Thermal Decomposition of [AH][M(HCOO)3] Perovskite-Like Formates
Abstract
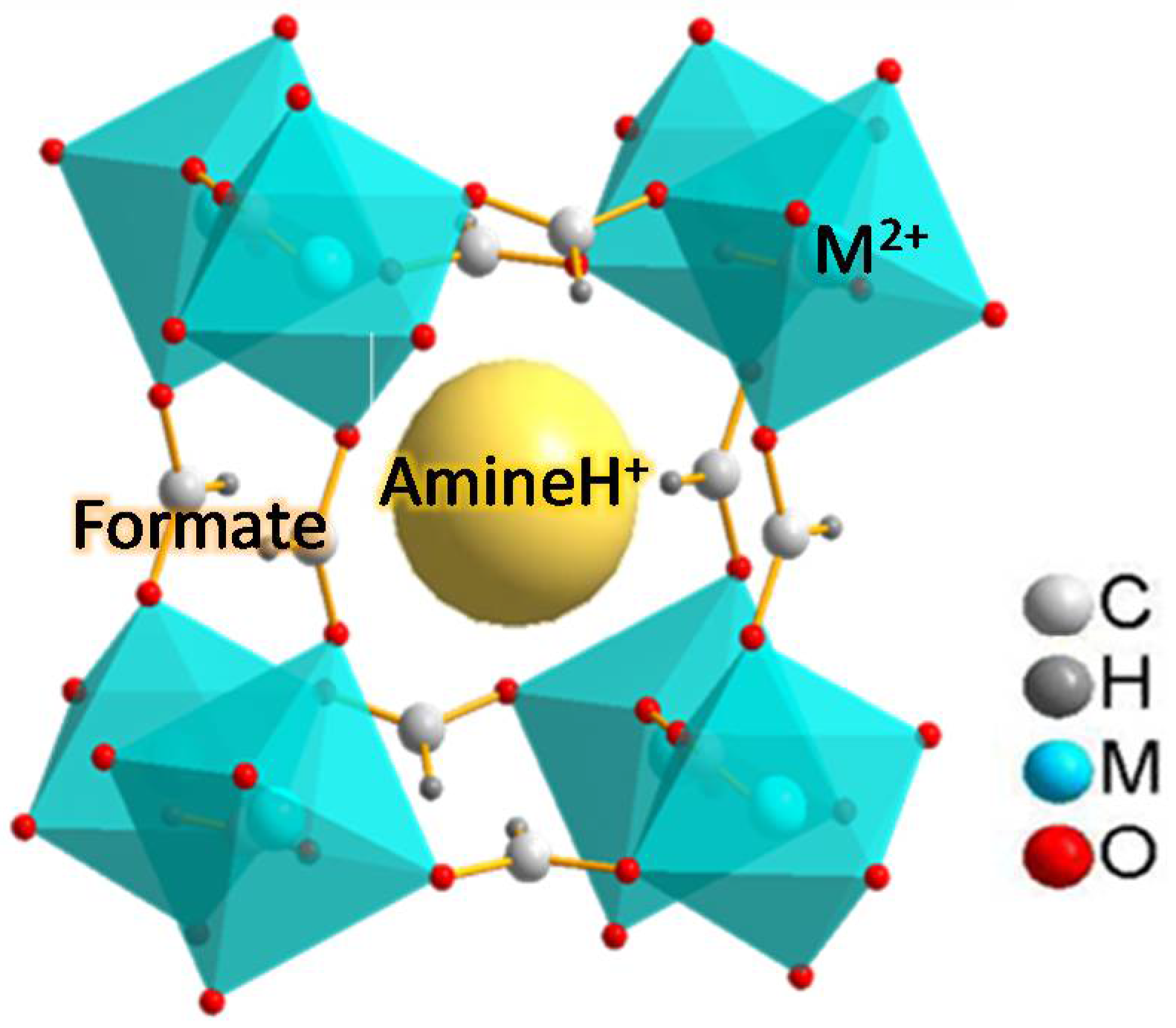
1. Introduction
2. Results
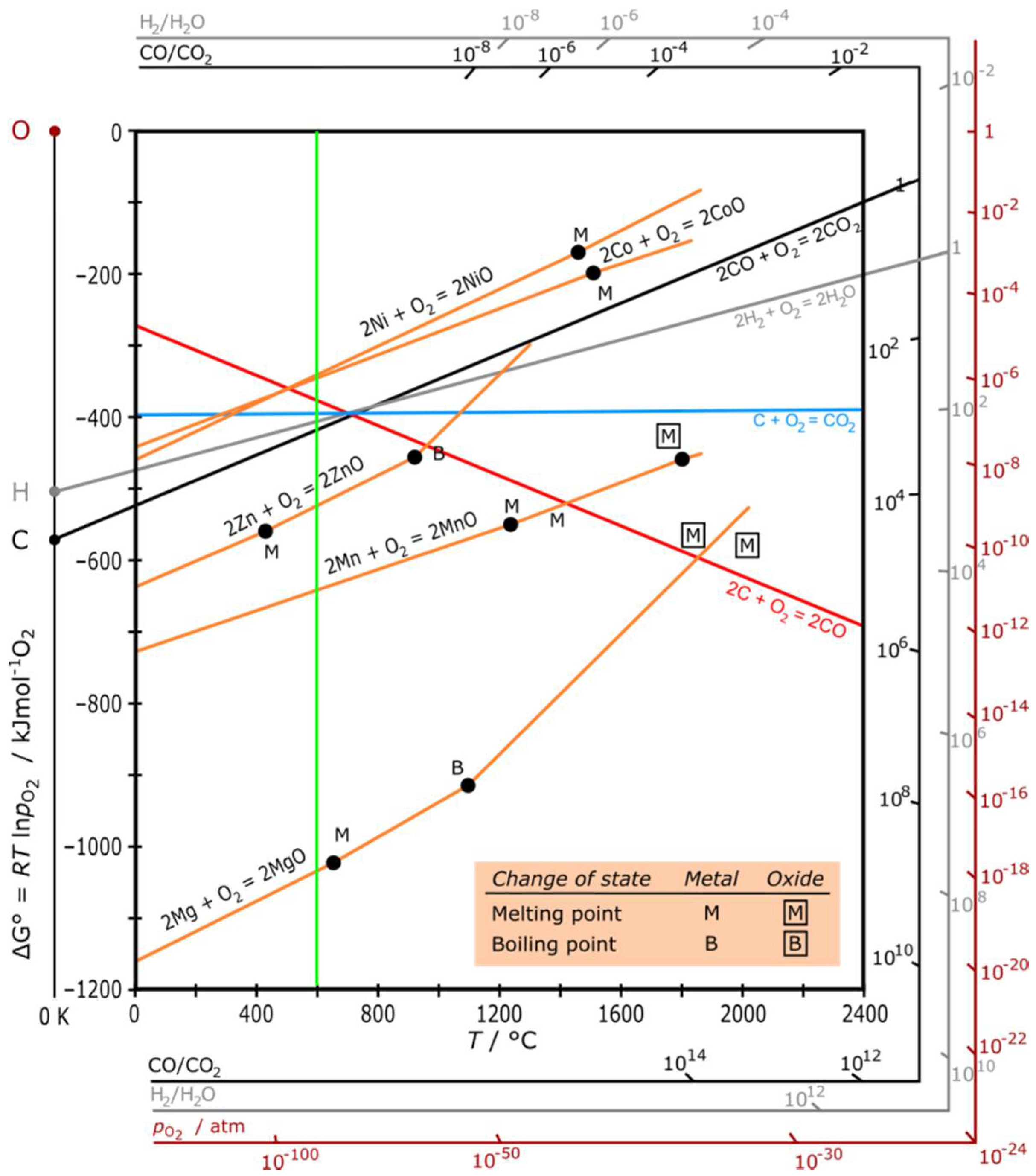
2.1. Role of Metal Cations on the Thermal Decomposition of [CH3NH3][M(HCOO)3] Perovskites
2.2. Role of A-Site Cation on the Thermal Decomposition of [AH][Cd(HCOO)3] Materials
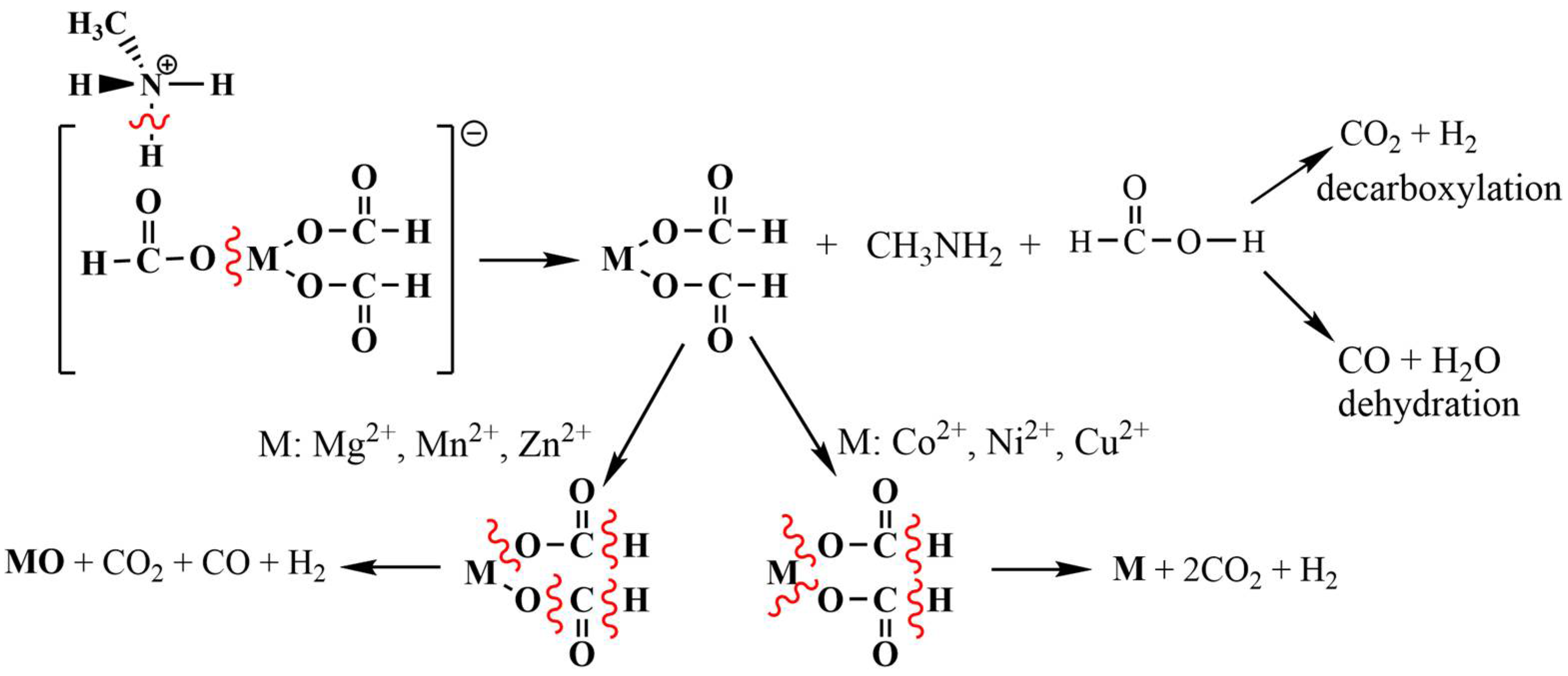
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, thermal and mechanical stabilities of metal-organic frameworks. Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Zhao, X.; Bu, X.; Wu, T.; Zheng, S.T.; Wang, L.; Feng, P. Selective anion exchange with nanogated isoreticular positive metal-organic frameworks. Nat. Commun. 2013, 4, 2344. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Demir, N.K.; Wu, Z.; Li, K. Highly Water-Stable Zirconium Metal-Organic Framework UiO-66 Membranes Supported on Alumina Hollow Fibers for Desalination. J. Am. Chem. Soc. 2015, 137, 6999–7002. [Google Scholar] [CrossRef] [PubMed]
- Henninger, S.K.; Habib, H.A.; Janiak, C. MOFs as adsorbents for low temperature heating and cooling applications. J. Am. Chem. Soc. 2009, 131, 2776–2777. [Google Scholar] [CrossRef]
- Cadiau, A.; Lee, J.S.; Damasceno Borges, D.; Fabry, P.; Devic, T.; Wharmby, M.T.; Martineau, C.; Foucher, D.; Taulelle, F.; Jun, C.H.; et al. Design of Hydrophilic Metal Organic Framework Water Adsorbents for Heat Reallocation. Adv. Mater. 2015, 27, 4775–4780. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhang, J.; Xue, Z.; Han, B.; Li, J.; Yang, G. Large-pore mesoporous Mn3O4 crystals derived from metal-organic frameworks. Chem. Commun. 2013, 49, 11695–11697. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Park, S.; Oh, M. Coordination polymer nanorods of Fe-MIL-88B and their utilization for selective preparation of hematite and magnetite nanorods. Chem. Commun. 2011, 47, 4138–4140. [Google Scholar] [CrossRef]
- Li, J.; Xia, W.; Tang, J.; Tan, H.; Wang, J.; Kaneti, Y.V.; Bando, Y.; Wang, T.; He, J.; Yamauchi, Y. MOF nanoleaves as new sacrificial templates for the fabrication of nanoporous Co-N:X/C electrocatalysts for oxygen reduction. Nanoscale Horiz. 2019, 4, 1006–1013. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Sun, L.; Wang, L. Metal/metal oxide nanostructures derived from metal-organic frameworks. RSC Adv. 2015, 5, 7267–7279. [Google Scholar] [CrossRef]
- Gómez-Aguirre, L.C.; Castro-García, S.; Sánchez-Andújar, M.; Yáñez-Vilar, S.; Mira, J.; Bermúdez-García, J.M.; Centeno, T.A.; Señarís-Rodríguez, M.A. A Facile Synthesis of Co3O4 Hollow Microtubes by Decomposition of a Cobalt Metal-Organic Framework. Eur. J. Inorg. Chem. 2016, 2016, 4463–4469. [Google Scholar] [CrossRef]
- Oar-Arteta, L.; Wezendonk, T.; Sun, X.; Kapteijn, F.; Gascon, J. Metal organic frameworks as precursors for the manufacture of advanced catalytic materials. Mater. Chem. Front. 2017, 1, 1709–1745. [Google Scholar] [CrossRef]
- Sun, J.K.; Xu, Q. Functional materials derived from open framework templates/precursors: Synthesis and applications. Energy Environ. Sci. 2014, 7, 2071–2100. [Google Scholar] [CrossRef]
- Ansari, S.N.; Saraf, M.; Gupta, A.K.; Mobin, S.M. Functionalized Cu-MOF@CNT Hybrid: Synthesis, Crystal Structure and Applicability in Supercapacitors. Chem. Asian J. 2019, 14, 3566–3571. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Z.; Li, X.; Sun, Q.; Cheng, N.; Lawes, S.; Sun, X. Metal organic frameworks for energy storage and conversion. Energy Storage Mater. 2016, 2, 35–62. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Hu, H.; Yang, Q.; Cai, J. From metal-organic frameworks to porous carbon materials: Recent progress and prospects from energy and environmental perspectives. Nanoscale 2020, 12, 4238–4268. [Google Scholar] [CrossRef]
- Jain, P.; Dalal, N.S.; Toby, B.H.; Kroto, H.W.; Cheetham, A.K. Order-disorder antiferroelectric phase transition in a hybrid inorganic-organic framework with the perovskite architecture. J. Am. Chem. Soc. 2008, 130, 10450–10451. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Andújar, M.; Presedo, S.; Yáñez-Vilar, S.; Castro-García, S.; Shamir, J.; Señarís-Rodríguez, M.A. Characterization of the order-disorder dielectric transition in the hybrid organic-inorganic perovskite-like formate Mn(HCOO)3[(CH3)2NH2]. Inorg. Chem. 2010, 49, 1510–1516. [Google Scholar] [CrossRef]
- Pato-Doldán, B.; Sánchez-Andújar, M.; Gómez-Aguirre, L.C.; Yáñez-Vilar, S.; López-Beceiro, J.; Gracia-Fernández, C.; Haghighirad, A.A.; Ritter, F.; Castro-García, S.; Senaris-Rodriguez, M.A. Near room temperature dielectric transition in the perovskite formate framework [(CH3)2NH2][Mg(HCOO)3]. Phys. Chem. Chem. Phys. 2012, 14, 8498–8501. [Google Scholar] [CrossRef]
- Pato-Doldán, B.; Gómez-Aguirre, L.C.; Hansen, A.P.; Mira, J.; Castro-García, S.; Sánchez-Andújar, M.; Señarís-Rodríguez, M.A.; Zapf, V.S.; Singleton, J. Magnetic transitions and isotropic: Versus anisotropic magnetic behaviour of [CH3NH3][M(HCOO)3] M = Mn2+, Co2+, Ni2+, Cu2+ metal-organic perovskites. J. Mater. Chem. C 2016, 4, 11164–11172. [Google Scholar] [CrossRef]
- Hu, K.-L.; Kurmoo, M.; Wang, Z.; Gao, S. Metal-organic perovskites: Synthesis, structures, and magnetic properties of [C(NH2)3][M(II)(HCOO)3] (M = Mn, Fe, Co, Ni, Cu, and Zn; C(NH2)3 = guanidinium). Chem. Eur. J. 2009, 15, 12050–12064. [Google Scholar] [CrossRef] [PubMed]
- Rogez, G.; Viart, N.; Drillon, M. Multiferroic materials: The attractive approach of metal-organic frameworks (MOFs). Angew. Chem. Int. Ed. 2010, 49, 1921–1923. [Google Scholar] [CrossRef] [PubMed]
- Pato-Doldán, B.; Gómez-Aguirre, L.C.; Bermúdez-García, J.M.; Sánchez-Andújar, M.; Fondado, A.; Mira, J.; Castro-García, S.; Señaris-Rodriguez, M.A. Coexistence of magnetic and electrical order in the new perovskite-like (C3N2H5)[Mn(HCOO)3] formate. RSC Adv. 2013, 3, 22404–22411. [Google Scholar] [CrossRef]
- Gómez-Aguirre, L.C.; Pato-Doldán, B.; Mira, J.; Castro-García, S.; Señarís-Rodríguez, M.A.; Sánchez-Andújar, M.; Singleton, J.; Zapf, V.S.; Senñarís-Rodríguez, M.A.; Sánchez-Andújar, M.; et al. Magnetic Ordering-Induced Multiferroic Behavior in [CH3NH3][Co(HCOO)3] Metal-Organic Framework. J. Am. Chem. Soc. 2016, 138, 1122–1125. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Stroppa, A.; Wang, Z.; Gao, S. Hybrid Organic-Inorganic Perovskites; Wiley-VCH Verlag: Weinheim, Germany, 2020; ISBN 978-3-527-34431-4. [Google Scholar]
- Baraldi, P. Thermal behaviour of metal carboxylates: Metal formates. Spectrochim. Acta Part A Mol. Spectrosc. 1979, 35, 1003–1007. [Google Scholar] [CrossRef]
- Dollimore, D.; Tonge, K.H. The thermal decomposition of zinc and manganous formates. J. Inorg. Nucl. Chem. 1967, 29, 621–627. [Google Scholar] [CrossRef]
- Dollimore, D.; Gupta, J.P.; Nowell, D.V. The thermal decomposition of metal formates. II. Solid state thermal decomposition studies on magnesium formate dihydrate. Thermochim. Acta 1979, 30, 339–350. [Google Scholar] [CrossRef]
- Rozenberg, A.S.; Aleksandrova, E.I. Thermal decomposition of transition metal carboxylates: 1. Decomposition of anhydrous copper(II) formate. Morphology and regularities of gas evolution. Russ. Chem. Bull. 1996, 45, 64–68. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: webbook.nist.gov/chemistry/ (accessed on 14 April 2020).
- Shishido, S.; Masuda, Y. The Gaseous Products formed in the Thermal Decompositions of Formates. Nippon Kagaku Kaishi 1973, 1973, 185–188. [Google Scholar] [CrossRef][Green Version]
- Małecka, B.; Łącz, A. Thermal decomposition of cadmium formate in inert and oxidative atmosphere. Thermochim. Acta 2008, 479, 12–16. [Google Scholar] [CrossRef]
- McBreen, P.H.; Serghini-Monim, S.; Roy, D.; Adnot, A. Decomposition of formic acid on FeTi. Surf. Sci. 1988, 195. [Google Scholar] [CrossRef]
- Das, R.; Pachfule, P.; Banerjee, R.; Poddar, P. Metal and Metal Oxide Nanoparticle Synthesis from Metal Organic Frameworks (MOFs): Finding the Border of Metal and Metal Oxides. Nanoscale 2012, 4, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aguirre, L.C.; Pato-Doldán, B.; Stroppa, A.; Yáñez-Vilar, S.; Bayarjargal, L.; Winkler, B.; Castro-García, S.; Mira, J.; Sánchez-Andújar, M.; Señarís-Rodríguez, M.A. Room-Temperature Polar Order in [NH4][Cd(HCOO)3]—A Hybrid Inorganic–Organic Compound with a Unique Perovskite Architecture. Inorg. Chem. 2015, 54, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Takeuchi, Y.; Fujino, K. X-Ray Determination of Electron-Density Distributions in Oxides, MgO, MnO, CoO, and NiO, and Atomic Scattering Factors of their Constituent Atoms. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 1979, 55, 43–48. [Google Scholar] [CrossRef]
- Kihara, K.; Donnay, G. Anharmonic thermal vibrations in ZnO. Can. Mineral. 1985, 23, 647–654. [Google Scholar]
- Owen, E.A.; Yates, E.L. LXVI. X-ray measurement of the thermal expansion of pure nickel. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1936, 21, 809–819. [Google Scholar] [CrossRef]
- Rutt, O.J.; Williams, G.R.; Clarke, S.J. Reversible lithium insertion and copper extrusion in layered oxysulfides. Chem. Commun. 2006, 2869–2871. [Google Scholar] [CrossRef] [PubMed]
- Wyckoff, R.W.G. Cubic closest packed, ccp. Struct. Cryst. Struct. 1963, 1, 33. [Google Scholar]
- Viertelhaus, M.; Anson, C.E.; Powell, A.K. Solvothermal synthesis and crystal structure of one-dimensional chains of anhydrous zinc and magnesium formate. Zeitschrift Anorg. Allg. Chemie 2005, 631, 2365–2370. [Google Scholar] [CrossRef]
- Viertelhaus, M.; Henke, H.; Anson, C.E.; Powell, A.K. Solvothermal synthesis and structure of anhydrous manganese(II) formate, and its topotactic dehydration from manganese(II) formate dihydrate. Eur. J. Inorg. Chem. 2003, 2003, 2283–2289. [Google Scholar] [CrossRef]
- Zachariasen, W.H. Untersuchungen über die Kristallstruktur von Sesquioxyden und Verbindungen ABO3. In Proceedings of the Skrifter Utgitt av Det Norske Videnskaps-Akademi i Oslo 1: Matematisk- Naturvidenskapelig Klasse; i kommisjon HJ Dybwad; Det Norske Videnskaps-Akademi: Oslo, Norway, 1928. [Google Scholar]
- Walmsley, H.P. The structure of the smoke particles from a cadmium arc. Proc. Phys. Soc. 1927, 40, 7. [Google Scholar] [CrossRef]
- Post, M.L.; Trotter, J. Cadmium(II) formate dihydrate. Acta Crystallogr. Sect. B 1974, 30, 1880–1882. [Google Scholar] [CrossRef]
- Weber, G. The structure of anhydrous cadmium formate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1980, 36, 1947–1949. [Google Scholar] [CrossRef]
- Graulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist crystal structure database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Linstrom, P.; Mallard, W. The NIST Chemistry WebBook: A Chemical Data Resource on the Internet. J. Chem. Eng. Data 2001, 46, 1059–1063. [Google Scholar] [CrossRef]
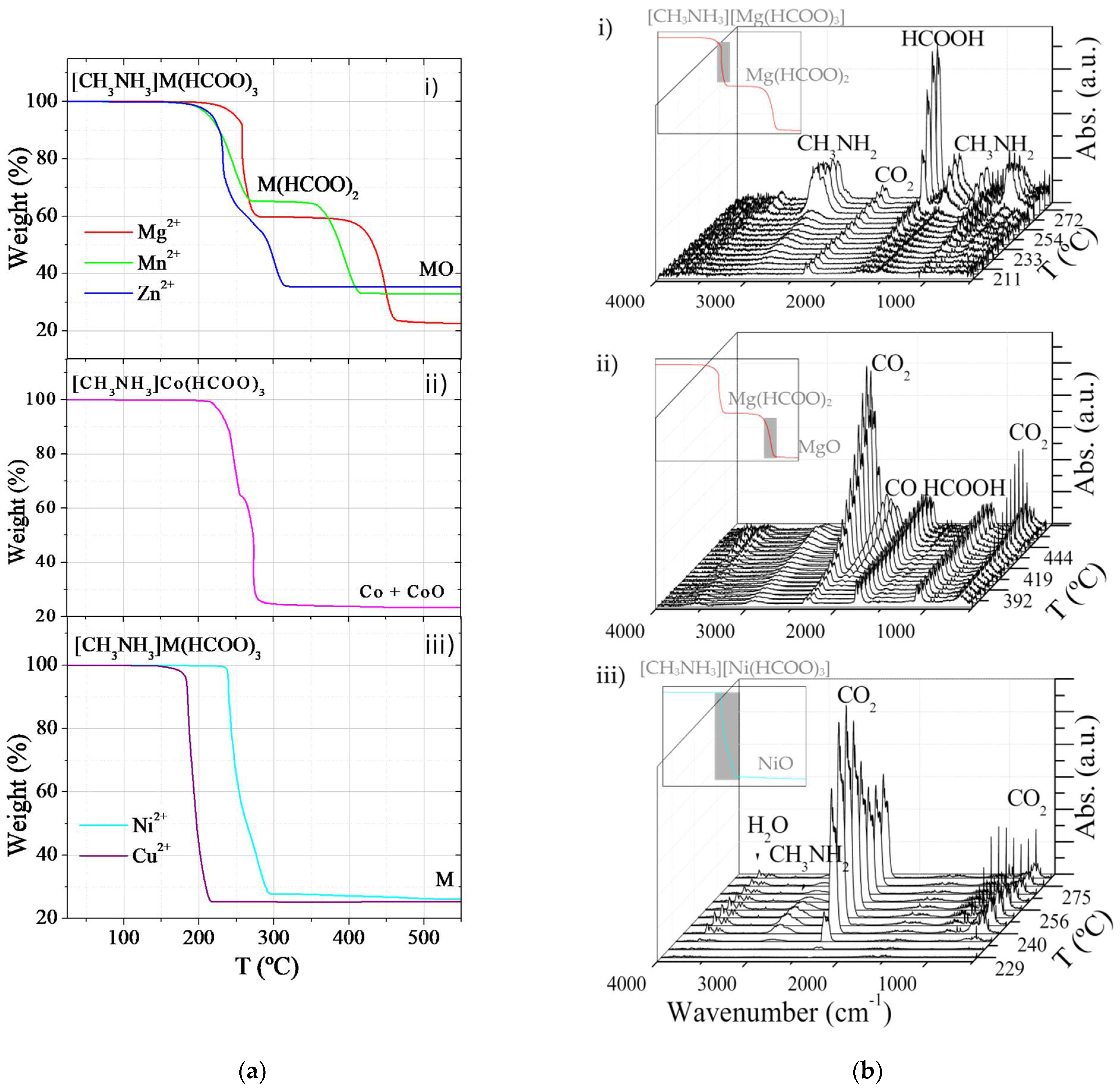


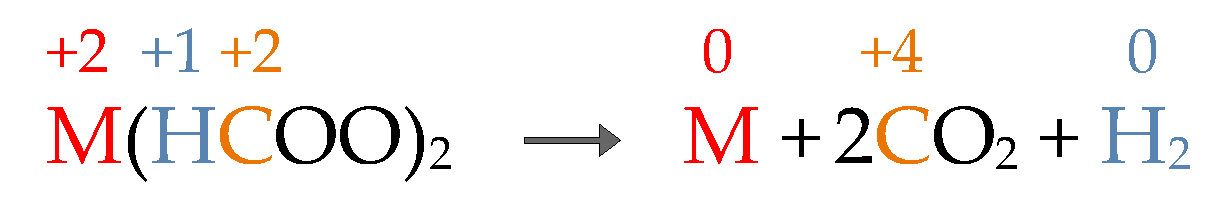
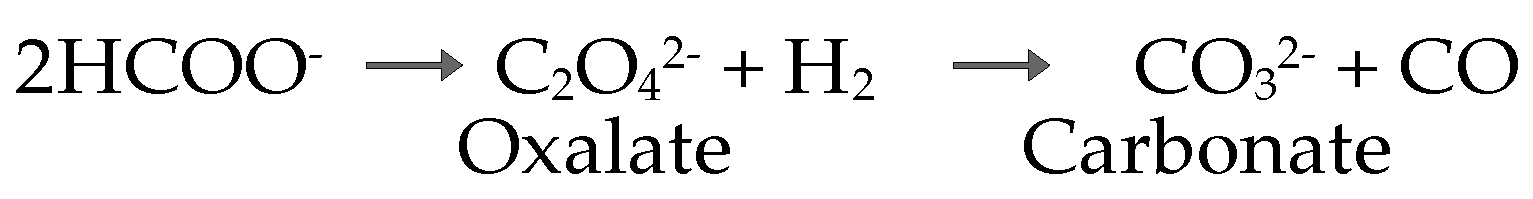
| M2+ | Step | T (°C) | Weight Loss (%) | Product | |
|---|---|---|---|---|---|
| Exp. | Calc. | ||||
| Mg2+ | 1 | 200–280 | 40.4 | 40.3 | Mg(HCOO)2 |
| 2 | 370–470 | 35.7 | 38.7 | MgO | |
| Mn2+ | 1 | 180–280 | 34.8 | 34.7 | Mn(HCOO)2 |
| 2 | 340–423 | 32.3 | 33.3 | MnO | |
| Co2+ | 1 | 215–254 | 33.8 | 34.1 | Co(HCOO)2* |
| 2 | 254–290 | 41.1 | 37.8 | 0.29Co + 0.71CoO | |
| Ni2+ | 1 | 230–280 | 72.2 | 74.0 | Ni |
| Cu2+ | 1 | 150–220 | 74.3 | 72.2 | Cu |
| Zn2+ | 1 | 160–240 | 32.8 | 33.2 | Zn(HCOO)2* |
| 2 | 240–320 | 32.0 | 31.8 | ZnO | |
| M2+ | Step No. | Main Released Gases |
|---|---|---|
| Mg2+ | 1 | CH3NH2, HCOOH, CO2 |
| 2 | CO2, CO, HCOOH | |
| Mn2+ | 1 | CH3NH2, CO2, HCOOH |
| 2 | CO2, CO, H2O | |
| Zn2+ | 1 | CH3NH2, CO2, H2O |
| 2 | CH3NH2, CO2, CO, HCOOH, H2O | |
| Co2+ | 1 | CO2, CH3NH2, H2O |
| 2 | CH3NH2, CO2, H2O, CO | |
| Ni2+ | 1 | CH3NH2, CO2, H2O |
| Cu2+ | 1 | CH3NH2, CO2, H2O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Aguirre, L.C.; Otero-Canabal, J.; Sánchez-Andújar, M.; Señarís-Rodríguez, M.A.; Castro-García, S.; Pato-Doldán, B. Thermal Decomposition of [AH][M(HCOO)3] Perovskite-Like Formates. Solids 2021, 2, 165-176. https://doi.org/10.3390/solids2020011
Gómez-Aguirre LC, Otero-Canabal J, Sánchez-Andújar M, Señarís-Rodríguez MA, Castro-García S, Pato-Doldán B. Thermal Decomposition of [AH][M(HCOO)3] Perovskite-Like Formates. Solids. 2021; 2(2):165-176. https://doi.org/10.3390/solids2020011
Chicago/Turabian StyleGómez-Aguirre, Lilián Claudia, Jorge Otero-Canabal, Manuel Sánchez-Andújar, María Antonia Señarís-Rodríguez, Socorro Castro-García, and Breogán Pato-Doldán. 2021. "Thermal Decomposition of [AH][M(HCOO)3] Perovskite-Like Formates" Solids 2, no. 2: 165-176. https://doi.org/10.3390/solids2020011
APA StyleGómez-Aguirre, L. C., Otero-Canabal, J., Sánchez-Andújar, M., Señarís-Rodríguez, M. A., Castro-García, S., & Pato-Doldán, B. (2021). Thermal Decomposition of [AH][M(HCOO)3] Perovskite-Like Formates. Solids, 2(2), 165-176. https://doi.org/10.3390/solids2020011







