Evaluation of the Molecular Conformation of Surface Alkyl Chains of Alkylsilane-Derived Hybrid Films Using Sum-Frequency Generation Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Alkylsilane-Derived Hybrid Film
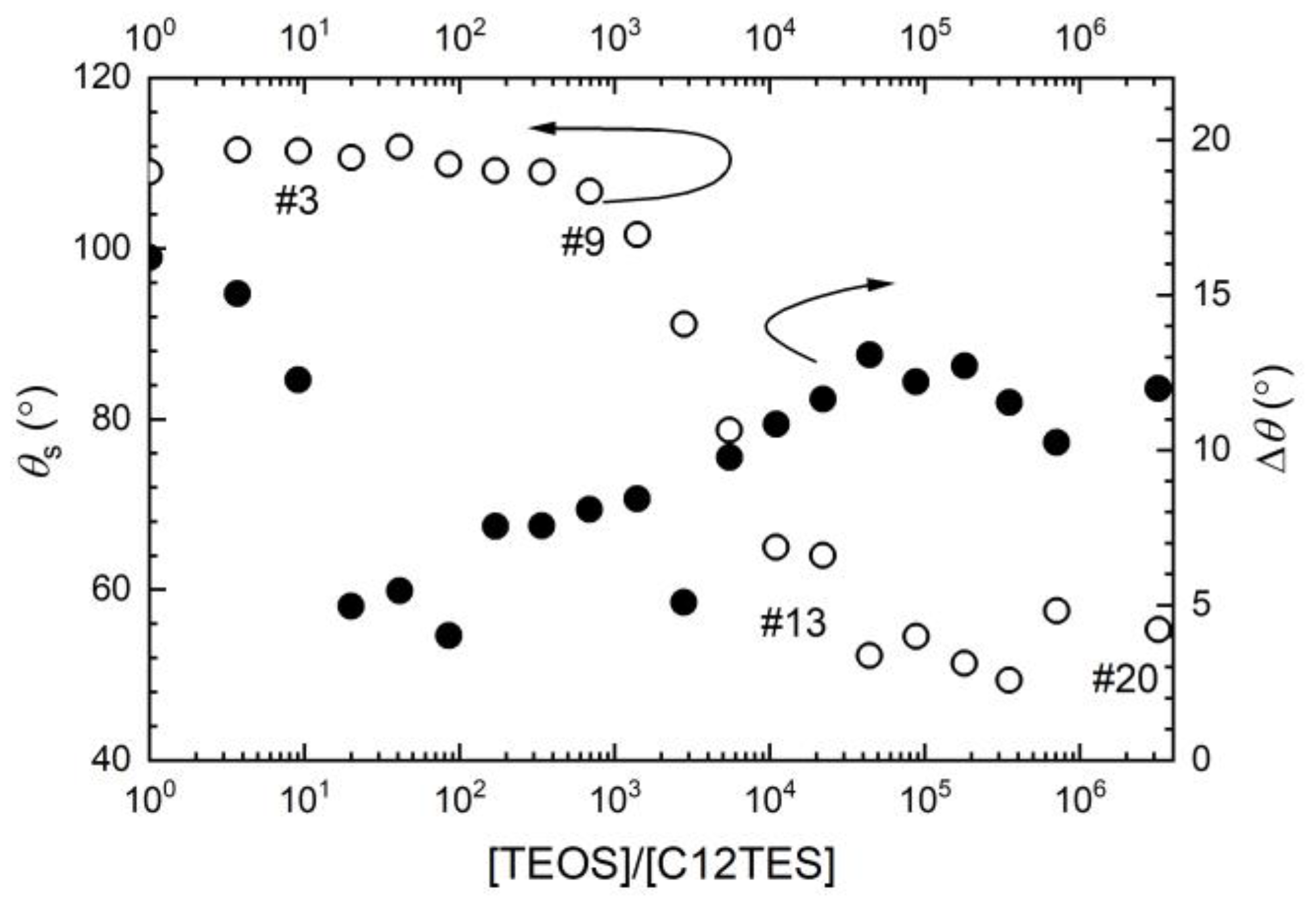
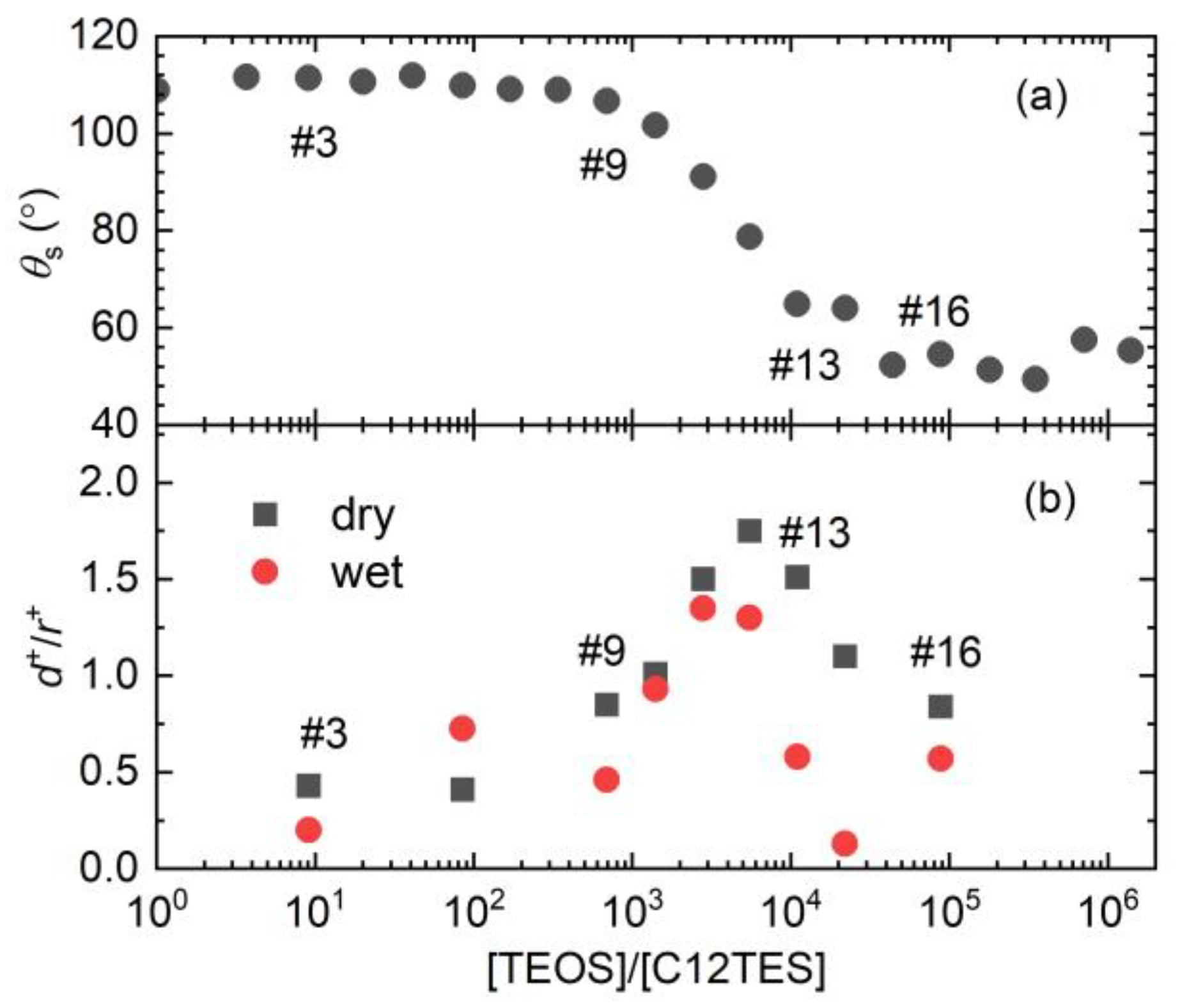
2.3. The Static/Dynamic Water Contact Angles
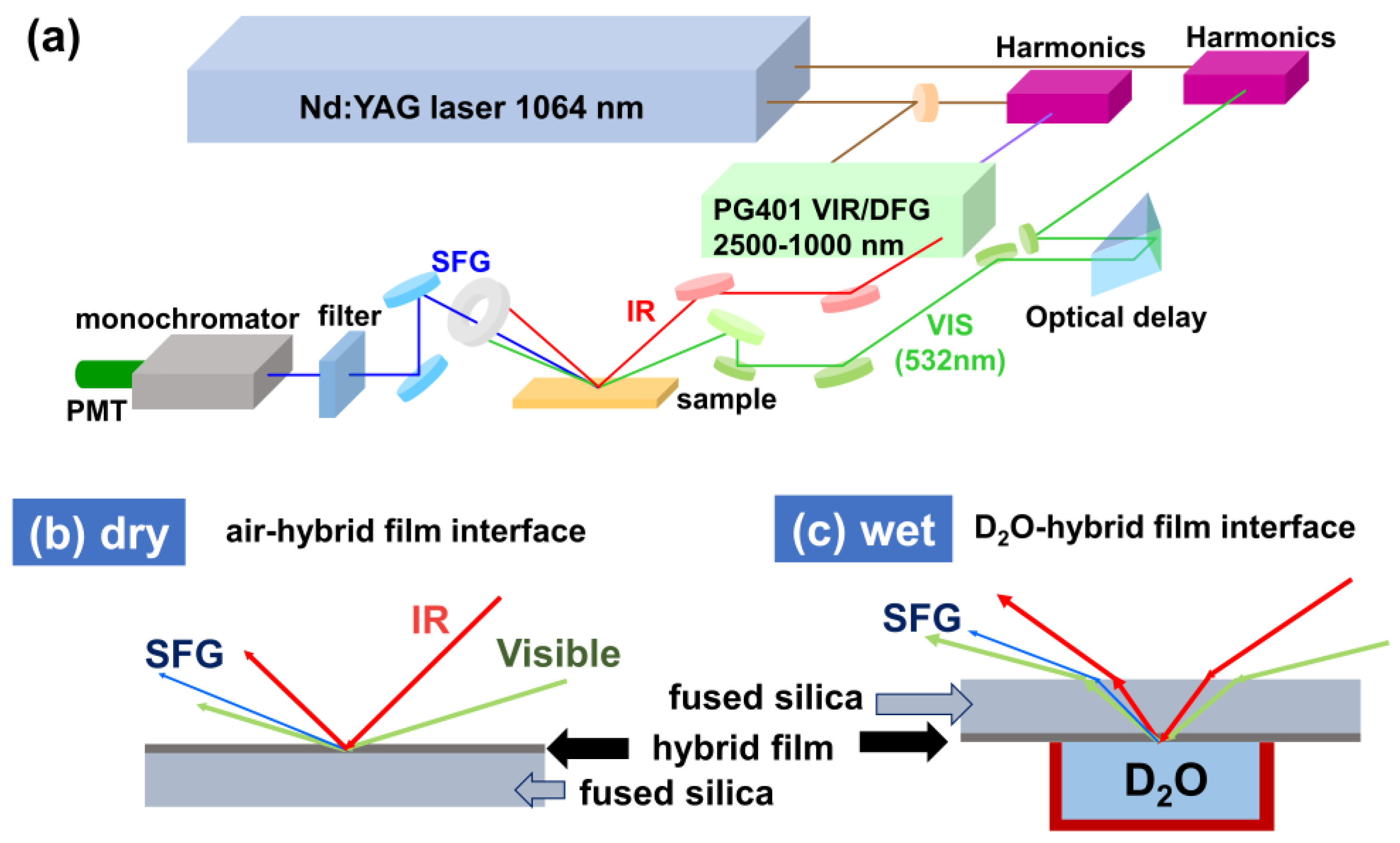
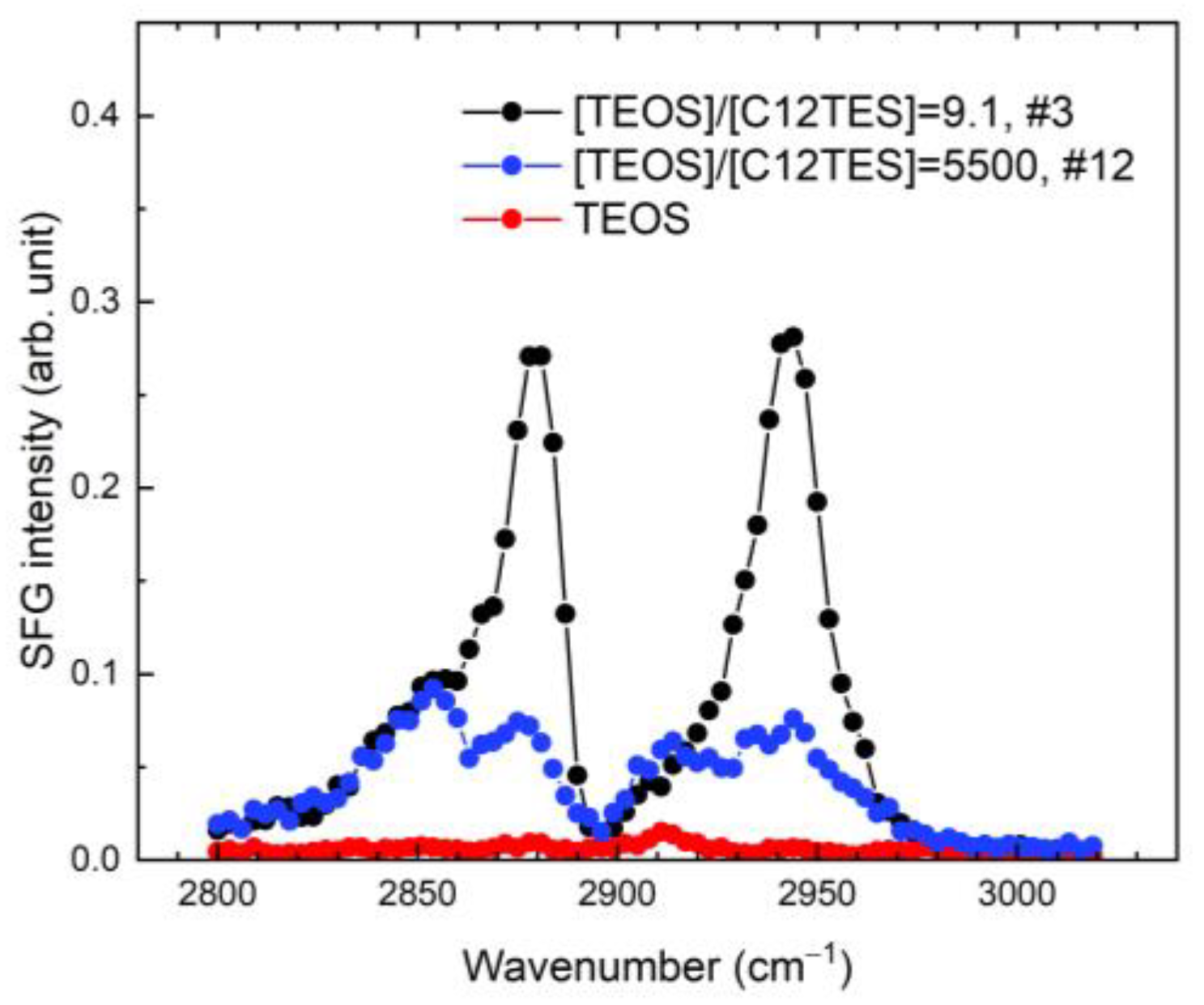
2.4. Sum-Frequency Generation (SFG) Spectroscopy
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, T.-S.; Sun, T.; Feng, L.; Aizenberg, J. Interfacial Materials with Special Wettability. MRS Bull. 2013, 38, 366–371. [Google Scholar] [CrossRef] [Green Version]
- Kota, A.K.; Choi, W.; Tuteja, A. Superomniphobic Surfaces: Design and Durability. MRS Bull. 2013, 38, 383–390. [Google Scholar] [CrossRef]
- Urata, C.; Masheder, B.; Cheng, D.F.; Miranda, D.F.; Dunderdale, G.J.; Miyamae, T.; Hozumi, A. Why Can Organic Liquids Move Easily on Smooth Alkyl-Terminated Surfaces? Langmuir 2014, 30, 4049–4055. [Google Scholar] [CrossRef]
- Tsujii, K.; Yamamoto, T. Super Oil-Repellent Surfaces. Angew. Chem. 1997, 36, 1011–1012. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W. Designing superoleophobic surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.P.; Seeger, S. Superoleophobic Coatings with Ultralow Sliding Angles Based on Silicone Nanofilaments. Angew. Chem. 2011, 50, 6652–6656. [Google Scholar] [CrossRef]
- Deng, X.; Mammen, L. Candle soot as a template for a transparent robust superamphiphobic coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.L.; Kim, C.J. Turning a surface superrepellent even to completely wetting liquids. Science 2014, 346, 1096–1100. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Hong, S.C.; Lvovsky, A.I.; Held, H.; Shen, Y.R. Evaluation of Surface vs Bulk Contributions in Sum-Frequency Vibrational Spectroscopy Using Reflection and Transmission Geometries. J. Phys. Chem. B 2000, 104, 3349–3354. [Google Scholar] [CrossRef]
- Mori, F.; Kabashima, S.; Kawakami, T.; Yamamoto, T.; Miyamae, T.; Iimura, K.; Tobori, N. Effect of the interfacial structures of alkyl-side-chain polymer films on the peel force. Int. J. Adhes. Adhes. 2018, 82, 166–172. [Google Scholar] [CrossRef]
- Tong, Y.; Tyrode, E.; Osawa, M.; Yoshida, N.; Watanabe, T.; Nakajima, A.; Ye, S. Preferential Adsorption of Amino-Terminated Silane in a Binary Mixed Self-Assembled Monolayer. Langmuir 2011, 27, 5420–5426. [Google Scholar] [CrossRef] [PubMed]
- Urata, C.; Cheng, D.F.; Masheder, B.; Hozumi, A. Smooth, Transparent and Nonperfluorinated Surfaces Exhibiting Unusual Contact Angle Behavior toward Organic Liquids. RSC Adv. 2012, 2, 9805–9808. [Google Scholar] [CrossRef]
- Urata, C.; Masheder, B.; Cheng, F.D.; Hozumi, A. How to Reduce Resistance to Movement of Alkane Liquid Drops Across Tilted Surfaces Without Relying on Surface Roughening and Perfluorination. Langmuir 2012, 28, 17681–17689. [Google Scholar] [CrossRef]
- Miyamae, T.; Tsukagoshi, K.; Matsuoka, O.; Yamamoto, S.; Nozoye, H. Surface Characterization of Polyamic Acid and Polyimide Films Prepared by Vapor Deposition Polymerization by Using Sum-Frequency Generation. Langmuir 2001, 17, 8125–8130. [Google Scholar] [CrossRef]
- Chiang, C.H.; Ishida, H.; Koening, J.L. The structure of γ-aminopropyltriethoxysilane on glass surfaces. J. Coll. Interface Sci. 1980, 74, 396–404. [Google Scholar] [CrossRef]
- Ye, S.; Nihonyanagi, S.; Uosaki, K. Sum frequency generation (SFG) study of the pH-dependent water structure on a fused quartz surface modified by an octadecyltrichlorosilane (OTS) monolayer. Phys. Chem. Chem. Phys. 2001, 3, 3463–3469. [Google Scholar] [CrossRef]
- Lahorija, B.; Vensa, V.; Vladimir, D. Conformational and vibrational analysis of γ-aminopropyltriethoxysilane. J. Mol. Struct. 2007, 834, 355–363. [Google Scholar]
- Ye, S.; Noda, H.; Nishida, T.; Morita, S.; Osawa, M. Cd2+-Induced Interfacial Structural Changes of Langmuir–Blodgett Films of Stearic Acid on Solid Substrates: A Sum Frequency Generation Study. Langmuir 2004, 20, 357–365. [Google Scholar] [CrossRef]
- Wang, H.-F.; Gan, W.; Rao, Y.; Wu, B.H. Quantitative Spectral and Orientational Analysis in Surface Sum Frequency Generation Vibrational Spectroscopy (SFG-VS). Int. Rev. Phys. Chem. 2005, 24, 191–256. [Google Scholar] [CrossRef]
- Tyrode, E.; Liljeblad, J.F.D. Water Structure next to Ordered and Disordered Hydrophobic Silane Monolayers: A Vibrational Sum Frequency Spectroscopy Study. J. Phys. Chem. C 2013, 117, 1780–1790. [Google Scholar] [CrossRef]
- Liu, Y.; Wolf, L.K.; Messmer, M.C. A Study of Alkyl Chain Conformational Changes in Self-Assembled N-Octadecyltrichlorosilane Monolayers on Fused Silica Surfaces. Langmuir 2001, 17, 4329–4335. [Google Scholar] [CrossRef]
- Wolf, L.K.; Yang, Y.J.; Pizzolatto, R.L.; Messmer, M.C. Microscopic Structure of Chromatographic Interfaces as Studied by Sum-Frequency Generation Spectroscopy. ACS Symp. Ser. 2001, 781, 293–305. [Google Scholar]
- Offord, D.A.; Griffin, J.H. Kinetic Control in the Formation of Self-Assembled Mixed Monolayers on Planar Silica Substrates. Langmuir 1993, 9, 3015–3025. [Google Scholar] [CrossRef]
- Pizzolatto, R.L.; Yang, Y.J.; Messmer, M.C. Conformational Aspects of Model Chromatographic Surfaces Studied by Sum-Frequency Generation. Anal. Chim. Acta 1999, 397, 81–92. [Google Scholar] [CrossRef]

| Sample No. | TEOS (mmol) | C12TES (mmol) | [TEOS]/[C12TES] | Water (mmol) | EtOH (mmol) |
|---|---|---|---|---|---|
| #1 | 1.7 | 1.7 | 1 | 44 | 69 |
| #2 | 3.1 | 8.3 × 10−1 | 3.7 | 44 | 69 |
| #3 | 3.8 | 4.2 × 10−1 | 9.1 | 44 | 69 |
| #4 | 4.1 | 2.1 × 10−1 | 2.0 × 101 | 44 | 69 |
| #5 | 4.3 | 1.0 × 10−1 | 4.1 × 101 | 44 | 69 |
| #6 | 4.4 | 5.2 × 10−2 | 8.5 × 101 | 44 | 69 |
| #7 | 4.4 | 2.6 × 10−2 | 1.7 × 102 | 44 | 69 |
| #8 | 4.5 | 1.3 × 10−2 | 3.4 × 102 | 44 | 69 |
| #9 | 4.5 | 6.5 × 10−3 | 6.9 × 102 | 44 | 69 |
| #10 | 4.5 | 3.2 × 10−3 | 1.4 × 103 | 44 | 69 |
| #11 | 4.5 | 1.6 × 10−3 | 2.8 × 103 | 44 | 69 |
| #12 | 4.5 | 8.1 × 10−4 | 5.5 × 103 | 44 | 69 |
| #13 | 4.5 | 4.1 × 10−4 | 1.1 × 104 | 44 | 69 |
| #14 | 4.5 | 2.0 × 10−4 | 2.2 × 104 | 44 | 69 |
| #15 | 4.5 | 1.0 × 10−4 | 4.4 × 104 | 44 | 69 |
| #16 | 4.5 | 5.1 × 10−5 | 8.8 × 104 | 44 | 69 |
| #17 | 4.5 | 2.5 × 10−5 | 1.8 × 105 | 44 | 69 |
| #18 | 4.5 | 1.3 × 10−5 | 3.5 × 105 | 44 | 69 |
| #19 | 4.5 | 6.3 × 10−6 | 7.1 × 105 | 44 | 69 |
| #20 | 4.5 | 3.2 × 10−6 | 1.4 × 106 | 44 | 69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuno, R.; Urata, C.; Watanabe, K.; Hozumi, A.; Miyamae, T. Evaluation of the Molecular Conformation of Surface Alkyl Chains of Alkylsilane-Derived Hybrid Films Using Sum-Frequency Generation Spectroscopy. Solids 2022, 3, 147-154. https://doi.org/10.3390/solids3010010
Mizuno R, Urata C, Watanabe K, Hozumi A, Miyamae T. Evaluation of the Molecular Conformation of Surface Alkyl Chains of Alkylsilane-Derived Hybrid Films Using Sum-Frequency Generation Spectroscopy. Solids. 2022; 3(1):147-154. https://doi.org/10.3390/solids3010010
Chicago/Turabian StyleMizuno, Rika, Chihiro Urata, Ken Watanabe, Atsushi Hozumi, and Takayuki Miyamae. 2022. "Evaluation of the Molecular Conformation of Surface Alkyl Chains of Alkylsilane-Derived Hybrid Films Using Sum-Frequency Generation Spectroscopy" Solids 3, no. 1: 147-154. https://doi.org/10.3390/solids3010010
APA StyleMizuno, R., Urata, C., Watanabe, K., Hozumi, A., & Miyamae, T. (2022). Evaluation of the Molecular Conformation of Surface Alkyl Chains of Alkylsilane-Derived Hybrid Films Using Sum-Frequency Generation Spectroscopy. Solids, 3(1), 147-154. https://doi.org/10.3390/solids3010010






