Protonated Forms of Layered Perovskite-Like Titanate NaNdTiO4: Neutron and X-ray Diffraction Structural Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Instrumentation
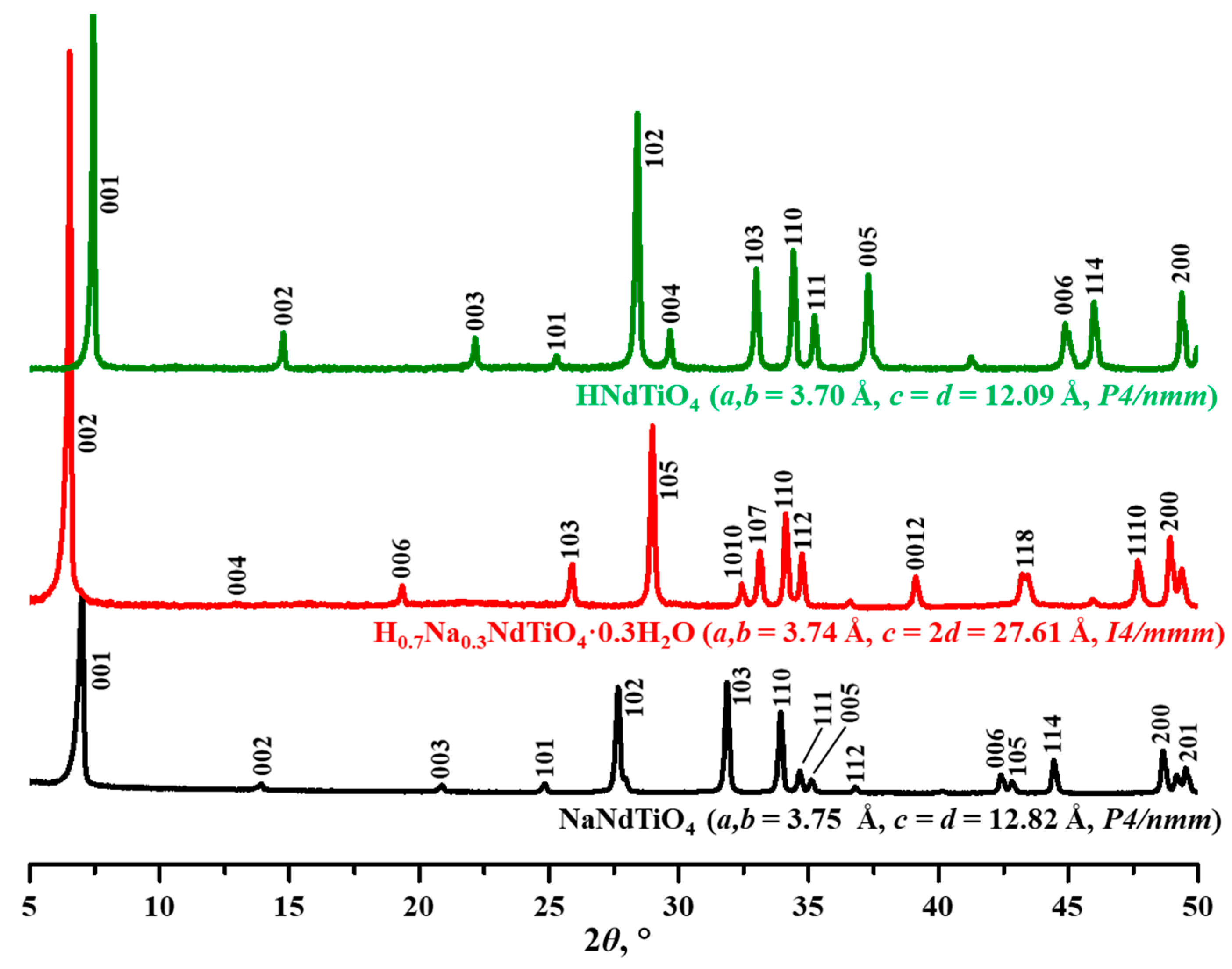
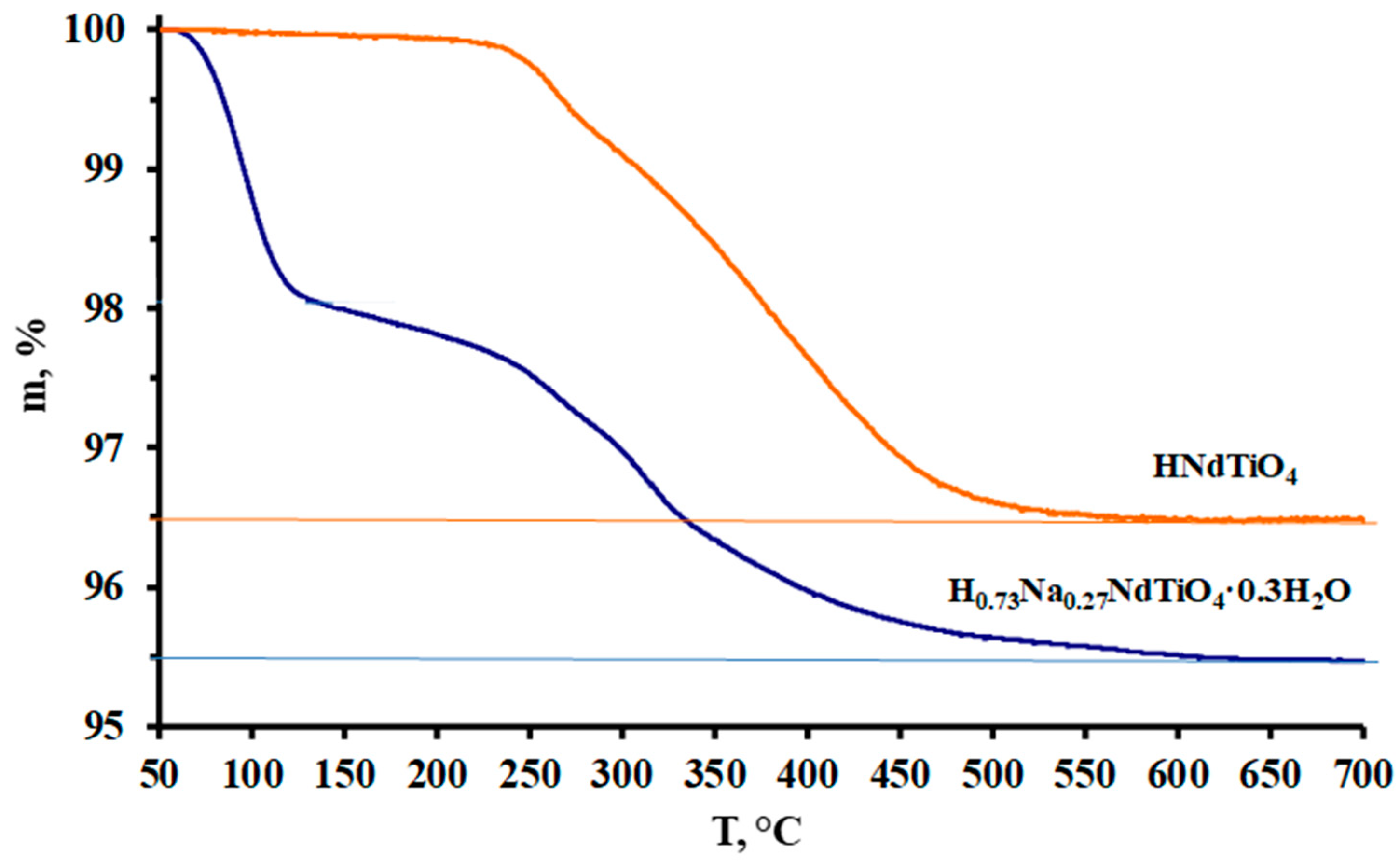
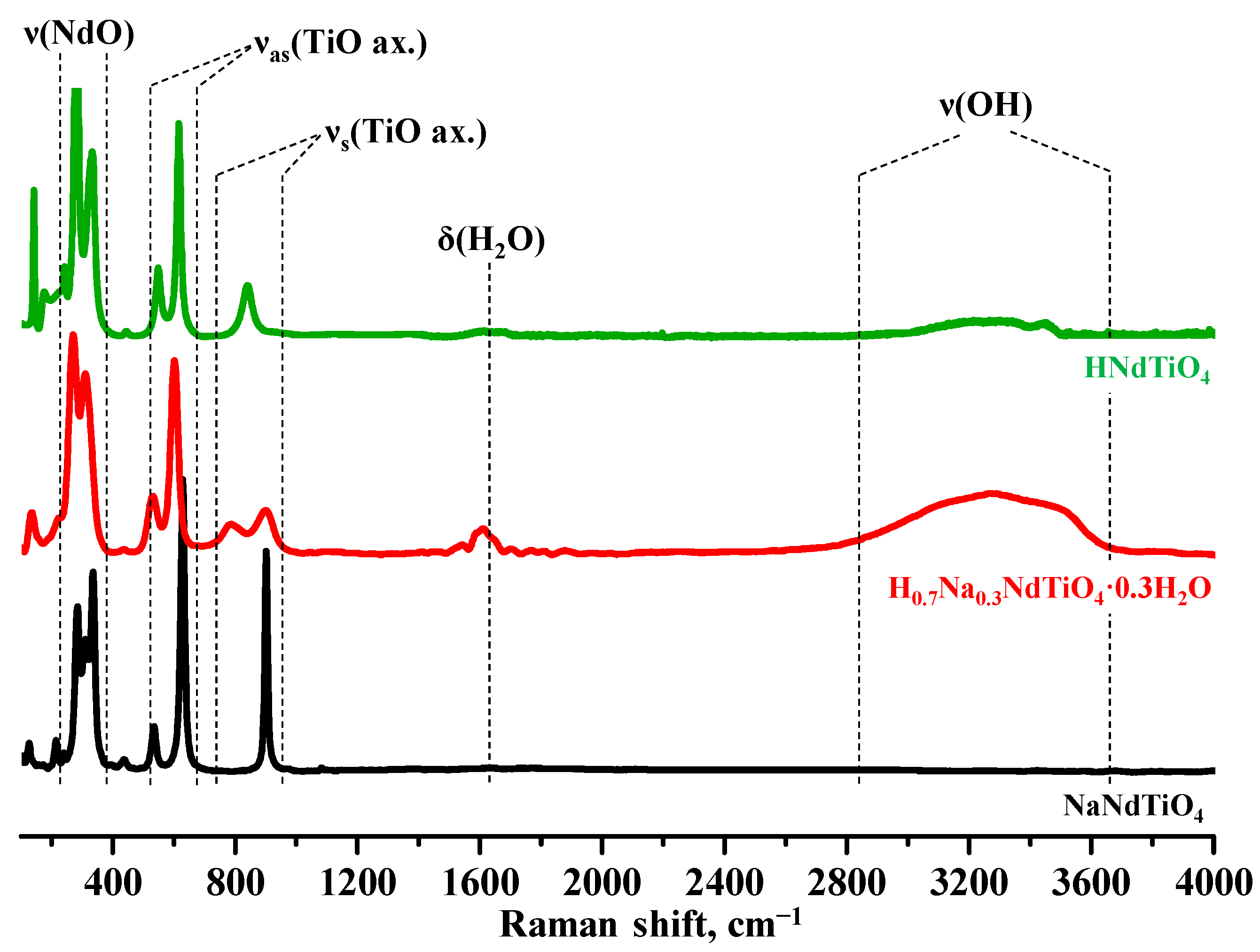
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thangadurai, V.; Shukla, A.; Gopalakrishnan, J. Proton conduction in layered perovskite oxides. Solid State Ion. 1994, 73, 9–14. [Google Scholar] [CrossRef]
- Toda, K.; Watanabeb, J.; Satob, M. Synthesis and ionic conductivity of new layered perovskite compound, Ag2La2Ti3O10. Solid State Ion. 1996, 90, 15–19. [Google Scholar] [CrossRef]
- Sato, M.; Abo, J.; Jin, T.; Ohta, M. Structure and ionic conductivity of MLaNb2O7 (M-K, Na, Li, H). J. Alloys Compd. 1993, 192, 81–83. [Google Scholar] [CrossRef]
- Toda, K.; Suzuki, T.; Sato, M. Synthesis and high ionic conductivity of new layered perovskite compounds, AgLaTa2O7 and AgCa2Ta3O10. Solid State Ion. 1997, 93, 177–181. [Google Scholar] [CrossRef]
- Sato, M.; Watanabe, J.; Kazuyoshi, U. Crystal Structure and Ionic Conductivity of a Layered-Perovskite AgLaNb2O7. J. Solid State Chem. 1993, 107, 460–470. [Google Scholar] [CrossRef]
- Campbell, K.D. Layered and double perovskites as methane coupling catalysts. Catal. Today 1992, 13, 245–253. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Zvereva, I.A. Photocatalytic activity of layered perovskite-like oxides in practically valuable chemical reactions. Russ. Chem. Rev. 2016, 85, 248–279. [Google Scholar] [CrossRef]
- Hinterding, R.; Feldhoff, A. Two-Dimensional Oxides: Recent Progress in Nanosheets. Z. Phys. Chem. 2018, 233, 117–165. [Google Scholar] [CrossRef]
- Hu, Y.; Mao, L.; Guan, X.; Tucker, K.A.; Xie, H.; Wu, X.; Shi, J. Layered perovskite oxides and their derivative nanosheets adopting different modification strategies towards better photocatalytic performance of water splitting. Renew. Sustain. Energy Rev. 2020, 119, 109527. [Google Scholar] [CrossRef]
- Tahara, S.; Ichikawa, T.; Kajiwara, G.; Sugahara, Y. Reactivity of the Ruddlesden−Popper Phase H2La2Ti3O10 with Organic Compounds: Intercalation and Grafting Reactions. Chem. Mater. 2007, 19, 2352–2358. [Google Scholar] [CrossRef]
- Minich, I.A.; Silyukov, O.I.; Gak, V.V.; Borisov, E.V.; Zvereva, I.A. Synthesis of Organic–Inorganic Hybrids Based on Perovskite-like Bismuth Titanate H2K0.5Bi2.5Ti4 O13·H2O and n-Alkylamines. ACS Omega 2020, 5, 8158–8168. [Google Scholar] [CrossRef] [PubMed]
- Silyukov, O.I.; Kurnosenko, S.A.; Zvereva, I.A. Intercalation of Methylamine into the Protonated Forms of Layered Perovskite-Like Oxides HLnTiO4 (Ln = La and Nd). Glas. Phys. Chem. 2018, 44, 428–432. [Google Scholar] [CrossRef]
- Kurnosenko, S.A.; Silyukov, O.I.; Mazur, A.S.; Zvereva, I.A. Synthesis and thermal stability of new inorganic-organic perovskite-like hybrids based on layered titanates HLnTiO4 (Ln = La, Nd). Ceram. Int. 2020, 46, 5058–5068. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Lushpinskaya, I.P.; Kurnosenko, S.A.; Silyukov, O.I.; Zvereva, I.A. Identification of Intercalates and Grafted Organic Derivatives of H2La2Ti3O10 by Multinuclear NMR. Russ. J. Gen. Chem. 2020, 90, 760–761. [Google Scholar] [CrossRef]
- Kurnosenko, S.A.; Silyukov, O.I.; Minich, I.A.; Zvereva, I.A. Exfoliation of methylamine and n-butylamine derivatives of layered perovskite-like oxides HLnTiO4 and H2Ln2Ti3O10 (Ln = La, Nd) into nanolayers. Russ. J. Gen. Chem. 2020, 44, 428–432. [Google Scholar]
- Rodionov, I.A.; Maksimova, E.A.; Pozhidaev, A.Y.; Kurnosenko, S.A.; Silyukov, O.I.; Zvereva, I.A. Layered Titanate H2Nd2Ti3O10 Intercalated with n-Butylamine: A New Highly Efficient Hybrid Photocatalyst for Hydrogen Production from Aqueous Solutions of Alcohols. Front. Chem. 2019, 7, 863. [Google Scholar] [CrossRef] [PubMed]
- Voytovich, V.V.; Kurnosenko, S.A.; Silyukov, O.I.; Rodionov, I.A.; Minich, I.A.; Zvereva, I.A. Study of n-alkylamine Intercalated Layered Perovskite-Like Niobates HCa2Nb3O10 as Photocatalysts for Hydrogen Production from an Aqueous Solution of Methanol. Front. Chem. 2020, 8, 300. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Silyukov, O.I.; Lushpinskaia, I.P.; Kurnosenko, S.A.; Mazur, A.S.; Shenderovich, I.G.; Zvereva, I.A. NMR Study of Intercalates and Grafted Organic Derivatives of H2La2Ti3O10. Molecules 2020, 25, 5229. [Google Scholar] [CrossRef]
- Osada, M.; Sasaki, T. Exfoliated oxide nanosheets: New solution to nanoelectronics. J. Mater. Chem. 2009, 19, 2503–2511. [Google Scholar] [CrossRef]
- Peláiz-Barranco, A.; González-Abreu, Y. Ferroelectric ceramic materials of the Aurivillius family. J. Adv. Dielectr. 2013, 3, 1330003. [Google Scholar] [CrossRef] [Green Version]
- Moure, A. Review and perspectives of Aurivillius structures as a lead-free Piezoelectric system. Appl. Sci. 2018, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Ida, S.; Ogata, C.; Eguchi, M.; Youngblood, W.J.; Mallouk, T.E.; Matsumoto, Y. Photoluminescence of perovskite nanosheets prepared by exfoliation of layered oxides, K2Ln2Ti3O10, KLnNb2O7, and RbLnTa2O7 (Ln: Lanthanide ion). J. Am. Chem. Soc. 2008, 130, 7052–7059. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Tress, W.; Dar, M.I.; Gao, P.; Luo, J.; Renevier, C.; Schenk, K.; Abate, A.; Giordano, F.; Baena, J.C.; et al. Efficient luminescent solar cells based on tailored mixed-cation perovskites. Sci. Adv. 2016, 2, e1501170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczepanski, F.; Bayart, A.; Katelnikovas, A.; Blach, J.F.; Rousseau, J.; Saitzek, S. Luminescence and up-conversion properties in La2Ti2O7:Eu3+,Er3+ oxides under UV and NIR radiations towards a two-color sensor. J. Alloys Compd. 2020, 826, 154157. [Google Scholar] [CrossRef]
- Yuan, M.; Dong, W.; Wei, L.; Liu, Q.; Meng, Y.; Wang, X.; Wang, B.; Zhu, B. Stability study of SOFC using layered perovskite oxide La1.85Sr0.15CuO4 mixed with ionic conductor as membrane. Electrochim. Acta 2020, 332, 135487. [Google Scholar] [CrossRef]
- Schaak, R.E.; Mallouk, T.E. Perovskites by Design: A Toolbox of Solid-State Reactions. Chem. Mater. 2002, 14, 1455–1471. [Google Scholar] [CrossRef]
- Uppuluri, R.; Sen Gupta, A.; Rosas, A.S.; Mallouk, T.E. Soft chemistry of ion-exchangeable layered metal oxides. Chem. Soc. Rev. 2018, 47, 2401–2430. [Google Scholar] [CrossRef] [PubMed]
- Dion, M.; Ganne, M.; Tournoux, M. Nouvelles familles de phases MIMII2Nb3O10 a feullets “perovskites”. Mater. Res. Bull. 1981, 16, 1429–1435. [Google Scholar] [CrossRef]
- Jacobson, A.J.; Johnson, J.W.; Lewandowski, J.T. Interlayer Chemistry between Thick Transition-Metal Oxide Layers: Synthesis and Intercalation Reactions of K[Ca2Nan−3NbnO3n+1] (3 ≤ n ≤ 7). Inorg. Chem. 1985, 24, 3727–3729. [Google Scholar] [CrossRef]
- Gopalakrishnan, J.; Bhat, V. A2Ln2Ti3O10 (A = potassium or rubidium; Ln = lanthanum or rare earth): A new series of layered perovskites exhibiting ion exchange. Inorg. Chem. 1987, 26, 4299–4301. [Google Scholar] [CrossRef]
- Gopalakrishnan, J.; Sivakumar, T.; Ramesha, K.; Thangadurai, V.; Subbanna, G.N. Transformations of Ruddlesden−Popper Oxides to New Layered Perovskite Oxides by Metathesis Reactions. Chem. Phys. 2000, 122, 6237–6241. [Google Scholar] [CrossRef]
- Jacobson, A.J.; Lewandowski, J.T.; Johnson, J.W. Ion exchange of the layered perovskite KCa2Nb3O10 by protons. J. Less Common Met. 1986, 116, 137–146. [Google Scholar] [CrossRef]
- Jacobson, A.J.; Johnson, J.W.; Lewandowski, J. Intercalation of the layered solid acid HCa2Nb3O10 by organic amines. Mater. Res. Bull. 1987, 22, 45–51. [Google Scholar] [CrossRef]
- Tahara, S.; Sugahara, Y. Interlayer Surface Modification of the Protonated Triple-Layered Perovskite HCa2Nb3O10·xH2O with n-Alcohols. Langmuir 2003, 19, 9473–9478. [Google Scholar] [CrossRef]
- Yafarova, L.V.; Silyukov, O.I.; Myshkovskaya, T.D.; Minich, I.A.; Zvereva, I.A. New data on protonation and hydration of perovskite-type layered oxide KCa2Nb3O10. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Silyukov, O.I.; Utkina, T.D.; Chislov, M.V.; Sokolova, Y.P.; Zvereva, I.A. Photocatalytic properties and hydration of perovskite-type layered titanates A2Ln2Ti3O10 (A = Li, Na, K.; Ln = La, Nd). Russ. J. Gen. Chem. 2012, 82, 1191–1196. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Mechtaeva, E.V.; Burovikhina, A.A.; Silyukov, O.I.; Toikka, M.A.; Zvereva, I.A. Effect of protonation on the photocatalytic activity of the K2La2Ti3O10 layered oxide in the reaction of hydrogen production. Mon. Chem. Chem. Mon. 2018, 149, 475–482. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Fateev, S.A.; Zvereva, I.A. Synthesis of a New Layered Rb2Nd2Ti3O10 Oxide, Its Hydration and Protonation. Glas. Phys. Chem. 2017, 43, 593–596. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Sokolova, I.P.; Silyukov, O.I.; Burovikhina, A.A.; Fateev, S.A.; Zvereva, I.A. Protonation and Photocatalytic Activity of the Rb2La2Ti3O10 Layered Oxide in the Reaction of Hydrogen Production. Int. J. Photoenergy 2017, 2017, 9628146. [Google Scholar] [CrossRef] [Green Version]
- Silyukov, O.I.; Minich, I.A.; Zvereva, I.A. Synthesis of Protonated Derivatives of Layered Perovskite-Like Bismuth Titanates. Glas. Phys. Chem. 2018, 44, 115–119. [Google Scholar] [CrossRef]
- Kurnosenko, S.A.; Silyukov, O.I.; Zvereva, I.A. Preparation of Porous Particles of Layered Perovskite-Like Titanate HLaTiO4. Glas. Phys. Chem. 2020, 46, 272–276. [Google Scholar] [CrossRef]
- Byeon, S.; Kileung, P.; Park, K. Structure and Ionic Conductivity of NaLnTiO4, Comparison with Those of Na2Ln2Ti3O10 (Ln = La, Nd, Sm, and Gd). J. Solid State Chem. 1996, 121, 430–436. [Google Scholar] [CrossRef]
- Cui, W.; Liu, L.; Ma, S.; Liang, Y.; Zhang, Z. CdS-sensitized K2La2Ti3O10 composite: A new photocatalyst for hydrogen evolution under visible light irradiation. Catal. Today 2013, 207, 44–49. [Google Scholar] [CrossRef]
- Takahashi, S.; Nakato, T.; Hayashi, S.; Sugahara, Y.; Kuroda, K. Formation of Methoxy-Modified Interlayer Surface via the Reaction between Methanol and Layered Perovskite HLaNb2O7.cntdot.xH2O. Inorg. Chem. 1995, 34, 5065–5069. [Google Scholar] [CrossRef]
- Blasse, G. Crystallographic data of sodium lanthanide titanates (NaLnTiO4). J. Inorg. Nucl. Chem. 1968, 30, 656–658. [Google Scholar] [CrossRef]
- Byeon, S.; Yoon, J.J.; Lee, S.O. A New Family of Protonated Oxides HLnTiO4 (Ln = La, Nd, Sm, and Gd). J. Solid State Chem. 1996, 127, 119–122. [Google Scholar] [CrossRef]
- Pradhan, D.K.; Samantaray, B.K.; Choudhary, R.N.; Thakur, A.K. Complex impedance studies on a layered perovskite ceramic oxide—NaNdTiO4. Mater. Sci. Eng. B 2005, 116, 7–13. [Google Scholar] [CrossRef]
- Pradhan, D.K.; Samantaray, B.K.; Choudhary, R.N.; Thakur, A.K. Complex impedance analysis of NaLaTiO4 electroceramics. J. Mater. Sci. Mater. Electron. 2006, 17, 157–164. [Google Scholar] [CrossRef]
- Petrov, A.A.; Melnikova, N.A.; Petrov, A.V.; Silyukov, O.I.; Murin, I.V.; Zvereva, I.A. Experimental investigation and modelling of the Na+ mobility in NaLnTiO4 (Ln = La, Nd) ceramics. Ceram. Int. 2017, 43, 10861–10865. [Google Scholar] [CrossRef]
- Toda, K.; Kurita, S.; Sato, M.; Todaa, K.; Kuritab, S.; Satob, M. Synthesis and ionic conductivity of novel layered perovskite compounds, AgLaTiO4 and AgEuTiO4. Solid State Ion. 1995, 81, 267–271. [Google Scholar] [CrossRef]
- Toda, K.; Kameo, Y.; Kurita, S.; Sato, M. Crystal structure determination and ionic conductivity of layered perovskite compounds NaLnTiO4 (Ln = rare earth). J. Alloys Compd. 1996, 234, 19–25. [Google Scholar] [CrossRef]
- Singh, S.J.; Jayaram, R.V. Chemoselective O-tert-butoxycarbonylation of hydroxy compounds using NaLaTiO4 as a heterogeneous and reusable catalyst. Tetrahedron Lett. 2008, 49, 4249–4251. [Google Scholar] [CrossRef]
- Silyukov, O.I.; Abdulaeva, L.D.; Burovikhina, A.A.; Rodionov, I.A.; Zvereva, I.A. Phase transformations during HLnTiO4 (Ln = La, Nd) thermolysis and photocatalytic activity of obtained compounds. J. Solid State Chem. 2015, 226, 101–106. [Google Scholar] [CrossRef]
- Reddy, V.; Hwang, D.; Lee, J. Effect of Zr substitution for Ti in KLaTiO4 for photocatalytic water splitting. Catal. Lett. 2003, 90, 39–44. [Google Scholar] [CrossRef]
- Kawashima, K.; Hojamberdiev, M.; Chen, S.; Yubuta, K.; Wagata, H.; Domen, K.; Teshima, K. Understanding the effect of partial N3−-to-O2− substitution and H+-to-K+ exchange on photocatalytic water reduction activity of Ruddlesden–Popper layered perovskite KLaTiO4. Mol. Catal. 2017, 432, 250–258. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Silyukov, O.I.; Zvereva, I.A. Study of photocatalytic activity of layered oxides: NaNdTiO4, LiNdTiO4, and HNdTiO4 titanates. Russ. J. Gen. Chem. 2012, 82, 635–638. [Google Scholar] [CrossRef]
- Berdowski, P.A.; Blasse, G. Luminescence and energy migration in a two-dimensional system: NaEuTiO4. J. Lumin. 1984, 29, 243–260. [Google Scholar] [CrossRef]
- Ozawa, T.C.; Ikoshi, A.; Taniguchi, T.; Mizusaki, S.; Nagata, Y.; Noro, Y.; Samata, H.; Takayanagi, S. Low temperature magnetic properties of layered compounds: NaLnTiO4 (Ln = Sm, Eu, Gd, Tb, Dy, Ho and Er). J. Alloys Compd. 2008, 448, 38–43. [Google Scholar] [CrossRef]
- Ozawa, T.C.; Ikoshi, A.; Taniguchi, T.; Mizusaki, S.; Nagata, Y.; Noro, Y.; Samata, H.; Takayanagi, S. Magnetic spin interactions observed by heat capacity measurements for layered compounds: NaLnTiO4 (Ln = Sm, Eu, Gd, Tb, Dy, Ho and Er). J. Alloys Compd. 2008, 448, 64–68. [Google Scholar] [CrossRef]
- Tezuka, K.; Hinatsu, Y.; Preparation, S. Magnetic Properties of Layered Perovskites NaLnTiO4 (Ln, Sm, Eu, and Gd). J. Solid State Chem. 1998, 346, 342–346. [Google Scholar] [CrossRef]
- Silyukov, O.; Chislov, M.; Burovikhina, A.; Utkina, T.; Zvereva, I. Thermogravimetry study of ion exchange and hydration in layered oxide materials. J. Therm. Anal. Calorim. 2012, 110, 187–192. [Google Scholar] [CrossRef]
- Zvereva, I.A.; Silyukov, O.I.; Chislov, M.V. Ion-exchange reactions in the structure of perovskite-like layered oxides: I. Protonation of NaNdTiO4 complex oxide. Russ. J. Gen. Chem. 2011, 81, 1434–1441. [Google Scholar] [CrossRef]
- Nishimoto, S.; Matsuda, M.; Harjo, S.; Hoshikawa, A.; Ishigaki, T.; Kamiyama, T.; Miyake, M. Neutron diffraction study on protonated and hydrated layered perovskite. J. Solid State Chem. 2006, 179, 3308–3313. [Google Scholar] [CrossRef]
- Nishimoto, S.; Matsuda, M.; Harjo, S.; Hoshikawa, A.; Kamiyama, T.; Ishigaki, T.; Miyake, M. Structural change in a series of protonated layered perovskite compounds, HLnTiO4 (Ln = La, Nd and Y). J. Solid State Chem. 2006, 179, 1892–1897. [Google Scholar] [CrossRef]
- Abdulaeva, L.D.; Silyukov, O.I.; Petrov, Y.V.; Zvereva, I.A. Low-Temperature Transformations of Protonic Forms of Layered Complex Oxides HLnTiO4 and H2Ln2Ti3O10 (Ln = La, Nd). J. Nanomater. 2013, 2013, 514781. [Google Scholar] [CrossRef] [Green Version]
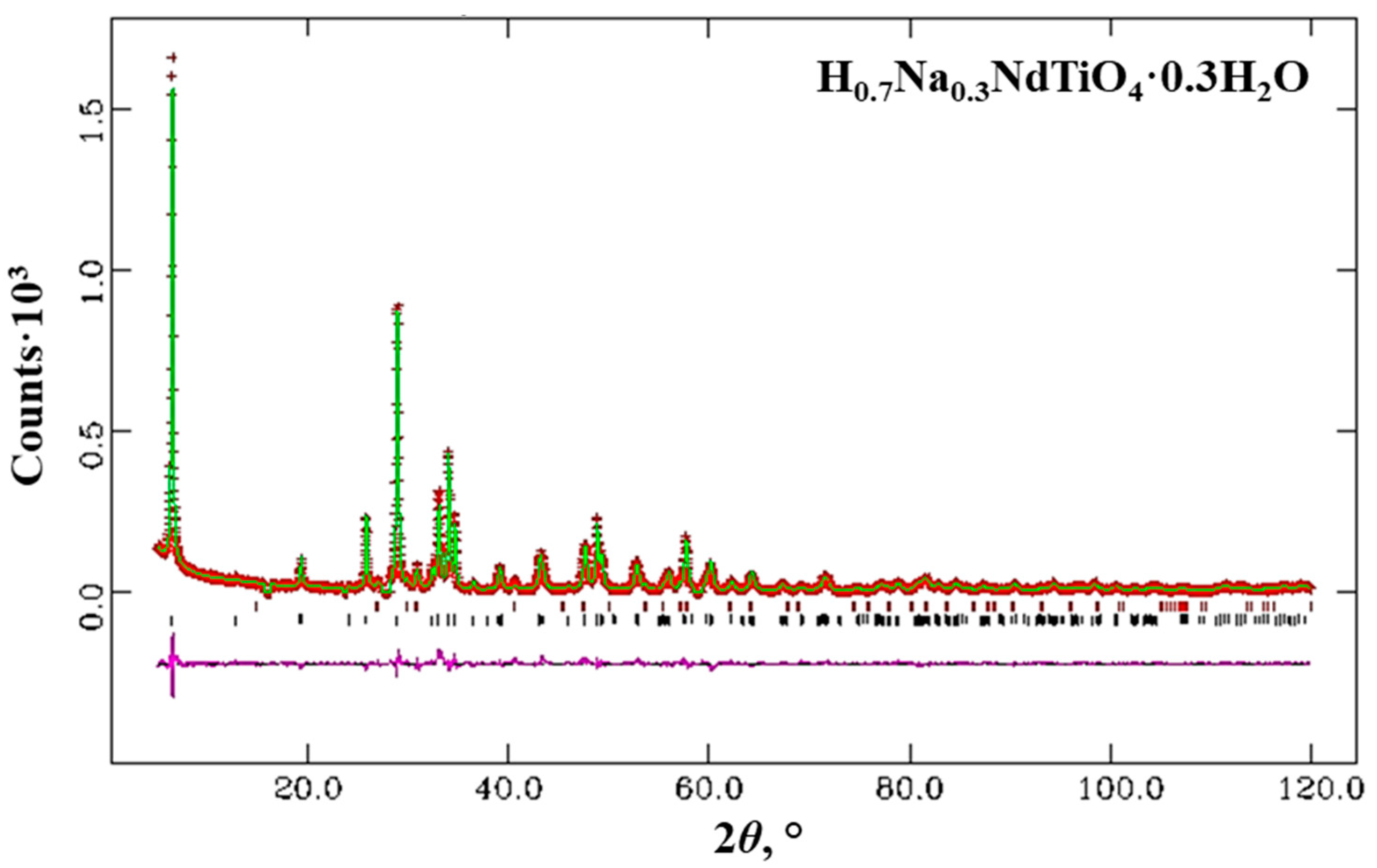

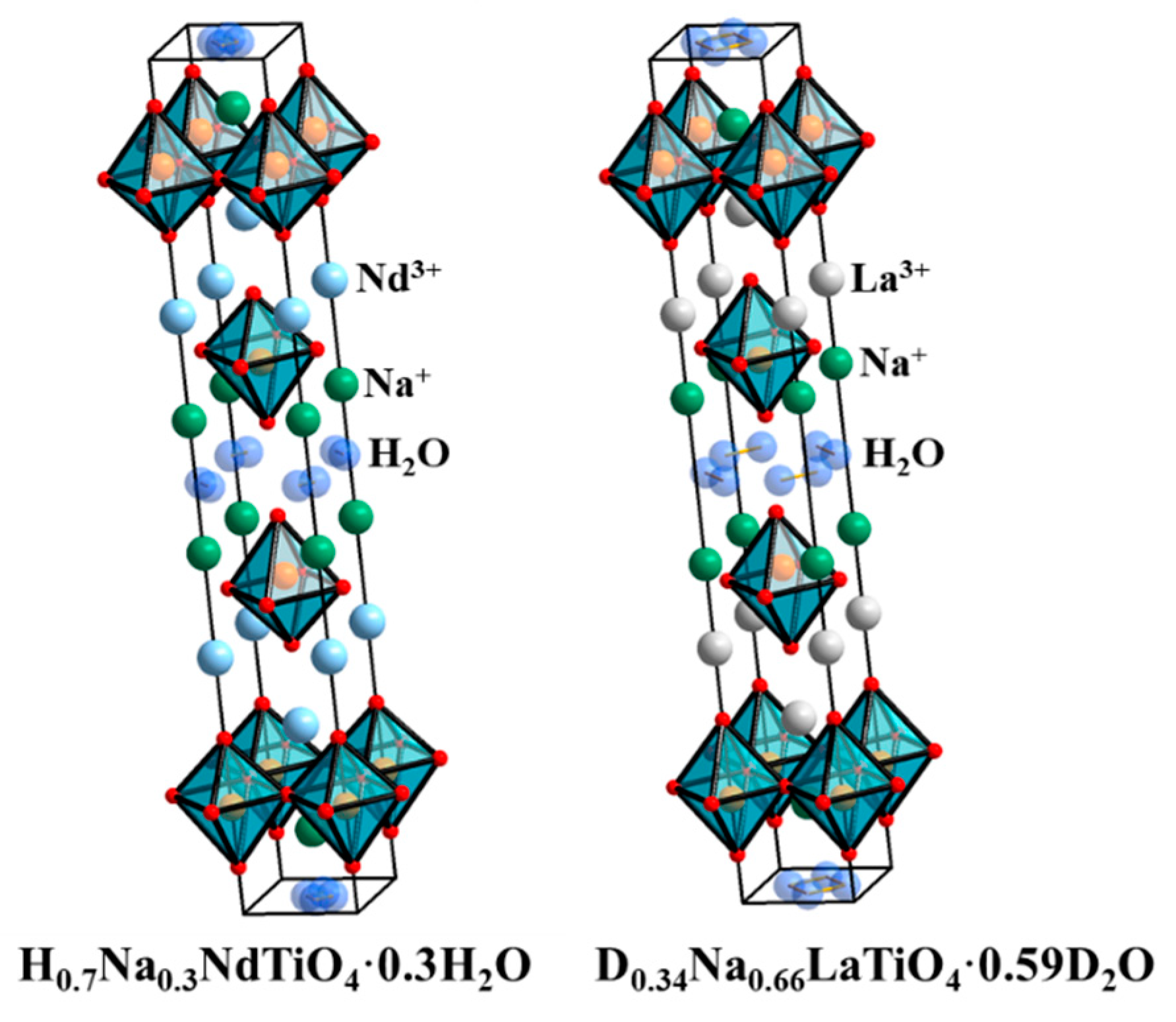
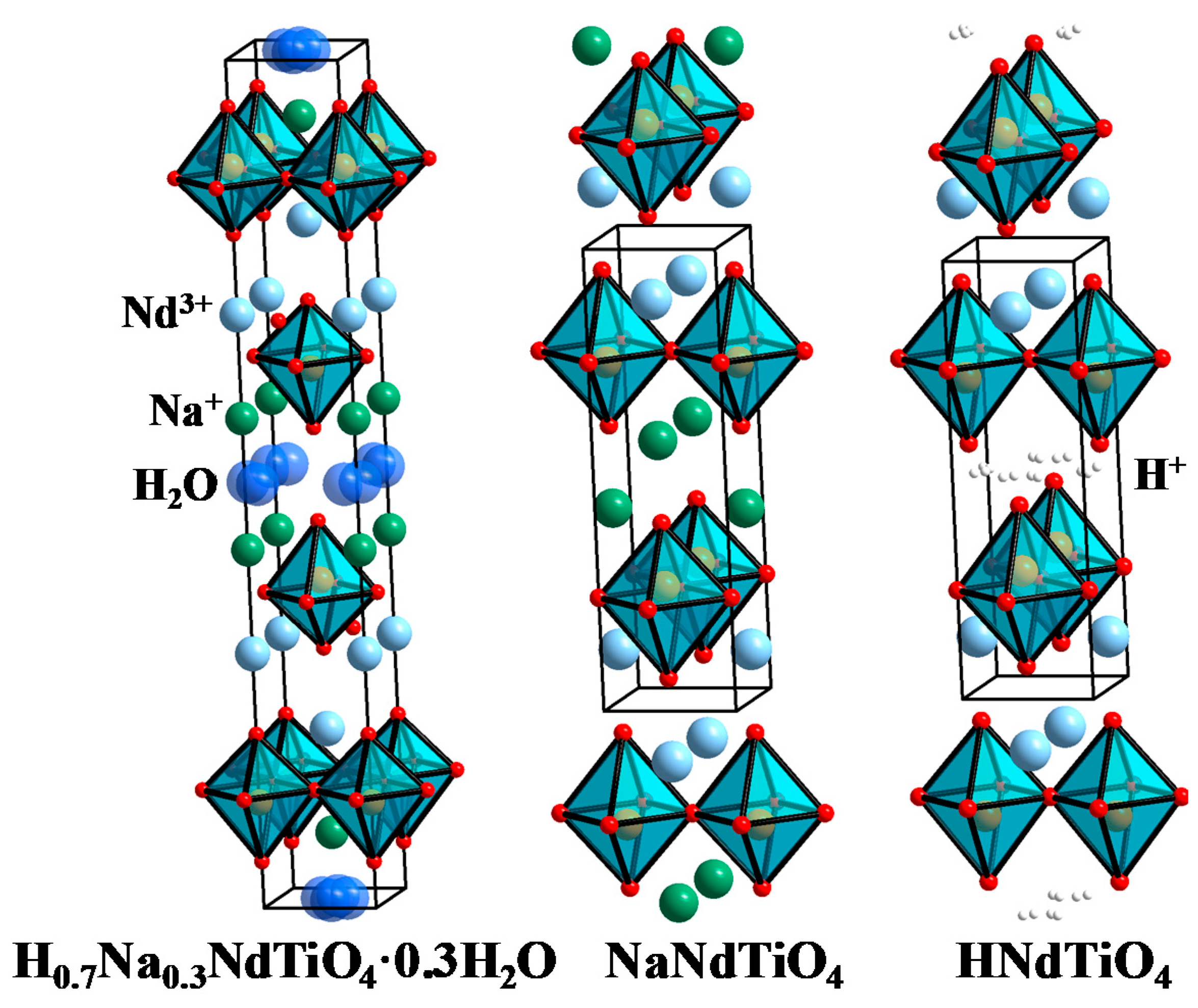
| H0.7Na0.3NdTiO4·0.3H2O Tetragonal System, I4/mmm, a = b = 3.7359 Å, c = 27.613 Å, Rwp = 0.1137, R(F2) = 0.0854 | ||||||
|---|---|---|---|---|---|---|
| Ion | Position | x | y | z | Uiso | Occupancy |
| Na | 4e | 0 | 0 | 0.4225 (25) | 0.043 (10) | 0.265 (14) |
| Nd | 4e | 0 | 0 | 0.30010 (7) | 0.0006 (25) | 1 |
| Ti | 4e | 0 | 0 | 0.12489 (17) | 0.004 (5) | 1 |
| O1 | 4e | 0 | 0 | 0.0551 (7) | 0.0036 (34) | 1 |
| O2 | 8g | 0 | 0.5 | 0.13925 (34) | 0.0084 (26) | 1 |
| O3 | 4e | 0 | 0 | 0.2048 (9) | 0.015 (4) | 1 |
| Ow | 8j | 0.388 (17) | 0.5 | 0.0 | 0.025 | 0.314 (14) |
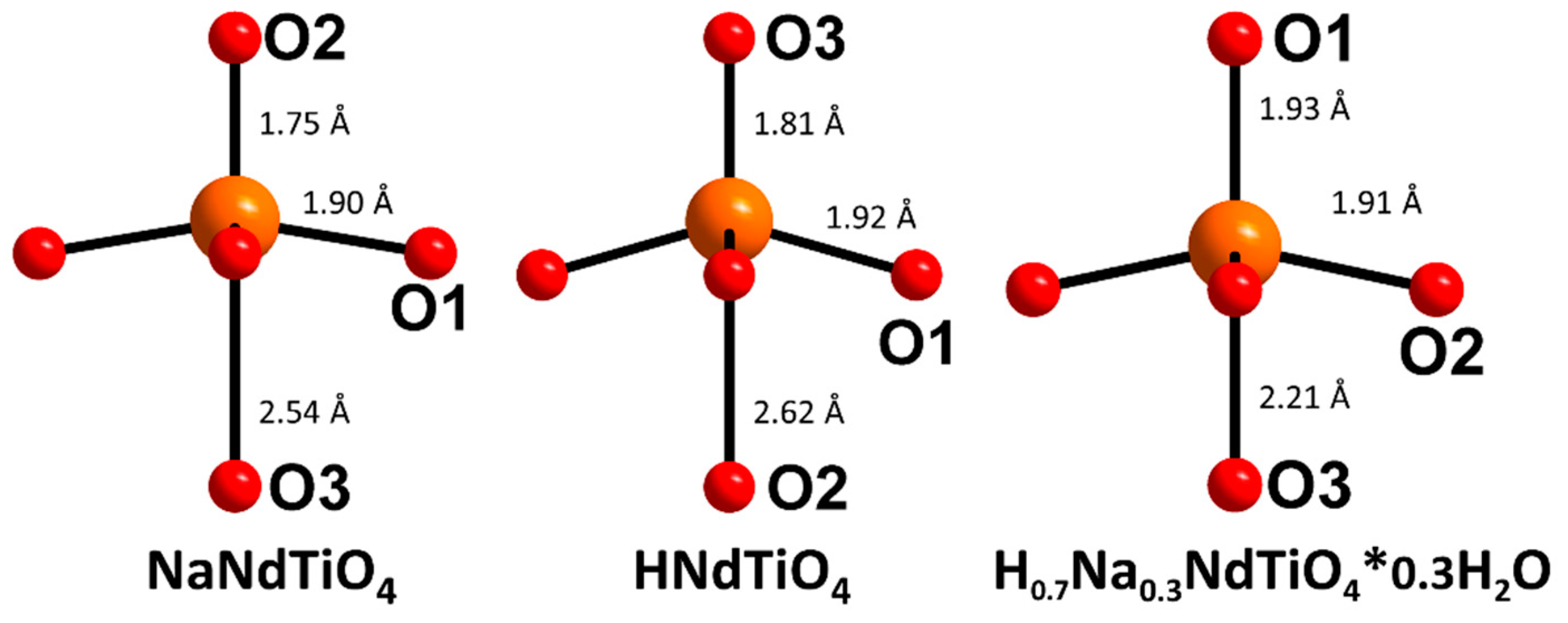
| HNdTiO4 Tetragonal system, P4/nmm a = b = 3.6983 Å, c = 12.0928 Å, Rwp = 0.0253, R(F2) = 0.0627 | ||||||
|---|---|---|---|---|---|---|
| Ion | Position | x | y | z | Uiso | Occupancy |
| H | 16e | 0.434 (12) | 0.133 (12) | 0.4966 (25) | 0.045 (13) | 0.125 |
| Nd | 2e | 0.25 | 0.25 | 0.8834 (5) | 0.0001 (13) | 1 |
| Ti | 2e | 0.25 | 0.25 | 0.2928 (8) | 0.0152 (35) | 1 |
| O1 | 4g | 0.25 | 0.75 | 0.2490 (5) | 0.0093 (15) | 1 |
| O2 | 2e | 0.25 | 0.25 | 0.0759 (6) | 0.0093 (15) | 1 |
| O3 | 2e | 0.25 | 0.25 | 0.4425 (8) | 0.0177 (23) | 1 |
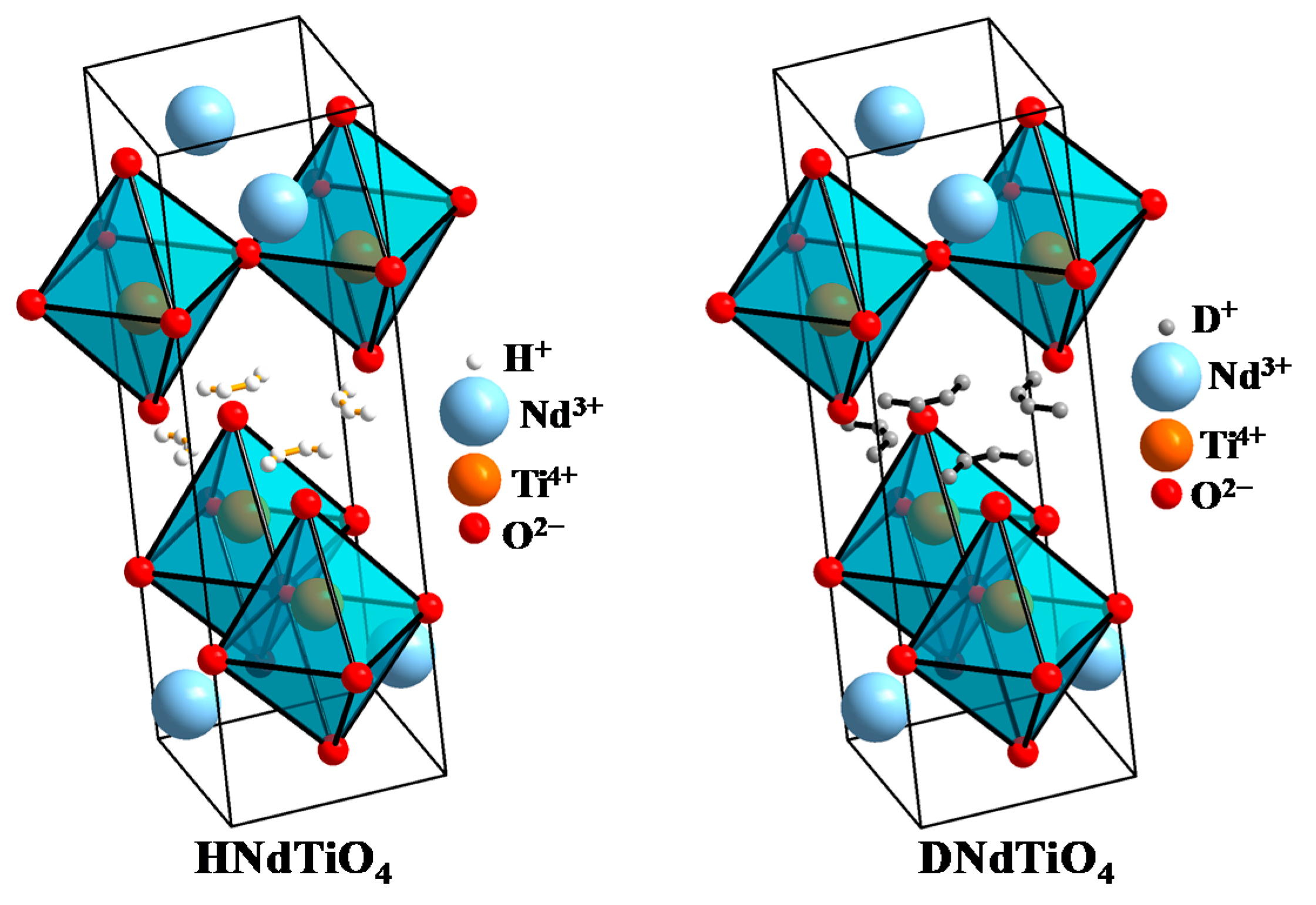
| HNdTiO4 | DNdTiO4 | ||
|---|---|---|---|
| Bond | Length, Å | Bond | Length, Å |
| H–O3 8x | 0.919 | D–O3 8x | 0.932 |
| Ti–O3 1x | 1.810 | Ti–O3 1x | 1.789 |
| Ti–O1 4x | 1.923 | Ti–O1 4x | 1.919 |
| Ti–O2 1x | 2.623 | Ti–O2 1x | 2.633 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silyukov, O.I.; Kurnosenko, S.A.; Minich, I.A.; Rodionov, I.A.; Zvereva, I.A. Protonated Forms of Layered Perovskite-Like Titanate NaNdTiO4: Neutron and X-ray Diffraction Structural Analysis. Solids 2021, 2, 265-277. https://doi.org/10.3390/solids2030017
Silyukov OI, Kurnosenko SA, Minich IA, Rodionov IA, Zvereva IA. Protonated Forms of Layered Perovskite-Like Titanate NaNdTiO4: Neutron and X-ray Diffraction Structural Analysis. Solids. 2021; 2(3):265-277. https://doi.org/10.3390/solids2030017
Chicago/Turabian StyleSilyukov, Oleg I., Sergey A. Kurnosenko, Iana A. Minich, Ivan A. Rodionov, and Irina A. Zvereva. 2021. "Protonated Forms of Layered Perovskite-Like Titanate NaNdTiO4: Neutron and X-ray Diffraction Structural Analysis" Solids 2, no. 3: 265-277. https://doi.org/10.3390/solids2030017
APA StyleSilyukov, O. I., Kurnosenko, S. A., Minich, I. A., Rodionov, I. A., & Zvereva, I. A. (2021). Protonated Forms of Layered Perovskite-Like Titanate NaNdTiO4: Neutron and X-ray Diffraction Structural Analysis. Solids, 2(3), 265-277. https://doi.org/10.3390/solids2030017







