dots and its application as a photocatalyst for the degradation of dyes from aqueous phase. J. Colloid Interface Sci. 2015, 448, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Sik Choi, M.; Ahn, J.; Young Kim, M.; Mirzaei, A.; Choi, S.M.; Won Chun, D.; Jin, C.; Hyoung Lee, K. Changes in the crystal structure of SnO2 nanoparticles and improved H2S gas-sensing characteristics by Al doping. Appl. Surf. Sci. 2021, 565, 150493. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Rafiqul Bari, G.A.K.M.; Vattikuti, S.V.P.; Kim, H. Synthesis of carbon-doped SnO2 nanostructures for visible-light-driven photocatalytic hydrogen production from water splitting. Int. J. Hydrogen Energy 2020, 45, 32789–32796. [Google Scholar] [CrossRef]
- Entradas, T.; Cabrita, J.F.; Dalui, S.; Nunes, M.R.; Monteiro, O.C.; Silvestre, A.J. Synthesis of sub-5 nm Co-doped SnO2 nanoparticles and their structural, microstructural, optical and photocatalytic properties. Mater. Chem. Phys. 2014, 147, 563–571. [Google Scholar] [CrossRef]
- Reddy, C.V.; Babu, B.; Shim, J. Synthesis of Cr-doped SnO2 quantum dots and its enhanced photocatalytic activity. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2017, 223, 131–142. [Google Scholar] [CrossRef]
- Sagadevan, S.; Chowdhury, Z.Z.; Bin Johan, M.R.; Aziz, F.A.; Roselin, L.S.; Podder, J.; Lett, J.A.; Selvin, R. Cu-Doped SnO2 Nanoparticles: Synthesis and Properties. J. Nanosci. Nanotechnol. 2019, 19, 7139–7148. [Google Scholar] [CrossRef]
- Kaur, H.; Bhatti, H.S.; Singh, K. Europium doping effect on 3D flower-like SnO2 nanostructures: Morphological changes, photocatalytic performance and fluorescence detection of heavy metal ion contamination in drinking water. RSC Adv. 2019, 9, 37450–37466. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, J.; Chen, H.; Qu, Y.; Deng, Q.; Lin, Z. One-pot synthesis of echinus-like Fe-doped SnO2 with enhanced photocatalytic activity under simulated sunlight. J. Alloys Compd. 2017, 695, 3318–3323. [Google Scholar] [CrossRef]
- Arif, H.S.; Murtaza, G.; Hanif, H.; Ali, H.S.; Yaseen, M.; Khalid, N.R. Effect of La on structural and photocatalytic activity of SnO2 nanoparticles under UV irradiation. J. Environ. Chem. Eng. 2017, 5, 3844–3851. [Google Scholar] [CrossRef]
- Song, J.; Xu, X.; Wu, J.; Lan, Z. Low-temperature solution-processing high quality Nb-doped SnO2 nanocrystals-based electron transport layers for efficient planar perovskite solar cells. Funct. Mater. Lett. 2019, 12, 1850091. [Google Scholar] [CrossRef]
- Chen, H.; Ding, L.; Sun, W.; Jiang, Q.; Hu, J.; Li, J. Synthesis and characterization of Ni doped SnO2 microspheres with enhanced visible-light photocatalytic activity. RSC Adv. 2015, 5, 56401–56409. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Yao, Y.W.; Gao, J.J.; Liu, Z.D.; Zhou, J. Photocatalytic Activity of Pd Doped Tin Dioxide Inverse Opal Films. Adv. Mater. Res. 2012, 534, 135–140. [Google Scholar] [CrossRef]
- Ma, L.; Xu, L.; Xu, X.; Zhou, X.; Zhang, L. One-Pot Hydrothermal Synthesis of Sulfur-Doped SnO2 Nanoparticles and their Enhanced Photocatalytic Properties. Nano 2016, 11, 1650035. [Google Scholar] [CrossRef]
- Letifi, H.; Dridi, D.; Litaiem, Y.; Ammar, S.; Dimassi, W.; Chtourou, R. High efficient and cost effective titanium doped tin dioxide based photocatalysts synthesized via co-precipitation approach. Catalysts 2021, 11, 803. [Google Scholar] [CrossRef]
- Reddy, C.V.; Babu, B.; Vattikuti, S.V.P.; Ravikumar, R.V.S.S.N.; Shim, J. Structural and optical properties of vanadium doped SnO2 nanoparticles with high photocatalytic activities. J. Lumin. 2016, 179, 26–34. [Google Scholar] [CrossRef]
- Ullah, I.; Munir, A.; Muhammad, S.; Ali, S.; Khalid, N.; Zubair, M.; Sirajuddin, M.; Hussain, S.Z.; Ahmed, S.; Khan, Y.; et al. Influence of W-doping on the optical and electrical properties of SnO2 towards photocatalytic detoxification and electrocatalytic water splitting. J. Alloys Compd. 2020, 827, 154247. [Google Scholar] [CrossRef]
- Ali Baig, A.B.; Rathinam, V.; Palaninathan, J. Photodegradation activity of yttrium-doped SnO2 nanoparticles against methylene blue dye and antibacterial effects. Appl. Water Sci. 2020, 10, 76. [Google Scholar] [CrossRef]
- Ben Soltan, W.; Ammar, S.; Olivier, C.; Toupance, T. Influence of zinc doping on the photocatalytic activity of nanocrystalline SnO2 particles synthesized by the polyol method for enhanced degradation of organic dyes. J. Alloys Compd. 2017, 729, 638–647. [Google Scholar] [CrossRef]
- Suthakaran, S.; Dhanapandian, S.; Krishnakumar, N.; Ponpandian, N.; Dhamodharan, P.; Anandan, M. Surfactant-assisted hydrothermal synthesis of Zr doped SnO2 nanoparticles with photocatalytic and supercapacitor applications. Mater. Sci. Semicond. Process. 2020, 111, 104982. [Google Scholar] [CrossRef]
- Costa, I.M.; Colmenares, Y.N.; Pizani, P.S.; Leite, E.R.; Chiquito, A.J. Sb doping of VLS synthesized SnO2 nanowires probed by Raman and XPS spectroscopy. Chem. Phys. Lett. 2018, 695, 125–130. [Google Scholar] [CrossRef]
- Parthibavarman, M.; Sathishkumar, S.; Prabhakaran, S.; Jayashree, M.; BoopathiRaja, R. High visible light-driven photocatalytic activity of large surface area Cu doped SnO2 nanorods synthesized by novel one-step microwave irradiation method. J. Iran. Chem. Soc. 2018, 15, 2789–2801. [Google Scholar] [CrossRef]
- Zhao, Q.; Ju, D.; Deng, X.; Huang, J.; Cao, B.; Xu, X. Morphology-modulation of SnO2 hierarchical architectures by Zn doping for glycol gas sensing and photocatalytic applications. Sci. Rep. 2015, 5, 2–10. [Google Scholar] [CrossRef]
- Stroppa, D.G.; Montoro, L.A.; Beltrán, A.; Conti, T.G.; Da Silva, R.O.; Andrés, J.; Longo, E.; Leite, E.R.; Ramirez, A.J. Unveiling the chemical and morphological features of Sb-SnO2 nanocrystals by the combined use of high-resolution transmission electron microscopy and ab initio surface energy calculations. J. Am. Chem. Soc. 2009, 131, 14544–14548. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, J.; Xu, F.; Wang, A.; Meng, D. Zn-doped SnO2 hierarchical structures formed by a hydrothermal route with remarkably enhanced photocatalytic performance. Ceram. Int. 2018, 44, 15145–15152. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Xu, M.; Cui, Y.; Ren, W.; Zhang, J.; Zhao, H.; Liang, B. Recent intensification strategies of SnO2-based photocatalysts: A review. Chem. Eng. J. 2022, 427, 131564. [Google Scholar] [CrossRef]
- Crippa, M.; Oreggioni, G.D.G.; Muntean, M.; Schaaf, E.; Lo Vullo, E.; Solazzo, E.; Monforti-Ferrario, F.; Olivier, J.G.; Vignati, E. Fossil CO2 and GHG Emissions of all World Countries—2019 Report Publications Office of the EU. JRC Sci. Policy Rep. 2019, 251. [Google Scholar] [CrossRef]
- Júnior, R.C.d.S.; Nogueira, A.E.; Giroto, A.S.; Torres, J.A.; Ribeiro, C.; Siqueira, K.P.F. Microwave-assisted synthesis of Ca1-xMnxMoO4 (x = 0, 0.2, 0.7, and 1) and its application in artificial photosynthesis. Ceram. Int. 2021, 47, 5388–5398. [Google Scholar] [CrossRef]
- Nogueira, A.E.; da Silva, G.T.S.T.; Oliveira, J.A.; Torres, J.A.; da Silva, M.G.S.; Carmo, M.; Ribeiro, C. Unveiling CuO role in CO2 photoreduction process—Catalyst or reactant? Catal. Commun. 2020, 137, 105929. [Google Scholar] [CrossRef]
- Studt, F.; Sharafutdinov, I.; Abild-Pedersen, F.; Elkjær, C.F.; Hummelshøj, J.S.; Dahl, S.; Chorkendorff, I.; Nørskov, J.K. Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nat. Chem. 2014, 6, 320–324. [Google Scholar] [CrossRef]
- Li, N.; Jiang, R.; Li, Y.; Zhou, J.; Ma, Q.; Shen, S.; Liu, M. Plasma-Assisted Photocatalysis of CH4 and CO2 into Ethylene. ACS Sustain. Chem. Eng. 2019, 7, 11455–11463. [Google Scholar] [CrossRef]
- Hu, X.; Yang, H.; Guo, M.; Gao, M.; Zhang, E.; Tian, H.; Liang, Z.; Liu, X. Synthesis and Characterization of (Cu, S) Co-doped SnO2 for Electrocatalytic Reduction of CO2 to Formate at Low Overpotential. ChemElectroChem 2018, 5, 1330–1335. [Google Scholar] [CrossRef]
- Hu, B.; Guild, C.; Suib, S.L. Thermal, electrochemical, and photochemical conversion of CO2 to fuels and value-added products. J. CO2 Util. 2013, 1, 18–27. [Google Scholar] [CrossRef]
- Ferreira de Brito, J.; Corradini, P.G.; Silva, A.B.; Mascaro, L.H. Reduction of CO2 by Photoelectrochemical Process Using Non-Oxide Two-Dimensional Nanomaterials—A Review. ChemElectroChem 2021, 8, 4305–4320. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Das, A.; Riyajuddin, S.; Ghosh, K.; Islam, S.M. Reduction of carbon dioxide with mesoporous SnO2 nanoparticles as active photocatalysts under visible light in water. Catal. Sci. Technol. 2019, 9, 6566–6569. [Google Scholar] [CrossRef]
- Torres, J.A.; Da Silva, G.T.S.T.; Barbosa de Freitas Silva, F.; Ribeiro, C. Experimental Evidence of CO2 Photoreduction Activity of SnO2 Nanoparticles. ChemPhysChem 2020, 21, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Baruch, M.F.; Pander, J.E.; White, J.L.; Bocarsly, A.B. Mechanistic Insights into the Reduction of CO2 on Tin Electrodes using in Situ ATR-IR Spectroscopy. ACS Catal. 2015, 5, 3148–3156. [Google Scholar] [CrossRef]
- Matussin, S.N.; Tan, A.L.; Harunsani, M.H.; Mohammad, A.; Cho, M.H.; Khan, M.M. Effect of Ni-doping on properties of the SnO2 synthesized using Tradescantia spathacea for photoantioxidant studies. Mater. Chem. Phys. 2020, 252, 123293. [Google Scholar] [CrossRef]
- Bhawna; Gupta, A.; Kumar, P.; Tyagi, A.; Kumar, R.; Kumar, A.; Singh, P.; Singh, R.P.; Kumar, V. Facile Synthesis of N-Doped SnO2 Nanoparticles: A Cocatalyst-Free Promising Photocatalyst for Hydrogen Generation. ChemistrySelect 2020, 5, 7775–7782. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Wongsakulphasatch, S.; Vo, D.V.N. Understanding the role of surface basic sites of catalysts in CO2 activation in dry reforming of methane: A short review. Catal. Sci. Technol. 2020, 10, 35–45. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Fan, M.; Wang, X.; Walbridge, M.L.; Nong, Q.; Wu, Y.; Zhao, L. Z-scheme SnO2-x/g-C3N4 composite as an efficient photocatalyst for dye degradation and photocatalytic CO2 reduction. Sol. Energy Mater. Sol. Cells 2015, 137, 175–184. [Google Scholar] [CrossRef]
- Ye, J.; Xu, J.; Tian, D.; Zhao, X.; Wang, Q.; Wang, J.; Li, Y.; Zhao, C.; Liu, Z.; Fu, Y. Efficient photocatalytic reduction of CO2 by a rhenium-doped TiO2-x/SnO2 inverse opal S-scheme heterostructure assisted by the slow-phonon effect. Sep. Purif. Technol. 2021, 277, 119431. [Google Scholar] [CrossRef]
- Lei, M.; Wu, W.; Sun, L.; Tian, Q.; Jiang, C.; Xiao, X. Controlled preparation of hollow SnO2@M (M = Au, Ag) heterostructures through template-assist method for enhanced photocatalysis. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 482, 276–282. [Google Scholar] [CrossRef]
- Li, Q.; Fu, J.; Zhu, W.; Chen, Z.; Shen, B.; Wu, L.; Xi, Z.; Wang, T.; Lu, G.; Zhu, J.J.; et al. Tuning Sn-Catalysis for Electrochemical Reduction of CO2 to CO via the Core/Shell Cu/SnO2 Structure. J. Am. Chem. Soc. 2017, 139, 4290–4293. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Lee, J.; Cho, M.H. Visible light-driven photocatalytic and photoelectrochemical studies of Ag-SnO2 nanocomposites synthesized using an electrochemically active biofilm. RSC Adv. 2014, 4, 26013–26021. [Google Scholar] [CrossRef]
- Betiha, M.A.; Rabie, A.M.; Ahmed, H.S.; Abdelrahman, A.A.; El-Shahat, M.F. Oxidative desulfurization using graphene and its composites for fuel containing thiophene and its derivatives: An update review. Egypt. J. Pet. 2018, 27, 715–730. [Google Scholar] [CrossRef]
- Muzic, M.; Sertic-Bionda, K.; Gomzi, Z.; Podolski, S.; Telen, S. Study of diesel fuel desulfurization by adsorption. Chem. Eng. Res. Des. 2010, 88, 487–495. [Google Scholar] [CrossRef]
- Chandra Srivastava, V. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv. 2012, 2, 759–783. [Google Scholar] [CrossRef]
- Environmental Protection Agency Control of Air Pollution From Motor Vehicles: Tier 3 Motor Vehicle Emission and Fuel Standards. Fed. Regist. 2014, 79, 23414–23886.
- World Health Organization. Health Effects of Transport-Related Air Pollution [Internet]; Krzyzanowski, M., Kuna-Dibbert, B., Schneider, J., Eds.; World Health Organization: Copenhagen Ø, Denmark, 2005; pp. 1–190. Available online: https://www.euro.who.int/__data/assets/pdf_file/0006/74715/E86650.pdf (accessed on 1 June 2022).
- Rajendran, A.; Cui, T.Y.; Fan, H.X.; Yang, Z.F.; Feng, J.; Li, W.Y. A comprehensive review on oxidative desulfurization catalysts targeting clean energy and environment. J. Mater. Chem. A 2020, 8, 2246–2285. [Google Scholar] [CrossRef]
- Liu, A.; Zhu, M.; Dai, B. A novel high-performance SnO2 catalyst for oxidative desulfurization under mild conditions. Appl. Catal. A Gen. 2019, 583, 117134. [Google Scholar] [CrossRef]
- Piao, W.; Li, Z.; Li, C.; Park, J.S.; Lee, J.H.; Li, Z.; Kim, K.Y.; Jin, L.Y.; Kim, J.M.; Jin, M. Efficient and reusable ordered mesoporous WOx/SnO2catalyst for oxidative desulfurization of dibenzothiophene. RSC Adv. 2021, 11, 27453–27460. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, A.; Fan, H.X.; Cui, T.Y.; Feng, J.; Li, W.Y. Enrichment of polymeric WOx species in WOx@SnO2 catalysts for ultra-deep oxidative desulfurization of liquid fuels. Fuel 2021, 290, 120036. [Google Scholar] [CrossRef]
- Zhang, G.; Ren, J.; Liu, B.; Tian, M.; Zhou, H.; Zhao, J. In situ hydrothermal preparation and photocatalytic desulfurization performance of metallophthalocyanine sensitized SnO2. Inorganica Chim. Acta 2018, 471, 782–787. [Google Scholar] [CrossRef]
- Ko, T.H.; Chu, H.; Chaung, L.K. The sorption of hydrogen sulfide from hot syngas by metal oxides over supports. Chemosphere 2005, 58, 467–474. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Y.; Cai, N. Simultaneous removal of COS and H2S from hot syngas by rare earth metal-doped SnO2 sorbents. Fuel 2016, 181, 1020–1026. [Google Scholar] [CrossRef]
- Babich, I.V.; Moulijn, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Mohanta, D.; Nath, A. Environmentally benign fabrication of SnO2-CNT nanohybrids and their multifunctional efficiency as an adsorbent, catalyst and antimicrobial agent for water decontamination. Sci. Rep. 2019, 9, 12935. [Google Scholar] [CrossRef]
- Nasir, Z.; Shakir, M.L.; Wahab, R.; Shoeb, M.; Alam, P.; Khan, R.H.; Mobin, M. Co-precipitation synthesis and characterization of Co doped SnO2 NPs, HSA interaction via various spectroscopic techniques and their antimicrobial and photocatalytic activities. Int. J. Biol. Macromol. 2017, 94, 554–565. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Khan, S.A.; Zaman, S.; Sarwar, M.N. Synthesis characterization, optical and antibacterial studies of Co-Doped SnO2 nanoparticles. Dig. J. Nanomater. Biostruct. 2017, 12, 1127–1135. [Google Scholar]
- Amutha, T.; Rameshbabu, M.; Sasi Florence, S.; Senthilkumar, N.; Vetha Potheher, I.; Prabha, K. Studies on structural and optical properties of pure and transition metals (Ni, Fe and co-doped Ni–Fe) doped tin oxide (SnO2) nanoparticles for anti-microbial activity. Res. Chem. Intermed. 2019, 45, 1929–1941. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Bhawna; Choudhary, A.K.; Gupta, A.; Kumar, S.; Kumar, P.; Singh, R.P.; Singh, P.; Kumar, V. Synthesis, Antimicrobial Activity, and Photocatalytic Performance of Ce Doped SnO2 Nanoparticles. Front. Nanotechnol. 2020, 2, 595352. [Google Scholar] [CrossRef]
- Ali Baig, A.B.; Rathinam, V.; Palaninathan, J. Fabrication of Zr-doped SnO2 nanoparticles with synergistic influence for improved visible-light photocatalytic action and antibacterial performance. Appl. Water Sci. 2020, 10, 54. [Google Scholar] [CrossRef]
- Roychaudhury, A.M.; Debnath, U.; Munshi, S.; Basak, G.K.; Dutta, A.; Masanta, S.; Singha, A.; Banerjee, A.; Ghosh, D.; Chatterjee, P.; et al. Synthesis Structural and Anti-microbial Characterization of Nanostructured Doped Tin Oxide. J. Theor. Appl. Phys. 2022, 16, 1–8. [Google Scholar]
- Thamiz Selvi, R.; Nallaiyan, M.; Nisha, S.; Sagadevan, S.; Mohammad, F.; Sadaiyandi, K.; Pradeev Raj, K.; Ashokkumar, L.; Latha, M.B.; Lett, J.A. Comparative studies on structural, optical, and biological properties of SnO2 and Ni-doped SnO2 nanocrystals. Mater. Res. Express 2019, 6, 125099. [Google Scholar] [CrossRef]
- John, N.; Somaraj, M.; Tharayil, N.J. Synthesis, Characterization and Anti—Bacterial Activities of Pure and Ag—Doped SnO2Nanoparticles. Mater. Today Proc. 2017, 4, 4351–4357. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Geethalakshmi, S. Enhanced photocatalytic and antibacterial activity of Cu:SnO2 nanoparticles synthesized by microwave assisted method. Mater. Today Proc. 2020, 20, 54–63. [Google Scholar] [CrossRef]
- Mala, N.; Ravichandran, K.; Pandiarajan, S.; Srinivasan, N.; Ravikumar, B.; Nithiyadevi, K. Enhanced antibacterial and photocatalytic activity of (Mg+Co) doped tin oxide nanopowders synthesised using wet chemical route. Mater. Technol. 2017, 32, 686–694. [Google Scholar] [CrossRef]
- Ali Baig, A.B.; Rathinam, V.; Ramya, V. Synthesis and Investigation of Fe doped SnO2 Nanoparticles for Improved Photocatalytic Activity under Visible Light and Antibacterial performances. Mater. Technol. 2021, 36, 623–635. [Google Scholar] [CrossRef]
- Vega-Jiménez, A.L.; Vázquez-Olmos, A.R.; Acosta-Gío, E.; Antonio Álvarez-Pérez, M. In vitro Antimicrobial Activity Evaluation of Metal Oxide Nanoparticles. In Nanoemulsions—Properties, Fabrications and Applications; IntechOpen: London, UK, 2019; p. 13. [Google Scholar]
- Pandey, M.; Wasnik, K.; Gupta, S.; Singh, M.; Patra, S.; Gupta, P.; Pareek, D.; Maity, S.; Tilak, R.; Paik, P. Targeted specific inhibition of bacterial and: Candida species by mesoporous Ag/Sn-SnO2composite nanoparticles: In silico and in vitro investigation. RSC Adv. 2022, 12, 1105–1120. [Google Scholar] [CrossRef]
- Khan, S.A.; Kanwal, S.; Rizwan, K.; Shahid, S. Enhanced antimicrobial, antioxidant, in vivo antitumor and in vitro anticancer effects against breast cancer cell line by green synthesized un-doped SnO2 and Co-doped SnO2 nanoparticles from Clerodendrum inerme. Microb. Pathog. 2018, 125, 366–384. [Google Scholar] [CrossRef]
- Obeizi, Z.; Benbouzid, H.; Bouarroudj, T. Excellent antimicrobial and anti-bio fi lm activities of Fe—SnO2 nanoparticles as promising antiseptics and disinfectants. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 1. [Google Scholar]
- Vignesh, K.; Hariharan, R.; Rajarajan, M.; Suganthi, A. Photocatalytic performance of Ag doped SnO2 nanoparticles modified with curcumin. Solid State Sci. 2013, 21, 91–99. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Neogi, S. Antibacterial properties of doped nanoparticles. Rev. Chem. Eng. 2019, 35, 861–876. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Gökçeli, G.; Karatepe, N. Improving the properties of indium tin oxide thin films by the incorporation of carbon nanotubes with solution-based techniques. Thin Solid Films 2020, 697, 137844. [Google Scholar] [CrossRef]
- Graužinyte, M.; Goedecker, S.; Flores-Livas, J.A. Computational Screening of Useful Hole-Electron Dopants in SnO2. Chem. Mater. 2017, 29, 10095–10103. [Google Scholar] [CrossRef]
- Williamson, B.A.D.; Featherstone, T.J.; Sathasivam, S.S.; Swallow, J.E.N.; Shiel, H.; Jones, L.A.H.; Smiles, M.J.; Regoutz, A.; Lee, T.L.; Xia, X.; et al. Resonant Ta Doping for Enhanced Mobility in Transparent Conducting SnO2. Chem. Mater. 2020, 32, 1964–1973. [Google Scholar] [CrossRef]
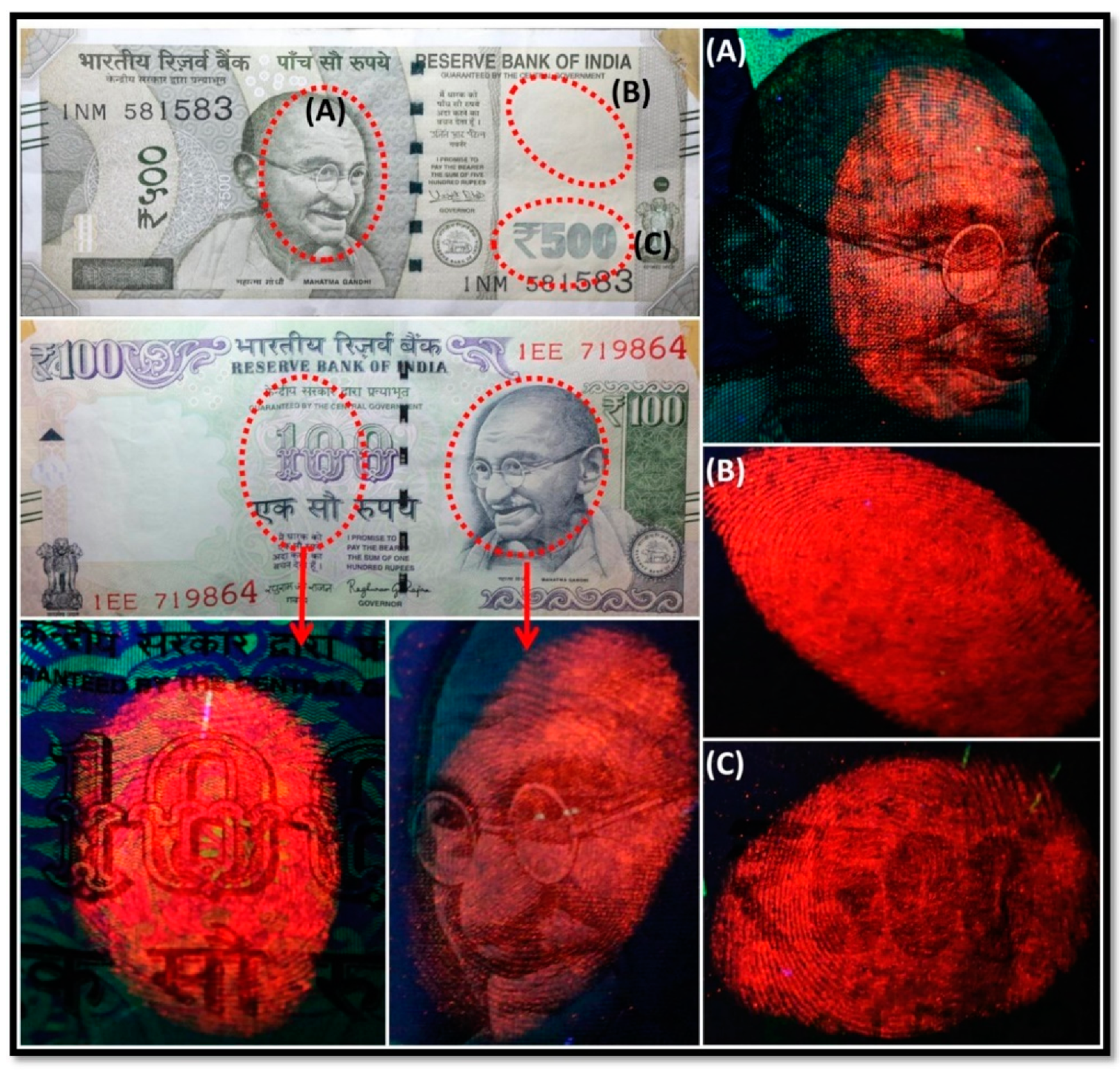
- Bose, P.K.; Kabir, M.J. Fingerprint: A Unique and Reliable Method for Identification. J. Enam Med. Coll. 2017, 7, 29–34. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Shu, C.; Hou, X.; Wu, P. Simultaneous extraction of level 2 and level 3 characteristics from latent fingerprints imaged with quantum dots for improved fingerprint analysis. Chin. Chem. Lett. 2017, 28, 1961–1964. [Google Scholar] [CrossRef]
- Figueroa, B.; Chen, Y.; Berry, K.; Francis, A.; Fu, D. Label-Free Chemical Imaging of Latent Fingerprints with Stimulated Raman Scattering Microscopy. Anal. Chem. 2017, 89, 4468–4473. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, N.H.; Darshan, G.P.; Basavaraj, R.B.; Prasad, B.D.; Sharma, S.C.; Kavyashree, D.; Nagabhushana, H. Nanostructured Stannic Oxides for White Light Emitting Diodes Provides Authentication for Latent Fingerprints Visualization under Diverse Environmental Conditions. ACS Sustain. Chem. Eng. 2019, 7, 578–591. [Google Scholar] [CrossRef]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef]
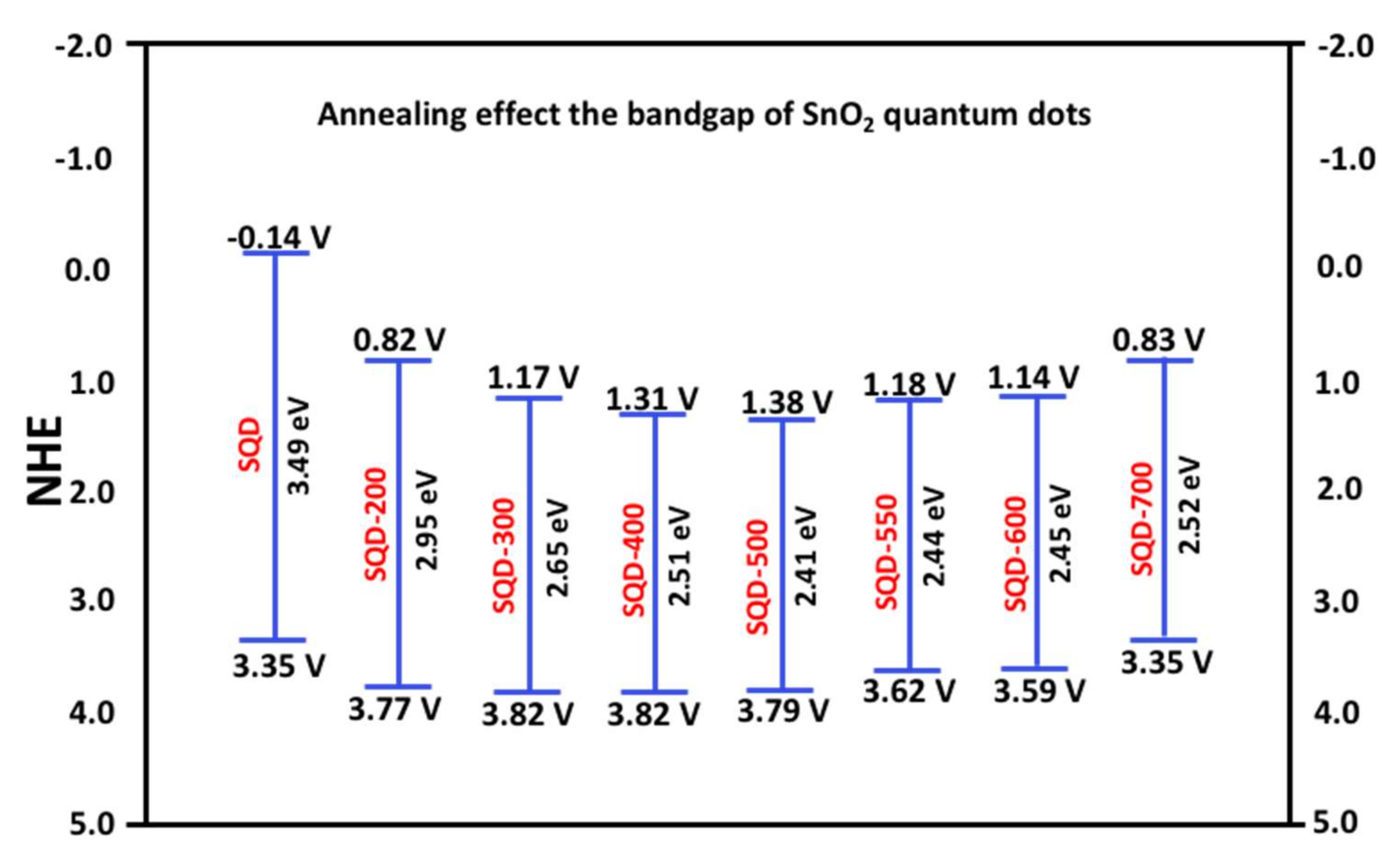
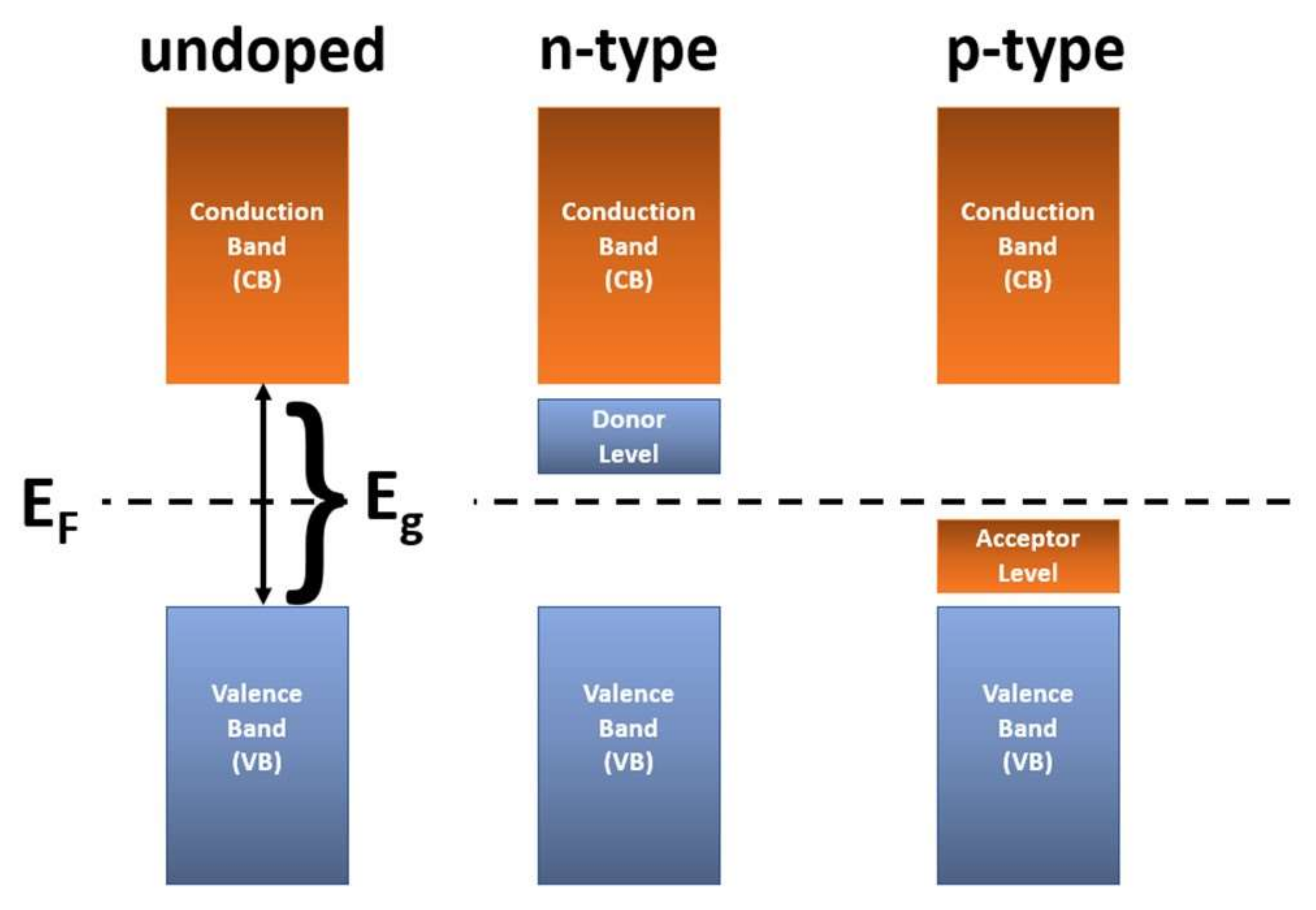
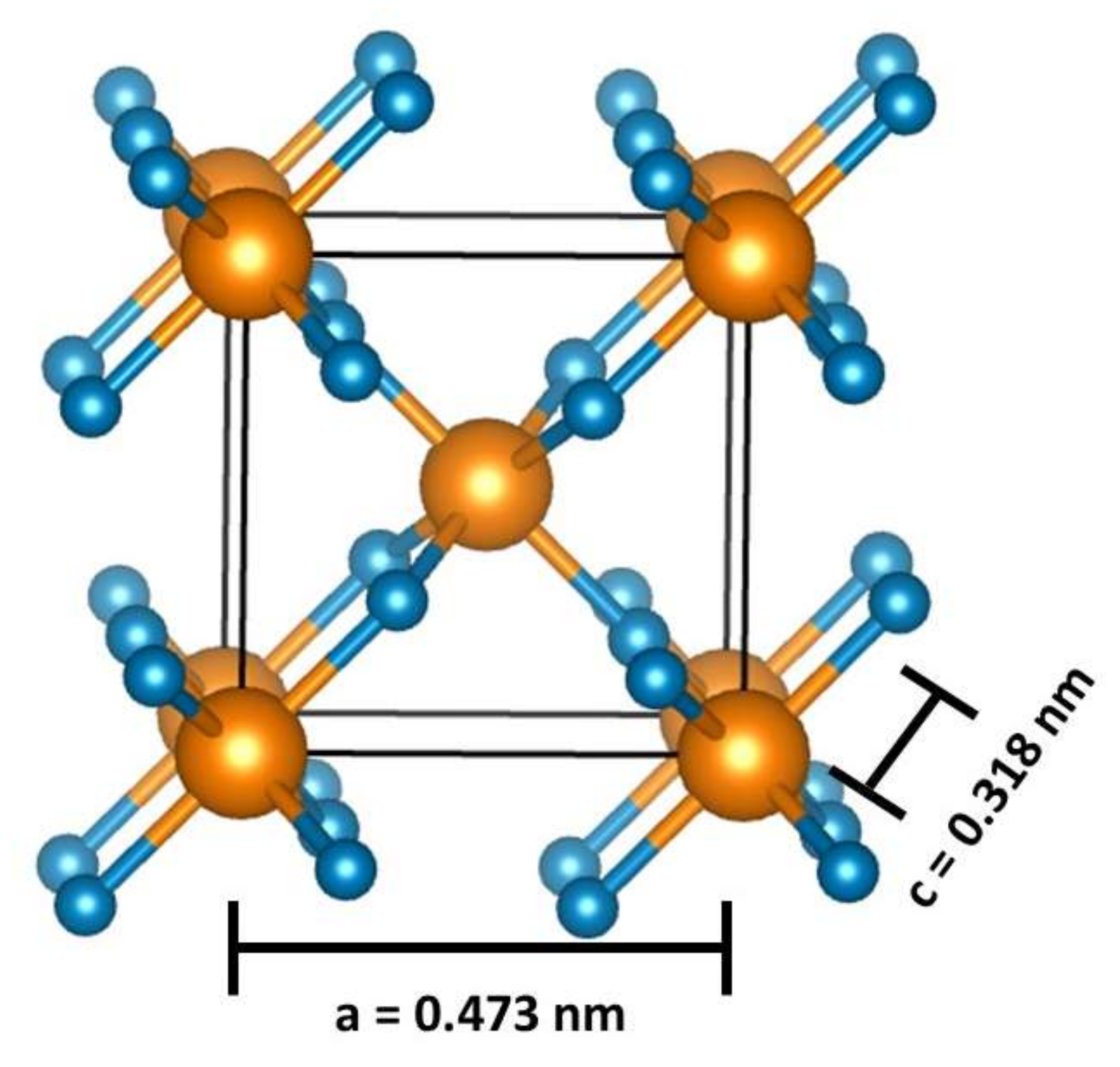
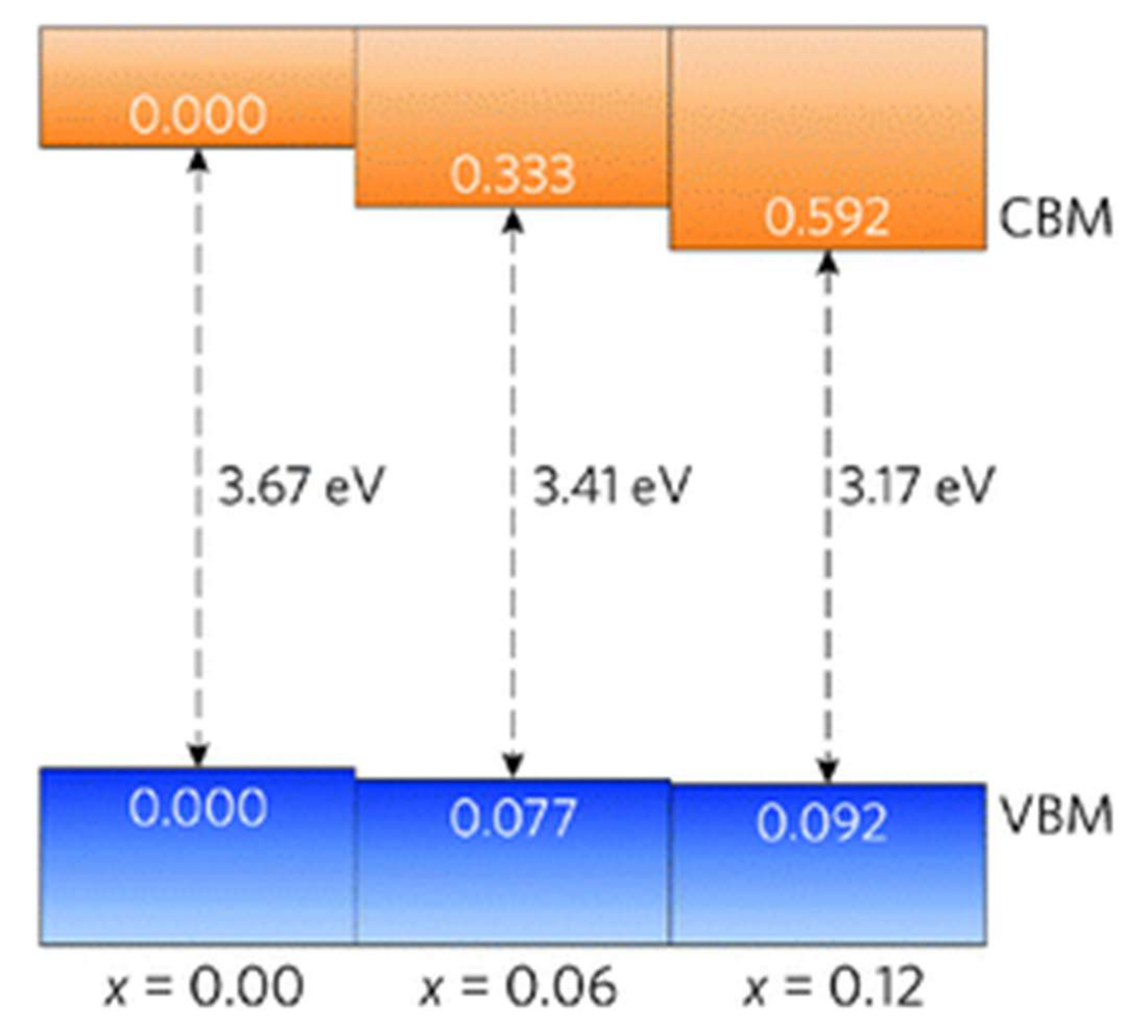
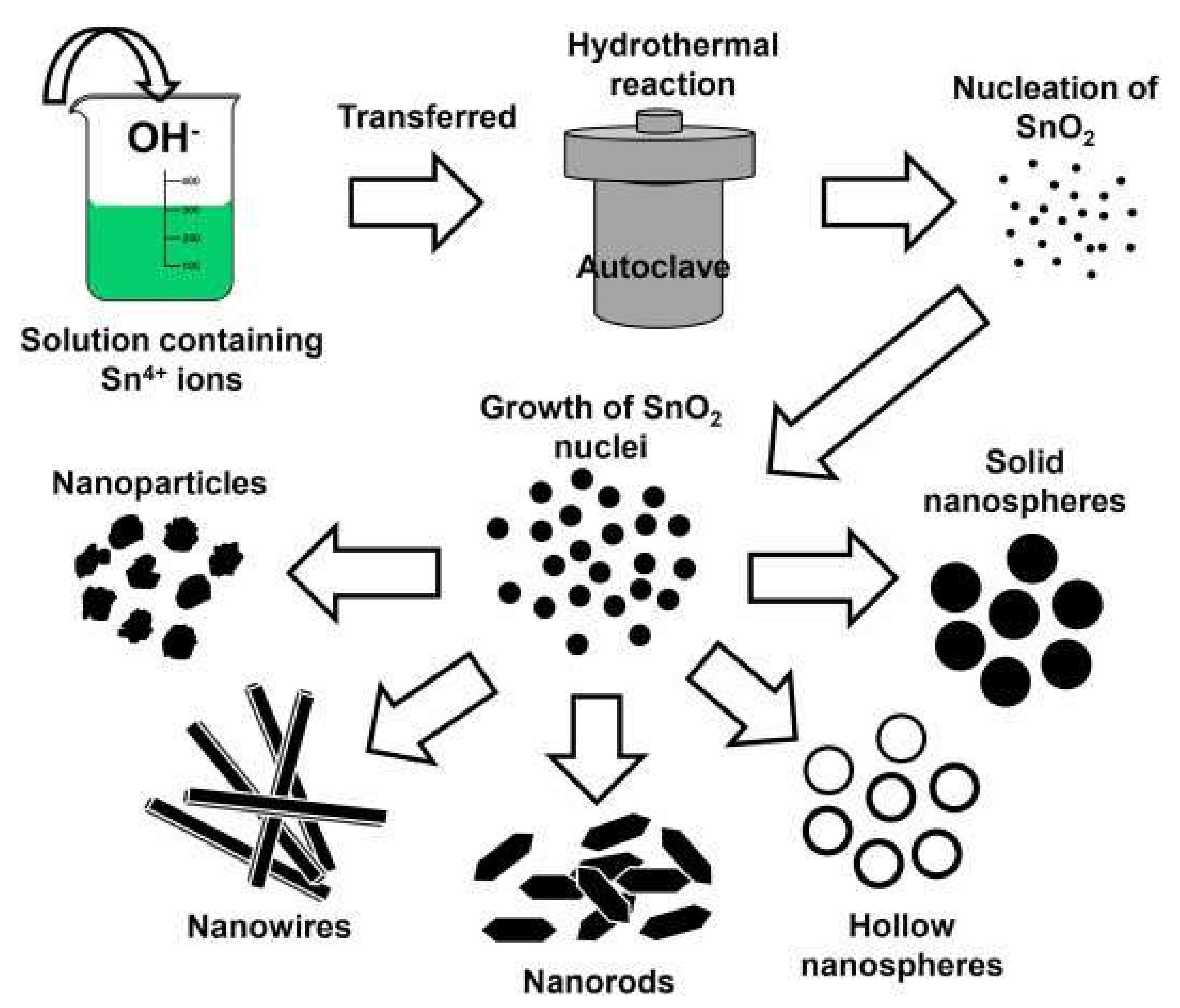
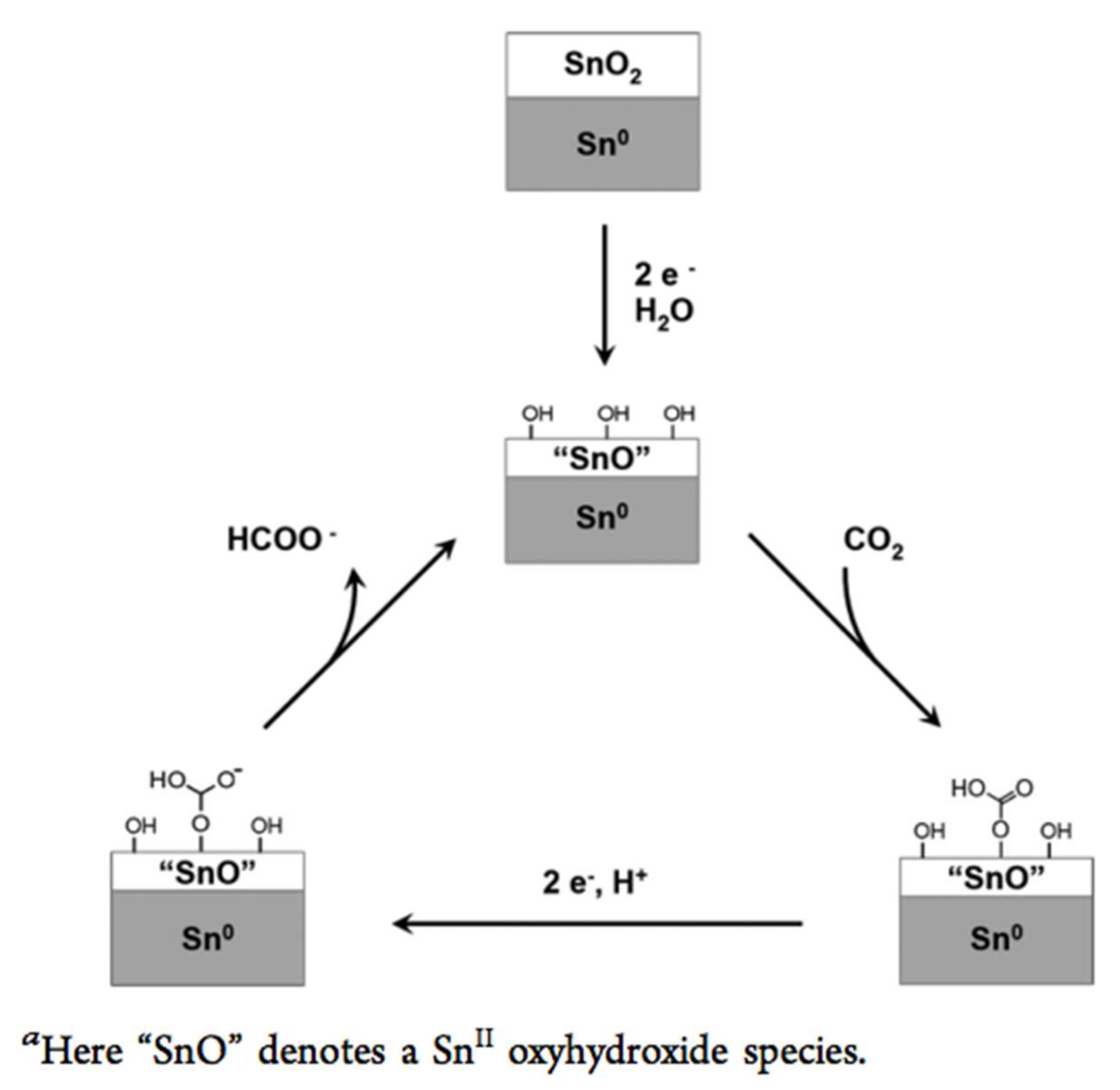
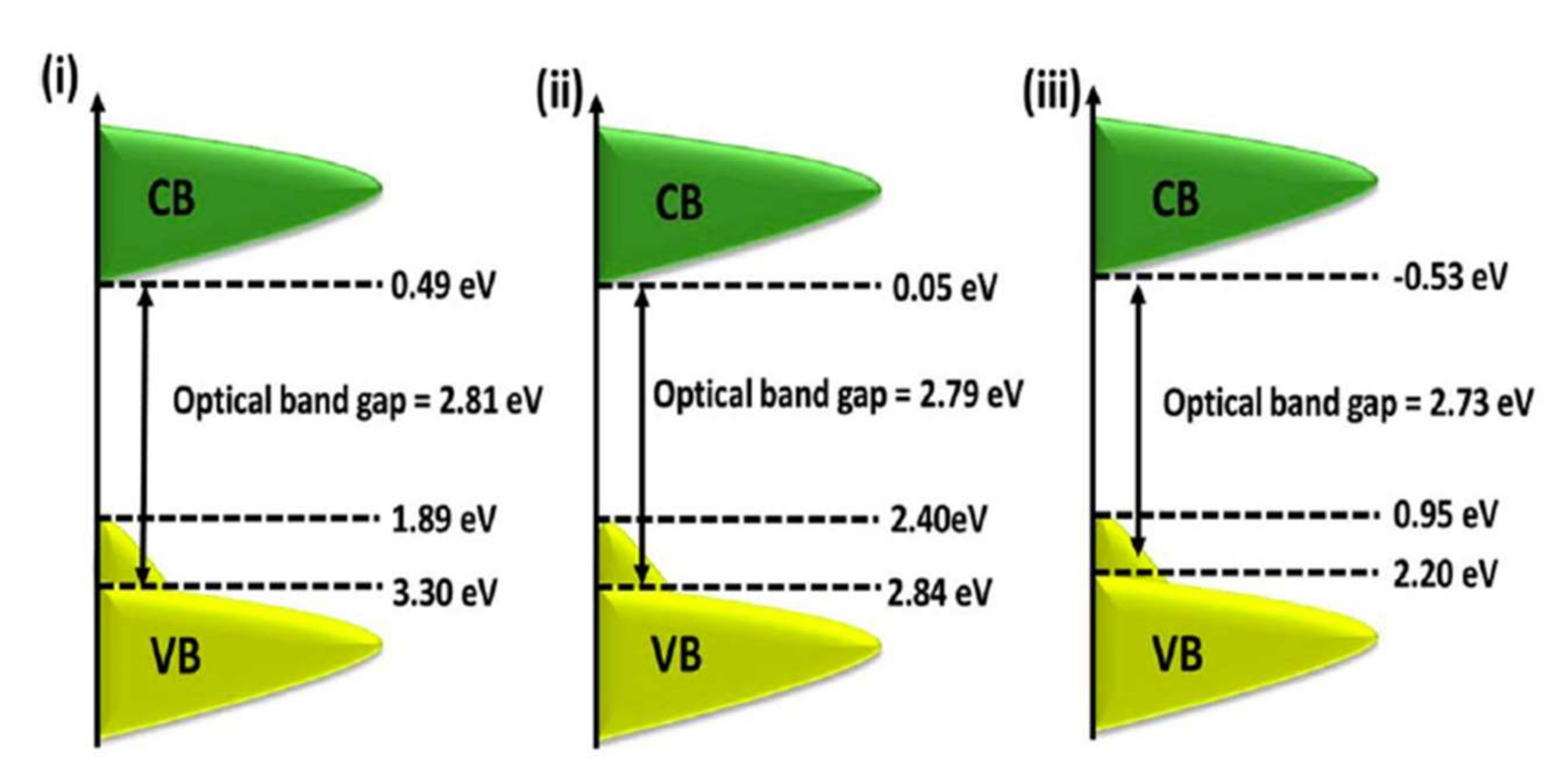
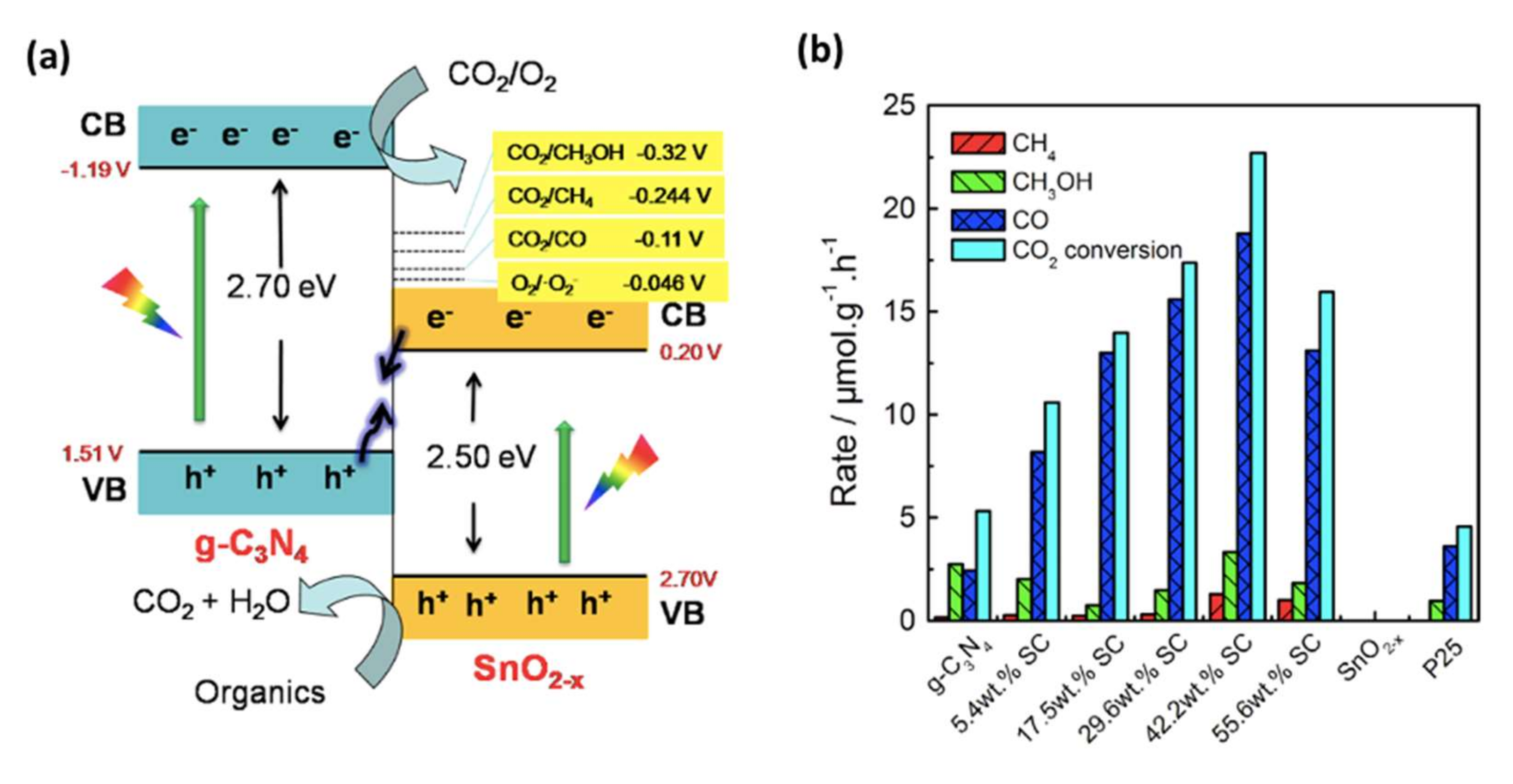
| Doping | Synthesis Method | Morphology—Primary Structures to Hierarchical | Reference |
|---|---|---|---|
| Ag | Sol-gel/electrospinning and calcination | Nanoparticles to Hollow nanofibres | [87] |
| Al | Hydrothermal | Nanoparticles | [95] |
| Bi | Hydrothermal | Nanoparticles | [4] |
| Ce | Hydrothermal | Hollow spheres | [86] |
| C | Combustion | faceted nanocrystals | [96] |
| Co | Precipitation and hydrothermal | Nanoparticles | [97] |
| Cr | Combustion | Nanoparticles | [98] |
| Cu | Precipitation and calcination | Faceted nanocrystals | [99] |
| Eu | Hydrothermal | Nanorods to flower-like | [100] |
| Fe | Hydrolyses and hydrothermal | Nanoparticles to echinus-like | [101] |
| La | Microemulsion and calcination | Nanoparticles | [102] |
| Nb | Solvothermal | Faceted nanocrystals | [103] |
| Ni | Solvothermal | Particles to microspheres | [104] |
| Pd | Sol-gel and calcination | Particles to microspheres | [105] |
| S | Thermal oxidation in air | Nanosheets to flower-like | [106] |
| Sr | Sol-gel precipitation and calcination | Nanoparticles | [73] |
| Ti | Precipitation and calcination | Nanoparticles | [107] |
| V | Combustion | Nanoparticles | [108] |
| W | Sol-gel and calcination | Nanoparticles | [109] |
| Y | Hydrothermal | Nanoparticles | [110] |
| Zn | Polyol and calcination | Nanoparticles | [111] |
| Zr | Hydrothermal | Nanoparticles | [112] |
| d-SnO2 Sample | Bacteria Species | ZOI (mm) | ZOI of Undoped Sample (mm) | Reference |
|---|---|---|---|---|
| Co-doped SnO2 NPs | Enterococcus faecalis Staphylococcus aureus Escherichia coli Enterobacter spp. Pseudomonas aeruginosa | 26 19 28 24 20 | inactive inactive 10 inactive inactive | [19] |
| Ce-doped SnO2 NPs | Escherichia coli | - | unanalysed | [156] |
| Zr-doped SnO2 NPs | Staphylococcus aureus Escherichia coli | 5 ± 0.5 7 ± 0.5 | 3 ± 1 4 ± 0.5 | [157] |
| Zn-doped SnO2 NPs | Staphylococcus aureus Escherichia coli | 3 ± 0.5 8 ± 0.3 | 1 ± 1 4 ± 0.5 | [66] |
| Ce-doped SnO2 NPs | Staphylococcus aureus Escherichia coli | 9 ± 1 5 ± 1 | 7 ± 0.5 4 ± 0.5 | [83] |
| B-doped and B-Ag co-doped SnO2 NPs | Staphylococcus aureus Escherichia coli | 6–13 13–15 | unanalysed unanalysed | [158] |
| Ni-doped SnO2 NPs | Staphylococcus aureus Escherichia coli | 14–18 16–20 | 13 14 | [159] |
| Co-doped SnO2 NPs | Escherichia coli Bacillus subtilis | 16 ± 0.8 22 ± 1.6 | unanalysed unanalysed | [153] |
| Ag-doped SnO2 NPs | Staphylococcus aureus Escherichia coli Aeronomous hydrophila Shigella flexineri | 8 12–13 6–8 10–15 | unanalysed unanalysed unanalysed unanalysed | [160] |
| Cu-doped SnO2 NPs | Pseudomonas aeruginosa Staphylococcus aureus | 12–19 10–18 | unanalysed unanalysed | [161] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, A.H.; Nogueira, A.E.; Dalmaschio, C.J.; Frigini, I.N.; de Almeida, J.C.; Ferrer, M.M.; Berengue, O.M.; Gonçalves, R.A.; de Mendonça, V.R. Doped Tin Dioxide (d-SnO2) and Its Nanostructures: Review of the Theoretical Aspects, Photocatalytic and Biomedical Applications. Solids 2022, 3, 327-360. https://doi.org/10.3390/solids3020024
Pinto AH, Nogueira AE, Dalmaschio CJ, Frigini IN, de Almeida JC, Ferrer MM, Berengue OM, Gonçalves RA, de Mendonça VR. Doped Tin Dioxide (d-SnO2) and Its Nanostructures: Review of the Theoretical Aspects, Photocatalytic and Biomedical Applications. Solids. 2022; 3(2):327-360. https://doi.org/10.3390/solids3020024
Chicago/Turabian StylePinto, Alexandre H., Andre E. Nogueira, Cleocir J. Dalmaschio, Iago N. Frigini, Jéssica C. de Almeida, Mateus M. Ferrer, Olivia M. Berengue, Rosana A. Gonçalves, and Vagner R. de Mendonça. 2022. "Doped Tin Dioxide (d-SnO2) and Its Nanostructures: Review of the Theoretical Aspects, Photocatalytic and Biomedical Applications" Solids 3, no. 2: 327-360. https://doi.org/10.3390/solids3020024
APA StylePinto, A. H., Nogueira, A. E., Dalmaschio, C. J., Frigini, I. N., de Almeida, J. C., Ferrer, M. M., Berengue, O. M., Gonçalves, R. A., & de Mendonça, V. R. (2022). Doped Tin Dioxide (d-SnO2) and Its Nanostructures: Review of the Theoretical Aspects, Photocatalytic and Biomedical Applications. Solids, 3(2), 327-360. https://doi.org/10.3390/solids3020024






